- 1Department of Pharmaceutical Biology, Institute of Pharmacy and Biochemistry, University of Mainz, Mainz, Germany
- 2Department of Biochemistry, Faculty of Science, University of Dschang, Dschang, Cameroon
Cancer remains a major health hurdle worldwide and has moved from the third leading cause of death in the year 1990 to second place after cardiovascular disease since 2013. Chemotherapy is one of the most widely used treatment modes; however, its efficiency is limited due to the resistance of cancer cells to cytotoxic agents. The present overview deals with the potential of the flora of Central, Eastern and Western African (CEWA) regions as resource for anticancer drug discovery. It also reviews the molecular targets of phytochemicals of these plants such as ABC transporters, namely P-glycoprotein (P-gp), multi drug-resistance-related proteins (MRPs), breast cancer resistance protein (BCRP, ABCG2) as well as the epidermal growth factor receptor (EGFR/ErbB-1/HER1), human tumor suppressor protein p53, caspases, mitochondria, angiogenesis, and components of MAP kinase signaling pathways. Plants with the ability to preferentially kills resistant cancer cells were also reported. Data compiled in the present document were retrieved from scientific websites such as PubMed, Scopus, Sciencedirect, Web-of-Science, and Scholar Google. In summary, plant extracts from CEWA and isolated compounds thereof exert cytotoxic effects by several modes of action including caspases activation, alteration of mitochondrial membrane potential (MMP), induction of reactive oxygen species (ROS) in cancer cells and inhibition of angiogenesis. Ten strongest cytotoxic plants from CEWA recorded following in vitro screening assays are: Beilschmiedia acuta Kosterm, Echinops giganteus var. lelyi (C. D. Adams) A. Rich., Erythrina sigmoidea Hua (Fabaceae), Imperata cylindrical Beauv. var. koenigii Durand et Schinz, Nauclea pobeguinii (Pobég. ex Pellegr.) Merr. ex E.M.A., Piper capense L.f., Polyscias fulva (Hiern) Harms., Uapaca togoensis Pax., Vepris soyauxii Engl. and Xylopia aethiopica (Dunal) A. Rich. Prominent antiproliferative compounds include: isoquinoline alkaloid isotetrandrine (51), two benzophenones: guttiferone E (26) and isoxanthochymol (30), the isoflavonoid 6α-hydroxyphaseollidin (9), the naphthyl butenone guieranone A (25), two naphthoquinones: 2-acetylfuro-1,4-naphthoquinone (4) and plumbagin (37) and xanthone V1 (46). However, only few research activities in the African continent focus on cytotoxic drug discovery from botanicals. The present review is expected to stimulate further scientific efforts to better valorize the African flora.
Introduction
Cancer is a term for a series of malign diseases characterized by abnormal cell proliferation, leading to invasion and metastasis, the ultimate causes of deaths by cancer. The burden of neoplastic diseases affects the entire world population. Over the past two decades, there has been a slight improvement in cancer statistics due to diagnostic and therapeutic progresses and a better understanding of tumor biology (Siegel et al., 2014). However, cancer remains associated with very high mortality rates, which indicate still existing difficulties of effective treatment. Chemotherapy is one of the most widely used modes of anti-cancer therapy. However, the development of resistance of cancer cells to cytotoxic agents represents a main factor, which is responsible for the non-satisfactory treatment outcomes associated with malignant diseases (Singh and Settleman, 2010). In fact, most types of cancer cells reveal variable degrees of resistance to antineoplastic agents (Luqmani, 2005). In 2008, men in Africa had more than double of the rate of world liver cancer cases, whilst women had the highest incidence of cervical cancer of the world. Medicinal plants have long been used to fight against cancer. Several natural products isolated from medicinal plants including: terpenoids, phenolics, and alkaloids play an important role in cancer treatment (Kaur et al., 2011). More than 3,000 plants worldwide have been reported to exert cytotoxicity toward cancer cells (Graham et al., 2000; Solowey et al., 2014). About 80% of the rural African population almost exclusively uses traditional medicine for its primary health care needs (Farnsworth et al., 1985). For cultural and economic reasons, medicinal plants constitute the major part of traditional medicine. In the recent years, numerous African medicinal plants have been screened for their cytotoxic potential. This review deals with plants and derived molecules from Central, Eastern and Western Africa (CEWA) as potential resource for cancer chemotherapy with emphasis on their molecular targets. Countries of Central Africa include: Cameroon, Gabon, Equatorial Guinea, Central African Republic, Congo, Democratic Republic of Congo, São Tomé and Príncipe, Chad, Angola. East Africa comprises of Kenya, Uganda, Tanzania, Rwanda, Burundi, Sudan, Eritrea, Djibouti, Ethiopia, Somalia, Seychelles, Comoros, Mauritius Island, Madacascar, Mozambique, and Malawi. Western African countries include Benin, Burkina Faso, Ivory Coast, Gambia, Ghana, Guinea, Guinea- Bissau, Cape Verde, Nigeria, Mali, Mauritania, Niger, Liberia, Senegal, Sierra Leone, and Togo. Hence, the medicinal plants of CEWA described in the present review cover a considerable portion of the African continent.
Overview of Cancer Burdenin Africa
Cancer moved from the third leading cause of death worldwide in 1990 to the second leading cause of death after cardiovascular disease since 2013, with more than 8 million deaths in 2013 (Murray and Lopez, 1997; Lozano et al., 2012). Although significant progress has been made in recent years in cancer prevention and treatment (Edwards et al., 2014; Allemani et al., 2015), the burden of cancer is increasing as a result of a growing and aging population worldwide, in addition to risk factors such as smoking, obesity and diet. To adequately allocate resources for prevention, screening, diagnosis, treatment and palliative care, and to monitor its effectiveness, there is an urgent need for timely information on the burden of cancer for each country. It is worth noting that in several African countries, the cancer burden still remains unclear in terms of reliable epidemiological data, though most practicing physicians recognize that the number of cases among patients visiting local health facilities continuously increases (Omosa et al., 2015). By 2020, 15 million new cancer cases are annually expected, 70% of which will be from developing countries. African countries will account for more than a million new cancer cases per year and have to cope with them despite few cancer care services (Vorobiof and Abratt, 2007). In Africa, about a third of cancer deaths are potentially preventable. In sub-Saharan Africa in 2002, more than half a million deaths from cancer were reported, with nearly 40% related to chronic infections and smoking (Vorobiof and Abratt, 2007). Due to the lack of basic resources and infrastructure, most Africans, including those in CEWA, do not have access to cancer screening, early diagnosis, appropriate treatment or palliative care. For example, radiotherapy is available in only 21 of the 53 African countries, reaching less than 5% of the population, and consequently patients are deprived of life-saving treatment (Vorobiof and Abratt, 2007).
Molecular Targets of Phytochemicals and Their Role in the Resistance of Tumors to Cytotoxic Drugs
The role of phytochemicals as cytotoxic agents against cancer cell lines has frequently been reported. Various plant molecules including nutraceuticals, such as allicin, apigenin, berberine, catechin gallate, celastrol, curcumin, epigallocatechin gallate, fisetin, flavopiridol, gambogicacid, genistein, plumbagin, quercetin, resveratrol, silibinin, taxol, etc. derived from spices, legumes, fruits, nuts, and vegetables have been shown to modulate inflammatory pathways and exert inhibitory effects against tumor cells (Chirumbolo, 2012). Several other molecules from medicinal plants are already clinically established for cancer treatment, for example alkaloids such as vinblastine and vincristine isolated from Catharanthus roseus (Gullett et al., 2010), combretastatins isolated from Combretum caffrum (Cirla and Mann, 2003), paclitaxel, obtained from Taxus brevifolia (Luduena, 1998), camptothecin isolated from Camptotheca acuminata and homoharringtonine isolated from Cephalotaxus harringtonia (Aboul-Enein et al., 2014). Phytochemicals and nutraceuticals have frequently many molecular targets. Targets of natural products include: Aurora-A, Cdc2, Cdc25a, Cyclin B1, Cyclin D1, E2F4, RB, FoxM1, Skp2, p16, p21, p27 (cell cycle), EGFR, IGF-I, IGF-II, IGF-1R, IGFBP-1, IGFBP-2, IGFBP-3, IGFBP-5, ERK, JNK/c-Jun, p38, Akt, mTOR, PI3K, PTEN, 4E-BP1, G3BP1, Ras, ErbB2 (growth factor signaling), androgen receptor, estrogen receptors (ERα, ERβ), (hormone signaling), FOXO, C/EBPα, BTG3, PHB, Pin1, PKCα, PKCδ, RARα, RARβ, VDR, telomerase (Non-classified targets). Targets involving apoptotic pathways include Apaf-1, GDF15, BAD, Bax, Bcl-2, Bcl-xL, Bcl-xS, caspases 3, 8, 9, and 10, cIAP1, XIAP, DR5, Fas, Hsp70 andsurvivin. Phytochemicals are also involved in other cell activities such as cell metabolism modification (SphK1, HIF-1α, FASN, HMG-CoA reductase, AMPK, PFKFB4), drug resistance inhibition (MRP5, BCRP, P-glycoprotein), genome stability (ATM/Chk1, BRCA1, BRCA2, p53, topoisomerase-II), inhibition of immune evasion (IL-10, IDO, TGFβ), inhibition of invasion, metastasis and angiogenesis (E-cadherin, CXCL1, CXCL2, CXCL12, CXCR4, EMMPRIN, connexin 43, KAI1, c-Met, endoglin, VEGF/VEGFR, vimentin, ZEB1, MMP-2, -7, -9, PAK1) and stemness inhibition (Gli1, WIF-1, Wnt/β-catenin, Notch-1, Notch-2, Twist-1), antioxidant/carcinogen metabolism (hSULT1A1, hSULT2A1, UGT1A, QR, GST, Nrf2, ARE, CYP1A1, metallothionein), anti-inflammation (IL-1RI, CCL2, NF-κB, IKK, COX-1, COX-2, PGE2, iNOS, PPARγ) (Gonzalez-Vallinas et al., 2013). However, in this section, we will discuss the most currently investigated targets of plants and their derived molecules as well as those involved in cancer drug resistance.
ABC Transporters and Drug Resistance
The adenosine triphosphate (ATP)-binding cassette (ABC) proteins are amongst the largest protein families found in all living organisms from microbes to humans (Efferth and Volm, 2017). The roles of ABC transporters include binding to and hydrolysis of ATP to fuel energy-dependent efflux of specific compounds across the membrane or to return them from the inner to the outer surface of membranes (Dean, 2009). Malignant cells resist to anticancer drugs by mutation or overexpression of drug targets, as well as by inactivation or efflux of the compounds to prevent cytotoxic drug concentrations sufficient to kill tumor cells (Gottesman et al., 2006). Human ABC transporters involved in drug resistance include ABCA3 or ABC3/ABCC (ABCA family), ABCB1 or MDR1/P-glycoprotein (P-gp) (ABCB family), ABCC1 or MRP1 and ABCC3 or MRP3/cMOAT-2 (ABCC family), ABCG2 or ABCP/MXR/BCRP (ABCG family) (Glavinas et al., 2004). The roles of P-gp, multidrug-resistance-proteins (MRPs) and breast cancer resistance protein (BCRP) in cancer drug resistance have been intensively investigated (Efferth, 2001; Gillet et al., 2007).
P-glycoprotein (P-gp)
P-gp is encoded by the ABCB1/MDR1 gene and was identified as the first ABC transporter to be overexpressed in multidrug resistant cancer cell lines (Kartner et al., 1985). P-gptransports and/or secretes substrates in normal tissues such as the kidney, liver, colon, and adrenal gland as well as in the blood-brain, blood-placenta, and blood-testis barriers to protect these tissues from harmful compounds (Katayama et al., 2014). P-gp is involved in the efflux of doxorubicin, daunorubicin, vincristine, etoposide, colchicine, camptothecins and methotrexate, leading to resistance of cancer cells to these molecules (Dean, 2009). Clinical trials with synthetic drugs undertaken since 1994 have not resulted in significant progress in the discovery of new blockbusters for chemotherapy (Dean et al., 2005). Combating cancer-drug-resistance with phytochemicals inhibiting ABCB1 could therefore be a more promising strategy to overcome multi-drug resistance (MDR). Additionally, other ABC transporters such as ABCC1/MRP1 (Cole et al., 1992) and ABCG2 (Kim et al., 2002) are also overexpressed in cancer cells and could be targeted by plant products.
Multidrug-Resistance-Related Proteins (MRPs)
MRPs comprise of at least 9 types of transporters termed MRP1-9. They transport a wide array of structurally diverse molecules across cell membranes. They are involved in the absorption, disposition, and elimination of compounds in the body (Tian et al., 2005). MRPs are ATP-dependent efflux pumps having broad substrate specificity for the transport of endogenous substances such as glutathione conjugates (leukotriene C4 for MRP1, MRP2, and MRP4), bilirubin glucuronosides (MRP2 and MRP3), and cyclic AMP and cyclic GMP (MRP4, MRP5, and MRP8) as well as xenobiotic anionic substances localized in cellular plasma membranes (Keppler, 2011). Their overexpression in malignant cells is associated with resistance to a number of important cytotoxic drugs. MRPs are involved in the efflux of several anticancer drugs such as doxorubicin, daunorubicin, vincristine, etoposide, colchicine, camptothecins, methotrexate (MRP1), vinblastine, cisplatin, doxorubicin, methotrexate (MRP2), methotrexate, etoposide (MRP3), 6-mercaptopurine (6-MP), 6-thioguanine (6-TG), methotrexate (MRP4), 6-MP and 6-TG (MRP5), etoposide (MRP6) and 5-fluorouracil (MRP8) (Dean, 2009).
Breast Cancer Resistance Protein (BCRP, ABCG2)
MXR alias BCRP is an ABC transporter that plays a role in absorption, distribution, metabolism and excretion in normal tissues (Natarajan et al., 2012). Its overexpression in tumor cells confers resistance to chemotherapy by active extrusion of cytotoxic compounds. BCRP is involved in the efflux of mitoxantrone, topotecan, doxorubicin, daunorubicin, irinotecan, imatinib, and methotrexate (Dean, 2009). This receptor protein is involved in MDR of several tumor types including: acute leukemia and other hematological malignancies, head and neck carcinoma, breast cancer, lung cancer, brain tumors, hepatocellular carcinoma, gastrointestinal cancers such as pancreatic, colon, gastric and esophageal carcinomas (Natarajan et al., 2012).
Epidermal Growth Factor Receptor (EGFR/ErbB-1/HER1)
The epidermal growth factor receptor (EGFR; ErbB-1; HER1), a signal transducer for cell growth and differentiation, is the cell-surface receptor belonging to the ErbB family of receptors. This family consists of four closely related receptor tyrosine kinases, namely EGFR/HER1/ErbB-1, HER2/c-neu/ErbB-2, HER3/ErbB-3, and HER4/ErbB-4. Mutations affecting the activity or expression of EGFR can contribute to carcinogenesis (Zhang et al., 2007). Upon stimulation by ligands, EGFR is activated through homodimerization or heterodimerization and transmit signals to downstream substrates such as PI3K/AKT, RAS/RAF/MAPK, and STAT3/5 pathways, leading to cell proliferation and cell survival (Ji, 2010). Downstream substrates of EGFR have been found responsible for drug resistance meanwhile activation of PI3K/AKT pathway is essential for cancer cell survival (Ji, 2010). ErbB family receptors represent important targets of anticancer therapeutics such as tyrosine kinase inhibitors (TKIs; for example gefitinib and erlotinib) (Zhang et al., 2007).
Human Tumor Suppressor Protein p53
The tumor suppressor protein p53 is encoded by the TP53 gene in human beings and Trp53 gene in mice. It is crucial in multicellular organisms, where it prevents cancer formation, thus, functions as a tumor suppressor (Surget et al., 2013). The gene p53 is involved in the regulation of cell fate in response to different stresses in normal cells through the differential regulation of gene expression. Abnormal p53 expression actively contributes to cancer formation and progression in malignant cells. The gene p53 is also associated with response to cancer treatment by regulating apoptosis, genomic stability, and angiogenesis. Overexpression of mutated p53 with reduced or abolished function is often associated with resistance to various cytotoxic drug such as cisplatin, temozolomide, doxorubicin, gemcitabine, tamoxifen, and cetuximab (Hientz et al., 2017).
Caspases as Anticancer Drug Target
Caspases or cysteine-aspartic proteases are a family of protease enzymes essential for programmed cell death and inflammation. There are 14 mammalian caspases, 12 of which are of human origin (caspases 1–10, 12, and 14). They can be classified into three main types, that are initiator caspase (2, 8, 9, and 10), executioner or effector caspases (3, 6, and 7) and inflammatory caspases (1, 4, 5, 11, and 12) (Galluzzi et al., 2016). Caspase-14 plays a role in epithelial cell keratinocyte differentiation, and forms an epidermal barrier that protects against dehydration and UVB radiation (Denecker et al., 2008). Upon activation, caspases cleave a variety of substrates including: proteins involved in signal transduction (apoptosis regulators, cytokines, serine/threonine kinases), structural proteins (cytoskeletal and nuclear) and proteins involved in regulation of transcription, translation and RNA editing (Howley and Fearnhead, 2008). Deregulation of caspase activation or expression also leads to neurodegenerative and autoinflammatory disorders (Howley and Fearnhead, 2008). Initiator caspase-9 is activated in the apoptosome, while caspase-2 is activated in the PIDDosome and caspase-8 or -10 in the death-inducing signaling complex (DISC) (Howley and Fearnhead, 2008). Activated initiator caspases activate effector caspases, which in turn cleave structural and regulatory proteins culminating in the features of apoptosis. The search for caspase modulators is a novel attractive therapeutic approach in cancer research (Howley and Fearnhead, 2008).
Mitochondria as Anticancer Drug Target
Mitochondria play a central role in cellular metabolism, calcium homeostasis, redox signaling, and cell fate as main ATP source. During ATP biosynthesis, reactive oxygen species (ROS) are generated. In many cancer cells, mitochondria appear to be dysfunctional (due to a variety of factors, such as oncogenic signals and mitochondrial DNA mutations), with a shift in energy metabolism from oxidative phosphorylation to active glycolysis and an increase in the generation of ROS (Wen et al., 2013). The energy metabolism is different between normal and cancer cells, providing a scientific basis for development of strategies to selectively target malignant cells. As a result of mitochondrial dysfunction, cancer cells rely more on the glycolytic pathway in the cytosol to generate ATP. Key enzymes in this pathway such as hexokinase II, glyceraldehyde 3-phosphate dehydrogenase (overexpressed in malignant cells) therefore became potential therapeutic targets (Wen et al., 2013). Mitochondria-targeting compounds can kill drug-resistant cancer cells due to their ability to initiate mitochondrial outer membrane permeabilization in mitochondria, independently of other upstream signaling processes that may be impaired in cancer cells (Fulda and Kroemer, 2011). Some potential therapeutic targets associated with mitochondria include NADPH oxidases (NOX), the translocator protein (TSPO), the mitochondrial protein known as complement component 1, q subcomponent-binding protein (C1qBP) and the monocarboxylate transporters (MCTs) (Wen et al., 2013). Compounds known to target the mitochondrial membrane potential are for instance the natural alkaloid pancratistatin, rhodamine-123, 4-phenyl-2,7-di(piperazin-1-yl)-1,8-naphthyridine, 2,5-diaziridinyl-3- (hydroxymethyl)-6-methyl-1,4-benzoquinone and edelfosine (Wen et al., 2013). Natural products such ascurcumin, resveratrol, berberine and cerulenin target mitochondrial apoptotic pathway (Wen et al., 2013).
