
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 10 November 2017
Sec. Neuropharmacology
Volume 8 - 2017 | https://doi.org/10.3389/fphar.2017.00794
 Elham A. Afify1,2*
Elham A. Afify1,2* Najlaa M. Andijani1
Najlaa M. Andijani1Tolerance to the analgesic effect of morphine is a major clinical problem which can be managed by co-administration of another drug. This study investigated the ability of propranolol to potentiate the antinociceptive action of morphine and the possible mechanisms underlying this effect. Antinociception was assessed in three nociceptive tests (thermal, hot plate), (visceral, acetic acid), and (inflammatory, formalin test) in mice and quantified by measuring the percent maximum possible effect, the percent inhibition of acetic acid-evoked writhing response, and the area under the curve values of number of flinches for treated mice, respectively. The study revealed that propranolol (0.25–20 mg/Kg, IP) administration did not produce analgesia in mice. However, 10 mg/Kg propranolol, enhanced the antinociceptive effect of sub-analgesic doses of morphine (0.2, 1, and 2 mg/Kg, IP) in the three nociceptive tests. It also shifted the dose response curve of morphine to the left. The combined effect of propranolol and morphine was attenuated by haloperidol (D2 receptor antagonist, 1.5 mg/Kg, IP), and bicuculline (GABAA receptor antagonist, 2 mg/Kg, IP). Repeated daily administration of propranolol (10 mg/Kg, IP) did not alter the nociceptive responses in the three pain tests, but it significantly potentiated morphine-induced antinociception in the hot plate, acetic acid-evoked writhing, and in the second phase of formalin tests. Together, the data suggest that a cross-talk exists between the opioidergic and adrenergic systems and implicate dopamine and GABA systems in this synergistic effect of morphine-propranolol combination. Propranolol may serve as an adjuvant therapy to potentiate the effect of opioid analgesics.
Pain is an important reflex that warns against a potential damage or injury, and has been the subject of intense study and research. Until today, morphine is believed to be the most effective pain killer. However, its use is hampered by the development of tolerance, dependence and respiratory depression (Garcely, 1995; Sehgal et al., 2013). Catecholaminergic system has a pivotal role in regulating the opioid activity. Local and subcutaneous application of β-blockers has antinociceptive effect. For example, blockade of β-adrenoceptors in the joints connecting the jawbone to the skull induced antinociception in experimental animals (Fávaro-Moreira et al., 2012). In clinical studies, esmolol (Chia et al., 2004; Collard et al., 2007; Haghighi et al., 2015), atenolol (Zaugg et al., 1999), and labetalol (Xiao et al., 2008) demonstrated analgesic properties and reduced the recurrent postoperative pain. Coloma et al. (2001) suggested that perioperative β-antagonist administration was an alternative to opioids to maintain postoperative analgesia. Additionally, non-selective β-adrenergic receptor blockers inhibited the development of morphine tolerance in mice (Kihara and Kaneto, 1986; Kaneto and Inoue, 1990); reduced naloxone precipitated opioid withdrawal and might be effective in the treatment of opiate addiction (Harris and Aston-Jones, 1993). However, the specific mechanisms by which β-blockers potentiate the analgesic effect of opioids remains controversial.
Propranolol is one of the prototype clinically prescribed cardioprotective β-adrenergic receptor blockers primarily used in treatment of arrhythmia and hypertension (Degoute, 2007; Koelemay and Legemate, 2008). It showed cutaneous analgesia due to membrane stabilizing activity, either alone (Chen et al., 2012) or combined with dopamine (Chen et al., 2015) against nociceptive stimuli in rats. It is reasonable that in certain painful situations propranolol can modulate nociceptive signals and may be effective on occasion as a combined analgesic medication (Smith, 2006). These reported studies evaluated the effect of propranolol on cutaneous skin insults. Whether propranolol administered systemically will show some promise as an agent to enhance morphine analgesia has not been studied.
In this study we explored the ability of propranolol to modify the analgesic response to morphine and the possible mechanisms underlying this effect in three models of pain. To this purpose, morphine and propranolol were administered alone or combined to female mice. To assess antinociception, the hot plate, acetic acid-evoked writhing, and formalin tests were employed and quantified by measuring the percent maximum possible effect, the percent inhibition of acetic acid-evoked writhing and the area under the curve values of formalin-induced flinches for treated mice. Additionally, pharmacological antagonists were used to define the possible role of dopamine and GABA receptors in the antinociceptive effect of propranolol-morphine combination in mice.
Female Swiss mice (30–40 g: Animal care facility of King Fahd Medical Research Center, King Abdulaziz University in Jeddah) were employed in the present study. Mice were housed in cages under standard conditions (temperature: 22 ± 2°C, 12/12 h light/dark cycle, free access to water and standard chow). All experiments were approved by the institutional research unit of the biomedical ethics for the care and handling of experimental animals (Reference No. 278–17). The experimental animals were utilized for one behavior test and were sacrificed under anesthesia at the end of the experiment. The observer of the performed experiments was blind to the treatment type.
The following drugs were used: Morphine sulfate (10 mg/ml) (Laboratoire Renaudin, Saint-Cloud, France), propranolol (Fluke, Chemie GmbH, Basel, Switzerland), formaldehyde solution (Riedel-de Haën, Seelze, Germany), bicuculline (Sigma Chemical Co., St Louis, MO, United States), and haloperidol (Jamjoom pharma factory, Jeddah, Kingdom of Saudi Arabia). Drugs were dissolved in 0.9% NaCl. Two drops of concentrated acetic acid 97% was added to bicuculline solution for stability purpose (Hemnani et al., 1983).
Propranolol was administered intraperitoneally (IP) at doses of 0.5–20 mg/Kg in mice. The 10 mg/Kg dose of the drug was then used for all of the subsequent experiments. Morphine was administered at 0.2–8 mg/Kg, IP and propranolol was administered 15 min before morphine, which was administered 30 min prior to the pain test. Control mice were injected with saline and the drugs were administered to the mice with the total volume of 0.1 mL/10 g of body weight. Figure 1 illustrates the treatment regimen in the pain models.
After treatment with propranolol or morphine, mice (n = 6–8/group) were administered acetic acid solution (0.6%, 10 mL/kg, IP). Injection of acetic acid produced typical abdominal contractions in mice in the form of waves of muscles contractions accompanied by extension of the hind limb. The potency of the nociceptive stimulus was evaluated by recording the number of writhes for 30 min following acetic acid injection and the percentage of inhibition of acetic acid-evoked writhing was quantified according to Koster et al. (1959) using the following formula:
The hot plate apparatus (Ugo Basile Comerio, Italy) was heated at 50 ± 1°C. Thirty minutes after morphine administration, each mouse was positioned on the preheated metallic base of the apparatus till the appearance of the painful symptoms as lifting or licking of the hind paws or escaping out the chamber. The time in seconds between placing the mouse on the hot surface of the plate and the appearance of nociceptive signs was recorded using stop watch with a cut off time of 30 s as described by Woolfe and MacDonald (1944). Antinociception was quantified as percent maximum possible effect (%MPE) induced by the drugs according to the equation:
The test was done by introducing the mice in 25 cm × 15 cm × 20 cm translucent plastic cage for a period of 30 min to get used to the surroundings provided in the experiment. A 27-gauge needle was used to inject 50 μl of 1% formalin solution into the plantar exterior of the right hind paw of the mice. The Mice were then positioned in the cage, and pain associated behaviors were evaluated and analyzed by calculating the frequency of the flinching of the injected paw in which the solution was injected (Hunskaar and Hole, 1987). Flinches were calculated and analyzed for a whole 60 min after IP injection at intervals of 5 min. Intraplantar injections of formalin solution elicited a biphasic reaction. The first phase started immediately following formalin injection, lasted for 5–10 min and indicated acute nociceptive response. The second phase referred as the prolonged tonic phase started 10–15 min subsequent to the formalin injection and its effect remained evident till 60 min. For comparative reason, graphical presentations were created for the AUC of the number of flinches in opposition to the time period by the Graph Pad Prism Version 5.2 for the Windows based on the trapezoidal rule.
