
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 18 December 2017
Sec. Experimental Pharmacology and Drug Discovery
Volume 8 - 2017 | https://doi.org/10.3389/fphar.2017.00903
This article is part of the Research Topic Purinergic Pharmacology, Volume I View all 62 articles
 Masahiro Mishina1,2,3*
Masahiro Mishina1,2,3* Yuichi Kimura2,4
Yuichi Kimura2,4 Muneyuki Sakata2
Muneyuki Sakata2 Kenji Ishii2
Kenji Ishii2 Keiichi Oda2,5
Keiichi Oda2,5 Jun Toyohara2
Jun Toyohara2 Kazumi Kimura3
Kazumi Kimura3 Kiichi Ishiwata2,6,7
Kiichi Ishiwata2,6,7Adenosine A1 receptors (A1Rs) are widely distributed throughout the entire human brain, while adenosine A2A receptors (A2ARs) are present in dopamine-rich areas of the brain, such as the basal ganglia. A past study using autoradiography reported a reduced binding ability of A1R in the striatum of old rats. We developed positron emission tomography (PET) ligands for mapping the adenosine receptors and we successfully visualized the A1Rs using 8-dicyclopropylmethyl-1-11C-methyl-3-propylxanthine (11C-MPDX). We previously reported that the density of A1Rs decreased with age in the human striatum, although we could not observe an age-related change in A2ARs. The aim of this study was to investigate the age-related change of the density of A1Rs in the thalamus and cerebral cortices of healthy participants using 11C-MPDX PET. We recruited eight young (22.0 ± 1.7 years) and nine elderly healthy male volunteers (65.7 ± 8.0 years). A dynamic series of decay-corrected PET scans was performed for 60 min starting with the injection of 11C-MPDX. We placed the circular regions of interest of 10 mm in diameter in 11C-MPDX PET images. The values for the binding potential (BPND) of 11C-MPDX in the thalamus, and frontal, temporal, occipital, and parietal cortices were calculated using a graphical analysis, wherein the reference region was the cerebellum. BPND of 11C-MPDX was significantly lower in elderly participants than young participants in the thalamus, and frontal, temporal, occipital, and parietal cortices. In the human brain, we could observe the age-related decrease in the distribution of A1Rs.
Medical development has increased the average human lifespan (Vaupel, 2010). Cognitive functions such as memory often decline as humans age (van Geldorp et al., 2015), and aging is the major risk factor for Alzheimer’s disease (Fjell et al., 2014a). Human brain becomes atrophied with the aging (Fjell et al., 2014b), although brain atrophy remains mild in some elderly people called “superager” or “successful aging” whose cognitive functions remain intact with age (Depp and Jeste, 2006; Harrison et al., 2012; Sun et al., 2016).
Neuronal systems responsible for brain function are known to decline with age (Morrison and Baxter, 2012). In the human brain, neuroimaging studies revealed that endogenous dopamine, and dopamine transporter, D1 and D2 receptors and aromatic L-amino acid decarboxylase decrease with age (Suhara et al., 1991; Reeves et al., 2002; Ishibashi et al., 2009), while monoamine oxidase B increases with age (Reeves et al., 2002). Such age-related decrease has also been reported in the cholinergic, glutamatergic and γ-aminobutyric acid (GABA)ergic systems (Segovia et al., 2001; Rissman et al., 2007; Schliebs and Arendt, 2011).
In the adenosinergic system, animal studies reported that the age-related changes differ in the subtypes: adenosine A1 (A1R) and A2A receptors (A2AR) (Cunha et al., 1995, 2001; Lopes et al., 1999; Rebola et al., 2003; Meerlo et al., 2004). We developed ligands for positron emission tomography (PET) to map the adenosine receptors, and successfully visualized the A1Rs using 8-dicyclopropylmethyl-1-11C-methyl-3-propylxanthine (11C-MPDX, Figure 1) (Fukumitsu et al., 2005) and the A2ARs using [7-methyl-11C]-(E)-8-(3,4,5-trimethoxystyryl)-1,3,7-trimethylxanthine (11C-TMSX) (Ishiwata et al., 2000a,b, 2002). Using 11C-MPDX and 11C-TMSX PET, we previously reported that the density of A1Rs decreased with age in the human striatum, although we could not observe an age-related change in A2ARs (Mishina et al., 2012). In order to compare A1R and A2AR, we did not study the density of A1Rs other than striatum in the past paper (Mishina et al., 2012). Because A2ARs are enriched in the striatum (Fredholm and Svenningsson, 2003; Mishina et al., 2007), while A1Rs are widely distributed throughout the entire human brain (Fukumitsu et al., 2005). Another human PET study reported that the binding ability of 18F-8-cyclopentyl-3-(3-fluoropropyl)-1-propylxanthine (18F-CPFPX), an A1R ligand, was negatively correlated with age in the cerebral cortices and thalamus in addition to the striatum (Meyer et al., 2007). The A1Rs in the cerebral cortex are thought to help regulate the GABAergic and glutamatergic systems (Albasanz et al., 2002; Cunha-Reis et al., 2008; Ferreira et al., 2014), while A1Rs in the striatum are mainly responsible for the regulation of the D1 receptor in medium spiny neurons (Ferre et al., 1994; Yabuuchi et al., 2006). We hypothesized that the A1Rs may decrease with age in the thalamus and cerebral cortices. The aim of this study was to investigate the age-related change in the density of A1Rs in the thalamus and cerebral cortices of healthy participants using 11C-MPDX PET.