Reactive Oxygen Species and Cancer Chemotherapy
Reactive oxygen species are chemically reactive chemical species containing oxygen such as hydroxyl radical, peroxides, superoxide, and singlet oxygen. They are produced through multiple mechanisms depending on cell and tissue types by NOX complexes in cell membranes, mitochondria, peroxisomes and endoplasmic reticulum (Muller, 2000; Han et al., 2001). ROS not only induce apoptosis, but also regulate host defense genes or airway homeostasis (Conner et al., 2002; Rada and Leto, 2008). In malignant cells, ROS induce changes in cellular functions such as cell death, cell proliferation, migration and differentiation (Wen et al., 2013). Increased ROS levels and mitochondrial dysfunction make cancer cells more vulnerable than normal cells.
Angiogenesis as Anticancer Drug Target
Angiogenesis is a physiological process in embryogenesis, in wound healing and in the female reproductive cycle leading to the formation of new blood vessels from pre-existing ones (Kumaran et al., 2008; Birbrair et al., 2015). Angiogenesis is critical in cancer for growth and metastasis, as tumors cannot grow beyond 200–300 μm in diameter without recruitment of new blood vessels to maintain nutrients and oxygen supply (Kumaran et al., 2008). This also makes angiogenesis an ideal target for cancer treatment. Some established therapeutic strategies targeting angiogenesis include bevacizumab [antibody to vascular endothelial growth factor (VEGF)], sorafenib and sunitinib (tyrosine kinase inhibitors). Combretastatin (vascular disruptive agents) and endostatin (endogenous inhibitor) are currently in clinical trials (Kumaran et al., 2008).
MAP Kinase Signaling Pathways in Cancer Chemotherapy
The mitogen-activated protein kinases/extracellular signal-regulated kinases (MAPK/ERK) pathway or Ras-Raf-MEK-ERK pathway is one of the most important signal transduction pathways. The MAPK/ERK pathway regulates growth, proliferation, differentiation and survival of the cells. Its deregulation is observed in various diseases such as cancer, degenerative syndromes, immunological and inflammatory diseases, making it an important drug target (Orton et al., 2005). The activation of a MAPK employs a core three-kinase cascade consisting of a MAPK kinase kinase (MAP3K or MAPKKK), which phosphorylates/activates another MAPK kinase (MAP2K, MEK, or MKK), which in turn phosphorylates and activates more MAPKs. Upon activation, MAPKs can phosphorylate a variety of intracellular targets such as cytoskeletal elements, nuclear pore proteins, membrane transporters, transcription factors, and other protein kinases (Avruch et al., 2001). Mutations in proteins of this pathway, for example in Ras and B-Raf lead to carcinogenesis. Compounds targeting MAPK pathways are therefore investigated as potential cancer drugs (Orton et al., 2005). In fact, the role of stress-activated pathways such as Jun N-terminal kinase and p38 in the prevention of malignant transformation has been shown (Dhillon et al., 2007).
Central, Eastern and Western Africa Plants and Derived Molecules and Their Anticancer Targets
During the past decade, intensive investigations of African medicinal plants as potential anticancer drug candidates have been carried out by African scientists in collaboration with various research teams throughout the world. However, this work should be strenghtened with particular emphasis on the study of mechanisms of action and the identification of the different molecular targets of bioactive substances. Here, we give an overview of the studies published so far on plants and products derived from CEWA as far as their molecular target are available. A synopsis of phytochemicals acting preferentially on cancer cell lines actively expressing drug targets such ABC transporters, EGFR, p53 and BCRP (Figures 1–3) will also be given. For instance the degree of resistance (DR) determined as the ratio of IC50 value of the resistant/IC50 sensitive cell line will be taken into account to consider samples with potential therapeutic values to combat MDR phenotypes. Hence, samples with hypersensitivity or collateral sensitivity (more active on resistant than on parental sensitive cells line with DRs below 0.90 as well as samples with regular sensitivity (DR between 0.91 and 1.19) will be discussed. According to the criteria of the American National Cancer Institute, 20 μg/mL is the upper IC50 limit to be considered as promising for cytotoxic crude extracts (Suffness and Pezzuto, 1990). Meanwhile, a threshold of 4 μg/ml or 10 μM (Boik, 2001; Brahemi et al., 2010) after 48–72 h incubation has been set to identify compounds with considerable cytotoxic activity.
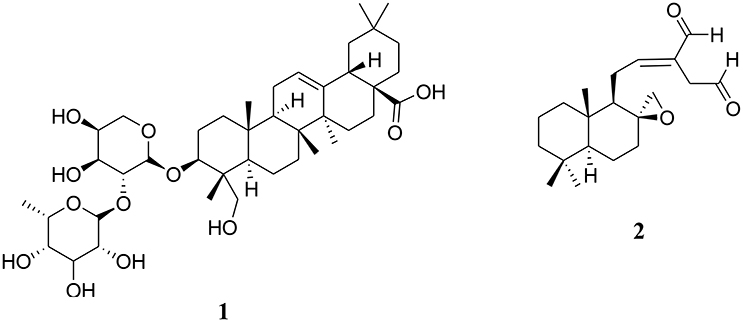
Figure 1. Chemical structures of two cytotoxic terpenoids [alpha-hederin (1) and galanal A (2)] isolated from Central, East and West African plants.
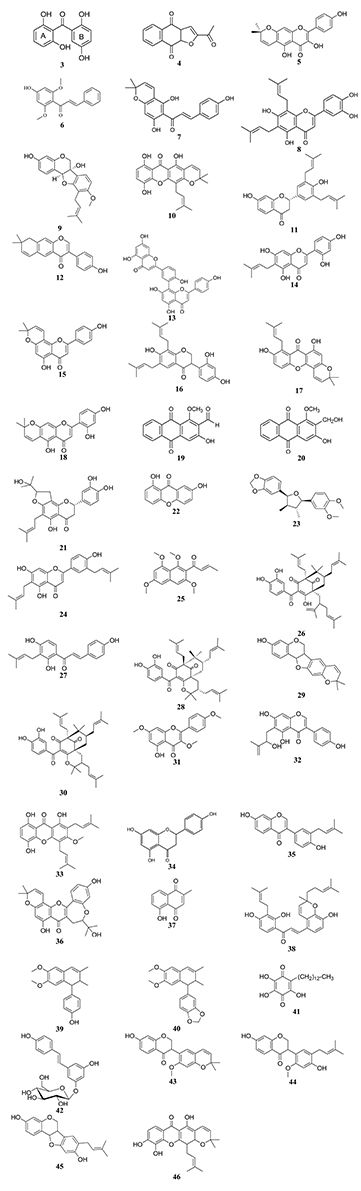
Figure 2. Chemical structures of hit cytotoxic phenolics isolated from Central, East and West African plants. 2,2′,5,6′-tetrahydroxybenzophenone 3); 2-acetylfuro-1,4-naphthoquinone (4); 3,4′,5-trihydroxy-6″,6″-dimethylpyrano[2,3-g]flavone (5); 4′-hydroxy-2′,6′-dimethoxychalcone (6); 4-hydroxylonchocarpin (7); 6,8-diprenyleriodictyol (8); 6α-hydroxyphaseollidin (9); 8-hydroxycudraxanthone G (10); abyssinone IV (11); alpinumisoflavone (12); amentoflavone (13); artocarpesin (14); atalantoflavone (15); bidwillon A (16); cudraxanthone I (17); cycloartocapesin (18); damnacanthal (19); damnacanthol (20); dorsmanin F (21); euxanthone (22); futokadsurin B (23); gancaonin Q (24); guieranone A (25); guttiferone E (26); isobavachalcone (27); isogarcinol (28); isoneorautenol (29); isoxanthochymol (30); kaempferol-3,7,4′-trimethylether (31); laburnetin (32); morusignin I (33); naringenin (34); neobavaisoflavone (35); neocyclomorusin (36); plumbagin (37); poinsettifolin B (38); pycnanthulignene A (39); pycnanthulignene B (40); rapanone (41); resveratrol β-D-glucopyranoside (42); sigmoidin H (43); sigmoidin I (44); sophorapterocarpan A (45); xanthone V1 (46).
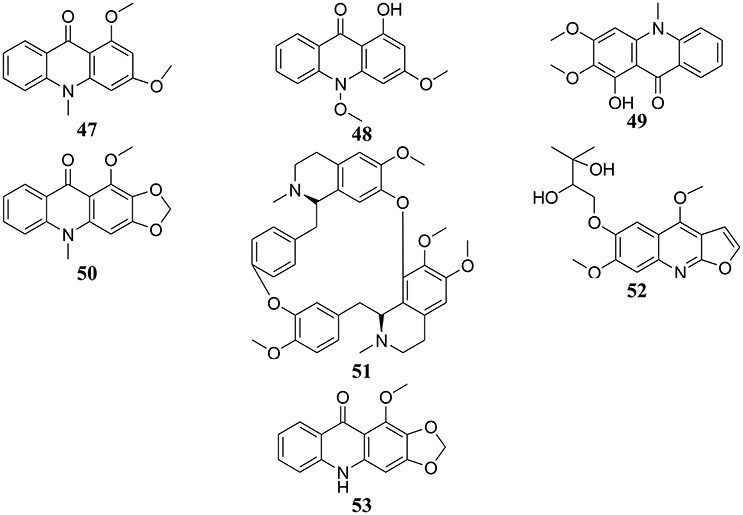
Figure 3. Chemical structures of hit cytotoxic alkaloids isolated from plants of Central, East and West Africa. 1,3-dimethoxy-10-methylacridone (47); 1-hydroxy-3-methoxy-10-methylacridone (48); arborinin (49); evoxanthine (50); isotetrandrine (51); montrifoline (52); norevoxanthine (53).
Caspases Activators
Although many African plant extracts were poor caspase activators (Kuete and Efferth, 2015), several phytochemicals from the flora of CEWA were reported as caspase activators (Tables 1, 2). Some documented caspase 3/7 activators included: benzophenones: guttiferone E (26) (Kuete et al., 2013d) and isoxanthochymol (30) from Garcinia punctata Oliv. (Kuete et al., 2013d), flavonoids: 4-hydroxylonchocarpin (7) and isobavachalcone (27) isolated from Dorstenia barteri Bureau (Kuete et al., 2011b, 2015c), 6,8-diprenyleriodictyol (8) isolated from Dorstenia mannii Hook.f. (Kuete et al., 2011b), cycloartocarpesin (18) from Morus mesozygia Stapf. (Kuete et al., 2015c), gancaonin Q (24) from Dorstenia angusticornis Engl. (Kuete et al., 2011b), isoflavonoids: 6α-hydroxyphaseollidin (9) from Erythrina sigmoidea Hua (Kuete et al., 2014c), isoneorautenol (29) from Erythrina excelsa Baker (Kuete et al., 2014d), xanthones: cudraxanthone I (17) from Milicia excelsa Welw C.C. Berg. (Kuete et al., 2013b) and xanthone V1 (46) from Vismia laurentii De Wild. (Kuete et al., 2011d). Activators of initiator caspases 8 and 9 include benzophenone 26 (Kuete et al., 2013d) and 30 (Kuete et al., 2013d), flavonid 18 (Kuete et al., 2015c), isoflavonoid 9 (Kuete et al., 2014c), or xanthone 17 (Kuete et al., 2013b).
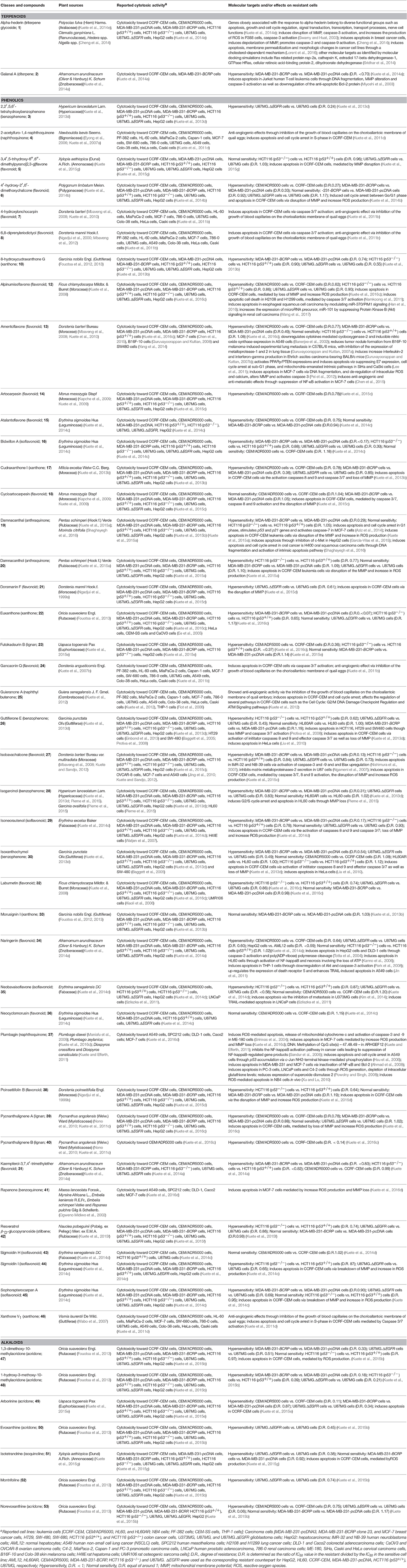
Table 2. Bioactive compounds identified in cytotoxic plants of Central, East and West Africa and their molecular targets.
Plants and Derived Compounds Targeting the Mitochondria of Cancer Cells
Several crude extracts and isolated compounds from CEWA plants targeted mitochondria to induce apoptosis in cancer cells (Tables 1, 2). Plant extracts inducing MMP alterations include: the Annonaceae plants Annona muricata Lin. (Kuete et al., 2016b), Anonidium mannii (oliv) Engl. et Diels. (Kuete et al., 2013a) and Xylopia aethiopica (Dunal) A. Rich. (Kuete et al., 2013c), a plant of Araliaceae family, Polyscias fulva (Hiern) Harms. (Kuete et al., 2014e), of Bignoniaceae, Markhamia tomentosa (Benth.) K.Schumex.Engl. (Ibrahim et al., 2013), of Compositae, Echinops giganteus var. lelyi (C. D. Adams) A. Rich. (Kuete et al., 2013c), the Euphorbiaceae, Alchornea cordifolia (Schum. & Thonn.) Müll.-Arg. (Kuete et al., 2016e) and Uapaca togoensis Pax. (Kuete et al., 2015e), the Fabaceae, Albizia adianthifolia (Schum.) (Kuete et al., 2016e) and Erythrina sigmoidea Hua (Kuete et al., 2016a), the Moraceae, Dorstenia psilurus Welwitch (Pieme et al., 2013), the Passifloraceae, Passiflora edulis Sims (Kuete et al., 2016b), the Piperaceae, Piper capense L.f. (Kuete et al., 2013c), the Poaceae, Imperata cylindrica Beauv. var. koenigii Durand et Schinz (Kuete et al., 2013c) and the Rutaceae, Vepris soyauxii Engl. (Kuete et al., 2013a). Several molecules isolated from plants of CEWA also targeted mitochondria. Some of them include: anthraquinones, damnacanthal (19) and damnacanthol (20) from Pentas schimperi (Hook f.) Verde (Kuete et al., 2015a), the benzoquinone, rapanone (41) from Maesa lanceolata Forssk., Myrsine africana L., Embelia keniensis R.E.Fr., Embelia schimperi Vatke and Rapanea pulchra Gilg & Schellenb. and the naphthoquinone, plumbagin (37) from Plumbago and Diospyros species (Kuete et al., 2016d), benzophenones, 26 and 30 (Kuete et al., 2013d), flavonoids, 3,4′,5-trihydroxy-6″,6″-dimethylpyrano[2,3-g]flavone (5) isolated Xylopia aethiopica (Dunal) A.Rich. (Kuete et al., 2015g), 4′-hydroxy-2′,6′-dimethoxychalcone (6) from Polygonum limbatum Meisn (Kuete et al., 2014b), abyssinone IV (11) from Erythrina sigmoidea Hua (Kuete et al., 2014c), alpinumisoflavone (12) from Ficus chlamydocarpa Mildbr. & Burret (Kuete et al., 2014c), dorsmanin F (21) from Dorstenia mannii Hook.f. (Kuete et al., 2015d), compound 27 (Kuete et al., 2015c) and poinsettifolin B (38) from Dorstenia poinsettifolia Engl. (Kuete et al., 2015d), isoflavonoids, 6α-hydroxyphaseollidin (9) (Kuete et al., 2014c), isoneorautenol (29) (Kuete et al., 2014d), sigmoidin I (44) and sophorapterocarpan A (45) (Kuete et al., 2014c) from Erythrina sigmoidea Hua, a lignan, pycnanthulignene A (39) from Pycnanthus angolensis (Welw.) Ward (Kuete et al., 2016c) and a xanthone, cudraxanthone I (17) from Milicia excelsa Welw C.C. Berg. (Kuete et al., 2013b).
Plants and Derived Compounds Inducing ROS Increase in Cancer Cells
The induction of apoptosis in cancer cells by some plants and derived molecules of CEWA was due to induced-ROS increase (Tables 1, 2). Plants that exert their anticancer activities via this mechanism include: Alchornea cordifolia (Schum. & Thonn.) Müll.-Arg. (Kuete et al., 2016e), Anonidium mannii (oliv) Engl. et Diels. (Kuete et al., 2013a), Dorstenia psilurus Welwitch (Pieme et al., 2013), Piper capense L.f. (Kuete et al., 2013c), Polyscias fulva (Hiern) Harms. (Kuete et al., 2014e), Sclerocarya birrea (A. Rich.) Hochst (Anacardiaceae) (Armentano et al., 2015). Compounds inducing increase in ROS production include: acridone alkaloid 1,3-dimethoxy-10-methylacridone (47) and isoquinoline alkaloid isotetrandrine (51), anthraquinones 19 and 20 (Kuete et al., 2015a), benzoquinone 41 (Kuete et al., 2016d) and naphthoquinone 37 (Kuete et al., 2016d), flavonoids 6 (Kuete et al., 2014b), 11 (Kuete et al., 2014c), 12 (Kuete et al., 2016c), 27 (Kuete et al., 2015c), 38 (Kuete et al., 2015d), isoflavonoids 9 (Kuete et al., 2014c), 29 (Kuete et al., 2014d), 44 and 45 (Kuete et al., 2014c), and lignan 39 (Kuete et al., 2016c).