Further, mechanisms by which propranolol potentiated morphine antinociception in mice in the three pain tests were performed using antagonists. For each pain test, eight groups of mice were used. I: (Control group) injected with equal volumes of 0.9% NaCl, II: propranolol (10 mg/Kg, IP), III: morphine (dose range: 0.2–4 mg/Kg according to pain test), IV: propranolol + morphine, V: haloperidol (1.5 mg/Kg, IP) as dopamine D2 receptor antagonist, VI: bicuculline (2 mg/Kg, IP) as GABAA receptor antagonist, VII (propranolol/morphine/haloperidol), and Group VIII (propranolol/morphine/bicuculline). Doses of blockers were chosen based on previous work done in our lab and by others (Omar, 2007; Afify et al., 2017). Treatment with blockers was started 15 min prior to IP administration of propranolol. Propranolol was administered 15 min before morphine, which was administered 30 min prior to the nociceptive test. The antinociceptive response was measured for 30 min (hot plate, acetic acid-evoked writhing) or 60 min (formalin test) after morphine treatment as previously described.
Since the 10 mg/Kg dose of propranolol potentiated the antinociceptive effect of morphine in the three pain models, it was appreciated to test this dose further to investigate the effect of repeated administration of propranolol. Mice were divided into four groups in each pain test and injections started at 8AM for four consecutive days as follows: I: (Control group) received equal volumes of the vehicle (saline), II: propranolol (10 mg/Kg, IP), III: morphine (0.2 mg/Kg, IP), IV: propranolol-morphine combination. Propranolol was administered at zero time and followed by, 15 min later, morphine and animals were tested 30 min after morphine. On the 4th day, the antinociceptive response was measured for 30 or 60 min after morphine treatment according to the pain test, as stated previously.
Values are presented as mean ± SEM Data were analyzed by one-way analysis of variance (ANOVA) followed by Bonferroni’s post hoc test with the level of significance set at P < 0.05 using the Graph Pad Prism (Version 5.2) for Windows.
Acetic acid injection to mice (0.6%, 0.1 mL/10 g) produced a typical abdominal constriction-stretching response between 0 and 30 min. Morphine dose dependently caused inhibition of the abdominal constrictions but propranolol (0.25–20 mg/Kg, IP) failed to elicit antinociceptive response compared to saline control group. IP administration of sub-antinociceptive doses of morphine (0.2, 1, and 2 mg/Kg) resulted in antinociception levels that does not exceed 27.5% inhibition of HAC evoked writhing. By contrast, mice receiving morphine and propranolol showed a significantly enhanced inhibition of HAC evoked writhing compared to mice treated with morphine alone and saline control (P < 0.05). Propranolol caused a parallel leftward shift in morphine dose response curve (ED50 = 1.342 mg, vs. 4.119 mg, for morphine group) (Figure 2A). The antinociception obtained with 10 mg/Kg propranolol plus 1 mg/Kg morphine was statistically not different from that obtained with 2 mg/Kg morphine administered alone to mice (%HAC inhibtion were 34.75 and 27.5%), respectively, (Figure 2B). The antinociception obtained with 10 mg/Kg propranolol combined with 2 mg/Kg morphine was higher than that obtained with 4 mg/Kg morphine administered alone to mice (%HAC inhibtion were 60.67 and 43.70%), respectively, (Figure 2C). Similarily, the antinociception obtained with 10 mg/Kg propranolol plus 4 mg/Kg morphine was equal to that induced by 8 mg/Kg of morphine administered alone (%HAC inhibtion were 76% and 80%), respectively, (Figure 2D). Moreover, IP administration of morphine (8 mg/Kg) plus propranolol (10 mg/Kg) to mice resulted in maximum possible antinociception (100% inhibition of HAC evoked writhing) compared to 80% in mice treated with morphine alone. The effect of administration of haloperidol and bicuculline was studied using HAC evoked writhing response (Figure 2E). One-way ANOVA showed a significant effect of treatment [F(5,24) = 101.6, P < 0.0001]. The antinociceptive response of morphine-propranolol combination was significantly reduced by haloperidol or bicuculline treatment (% inhibition of HAC induced writhing were 76 ± 4, 34 ± 3, and 38 ± 2, respectively. Levels of antinociception observed in haloperidol or bicuculline treated groups were not significantly different from the corresponding values obtained in saline control group (data not shown).
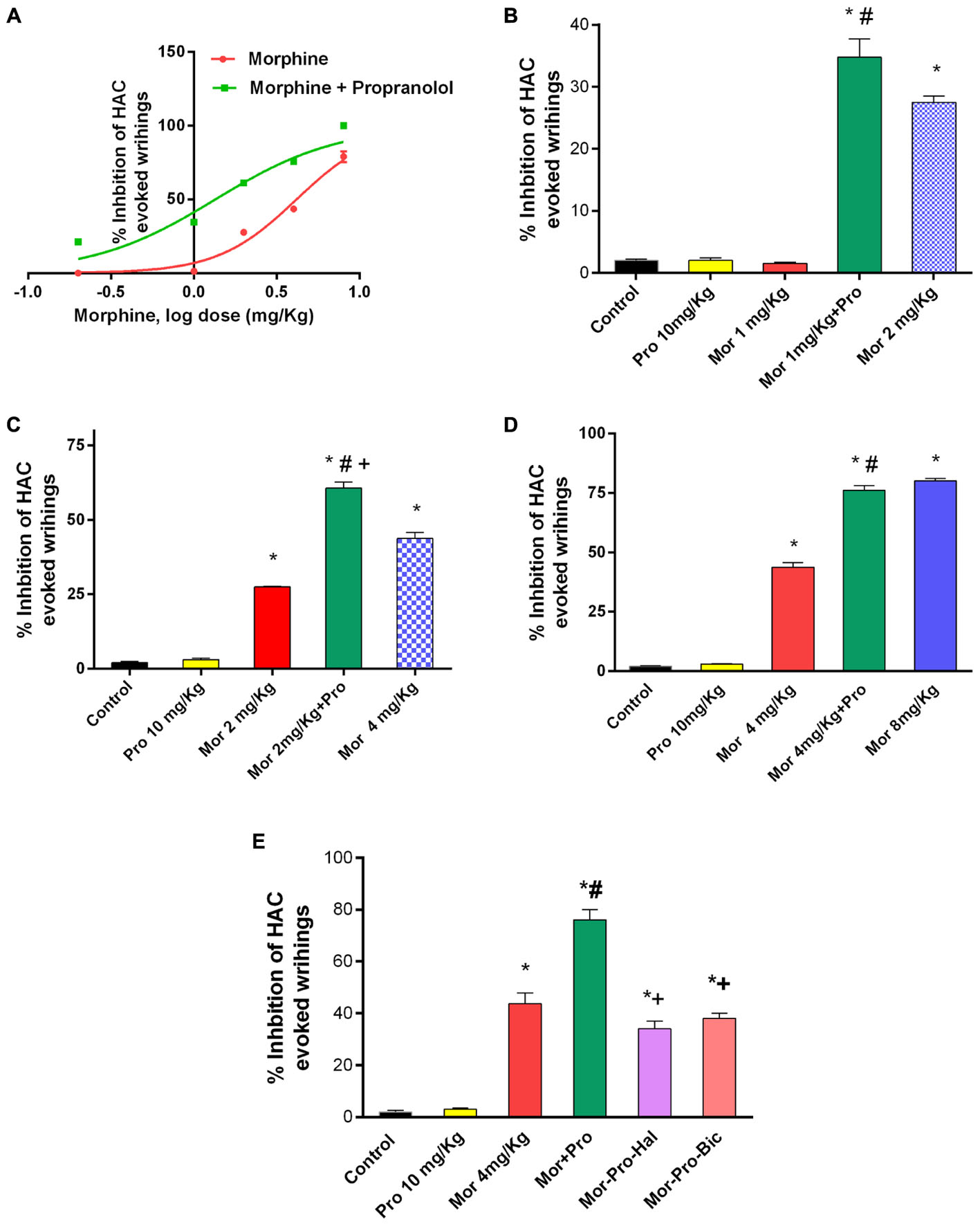
FIGURE 2. Effects of administration of morphine (Mor) and propranolol [Pro, 10 mg/Kg, intraperitoneally (IP)] either alone or in combination on acetic acid-evoked writhing in mice. (A) The dose-response curve for the antinociceptive effect of morphine alone (0.2–8 mg/Kg, IP) and morphine-propranolol combination. (B–D) The antinociceptive effect of Mor, Pro and their combination. (E) The effect of pretreatment with the dopamine receptor antagonist haloperidol (Hal, 1.5 mg/Kg, IP) and GABAA receptor antagonist bicuculline (Bic, 2 mg/Kg, IP) on the antinociceptive effect of morphine-propranolol combination. Each point represents the mean of % inhibition of acetic acid-evoked writhing ± SE for 6–8 mice. ∗P < 0.05 compared with control, #P < 0.05 compared with Mor 1 mg/Kg (B), Mor 2 mg/Kg (C), Mor 4 mg/Kg (D,E), +P < 0.05 compared with Mor-Pro group, by one-way ANOVA and Bonferroni’s post hoc test.
