- 1Drug Discovery Lab, Division of Life Sciences, Institute of Advanced Study in Science and Technology, Guwahati, India
- 2Department of Pathology, Hayat Hospital, Guwahati, India
- 3Girijananda Chowdhury Institute of Pharmaceutical Science, Guwahati, India
Randia dumetorum Lam. (RD) (Rubiaceae) is traditionally used by some tribes of Assam and Manipur of North East India for the treatment of liver ailments. In this context, to scientifically validate this indigenous traditional knowledge, we have evaluated the antioxidant and hepatoprotective activity of RD leaf and bark. The methanol extracts of RD leaf and bark were evaluated for in vitro antioxidant activity which exhibited good antioxidant activity in terms of reducing power assay, total antioxidant assay and DPPH (1,1-diphenyl-2-picrylhydrazyl) radical scavenging assay. Total phenolic and flavonoid content were found to be 112 ± 3.24 mg and 138 ± 2.46 mg gallic acid equivalents/g extract and 2.6 ± 0.26 mg and 3.34 ± 0.31 mg rutin equivalents/g extract respectively for RD leaf and bark methanol extracts. The in vivo hepato protective activity of the RD leaf and bark extract was evaluated against carbon tetrachloride (CCl4) induced hepatic damage in male wistar rats. CCl4 administration induced hepatic damage in rats resulted in increased levels of aspartate transaminase, alanine transaminase, alkaline phosphatase, lactate dehydrogenase, thiobarbituric acid reacting substances, albumin, bilirubin, TNF-α, IL-1β and decreased levels of total protein and antioxidant enzymes like superoxide dismutase, catalase, and glutathione reductase. RD leaf and bark methanol extracts pre-treatment exhibited protection against CCl4 induced hepatotoxicity by reversing all the abnormal parameters to significant levels. Histopathological results revealed that RD leaf and bark extracts at 400 mg/kg protects the liver from damage induced by CCl4. The results of this study scientifically validate the traditional use of RD leaf and bark for the treatment of liver ailments.
Introduction
Oxidation is the key process for production of energy in an organism to fuel biological processes (Kong et al., 2010). Over production of oxygen free radicals increase the oxidative stress in organisms by causing deleterious effects to cell structures. Many disease conditions like cancer, diabetes, atherosclerosis, hypertension, neurodegenerative diseases, aging, inflammation, and acute and chronic liver diseases precipitate directly or indirectly through reactive oxygen species (ROS) mediated mechanisms (Liang et al., 2011; Sarwar et al., 2015). Antioxidants are the compounds that neutralize the free radicles produced in the body (Fu et al., 2010). Liver is second largest organ in human body which plays a major role in metabolism. Impairment of liver leads to serious health consequences and in the majority of cases it is life threatening. Management of liver diseases is still a challenge to the modern medicine with a few treatment options are available. Oxidative stress on liver affects the antioxidant enzymes like superoxide dismutase (SOD), catalase (CAT), and glutathione reductase (GSH) and increase the lipid peroxidation (LPO) in liver (Banu et al., 2012; Habib et al., 2015). Different poly-herbal formulations are often prescribed by traditional practitioners as hepatoprotective agents. These formulations contain combination of natural antioxidants which act synergistically together to protect the liver cells.
Randia dumetorum Lam. (RD) is a large thorny shrub belongs to the family Rubiaceae. This plant is widely available throughout India and African subcontinent up to 4000 feet altitude. RD was reported to contain mannitol, iridoid-10-methylixoside, coumarin glycosides, triterpenoid glycosides, randianin and saponins named as dumentoronin A, B, C, D, E, F, etc. It also used in ayurvedic medicines for having properties like rasa, guna, virya, vipaka, etc. RD was also reported for its immunomodulatory, analgesic, antiinflammatory, antiallergic, and antibacterial activities (Patel et al., 2011). In North Eastern region of India some tribes of Assam and Manipur uses RD fruit, bark and leaf for treatment of different liver ailments. It is the need of the hour to scientifically validate this traditional knowledge before it gets lost. In this present study we evaluated the antioxidant and hepatoprotective activities of RD leaf and bark methanol extracts on CCl4 induced acute liver damage in rat. Successful validation of the claimed properties of RD can potentially lead to development of therapeutics for liver ailments.
Materials and Methods
Drugs and Chemicals
1,1-Diphenyl-2-picrylhydrazyl was obtained from Sigma (Sigma-Aldrich, USA). CCl4, BHT and Ascorbic acid were obtained from Merck, India. Silymarin was obtained from Micro Labs Limited. Biological kits were obtained from Accurex, India. The entire chemicals used in the experiments were of analytical grade and Millipore water was used throughout the experiments.
