- Department of Pharmacy, Sichuan Academy of Medical Sciences & Sichuan Provincial People's Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, China
Objective: Telavancin is approved to treat complicated skin and skin structure infections, hospital-acquired, and ventilator-associated bacterial pneumonia caused by Staphylococcus aureus. A previous meta-analysis of randomized controlled trials suggested that it might be an alternative to vancomycin in cases of difficult-to-treat meticillin-resistant S. aureus infections. We did a meta-analysis including one new trial to access the efficacy and safety of telavancin.
Methods: We searched PubMed, Cochrane Central Register of Controlled Trials, EMBASE and ClinicalTrials.gov up to December 30, 2015 to identify randomized controlled trials that assessed the clinical efficacy, eradication efficiency, adverse events and laboratory abnormalities of telavancin vs. other antibiotic agents for bacterial infection. Meta-analysis was performed using Review Manager 5.3.0.
Results: Five studies (3790 participants) were included in the meta-analysis. There was no significant difference in treatment success with telavancin than with control antibiotic agents. The pooled pathogen eradication for the telavancin group was numerically higher than that for the control groups, but there was no significant difference. While all-cause mortalities and serious adverse events were comparable between telavancin and control antibiotic agents, adverse event-related withdrawals (OR 1.47, 95% CI 1.13–1.91) were higher in telavancin group. The total number adverse events were more in the telavancin group than in the control groups, especially in the digestive system (OR 1.57, 95% CI 1.37–1.79), nervous system (OR 2.14, 95% CI 1.86–2.47) and urogenital system (OR 2.54, 95% CI 1.99–3.25). Serum creatinine increase (OR 2.25, 95% Cl 1.78–2.85) and hypokalemia (OR 1.74, 95% CI 1.19–2.53) occurred more frequently in telavancin group compared to control groups.
Conclusion: Telavancin may be as effective as but no better than the comparison therapy for S. aureus infection. However, because of the high risk of adverse event-related withdrawals and potential nephrotoxicity, prudence with the clinical use of telavancin in infections is required.
Introduction
Staphylococcus aureus is one of the most common and virulent clinically encountered Gram-positive bacteria (Spink and Ferris, 1945). This pathogen causes serious invasive infections, such as community acquired and nosocomial pneumonia, endocarditis, soft tissue infections, and bacteremia (Drew, 2007). In 2006, results of the Surveillance Network USA showed that nearly 60% of hospital-derived S. aureus isolates were meticillin-resistant S. aureus (Styers et al., 2006). Staphylococcus aureus has become a major cause of hospital-acquired pneumonia (HAP) with meticillin-resistant S. aureus (MRSA) as the predominant pathogen. Currently, glycopeptide antibiotics such as vancomycin and teicoplanin are the gold standard for the treatment of serious infections caused by Gram-positive bacteria, especially MRSA. The emergence and prevalence of multidrug-resistant Gram-positive pathogens underscores the urgent need for development of new antimicrobials.
Telavancin is a novel lipoglycopeptide antibiotic derived from vancomycin. Telavancin exhibited concentration-dependent bactericidal effects by at least two mechanisms. It not only inhibited late-stage peptidoglycan biosynthesis in a substrate-dependent fashion, but also perturbed bacterial cell membrane potential and permeability (Higgins et al., 2005). It is intended for use to combat infections caused by S. aureus and other Gram-positive bacteria, including methicillin resistant and vancomycin-intermediate strains of S. aureus (MRSA and VISA, respectively). In the US, telavancin was approved for complicated skin and skin structure infections (cSSSI) in September 2009 and for hospital-acquired and ventilator-associated bacterial pneumonia caused by S. aureus in June 2013. In Europe, telavancin had been approved as second-line treatment for hospital-acquired pneumonia, including ventilator-associated pneumonia (VAP), known or suspected to be caused by MRSA when other alternatives are not suitable (Rubinstein et al., 2011a).
So far, several studies have suggested that telavancin had comparable efficacy and higher MRSA eradication rate comparing with other antibiotics for the treatment of gram-positive bacteria (Stryjewski et al., 2005, 2006, 2008, 2014; Rubinstein et al., 2011b). However, Theravance Inc., a pharmaceutical company producing telavancin (brand name as VIBATIV), was one of the affiliations of all aforementioned studies, thus creating a potential factor of biase. Some retrospective reviews and traditional reviews also studied the efficacy and safety of telavancin. However, without quality evaluation of including articles and statistical calculation of the data, the results were often influenced by subjective factors and biases (Dunbar et al., 2008; Chang et al., 2010; Hooper and Smith, 2012; Rubinstein et al., 2014; Nnedu and Pankey, 2015). Konstantinos A. Polyzos and colleagues did a meta-analysis to synthetically assess the efficacy and safety of telavancin, but it was limited to six published randomized controlled trials up to March 2012 (Polyzos et al., 2012). In addition, they only focused on the eradication of MRSA for cSSSI, but did not report the eradication of total S. aureus and methicillin-susceptible S. aureus (MSSA).
Therefore, we aimed to update Konstantinos A. Polyzos and colleagues' meta-analysis involving efficacy and safety of telavancin comparing with other antibiotics, and to give a clear insight of its clinical efficacy, adverse events and laboratory abnormalities by synthesis of the results of existing trials.
Materials and Methods
This meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist.