Mice treated with saline did not exhibit antinociceptive response using the hot plate test. Propranolol (0.25–20 mg/Kg, IP) did not produce significant antinociceptive response (P > 0.05). On the other hand, morphine (0.2–8mg/Kg, IP) produced a dose-dependent antinociception (F = 34.73, P < 0.05, Figure 3A). Although the lowest tested dose of morphine (0.2 mg/Kg, IP) did not induce a signifcant antinociception, the combination of propranolol (10 mg/Kg, IP) and morphine (0.2–8 mg/Kg, IP) induced a dose-dependent antinociceptive response [F(5,24) = 105.4, P < 0.05]. Animals receiving 10 mg/Kg propranolol and 1 mg/Kg morphine (%MPE 54.12 ± 2) showed a significant increase in antinociceptive response compared to 1 mg/Kg morphine given alone and saline control groups (P < 0.05). The response was comparable to that induced by 2 mg/Kg of morphine given alone (Figure 3B). Similarily, combined administration of 10 mg/Kg propranolol and 2 mg/Kg morphine produced effect similar to that produced by 4 mg/Kg morphine administered alone (Figure 3C). In the receptor antagonism tests, one way ANOVA showed a significant effect of treatment [F(5,24) = 34.27, P < 0.001, Figure 3D]. Antinociception induced by combined propranolol (10 mg/Kg) – morphine (0.2 mg/Kg) therapy was abolished by haloperidol (%MPE = 4 ± 0.3) or bicuculline treatment (%MPE = 6 ± 0.3). The %MPE was not affected by treatment with either haloperidol or bicuculline alone compared with saline control group (data not shown).
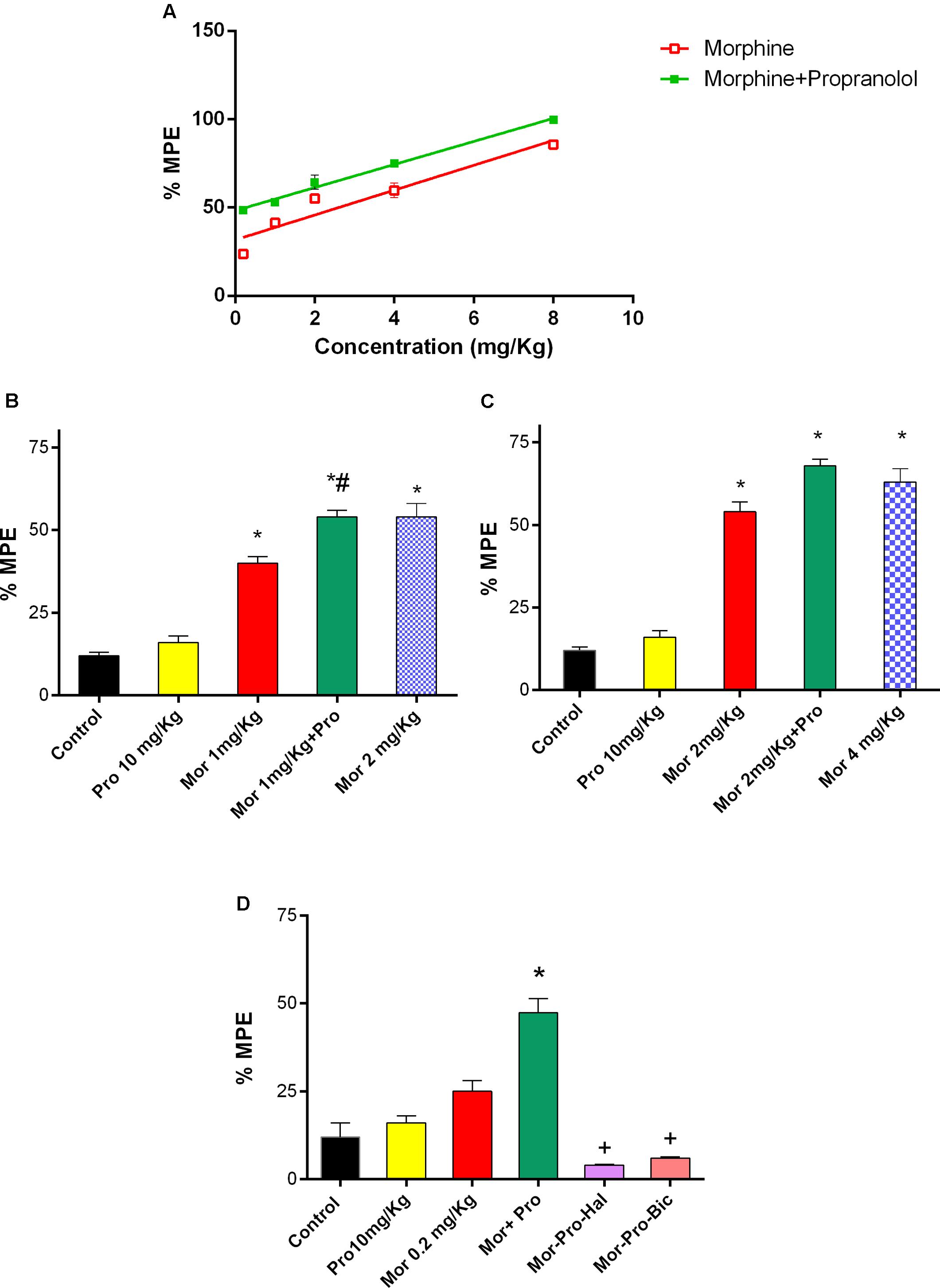
FIGURE 3. Effect of administration of propranolol (Pro, 10 mg/Kg, IP) on the antinociceptive effect of morphine (Mor) in the hot plate test represented by percent maximum possible effect (%MPE). (A) The dose–response curve for the antinociceptive effect of morphine alone (0.2–8 mg/Kg, IP) and morphine-propranolol combination. (B,C) The antinociceptive effect of different doses of morphine, propranolol and their combination. (D) The effect of pretreatment with dopamine receptor antagonist haloperidol (Hal, 1.5 mg/Kg, IP) and bicuculline, GABAA receptor antagonist (Bic, 2 mg/Kg, IP) on the antinociceptive effect of morphine-propranolol combination. Each point indicated the mean %MPE ± SE for 6–8 mice. ∗P < 0.05 compared with control, #P < 0.05 compared with Mor 1 mg/Kg, +P < 0.05 compared with Mor-Pro group, by one-way ANOVA and Bonferroni’s post hoc test.
Intraplantar injection of 1% formalin to the mice evoked a typical biphasic flinching response. The AUC of the number of flinches for the first phase (0–15 min) was 600 ± 45 and for the second phase (15–60 min) was 2000 ± 160 in control group. Administration of morphine (0.2 mg/Kg) or propranolol (10 mg/Kg) had no effect on pain response as evident by insignificant changes in the AUC of number of flinches of either first (633 ± 40, 730 ± 40, and 600 ± 45) or second phase (1285 ± 67, 2056 ± 150, and 2000 ± 160) of the formalin test compared with saline control (P > 0.05, Figures 4A,B). Propranolol-morphine combination significantly reduced the number of flinches during the first phase of formalin test compared to saline control. Animals receiving 10 mg/Kg propranolol and 0.2 mg/Kg morphine showed a significantly enhanced antinociceptive response compared to 0.2 mg/Kg morphine alone and saline control treated mice (447 ± 34, 633 ± 40, and 600 ± 45), respectively, (P < 0.05). The response was comparable to that induced by 4 mg/Kg of morphine (423 ± 23) given alone (Figure 4A). Similar potentiation of morphine reponse was observed during the second phase of formalin test (Figure 4B). Administration of haloperidol significantly antagonized the antinociceptive effect of propranolol-morphine combination in the first but not the second phase of the test. The number of flinches during the first phase was 600 ± 10 vs. 447 ± 23 for morphine-propranolol group (Figure 4C, P < 0.05) and second phase was 740 ± 34 vs. 880 ± 34 for morphine-propranolol group (Figure 4D, P < 0.05).