Preparation of the Plant Extract
Leaf and bark of RD were collected from Kamrup district of Assam, India in the month of January. Plant was identified by a botanist at North East India Ayurvedic Institute (NEIAI), Guwahati and a voucher Specimen (no IASST/1016) was deposited in IASST, herbarium library. Plant material was dried under shade and after drying leaf and bark grinded into a coarse powder. Plant material (100 g) was soaked in methanol (500 ml) for 4 days and filtered using Whatman filter paper. The methanol used for extraction was completely evaporated by rotary evaporator at 45°C to avoid toxicity. The percentage yield was calculated 10–15% for RD leaf and 5–7% for RD bark.
Determination of Total Phenolic and Flavonoid Content
The total phenolic content of the extracts were determined using the Folin-Ciocalteu assay (Sabir and Rocha, 2008). The results are expressed as grams of gallic acid equivalents per 100 g of dry extract. The total flavonoid content was determined with a colorimetric assay (Moreno et al., 2000) using rutin as a standard. The results are expressed as grams of rutin equivalents per 100 g of dry extract.
In Vitro Antioxidant Activity
DPPH Radicle Scavenging Assay
The radicle scavenging activity was evaluated by modified DPPH assay (He et al., 2012). Briefly to 2.7 mL of 0.2 mM DPPH, 0.3 mL of the extract solution at various concentrations was added. The mixture was shaken vigorously and incubated at room temperature for 1 h before the absorbance was measured at 517 nm. The radical scavenging activity was calculated as follows: scavenging rate = [(As - Ai)/As] × 100, where As is the absorbance of pure DPPH and Ai is the absorbance of DPPH in the presence of various extracts. Ascorbic acid at different concentrations identical to the experimental samples was used as reference.
Reducing Power Assay
The reducing power of the extracts was estimated using the standard method (Oyaizu, 1986). Briefly, increasing concentrations of 0.2 mL of extracts were mixed with 2.5 mL of phosphate buffer (0.2 M, pH 6.6) and 2.5 mL of potassium ferricyanide (1%). After incubation at 50°C for 20 min, 2.5 mL of trichloroacetic acid (10%) was added and each mixture was centrifuged at 1000 rpm for 10 min. Then, 2.5 mL of the supernatant was collected and mixed with 2.5 mL of deionized water and 0.5 mL of ferric chloride (0.1%). The absorbance was measured at 700 nm. The increased absorbance of the reaction mixture indicated increased reducing power. BHT was used as a standard for comparison.
Total Antioxidant Activity of Plant Extracts
Measurement of total antioxidant capacity of plant extracts were performed by photochemiluminescence method in the Photochem instrument, Germany. Lipid soluble antioxidant capacity assay estimate the photochemical generation of free radicals with a sensitive detector by using chemiluminescence. Free radicals were produced from the luminal, which worked partly as a photosensitizer and oxygen radical detection reagent. The lipophilic antioxidants were measured with the kits commercially available from Photochem (Germany). Total antioxidant activity of plant extracts was expressed in trolox equivalents (Kalita et al., 2016).
Acute Toxicity Studies
Acute oral toxicity studies were performed according to OECD guidelines to test chemicals. Adult swiss albino mice of either sex (six animals) were used for this study. Animals were kept fasting overnight with free access to water. Next day morning single dose of RD methanol leaf and bark extracts at 2000 mg/kg body weight were administered orally to three animals each. After administrating the single large dose of plant extract, animals were observed for different adverse effects for 14 days. If mortality was observed in two out of three animals, then the dose was identified as toxic dose. If mortality was observed in 1 animal, experiment was repeated again with the same dose to confirm the toxic dose. If mortality observed again experiment was continued with low doses (300, 50, and 5 mg/kg body weight).
Hepato Protective Effect of Methanol Leaf and Bark Extracts of RD
Animals
Adult male wistar rats (150–200 g) were obtained from the institutional animal house of Institute of Advanced Study in Science and Technology (IASST), Guwahati (India). Animals were maintained at 24°C ± 1°C, with relative humidity of 45–55% and 12:12 h dark/light cycle. The animals had free access to standard pellet diet (Provimi Animal Nutrition, Pvt, Ltd, India) and water throughout the experimental protocol. All experiments were carried out between 09:00 and 17:00 h. The experimental protocol was approved by the Institutional Animal Ethics Committee (IAEC) of IASST, Guwahati (CPCSEA/44/2014) and performed in accordance with the guidelines of Committee for Control and Supervision of Experimentation on Animals (CPCSEA), Government of India on animal experimentation.
Experimental Design
Total of 42 animals were divided into seven groups of six animals each.
• Group I served as normal control and received D.W for 14 days orally and on 14th day olive oil (1.5 ml/kg, i.p.).
• Group II served as toxic control and received 0.3% CMC for 14 days orally and on 14th day CCl4 (1.5 ml/kg, i.p.) in 1:1 dilution with olive oil.
• Group III served as standard group and received silymarin (100 mg/kg) for 14 days orally and on 14th day CCl4 (1.5 ml/kg, i.p.) in 1:1 dilution with olive oil.