Angiogenesis
Angiogenesis as treatment target against cancer was merely studied for botanicals from Africa. The Fabaceae Calliandra portoricensis (Jacq.) (Adaramoye et al., 2015) was reported as angiogenesis inhibitor through inhibition of the growth of blood capillaries on the chorioallantoic membrane of quail eggs. Compounds with similar effects include naphthoquinone 2-acetylfuro-1,4-naphthoquinone (4) (Kuete et al., 2011d), flavonoids 7, 8, and 24 (Kuete et al., 2011b), the naphthyl butenone guieranone A (25) and the xanthone 46 (Kuete et al., 2011d).
African Plants and Compounds with Regular Sensitivity and Collateral Sensitivity in Drug Resistant Cancer Cells
The investigation of the mode of action of botanicals and phytochemicals from the flora of Africa is not yet done in a systematic manner due to the lack of facilities and appropriate technology in research centers throughout the continent. However, the fight against MDR in cancer will provide conceptual clues on the molecular targets of the active samples. In collaborations with more equiped research institutes in Western countries, plants and isolated compounds from the flora of CEWA were tested on cancer cells expressing well-known drug resistance phenotypes. In Tables 1, 2, results on samples are documented, that inhibited resistant cell lines with similar efficacy than sensitive ones (regular sensitivity). In some cases, it was observed that resistant cells were killed with even better efficacy than sensitive cells (hyper-sensitivity or collateral sensitivity). These plant extracts and phytochemicals could be especially useful to fight MDR in cancer. In this section, we will focus on plants and compounds exerting hypersensitivity on cell lines over-expressing ABC transporters, EGFR and with p53 knock out genes.
Plants and Compounds Acting in Cancer Cells Over-Expressing ABC Transporters
Some botanicals and phytochemicals from CEWA were screened against ABC transporters-expressing cell lines. The most investigated cell lines included the P-gp-overexpressing CEM/ADR5000 leukemia cell line, the MRP1-expressing HL60/AR leukemia cell line and BCRP-expressing MDA-MB-231/BCRP breast adenocarcinoma cell line. The studies were mainly conducted by the team of Professor Thomas Efferth (University of Mainz, Germany). Plants and compounds inducing hypersensitivity in these cell lines are summarized in Tables 1, 2.
The hypersensitivity of CEM/ADR5000 cells compared to its parental cell line CCRF-CEM was induced by Aframomum arundinaceum (Oliver & Hanbury) K. Schum (Zingiberaceae) (Kuete et al., 2014a), Imperata cylindrica Beauv. var. koenigii Durand et Schinz (Poaceae) (Kuete et al., 2013c), Nauclea pobeguinii (Pobég. ex Pellegr.) Merr. ex E.M.A. (Rubiaceae) (Kuete et al., 2015f), Pachypodanthium staudtii Engl & Diels (Annonaceae) (Kuete et al., 2016b), Piper capense L.f. (Piperaceae) (Kuete et al., 2013c) and Zinziber officinale Roscoe (Zingiberaceae) (Kuete et al., 2011a). Compunds with similar activity included: acridone alkaloids, 49 and 53 (Kuete et al., 2015b), anthraquinone, 19 (Kuete et al., 2015a), flavonoids, 4′-hydroxy-2′,6′-dimethoxychalcone (6) from Polygonum limbatum Meisn (Kuete et al., 2014b), 12 (Kuete et al., 2016c), amentoflavone (13) from Dorstenia barteri (Kuete et al., 2016c), atalantoflavone (15) from Erythrina sigmoidea Hua (Kuete et al., 2014c), naringenin (34) from Aframomum arundinaceum (Oliver & Hanbury) K. Schum (Kuete et al., 2014a), lignans, futokadsurin B (23) from Uapaca togoensis Pax. (Kuete et al., 2016c), 39 and 40 (Kuete et al., 2016c) and xanthone 17 (Kuete et al., 2013b).
Plant extracts inducing hypersensitivity in MDA-MB-231-BCRP clone 23 cells compared to its sensitive counterparts MDA-MB-231 cells include: Aframomum polyanthum K. Schum (Zinziberaceae) (Kuete et al., 2014a), Nauclea latifolia Smith. (Rubiaceae) (Kuete et al., 2014a), Nauclea pobeguinii (Pobég. ex Pellegr.) Merr. ex E.M.A. (Kuete et al., 2015f), Pachypodanthium staudtii Engl & Diels (Kuete et al., 2016b) and Uapaca togoensis Pax. (Euphorbiaceae) (Kuete et al., 2015e) (Table 1). Compounds exerting similar activity included: alkaloids, 47, 48, and 49 (Kuete et al., 2015b), diterpene, galanal A (2) isolated from Aframomum arundinaceum (Oliver & Hanbury) K. Schum (Kuete et al., 2014a), benzophenone, isogarcinol (28) from Hypericum lanceolatum Lam. (Kuete et al., 2013d), 30 (Kuete et al., 2013d), anthraquinones, 19 and 20 (Kuete et al., 2015a), flavonoids, 6 (Kuete et al., 2014b), 13 (Kuete et al., 2016c), 27 (Kuete et al., 2015c), kaempferol-3,7,4′-trimethylether (31) from Aframomum arundinaceum (Oliver & Hanbury) K. Schum (Kuete et al., 2014a), isoflavonoids, bidwillon A (16) from Erythrina sigmoidea Hua (Kuete et al., 2014c), 29 (Kuete et al., 2014d), 44 and 45 (Kuete et al., 2014c), lignan, 39 (Kuete et al., 2016c) and xanthones, 8-hydroxycudraxanthone G (10) from Garcinia nobilis Engl. (Kuete et al., 2013b), 17 (Kuete et al., 2013b) and euxanthone (22) (Kuete et al., 2016c).
Plants and Compounds Acting in EGFR Over-Expressing Cancer Cells
Several plants extracts and compounds were more active in the resistant gliobastoma U87MG.ΔEGFR cells than in its normal counterpart U87MG cells (D.R. < 0.90). They included: Albizia adianthifolia (Schum.) and Alchornea cordifolia (Schum. & Thonn.) Müll.-Arg. (Kuete et al., 2016e), Anonidium mannii Engl. et Diels. (Anonaceae) (Kuete et al., 2013a), Elaeophorbia drupifera (Thonn.) Stapf. (Euphorbiaceae) (Kuete et al., 2013e), Erythrina sigmoidea Hua (Kuete et al., 2014a), Gladiolus quartinianus A. Rich (Iridaceae) (Kuete et al., 2013a), Nauclea pobeguinii (Pobég. ex Pellegr.) Merr. ex E.M.A. (Kuete et al., 2015f), Vepris soyauxii Engl. (Rutaceae) (Kuete et al., 2013a) and Xylopia aethiopica (Dunal) A.Rich. (Annonaceae) (Kuete et al., 2013c). Compounds with similar activity include: the alkaloids, 47-53 (Kuete et al., 2015b,g), anthraquinone, 19 (Kuete et al., 2015a), benzophenones, 2,2′,5,6′-tetrahydroxybenzophenone (3) from Hypericum lanceolatum Lam. (Kuete et al., 2013d), 26 (Kuete et al., 2013d), 28 (Kuete et al., 2013d), 30 (Kuete et al., 2013d), flavonoids, 12 (Kuete et al., 2016c), dorsmanin F (21) (Kuete et al., 2015d), 27 (Kuete et al., 2015c), 34 (Kuete et al., 2014a), isoflavonoids, 16 (Kuete et al., 2014c), 35 (Kuete et al., 2014d), 44 and 45 (Kuete et al., 2014c), xanthones, 10 (Kuete et al., 2013b), and 17 (Kuete et al., 2013b).
Plants and Compounds Acting in p53 Knockout Cancer Cells
Botanicals inducing hypersensitivity in p53 knockout cell line HCT116 (p53−/−) compared to its sensitive counterpart HCT116 (p53+/+) cell line included: Beilschmiedia acuta Kosterm (Lauraceae) (Kuete et al., 2014e), Echinops giganteus var. lelyi (C. D. Adams) A. Rich. (Compositae) (Kuete et al., 2013c), Erythrina sigmoidea Hua (Fabaceae) (Kuete et al., 2014a), Nauclea latifolia Smith. (Kuete et al., 2014a), Nauclea pobeguinii (Pobég. ex Pellegr.) Merr. ex E.M.A. (Kuete et al., 2015f), Polyscias fulva (Hiern) Harms. (Araliaceae) (Kuete et al., 2014e) and Uapaca togoensis Pax. (Kuete et al., 2015e). Compounds acting in p53 knockout cancer cells included: alkaloid, 48 (Kuete et al., 2015b), benzophenone, 26 (Kuete et al., 2013d), flavonoids, 12 (Kuete et al., 2016c), 27 (Kuete et al., 2015c), laburnetin (32) (Kuete et al., 2016c), 38 (Kuete et al., 2015d), isoflavonoids, 9 (Kuete et al., 2014c), 11 (Kuete et al., 2014c), 16 (Kuete et al., 2014c), 29 (Kuete et al., 2014d), 44 (Kuete et al., 2014c), neobavaisoflavone (35) from Erythrina senegalensis DC (Kuete et al., 2014d), lignan, 23 (Kuete et al., 2016c), xanthones, 10 (Kuete et al., 2013b), and 22 (Kuete et al., 2016c).
Hit Cytotoxic Plants of Central, East and West Africa
Some African plant extracts displayed very interresting cytotoxic effects with IC50 values below 20 μg/mL in the majority of cancer cell lines tested. In this section, the synopsis of 10 strongest cytotoxic plants of CEWA as observed with in vitro screening assays is provided.
Beilschmiedia acuta Kosterm (Lauraceae)
Beilschmiedia acuta [Synonyms: Beilschmiedia acutifolia (Engl. & K. Krause) Robyns & Wilczek or Tylostemon acutifolius Engl. & K. Krause] belongs to the family Lauraceae. The plant is mainly found in Cameroon and Central African Republic, where it is traditionally used to treat cancer and gastrointestinal infections (Kuete et al., 2014e). The methanol extracts of leaves and roots of the plant were tested on a panel of cancer cell lines, including MDR phenotypes. Both leaves and roots extracts displayed good antiproliferative effects with respective IC50 values of 8.22 and 14.72 μg/mL in leukemia CCRF-CEM cells, 19.76 and 26.74 μg/mL in its resistant subline CEM/ADR5000 cells, 6.45 and 6.66 μg/mL in breast adenocarcinoma MDA-MB-231 cells and 21.09 and 22.75 μg/mL in its resistant counterparts MDA-MB-231/BCRP, 21.12 and 11.62 μg/mL in colon adenocarcinoma HCT116 p53+/+and its resistant counterparts HCT116 p53−/−, 7.46 and 7.27 μg/mL in gliobastoma U87MG cells and its resistant counterparts U87MG.ΔEGFR cells and 23.09 μg/mL for leaves extract in HepG2 cells (Kuete et al., 2014e). Interestingly, the two extracts were less toxic toward normal AML12 hepatocytes with IC50 values above 40 μg/mL (Kuete et al., 2014e). Both leaves and roots extracts induced apoptosis in CCRF-CEM cells. However, the mode of induction of apoptosis was not dectected when MMP and ROS production were investigated (Kuete et al., 2014e).
Echinops giganteus var. lelyi (C. D. Adams) A. Rich. (Composiatae)
Echinops giganteus is a medicinal spicy plant of the family Compositae mainly found in Cameroon, Ethiopia, Rwanda, Sudan, Tanzania, Uganda, DR Congo. The plant is traditionally used to treat cancer, as well as heart and gastric troubles (Tene et al., 2004; Kuete et al., 2011a). The methanol extract of the rhizomes of the plant displayed good antiproliferative effects toward leukemia CCRF-CEM cells (IC50: 6.68μg/mL),CEM/ADR5000 cells (IC50: 7.96 μg/mL) (Kuete et al., 2011a), HL60 cells (IC50: 6.38 μg/mL) and HL60AR cells (IC50: 9.24 μg/mL), MDA-MB-231-pcDNA cells (IC50: 8.61 μg/mL), MDA-MB-231-BCRP cells (IC50: 6.52 μg/mL), colon carcinoma HCT116 (p53+/+) cells (IC50: 3.58 μg/mL), HCT116 (p53−/−) cells (IC50: 3.29 μg/mL), gliobastoma U87MG cells (IC50: 13.55 μg/mL) and U87MG.ΔEGFR cells (IC50: 11.15 μg/mL), hepatocarcinoma HepG2 cells (IC50: 14.32 μg/mL) (Kuete et al., 2013c). Importantly, this extract was less toxic to the normal human umbilical vein endothelial cells (HUVECs; IC50> 80 μg/mL) (Kuete et al., 2011a) and to normal AML12 hepatocytes with less than 50% proliferation at 40 μg/mL (Kuete et al., 2013c). This extract induced apoptosis in CCRF-CEM cells by loss of MMP (Kuete et al., 2013c).
Erythrina Sigmoidea Hua (Fabaceae)
Erythrina sigmoidea (synonyms: Erythrina dybowskii Hua; Erythrinaeriotricha Harms; Erythrina lanata Taub. ex Gilg; Erythrina sudanica Baker f.) is a tree of 3–6 m, or 10–20 m belonging to the Fabaceae family. The plant is mainly found in Cameroon and Chad, where it is used as antidote (venomous stings, bites, etc.), diuretic, febrifuge and to treat arthritis, rheumatism, pulmonary troubles, stomach troubles, infectious diseases and kidney diseases (Burkill, 1985), gastrointestinal infections, venereal diseases and leprosy (Mabeku et al., 2011). The cytotoxic constituents of the plant include 6α-hydroxyphaseollidin (9), atalantoflavone (15), bidwillon A (16), neobavaisoflavone (35), neocyclomorusin (36), and sigmoidin I (44) (Kuete et al., 2014c). The cytotoxicity of bark methanol extract was reported toward CCRF-CEM cells (IC50: 18.50 μg/mL), CEM/ADR5000 cells (IC50: 20.06 μg/mL), MDA-MB-231-pcDNA cells (IC50: 22.37 μg/mL), MDA-MB-231-BCRP cells (IC50: 27.42 μg/mL), HCT116 (p53+/+) cells (IC50: 19.63 μg/mL), HCT116 (p53−/−) cells (IC50: 16.22 μg/mL), U87MG cells (IC50: 45 μg/mL), U87MG.ΔEGFR cells (IC50: 29.80 μg/mL), and HepG2 cells (IC50:22.34 μg/mL) (Kuete et al., 2016a). This extract had low cytotoxicity toward normal AML12 hepatocytes, inducing less than 50% proliferation at 80 μg/mL (Kuete et al., 2016a). It induced apoptosis in CCRF-CEM leukemia cells by disruption of the MMP (Kuete et al., 2014a).
Imperata cylindrica Beauv. var. koenigii Durand et Schinz (Poaceae)
Imperata cylindrica commonly known as cogon grass is a perennial rhizomatous grass of the Poaceae family. The plant is native to East and south East Asia, India, Micronesia, Melanesia, Australia, and Eastern and Southern Africa. In CEWA, the plant is found in Benin, Burkina Faso, Congo, Ivory Cost, Gambia, Ghana, Guinea, Kenya, Liberia, Mali, Mozambique, Niger, Nigeria, Senegal, Tanzania, Togo, Uganda. The plant is traditionally used as diuretic and anti-inflammatory agents and to treat cancer (Nishimoto et al., 1968; Kuete et al., 2011a). The cytotoxicity of roots methanol extract of the plant was reported toward CCRF-CEM cells (IC50: 8.4 μg/mL) and CEM/ADR5000 cells (IC50: 7.18 μg/mL), pancreatic MiaPaca-2cells (IC50: 12.11 μg/mL) (Kuete et al., 2011a), HL60 cells (IC50: 7.94 μg/mL), HL60AR cells (IC50: 30.60 μg/mL), MDA-MB-231-pcDNA cells (IC50: 5.19 μg/mL), MDA-MB-231-BCRP cells IC50: 10.04 μg/mL), HCT116 (p53+/+) cells (IC50: 4.37 μg/mL), HCT116 (p53−/−) cells (IC50: 4.60 μg/mL), U87MG cells (IC50: 19.99 μg/mL), U87MG.ΔEGFR cells (IC50: 10.68 μg/mL), and HepG2 cells (IC50: 18.28 μg/mL) (Kuete et al., 2013c). Less than 50% proliferation of CCRF-CEM cells was induced by this extract in normal AML12 hepatocytes (Kuete et al., 2013c) meanwhile the IC50 value as high as 47.73 μg/mL was obtained in HUVEC cells. This extract induced apoptosis in CCRF-CEM cells by loss of MMP (Kuete et al., 2013c).
Nauclea pobeguinii (Pobég. ex Pellegr.) Merr. ex E.M.A. (Rubiaceae)
Nauclea pobeguinii (synonym: Sarcocephalus pobeguinii Pobég. ex Pellegr.) is a deciduous, small to medium-sized tree growing up to 30 m tall, sometimes a shrub. In CEWA, the plant is distributed in South Tropical Africa especially in Angola, Zambia, West Tropical Africa: Burkina, Ghana, Guinea, Guinea-Bissau, Ivory Coast, Nigeria, Senegal, Sierra Leone, West-Central Tropical Africa: Cameroon, Central African Republic, Congo, DR Congo, Gabon. The plant is used in traditional medicine as abortive and to treat stomach ache and infectious diseases (Karou et al., 2011), jaundice (Kadiri et al., 2007), fever, diarrhea, worm, and malaria (Mesia et al., 2005). The cytotoxicity of the methanol extract from bark and leaves was reported toward CCRF-CEM cells (IC50: 14.62 and 25.84 μg/mL, respectively), CEM/ADR5000 cells (IC50: 11.56 and 25.55 μg/mL, respectively), HCT116 (p53+/+) cells (IC50: 16.19 and 32.72 μg/mL, respectively) and HCT116 (p53−/−) cells (IC50: 8.70 and 19.39 μg/mL, respectively) (Kuete et al., 2015f). Resveratrol was identified as the major cytotoxic constituent of this extract (Kuete et al., 2015f).