We recruited eight young healthy (mean age ± standard deviation [SD], 22.0 ± 1.7 years, age range, 20–25 years) and nine elderly male volunteers (65.7 ± 8.0 years, age range, 51–77 years). The participants were all Japanese and right-handed. None of the participants had a history of neurological diseases or any abnormalities upon physical or neurological examinations. Additionally, none took any medications known to affect the brain function or had a history of alcoholism. They had no medical history of bronchial asthma, and did not regularly use theophylline, a nonselective A1R and A2AR antagonist.
This study was approved by the Ethics Committee of Tokyo Metropolitan Institute of Gerontology. Written, informed consent was obtained from all participants in this study.
MRI was performed in the Tokyo Metropolitan Geriatric Hospital with three-dimensional spoiled gradient-recalled echo (SPGR) imaging and a SIGNA 1.5 Tesla machine (General Electric, Waukesha, WI, United States). The MRI images validated that the participants had no neurological diseases, such as stroke or brain tumors, and were used as a reference for placing the regions of interest (ROIs) on the PET images.
Positron emission tomography was performed in the Positron Medical Center, Tokyo Metropolitan Institute of Gerontology with a SET-2400W PET scanner (Shimadzu, Kyoto, Japan). The scanner had an axial field-of-view of 20 cm, acquired 63 slices at a center-to-center interval of 3.125 mm, and had a spatial resolution of 4.4 mm full width at half maximum (FWHM) and a z-axis resolution of 6.5 mm FWHM (Fujiwara et al., 1997). All participants were asked to abstain from caffeinated beverages, such as tea and coffee, and foods containing chocolate, for 12 h prior to undergoing the 11C-MPDX PET, because caffeine is a non-selective adenosine receptor antagonist (Statland and Demas, 1980). 11C-MPDX was prepared as described previously (Fukumitsu et al., 2005). To obtain an attenuation map to correct for photon attenuation, an 8-min transmission scan with a rotating 68Ga/68Ge line source was recorded before the radiotracer injection. Starting at the time of injection, a dynamic series of decay-corrected PET scans was performed for 60 min in a two-dimensional scanning mode. The injected dose of 11C-MPDX was 639 ± 77 MBq (16.0 ± 11.6 nmol). Specific activity at the time of injection ranged from 14.6 to 129.5 TBq/mmol (59.5 ± 36.8 TBq/mmol). The total number of frames was 27 and the frame arrangements were 6 × 10 s, 3 × 30 s, 5 × 1 min, 5 × 2.5 min, and 8 × 5 min.
Image analyses were carried out with the medical image processing software Dr. View/Linux R2.5 (AJS, Tokyo, Japan) implemented in CentOS 5.4 (The CentOS Project1) and Parallels Desktop 5.0.9344 (Parallels Holdings, Renton, WA, United States).
We generated early images, which were considered similar to images for cerebral blood flow, by summing frames from 0 to 10 min (Mishina et al., 2000). The MRI image was three-dimensionally registered to the early image of each participant. The early images and the registered MRI images were used as references for placing each ROI on the PET images from the dynamic scans. Circular ROIs with 10 mm in diameter were placed bilaterally on the PET images over the thalamus, frontal, temporal, occipital, and parietal cortex. We also placed the circular ROI over the cerebellar hemisphere as a reference region for kinetic analysis. Averaged tissue time activity curves (tTACs) were derived from the dynamic data and ROI, and data were used to calculate the standardized uptake value.
Kinetic analyses of the tTACs were performed using programs implemented on MATLAB version 7.04 (The Mathworks, Natick, MA, United States) and a General Kinetic Modeling Tool in PMOD 3.0 (PMOD Technologies, Zurich, Switzerland). The values for the binding potential (BPND) of 11C-MPDX (Kimura et al., 2004) in the regions were calculated using an averaged tTAC and a graphical analysis with the cerebellum as the reference region (Logan, 2003), where the k2 of the reference region was 0.23/min that was the averaged k2 as presented in the Table 1 of a past paper (Kimura et al., 2004) and the starting time for the analysis was 10 min after the administration. We confirmed that the BPND of 11C-MPDX was suitable for evaluating the distribution of A1Rs (Kimura et al., 2004).
Statistical computations were performed using the software package JMP Pro version 13.2.0 (SAS Institute, Cary, NC, United States). If the variance had a significant difference between young and elderly groups with Bartlett’s test, Welch’s t-test were used to compare the BPND of 11C-MPDX. If not, unpaired t-tests were used instead. We also used the regression analysis to compare the age with the BPND of participants. The level of significance was set at p < 0.05.
Figure 2 shows representative 11C-MPDX PET images. BPND of 11C-MPDX was significantly smaller in elderly participants than young participants in the thalamus (young vs. elderly; 0.43 ± 0.07 vs. 0.33 ± 0.06, p < 0.01; unpaired t-test), frontal (0.19 ± 0.04vs. 0.12 ± 0.04, p < 0.001; unpaired t-test), temporal (0.29 ± 0.05 vs. 0.21 ± 0.06, p < 0.05; unpaired t-test), occipital (0.31 ± 0.05 vs. 0.23 ± 0.06, p < 0.05; unpaired t-test), and parietal cortices (0.27 ± 0.05 vs. 0.20 ± 0.06, p < 0.05; unpaired t-test, Figure 3).