Data Sources
A computerized search in PubMed, Cochrane Central Register of Controlled Trials and EMBASE up to December 30, 2015 was conducted independently by two individuals (Zhu and Zhong) with the search terms “telavancin” or “TD-6424.” We also did a search in ClinicalTrials.gov up to December 30, 2015 to screen completed trials about telavancin but with no published results. Furthermore, the references of retrieved literatures were manually screened for more eligible studies.
Selection of Studies
Studies that met the following criteria were considered as eligible for this meta-analysis (1) they were randomized controlled trials (RCTs); (2) studies compared the outcomes of telavancin and other antibiotic agents in infection treatment; (3) studies assessed the clinical efficacy, efficiency for eradication of pathogens, adverse events and laboratory abnormalities of both therapeutic regimens. Two independent reviewers searched the databases and screened all retrieved articles according to the inclusion criteria. Studies were excluded if they were animal studies, retrospective studies, post-hoc analyses, bactericidal activity studies, pharmacokinetic, or pharmacodynamic studies.
Data Extraction
Two of our authors (Zhang and He) independently extracted data for each eligible study. In cases of discrepancy, a third author (Chuan) was consulted. The following information was recorded for the included trials: study title, name of first author, year of publication, study design, type of infection, drug regimens, treatment duration, time to test of cure (TOC), number of patients, clinical and microbiological outcomes, and data on safety.
The Intention-to-Treat (ITT) population included all randomized patients in the group to which they were randomly assigned, regardless of the treatment they actually received, and regardless of subsequent withdrawl from treatment or deviation from the protocol (Fisher et al., 1989). The modified Intention-to-Treat (mITT) population referred to all patients that received at least one dose of study drug. The clinically evaluable (CE) population was patients in the mITT population who complied with all exclusion and inclusion criteria and had a clinical response of either cure or failure as assessed at the TOC visit. The microbiologically evaluable (ME) population consisted of CE patients who had a gram-positive pathogen recovered from baseline respiratory specimens or blood cultures (Stryjewski et al., 2006).
Assessment of Risk of Bias
The quality of included studies was evaluated according to The Cochrane Collaboration's tool for assessing risk of bias: random sequence generation; allocation concealment; blinding of participants, personnel and outcome assessment; incomplete outcome data or selective reporting; and other sources of bias (Higgins and Green, 2011). One point was awarded for each criterion, with a maximum score of 5. Studies scored 3 or more were considered as high quality studies, whereas studies scored 2 or fewer points were classified into low-quality studies (Tasina et al., 2011).
Outcomes
The primary outcome examined in the meta-analysis was treatment success in the mITT, CE, and ME populations, which was defined as resolution of clinically significant signs and symptoms associated with cSSSI, HAP or other infection diseases, or was defined as improvement to the extent that the infectious process had been controlled and no further antimicrobial therapy was necessary. Secondary outcomes included the pathogen eradication, adverse effects (AEs) and laboratory abnormalities. Eradication of pathogens was based on ME populations. Adverse effects and laboratory abnormalities were assessed in mITT populations.
Statistical Analysis
The statistical analyses were carried out in Review Manager (version 5.3.0) (Cochrane Collaboration, Oxford, United Kingdom). Random effects model (REM) was chosen since the included studies involving different infections, different control regimens, different sample size, which introduced obvious heterogeneity across the trials. Odds ratio (OR), with 95% confidence interval (CI), was used for all primary and secondary outcomes by Mantel-Haenszel method. The publication bias was not assessed due to the small number of the included studies. Sensitivity analysis was performed to investigate the influence on the overall results by omitting a single trial at a time.
Results
Selected Studies and Their Characteristics
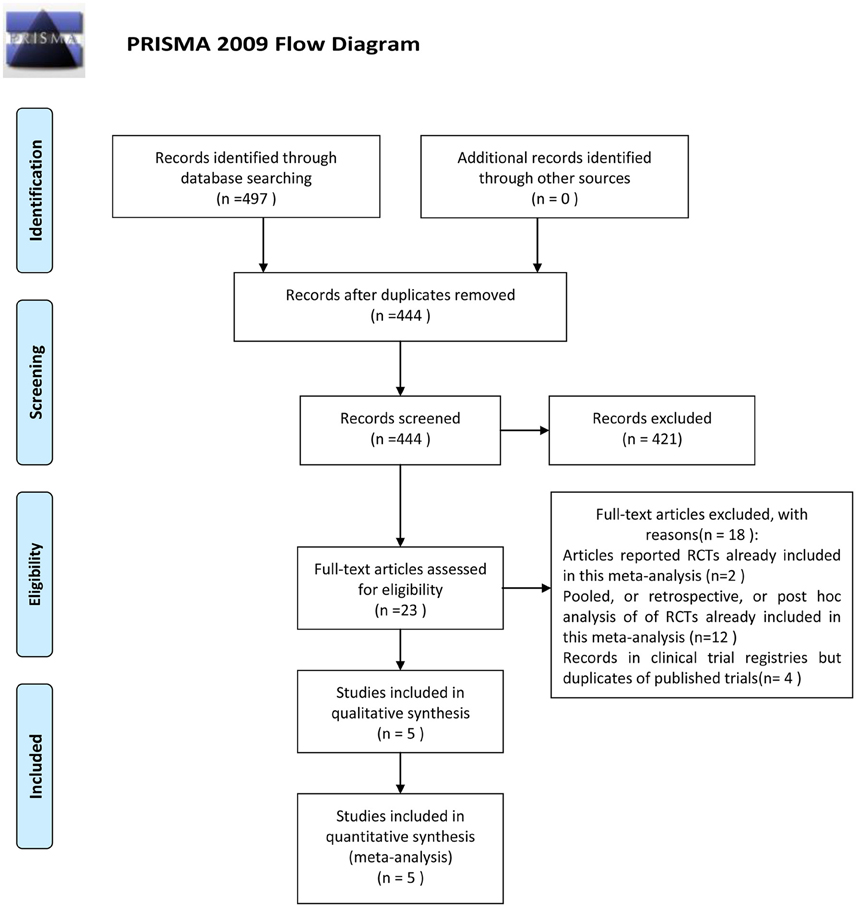
The outcome of the search was shown in Figure 1. Four hundred and ninety seven papers were identified after initial searching. Fifty three articles were excluded because of repeated reports. Among the remaining studies, 421 studies were excluded after reading titles, abstracts or texts. Only 23 were retrieved full text for eligibility, of which 18 studies were excluded (Corey et al., 2007b,a, 2014; Stryjewski et al., 2008, 2012, 2013; Wilson et al., 2009; Barriere, 2010, 2014; Rubinstein et al., 2011a, 2014; Hooper and Smith, 2012; Nannini et al., 2012; Barriere et al., 2014a,b; Torres et al., 2014; Lacy et al., 2015). Thus, five studies comparing telavancin with control regimens were included in the meta-analysis (Stryjewski et al., 2005, 2006, 2008, 2014; Rubinstein et al., 2011b; Figure 1).