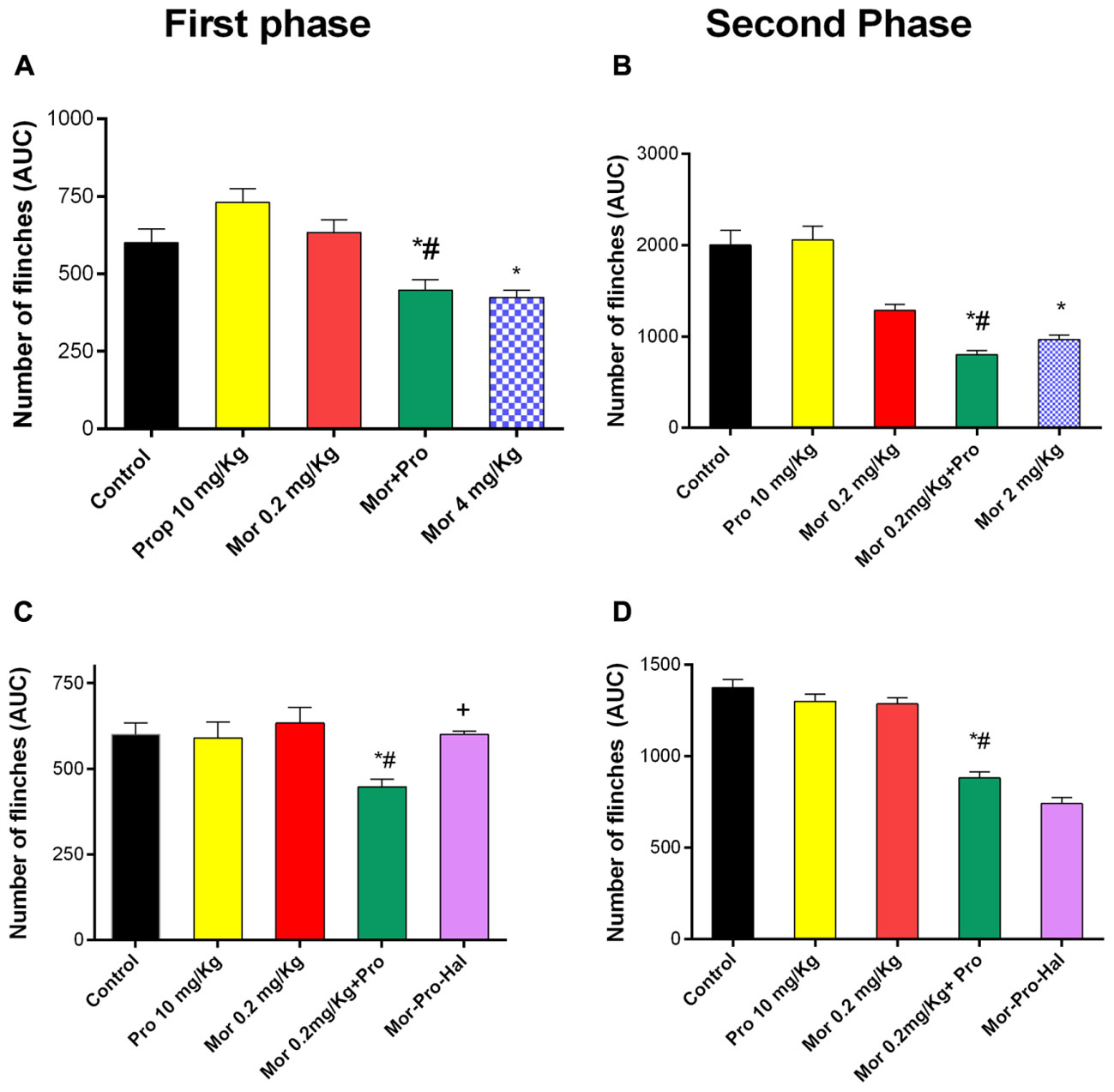
FIGURE 4. Effect of administration of morphine (Mor, 0.2 mg/Kg, IP) and propranolol (Pro, 10 mg/Kg, IP) on the hind paw flinches quantified during the two phases of the formalin test in mice during the first phase (A) and second phase (B) of the test. The effect of pretreatment with haloperidol, dopamine receptor antagonist (Hal, 1.5 mg/Kg, IP) on the antinociceptive effect of morphine-propranolol combination during both first and second phases (C,D), respectively. Each point represents the mean area under the curve (AUC) for the number of flinches ± SE for 6–8 mice. ∗P < 0.05 compared with control, #P < 0.05 compared with Mor 0.2 mg/Kg, +P < 0.05 compared with Mor-Pro group, by one-way ANOVA and Bonferroni’s post hoc test.
In the acetic acid-evoked writhing, hot plate, and the formalin tests, mice treated with propranolol 10 mg/Kg IP for 4 days did not exhibit antinociceptive response compared to saline treated group (Table 1). However, combined treatment with propranolol and morphine for 4 days significantly potentiated morphine induced antinociception in the three pain tests. The % inhibition of writhes of propranolol-morphine combination was 71%, P < 0.05. Table 1 indicated that animals treated with morphine-propranolol combination exhibited significant increase in % MPE of 26. 30 ± 3.40 compared to 2 ± 0.05 for the saline control group (P < 0.05) in the hot plate test. In the second phase of formalin test, combined treatment with propranolol-morphine had significant antinociceptive effect (39.03% inhibition, P < 0.05). The AUC for the number of flinches of morphine-propranolol combination were 550.5 ± 31.30 in the first phase and 838.1 ± 82.22 in the second phase compared to 477.5 ± 30 and 1374.62 ± 57 for the saline control group, respectively.
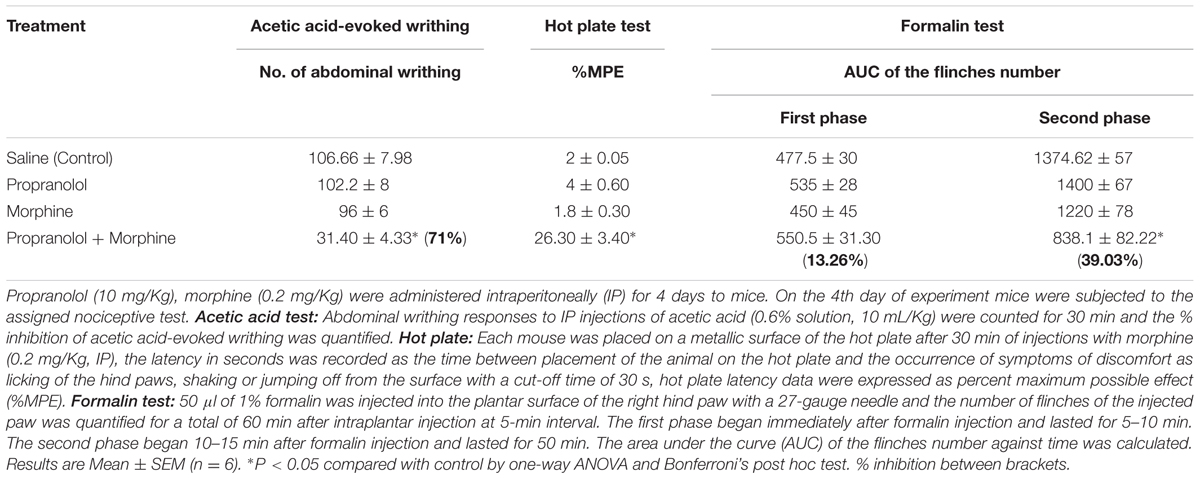
TABLE 1. Effect of repeated daily treatment with propranolol and morphine on the antinociceptive responses of mice.