Piper capense L.f. (Piperaceae)
Piper capense is a rather variable spicy plant ranging from a weakly erect, aromatic, evergreen shrub or subshrub, to a more or less herbaceous perennial and sometimes a straggling plant that scrambles into other plants for support. Piper capense is found from Guinea to Ethiopia, Angola and Mozambique. Traditionally, the plant is used as sleep inducing remedy, anthelmintic and to treat cancer (Kokowaro, 1976; Van Wyk and Gericke, 2000; Kuete et al., 2011a). The cytotoxicity of seeds methanol extract was reported toward CCRF-CEM cells (IC50: 7.03 μg/mL), CEM/ADR5000 (IC50: 6.56 μg/mL) and MiaPaca-2 cells (IC50: 8.92 μg/mL) (Kuete et al., 2011a), HL60 cells (IC50: μg/mL), HL60AR cells (IC50: μg/mL), MDA-MB-231-pcDNA cells (IC50:4.17 μg/mL), MDA-MB-231-BCRP cells (IC50: 19.45 μg/mL), HCT116 (p53+/+) cells (IC50: 4.64 μg/mL), HCT116 (p53−/−) cells (IC50: 4.62 μg/mL), U87MG cells (IC50: 13.48 μg/mL), U87MG.ΔEGFR cells (IC50: 7.44 μg/mL), HepG2 cells (IC50: 16.07 μg/mL) (Kuete et al., 2013c). This extract was less toxic toward normal AML12 hepatocytes and HUVEC cells inducing less than 50% cell proliferation at 40 μg/mL and 80 μg/mL respectively (Kuete et al., 2011a, 2013c). This extract induced apoptosis in CCRF-CEM cells by the loss of MMP and increase ROS production (Kuete et al., 2013c).
Polyscias fulva (Hiern) Harms. (Araliaceae)
Polyscias fulva is a deciduous to evergreen tree of the family Araliaceae. The plant is found in Tropical Africa, from Sierra Leone to Sudan, Ethiopia, and Yemen; in Angola, Zambia, Zimbabwe, and Mozambique. Traditionally, Polyscias fulva is used to treat malaria, fever, mental illness (Tshibangu et al., 2002), venereal infections and obesity (Jeruto et al., 2007; Focho et al., 2009), and cancer (Kuete et al., 2014e). The phytochemical investigations of the plant led to the isolation of polysciasoside A, kalopanax-saponin B, α-hederin (Bedir et al., 2001; Kuete and Efferth, 2011). Investigation of the cytotoxic potential of various parts of the plant demonstrated that the roots were more active than the leaves and bark (Kuete et al., 2014e). Roots methanol extract had good cytotoxic effects on a panel of human cancer cell lines with the IC50 values of 7.79 μg/mL (CCRF-CEM cells), 22.63 μg/mL (CEM/ADR5000 cells), 3.27 μg/mL (MDA-MB-231 cells), 16.67 μg/mL (MDA-MB-231/BCRP cells), 14.66 μg/mL (HCT116 p53+/+cells), 5.98 μg/mL (HCT116 p53−/−cells), 4.15 μg/mL (U87MG cells), 16.35 μg/mL (U87MG.ΔEGFR cells), and 12.99 μg/mL (HepG2 cells) (Kuete et al., 2014e). Lower cytotoxicity of this extract was shown in normal AML12 hepatocytes with less than 50% cells proliferation at 40 μg/mL (Kuete et al., 2014e). The active constituent of the plant was reported as α-hederin and this compound had moderate antiproliferative effects (IC50 values ranged from 7.43 μM in CCRF-CEM cells to 43.98 μM in U87MG.ΔEGFR cells) against the above cancer cell lines (Kuete et al., 2014e). The roots methanol extract of Polyscias fulva induced apoptosis in CCRF-CEM cells, mediated by MMP alterations and increased ROS production (Kuete et al., 2014e).
Uapaca togoensis Pax (Euphorbiaceae)
Uapaca togoensis (Synonyms:Uapaca chevalieri Beille, Uapaca guignardii A.Chec. ex Beille, Uapaca guineensis sudanica (Beille) Hutch., Uapaca perrotii Beille Uapaca somon Aubrév. & Leandri) is an evergreen tree. The plant grows in tropical Africa, from Senegal to southern Chad and Central African Republic and from Gabon to DR Congo and northern Angola. In traditional medicine, the plant is used as antiemetic, lotion for skin disorders (Mengome et al., 2010), remedy for pneumonia, cough, fever, rheumatism, vomiting, epilepsy (Kone et al., 2006) and bacterial diseases (Kone et al., 2004). The cytotoxicity of the methanol extract from fruit was reported toward CCRF-CEM cells (IC50: 4.23 μg/mL), CEM/ADR5000 cells (IC50: 4.44 μg/mL), MDA-MB-231-pcDNA cells (IC50: 25.85 μg/mL), MDA-MB-231-BCRP cells (IC50: 4.17 μg/mL), HCT116 (p53+/+) cells (IC50: 3,69 μg/mL), HCT116 (p53−/−) cells (IC50: 3.09 μg/mL), U87MG cells (IC50: 8.01 μg/mL), U87MG.ΔEGFR cells (IC50: 8.68 μg/mL), and HepG2 cells (IC50: 19.90 μg/mL) (Kuete et al., 2015e). This extract induced apoptosis in CCRF-CEM cells mediated by MMP loss (Kuete et al., 2015e). The cytotoxic consitutuents of the extract were identified as a terpenoid, 11-oxo-α-amyryl acetate, a lignan, futokadsurin B (23) and an alkaloid,arborinin (49) (Kuete et al., 2015e).
Vepris soyauxii Engl. (Rutaceae)
Vepris soyauxii (synonym: Araliopsis soyauxii Engl) is a plant of the family Rutaceae mostly found throughout West Africa, from Sierra Leone, Liberia, Ivory Cost, Mali, Ghana to Nigeria, and Cameroon. In traditional medicine, the plant is used as anti-fibriomyoma and to treat stomachache, malaria (Momeni et al., 2010) and cancer (Kuete et al., 2013a). The antiproliferative effects of the methanol extract from leaves was reported toward CCRF-CEM cells (IC50: 9.28 μg/mL), CEM/ADR5000 cells (IC50: 11.72 μg/mL), MDA-MB-231-pcDNA cells (IC50: 7.52 μg/mL), MDA-MB-231-BCRP cells (IC50: 12.93 μg/mL), HCT116 (p53+/+) cells (IC50: 8.59 μg/mL), HCT116 (p53−/−) cells (IC50:9.70 μg/mL), U87MG cells (IC50: 8.75 μg/mL), U87MG.ΔEGFR cells (IC50: 4.09 μg/mL) and HepG2 cells (IC50: 13.60 μg/mL) (Kuete et al., 2013a). This extract induced apoptosis in CCRF-CEM cells mediated by disruption of MMP (Kuete et al., 2013a). Besides, this extract had low cytotoxic effect toward normal AML12 hepacytes, inducing less than 50% proliferation at 40 μg/mL (Kuete et al., 2013a).
Xylopia aethiopica (Dunal) A. Rich. (Annonaceae)
Xylopia aethiopica is an aromatic tree of the family Annonaceae. The plant is native to the lowland rainforest and moist fringe forests in the savanna zones of Africa. The plant grows in Angola, Benin, Burkina Faso, Cameroon, Central African Republic, DR Congo, Ethiopia, Gabon, Gambia, Ghana, Guinea, Guinea-Bissau, Ivory Coast, Kenya, Liberia, Mozambique, Nigeria, São Tomé and Príncipe, Senegal, Sierra Leone, Sudan, South Sudan, Tanzania, Togo, and Uganda. Traditionally, this plant is used to treat cancer, constipation, uterine hemorrhage, fever and as diuretic (Iwu, 1993; Kuete et al., 2011a; Okafor, 2012). The cytotoxicity of seeds methanol extract was demonstrated toward CCRF-CEM cells (IC50: 3.91 μg/mL), CEM/ADR5000 cells (IC50:7.4 μg/mL)and Mia PaCa-2 cells (IC50: 6.86 μg/mL) (Kuete et al., 2011a), human cervical cancer cell line C-33A (IC50:30.8μg/mL), breast adonocarcinoma MCF7 cells (IC50: 60.2 μg/mL), human oral squamous carcinoma KB cells (IC50: 62.5 μg/mL) (Adaramoye et al., 2011), HL60 cells (IC50: 7.94 μg/mL), HL60AR cells (IC50: 30.60 μg/mL), MDA-MB-231-pcDNA cells (IC50: 5.19 μg/mL), MDA-MB-231-BCRP cells (IC50: 10.04 μg/mL), HCT116 (p53+/+) cells (IC50: 4.37 μg/mL), HCT116 (p53−/−) cells (IC50: 4.60 μg/mL), U87MG cells (IC50: 19.99 μg/mL), U87MG.ΔEGFR cells (IC50: 10.68 μg/mL) and HepG2 cells (IC50: 18.28 μg/mL) (Kuete et al., 2013c). This extract was less toxic against AML12 hepatocytes and HUVEC cells inducing less than 50% cell proliferation at 40 and 80 μg/mL, respectively (Kuete et al., 2011a, 2013c). Its also induced apoptosis in C-33A cells, nuclear fragmentation, cells accumulation in sub-G0/G1, cycle arrest in G2, up-regulation of p53 and p21 genes, and an increase in the Bax/Bcl-2 ratio (Adaramoye et al., 2011). It also induced apoptosis in CCRF-CEM cells by loss of MMP (Kuete et al., 2013c). The cytotoxic constituents of this extract were identified as 16α-hydroxy-ent-kauran-19-oic acid, 3,4′,5-trihydroxy-6″,6″-dimethylpyrano[2,3-g]flavone, isotetrandrine (51) and trans-tiliroside (Kuete et al., 2015g).
Hit Cytotoxic Compounds from Plants of Central, Eastern and Western Africa
Several bioactive consituents of African medicinal plants were identified. They include: terpenoids, phenolics and alkaloids (Table 2). However, phenolics were the best cytotoxic ingredients isolated from CEWA plants. In this section, a summary of the prominent antiproliferative phytochemicals identified in CEWA plant will be given.
Alkaloids
The isoquinoline alkaloid, isotetrandrine (51) isolated from Xylopia aethiopica was amongst the most active alkaloids reported in CEWA plants. This compound displayed interesting cytotoxic effects with IC50 values below 10 μM toward a panel of sensitive and MDR cancer cell lines. These cell lines included: CCRF-CEM cells (IC50: 1.53 μM), CEM/ADR5000 cells (IC50: 2.36 μM), MDA-MB-231-pcDNA cells (IC50: 7.28 μM), MDA-MB-231-BCRP cells IC50:6.70 μM), HCT116 (p53+/+) cells (IC50: 2.39 μM), HCT116 (p53−/−) cells(IC50: 4.55 μM), U87MG cells (IC50: 3.89 μM), U87MG.ΔEGFR cells (IC50: 1.45 μM) and HepG2 cells (IC50: 3.28 μM) (Kuete et al., 2015g). Alkaloid, 51 was less toxic against the normal AML12 hepatocytes, inducing less than 50% proliferation at up to 64.27 μM (Kuete et al., 2015g). This compound did not alter the integrity of the mitochondrial membrane in CCRF-CEM cells, and its mode of induction of apoptosis was mainly by increased ROS production (Kuete et al., 2015g).
Phenolic Compounds
Phenolics have been so far the most represented group of secondary metabolites isolated from CEWA medicinal plants. Several compounds with interesting cytotoxic activities were identified within benzophenones, flavonoids and isoflavonoids, naphthyl butenone, quinones and xanthones.
Benzophenones
Guttiferone E (26) and isoxanthochymol (30) isolated from Garcinia punctata Oliv. (Guttiferae) (Kuete et al., 2013d) showed good cytotoxic effects against a panel of human cancer cell lines. Compounds 26 and 30 have also been isolated from various Garcinia species such as Garcinia pyrifera (Roux et al., 2000), Garcinia xanthochymus (Baggett et al., 2005), Garcinia virgata (Merza et al., 2006), Garcinia afzelii (Lannang et al., 2010), Garcinia livingstonei (Yang et al., 2010), Garcinia multiflora (Liu et al., 2010) and from Rheedia edulis (Acuna et al., 2010). Compound 26 had an IC50 value of 7.5 μM toward colon carcinoma SW-480 cells, meanwhile 30 was less active in this cell line (IC50: 16.6 μM) (Baggett et al., 2005). Benzophenones 26 and 30 displayed good cytotoxic effects toward CCRF-CEM cells (IC50: 6.86 and 9.55 μM, respectively), CEM/ADR5000 cells(IC50:13.57 and 10.33 μM, respectively), HL60 cells (IC50: 11.69 and 8.92 μM, respectively), HL60AR cells (IC50: 11.69 and 8.92 μM, respectively), MDA-MB-231-pcDNA cells (IC50:11.69 and 6.30 μM, respectively), MDA-MB-231-BCRP cells (IC50: 13.92 and 3.42 μM, respectively), HCT116 (p53+/+) cells (IC50:12.74 and 3.24 μM, respectively), HCT116 (p53−/−) cells (IC50: 7.87 and 3.62 μM, respectively), U87MG cells (IC50: 7.87 and 6.40 μM, respectively), U87MG.ΔEGFR cells (IC50: 3.39 and 3.12 μM, respectively) and HepG2 cells (IC50: 11.13 and 8.34 μM, respectively) (Kuete et al., 2013d). Both 26 and 30 induced apoptosis in cervix adenocarcinoma HeLa cells (Liu et al., 2010). The two compounds also induced apoptosis in CCRF-CEM cells by activation of caspases 3/7, 8 and 9 and loss of MMP (Kuete et al., 2013d).
Flavonoids and Isoflavonoids
Flavonoids and isoflavonoids are amongst the most isolated and the most active phytochemicals identified in African medicinal plants (Kuete and Efferth, 2015). Well studied flavonoids from CEWA plants having prominent cytotoxic effect against human cancer cell lines include 4′-hydroxy-2′,6′-dimethoxychalcone (6), isobavachalcone (27), neocyclomorusin (36), poinsettifolin B (38), 6α-hydroxyphaseollidin (9), isoneorautenol (29), neobavaisoflavone (35), sigmoidin I (44) and sophorapterocarpan A (45) (Table 2). Amongst them, isoflavonoid 9 revealed the best activity with IC50 values below 10 μM on a panel of cancer cell lines including CCRF-CEM cells (IC50: 3.36 μM), CEM/ADR5000 cells (IC50: 5.51 μM), MDA-MB-231-pcDNA cells (IC50: 5.70 μM), MDA-MB-231-BCRP cells (IC50: 5.87 μM), HCT116 (p53+/+) cells (IC50: 5.68 μM), HCT116 (p53−/−) cells (IC50: 4.60 μM), U87MG cells (IC50: 4.91 μM), U87MG.ΔEGFR cells (IC50: 4.91 μM) and HepG2 cells (IC50: 6.44 μM)(Kuete et al., 2014c). Compound 9 induced apoptosis in CCRF-CEM cells by the activation of caspases 3/7, 8 and 9 and breakdown of MMP as well as increased ROS production (Kuete et al., 2014c).
Naphthyl Butenone
Guieranone A (25), a major component of the leaves of Guiera senegalensis displayed good cytotoxic effects on a panel of human cancer cell lines. The cytotoxicity of 25 was documented toward CCRF-CEM cells (IC50: 2.31 μM), CEM/ADR5000 cells (IC50: 3.19 μM), MiaPaCa-2 cells (IC50: 12.39 μM), Capan-1 cells (IC50: 29.08 μM), MCF-7 cells (IC50: 3.42μM), 786-0 cells (IC50: 11.32 μM), U87MG cells (IC50: 7.78 μM), lung carcinoma A549 cells (IC50: 2.28 μM), skin melanoma Colo-38 cells (IC50: 7.69 μM), cervical carcinoma HeLa cells (IC50: 1.61 μM), and Caski cells (IC50: 3.73 μM) (Kuete et al., 2012) and leukemia THP-1 cells (IC50: 13.43 μM) (Fiot et al., 2006). Compound 25 showed anti-angiogenic activity via the inhibition of the growth of blood capillaries on the chorioallantoic membrane of quail embryo. It also induces apoptosis in CCRF-CEM cells. Meanwhile, microarray analysis demonstrated that it affected the regulation of several pathways in CCRF-CEM cells, including the cell cycle: G2/M DNA damage checkpoint regulation and ATM signaling pathways (Kuete et al., 2012).
Quinones
Two naphthoquinones isolated from African plants, 2-acetylfuro-1,4-naphthoquinone (4) and plumbagin (37), showed remarkable cytotoxic effects (Table 2). Compound 4 showed good cytotoxicity toward PF-382 cells (IC50: 2.36 μM), MiaPaCa-2 cells (IC50: 7.48 μM), MCF-7 cells (IC50: 6.68 μM), U87MG cells (IC50: 8.02 μM), Colo-38 cells (IC50: 2.77 μM), HeLa cells (IC50: 1.65 μM), and Caski cells (IC50: 0.70 μM) (Kuete et al., 2011d). Naphthoquinone 4 revealed anti-angiogenic effects through inhibition of the growth of blood capillaries on the chorioallantoic membrane of quail eggs and also induced apoptosis and cell cycle arrest in S-phase in CCRF-CEM (Kuete et al., 2011d). The cytotoxic potential of compound 37 has widely been reported (Srinivas et al., 2004; Kuo et al., 2006; Powolny and Singh, 2008; Sun and McKallip, 2011; Kawiak et al., 2012). It has been isolated from various species of Plumbaginaceae, Ebenaceae and Droseraceae (Sagar et al., 2014). Compound 37 induced ROS mediated apoptosis in human cervical cancer ME-180 cells (Srinivas et al., 2004), human prostate cancer PC-3 cells and LNCaP cells (Powolny and Singh, 2008), G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin (mTOR) pathway in MCF-7 cells and MDA-MD-231 cells (Kuo et al., 2006). Naphthoquinone 37 also induced apoptosis in Her2-overexpressing breast cancer cells through the mitochondrial-mediated pathway (Kawiak et al., 2012) as well as in human K562 leukemia cells through increased ROS and elevated tumor-necrosis-factor related apoptosis inducing ligand (TRAIL) receptor expression (Sun and McKallip, 2011).
Xanthones
Xanthone V1 (46) (Table 2) is one of the most active xanthones with prominent cytotoxic effects isolated from CEWA plants, displaying IC50 values below or around 10 μM against MCF-7 cells (IC50: 1.42 μM), 786-0 cells (IC50: 9.62 μM), U87MG cells (IC50: 9.64 μM), A549 cells (IC50:10.13 μM), Colo-38 cells (IC50: 3.02 μM), HeLa cells (IC50: 0.58 μM), and Caski cells (IC50: 0.61 μM) (Kuete et al., 2011d). This compound had anti-angiogenic effects, inhibiting the growth of blood capillaries on the chorioallantoic membrane of quail eggs. Compound 46 induced apoptosis and cell cycle arrest in S-phase in CCRF-CEM cells mediated by caspase 3/7 activation (Kuete et al., 2011d).
Conclusion
The present review paper aimed at compiling and summarizing relevant data on the potential of medicinal plant and isolated natural products from Central, Eastern and Western Africa to combat cancer with emphasis on their possible cellular targets. This report could not deliver medical results on the therapeutic capacities of the flora of these three African Regions as anticancer drugs. Nonetheless, in phytochemical and pharmacological basic sciences it clearly shows that efforts are being made by African scientists and their international collaborators to achieve this goal in the future. However, few research teams in the continent are already involved in the cytotoxic drug discovery from botanicals and it is expected that this review will stimulate other laboratories to undertake similar research projects to better valorize the African flora.