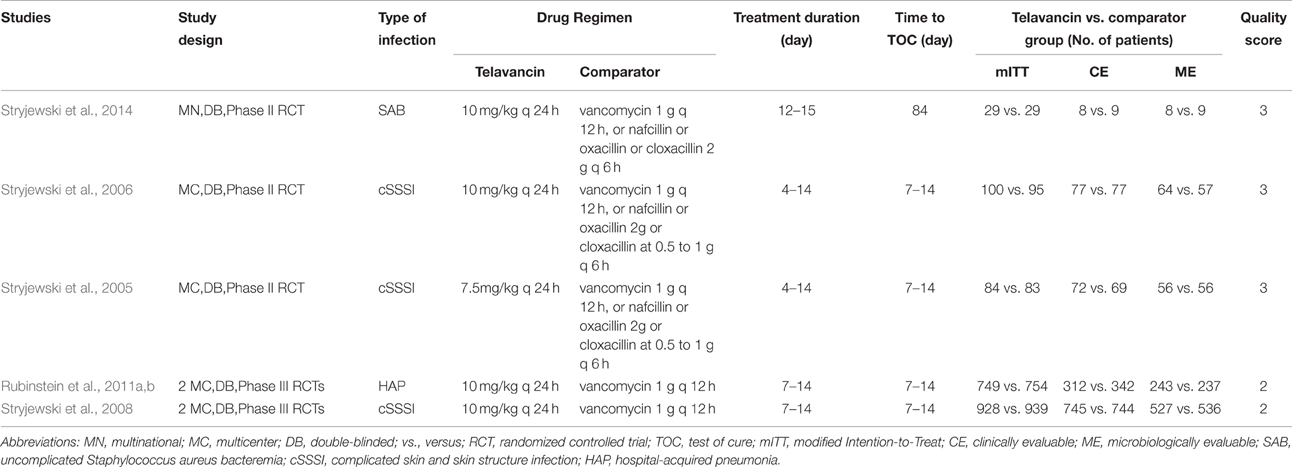
Baseline characteristics of the studies included in this analysis were presented in Table 1. All 5 studies were multicentre double-blind trials and privately funded by the pharmaceutical industry. The average age of participants was 42.3–60 years. Three studies received quality scores of 3 and the other two received 2 (Table 1). Three studies involved patients with complicated skin and skin structure infections (cSSSI) and compared telavancin intravenous (IV) at 10 mg/kg/24 h or 7.5 mg/kg/24 h with standard therapy (1 g of vancomycin every 12 h, 2 g of nafcillin or oxacillin every 6 h, or 0.5–1 g of cloxacillin every 6 h; Stryjewski et al., 2005, 2006, 2008). One study involved patients with hospital-acquired pneumonia and compared telavancin at 10 mg/kg/24 h with vancomycin at a dosage of 1 g IV every 12 h (Rubinstein et al., 2011b). One study involved patients with uncomplicated S. aureus bacteremia and compared telavancin IV at 10 mg/kg/24 h with standard therapy (vancomycin 1 g IV q 12 h, or nafcillin 2 g IV q 6 h, oxacillin 2 g IV q 6 h, or cloxacillin 2 g IV q 6 h; Stryjewski et al., 2014).
Treatment Success in mITT, CE, and ME Populations
Assessment of treatment success was based on mITT, CE, and ME population. There was no significant difference in treatment success in the mITT population between patients treated with telavancin and those treated with comparators (5 studies, 3790 participants, OR = 1.02, 95% CI = 0.89–1.18, P = 0.74; Figure 2). The same was true for ME population (5 studies, 2650 participants, OR = 1.46, 95% CI = 0.64–3.34, P = 0.37, Figure 2) and CE patients (5 studies, 1832 participants, OR = 1.23, 95% CI = 0.94–1.62, P = 0.36; Figure 2). The results of two largest study groups (Stryjewski et al., 2008; Rubinstein et al., 2011b) had no effect on the overall results when they were removed one by one or both.