The results of the present study are the first to demonstrate that propranolol; a non-selective β-adrenergic blocker potentiated the effect of sub-analgesic doses of morphine in animal models of thermal, visceral and inflammatory pain. Additionally, it reduced the doses of morphine required to achieve a maximum antinociceptive response as indicated by the reduced ED50 of morphine suggesting a greater effect of the combination therapy. The present study is also the first to implicate GABAergic and dopaminergic systems in the analgesic effect of morphine-propranolol combination. Data obtained from nociceptive experiments concerning the ability of β-blockers to potentiate morphine analgesia are conflicting. Propranolol alone (Chen et al., 2012) or combined with lidocaine or dopamine (Chen et al., 2015) intensified the analgesia against skin nociceptive stimuli in rats. Similar observations were reported for other β-adrenergic antagonists as atenolol (Zaugg et al., 1999), labetalol (Xiao et al., 2008), and esmolol (Chia et al., 2004; Collard et al., 2007; Haghighi et al., 2015). In another study, β2-adrenergic receptors modulated both opioid tolerance and physical dependence. Administration of butoxamine reversed morphine tolerance in mice (Liang et al., 2007). Left unclear the ability of propranolol to modify the analgesic effect of sub-analgesic doses of morphine and the mechanisms underlying this effect. In contrast to the observed potentiation of the antinociceptive effect of propranolol-morphine combination in our study, previous studies have shown that propranolol didn’t modify the dose response curve to the antinociceptive action of morphine or alter the ED50 of morphine in the tail flick test (Fennessy and Lee, 1970; Gorlitz and Frey, 1972; Richard et al., 1975). The reason for this discrepancy may be attributed to the difference in the pain model itself or the short time interval between the administration of propranolol and morphine.
Opioid receptors, β-adrenergic receptors and dopamine receptors belong to the G protein coupled receptor family (GPCR) (Zheng et al., 2010) that mediates antinociceptive effects via similar signal transduction pathways. β-adrenergic stimulation positively impacts adenylyl cyclase (AC) and stimulates protein kinase A (PKA) (Pepe et al., 2004; Grimm and Brown, 2010). Opioid receptors acting in an inverse way through G i/o proteins, which blunt the recruitment of PKA via inhibiting (AC) and reducing the level of cyclic adenosine monophosphate (cAMP) (Law and Loh, 1999). Remarkably, reported studies highlighted the role of opioids in facilitating β-adrenergic blockade. That said, a possible additive effect could exsist between blocking of β-adrenergic receptors and stimulation of opioid receptors in reducing cellular cAMP (Vamecq et al., 2015). The inhibition of cAMP signaling pathway alleviates nociceptive sensations in the pain memory (Shao et al., 2016), since the activation of the cAMP/PKA signaling pathway can improve the recognition function and generated hyperalgesia (Li et al., 2015). Moreover, the elevation of cAMP produces nociception in rodent pain models (Dolan and Nolan, 2001; Song et al., 2006). These findings provide evidence that links decreased cAMP to antinociception and increases to blockade of analgesia. Therefore, propranolol by blocking the adrenergic receptors inhibits the stimulatory action of Gs on the AC enzyme, blocks the generation of cAMP, augments the inhibitory effect of opioids on pain transmission and potentiates the antinociceptive response. This in turn supports the crosstalk between propranolol and morphine in potentiating morphine antinociception. In fact the analgesic effect of morphine has been linked to mechanisms more than changes in the level of cAMP such as reducing neurotransmitter release at the presynaptic levels via inhibition of calcium channels (Kumar et al., 2010). Morphine also can hyperpolarize nociceptors at the postsynaptic levels through activation of potassium channels (Khanna et al., 2011). The link between these pathways and β-adrenergic receptors warrants further investigation.
Interestingly, our results indicated that the D2 receptor blocker haloperidol antagonized the antinociceptive effect of propranolol-morphine combination. The role of dopamine receptors was widely accepted generally in analgesia and specifically in morphine antinociception. The painful symptoms observed in Parkinson’s disease and fibromyalgia are associated with decreased dopamine levels (Wood, 2008). Recently, it has been shown that D2 receptor agonists inhibited allodynic responses in rats (Cobacho et al., 2014) and mice (Almeida-Santos et al., 2015). In the same context blocking of dopamine D2 receptors attenuated morphine antinociceptive tolerance in mice (Dai et al., 2016). However, reports addressing the role of D2 receptors in morphine antinociception in combination therapy are scarce. One study reported that dopamine potentiated propranolol’s cutaneous analgesia (Chen et al., 2015). The observed attenuation of the antinociceptive response of propranolol-morphine combination by haloperidol in our study implicates the activation of dopamine receptors as a possible antinociceptive mechanism of the combination. There is a cooperative pathway between opioidergic and dopaminergic effects (Wood, 1983; Nestler, 1996). Furthermore blocking of the D2 receptors has been shown to prevent the inhibitory effect of dopamine on AC enzyme and the reduction of cAMP level (Rangel-Barajas et al., 2015) which in turn would antagonize the inhibitory effect of morphine and propranolol on reducing cAMP level and reversed their antinociceptive effect. The involvement of downstream signaling cascade could explain the potentiating effect of morphine-propranolol combination observed in the performed pharmacological studies. A proposed mechanism of the crosstalk between opioid, β-adrenergic and dopamine receptors is presented in Figure 5.
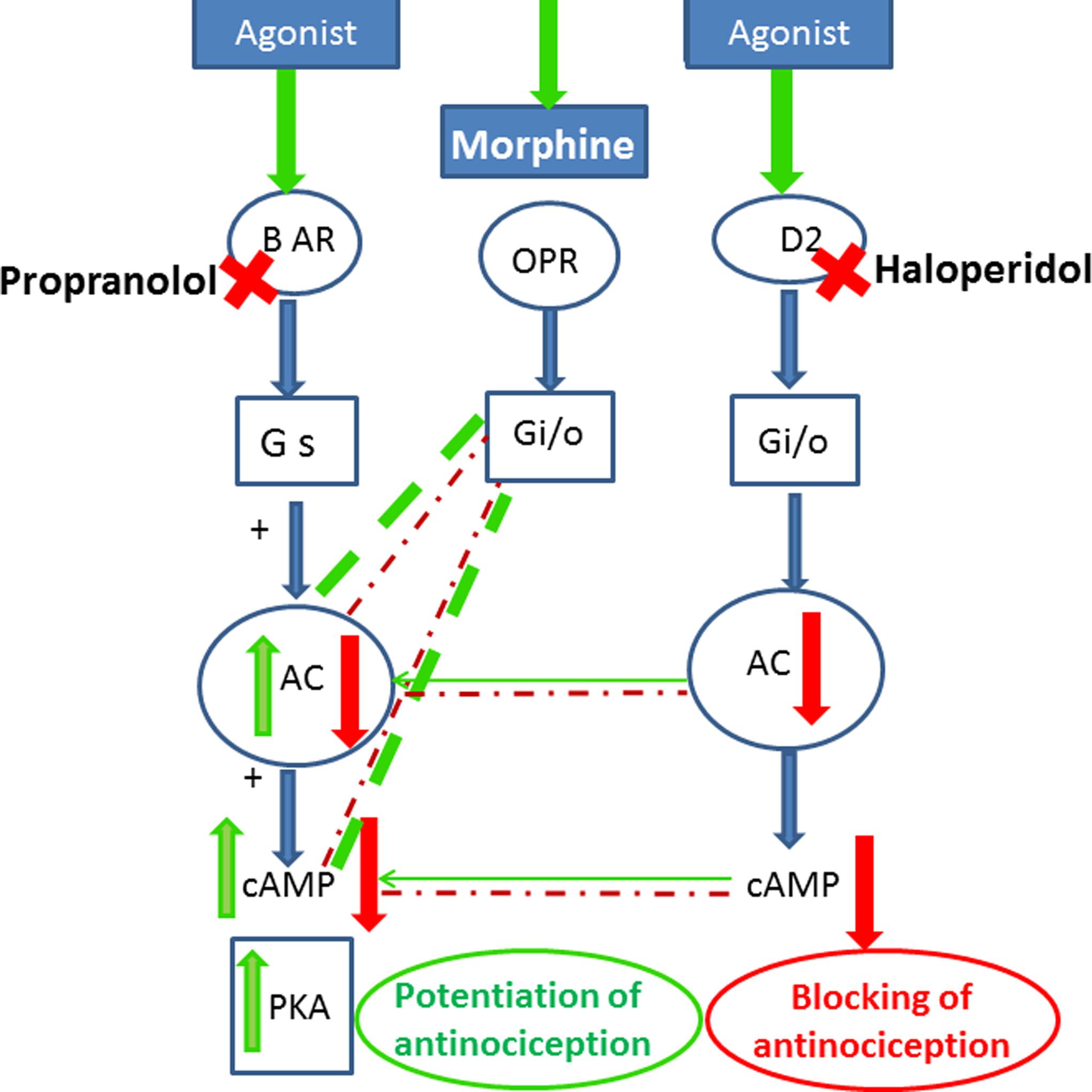
FIGURE 5. Scheme for cross-talk between opioid, adrenergic and D2 dopamine receptors that may lead to the potentiation of opioid antinociception after treatment with propranolol. Stimulatory effects are indicated by green arrows/lines, inhibitory effects in red arrows/lines, the potentiating effects are shown in thick dashed green lines. Agonist stimulation of β-adrenergic receptors activates (AC) and stimulates (PKA). Morphine acting on opioid receptors activates G i/o proteins, which blunts the recruitment of PKA via inhibiting (AC) and reducing the level of cAMP. Blocking of β-adrenergic receptors by propranolol and stimulation of opioid receptors by morphine reduce cellular cAMP and potentiate the antinociceptive response. Blocking of the D2 receptors by haloperidol prevents the inhibitory effect of dopamine on AC enzyme and the reduction in cAMP level and antagonizes the inhibitory effect of morphine and propranolol on reducing cAMP level and reversed their antinociceptive effect. BAR, β-adrenergic receptors; ORP, opioid receptors; Gs, stimulatory G-protein; Gi, inhibitory G protein; AC, adenylyl cyclase; cAMP, cyclic adenosine monophosphate; PKA, protein kinase A.