Author Contributions
AM and VK wrote the manuscript; VK and TE designed and corrected the work. All authors read and approved the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
AM is thankful to Alexander von Humboldt Foundation for an 18 months fellowship in Prof. Dr. Thomas Efferth's laboratory in Mainz, Germany through the “Georg Foster Research Fellowship for Experienced Researcher” program. VK is very grateful to the Alexander von Humboldt Foundation for the funding through the Linkage program (2015–2018).
Abbreviations
4E-BP1, 4E-binding protein 1; ABC transporters, ATP binding cassette transporters; Akt, protein kinase B; AMPK, AMP-activated protein kinase; Apaf-1, apoptotic peptidase activating factor 1; AR, androgen receptor; ARE, antioxidant response element; ATM/Chk1, ataxia–telangiectasia-mutated/check point kinase-1; BAD, Bcl-2-associated agonist of cell death; Bax, Bcl-2-associated X protein; BCRP, breast cancer resistance protein; BRCA, breast cancer; BTG3, B-cell translocation gene 3; C/EBPα, CAAT-enhancer binding protein α;CCL2, CC motif ligand 2; Cdc2, cyclin-dependent kinase 1; Cdc25a, cell division cycle 25 homolog A; cIAP, cellular inhibitor of apoptosis protein; COX, cyclooxygenase; CXCL, chemokine (C-X-C motif) ligand; CXCR4, chemokine (C-X-C motif) receptor 4; CYP1A1, cytochrome P450 1A1; DR5, death receptor 5; EGFR, epidermal growth factor receptor; EMMPRIN, extracellular matrix metalloproteinase inducer; ErbB2, Receptor tyrosine-protein kinase; ERK, extracellular signal-regulated kinase; ER-α, -β, estrogen receptor -α, -β; Fas, Fatty acid synthase; FoxM1, forkhead box M1; FOXO, forkhead transcription-factor O; G3BP1, GTPaseactivating protein (SH3 domain) binding protein 1; GDF15, growth differentiation factor 15; Gli1, glioma-associated oncogene 1; GST, glutathione transferase; HIF-1α, hypoxia inducible factor 1, alpha; HMG-CoA, hydroxy-methylglutaryl-coenzyme A; Hsp70, heat shock 70 kDa protein; hSULT1A1, human simple phenol sulfotransferase; hSULT2A1, human dehydroepiandrosterone sulfotransferase; IDO, indole amine 2,3-dioxygenase; IGF-1R, insulin-like growth factor -1 receptor; IGFBP, insulin-like growth factor binding protein; IGF, insulin-like growth factor; IKK, Ikappa beta kinase; IL, interleukin; IL-1RI, IL-1 receptor type I; iNOS, inducible nitric oxide synthase; JNK, c-Jun NH(2)-terminal kinase; MAPK, mitogen activated protein kinase; MDR, multi-drug resistance; MMP-2, -7, -9, matrix metallopeptidase-2, -7, -9; MMP, mitochondrial membrane potential; MRPs, multi drug resistance proteins; mTOR, mammalian target of rapamycin; NF-κB, nuclear factor-kappaB; Nrf2, nuclearfactor erythroid 2 related factor 2; PAK1, p21-activated protein kinase 1; PFKFB4, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4; PGE2, prostaglandin-E2;P-gp, P-glycoprotein; PHB, prohibitin; PI3K, phosphatidylinositol 3-kinase; Pin1, peptidyl prolyl cis/trans isomerase; PKC-α, -δ, protein kinase C -α, -δ; PPARγ, peroxisome proliferator-activated receptor-gamma; PTEN, phosphatase and tensin homolog; QR, quinone reductase; RAR-α, -β, retinoic acid receptor-α, -β; RB, retinoblastoma; Skp2, S-phase kinase-associated protein 2; SphK1, sphingosine kinase 1; TGFβ, Transforming growth factor-β; TNF, tumor necrosis factor; TRAIL, TNF-related apoptosis-inducing ligand; UGT1A, uridine 5′-diphosphate-glucuronosyltransferase 1A; VDR, vitamin D receptor; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor; WIF-1, Wnt inhibitory factor-1; XIAP, X-linked inhibitor of apoptosis; ZEB1, zinc finger E-box binding homeobox 1.
References
Abbiw, D. K. (1990). Useful Plants of Ghana: West African Uses of Wild and Cultivated Plants. London: Intermediate Technology Publications. doi: 10.3362/9781780443737
Aboul-Enein, A. M., Shanab, S. M., Shalaby, E. A., Zahran, M. M., Lightfoot, D. A., and El-Shemy, H. A. (2014). Cytotoxic and antioxidant properties of active principals isolated from water hyacinth against four cancer cells lines. BMC Complement. Altern. Med. 14:397. doi: 10.1186/1472-6882-14-397
Acuna, U. M., Figueroa, M., Kavalier, A., Jancovski, N., Basile, M. J., and Kennelly, E. J. (2010). Benzophenones and biflavonoids from Rheedia edulis. J. Nat. Prod. 73, 1775–1779. doi: 10.1021/np100322d
Adaramoye, O. A., Sarkar, J., Singh, N., Meena, S., Changkija, B., Yadav, P. P., et al. (2011). Antiproliferative action of Xylopia aethiopica fruit extract on human cervical cancer cells. Phytother. Res. 25, 1558–1563. doi: 10.1002/ptr.3551
Adaramoye, O., Erguen, B., Oyebode, O., Nitzsche, B., Hopfner, M., Jung, K., et al. (2015). Antioxidant, antiangiogenic and antiproliferative activities of root methanol extract of Calliandra portoricensis in human prostate cancer cells. J. Integr. Med. 13, 185–193. doi: 10.1016/S2095-4964(15)60175-3
Adeneye, A. A., Oreagba, A. I., Ishola, I. O., and Kalejaiye, H. A. (2014). Evaluation of the anti-arthritic activity of the hydroethanolic leaf extract of Alchornea cordifolia in rats. Afr. J. Tradit. Complement. Altern. Med. 11, 402–410. doi: 10.4314/ajtcam.v11i2.26
Adjanohoun, J., Aboubakar, N., Dramane, K., Ebot, M., Ekpere, J., Enow-Orock, E., et al. (1996). Traditional Medicine and Pharmacopoeia: Contribution to Ethnobotanical and Floristic Studies in Cameroon. Lagos: OUA/STRC.
Aguwa, C. N., and Lawal, A. M. (1988). Pharmacologic studies on the active principles of Calliandra portoticensis leaf extracts. J. Ethnopharmacol. 22, 63–71. doi: 10.1016/0378-8741(88)90231-0
Ahiahonu, P. W., and Goodenowe, D. B. (2007). Triterpenoids from leaves of Elaeophorbia drupifera. Fitoterapia 78, 337–341. doi: 10.1016/j.fitote.2007.02.002
Ahmad, A., Banerjee, S., Wang, Z., Kong, D., and Sarkar, F. H. (2008). Plumbagin-induced apoptosis of human breast cancer cells is mediated by inactivation of NF-kappaB and Bcl-2. J. Cell Biochem. 105, 1461–1471. doi: 10.1002/jcb.21966
Akabue, P., and Mittal, H. (1982). Clinical evaluation of a traditional herbal practice in Nigeria: a preliminary report. J. Ethnopharmacol. 6, 355–359. doi: 10.1016/0378-8741(82)90056-3
Akoachere, J., Ndip, R., Chenwi, E., Ndip, L., Njock, T., and Anong, D. (2002). Antibacterial effect of Zingiber officinale and Garcinia kola on respiratory tract pathogens. East Afr. Med. J. 79, 588–592. doi: 10.4314/eamj.v79i11.8804
Ali, B. H., Blunden, G., Tanira, M. O., and Nemmar, A. (2008). Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): a review of recent research. Food Chem. Toxicol. 46, 409–420. doi: 10.1016/j.fct.2007.09.085
Allemani, C., Weir, H. K., Carreira, H., Harewood, R., Spika, D., Wang, X. S., et al. (2015). Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet 385, 977–1010. doi: 10.1016/S0140-6736(14)62038-9
Anowi, C. F., Nnabuife, C. C., Mbah, C., and Onyekaba, T. (2012). Antimicrobial properties of the methanolic extract of the leaves of Nauclea latifolia. Int. J. Drug Res. Technol. 2, 45–55.
Armentano, M. F., Bisaccia, F., Miglionico, R., Russo, D., Nolfi, N., Carmosino, M., et al. (2015). Antioxidant and proapoptotic activities of Sclerocarya birrea [(A. Rich.) Hochst.] methanolic root extract on the hepatocellular carcinoma cell line HepG2. Biomed. Res. Int. 2015:561589. doi: 10.1155/2015/561589
Avruch, J., Khokhlatchev, A., Kyriakis, J. M., Luo, Z., Tzivion, G., Vavvas, D., et al. (2001). Ras activation of the Raf kinase: tyrosine kinase recruitment of the MAP kinase cascade. Recent Prog. Horm. Res. 56, 127–155. doi: 10.1210/rp.56.1.127
Ayafor, J. F., Tchuendem, M. H. K., Nyasse, B., Tillequin, F., and Anke, H. (1994). Aframodial and other bioactive diterpenoids from Aframomum species. Pure Appl. Chem. 66, 2327–2330. doi: 10.1351/pac199466102327
Aziz, M. Y., Omar, A. R., Subramani, T., Yeap, S. K., Ho, W. Y., Ismail, N. H., et al. (2014). Damnacanthal is a potent inducer of apoptosis with anticancer activity by stimulating p53 and p21 genes in MCF-7 breast cancer cells. Oncol. Lett. 7, 1479–1484. doi: 10.3892/ol.2014.1898
Baggett, S., Protiva, P., Mazzola, E. P., Yang, H., Ressler, E. T., Basile, M. J., et al. (2005). Bioactive benzophenones from Garcinia xanthochymus fruits. J. Nat. Prod. 68, 354–360. doi: 10.1021/np0497595
Banerjee, T., Van der Vliet, A., and Ziboh, V. A. (2002). Downregulation of COX-2 and iNOS by amentoflavone and quercetin in A549 human lung adenocarcinoma cell line. Prostaglandins Leukot. Essent. Fatty Acids 66, 485–492. doi: 10.1054/plef.2002.0387
Bedir, E., Toyang, N. J., Khan, I. A., Walker, L. A., and Clark, A. M. (2001). A new dammarane-type triterpene glycoside from Polyscias fulva. J. Nat. Prod. 64, 95–97. doi: 10.1021/np0003589
Betti, J. (2004). An ethnobotanical study of medicinal plants among the Baka Pygmies in the Dja Biosphere Reserve, Cameroon. Afr. Stud. Monogr. 25, 1–27.
Birbrair, A., Zhang, T., Wang, Z. M., Messi, M. L., Mintz, A., and Delbono, O. (2015). Pericytes at the intersection between tissue regeneration and pathology. Clin. Sci. 128, 81–93. doi: 10.1042/CS20140278
Braca, A., Politi, M., Sanogo, R., Sanou, H., Morelli, I., Pizza, C., et al. (2003). Chemical composition and antioxidant activity of phenolic compounds from wild and cultivated Sclerocarya birrea (Anacardiaceae) leaves. J. Agric. Food Chem. 51, 6689–6695. doi: 10.1021/jf030374m
Brahemi, G., Kona, F. R., Fiasella, A., Buac, D., Soukupova, J., Brancale, A., et al. (2010). Exploring the structural requirements for inhibition of the ubiquitin E3 ligase breast cancer associated protein 2 (BCA2) as a treatment for breast cancer. J. Med. Chem. 53, 2757–2765. doi: 10.1021/jm901757t
Chen, J. H., Chen, W. L., and Liu, Y. C. (2015). Amentoflavone induces anti-angiogenic and anti-metastatic effects through suppression of NF-kappaB sctivation in MCF-7 cells. Anticancer Res. 35, 6685–6693.
Cheng, L., Xia, T. S., Wang, Y. F., Zhou, W., Liang, X. Q., Xue, J. Q., et al. (2014). The anticancer effect and mechanism of α-hederin on breast cancer cells. Int. J. Oncol. 45, 757–763. doi: 10.3892/ijo.2014.2449
Chirumbolo, S. (2012). Plant phytochemicals as new potential drugs for immune disorders and cancer therapy: really a promising path? J. Sci. Food Agric. 92, 1573–1577. doi: 10.1002/jsfa.5670
Chrubasik, S., Pittler, M. H., and Roufogalis, B. D. (2005). Zingiberis rhizoma: a comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 12, 684–701. doi: 10.1016/j.phymed.2004.07.009
Cirla, A., and Mann, J. (2003). Combretastatins: from natural products to drug discovery. Nat. Prod. Rep. 20, 558–564. doi: 10.1039/b306797c
Cole, S. P., Bhardwaj, G., Gerlach, J. H., Mackie, J. E., Grant, C. E., Almquist, K. C., et al. (1992). Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258, 1650–1654. doi: 10.1126/science.1360704
Conner, G. E., Salathe, M., and Forteza, R. (2002). Lactoperoxidase and hydrogen peroxide metabolism in the airway. Am. J. Respir. Crit. Care Med. 166, S57–S61. doi: 10.1164/rccm.2206018
Dean, M. (2009). ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol. Neoplasia 14, 3–9. doi: 10.1007/s10911-009-9109-9
Dean, M., Fojo, T., and Bates, S. (2005). Tumour stem cells and drug resistance. Nat. Rev. Cancer 5, 275–284. doi: 10.1038/nrc1590
Denecker, G., Ovaere, P., Vandenabeele, P., and Declercq, W. (2008). Caspase-14 reveals its secrets. J. Cell. Biol. 180, 451–458. doi: 10.1083/jcb.200709098
Dhillon, A. S., Hagan, S., Rath, O., and Kolch, W. (2007). MAP kinase signalling pathways in cancer. Oncogene 26, 3279–3290. doi: 10.1038/sj.onc.1210421
Dimo, T., Rakotonirina, A., Tan, P., Dongo, E., Dongmo, A., Kamtchouing, P., et al. (2001). Antihypertensive effects of Dorstenia psilurus extract in fructose-fed hyperinsulinemic, hypertensive rats. Phytomedicine 8, 101–106. doi: 10.1078/0944-7113-00014
Edwards, B. K., Noone, A. M., Mariotto, A. B., Simard, E. P., Boscoe, F. P., Henley, S. J., et al. (2014). Annual Report to the Nation on the status of cancer, 1975-2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 120, 1290–1314. doi: 10.1002/cncr.28509
Ee, G. C., Lim, C. K., Rahmat, A., and Lee, H. L. (2005). Cytotoxic activities of chemical constituents from Mesua daphnifolia. Trop. Biomed. 22, 99–102.
Efferth, T. (2001). The human ATP-binding cassette transporter genes: from the bench to the bedside. Curr. Mol. Med. 1, 45–65. doi: 10.2174/1566524013364194
Efferth, T., and Volm, M. (2017). Multiple resistance to carcinogens and xenobiotics: P-glycoproteins as universal detoxifiers. Arch. Toxicol. doi: 10.1007/s00204-017-1938-5. [Epub ahead of print].
Einbond, L. S., Mighty, J., Kashiwazaki, R., Figueroa, M., Jalees, F., Acuna, U. M., et al. (2013). Garcinia benzophenones inhibit the growth of human colon cancer cells and synergize with sulindac sulfide and turmeric. Anticancer Agents Med. Chem. 13, 1540–1550. doi: 10.2174/18715206113139990095
Elujoba, A. (1995). Female infertility in the hands of traditional birth attendants in South-West Nigeria. Fitoterapia 66, 239–248.
Eno, A., and Azah, N. (2004). Effect of ethanolic extract from Elaeophorbia drupifera leaves on the gastrointestinal smooth muscle of the rabbit. Nigerian J. Physiol. Sci. 19, 60–68. doi: 10.4314/njps.v19i1.32637
Eyong, K. O., Folefoc, G. N., Kuete, V., Beng, V. P., Krohn, K., Hussain, H., et al. (2006). Newbouldiaquinone A: a naphthoquinone-anthraquinone ether coupled pigment, as a potential antimicrobial and antimalarial agent from Newbouldia laevis. Phytochemistry 67, 605–609. doi: 10.1016/j.phytochem.2005.12.019
Farnsworth, N. R., Akerele, O., Bingel, A. S., Soejarto, D. D., and Guo, Z. (1985). Medicinal plants in therapy. Bull. World Health Organ. 63, 965–981.
Fiot, J., Sanon, S., Azas, N., Mahiou, V., Jansen, O., Angenot, L., et al. (2006). Phytochemical and pharmacological study of roots and leaves of Guiera senegalensis J. F. Gmel (Combretaceae). J. Ethnopharmacol. 106, 173–178. doi: 10.1016/j.jep.2005.12.030
Focho, D., Wt, N., and Fonge, B. (2009). Medicinal plants of Aguambu - Bamumbu in the Lebialem highlands, southwest province of Cameroon. Afr. J. Pharm. Pharmacol. 3, 1–13.
Fouotsa, H., Mbaveng, A., Mbazoa, C., Nkengfack, A., Farzana, S., Iqbal, C., et al. (2013). Antibacterial constituents of three Cameroonian medicinal plants: Garcinia nobilis, Oricia suaveolens and Balsamocitrus camerunensis. BMC Complement. Altern. Med. 13:81. doi: 10.1186/1472-6882-13-81
Fouotsa, H., Meli Lannang, L., Djama Mbazoa, C., Rasheed, S., Marasini, B., Ali, Z., et al. (2012). Xanthones inhibitors of α-glucosidase and glycation from Garcinia nobilis. Phytochem. Lett. 5, 236–239. doi: 10.1016/j.phytol.2012.01.002
Fulda, S., and Kroemer, G. (2011). Mitochondria as therapeutic targets for the treatment of malignant disease. Antioxid. Redox. Signal. 15, 2937–2949. doi: 10.1089/ars.2011.4078
Galluzzi, L., Lopez-Soto, A., Kumar, S., and Kroemer, G. (2016). Caspases connect cell-death signaling to organismal homeostasis. Immunity 44, 221–231. doi: 10.1016/j.immuni.2016.01.020
García-Vilas, J. A., Quesada, A. R., and Medina, M. A. (2015). Damnacanthal, a noni anthraquinone, inhibits c-Met and is a potent antitumor compound against Hep G2 human hepatocellular carcinoma cells. Sci. Rep. 5:8021. doi: 10.1038/srep08021
Gillet, J. P., Efferth, T., and Remacle, J. (2007). Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochim. Biophys. Acta 1775, 237–262. doi: 10.1016/j.bbcan.2007.05.002
Glavinas, H., Krajcsi, P., Cserepes, J., and Sarkadi, B. (2004). The role of ABC transporters in drug resistance, metabolism and toxicity. Curr. Drug Deliv. 1, 27–42. doi: 10.2174/1567201043480036
Gonzalez-Vallinas, M., Gonzalez-Castejon, M., Rodriguez-Casado, A., and Ramirez De Molina, A. (2013). Dietary phytochemicals in cancer prevention and therapy: a complementary approach with promising perspectives. Nutr. Rev. 71, 585–599. doi: 10.1111/nure.12051
Gottesman, M. M., Ludwig, J., Xia, D., and Szakacs, G. (2006). Defeating drug resistance in cancer. Discov. Med. 6, 18–23.