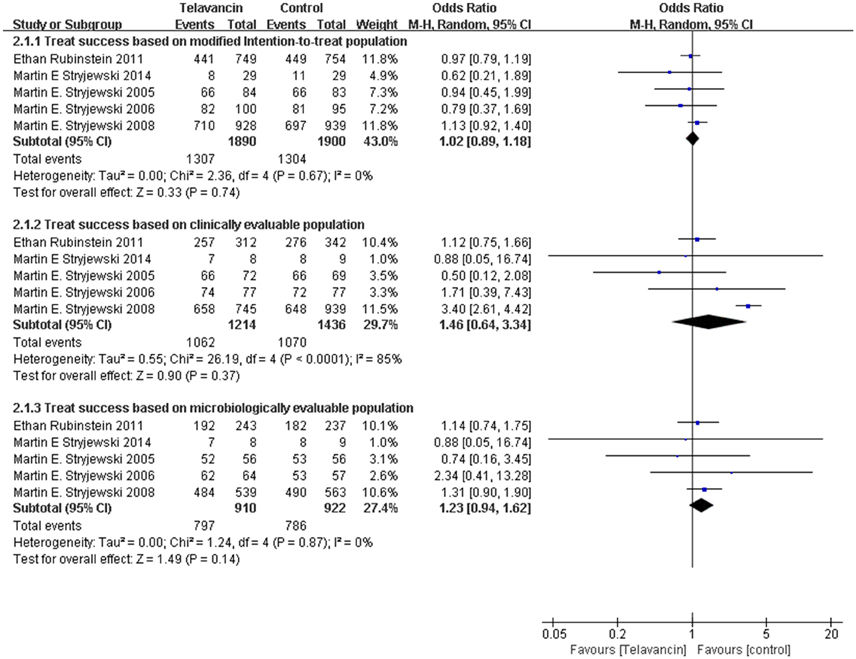
Figure 2. Treatment success based on mITT, CE, and ME populations. Df, degrees of freedom; M-H, Mantel-Haenszel method.
Pathogen Eradication in ME Population
The total pathogen eradication for the telavancin group was numerically higher than that for the comparator group in the ME population at the TOC visit, but there was no significant difference (4 studies, 1313 participants, OR = 1.30, 95% CI = 0.88–1.94, P = 0.19, Figure 3). More specifically, treatment with telavancin was associated with numerically higher eradication rate for total S. aureus, MSSA (for S. aureus, 1477 strains, OR = 1.31, 95% CI = 0.99–1.75, P = 0.06; for MSSA, 459 strains, OR = 1.25, 95% CI = 0.66–2.38, P = 0.50, Figure 3). Treatment with telavancin was associated with almost the same eradication rate for MRSA and Streptococcus pneumoniae (for MRSA, 964 strains, OR = 1.42, 95% CI = 0.94–2.14, P = 0.10; for Streptococcus pneumoniae, 146 strains, OR = 0.99, 95% CI = 0.30–3.29, P = 0.98, Figure 3). However, there were no significant differences in eradication for all these species.
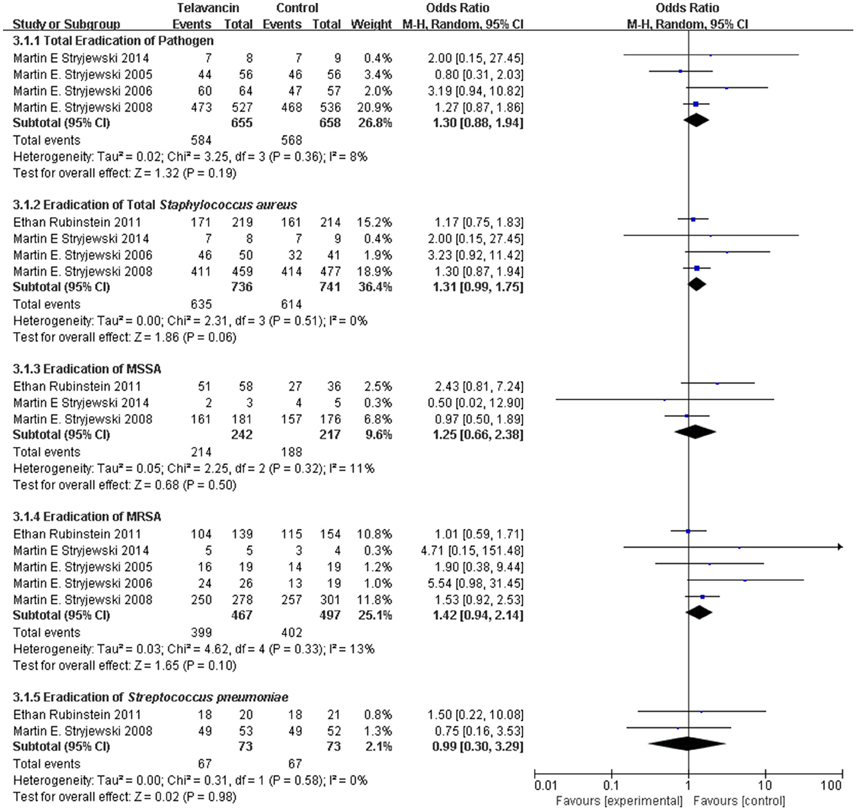
Figure 3. Pathogen eradication in total and for total Staphylococcus aureus, MSSA, MRSA, and Streptococcus pneumonia. Df, degrees of freedom; M-H, Mantel-Haenszel method.