• Groups IV and V served as treatment groups and received RD methanol leaf extract (200 and 400 mg/kg) for 14 days orally and on 14th day CCl4 (1.5 ml/kg, i.p.) in 1:1 dilution with olive oil.
• Groups VI and VII served as treatment groups and received RD methanol bark extract (200 and 400 mg/kg) for 14 days orally and on 14th day CCl4 (1.5 ml/kg, i.p.) in 1:1 dilution with olive oil.
After 48 h of CCl4 administration all the animals were sacrificed and blood and liver was collected for biochemical estimation and histopathology analysis.
Serum Biochemical Estimation
Blood was collected from retro-orbital route under mild anesthesia and allowed to clot. Serum was separated by centrifugation at 3000 rpm for 10 min for estimation of aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), lactate dehydrogenase (LDH), albumin, total protein, urea, total and direct bilirubin by commercially available kits as per the instructions given by the manufacturer (Accurex, India). ELISA kits from R&D systems, USA were used to measure the serum pro inflammatory cytokines TNF-α and IL-1β. Each sample was done in duplicate and results were expressed in Pg/ml (Kandimalla et al., 2016b,c).
Tissue Biochemical Assays
All the treatments were done at 4°C. Liver homogenate was prepared with 50 mM cold potassium phosphate buffer (pH 7.4). The resulting suspension was centrifuged at 3000 rpm for 15 min and supernatant was collected and analyzed for SOD (Marklund and Marklund, 1974), CAT (Aebi, 1974), GSH (Ellman, 1959), and thiobarturic acid reacting substances (TBARSs; Xia et al., 2013).
Histopathology Examination
Livers was collected in 10% buffered formaldehyde and preserved for at least 24 h. Further liver samples were dehydrated gradually with ethanol (70–100%), cleared in xylene and embedded in paraffin. Sections of 5 μm were prepared by microtome assembly (Leica, Germany) and stained with hematoxylin and eosin to examine under microscope (10x) for histopathological changes (Kalita et al., 2015; Bhardwaj et al., 2016; Choudhury A.J. et al., 2016).
Statistical Analysis
All the results were expressed as mean ± SD. One way ANOVA followed by Tukey’s multiple comparison test was used to compare the different parameters between the groups. A p-value < 0.05 was considered as significant.
Results
Total Phenolic and Flavonoid Content
Presence of phenolic type compounds can be correlates with the antioxidant activity of medicinal plants (Bravo, 1998; Huang et al., 2010; Chen et al., 2011). In this study, the total phenolic and flavonoid content of the methanol extract of RD leaf and bark was determined (Table 1). Total phenolic and flavonoid content were found to be 112 ± 3.24 mg and 138 ± 2.46 mg gallic acid equivalents/g extract and 2.6 ± 0.26 mg and 3.34 ± 0.31 mg rutin equivalents/g extract respectively for RD leaf and bark methanol extracts. It is observed that RD bark methanol extract contain more total phenolic and flavonoid content than RD leaf methanol extract.
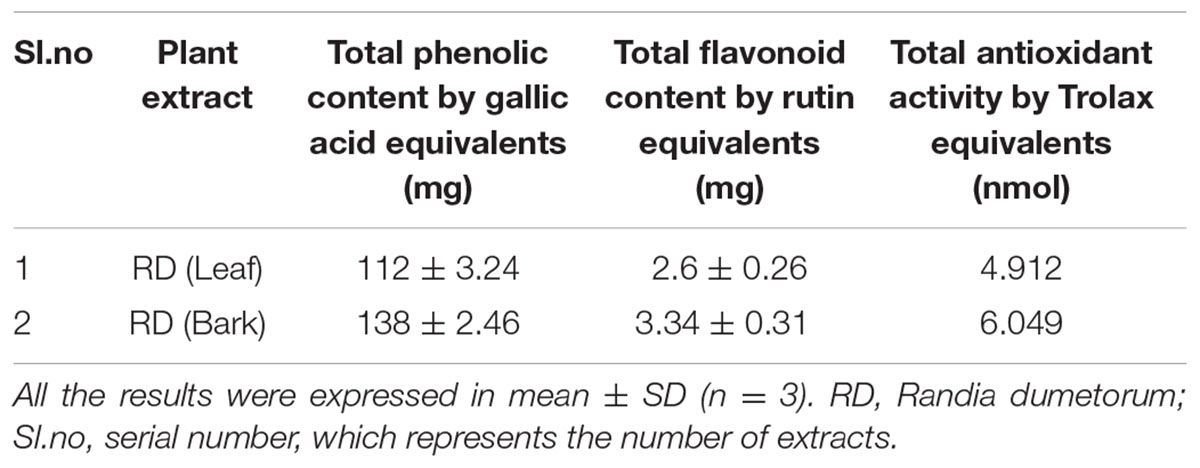
TABLE 1. Total phenolic, flavonoid, and antioxidant content of methanol extracts of RD leaf and bark.