Gouwakinnou, G. N., Lykke, A. M., Assogbadjo, A. E., and Sinsin, B. (2011). Local knowledge, pattern and diversity of use of Sclerocarya birrea. J. Ethnobiol. Ethnomed. 7:8. doi: 10.1186/1746-4269-7-8
Graham, J. G., Quinn, M. L., Fabricant, D. S., and Farnsworth, N. R. (2000). Plants used against cancer - an extension of the work of Jonathan Hartwell. J. Ethnopharmacol. 73, 347–377. doi: 10.1016/S0378-8741(00)00341-X
Gullett, N. P., Ruhul Amin, A. R., Bayraktar, S., Pezzuto, J. M., Shin, D. M., Khuri, F. R., et al. (2010). Cancer prevention with natural compounds. Semin. Oncol. 37, 258–281. doi: 10.1053/j.seminoncol.2010.06.014
Guruvayoorappan, C., and Kuttan, G. (2007a). Amentoflavone, a biflavonoid from Biophytum sensitivum augments lymphocyte proliferation, natural killer cell and antibody dependent cellular cytotoxicity through enhanced production of IL-2 and IFN-gamma and restrains serum sialic acid and gamma glutamyl transpeptidase production in tumor - bearing animals. J. Exp. Ther. Oncol. 6, 285–295. doi: 10.1177/1534735407302345
Guruvayoorappan, C., and Kuttan, G. (2007b). Effect of amentoflavone on the inhibition of pulmonary metastasis induced by B16F-10 melanoma cells in C57BL/6 mice. Integr. Cancer Ther. 6, 185–197. doi: 10.1177/1534735407302345
Guruvayoorappan, C., and Kuttan, G. (2008). Amentoflavone stimulates apoptosis in B16F-10 melanoma cells by regulating bcl-2, p53 as well as caspase-3 genes and regulates the nitric oxide as well as proinflammatory cytokine production in B16F-10 melanoma cells, tumor associated macrophages and peritoneal macrophages. J. Exp. Ther. Oncol. 7, 207–218.
Haddad, M., Khan, I. A., and Lacaille-Dubois, M. A. (2002). Two new prosapogenins from Albizia adianthifolia. Pharmazie 57, 705–708.
Haddad, M., Laurens, V., and Lacaille-Dubois, M. A. (2004). Induction of apoptosis in a leukemia cell line by triterpene saponins from Albizia adianthifolia. Bioorg. Med. Chem. 12, 4725–4734. doi: 10.1016/j.bmc.2004.06.025
Haddad, M., Miyamoto, T., Laurens, V., and Lacaille-Dubois, M. A. (2003). Two new biologically active triterpenoidal saponins acylated with salicylic acid from Albizia adianthifolia. J. Nat. Prod. 66, 372–377. doi: 10.1021/np020391q
Han, D., Williams, E., and Cadenas, E. (2001). Mitochondrial respiratory chain-dependent generation of superoxide anion and its release into the intermembrane space. Biochem. J. 353, 411–416. doi: 10.1042/bj3530411
Han, Y., Yang, X., Zhao, N., Peng, J., and Gao, H. (2016). Alpinumisoflavone induces apoptosis in esophageal squamous cell carcinoma by modulating miR-370/PIM1 signaling. Am. J. Cancer Res. 6, 2755–2771.
He, W., Van Puyvelde, L., De Kimpe, N., Verbruggen, L., Anthonissen, K., Van Der Flaas, M., et al. (2002). Chemical constituents and biological activities of Zanthoxylum usambarense. Phytother. Res. 16, 66–70. doi: 10.1002/ptr.849
Hientz, K., Mohr, A., Bhakta-Guha, D., and Efferth, T. (2017). The role of p53 in cancer drug resistance and targeted chemotherapy. Oncotarget 8, 8921–8946. doi: 10.18632/oncotarget.13475
Howley, B., and Fearnhead, H. O. (2008). Caspases as therapeutic targets. J. Cell. Mol. Med. 12, 1502–1516. doi: 10.1111/j.1582-4934.2008.00292.x
Hsu, Y. L., Cho, C. Y., Kuo, P. L., Huang, Y. T., and Lin, C. C. (2006). Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) induces apoptosis and cell cycle arrest in A549 cells through p53 accumulation via c-Jun NH2-terminal kinase-mediated phosphorylation at serine 15 in vitro and in vivo. J. Pharmacol. Exp. Ther. 318, 484–494. doi: 10.1124/jpet.105.098863
Ibrahim, B., Sowemimo, A., Spies, L., Koekomoer, T., Van De Venter, M., and Odukoya, O. A. (2013). Antiproliferative and apoptosis inducing activity of Markhamia tomentosa leaf extract on HeLa cells. J. Ethnopharmacol. 149, 745–749. doi: 10.1016/j.jep.2013.07.040
Ibrahim, M. B., Kaushik, N., Sowemimo, A. A., and Odukoya, O. A. (2016). Review of the phytochemical and pharmacological studies of the genus Markhamia. Pharmacogn. Rev. 10, 50–59. doi: 10.4103/0973-7847.176547
Ichimura, T., Yamanaka, A., Ichiba, T., Toyokawa, T., Kamada, Y., Tamamura, T., et al. (2006). Antihypertensive effect of an extract of Passiflora edulis rind in spontaneously hypertensive rats. Biosci. Biotechnol. Biochem. 70, 718–721. doi: 10.1271/bbb.70.718
Jeruto, P., Lukhoba, C., Ouma, G., Otieno, D., and Mutai, C. (2007). Herbal treatments in Aldai and Kaptumo divisions in Nandi district, Rift valley province, Kenya. Afr. J. Tradit. Complement. Altern. Med. 5, 103–105.
Ji, H. (2010). Mechanistic insights into acquired drug resistance in epidermal growth factor receptor mutation-targeted lung cancer therapy. Cancer Sci. 101, 1933–1938. doi: 10.1111/j.1349-7006.2010.01629.x
Jin, C. Y., Park, C., Hwang, H. J., Kim, G. Y., Choi, B. T., Kim, W. J., et al. (2011). Naringenin up-regulates the expression of death receptor 5 and enhances TRAIL-induced apoptosis in human lung cancer A549 cells. Mol. Nutr. Food Res. 55, 300–309. doi: 10.1002/mnfr.201000024
Jing, H., Zhou, X., Dong, X., Cao, J., Zhu, H., Lou, J., et al. (2010). Abrogation of akt signaling by isobavachalcone contributes to its anti-proliferative effects towards human cancer cells. Cancer Lett. 294, 167–177. doi: 10.1016/j.canlet.2010.01.035
Kadiri, H., Adegor, E., and Asagba, S. (2007). Effect of aqueous Nauclea pobeguinii leaf extract on rats induced with hepatic injury. Res. J. Med. Plants 1, 139–143. doi: 10.3923/rjmp.2007.139.143
Kannan, S., Parimala, B., and Jayakar, B. (2011). Antibacterial evaluation of the methanolic extract of Passiflora edulis. Hygeia. J. Drugs Med. 3, 46–49.
Kanno, S., Tomizawa, A., Ohtake, T., Koiwai, K., Ujibe, M., and Ishikawa, M. (2006). Naringenin-induced apoptosis via activation of NF-kappaB and necrosis involving the loss of ATP in human promyeloleukemia HL-60 cells. Toxicol. Lett. 166, 131–139. doi: 10.1016/j.toxlet.2006.06.005
Kaou, A., Mahiou-Leddet, V., Canlet, C., Debrauwer, L., Hutter, S., Azas, N., et al. (2010). New amide alkaloid from the aerial part of Piper capense L.f. (Piperaceae). Fitoterapia 81, 632–635. doi: 10.1016/j.fitote.2010.03.006
Kapche, G. D., Fozing, C. D., Donfack, J. H., Fotso, G. W., Amadou, D., Tchana, A. N., et al. (2009). Prenylated arylbenzofuran derivatives from Morus mesozygia with antioxidant activity. Phytochemistry 70, 216–221. doi: 10.1016/j.phytochem.2008.12.014
Karou, S. D., Tchacondo, T., Ilboudo, D. P., and Simpore, J. (2011). Sub-Saharan Rubiaceae: a review of their traditional uses, phytochemistry and biological activities. Pak. J. Biol. Sci. 14, 149–169. doi: 10.3923/pjbs.2011.149.169
Kartner, N., Evernden-Porelle, D., Bradley, G., and Ling, V. (1985). Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. Nature 316, 820–823. doi: 10.1038/316820a0
Katayama, K., Noguchi, K., and Sugimoto, Y. (2014). Regulations of P-glycoprotein/ABCB1/MDR1 in human cancer cells. New. J. Sci. 2014, 10. doi: 10.1155/2014/476974
Kato, A., Higuchi, Y., Goto, H., Kizu, H., Okamoto, T., Asano, N., et al. (2006). Inhibitory effects of Zinziber officinale Roscoe derived components on aldose reductase activity in vitro and in vivo. J. Agric. Food Chem. 54, 6640–6644. doi: 10.1021/jf061599a
Kaur, R., Kapoor, K., and Kaur, H. (2011). Plants as a source of anticancer agents. J. Nat. Prod. Plant. Resour. 1, 119–124.
Kawiak, A., Zawacka-Pankau, J., and Lojkowska, E. (2012). Plumbagin induces apoptosis in Her2-overexpressing breast cancer cells through the mitochondrial-mediated pathway. J. Nat. Prod. 75, 747–751. doi: 10.1021/np3000409
Keppler, D. (2011). Multidrug resistance proteins (MRPs, ABCCs): importance for pathophysiology and drug therapy. Handb. Exp. Pharmacol. 201, 299–323. doi: 10.1007/978-3-642-14541-4_8
Keshava, R., Muniyappa, N., Gope, R., and Ramaswamaiah, A. S. (2016). Anti-cancer effects of Imperata cylindrica leaf extract on human oral squamous carcinoma cell line SCC-9 in vitro. Asian Pac. J. Cancer Prev. 17, 1891–1898. doi: 10.7314/APJCP.2016.17.4.1891
Kim, J., Lee, S., Park, H., Yang, J., Shin, T., Kim, Y., et al. (2008). Cytotoxic components from the dried rhizomes of Zingiber officinale Roscoe. Arch. Pharm. Res. 31, 415–418. doi: 10.1007/s12272-001-1172-y
Kim, M., Turnquist, H., Jackson, J., Sgagias, M., Yan, Y., Gong, M., et al. (2002). The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin. Cancer Res. 8, 22–28.
Kim, Y. J., Choi, W. I., Ko, H., So, Y., Kang, K. S., Kim, I., et al. (2014). Neobavaisoflavone sensitizes apoptosis via the inhibition of metastasis in TRAIL-resistant human glioma U373MG cells. Life Sci. 95, 101–107. doi: 10.1016/j.lfs.2013.10.035
Kinghorn, A., and Evans, F. (1974). Occurrence of ingenol in Elaeophorbia species. Planta Med. 26, 150–154. doi: 10.1055/s-0028-1097982
Kone, W. M., Atindehou, K. K., Kacou-N'douba, A., and Dosso, M. (2006). Evaluation of 17 medicinal plants from Northern Cote d'Ivoire for their in vitro activity against Streptococcus pneumoniae. Afr. J. Tradit. Complement. Altern. Med. 4, 17–22.
Kone, W. M., Atindehou, K. K., Terreaux, C., Hostettmann, K., Traore, D., and Dosso, M. (2004). Traditional medicine in north Cote-d'Ivoire: screening of 50 medicinal plants for antibacterial activity. J. Ethnopharmacol. 93, 43–49. doi: 10.1016/j.jep.2004.03.006
Kuete, V., Ango, P. Y., Yeboah, S. O., Mbaveng, A. T., Mapitse, R., Kapche, G. D., et al. (2014a). Cytotoxicity of four Aframomum species (A. arundinaceum, A. alboviolaceum, A. kayserianum and A. polyanthum) towards multi-factorial drug resistant cancer cell lines. BMC Complement. Altern. Med. 14:340. doi: 10.1186/1472-6882-14-340
Kuete, V., Djeussi, D. E., Mbaveng, A. T., Zeino, M., and Efferth, T. (2016a). Cytotoxicity of 15 Cameroonian medicinal plants against drug sensitive and multi-drug resistant cancer cells. J. Ethnopharmacol. 186, 196–204. doi: 10.1016/j.jep.2016.04.001
Kuete, V., Donfack, A. R., Mbaveng, A. T., Zeino, M., Tane, P., and Efferth, T. (2015a). Cytotoxicity of anthraquinones from the roots of Pentas schimperi towards multi-factorial drug-resistant cancer cells. Invest. New Drugs 33, 861–869. doi: 10.1007/s10637-015-0268-9
Kuete, V., Dzotam, J. K., Voukeng, I. K., Fankam, A. G., and Efferth, T. (2016b). Cytotoxicity of methanol extracts of Annona muricata, Passiflora edulis and nine other Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. Springerplus 5, 1666. doi: 10.1186/s40064-016-3361-4
Kuete, V., and Efferth, T. (2010). Cameroonian medicinal plants: pharmacology and derived natural products. Front. Pharmacol. 1:123. doi: 10.3389/fphar.2010.00123
Kuete, V., and Efferth, T. (2011). Pharmacogenomics of Cameroonian traditional herbal medicine for cancer therapy. J. Ethnopharmacol. 137, 752–766. doi: 10.1016/j.jep.2011.06.035
Kuete, V., and Efferth, T. (2015). African flora has the potential to fight multidrug resistance of cancer. BioMed. Res. Int. 2015:914813. doi: 10.1155/2015/914813
Kuete, V., Eichhorn, T., Wiench, B., Krusche, B., and Efferth, T. (2012). Cytotoxicity, anti-angiogenic, apoptotic effects and transcript profiling of a naturally occurring naphthyl butenone, guieranone A. Cell Div. 7:16. doi: 10.1186/1747-1028-7-16
Kuete, V., Eyong, K. O., Folefoc, G. N., Beng, V. P., Hussain, H., Krohn, K., et al. (2007a). Antimicrobial activity of the methanolic extract and of the chemical constituents isolated from Newbouldia laevis. Pharmazie 62, 552–556.