It is important to comment on the differential effect of haloperidol on the antinociceptive effect of combined propranolol-morphine regimen. Haloperidol antagonized the antinociceptive effect of propranolol-morphine only in the first phase of the formalin test. Similarly, sulpiride another D2 receptor antagonist reversed the antinociceptive effects of morphine only in the first phase of formalin-induced orofacial pain in rats (Reisi et al., 2014). The failure of haloperidol to attenuate the antinociceptive effect of propranolol-morphine in the second phase of formalin test is difficult to explain. Possible justification to this observation may be that the nociceptive component of the first phase of the formalin test reflects acute neurogenic origin that involves the Aδ fibers. However, direct stimulation of chemical nociceptors triggered the input from C fibers, which in turn provokes the second inflammatory response (Pajot et al., 2000). Therefore, it is conceivable that the differential implication of dopamine receptors in the antinociceptive response of propranolol-morphine during the two phases of formalin test may be attributed to the different nociceptive stimuli or the different origin of nerve input within the pain model itself. Similar conclusions were previously reported for morphine (Afify et al., 2013), khat extract (Afify et al., 2017), amphetamine (Clarke and Franklin, 1992), and nicotine (Damaj, 2007).
Other mechanisms, however, may contribute to the analgesic effect of the combination of propranolol with morphine in pain management. In the present study, bicuculline, a GABAA receptor antagonist, blocked the antinociceptive response of propranolol-morphine therapy in the hot plate and acetic acid models of nociception. The vast distribution of GABAergic neurons throughout the peripheral and central nervous system established the role of GABA in transmission and perception of pain impulses (Carlton et al., 1999). For example, the GABAA receptor agonists (Carlton et al., 1999; Motta et al., 2004), and the GABA-mimetic drugs (Carlton and Zhou, 1998) induced peripheral antinociception in the formalin test. Remarkably, β-agonists decreased GABA activity in isolated preparations through the activation of cAMP and PKA (Danielsson et al., 2016) whereas the β-adrenergic blocker, propranolol can stimulate GABA benzodiazepine receptor coupling (Benistant et al., 1988). Thus the current results indicated that propranolol by blocking the β-receptors and negatively modulates cAMP activity may increase the release of GABA and augments the antinociceptive action of morphine. This action is blocked by the GABAA antagonist, bicuculline. Taken together, both gabaergic and dopaminergic receptors are involved, at least in part in the antinociceptive effect of propranolol-morphine combination.
We further investigated the effect of repeated propranolol administration on morphine antinociception. Administration of propranolol for 4 days potentiated morphine analgesia in the three nociceptive tests. In the inflammatory model, the potentiation was only noticeable in the second phase of the formalin test. It is well-established that the direct stimulation of nociceptors triggers inflammatory response in the late phase of formalin test (Pajot et al., 2000) that caused by the released cytokines and inflammatory mediators (Oprée and Kress, 2000). This in turn stimulates C-fibers (Ringkamp et al., 2011) and the nociceptive transmission in the second phase of formalin test. Propranolol and other β-blockers (Benish et al., 2008; Nguyen et al., 2008; Kato et al., 2009) abrogate the proinflammatory cytokines (Deten et al., 2003; Tang et al., 2008) and exert an anti-inflammatory response. Propranolol decreased the T helper type 1 cytokine profile in human leukemic T cells (Hajighasemi and Mirshafiey, 2016). Moreover, it inhibits the proliferative activity and the vascular endothelial growth factor production on peripheral blood mononuclear cells (Hajighasemi and Mirshafiey, 2010). Not surprisingly, chronic propranolol administration via its anti-inflammatory effect significantly potentiated morphine antinociception during the inflammatory second phase of formalin test.
The current study is the first to report on the antinociceptive mechansim of morphine-propranolol treatment. The inhibition of the antinociceptive effect of morphine-propranolol therapy in the presence of the GABAA blocker suggests a role for gabergic receptors in the antinociceptive effect. The inhibtion of dopamine receptor is another mechanism that might contribute to propranolol-morphine analgesic effect. Moreover, the antinociceptive effect of the combined propranolol-morphine regimen depends on the nature of the painful stimulus and the activated nerve fiber. The results suggest that propranolol may be useful as a new alternative add on therapy to morphine for controlling pain. More mechanistic studies are required, however, to elucidate the possible crosstalks between opioids, adrenergic and other receptors in modulating pain transmission. Interpretation of results of this work is expected to open new avenues for improving the efficacy of opioids in management of pain.
The submitted paper was orally presented at the ESPET 56th Annual Conference and Workshop on Behavioral pharmacology held at the British University in Egypt (BUE) at El-Shorooq City on January, 28th 2017. The abstract was published in “booklet Abstracts” in the ESPET conference manual. The actual manuscript, however, has not been submitted or published in any journal.
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
EA raised the idea, designed the experimental protocol, data analysis and interpretation of the results, manuscript preparation and work overseeing. NA performed the experiments and collected data.
This work was supported by the Deanship of Scientific Research (DSR), King Abdulaziz University, Jeddah, under grant number 166-374-D1435.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors, therefore gratefully acknowledge the DSR technical and financial support.