In Vitro Antioxidant Activity
DPPH Radical Scavenging Activity
1,1-Diphenyl-2-picrylhydrazyl is a stable, organic free radical widely used to detect the scavenging activity of the antioxidants (Debnath et al., 2015). The DPPH scavenging activity of methanol extracts of RD leaf and bark at different concentrations are presented in Figure 1A. Both the extracts show dose dependent DPPH scavenging activity. Apparently ascorbic acid showed the highest radical scavenging activity than methanol plant extracts.
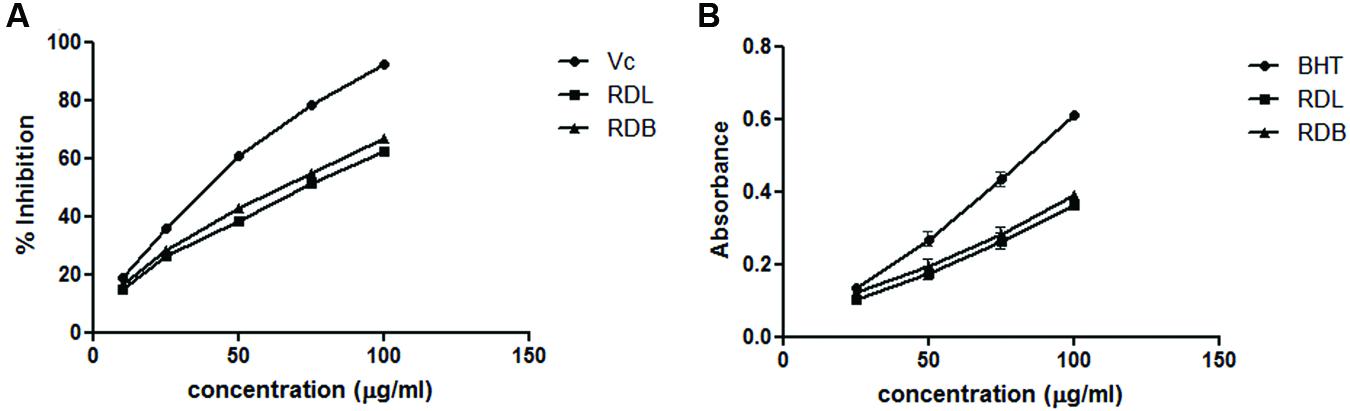
FIGURE 1. (A) 1,1-Diphenyl-2-picrylhydrazyl (DPPH) radicle scavenging assay of methanol extract of RD leaf and bark extracts: (RDL), RD leaf extract; (RDB), RD bark extract; (Vc), ascorbic acid. (B) Reducing power activity of methanol extract of RD leaf and bark extracts: RDL, RD leaf extract; RDB, RD bark extract; BHT, butylated hydroxytoluene.
Reducing Power Ability
Reducing power ability of the compounds is responsible for the potent antioxidant activity of the extracts (Choudhury B. et al., 2016). The ability of RD extracts to convert Fe3+ to Fe2+ was considered as reducing potential. Formation of Fe2+ can be monitored by measuring the prussian blue color formation at 700 nm. The reducing ability of methanol extracts of RD leaf and bark were increased with increasing the concentrations (Figure 1B). The order of reducing ability of the tested sample is BHT > RD bark > RD leaf.
Total Antioxidant Activity
Photo-chemi-luminescence method in the Photochem instrument was dependent on the antioxidant concentration based on the Guldberg-Waage law. This law expresses the magnitude of the reaction. Reactions of radicals and antioxidants give relatively stable products which are stable and measurable. Antioxidant capacity of the drug/substance depends on the concentration (Hic and Balic, 2012). Total antioxidant activity of methanol leaf and bark extracts of RD was presented in Table 1.
Acute Toxicity Studies
No observed adverse effect level (NOAEL) dose is the highest exposure drug dose at which there is no significant increase in adverse effects compared to non-treated animals. In this study RD leaf and bark methanol extracts at 2000 mg/kg did not show any effect on heart rate, respiratory rate, salivation, body temperature, locomotor activity, corneal reflex, body tone, skin tone, abdominal tone, grip strength. tremors, tail elevation, piloerection, twitches, and convulsions are also not observed. After 14 days of observation period no mortality was observed in tested mice. RD leaf and bark methanol extracts at 2000 mg/kg is considered as NOAEL. According to OECD guidelines 2000 mg/kg is the highest dose for acute toxicity studies. RD methanol leaf and bark extracts were safe at highest dose, so no further low dose was tested. 1/10th (200 mg/kg) and 1/5th (400 mg/kg) of NOAEL dose was selected for the present study.