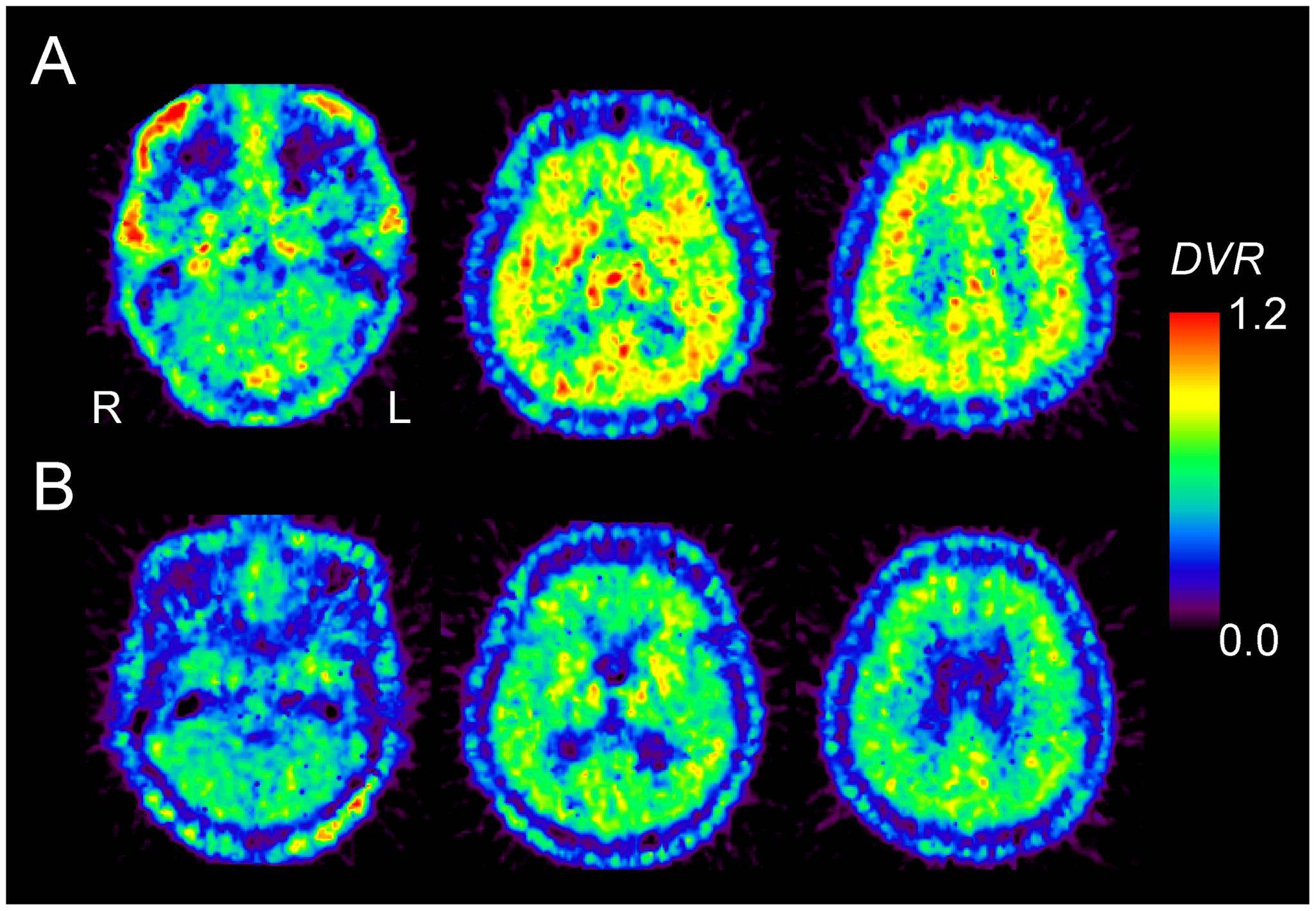
FIGURE 2. 11C-MPDX PET images for a 22-year-old male subject (A) and a 77-year-old male subject (B). The pixel values for the PET images of 11C-MPDX are visualized as the distribution volume ratio (DVR), because the brain anatomy is unclear in the BPND images of 11C-MPDX. Note that we use the values for the binding potential (BPND) in the kinetic analysis for 11C-MPDX PET in Figures 3 and 4.
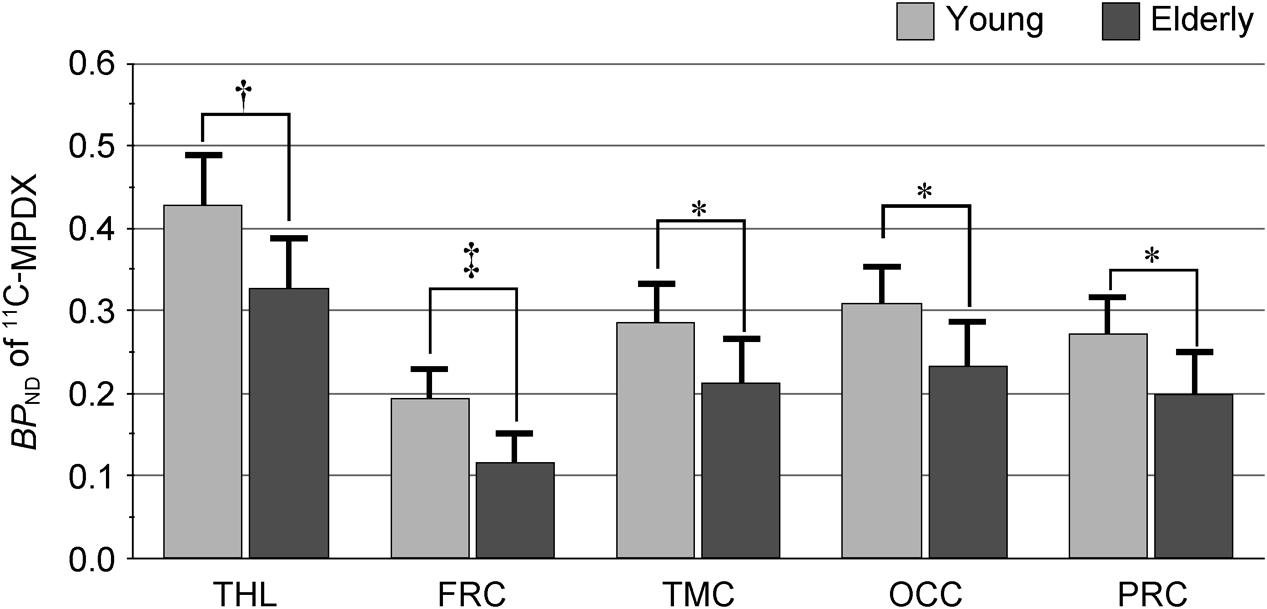
FIGURE 3. BPND of 11C-MPDX in the young and elderly participants. BPND of 11C-MPDX was significantly smaller in elderly participants than young participants in the thalamus, frontal, temporal, occipital, and parietal cortex. THL, thalamus; FRC, frontal cortex; TMC, temporal cortex; OCC, occipital cortex; PRC, parietal cortex. ∗p < 0.05, †p < 0.01, ‡p < 0.001, unpaired t-test.