Adverse Effects
The total number of adverse events in the telavancin groups was higher than the number in the comparators in the mITT population, but there was no significant difference (5 studies, 3790 participants, OR = 1.17, 95% CI = 0.89–1.54, P = 0.26; Figure 4). Overall, mortality rate (3 studies; 3428 participants, OR = 1.10, 95% CI = 0.86–1.41, P = 0.45; Figure 4) and serious adverse events (5 studies, 3790 participants, OR = 1.41, 95% CI = 0.99–1.99, P = 0.06; Figure 4) were comparable between telavancin and comparators. Discontinuance due to adverse events (5 studies, 3790 participants, OR = 1.47, 95% CI = 1.13–1.91, P = 0.004; Figure 4) was more common in the telavancin group based on the mITT population. Assessment of detailed adverse events showed a higher incidence in digestive system, nervous system and urogenital system in participants receiving telavancin than in control groups (Figure 5). There was no significant difference in the proportions of patients who developed adverse events in the metabolic and nutritional system, hemic and lymphatic system, cardiovascular system, respiratory system and body as a whole between the compared regimens. Significantly fewer episodes of adverse events in the skin/appendages were reported in the telavancin groups (Figure 5).
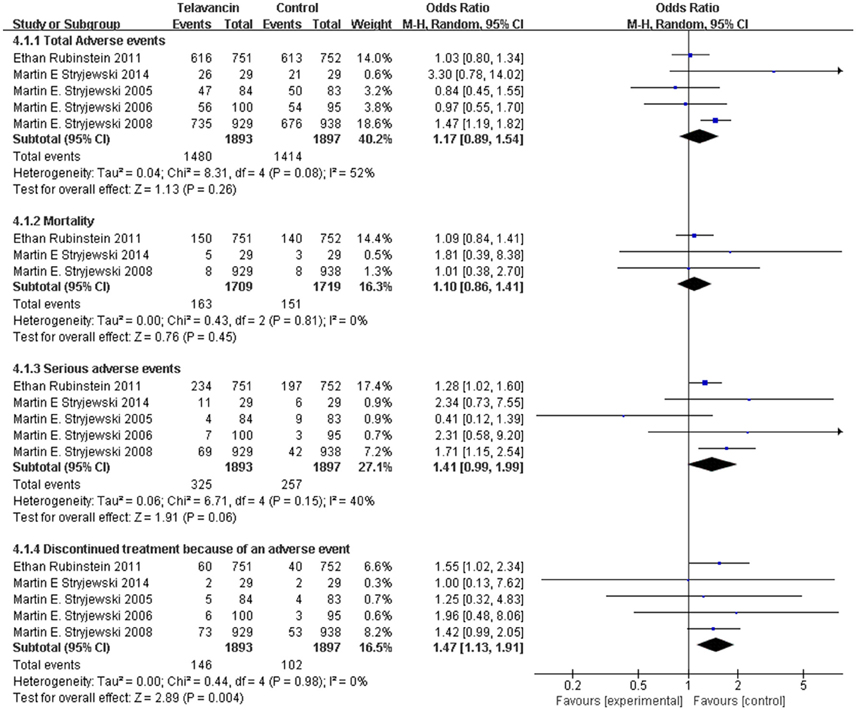
Figure 4. Total adverse events, mortality, serious adverse events and withdrawal related to studied medications. Df, degrees of freedom; M-H, Mantel-Haenszel method.
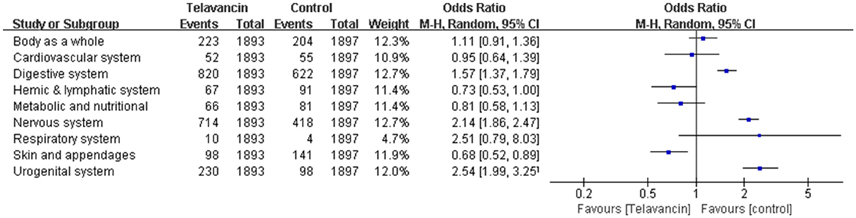
Figure 5. Detailed adverse events of telavancin vs. comparator antibiotics. Pooled odds ratios were calculated from random-effects models with the Mantel-Haenszel method.
Laboratory Abnormalities
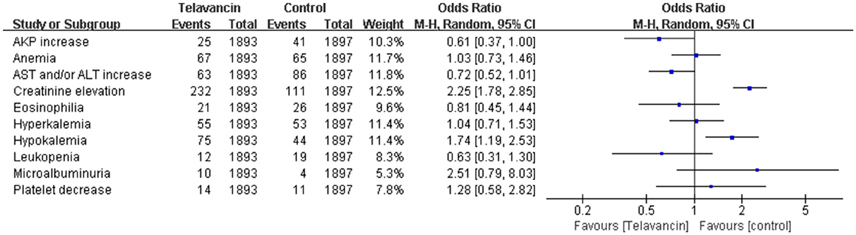
Significant increase in serum creatinine was more frequently observed in telavancin group compared to control group (OR = 2.25, 95% CI = 1.78–2.85; Figure 6). Moreover, hypokalemia also occurred more frequently in telavancin group than in control group (OR = 1.74, 95% CI = 1.19–2.53; Figure 6). There was no difference in alkaline phosphatase (AKP) increase, aspartate transaminase (AST) and/or alanine transaminase (ALT) increase, anemia, leukopenia, platelet decrease, eosinophilia, hyperkalemia and microalbuminuria between telavancin group and control group (Figure 6).