Effect of RD Leaf and Bark Methanol Extracts on Serum Biochemical Parameters
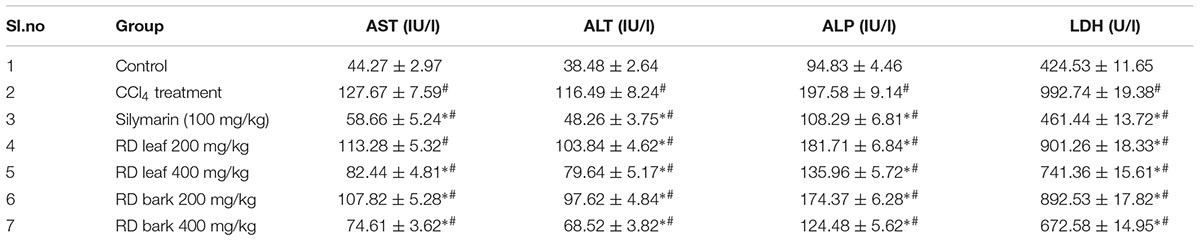
Carbon tetrachloride treatment in rats (Group II) showed significant increase in AST, ALT, ALP, LDH, albumin, total bilirubin, direct bilirubin and significant decrease in total protein levels compared to control animals (Group I). Pre-treatment with RD leaf and bark methanol extracts at 200 and 400 mg/kg for 14 days (Groups III–VII) before CCl4 intoxication showed significant protection against CCl4 by means of decreased serum AST, ALT, ALP, LDH, albumin, total and direct bilirubin and increased total protein levels compared to the CCl4 alone treated group (Group II). Though, this result indicated the hepatoprotective ability of test extracts and standard drug silymarin; but the biochemical parameters did not reach to that of control animals (Group I). RD leaf and bark at 200 mg/kg showed less protection than 400 mg/kg treated group. Order of showing hepatoprotective response by means of serum biochemical levels were Group 3 > Group 7 > Group 5 > Group 6 > Group 4 (Tables 2A,B).
Effect of RD Leaf and Bark Methanol Extract on Serum Cytokine Levels
Carbon tetrachloride induction activates inflammatory cytokines like TNF-α and IL-1β levels in in vivo system. Pre-treatment with standard drug silymarin, RD leaf and RD bark methanol extracts before CCl4 induction significantly inhibits the TNF-α (Figure 2), IL-1β (Figure 3) production dose dependently, but this reduction in levels did not reach up to that of control animals. RD bark extract showed potent response to RD leaf extract.
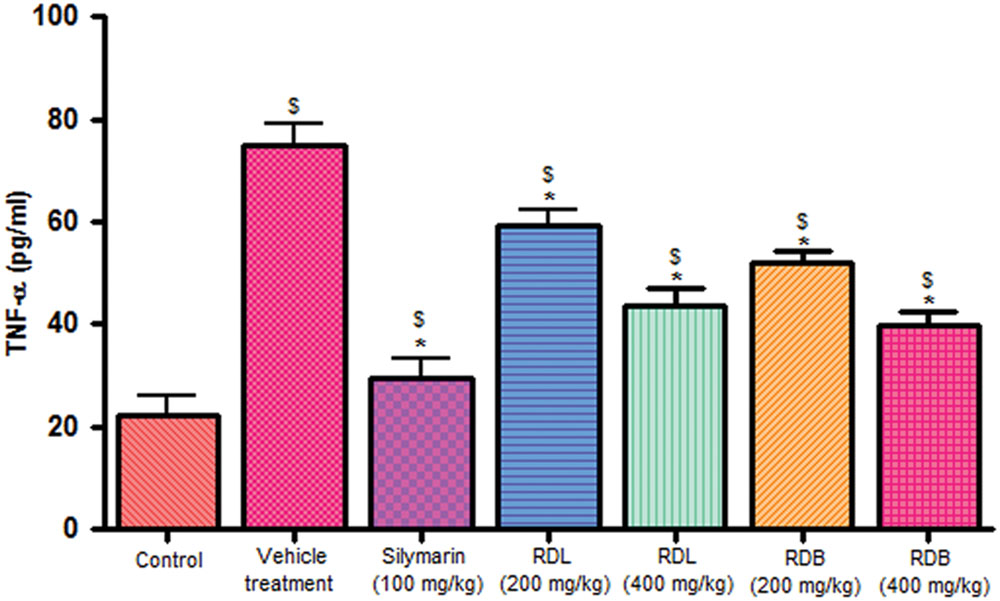
FIGURE 2. Effect of different drug treatment on serum TNF-α levels. All the results were expressed in mean ± SD (n = 6). $p < 0.05 in comparison with normal animals. ∗p < 0.05 in comparison with CCl4 alone treated animals. RDL, R. dumetorum leaf methanol extract; RDB, R. dumetorum bark methanol extract.