Regression analyses also showed that the BPND of 11C-MPDX was negatively correlated with age in the thalamus (R2 = 0.458; p < 0.005, Figure 4A), and frontal (R2 = 0.500; p < 0.005, Figure 4B), temporal (R2 = 0.402; p < 0.01, Figure 4C), occipital (R2 = 0.338; p < 0.05, Figure 4D), and parietal cortices (R2 = 0.349; p < 0.05, Figure 4E).
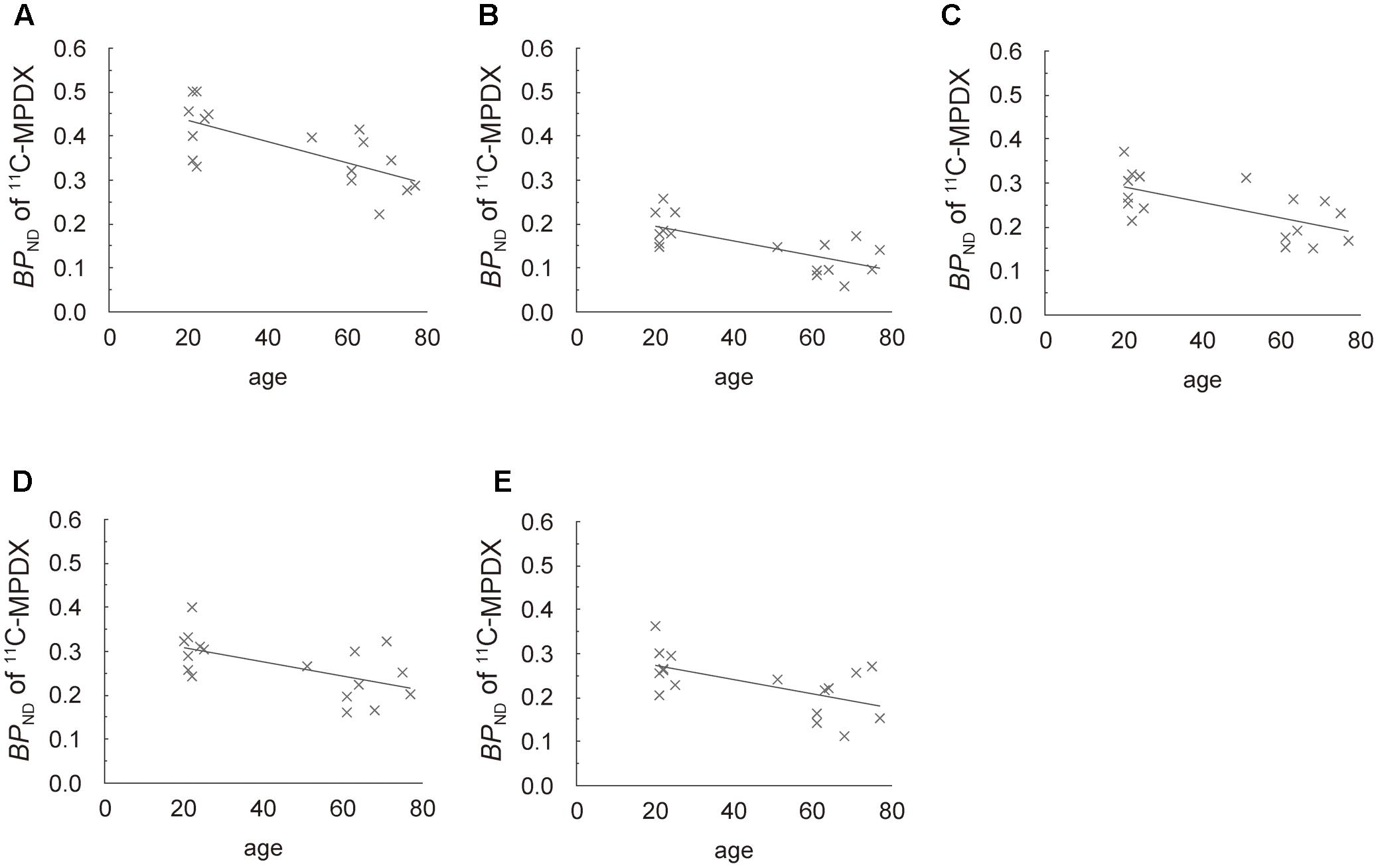
FIGURE 4. Scattergrams of age vs. BPND of 11C-MPDX. BPND of 11C-MPDX was negatively correlated with age in the thalamus (A), and frontal (B), temporal (C), occipital (D), and parietal cortices (E).