Kuete, V., Fankam, A. G., Wiench, B., and Efferth, T. (2013a). Cytotoxicity and modes of action of the methanol extracts of six Cameroonian medicinal plants against multidrug-mesistant tumor cells. Evid. Based Complement. Alternat. Med. 2013:285903. doi: 10.1155/2013/285903
Kuete, V., Fouotsa, H., Mbaveng, A. T., Wiench, B., Nkengfack, A. E., and Efferth, T. (2015b). Cytotoxicity of a naturally occurring furoquinoline alkaloid and four acridone alkaloids towards multi-factorial drug-resistant cancer cells. Phytomedicine 22, 946–951. doi: 10.1016/j.phymed.2015.07.002
Kuete, V., Fozing, D. C., Kapche, W. F., Mbaveng, A. T., Kuiate, J. R., Ngadjui, B. T., et al. (2009). Antimicrobial activity of the methanolic extract and compounds from Morus mesozygia stem bark. J. Ethnopharmacol. 124, 551–555. doi: 10.1016/j.jep.2009.05.004
Kuete, V., Krusche, B., Youns, M., Voukeng, I., Fankam, A. G., Tankeo, S., et al. (2011a). Cytotoxicity of some Cameroonian spices and selected medicinal plant extracts. J. Ethnopharmacol. 134, 803–812. doi: 10.1016/j.jep.2011.01.035
Kuete, V., Mbaveng, A. T., Nono, E. C., Simo, C. C., Zeino, M., Nkengfack, A. E., et al. (2016c). Cytotoxicity of seven naturally occurring phenolic compounds towards multi-factorial drug-resistant cancer cells. Phytomedicine 23, 856–863. doi: 10.1016/j.phymed.2016.04.007
Kuete, V., Mbaveng, A. T., Zeino, M., Fozing, C. D., Ngameni, B., Kapche, G. D., et al. (2015c). Cytotoxicity of three naturally occurring flavonoid derived compounds (artocarpesin, cycloartocarpesin and isobavachalcone) towards multi-factorial drug-resistant cancer cells. Phytomedicine 22, 1096–1102. doi: 10.1016/j.phymed.2015.07.006
Kuete, V., Mbaveng, A. T., Zeino, M., Ngameni, B., Kapche, G. D. W. F., Kouam, S. F., et al. (2015d). Cytotoxicity of two naturally occurring flavonoids (dorsmanin F and poinsettifolin B) towards multi-factorial drug-resistant cancer cells. Phytomedicine 22, 737–743. doi: 10.1016/j.phymed.2015.04.007
Kuete, V., Ngameni, B., Mbaveng, A. T., Ngadjui, B., Meyer, J. J., and Lall, N. (2010). Evaluation of flavonoids from Dorstenia barteri for their antimycobacterial, antigonorrheal and anti-reverse transcriptase activities. Acta Trop. 116, 100–104. doi: 10.1016/j.actatropica.2010.06.005
Kuete, V., Ngameni, B., Simo, C. C., Tankeu, R. K., Ngadjui, B. T., Meyer, J. J., et al. (2008). Antimicrobial activity of the crude extracts and compounds from Ficus chlamydocarpa and Ficus cordata (Moraceae). J. Ethnopharmacol. 120, 17–24. doi: 10.1016/j.jep.2008.07.026
Kuete, V., Ngameni, B., Wiench, B., Krusche, B., Horwedel, C., Ngadjui, B. T., et al. (2011b). Cytotoxicity and mode of action of four naturally occuring flavonoids from the genus Dorstenia: gancaonin Q, 4-hydroxylonchocarpin, 6-prenylapigenin, and 6,8-diprenyleriodictyol. Planta Med. 77, 1984–1989. doi: 10.1055/s-0031-1280023
Kuete, V., Nkuete, A. H. L., Mbaveng, A. T., Wiench, B., Wabo, H. K., Tane, P., et al. (2014b). Cytotoxicity and modes of action of 4′-hydroxy-2′,6′-dimethoxychalcone and other flavonoids toward drug-sensitive and multidrug-resistant cancer cell lines. Phytomedicine 21, 1651–1657. doi: 10.1016/j.phymed.2014.08.001
Kuete, V., Nono, E. C., Mkounga, P., Marat, K., Hultin, P. G., and Nkengfack, A. E. (2011c). Antimicrobial activities of the CH2Cl2-CH3OH (1:1) extracts and compounds from the roots and fruits of Pycnanthus angolensis (Myristicaceae). Nat. Prod. Res. 25, 432–443. doi: 10.1080/14786419.2010.522577
Kuete, V., Omosa, L. K., Tala, V. R., Midiwo, J. O., Mbaveng, A. T., Swaleh, S., et al. (2016d). Cytotoxicity of plumbagin, rapanone and 12 other naturally occurring quinones from Kenyan flora towards human carcinoma cells. BMC Pharmacol. Toxicol. 17:60. doi: 10.1186/s40360-016-0104-7
Kuete, V., Sandjo, L., Nantchouang Ouete, J., Fouotsa, H., Wiench, B., and Efferth, T. (2013b). Cytotoxicity and modes of action of three naturally occuring xanthones (8-hydroxycudraxanthone G, morusignin I and cudraxanthone I) against sensitive and multidrug-resistant cancer cell lines. Phytomedicine 21, 315–322. doi: 10.1016/j.phymed.2013.08.018
Kuete, V., and Sandjo, L. P. (2012). Isobavachalcone: an overview. Chin. J. Integr. Med. 18, 543–547. doi: 10.1007/s11655-012-1142-7
Kuete, V., Sandjo, L. P., Djeussi, D. E., Zeino, M., Kwamou, G. M., Ngadjui, B., et al. (2014c). Cytotoxic flavonoids and isoflavonoids from Erythrina sigmoidea towards multi-factorial drug resistant cancer cells. Invest. New Drugs 32, 1053–1062. doi: 10.1007/s10637-014-0137-y
Kuete, V., Sandjo, L. P., Kwamou, G. M., Wiench, B., Nkengfack, A. E., and Efferth, T. (2014d). Activity of three cytotoxic isoflavonoids from Erythrina excelsa and Erythrina senegalensis (neobavaisoflavone, sigmoidin H and isoneorautenol) toward multi-factorial drug resistant cancer cells. Phytomedicine 21, 682–688. doi: 10.1016/j.phymed.2013.10.017
Kuete, V., Sandjo, L. P., Mbaveng, A. T., Seukep, J. A., Ngadjui, B. T., and Efferth, T. (2015f). Cytotoxicity of selected Cameroonian medicinal plants and Nauclea pobeguinii towards multi-factorial drug-resistant cancer cells. BMC Complement. Altern. Med. 15:309. doi: 10.1186/s12906-015-0841-y
Kuete, V., Sandjo, L. P., Mbaveng, A. T., Zeino, M., and Efferth, T. (2015g). Cytotoxicity of compounds from Xylopia aethiopica towards multi-factorial drug-resistant cancer cells. Phytomedicine 22, 1247–1254. doi: 10.1016/j.phymed.2015.10.008
Kuete, V., Sandjo, L. P., Wiench, B., and Efferth, T. (2013c). Cytotoxicity and modes of action of four Cameroonian dietary spices ethno-medically used to treat Cancers: Echinops giganteus, Xylopia aethiopica, Imperata cylindrica and Piper capense. J. Ethnopharmacol. 149, 245–253. doi: 10.1016/j.jep.2013.06.029
Kuete, V., Sandjo, L., Seukep, J., Maen, Z., Ngadjui, B., and Efferth, T. (2015e). Cytotoxic compounds from the fruits of Uapaca togoensis towards multi-factorial drug-resistant cancer cells. Planta Med. 81, 32–38. doi: 10.1055/s-0034-1383362
Kuete, V., Simo, I. K., Ngameni, B., Bigoga, J. D., Watchueng, J., Kapguep, R. N., et al. (2007b). Antimicrobial activity of the methanolic extract, fractions and four flavonoids from the twigs of Dorstenia angusticornis Engl. (Moraceae). J. Ethnopharmacol. 112, 271–277. doi: 10.1016/j.jep.2007.03.008
Kuete, V., Tangmouo, J. G., Penlap Beng, V., Ngounou, F. N., and Lontsi, D. (2006). Antimicrobial activity of the methanolic extract from the stem bark of Tridesmostemon omphalocarpoides (Sapotaceae). J. Ethnopharmacol. 104, 5–11. doi: 10.1016/j.jep.2005.08.002
Kuete, V., Tankeo, S. B., Saeed, M. E., Wiench, B., Tane, P., and Efferth, T. (2014e). Cytotoxicity and modes of action of five Cameroonian medicinal plants against multi-factorial drug resistance of tumor cells. J. Ethnopharmacol. 153, 207–219. doi: 10.1016/j.jep.2014.02.025
Kuete, V., Tchakam, P. D., Wiench, B., Ngameni, B., Wabo, H. K., Tala, M. F., et al. (2013d). Cytotoxicity and modes of action of four naturally occuring benzophenones: 2,2′,5,6′-tetrahydroxybenzophenone, guttiferone E, isogarcinol and isoxanthochymol. Phytomedicine 20, 528–536. doi: 10.1016/j.phymed.2013.02.003
Kuete, V., Tchinda, C. F., Mambe, F. T., Beng, V. P., and Efferth, T. (2016e). Cytotoxicity of methanol extracts of 10 Cameroonian medicinal plants towards multi-factorial drug-resistant cancer cell lines. BMC Complement. Altern. Med. 16:267. doi: 10.1186/s12906-016-1253-3
Kuete, V., Voukeng, I. K., Tsobou, R., Mbaveng, A. T., Wiench, B., Beng, V. P., et al. (2013e). Cytotoxicity of Elaoephorbia drupifera and other Cameroonian medicinal plants against drug sensitive and multidrug resistant cancer cells. BMC Complement. Altern. Med. 13:250. doi: 10.1186/1472-6882-13-250
Kuete, V., Wabo, H. K., Eyong, K. O., Feussi, M. T., Wiench, B., Krusche, B., et al. (2011d). Anticancer activities of six selected natural compounds of some Cameroonian medicinal plants. PLoS ONE 6:e21762. doi: 10.1371/journal.pone.0021762
Kumaran, G., Clamp, A. R., and Jayson, G. C. (2008). Angiogenesis as a therapeutic target in cancer. Clin. Med. (Lond). 8, 455–458. doi: 10.7861/clinmedicine.8-4-455
Kuo, P. L., Hsu, Y. L., and Cho, C. Y. (2006). Plumbagin induces G2-M arrest and autophagy by inhibiting the AKT/mammalian target of rapamycin pathway in breast cancer cells. Mol. Cancer Ther. 5, 3209–3221. doi: 10.1158/1535-7163.MCT-06-0478
Kwok, A. H., Wang, Y., and Ho, W. S. (2016). Cytotoxic and pro-oxidative effects of Imperata cylindrica aerial part ethyl acetate extract in colorectal cancer in vitro. Phytomedicine 23, 558–565. doi: 10.1016/j.phymed.2016.02.015
Lannang, A. M., Louh, G. N., Biloa, B. M., Komguem, J., Mbazoa, C. D., Sondengam, B. L., et al. (2010). Cytotoxicity of natural compounds isolated from the seeds of Garcinia afzelii. Planta Med. 76, 708–712. doi: 10.1055/s-0029-1240627
Lee, S., Kim, H., Kang, J. W., Kim, J. H., Lee, D. H., and Kim, M. S. (2011). The biflavonoid amentoflavone induces apoptosis via suppressing E7 expression, cell cycle arrest at sub-G1Ą phase, and mitochondria-emanated intrinsic pathways in human cervical cancer cells. J. Med. Food 14, 808–816. doi: 10.1089/jmf.2010.1428
Liu, X., Yu, T., Gao, X. M., Zhou, Y., Qiao, C. F., Peng, Y., et al. (2010). Apoptotic effects of polyprenylated benzoylphloroglucinol derivatives from the twigs of Garcinia multiflora. J. Nat. Prod. 73, 1355–1359. doi: 10.1021/np100156w
Lorent, J. H., Léonard, C., Abouzi, M., Akabi, F., Quetin-Leclercq, J., and Mingeot-Leclercq, M. P. (2016). Planta Med. 82, 1532–1539. doi: 10.1055/s-0042-114780
Lozano, R., Naghavi, M., Foreman, K., Lim, S., Shibuya, K., Aboyans, V., et al. (2012). Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128. doi: 10.1016/S0140-6736(12)61728-0
Luduena, R. F. (1998). Multiple forms of tubulin: different gene products and covalent modifications. Int. Rev. Cytol. 178, 207–275. doi: 10.1016/S0074-7696(08)62138-5
Luqmani, Y. A. (2005). Mechanisms of drug resistance in cancer chemotherapy. Med. Princ. Pract. 14(Suppl. 1), 35–48. doi: 10.1159/000086183
Mabeku, L., Kuiate, J., and Oyono, E. (2011). Screening of some plants used in the Cameroonian folk medicine for the treatment of infectious diseases. Int. J. Biol. 3, 13–21. doi: 10.5539/ijb.v3n4p13
Madubunyi, I. (1995). Anti-hepatotoxic and trypanocidal activities of the ethanolic extract of nauclea latifolia root bark. J. Herbs Spices Med. Plants 3, 23–53. doi: 10.1300/J044v03n02_04
Maniafu, B. M., Wilber, L., Ndiege, I. O., Wanjala, C. C., and Akenga, T. A. (2009). Larvicidal activity of extracts from three Plumbago spp against Anopheles gambiae. Mem. Inst. Oswaldo Cruz. 104, 813–817. doi: 10.1590/S0074-02762009000600002
Matsunaga, K., Shibuya, M., and Ohizumi, Y. (1995). Imperanene, a novel phenolic compound with platelet aggregation inhibitory activity from Imperata cylindrica. J. Nat. Prod. 58, 138–139. doi: 10.1021/np50115a022
Mbaveng, A. T., and Kuete, V. (2017). “Chapter 30: Zingiber officinale,” in Medicinal Spices and Vegetables from Africa, ed V. Kuete (Amsterdam: Academic Press), 627–639. doi: 10.1016/B978-0-12-809286-6.00030-3
Mbaveng, A. T., Kuete, V., Ngameni, B., Beng, V. P., Ngadjui, B. T., Meyer, J. J., et al. (2012). Antimicrobial activities of the methanol extract and compounds from the twigs of Dorstenia mannii (Moraceae). BMC Complement. Altern. Med. 12:83. doi: 10.1186/1472-6882-12-83
Mbaveng, A. T., Ngameni, B., Kuete, V., Simo, I. K., Ambassa, P., Roy, R., et al. (2008). Antimicrobial activity of the crude extracts and five flavonoids from the twigs of Dorstenia barteri (Moraceae). J. Ethnopharmacol. 116, 483–489. doi: 10.1016/j.jep.2007.12.017
Mbouangouere, R., Tane, P., Ngamga, D., Khan, S., Choudhary, M., and Ngadjui, B. (2007). A New steroid and α-glucosidase inhibitors from Anthocleista schweinfurthii. J. Med. Plants Res. 1, 106. doi: 10.3923/rjmp.2007.106.111
Mengome, L. E., Akue, J. P., Souza, A., Feuya Tchoua, G. R., and Nsi Emvo, E. (2010). In vitro activities of plant extracts on human Loa loa isolates and cytotoxicity for eukaryotic cells. Parasitol. Res. 107, 643–650. doi: 10.1007/s00436-010-1910-2
Merza, J., Mallet, S., Litaudon, M., Dumontet, V., Seraphin, D., and Richomme, P. (2006). New cytotoxic guttiferone analogues from Garcinia virgata from New Caledonia. Planta Med. 72, 87–89. doi: 10.1055/s-2005-873185
Mesia, G. K., Tona, G. L., Penge, O., Lusakibanza, M., Nanga, T. M., Cimanga, R. K., et al. (2005). Antimalarial activities and toxicities of three plants used as traditional remedies for malaria in the Democratic Republic of Congo: Croton mubango, Nauclea pobeguinii and Pyrenacantha staudtii. Ann. Trop. Med. Parasitol. 99, 345–357. doi: 10.1179/136485905X36325
Miyoshi, N., Nakamura, Y., Ueda, Y., Abe, M., Ozawa, Y., and Uchida, K. (2003). Dietary ginger constituents, galanals A and B, are potent apoptosis inducers in Human T lymphoma Jurkat cells. Cancer Lett. 199, 113–119. doi: 10.1016/S0304-3835(03)00381-1
Mohamed, G., Abdel-Lateff, A., Fouad, M., Ibrahim, S., Elkhayat, E., and Okino, T. (2009). Chemical composition and hepato-protective activity of Imperata cylindrica Beauv. Pharmacogn. Mag. 5, 28–36.
Momeni, J., Djialeu Ntchatchoua, W. P., Fadimatou Akam, M. T., and Ngassoum, M. B. (2010). Antioxidant activities of some Cameroonian plants extracts used in the treatment of intestinal and infectious diseases. Indian J. Pharmaceut. Sci. 72, 140–144. doi: 10.4103/0250-474X.62236
Muller, F. (2000). The nature and mechanism of superoxide production by the electron transport chain: its relevance to aging. J. Am. Aging Assoc. 23, 227–253. doi: 10.1007/s11357-000-0022-9
Murray, C. J., and Lopez, A. D. (1997). Mortality by cause for eight regions of the world: global burden of disease study. Lancet 349, 1269–1276. doi: 10.1016/S0140-6736(96)07493-4
Namkoong, S., Kim, T. J., Jang, I. S., Kang, K. W., Oh, W. K., and Park, J. (2011). Alpinumisoflavone induces apoptosis and suppresses extracellular signal-regulated kinases/mitogen activated protein kinase and nuclear factor-kappaB pathways in lung tumor cells. Biol. Pharm. Bull. 34, 203–208. doi: 10.1248/bpb.34.203
Natarajan, K., Xie, Y., Baer, M. R., and Ross, D. D. (2012). Role of breast cancer resistance protein (BCRP/ABCG2) in cancer drug resistance. Biochem. Pharmacol. 83, 1084–1103. doi: 10.1016/j.bcp.2012.01.002
Ngadjui, B., Dongo, E., Happi, N., Bezabih, M., and Abegaz, B. (1998). Prenylated flavones and phenylpropanoid derivatives from roots of Dorstenia psilurus. Phytochemistry 48, 733–737. doi: 10.1016/S0031-9422(98)00017-X
Ngadjui, B. T., Dongo, E., Tamboue, H., Fogue, K., and Abegaz, B. M. (1999a). Prenylated flavanones from the twigs of Dorstenia mannii. Phytochemistry 50, 1401–1406. doi: 10.1016/S0031-9422(98)00324-0
Ngadjui, B. T., Kapche, G. W. F., Tamboue, H., Abegaz, B. M., and Connolly, J. D. (1999b). Prenylated flavonoids and a dihydro-4-phenylcoumarinfrom Dorstenia poinsettifolia. Phytochemistry 51, 119–123. doi: 10.1016/S0031-9422(98)00621-9
Ngadjui, B. T., Kouam, S. F., Dongo, E., Kapche, G. W., and Abegaz, B. M. (2000). Prenylated flavonoids from the aerial parts of Dorstenia mannii. Phytochemistry 55, 915–919. doi: 10.1016/S0031-9422(00)00215-6
Ngadjui, B. T., Lontsi, D., Ayafor, J. F., and Sondengam, B. L. (1989). Pachypophyllin and pachypostaudins A and B: three bisnorlignans from Pachypodanthium staudtii. Phytochemistry 28, 231–234. doi: 10.1016/0031-9422(89)85044-7
Ngameni, B., Touaibia, M., Belkaid, A., Ambassa, P., Watchueng, J., Patnama, R., et al. (2007). Inhibition of matrix metalloproteinase-2 secretion by chalcones from the twigs of Dorstenia barteri Bureau. Arkivoc 2007, 91–103. doi: 10.3998/ark.5550190.0008.911
Ngbolua, K.-T.-N., Mubindukila, R. E. N., Mpiana, P. T., Ashande, M. C., Baholy, R., Ekutsu, G. E., et al. (2014). In vitro assessment of antibacterial and antioxidant activities of a congolese medicinal plant species Anthocleista schweinfurthii Gilg (Gentianaceae). J. Modern Drug. Discov. Drug. Deliv. Res. 7, 1–6. doi: 10.15297/JMDDR.V1I3.03
Nicolle, E., Boccard, J., Guilet, D., Dijoux-Franca, M. G., Zelefac, F., Macalou, S., et al. (2009). Breast cancer resistance protein (BCRP/ABCG2): new inhibitors and QSAR studies by a 3D linear solvation energy approach. Eur. J. Pharm. Sci. 38, 39–46. doi: 10.1016/j.ejps.2009.05.012
Nishimoto, K., Ito, M., Natori, S., and Ohmoto, T. (1968). The structure of arundoin, cylindrin and fernenol triterpenoids of fernane and arborane groups of Imperata cylindrica var. Koenigii. Tetrahedron 24, 735–752. doi: 10.1016/0040-4020(68)88023-8
Nishimura, R., Tabata, K., Arakawa, M., Ito, Y., Kimura, Y., Akihisa, T., et al. (2007). Isobavachalcone, a chalcone constituent of Angelica keiskei induces apoptosis in neuroblastoma. Biol. Pharm. Bull. 30, 1878–1883. doi: 10.1248/bpb.30.1878
Njume, C., Afolayan, A. J., Green, E., and Ndip, R. N. (2011). Volatile compounds in the stem bark of Sclerocarya birrea (Anacardiaceae) possess antimicrobial activity against drug-resistant strains of Helicobacter pylori. Int. J. Antimicrob. Agents 38, 319–324. doi: 10.1016/j.ijantimicag.2011.05.002
Nono, E. C., Mkounga, P., Kuete, V., Marat, K., Hultin, P. G., and Nkengfack, A. E. (2010). Pycnanthulignenes A-D, antimicrobial cyclolignene derivatives from the roots of Pycnanthus angolensis. J. Nat. Prod. 73, 213–216. doi: 10.1021/np9007393
Noumi, E., and Eloumou, M. (2011). Syphilis ailment: prevalence and herbal remedies in Ebolowa subdivision (South region, Cameroon). Int. J. Biomed. Pharmaceut. Sci. 2, 20–28.
Ogungbamila, F. O., and Samuelsson, G. (1990). Smooth muscle relaxing flavonoids from Alchornea cordifolia. Acta Pharm. Nord. 2, 421–422.