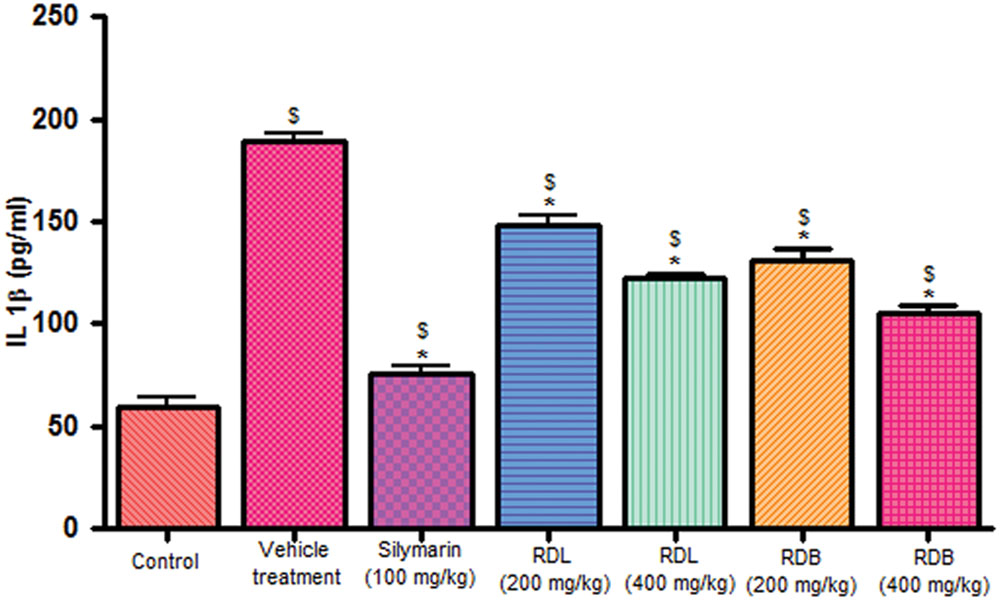
FIGURE 3. Effect of different drug treatment on serum IL-1β levels. All the results were expressed in mean ± SD (n = 6). $p < 0.05 in comparison with normal animals. ∗p < 0.05 in comparison with CCl4 alone treated animals. RDL, R. dumetorum leaf methanol extract; RDB, R. dumetorum bark methanol extract.
Effect of RD Leaf and Bark Methanol Extracts on Antioxidant Enzyme Levels
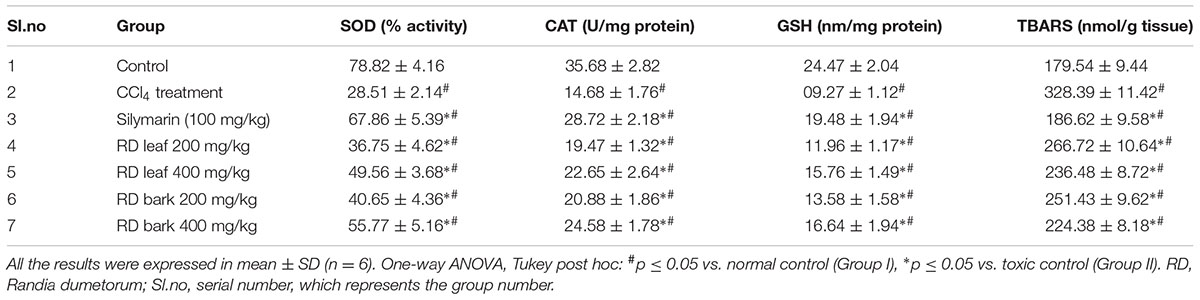
Administration of CCl4 significantly decreases the SOD, CAT, and GSH levels in comparison to control rats. Pre-treatment with RD leaf and bark methanol extracts showed significant increase in all the enzyme levels in comparison with CCl4 alone treated rats. But this observed rise in enzyme level was not as significant as standard drug silymarin treated rats (Table 2C). Standard drug and extracts treatment did not completely restore the abnormal parameters as that of control animals. RD bark methanol extract showed more significant results than that of leaf extract.
Effect of RD Leaf and Bark Methanol Extracts on Lipid Peroxidation
Carbon tetrachloride treated rats showed significantly increased TBARS levels when compared with control group. Pre-treatment with RD leaf and bark methanol extracts and standard drug silymarin before CCl4 intoxication showed significant decrease in TBARS levels compared with CCl4 alone treated rats (Table 2C), but this reduction did not reach to that of control animals.
Histopathology
Histopathology of livers provided supportive evidence for the biochemical analysis. Liver histopathology of rats in control group showed normal hepatocytes (Figure 4A). CCl4 intoxication causes demolishment of hepatocytes which was evidenced by formation of large septa, collagen accumulation, bridging necrosis, and periportal inflammation (Figure 4B). Treatment with standard drug silymarin, RD leaf and bark methanol extracts showed significant protection against CCl4 induced hepatic damage. Liver tissue of the treated group showed normal architecture with no necrosis and inflammation (Figures 4C–E).