In the human thalamus and cerebral cortices, we observed an age-related decrease of the BPND of 11C-MPDX, which is in line with previous findings in the striatum (Mishina et al., 2012). Two reasons are considered to have reduced the BPND of 11C-MPDX, namely (1) reduced binding site and (2) increased endogenous adenosine. If the concentration of extracellular adenosine is increased, the estimated apparent BPND using 11C-MPDX is decreased by competition at the A1R between endogenous adenosine and 11C-MPDX. Extracellular adenosine levels in the striatum were not affected by age (Burnstock and Dale, 2015), although no data are available on adenosine in the cerebral cortex. In addition, the affinity of 11C-MPDX is higher than that of adenosine (Noguchi et al., 1997; Müller and Jacobson, 2011; Mishina and Ishiwata, 2014). These findings weaken the counter-hypothesis that the age-related decrease in the BPND of 11C-MPDX involved an increase in adenosine. Therefore, the results involved an age-related decrease in A1Rs in the human brain.
Some studies have revealed differences in age-related changes between A1Rs and A2ARs (Burnstock and Dale, 2015). The age-related changes vary in different brain regions. Cunha et al. (1995) studied age-related changes in rats, using [3H]2-[4-(2-p-carboxyethyl)phenylamino]-5′-N-ethylcarboxamidoadenosine (3H-CGS 21680) for A1Rs and [3H]-1,3-dipropyl-8-cyclopentylxanthine (3H-DPCPX) for A2ARs. In their study, A1Rs were decreased in the cerebral cortex and hippocampus, but A2ARs were increased only in the cerebral cortex of aged rats. No significant changes were observed in A1R of the striatum and in A2AR of both the hippocampus and the striatum. An autoradiography study using [3H]N6-cyclohexyladenosine demonstrated that there was an age-dependent reduction in A1Rs in most of the brain areas of rats, but that the degree of the reduction varied among regions (Meerlo et al., 2004). Efficiency of A2ARs to modulate synaptic transmission in the hippocampus was decreased in aged rats (Sebastiao et al., 2000), although the efficiency of A2ARs was increased by aging (Lopes et al., 1999; Rebola et al., 2003). It seems that there are age-related changes in the balance between inhibitory A1R- and excitatory A2AR-mediated actions.
A limitation the lack of data on participants’ daily sleep state. A1R and A2AR play an important role in regulating sleep (Basheer et al., 2000; Scammell et al., 2001; Urade et al., 2003; Elmenhorst et al., 2007; Oishi et al., 2008). Some studies showed that sleep deprivation increased A1R (Basheer et al., 2000; Elmenhorst et al., 2007). Another study suggested that endogenous adenosine suppressed the histaminergic system via A1R to promote non-rapid eye movement sleep (Oishi et al., 2008). Many elderly people are suffering from insomnia (Dijk et al., 2000; Ohayon et al., 2004; Colrain, 2011). The age-related changes to A1R may be associated with insomnia in elderly people.
Another limitation of this study was the lack of data on participants’ daily caffeine intake. In this study, we restricted caffeine consumption in the 12 h prior to performing PET scans, because caffeine is a non-selective adenosine receptor antagonist. Many elderly Japanese people habitually drink green tea after meals (Kuriyama et al., 2006), although the overall caffeine consumption is attributed more to coffee than to tea in Japan (Fredholm et al., 1999). Animal studies reported that chronic administration of caffeine increases the density of adenosine receptors (Green and Stiles, 1986; Nehlig et al., 1992; Li et al., 2008), although human data are sparse. Another limitation the lack of data on participants’ daily sleep state. Adenosine is involved in circadian rhythm and sleep (Bjorness and Greene, 2009), and adenosine inhibits the arousal system via A1R and induces sleep (Oishi et al., 2008). Elderly people often have sleep disorders. Further studies are needed to reveal the relationship between chronic caffeine consumption and A1R density.
Our study was only comprised of males. A post-mortem study reported adenosine level in the cerebral cortex was higher in male than in female (Kovacs et al., 2010). However, some papers showed that there was no significant gender effect on A1Rs in the human brain (Ulas et al., 1993; Glass et al., 1996; Meyer et al., 2007).
MM wrote the first draft of the manuscript. MM, KeI, KO, JT, and KiI performed PET examinations. YK and MS performed kinetic analyses. KK and KiI supervised the study. All authors reviewed, commented on, and approved the final report.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was funded by Grants-in-Aid for Scientific Research (B) No. 16390348, (B) No. 20390334, (C) No. 17590901, (C) No. 20591033, and (C) No. 23591287 from the Japan Society for the Promotion of Science.