Ogweno Midiwo, J., Yenesew, A., Juma, B. F., Derese, S., Ayoo, J. A., Aluoch, A. O., et al. (2002). Bioactive compounds from some Kenyan ethnomedicinal plants: Myrsinaceae, Polygonaceae and Psiadia punctulata. Phytochem. Rev. 1, 311–323. doi: 10.1023/A:1026029609500
Okafor, C. I. (2012). The metabolic syndrome in Africa: current trends. Indian J. Endocrinol. Metab. 16, 56–66. doi: 10.4103/2230-8210.91191
Omosa, L. K., Midiwo, J. O., Masila, V. M., Gisacho, B. M., Munayi, R., Francisca, K., et al. (2015). Cytotoxicity of 91 Kenyan indigenous medicinal plants towards human CCRF-CEM leukemia cells. J. Ethnopharmacol. 179, 177–196. doi: 10.1016/j.jep.2015.12.028
Orton, R. J., Sturm, O. E., Vyshemirsky, V., Calder, M., Gilbert, D. R., and Kolch, W. (2005). Computational modelling of the receptor-tyrosine-kinase-activated MAPK pathway. Biochem. J. 392, 249–261. doi: 10.1042/BJ20050908
Osafo, N., and Obiri, D. D. (2016). Anti-inflammatory and anti-anaphylactic activity of xylopic acid isolated from the dried fruit of Xylopia aethiopica in mice. Planta Med. 81, S1–S381. doi: 10.1055/s-0036-1596165
Ozkan, M., Mutiso, P. B., Nahar, L., Liu, P., Brown, S., Wang, W., et al. (2013). Zanthoxylum usambarense (Engl.) Kokwaro (Rutaceae) extracts inhibit the growth of the breast cancer cell lines MDA-MB-231 and MCF-7, but not the brain tumour cell line U251 in vitro. Phytother. Res. 27, 787–790. doi: 10.1002/ptr.4775
Park, J. H., Jin, C. Y., Lee, B. K., Kim, G. Y., Choi, Y. H., and Jeong, Y. K. (2008). Naringenin induces apoptosis through downregulation of Akt and caspase-3 activation in human leukemia THP-1 cells. Food Chem. Toxicol. 46, 3684–3690. doi: 10.1016/j.fct.2008.09.056
Pedersen, M. E., Metzler, B., Stafford, G. I., Van Staden, J., Jager, A. K., and Rasmussen, H. B. (2009). Amides from Piper capense with CNS activity - a preliminary SAR analysis. Molecules 14, 3833–3843. doi: 10.3390/molecules14093833
Pei, J. S., Liu, C. C., Hsu, Y. N., Lin, L. L., Wang, S. C., Chung, J. G., et al. (2012). Amentoflavone induces cell-cycle arrest and apoptosis in MCF-7 human breast cancer cells via mitochondria-dependent pathway. In vivo 26, 963–970.
Pieme, C. A., Ambassa, P., Yankep, E., and Saxena, A. K. (2015). Epigarcinol and isogarcinol isolated from the root of Garcinia ovalifolia induce apoptosis of human promyelocytic leukemia (HL-60 cells). BMC Res. Notes 8:700. doi: 10.1186/s13104-015-1596-8
Pieme, C. A., Guru, S. K., Ambassa, P., Kumar, S., Ngameni, B., Ngogang, J. Y., et al. (2013). Induction of mitochondrial dependent apoptosis and cell cycle arrest in human promyelocytic leukemia HL-60 cells by an extract from Dorstenia psilurus: a spice from Cameroon. BMC Complement. Altern. Med. 13:223. doi: 10.1186/1472-6882-13-223
Plengsuriyakarn, T., Viyanant, V., Eursitthichai, V., Tesana, S., Chaijaroenkul, W., Itharat, A., et al. (2012). Cytotoxicity, toxicity, and anticancer activity of Zingiber officinale Roscoe against cholangiocarcinoma. Asian Pac. J. Cancer Prev. 13, 4597–4606. doi: 10.7314/APJCP.2012.13.9.4597
Powolny, A. A., and Singh, S. V. (2008). Plumbagin-induced apoptosis in human prostate cancer cells is associated with modulation of cellular redox status and generation of reactive oxygen species. Pharm. Res. 25, 2171–2180. doi: 10.1007/s11095-008-9533-3
Protiva, P., Hopkins, M. E., Baggett, S., Yang, H., Lipkin, M., Holt, P. R., et al. (2008). Growth inhibition of colon cancer cells by polyisoprenylated benzophenones is associated with induction of the endoplasmic reticulum response. Int. J. Cancer 123, 687–694. doi: 10.1002/ijc.23515
Rada, B., and Leto, T. L. (2008). Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib. Microbiol. 15, 164–187. doi: 10.1159/000136357
Rajeswari, D., Vijayalakshmi, S., and Gajalakshmi, S. (2012). Phytochemical and pharmacological properties of Annona muricata. Int. J. Pharm. Pharmaceut. Sci. 4, 3–6.
Roux, D., Hadi, H. A., Thoret, S., Guenard, D., Thoison, O., Pais, M., et al. (2000). Structure-activity relationship of polyisoprenyl benzophenones from Garcinia pyrifera on the tubulin/microtubule system. J. Nat. Prod. 63, 1070–1076. doi: 10.1021/np0000872
Rowe, C. A., Nantz, M. P., Deniera, C., Green, K., Talcott, S. T., and Percival, S. S. (2004). Inhibition of neoplastic transformation of benzo[alpha]pyrene-treated BALB/c 3T3 murine cells by a phytochemical extract of passionfruit juice. J. Med. Food. 7, 402–407. doi: 10.1089/jmf.2004.7.402
Ruppelt, B., Pereira, E., Goncalves, L., and Pereira, N. (1991). Pharmacological screening of plants recommended by folk medicine as anti-snake venom I. Analgesic and anti-inflammatory activities. Mem. Inst. Oswaldo Cruz 86, 203–205. doi: 10.1590/S0074-02761991000600046
Sagar, S., Esau, L., Moosa, B., Khashab, N. M., Bajic, V. B., and Kaur, M. (2014). Cytotoxicity and apoptosis induced by a plumbagin derivative in estrogen positive MCF-7 breast cancer cells. Anticancer Agents Med. Chem. 14, 170–180. doi: 10.2174/18715206113136660369
Sakpakdeejaroen, I., and Itharat, A. (2009). Cytotoxic compounds against breast adenocarcinoma cells (MCF-7) from Pikutbenjakul. J. Health Res. 23, 71–76.
Sandjo, L., Kuete, V., Poumale, P., and Efferth, T. (2016). Unprecedented brominated oleanolide and a new tetrahydrofurano-ceramide from Echinops giganteus. Nat. Prod. Res. 30, 2529–2537. doi: 10.1080/14786419.2015.1120724
Sandur, S. K., Ichikawa, H., Sethi, G., Ahn, K. S., and Aggarwal, B. B. (2006). Plumbagin (5-hydroxy-2-methyl-1,4-naphthoquinone) suppresses NF-kappaB activation and NF-kappaB-regulated gene products through modulation of p65 and IkappaBalpha kinase activation, leading to potentiation of apoptosis induced by cytokine and chemotherapeutic agents. J. Biol. Chem. 281, 17023–17033. doi: 10.1074/jbc.M601595200
Santos, P. A., Avanço, G. B., Nerilo, S. B., Marcelino, R. I., Janeiro, V., Valadares, M. C., et al. (2016). Assessment of cytotoxic activity of rosemary (Rosmarinus officinalis L.), turmeric (Curcuma longa L.), and ginger (Zingiber officinale R.) essential oils in cervical cancer cells (HeLa). ScientificWorldJournal 2016:9273078. doi: 10.1155/2016/9273078
Shaghayegh, G., Alabsi, A. M., Ali-Saeed, R., Ali, A. M., Vincent-Chong, V. K., and Zain, R. B. (2016). Cell cycle arrest and mechanism of apoptosis induction in H400 oral cancer cells in response to damnacanthal and nordamnacanthal isolated from Morinda citrifolia. Cytotechnology 68, 1999–2013. doi: 10.1007/s10616-016-0014-y
Shigemori, H., Kagata, T., Ishiyama, H., Morah, F., Ohsaki, A., and Kobayashi, J. (2003). Naucleamides, A-E, new monoterpene indole alkaloids from Nauclea latifolia. Chem. Pharm. Bull. 51, 58–61. doi: 10.1248/cpb.51.58
Siegel, R., Ma, J., Zou, Z., and Jemal, A. (2014). Cancer statistics, 2014. Cancer J. Clin. 64, 9–29. doi: 10.3322/caac.21208
Silva, J. R., Campos, A. C., Ferreira, L. M., Aranha Junior, A. A., Thiede, A., Zago Filho, L. A., et al. (2006). Extract of Passiflora edulis in the healing process of gastric sutures in rats: a morphological and tensiometric study. Acta. Cir. Bras. 21(Suppl. 2), 52–60. doi: 10.1590/S0102-86502006000800009
Singh, A., and Settleman, J. (2010). EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 29, 4741–4751. doi: 10.1038/onc.2010.215
Solowey, E., Lichtenstein, M., Sallon, S., Paavilainen, H., Solowey, E., and Lorberboum-Galski, H. (2014). Evaluating medicinal plants for anticancer activity. ScientificW. J. 2014:721402. doi: 10.1155/2014/721402
Sowemimo, A., Venables, L., Odedeji, M., Koekemoer, T., Van De Venter, M., and Hongbing, L. (2015). Antiproliferative mechanism of the methanolic extract of Enterolobium cyclocarpum (Jacq.) Griseb. (Fabaceae). J. Ethnopharmacol. 159, 257–261. doi: 10.1016/j.jep.2014.11.023
Sridhar, A., Saremy, S., and Bhattacharjee, B. (2014). Elucidation of molecular targets of bioactive principles of black cumin relevant to its anti-tumour functionality - An in silico target fishing approach. Bioinformation 10, 684–688. doi: 10.6026/97320630010684
Srinivas, P., Gopinath, G., Banerji, A., Dinakar, A., and Srinivas, G. (2004). Plumbagin induces reactive oxygen species, which mediate apoptosis in human cervical cancer cells. Mol. Carcinog. 40, 201–211. doi: 10.1002/mc.20031
Suffness, M., and Pezzuto, J. (eds.). (1990). Assays Related to Cancer Drug Discovery. London: Academic Press.
Sun, J., and McKallip, R. J. (2011). Plumbagin treatment leads to apoptosis in human K562 leukemia cells through increased ROS and elevated TRAIL receptor expression. Leuk. Res. 35, 1402–1408. doi: 10.1016/j.leukres.2011.06.018
Surget, S., Khoury, M. P., and Bourdon, J. C. (2013). Uncovering the role of p53 splice variants in human malignancy: a clinical perspective. Onco. Targets Ther. 7, 57–68. doi: 10.2147/OTT.S53876
Swamy, S. M., and Huat, B. T. (2003). Intracellular glutathione depletion and reactive oxygen species generation are important in alpha-hederin-induced apoptosis of P388 cells. Mol. Cell. Biochem. 245, 127–239. doi: 10.1023/A:1022807207948
Szliszka, E., Czuba, Z. P., Sedek, L., Paradysz, A., and Krol, W. (2011). Enhanced TRAIL-mediated apoptosis in prostate cancer cells by the bioactive compounds neobavaisoflavone and psoralidin isolated from Psoralea corylifolia. Pharmacol. Rep. 63, 139–148. doi: 10.1016/S1734-1140(11)70408-X
Tabopda, T. K., Ngoupayo, J., Awoussong, P. K., Mitaine-Offer, A. C., Ali, M. S., Ngadjui, B. T., et al. (2008). Triprenylated flavonoids from Dorstenia psilurus and their alpha-glucosidase inhibition properties. J. Nat. Prod. 71, 2068–2072. doi: 10.1021/np800509u
Tamokou, J. D. D., Simo Mpetga, D. J., Keilah Lunga, P., Tene, M., Tane, P., and Roger Kuiate, J. (2012). Antioxidant and antimicrobial activities of ethyl acetate extract, fractions and compounds from stem bark of Albizia adianthifolia (Mimosoideae). BMC Complement. Altern. Med. 12:99. doi: 10.1186/1472-6882-12-99
Tane, P., Tatsimo, S., Ayimele, G., and Connolly, J. (2005). “Bioactive metabolites from Aframomum species,” in 11th NAPRECA Symposium Book of Proceedings (Antananarivo).
Tatsadjieu, L., Essia Ngang, J., Ngassoum, M., and Etoa, F. (2003). Antibacterial and antifungal activity of Xylopia aethiopica, Monodora myristica, Zanthoxylum xanthoxyloides and Zanthoxylum leprieurii from Cameroon. Fitoterapia 74, 469–472. doi: 10.1016/S0367-326X(03)00067-4
Tene, M., Tane, P., Sondengam, B. L., and Connolly, J. D. (2004). Lignans from the roots of Echinops giganteus. Phytochemistry 65, 2101–2105. doi: 10.1016/j.phytochem.2004.05.014
Thomas, J., Bahuchets, S., Epelboin, A., and Furniss, S. (eds.). (2003). Encyclopédie des Pygmées Aka:Techniques, Langage et Société des Chasseurs-Cueilleurs de la Forêt Centrafricaine(Sud-Centrafrique et Nord-Congo). Paris: Peeters, 1981–2010.
Tian, Q., Zhang, J., Chan, E., Duan, W., and Zhou, S. (2005). Multidrug resistance proteins (MRPs) and implication in drug development. Drug Dev. Res. 64, 1–18. doi: 10.1002/ddr.10427
Torres, M. P., Rachagani, S., Purohit, V., Pandey, P., Joshi, S., Moore, E. D., et al. (2012). Graviola: a novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 323, 29–40. doi: 10.1016/j.canlet.2012.03.031
Totta, P., Acconcia, F., Leone, S., Cardillo, I., and Marino, M. (2004). Mechanisms of naringenin-induced apoptotic cascade in cancer cells: involvement of estrogen receptor alpha and beta signalling. IUBMB Life 56, 491–499. doi: 10.1080/15216540400010792
Tshibangu, J. N., Chifundera, K., Kaminsky, R., Wright, A. D., and Konig, G. M. (2002). Screening of African medicinal plants for antimicrobial and enzyme inhibitory activity. J. Ethnopharmacol. 80, 25–35. doi: 10.1016/S0378-8741(01)00409-3
Van Wyk, B., and Gericke, N. (2000). Peoples Plants: A Guide to Useful Plants in Southern Africa. Pretoria: Brizza Publications.
Voukeng, I. K., Nganou, B. K., Sandjo, L. P., Celik, I., Beng, V. P., Tane, P., et al. (2017). Antibacterial activities of the methanol extract, fractions and compounds from Elaeophorbia drupifera (Thonn.) Stapf. (Euphorbiaceae). BMC Complement. Altern. Med. 17:28. doi: 10.1186/s12906-016-1509-y
Wabo, H. K., Kouam, S. F., Krohn, K., Hussain, H., Tala, M. F., Tane, P., et al. (2007). Prenylated anthraquinones and other constituents from the seeds of Vismia laurentii. Chem. Pharm. Bull (Tokyo) 55, 1640–1642. doi: 10.1248/cpb.55.1640
Wang, T., Jiang, Y., Chu, L., Wu, T., and You, J. (2017). Alpinumisoflavone suppresses tumour growth and metastasis of clear-cell renal cell carcinoma. Am. J. Cancer Res. 7, 999–1015.
Watjen, W., Kulawik, A., Suckow-Schnitker, A. K., Chovolou, Y., Rohrig, R., Ruhl, S., et al. (2007). Pterocarpans phaseollin and neorautenol isolated from Erythrina addisoniae induce apoptotic cell death accompanied by inhibition of ERK phosphorylation. Toxicology 242, 71–79. doi: 10.1016/j.tox.2007.09.010
Watt, J., and Breyer-Brandwyk, M. (1962). The Medicinal and Poisonous Plants of Southern and Easthern Africa. London: Livingstone.
Wen, S., Zhu, D., and Huang, P. (2013). Targeting cancer cell mitochondria as a therapeutic approach. Future Med. Chem. 5, 53–67. doi: 10.4155/fmc.12.190
Woguem, V., Maggi, F., Fogang, H. P., Tapondjoua, L. A., Womeni, H. M., Luana, Q., et al. (2013). Antioxidant, antiproliferative and antimicrobial activities of the volatile oil from the wild pepper Piper capense used in Cameroon as a culinary spice. Nat. Prod. Commun. 8, 1791–1796.
Xiaoli, L., Naili, W., Sau, W. M., Chen, A. S., and Xinsheng, Y. (2006). Four new isoflavonoids from the stem bark of Erythrina variegata. Chem. Pharm. Bull. (Tokyo) 54, 570–573. doi: 10.1248/cpb.54.570
Xu, K. H., and Lu, D. P. (2010). Plumbagin induces ROS-mediated apoptosis in human promyelocytic leukemia cells in vivo. Leuk. Res. 34, 658–665. doi: 10.1016/j.leukres.2009.08.017
Yang, H., Figueroa, M., To, S., Baggett, S., Jiang, B., Basile, M. J., et al. (2010). Benzophenones and biflavonoids from Garcinia livingstonei fruits. J. Agric. Food Chem. 58, 4749–4755. doi: 10.1021/jf9046094
Yang, Y., Xu, W., Peng, K., and Sun, X. (2014). Amentoflavone induces apoptosis in SW480 human colorectal cancer cells via regulating beta-catenin and caspase-3 expressions. Nan Fang Yi Ke Da Xue Xue Bao 34, 1035–1038.
Yapi, T. A., Boti, J. B., Félix, T. Z., Ahibo, A. C., Tomi, F., and Bighelli, A. (2012). Pachypodanthium Staudtii Engl & Diels from Côte d'Ivoire: composition of leaf, stem bark and roots oils. Eur. J. Sci. Res. 69, 137–142.
Zeches, M., Richard, B., Gueye-M'bahia, L., Lemen-Olivier, L., and Delaude, C. (1985). Constituants des écorces de racine de Nauclea pobeguinii. J. Nat. Prod. 48, 42–46.
Zhang, H., Berezov, A., Wang, Q., Zhang, G., Drebin, J., Murali, R., et al. (2007). ErbB receptors: from oncogenes to targeted cancer therapies. J. Clin. Invest. 117, 2051–2058. doi: 10.1172/JCI32278
Zorofchian Moghadamtousi, S., Rouhollahi, E., Karimian, H., Fadaeinasab, M., Firoozinia, M., Ameen Abdulla, M., et al. (2015). The chemopotential effect of Annona muricata leaves against azoxymethane-induced colonic aberrant crypt foci in rats and the apoptotic effect of acetogenin Annomuricin E in HT-29 cells: a bioassay-guided approach. PLoS ONE 10:e0122288. doi: 10.1371/journal.pone.0122288
Keywords: Africa, cancer, plants, phytochemicals, molecular targets, resistance
Citation: Mbaveng AT, Kuete V and Efferth T (2017) Potential of Central, Eastern and Western Africa Medicinal Plants for Cancer Therapy: Spotlight on Resistant Cells and Molecular Targets. Front. Pharmacol. 8:343. doi: 10.3389/fphar.2017.00343
Received: 26 March 2017; Accepted: 19 May 2017;
Published: 02 June 2017.
Edited by:
Lyndy Joy McGaw, University of Pretoria, South AfricaReviewed by:
Leonidah Kerubo Omosa, University of Nairobi, KenyaLiselotte Krenn, University of Vienna, Austria
Copyright © 2017 Mbaveng, Kuete and Efferth. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Victor Kuete, a3VldGV2aWN0b3JAeWFob28uZnI=
Thomas Efferth, ZWZmZXJ0aEB1bmktbWFpbnouZGU=
 Armelle T. Mbaveng1,2
Armelle T. Mbaveng1,2 Victor Kuete
Victor Kuete