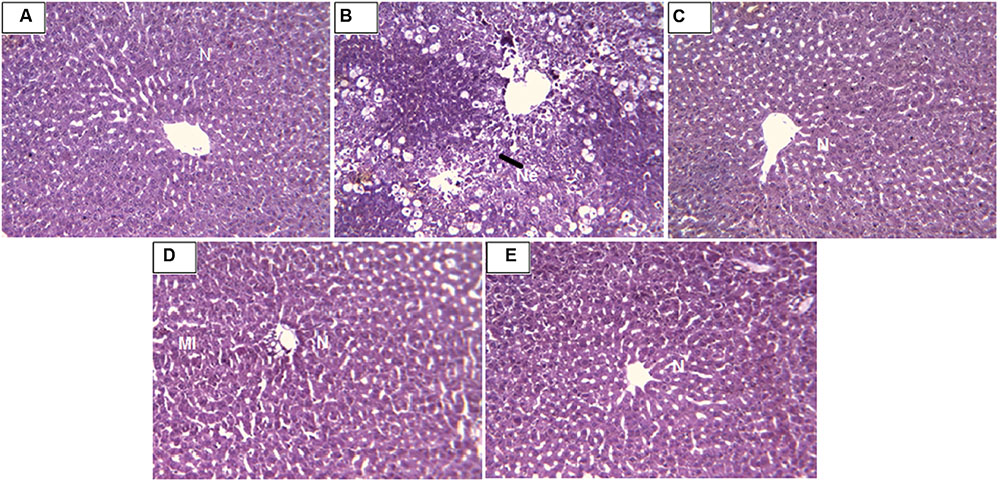
FIGURE 4. (A) Liver of control rat showing normal hepatocytes; x 10. (B) Liver of rat treated with CCl4 showing formation of septa, bridging necrosis and dense periportal inflammation; x 10. (C) Liver of rat treated with CCl4 and 100 mg/kg Silymarin showing complete absence of periportal inflammation or necrosis; x 400. (D) Liver of rat treated with CCl4 and 400 mg/kg of RD methanol leaf extract showing mild periportal inflammation; x 10. (E) Liver of rat treated with CCl4 and 400 mg/kg RD methanol bark extract showing reversal of changes with absence of periportal inflammation or necrosis; x 10. N, normal hepatocytes; Ne, necrotic cells; MI, mild inflammation.
Discussion
The present study deals with antioxidant and hepatoprotective activities of methanol extract of RD leaf and bark. Phenolic and flavonoid compounds are radical scavengers, metal chelators, reducing agents, hydrogen donors, and singlet oxygen quenchers (Khadri et al., 2010; Saeed and Farzaneh, 2010). Therefore, it is important to measure the total phenolic and flavonoid content of a plant extract to measure the antioxidant capacity. The in vitro antioxidant activity of the RD leaf and bark methanol extracts might be due to presence these phenolic and flavonoid component as demonstrated by DPPH scavenging activity, reducing power ability and total antioxidant activity. Liver is the second largest organ of our body involved in vital functions like detoxification of drugs and toxins through metabolism. CCl4 is the toxin which produce trichloromethyl radical (CCl3•) which is activated by cytochrome P450. This radicle mainly associated with CCl4 induced hepatic damage (Shakya et al., 2012; Vuda et al., 2012). These radicals reacts covalently with sulfhydryl-containing proteins in cells which leading to membrane lipid peroxidation and cell necrosis (Muriel et al., 2001).
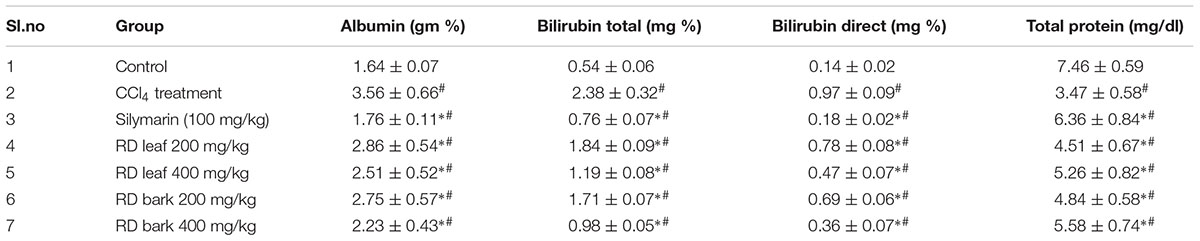
Serum enzyme levels are significant markers to determine the severity of damage to particular tissue (Tu et al., 2015). CCl4 administration cause significant increase in serum enzyme levels like AST, ALT, ALP, LDH, Albumin and decrease levels of total protein levels when compared with control animals which indicate the liver damage. Bilirubin is the product of heme in red blood cells and hyper-bilirubinemia is one of the markers of damaged liver. Treatment with standard drug silymarin and methanol leaf and bark extracts of RD restore the elevated serum levels of AST, ALT, ALP, LDH, Albumin, total and direct bilirubin with increase in total protein levels. Total protein levels also raised in drug treatment groups (Groups III–VII). Restoration of abnormal enzyme levels to normal indicating the protection of RD leaf and bark methanol extracts against the liver injury by CCl4.