Albasanz, J. L., Leon, D., Ruiz, M. A., Fernandez, M., and Martin, M. (2002). Adenosine A1 receptor agonist treatment up-regulates rat brain metabotropic glutamate receptors. Biochim. Biophys. Acta 1593, 69–75. doi: 10.1016/S0167-4889(02)00330-0
Basheer, R., Porkka-Heiskanen, T., Strecker, R. E., Thakkar, M. M., and McCarley, R. W. (2000). Adenosine as a biological signal mediating sleepiness following prolonged wakefulness. Biol. Signals Recept. 9, 319–327. doi: 10.1159/000014655
Bjorness, T. E., and Greene, R. W. (2009). Adenosine and sleep. Curr. Neuropharmacol. 7, 238–245. doi: 10.2174/157015909789152182
Burnstock, G., and Dale, N. (2015). Purinergic signalling during development and ageing. Purinergic Signal. 11, 277–305. doi: 10.1007/s11302-015-9452-9
Colrain, I. M. (2011). Sleep and the brain. Neuropsychol. Rev. 21, 1–4. doi: 10.1007/s11065-011-9156-z
Cunha, R. A., Constantino, M. C., Sebastiao, A. M., and Ribeiro, J. A. (1995). Modification of A1 and A2a adenosine receptor binding in aged striatum, hippocampus and cortex of the rat. Neuroreport 6, 1583–1588. doi: 10.1097/00001756-199507310-00029
Cunha, R. A., Constantino, M. D., Fonseca, E., and Ribeiro, J. A. (2001). Age-dependent decrease in adenosine A1 receptor binding sites in the rat brain. Effect of cis unsaturated free fatty acids. Eur. J. Biochem. 268, 2939–2947. doi: 10.1046/j.1432-1327.2001.02183.x
Cunha-Reis, D., Ribeiro, J. A., and Sebastiao, A. M. (2008). A1 and A2A receptor activation by endogenous adenosine is required for VIP enhancement of K+-evoked [3H]-GABA release from rat hippocampal nerve terminals. Neurosci. Lett. 430, 207–212. doi: 10.1016/j.neulet.2007.10.037
Depp, C. A., and Jeste, D. V. (2006). Definitions and predictors of successful aging: a comprehensive review of larger quantitative studies. Am. J. Geriatr. Psychiatry 14, 6–20. doi: 10.1097/01.JGP.0000192501.03069.bc
Dijk, D. J., Duffy, J. F., and Czeisler, C. A. (2000). Contribution of circadian physiology and sleep homeostasis to age-related changes in human sleep. Chronobiol. Int. 17, 285–311. doi: 10.1081/CBI-100101049
Elmenhorst, D., Meyer, P. T., Winz, O. H., Matusch, A., Ermert, J., Coenen, H. H., et al. (2007). Sleep deprivation increases A1 adenosine receptor binding in the human brain: a positron emission tomography study. J. Neurosci. 27, 2410–2415. doi: 10.1523/JNEUROSCI.5066-06.2007
Ferre, S., Popoli, P., Gimenez-Llort, L., Finnman, U. B., Martinez, E., Scotti de Carolis, A., et al. (1994). Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport 6, 73–76. doi: 10.1097/00001756-199412300-00020
Ferreira, D. D., Stutz, B., de Mello, F. G., Reis, R. A., and Kubrusly, R. C. (2014). Caffeine potentiates the release of GABA mediated by NMDA receptor activation: involvement of A1 adenosine receptors. Neuroscience 281, 208–215. doi: 10.1016/j.neuroscience.2014.09.060
Fjell, A. M., McEvoy, L., Holland, D., Dale, A. M., Walhovd, K. B., and Alzheimer’s Disease Neuroimaging Initiative (2014a). What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog. Neurobiol. 117, 20–40. doi: 10.1016/j.pneurobio.2014.02.004
Fjell, A. M., Westlye, L. T., Grydeland, H., Amlien, I., Espeseth, T., Reinvang, I., et al. (2014b). Accelerating cortical thinning: unique to dementia or universal in aging? Cereb. Cortex 24, 919–934. doi: 10.1093/cercor/bhs379
Fredholm, B. B., Battig, K., Holmen, J., Nehlig, A., and Zvartau, E. E. (1999). Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol. Rev. 51, 83–133.
Fredholm, B. B., and Svenningsson, P. (2003). Adenosine-dopamine interactions: development of a concept and some comments on therapeutic possibilities. Neurology 61(11 Suppl. 6), S5–S9. doi: 10.1212/01.WNL.0000095204.89871.FF
Fujiwara, T., Watanuki, S., Yamamoto, S., Miyake, M., Seo, S., Itoh, M., et al. (1997). Performance evaluation of a large axial field-of-view PET scanner: SET-2400W. Ann. Nucl. Med. 11, 307–313. doi: 10.1007/BF03165298
Fukumitsu, N., Ishii, K., Kimura, Y., Oda, K., Sasaki, T., Mori, Y., et al. (2005). Adenosine A1 receptor mapping of the human brain by PET with 8-dicyclopropylmethyl-1-11C-methyl-3-propylxanthine. J. Nucl. Med. 46, 32–37.
Glass, M., Faull, R. L., Bullock, J. Y., Jansen, K., Mee, E. W., Walker, E. B., et al. (1996). Loss of A1 adenosine receptors in human temporal lobe epilepsy. Brain Res. 710, 56–68. doi: 10.1016/0006-8993(95)01313-X
Green, R. M., and Stiles, G. L. (1986). Chronic caffeine ingestion sensitizes the A1 adenosine receptor-adenylate cyclase system in rat cerebral cortex. J. Clin. Invest. 77, 222–227. doi: 10.1172/JCI112280
Harrison, T. M., Weintraub, S., Mesulam, M. M., and Rogalski, E. (2012). Superior memory and higher cortical volumes in unusually successful cognitive aging. J. Int. Neuropsychol. Soc. 18, 1081–1085. doi: 10.1017/S1355617712000847
Ishibashi, K., Ishii, K., Oda, K., Kawasaki, K., Mizusawa, H., and Ishiwata, K. (2009). Regional analysis of age-related decline in dopamine transporters and dopamine D2-like receptors in human striatum. Synapse 63, 282–290. doi: 10.1002/syn.20603
Ishiwata, K., Noguchi, J., Wakabayashi, S., Shimada, J., Ogi, N., Nariai, T., et al. (2000a). 11C-labeled KF18446: a potential central nervous system adenosine A2A receptor ligand. J. Nucl. Med. 41, 345–354.