Pooled odds ratios were calculated from random-effects models with the Mantel-Haenszel method.
Discussion
The present meta-analysis demonstrated the noninferiority of telavancin comparing with comparator antibiotics for cSSSI, HAP and uncomplicated S. aureus bacteremia. Telavancin exhibited no significant difference in eradication rate for total S. aureus and MRSA comparing with control group. One issue that need to be addressed was that the American Thoracic Society and Infectious Diseases Society of America (IDSA) guidelines recommended 15–20 μg/mL of vancomycin for serious infections, such as pneumonia and severe skin and soft tissue infections (Liu et al., 2011). In fact, 34% participants had a trough vancomycin level ≤ 10 μg/mL in the trials for HAP and 14% participants had a trough vancomycin level ≤ 5 μg/mL in the trials for cSSSI. The number of patients with a level ≤ 15 μg/mL was conceivably much higher. A considerable proportion of participants from vancomycin group did not receive a reasonable trough level according to the IDSA guidelines. Thus, one can only state that telavancin was not inferior to underdosed vancomycin. But it was not sufficient to claim that telavancin and vancomycin have comparable efficacy since vancomycin was underdosed (Tarchini, 2011). Therefore, telavancin is no better than standard antimicrobial regimens, for the treatment of cSSSI, HAP and uncomplicated S. aureus bacteremia, since it is associated with a higher frequency of withdrawl due to adverse effects, especially in the urogenital system. Among HAP patients with kidney dysfunction and pre-existing moderate-to-severe renal impairment, 28-day survival rates for telavancin were lower than vancomycin (Corey et al., 2014; Nnedu and Pankey, 2015).
In Polyzos and colleagues' meta-analysis of telavancin (Polyzos et al., 2012), results were similar to those results in our analysis for all outcomes except for eradication of MRSA. Of all 5 studies including in our mete- analysis, three studies involved patients with cSSSI, one study involving patients with HAP and one study involving patients with uncomplicated S. aureus bacteremia. We analyzed the synthesis eradication of MRSA for all the trials while Polyzos and colleagues only focused on that for cSSSI. In Polyzos and colleagues' meta-analysis, eradication rate of MRSA was significantly higher with telavancin than that with comparator regimens (OR = 1.71, 95% CI 1.08–2.70), whereas the difference was not significant in our study (OR = 1.42, 95% CI 0.94–2.14). The results were the same between Polyzos's meta-analysis and our study when the data from trials involving HAP and uncomplicated S. aureus bacteremia were removed. Thus, these results suggested that telavancin showed higher eradication rate of MRSA for cSSSI but not for HAP and uncomplicated S. aureus bacteremia.
There were several limitations in our study. Only one new study assessing the treatment of telavancin for uncomplicated S. aureus bacteremia was added in this meta-analysis. It was a multinational, double-blind phase II randomized clinical trial with 58 participants. Its sample size was too small to affect the overall synthetic outcomes. We searched the ClinicalTrials.gov for completed trials with no published data. There was only one open-label, non-randomized phase I tiral and we failed to obtain the unpublished data. Finally, all of the five included studies (7 trials) were sponsored by Theravance Inc., a pharmaceutical company producing telavancin (brand name as VIBATIV). This could be considered a factor that might introduce bias in the assessment of outcomes.
Conclusions
In conclusion, telavancin has clinical efficacy and microbiological eradication rate similar to control antimicrobial regimens in S. aureus infection, including MRSA infection. However, telavancin is associated with a higher frequency of adverse events than the comparators, especially in the digestive system, nervous system and urogenital system. Because of significant serum creatinine increase and consequent potential nephrotoxicity, prudence with the clinical use of telavancin in infections is required.
Author Contributions
Conceived and designed the experiments: JC, YuaZ, and HX. Performed the experiments: JC, YuaZ, and XH. Searched the databases: YuxZ, LZ. Study inclusion: YuxZ, LZ. Data collection: JC, YuaZ, and XH. Analyzed the data: JC, YuaZ, and XH. Wrote the paper: JC, YuaZ, and HX. Critical revision of the manuscript for important intellectual content: YuaZ, DY.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was funded by the Science & Technology Program of Sichuan Province in (Grant number: 2009SZ0226, 2014FZ0103, 2015JQ0027, 2015ZR0160) and the health department of Sichuan province (Grant number: 100491, 120111) and Chengdu City Science and technology project (Grant number: 11PPYB010SF-289) and Young Scholars fundation of Sichuan Provincial People's Hospital (Grant number: 30305030606, 30305030859).