Afify, E., Alkreathy, H., Ali, A., Alfaifi, H., and Khan, L. (2017). Characterization of the antinociceptive mechanisms of khat extract (Catha edulis) in mice. Front. Neurol. 8:69. doi: 10.3389/fneur.2017.00069
Afify, E., Khedr, M., Omar, A., and Nasser, S. (2013). The involvement of K (ATP) channels in morphine-induced antinociception and hepatic oxidative stress in acute and inflammatory pain in rats. Fundam. Clin. Pharmacol. 27, 623–631. doi: 10.1111/fcp.12004
Almeida-Santos, A. F., Ferreira, R. C., Duarte, I. D., Aguiar, D. C., Romero, T. R., and Moreira, F. A. (2015). The antipsychotic aripiprazole induces antinociceptive effects: possible role of peripheral dopamine D2 and serotonin 5-HT1A receptors. Eur. J. Pharmacol. 765, 300–306. doi: 10.1016/j.ejphar.2015.08.053
Benish, M., Bartal, I., Goldfarb, Y., Levi, B., Avraham, R., Raz, A., et al. (2008). Perioperative use of beta-blockers and COX-2 inhibitors may improve immune competence and reduce the risk of tumor metastasis. Ann. Surg. Oncol. 15, 2042–2052. doi: 10.1245/s10434-008-9890-5
Benistant, C., Rey, C., Fonlupt, P., and Pacheco, H. (1988). Interaction of propranolol with GABA stimulated diazepam binding to rat brain membranes. Gen. Pharmacol. 19, 537–539. doi: 10.1016/0306-3623(88)90160-7
Carlton, S. M., and Zhou, S. (1998). Attenuation of formalin-induced nociceptive behaviours following local peripheral injection of gabapentin. Pain 76, 201–207. doi: 10.1016/S0304-3959(98)00043-8
Carlton, S. M., Zhou, S., and Coggeshall, R. E. (1999). Peripheral GABAA receptors: evidence for peripheral primary afferent depolarization. Neuroscience 93, 713–722. doi: 10.1016/S0306-4522(99)00101-3
Chen, Y. W., Chiu, C. C., Wei, Y. L., Hung, C. H., and Wang, J. J. (2015). Propranolol combined with dopamine has a synergistic action in intensifying and prolonging cutaneous analgesia in rats. Pharmacol. Rep. 67, 1224–1229. doi: 10.1016/j.pharep.2015.05.016
Chen, Y. W., Chu, C. C., Chen, Y. C., Hung, C. H., and Wang, J. J. (2012). Propranolol elicits cutaneous analgesia against skin nociceptive stimuli in rats. Neurosci. Lett. 524, 129–132. doi: 10.1016/j.neulet.2012.07.036
Chia, Y. Y., Chan, M. H., Ko, N. H., and Liu, K. (2004). Role of β-blockade in anaesthesia and postoperative pain management after hysterectomy. Br. J. Anaesth. 93, 799–805. doi: 10.1093/bja/aeh268
Clarke, P. B., and Franklin, K. B. (1992). Infusion of 6-hydroxydopamine into nucleus accumbens abolish the analgesic effect of amphetamine but not of MOR in the formalin test. Brain Res. 580, 106–110. doi: 10.1016/0006-8993(92)90932-Y
Cobacho, N., Calle, J. L., and Paíno, C. L. (2014). Dopaminergic modulation of neuropathic pain: analgesia in rats by a D2-type receptor agonist. Brain Res. Bull. 106, 62–71. doi: 10.1016/j.brainresbull.2014.06.003
Collard, V., Mistraletti, G., Asenjo, J. F., Feldman, L. S., Fried, G. M., and Carli, F. (2007). Intraoperative esmolol infusion in the absence of opioids spares postoperative fentanyl in patients undergoing ambulatory laparoscopic cholecystectomy. Anesth. Analg. 105, 1255–1262. doi: 10.1213/01.ane.0000282822.07437.02
Coloma, M., Chiu, J. W., White, P. F., and Armbruster, S. C. (2001). The use of esmolol as an alternative to remifentanil during desflurane anesthesia for fast-tract outpatient gynecologic laparoscopic surgery. Anesth. Analg. 92, 352–357. doi: 10.1213/00000539-200102000-00014
Dai, W. L., Xiong, F., Yan, B., Cao, Z. U., Liu, W. T., Liu, J. H., et al. (2016). Blockade of neuronal dopamine D2 receptor attenuates morphine tolerance in mice spinal cord. Sci. Rep. 6:38746. doi: 10.1038/srep38746
Damaj, M. (2007). Behavioral modulation of neuronal calcium/calmodulin-dependent protein kinase II activity: differential effects on nicotine-induced spinal and supraspinal antinociception in mice. Biochem. Pharmacol. 74, 1247–1252. doi: 10.1016/j.bcp.2007.07.008
Danielsson, J., Zaidi, S., Kim, B., Funayama, H., Yim, P. D., Xu, D., et al. (2016). Airway epithelial cell release of GABA is regulated by protein kinase A. Lung 194, 401–408. doi: 10.1007/s00408-016-9867-2
Degoute, C. S. (2007). Controlled hypotension: a guide to drug choice. Drugs. 67, 1053–1076. doi: 10.2165/00003495-200767070-00007
Deten, A., Volz, H. C., Holzl, A., Briest, W., and Zimmer, H. G. (2003). Effect of propranolol on cardiac cytokine expression after myocardial infarction in rats. Mol. Cell Biochem. 251, 127–137. doi: 10.1023/A:1025498319598
Dolan, S., and Nolan, A. M. (2001). Biphasic modulation of nociceptive processing by the cyclic AMP-protein kinase A signalling pathway in sheep spinal cord. Neurosci. Lett. 309, 157–160. doi: 10.1016/S0304-3940(01)02063-8
Fávaro-Moreira, N. C., Parada, C. A., and Tambeli, C. H. (2012). Blockade of β1-, β2- and β3-adrenoceptors in the temporomandibular joint induces antinociception especially in female rats. Eur. J. Pain 16, 1302–13010. doi: 10.1002/j.1532-2149.2012.00132.x
Fennessy, M. R., and Lee, J. R. (1970). Modification of morphine analgesia by drugs affecting adrenergic and tryptaminergic mechanisms. J. Pharm. Pharmac. 22, 930–935. doi: 10.1111/j.2042-7158.1970.tb08475.x
Garcely, R. (1995). “Studies of pain in normal man,” in Textbook of Pain, 3rd Edn, eds R. Melzack and P. Wall (London: Churchill Livingstone), 315–336.
Gorlitz, B. D., and Frey, H. H. (1972). Central monoamines and antinociceptive drug action. Eur. Z. Pharmac. 20, 171–180. doi: 10.1016/0014-2999(72)90146-X
Grimm, M., and Brown, J. H. (2010). β-Adrenergic receptor signaling in the heart: role of CaMKII. J. Mol. Cell Cardiol. 48, 322–330. doi: 10.1016/j.yjmcc.2009.10.016
Haghighi, M., Sedighinejad, A., Mirbolook, A., Nabi, B., Farahmand, M., Kazemnezhad, E., et al. (2015). Effect of intravenous intraoperative esmolol on pain management following lower limb orthopedic surgery. Korean J. Pain 28, 198–202. doi: 10.3344/kjp.2015.28.3.198
Hajighasemi, F., and Mirshafiey, A. (2010). In vitro effects of propranolol on T helper type 1 cytokine profile in human leukemic T cells. Int. J. Hematol. Oncol. Stem Cell Res. 10, 99–105.
Hajighasemi, F., and Mirshafiey, A. (2016). Propranolol effect on proliferation and vascular endothelial growth factor secretion in human immunocompetent cells. J. Clin. Immunol. Immunopathol. Res. 2, 22–27.
Harris, G. C., and Aston-Jones, G. (1993). Beta-adrenergic antagonists attenuate somatic and aversive signs of opiate withdrawal. Neuro Psychopharmacol. 9, 303–311. doi: 10.1038/npp.1993.66
Hemnani, T. J., Khan, I. M., Patki, V. P., and Dashputra, P. G. (1983). Effect of diphenyl-hydantoin with diazepam on electoseizure and chemoseizure susceptibility in mice. Indian J. Med. Res. 77, 521–524.