Ishiwata, K., Ogi, N., Shimada, J., Nonaka, H., Tanaka, A., Suzuki, F., et al. (2000b). Further characterization of a CNS adenosine A2A receptor ligand [11C]KF18446 with in vitro autoradiography and in vivo tissue uptake. Ann. Nucl. Med. 14, 81–89.
Ishiwata, K., Ogi, N., Hayakawa, N., Oda, K., Nagaoka, T., Toyama, H., et al. (2002). Adenosine A2A receptor imaging with [11C]KF18446 PET in the rat brain after quinolinic acid lesion: comparison with the dopamine receptor imaging. Ann. Nucl. Med. 16, 467–475. doi: 10.1007/BF02988643
Kimura, Y., Ishii, K., Fukumitsu, N., Oda, K., Sasaki, T., Kawamura, K., et al. (2004). Quantitative analysis of adenosine A1 receptors in human brain using positron emission tomography and [1-methyl-11C]8-dicyclopropylmethyl-1-methyl-3-propylxanthine. Nucl. Med. Biol. 31, 975–981. doi: 10.1016/j.nucmedbio.2004.06.005
Kovacs, Z., Juhasz, G., Dobolyi, A., Bobest, M., Papp, V., Takats, L., et al. (2010). Gender- and age-dependent changes in nucleoside levels in the cerebral cortex and white matter of the human brain. Brain Res. Bull. 81, 579–584. doi: 10.1016/j.brainresbull.2009.10.010
Kuriyama, S., Shimazu, T., Ohmori, K., Kikuchi, N., Nakaya, N., Nishino, Y., et al. (2006). Green tea consumption and mortality due to cardiovascular disease, cancer, and all causes in Japan: the Ohsaki study. JAMA 296, 1255–1265. doi: 10.1001/jama.296.10.1255
Li, W., Dai, S., An, J., Li, P., Chen, X., Xiong, R., et al. (2008). Chronic but not acute treatment with caffeine attenuates traumatic brain injury in the mouse cortical impact model. Neuroscience 151, 1198–1207. doi: 10.1016/j.neuroscience.2007.11.020
Logan, J. (2003). A review of graphical methods for tracer studies and strategies to reduce bias. Nucl. Med. Biol. 30, 833–844. doi: 10.1016/S0969-8051(03)00114-8
Lopes, L. V., Cunha, R. A., and Ribeiro, J. A. (1999). Increase in the number, G protein coupling, and efficiency of facilitatory adenosine A2A receptors in the limbic cortex, but not striatum, of aged rats. J. Neurochem. 73, 1733–1738. doi: 10.1046/j.1471-4159.1999.731733.x
Meerlo, P., Roman, V., Farkas, E., Keijser, J. N., Nyakas, C., and Luiten, P. G. (2004). Ageing-related decline in adenosine A1 receptor binding in the rat brain: an autoradiographic study. J. Neurosci. Res. 78, 742–748. doi: 10.1002/jnr.20314
Meyer, P. T., Elmenhorst, D., Boy, C., Winz, O., Matusch, A., Zilles, K., et al. (2007). Effect of aging on cerebral A1 adenosine receptors: a [18F]CPFPX PET study in humans. Neurobiol. Aging 28, 1914–1924. doi: 10.1016/j.neurobiolaging.2006.08.005
Mishina, M., and Ishiwata, K. (2014). Adenosine receptor PET imaging in human brain. Int. Rev. Neurobiol. 119, 51–69. doi: 10.1016/B978-0-12-801022-8.00002-7
Mishina, M., Ishiwata, K., Kimura, Y., Naganawa, M., Oda, K., Kobayashi, S., et al. (2007). Evaluation of distribution of adenosine A2A receptors in normal human brain measured with [11C]TMSX PET. Synapse 61, 778–784. doi: 10.1002/syn.20423
Mishina, M., Kimura, Y., Naganawa, M., Ishii, K., Oda, K., Sakata, M., et al. (2012). Differential effects of age on human striatal adenosine A1 and A2A receptors. Synapse 66, 832–839. doi: 10.1002/syn.21573
Mishina, M., Senda, M., Kimura, Y., Toyama, H., Ishiwata, K., Ohyama, M., et al. (2000). Intrasubject correlation between static scan and distribution volume images for [11C]flumazenil PET. Ann. Nucl. Med. 14, 193–198. doi: 10.1007/BF02987859
Morrison, J. H., and Baxter, M. G. (2012). The ageing cortical synapse: hallmarks and implications for cognitive decline. Nat. Rev. Neurosci. 13, 240–250. doi: 10.1038/nrn3200
Müller, C. E., and Jacobson, K. A. (2011). Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim. Biophys. Acta 1808, 1290–1308. doi: 10.1016/j.bbamem.2010.12.017
Nehlig, A., Daval, J. L., and Debry, G. (1992). Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res. Brain Res. Rev. 17, 139–170. doi: 10.1016/0165-0173(92)90012-B
Noguchi, J., Ishiwata, K., Furuta, R., Simada, J., Kiyosawa, M., Ishii, S., et al. (1997). Evaluation of carbon-11 labeled KF15372 and its ethyl and methyl derivatives as a potential CNS adenosine A1 receptor ligand. Nucl. Med. Biol. 24, 53–59. doi: 10.1016/S0969-8051(96)00161-8