References
Barriere, S. L. (2010). ATLAS trials: efficacy and safety of telavancin compared with vancomycin for the treatment of skin infections. Future Microbiol. 5, 1765–1773. doi: 10.2217/fmb.10.138
Barriere, S. L. (2014). The ATTAIN trials: efficacy and safety of telavancin compared with vancomycin for the treatment of hospital-acquired and ventilator-associated bacterial pneumonia. Future Microbiol. 9, 281–289. doi: 10.2217/fmb.14.4
Barriere, S. L., Farrell, D. J., Rhomberg, P. R., and Jones, R. N. (2014a). Serum inhibitory and bactericidal activity of telavancin in non-infected subjects with severe renal impairment or end-stage renal disease. Diagn. Microbiol. Infect. Dis. 80, 327–329. doi: 10.1016/j.diagmicrobio.2014.09.002
Barriere, S. L., Stryjewski, M. E., Corey, G. R., Genter, F. C., and Rubinstein, E. (2014b). Effect of vancomycin serum trough levels on outcomes in patients with nosocomial pneumonia due to Staphylococcus aureus: a retrospective, post hoc, subgroup analysis of the Phase 3 ATTAIN studies. BMC Infect Dis. 14:183. doi: 10.1186/1471-2334-14-183
Chang, M. H., Kish, T. D., and Fung, H. B. (2010). Telavancin: a lipoglycopeptide antimicrobial for the treatment of complicated skin and skin structure infections caused by gram-positive bacteria in adults. Clin Ther. 32, 2160–2185. doi: 10.1016/s0149-2918(11)00020-8
Corey, G. R., Kollef, M. H., Shorr, A. F., Rubinstein, E., Stryjewski, M. E., Hopkins, A., et al. (2014). Telavancin for hospital-acquired pneumonia: clinical response and 28-day survival. Antimicrob. Agents Chemother. 58, 2030–2037. doi: 10.1128/aac.02330-13
Corey, G. R., Stryjewski, M. E., Fowler, V. G. Jr., Teglia, O., Hopkins, A., Kitt, M. M., et al (2007b) ATLAS 2: a double-blind, randomised, active controlled, multinational Phase 3 study comparing telavancin with vancomycin for the treatment of patients with complicated skin skin structure infections. Int. J. Antimicrobial. Agents. 29, S217. doi: 10.1016/S0924-8579(07)70687-8
Corey, G. R., Stryjewski, M. E., O'Riordan, W. D., Fowler, V. G. Jr., Ross, D. P., Hopkins, A., et al (2007a) ATLAS 1: the first Phase 3 study evaluating the new lipoglycopeptide, telavancin, for the treatment of patients with complicated skin skin structure infections. In. J. Antimicrobial. Agents. 29, S216–S217. doi: 10.1016/S0924-8579(07)70686-6
Drew, R. H. (2007). Emerging options for treatment of invasive, multidrug-resistant Staphylococcus aureus infections. Pharmacotherapy. 27, 227–249. doi: 10.1592/phco.27.2.227
Dunbar, L. M., Tang, D. M., and Manausa, R. M. (2008). A review of telavancin in the treatment of complicated skin and skin structure infections (cSSSI). Ther. Clin. Risk Manag. 4, 235–244.
Fisher, L. D., Dixon, D. O., Herson, J., Frankowski, R. K., Hearron, M. S., and Peace, K. E. (1989). “Intention to treat in clinical trials,” in Statistical Issues in Pharmaceutical Drug Development, ed K. E. Peace (New York, NY: Marcel Dekker Inc.), 331–350.
Higgins, D. L., Chang, R., Debabov, D. V., Leung, J., Wu, T., Krause, K. M., et al. (2005). Telavancin, a multifunctional lipoglycopeptide, disrupts both cell wall synthesis and cell membrane integrity in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 49, 1127–1134. doi: 10.1128/aac.49.3.1127-1134.2005
Higgins, J. P. T., and Green, S. (eds.). (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. Available online at: http://handbook.cochrane.org
Hooper, C. Y., and Smith, W. J. (2012). Telavancin for the treatment of nosocomial pneumonia caused by methicillin-resistant Staphylococcus aureus (MRSA). Ther. Clin. Risk Manag. 8, 131–137. doi: 10.2147/tcrm.s23247
Lacy, M. K., Stryjewski, M. E., Wang, W., Hardin, T. C., Nogid, B., Luke, D. R., et al. (2015). Telavancin hospital-acquired pneumonia trials: impact of Gram-negative infections and inadequate Gram-negative coverage on clinical efficacy and all-cause mortality. Clin. Infect. Dis. 61, S87–S93. doi: 10.1093/cid/civ536
Liu, C., Bayer, A., Cosgrove, S. E., Daum, R. S., Fridkin, S. K., Gorwitz, R. J., et al. (2011). Clinical practice guidelines by the infectious diseases society of america for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin. Infect. Dis. 52, e18–e55. doi: 10.1093/cid/ciq146
Nannini, E. C., Corey, G. R., and Stryjewski, M. E. (2012). Telavancin for the treatment of hospital-acquired pneumonia: findings from the ATTAIN studies. Expert. Rev. Anti. Infect. Ther. 10, 847–854. doi: 10.1586/eri.12.81
Nnedu, O. N., and Pankey, G. A. (2015). Update on the emerging role of telavancin in hospital-acquired infections. Ther. Clin. Risk Manag. 11, 605–610. doi: 10.2147/tcrm.s57376