Hunskaar, S., and Hole, K. (1987). The formalin test in mice: dissociation between inflammatory and non-inflammatory pain. Pain 30, 103–114. doi: 10.1016/0304-3959(87)90088-1
Kaneto, H., and Inoue, M. (1990). Action site of adrenergic blockers to suppress the development of tolerance to morphine analgesia. Brain Res. 507, 35–39. doi: 10.1016/0006-8993(90)90518-G
Kato, H., Kawaguchi, M., Inoue, S., Hirai, K., and Furuya, H. (2009). The effects of beta-adrenoceptor antagonists on proinflammatory cytokine concentrations after subarachnoid hemorrhage in rats. Anesth. Analg. 108, 288–295. doi: 10.1213/ane.0b013e318187bb93
Khanna, N., Malhotra, R. S., Mehta, A. K., Garg, G. R., Halder, S., Sharma, K., et al. (2011). Interaction of morphine and potassium channel openers on experimental models of pain in mice. Fundam. Clin. Pharmacol. 25, 479–484. doi: 10.1111/j.1472-8206.2010.00880.x
Kihara, T., and Kaneto, H. (1986). Important role of adrenergic function in the development of analgesic tolerance to morphine in mice. Jpn. J. Pharmacol. 42, 419–423. doi: 10.1254/jjp.42.419
Koelemay, M. J., and Legemate, D. A. (2008). Perioperative beta-blockade for reduction of cardiovascular complications in non-cardiac surgery: advantages and disadvantages. Ned. Tijdschr. Geneeskd. 152, 2603–2605.
Koster, R., Anderson, M., and De Beer, E. J. (1959). Acetic acid for analgesic screening. Fed. Proc. 18, 412–416.
Kumar, R., Mehra, R. D., and Ray, B. S. (2010). L-type calcium channel blockers, morphine and pain: newer insights. Indian J. Anaesth. 54, 127–131. doi: 10.4103/0019-5049.63652
Law, P. Y., and Loh, H. H. (1999). Regulation of opioid receptor activities. J. Pharmacol. Exp. Ther. 289, 607–624.
Li, Q. Q., Shi, G. X., Yang, J. W., Li, Z. X., Zhang, Z. H., He, T., et al. (2015). Hippocampal cAMP/PKA/CREB is required for neuroprotective effect of acupuncture. Physiol. Behav. 139, 482–490. doi: 10.1016/j.physbeh.2014.12.001
Liang, D. Y., Shi, X., Li, X., Li, J., and Clark, D. (2007). The β2 adrenergic receptor regulates morphine tolerance and physical dependence. Behav. Brain Res. 181, 118–126. doi: 10.1016/j.bbr.2007.03.037
Motta, P. G., Veiga, A. P. C., Francischi, J. N., and Tatsuo, M. (2004). Evidence for participation of GABAA receptors in a rat model of secondary hyperalgesia. Eur. J. Pharmacol. 483, 233–239. doi: 10.1016/j.ejphar.2003.10.015
Nestler, E. J. (1996). Under siege: the brain on opiates. Neuron 16, 897–900. doi: 10.1016/S0896-6273(00)80110-5
Nguyen, L. P., Omoluabi, O., Parra, S., Frieske, J. M., Clement, C., Ammar- Aouchiche, Z., et al. (2008). Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am. J. Respir. Cell Mol. Biol. 38, 256–262. doi: 10.1165/rcmb.2007-0279RC
Omar, A. S. (2007). Modulation of visceral nociception, inflammation and gastric mucosal injury by cinnarazine. Drug Target Insights 2, 29–38.
Oprée, A., and Kress, M. (2000). Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J. Neurosci. 20, 6289–6293.
Pajot, J., Pelissier, T., Sierralta, F., Raboisson, P., and Dallel, R. (2000). Differential effects of trigeminal tractotomy on Adelta- and C-fiber-mediated nociceptive responses. Brain Res. 863, 289–292. doi: 10.1016/S0006-8993(00)02157-0
Pepe, S., Brink, O. W., Lakatta, E. G., and Xiao, R. P. (2004). Cross-talk of opioid peptide receptor and beta-adrenergic receptor signalling in the heart. Cardiovasc. Res. 63, 414–422. doi: 10.1016/j.cardiores.2004.04.022
Rangel-Barajas, C., Coronel, I., and Florán, B. (2015). Dopamine receptors and neurodegeneration. Aging Dis. 6, 349–368. doi: 10.14336/AD.2015.0330
Reisi, Z., Haghparast, A., Pahlevani, P., Shamsizadeh, A., and Haghparast, A. (2014). Interaction between the dopaminergic and opioidergic systems in dorsal hippocampus in modulation of formalin-induced orofacial pain in rats. Pharmacol. Biochem. Behav. 124, 220–225. doi: 10.1016/j.pbb.2014.06.015
Richard, E. C., William, L. D., Louis, S. H., and Werner, L. (1975). Effect of propranolol on antinociceptive and withdrawal characteristics of morphine. Pharmacol. Biochem. Behav. 3, 843–847. doi: 10.1016/0091-3057(75)90115-X
Ringkamp, M., Schepers, R. J., Shimada, S. G., Johanek, L. M., Hartke, T. V., Borzan, J., et al. (2011). A role for nociceptive, myelinated nerve fibers in itch sensation. J. Neurosci. 31, 14841–14849. doi: 10.1523/JNEUROSCI.3005-11.2011
Sehgal, N., Colson, J., and Smith, H. S. (2013). Chronic pain treatment with opioid analgesics: benefits versus harms of long-term therapy. Expert Rev. Neurother. 13, 1201–1220. doi: 10.1586/14737175.2013.846517
Shao, X. M., Sun, J., Jiang, Y. L., Liu, B. Y., Shen, Z., Fang, F., et al. (2016). Inhibition of the cAMP/PKA/CREB pathway contributes to the analgesic effects of electroacupuncture in the anterior cingulate cortex in a rat pain memory model. Neural Plast. 2016:5320641. doi: 10.1155/2016/5320641
Smith, H. (2006). beta blockers as analgesic adjuvants. J. Neuropathic Pain Symptom palliat. 1, 21–24. doi: 10.1300/J426v01n04_04
Song, X. J., Wang, Z. B., Gan, Q., and Walters, E. T. (2006). cAMP and cGMP contribute to sensory neuron hyperexcitability and hyperalgesia in rats with dorsal root ganglia compression. J. Neurophysiol. 95, 479–492. doi: 10.1152/jn.00503.2005
Tang, F. K., Hua, N., Lu, H., Xiao, J., Tang, X. Z., and Qi, Z. (2008). Effects of bisoprolol on serum interleukin-6 and tumor necrosis factor-alpha level in patients with congestive heart failure. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 24, 1177–1179.
Vamecq, J., Mention-Mulliez, K., Leclerc, F., and Dobbelaere, D. (2015). Opioid facilitation of β-adrenergic blockade: a new pharmacological condition? Pharmaceuticals 8, 664–674. doi: 10.3390/ph8040664
Wood, P. B. (2008). Role of central dopamine in pain and analgesia. Expert Rev. Neurother. 8, 781–797. doi: 10.1586/14737175.8.5.781
Wood, P. L. (1983). Opioid regulation of CNS dopaminergic pathways: a review of methodology, receptor types, regional variations and species differences. Peptides 4, 595–601. doi: 10.1016/0196-9781(83)90003-7
Woolfe, G., and MacDonald, A. D. (1944). The evaluation of the analgesic action of pethidine hydrochloride. J. Pharmacol. Exp. Ther. 80, 300–307.
Xiao, C., Zhou, C., Atlas, G., Delphin, E., and Ye, J. (2008). Labetalol facilitates GABAergic transmission in rat periaqueductal gray neurons via antagonizing B1- adrenergic receptors - possible mechanism underlying labetalol-induced analgesia. Brain Res. 198, 34–43. doi: 10.1016/j.brainres.2008.01.023
Zaugg, M., Tagliente, T., Lucchinetti, E., Jacobs, E., Krol, M., Bodian, C., et al. (1999). Beneficial effects from beta-adrenergic blockade in elderly patients undergoing noncardiac surgery. Anesthesiology 91, 1674–1686. doi: 10.1097/00000542-199912000-00020
Keywords: opioids, propranolol, antinociception, hot plate, formalin, acetic acid, D2 receptors, GABAA receptors
Citation: Afify EA and Andijani NM (2017) Potentiation of Morphine-Induced Antinociception by Propranolol: The Involvement of Dopamine and GABA Systems. Front. Pharmacol. 8:794. doi: 10.3389/fphar.2017.00794
Received: 08 August 2017; Accepted: 20 October 2017;
Published: 10 November 2017.
Edited by:
Robert M. Caudle, University of Florida, United StatesReviewed by:
Kabirullah Lutfy, Western University of Health Sciences, United StatesCopyright © 2017 Afify and Andijani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elham A. Afify, YWZpZnkwMDFAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.