Ohayon, M. M., Carskadon, M. A., Guilleminault, C., and Vitiello, M. V. (2004). Meta-analysis of quantitative sleep parameters from childhood to old age in healthy individuals: developing normative sleep values across the human lifespan. Sleep 27, 1255–1273. doi: 10.1093/sleep/27.7.1255
Oishi, Y., Huang, Z. L., Fredholm, B. B., Urade, Y., and Hayaishi, O. (2008). Adenosine in the tuberomammillary nucleus inhibits the histaminergic system via A1 receptors and promotes non-rapid eye movement sleep. Proc. Natl. Acad. Sci. U.S.A. 105, 19992–19997. doi: 10.1073/pnas.0810926105
Rebola, N., Sebastiao, A. M., de Mendonca, A., Oliveira, C. R., Ribeiro, J. A., and Cunha, R. A. (2003). Enhanced adenosine A2A receptor facilitation of synaptic transmission in the hippocampus of aged rats. J. Neurophysiol. 90, 1295–1303. doi: 10.1152/jn.00896.2002
Reeves, S., Bench, C., and Howard, R. (2002). Ageing and the nigrostriatal dopaminergic system. Int. J. Geriatr. Psychiatry 17, 359–370. doi: 10.1002/gps.606
Rissman, R. A., De Blas, A. L., and Armstrong, D. M. (2007). GABAA receptors in aging and Alzheimer’s disease. J. Neurochem. 103, 1285–1292. doi: 10.1111/j.1471-4159.2007.04832.x
Scammell, T. E., Gerashchenko, D. Y., Mochizuki, T., McCarthy, M. T., Estabrooke, I. V., Sears, C. A., et al. (2001). An adenosine A2a agonist increases sleep and induces Fos in ventrolateral preoptic neurons. Neuroscience 107, 653–663. doi: 10.1016/S0306-4522(01)00383-9
Schliebs, R., and Arendt, T. (2011). The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 221, 555–563. doi: 10.1016/j.bbr.2010.11.058
Sebastiao, A. M., Cunha, R. A., de Mendonca, A., and Ribeiro, J. A. (2000). Modification of adenosine modulation of synaptic transmission in the hippocampus of aged rats. Br. J. Pharmacol. 131, 1629–1634. doi: 10.1038/sj.bjp.0703736
Segovia, G., Porras, A., Del Arco, A., and Mora, F. (2001). Glutamatergic neurotransmission in aging: a critical perspective. Mech. Ageing Dev. 122, 1–29. doi: 10.1016/S0047-6374(00)00225-6
Statland, B. E., and Demas, T. J. (1980). Serum caffeine half-lives. Healthy subjects vs. patients having alcoholic hepatic disease. Am. J. Clin. Pathol. 73, 390–393. doi: 10.1093/ajcp/73.3.390
Suhara, T., Fukuda, H., Inoue, O., Itoh, T., Suzuki, K., Yamasaki, T., et al. (1991). Age-related changes in human D1 dopamine receptors measured by positron emission tomography. Psychopharmacology 103, 41–45. doi: 10.1007/BF02244071
Sun, F. W., Stepanovic, M. R., Andreano, J., Barrett, L. F., Touroutoglou, A., and Dickerson, B. C. (2016). Youthful brains in older adults: preserved neuroanatomy in the default mode and salience networks contributes to youthful memory in superaging. J. Neurosci. 36, 9659–9668. doi: 10.1523/JNEUROSCI.1492-16.2016
Ulas, J., Brunner, L. C., Nguyen, L., and Cotman, C. W. (1993). Reduced density of adenosine A1 receptors and preserved coupling of adenosine A1 receptors to G proteins in Alzheimer hippocampus: a quantitative autoradiographic study. Neuroscience 52, 843–854. doi: 10.1016/0306-4522(93)90533-L
Urade, Y., Eguchi, N., Qu, W. M., Sakata, M., Huang, Z. L., Chen, J. F., et al. (2003). Sleep regulation in adenosine A2A receptor-deficient mice. Neurology 61(11 Suppl. 6), S94–S96. doi: 10.1212/01.WNL.0000095222.41066.5E
van Geldorp, B., Heringa, S. M., van den Berg, E., Olde Rikkert, M. G., Biessels, G. J., and Kessels, R. P. (2015). Working memory binding and episodic memory formation in aging, mild cognitive impairment, and Alzheimer’s dementia. J. Clin. Exp. Neuropsychol. 37, 538–548. doi: 10.1080/13803395.2015.1037722
Keywords: adenosine A1 receptor, aging, positron emission tomography, humans, cerebral cortex, thalamus
Citation: Mishina M, Kimura Y, Sakata M, Ishii K, Oda K, Toyohara J, Kimura K and Ishiwata K (2017) Age-Related Decrease in Male Extra-Striatal Adenosine A1 Receptors Measured Using 11C-MPDX PET. Front. Pharmacol. 8:903. doi: 10.3389/fphar.2017.00903
Received: 30 August 2017; Accepted: 28 November 2017;
Published: 18 December 2017.
Edited by:
Francisco Ciruela, University of Barcelona, SpainReviewed by:
Vittoria Colotta, University of Florence, ItalyCopyright © 2017 Mishina, Kimura, Sakata, Ishii, Oda, Toyohara, Kimura and Ishiwata. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Masahiro Mishina, bWlzaGluYUBubXMuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.