Polyzos, K. A., Mavros, M. N., Vardakas, K. Z., Makris, M. C., Rafailidis, P. I., and Falagas, M. E. (2012). Efficacy and safety of telavancin in clinical trials: a systematic review and meta-analysis. PLoS ONE 7:e41870. doi: 10.1371/journal.pone.0041870
Rubinstein, E., Corey, G. R., Stryjewski, M. E., and Kanafani, Z. A. (2011a). Telavancin for the treatment of serious gram-positive infections, including hospital acquired pneumonia. Expert. Opin. Pharmacother. 12, 2737–2750. doi: 10.1517/14656566.2011.633511
Rubinstein, E., Lalani, T., Corey, G. R., Kanafani, Z. A., Nannini, E. C., Rocha, M. G., et al. (2011b). Telavancin versus vancomycin for hospital-acquired pneumonia due to gram-positive pathogens. Clin. Infect. Dis. 52, 31–40. doi: 10.1093/cid/ciq031
Rubinstein, E., Stryjewski, M. E., and Barriere, S. L. (2014). Clinical utility of telavancin for treatment of hospital-acquired pneumonia: focus on non-ventilator-associated pneumonia. Infect. Drug Resist. 7, 129–135. doi: 10.2147/idr.s25930
Spink, W. W., and Ferris, V. (1945). Quantitative action of penicillin inhibitor from penicillin-resistant strains of staphylococci. Science 102, 221–223. doi: 10.1126/science.102.2644.221
Stryjewski, M. E., Barriere, S. L., O'Riordan, W., Dunbar, L. M., Hopkins, A., Genter, F. C., et al. (2012). Efficacy of telavancin in patients with specific types of complicated skin and skin structure infections. J. Antimicrob. Chemother. 67, 1496–1502. doi: 10.1093/jac/dks081
Stryjewski, M. E., Barriere, S. L., Rubinstein, E., Genter, F. C., Lentnek, A. L., Magana-Aquino, M., et al. (2013). Telavancin versus vancomycin for bacteraemic hospital-acquired pneumonia. Int. J. Antimicrob. Agents 42, 367–369. doi: 10.1016/j.ijantimicag.2013.06.003
Stryjewski, M. E., Chu, V. H., O'Riordan, W. D., Warren, B. L., Dunbar, L. M., Young, D. M., et al. (2006). Telavancin versus standard therapy for treatment of complicated skin and skin structure infections caused by gram-positive bacteria: FAST 2 study. Antimicrob. Agents Chemother. 50, 862–867. doi: 10.1128/aac.50.3.862-867.2006
Stryjewski, M. E., Graham, D. R., Wilson, S. E., O'Riordan, W., Young, D., Lentnek, A., et al. (2008). Telavancin versus vancomycin for the treatment of complicated skin and skin-structure infections caused by gram-positive organisms. Clin. Infect. Dis. 46, 1683–1693. doi: 10.1086/587896
Stryjewski, M. E., Lentnek, A., O'Riordan, W., Pullman, J., Tambyah, P. A., Miro, J. M., et al. (2014). A randomized Phase 2 trial of telavancin versus standard therapy in patients with uncomplicated Staphylococcus aureus bacteremia: the ASSURE study. BMC Infect. Dis. 14:289. doi: 10.1186/1471-2334-14-289
Stryjewski, M. E., O'Riordan, W. D., Lau, W. K., Pien, F. D., Dunbar, L. M., Vallee, M., et al. (2005). Telavancin versus standard therapy for treatment of complicated skin and soft-tissue infections due to gram-positive bacteria. Clin. Infect. Dis. 40, 1601–1607. doi: 10.1086/429914
Styers, D., Sheehan, D. J., Hogan, P., and Sahm, D. F. (2006). Laboratory-based surveillance of current antimicrobial resistance patterns and trends among Staphylococcus aureus: status in the United States. Ann. Clin. Microbiol. Antimicrob. 5:2. doi: 10.1186/1476-0711-5-2
Tarchini, G. (2011). On a different level: telavancin versus vancomycin for hospital-acquired pneumonia. Clin. Infect. Dis. 52, 1390. doi: 10.1093/cid/cir189
Tasina, E., Haidich, A. B., Kokkali, S., and Arvanitidou, M. (2011). Efficacy and safety of tigecycline for the treatment of infectious diseases: a meta-analysis. Lancet Infect. Dis. 11, 834–844. doi: 10.1016/s1473-3099(11)70177-3
Torres, A., Rubinstein, E., Corey, G. R., Stryjewski, M. E., and Barriere, S. L. (2014). Analysis of Phase 3 telavancin nosocomial pneumonia data excluding patients with severe renal impairment and acute renal failure. J. Antimicrob. Chemother. 69, 1119–1126. doi: 10.1093/jac/dkt490
Keywords: systematic analysis, efficacy, safety, telavancin, infectious disease
Citation: Chuan J, Zhang Y, He X, Zhu Y, Zhong L, Yu D and Xiao H (2016) Systematic Review and Meta-Analysis of the Efficacy and Safety of Telavancin for Treatment of Infectious Disease: Are We Clearer? Front. Pharmacol. 7:330. doi: 10.3389/fphar.2016.00330
Received: 13 June 2016; Accepted: 07 September 2016;
Published: 23 September 2016.
Edited by:
Annalisa Bruno, University of Chieti-Pescara, ItalyReviewed by:
Catherine M. T. Sherwin, University of Utah, USAXue Liang, University of Pennsylvania, USA
Copyright © 2016 Chuan, Zhang, He, Zhu, Zhong, Yu and Xiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongtao Xiao, eGh0OTI3QDE2My5jb20=
 Junlan Chuan
Junlan Chuan Hongtao Xiao
Hongtao Xiao