By-products of LPO cause hepatic damage in hydrophobic core of bio-membranes (Maheswari et al., 2008). Elevated levels of TABRS are observed in CCl4 treated rats indicating the formation of free radicals in larger quantity due to failure of antioxidant defense mechanism and hepatic damage. Significant decline in LPO was observed in rats treated with standard drug silymarin and methanol leaf and bark extracts of RD.
We further studied the non-enzymatic antioxidants like GSH and endogenous enzymes like SOD and CAT which involves in neutralizing free radicals. Suppression of these enzyme levels is an indicator for hepatic damage (Ozer et al., 2008). CCl4 administration cause decline in all the hepatic antioxidant levels when compared with control animals. Standard drug silymarin and methanol leaf and bark extracts of RD pre-treatment restores the antioxidant levels, which is a positive sign of liver protection. CCl4 metabolism stimulated the Kupffer cells which activates this TNF-α and IL-1β. IL-1β is strong inflammatory cytokines which involves in the production of prostaglandins, macrophage activation, and neutrophil infiltration. Activation of inflammatory cytokine cascade produces significant damage to the cells especially to hepatocytes (Edwards et al., 1993; Ishiyama et al., 1995; Kandimalla et al., 2016a). RD leaf and bark treatment to animal before CCl4 cause significant protection, which might be due to stabilization of Kupffer cells and inhibition of macrophage activation and prostaglandin production. Histopathology results provided supportive evidence for biochemical analysis. Methanol leaf and bark extract of RD showed significant morphological changes in liver tissue compare to CCl4 treated group. Decreased periportal inflammation was also observed in silymarin and extract treated animals. In this study RD bark methanol extract showed highest protection against CCl4 induced liver damage than RD leaf methanol extract which is comparable to standard drug silymarin.
Conclusion
The present study provides a scientific rational for the traditional use of RD leaf and bark for the treatment of liver ailments. RD bark is more effective than the leaf extract in the treatment of liver ailments. Fractionation, isolation and screening of active chemical components from these extracts are in progress in our laboratory. Successful completion of this work can potentially contribute in the development of herbal remedy for liver disorders.
Author Contributions
Mr. RK is the first author and performed all the in vitro and in vivo experiments like animal experiments setup, biochemical estimation, and tissue processing etc. Mr. SK, Mr. BS, and Miss. BC helped the first author in different steps of experiments and manuscript preparation. Dr. KK performed the hisopathology of liver and examined for pathological changes. Prof. SD helped in literature survey and study design. Prof. JK contributed towards study design, experimental setup, results supervision, and manuscript correction.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors are thankful to Director, Institute of Advanced Study in Science and Technology, Guwahati, Assam for support and Department of Science and Technology (DST), New Delhi, India for financial assistance. We extend our gratitude for Dr. Sushmita Gupta for helping in preparation of manuscript.
References
Aebi, H. (1974). “Catalase,” in Methods of Enzymatic Analysis, Vol. 1, ed. H. U. Bergmeyer (New York: Academic Press), 673–684.
Banu, S., Bhaskar, B., and Balasekar, P. (2012). Hepatoprotective and antioxidant activity of Leucas aspera against d-galactosamine induced liver damage in rats. Pharm. Biol. 50, 1592–1595. doi: 10.3109/13880209.2012.685130
Bhardwaj, N., Singh, Y. P., Devi, D., Kandimalla, R., Kotoky, J., and Mandal, B. B. (2016). Potential of silk fibroin/chondrocyte constructs of muga silkworm Antheraea assamensis for cartilage tissue engineering. J. Mater. Chem. B. 4, 3670–3684. doi: 10.1039/C6TB00717A
Bravo, L. (1998). Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 56, 317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x
Chen, Y., Huang, B., He, J., Han, L., Zhan, Y., and Wang, Y. (2011). In vitro and in vivo antioxidant effects of the ethanolic extract of Swertia chirayita. J. Ethnopharmacol. 136, 309–315. doi: 10.1016/j.jep.2011.04.058
Choudhury, A. J., Gogoi, D., Chutia, J., Kandimalla, R., Kalita, S., Kotoky, J., et al. (2016). Controlled antibiotic-releasing Antheraea assama silk fibroin suture for infection prevention and fast wound healing. Surgery 159, 539–547. doi: 10.1016/j.surg.2015.07.022
Choudhury, B., Kandimalla, R., Bharali, R., Monisha, J., Kunnumakkara, A. B., Kalita, K., et al. (2016). Anticancer activity of garcinia morella on t-cell murine lymphoma via apoptotic induction. Front. Pharmacol. 7:3. doi: 10.3389/fphar.2016.00003
Debnath, T., Kim, D. H., Jo, J. E., Lee, J. J., Pyo, H. J., and Lim, B. O. (2015). Hepatoprotective activity of Haliotis discus hanniino extract on lipopolysaccharide-induced liver damage in rats. J. Food Biochem. 39, 310–315. doi: 10.1111/jfbc.12131
Edwards, M. J., Keller, B. J., Kauffman, F. C., and Thurman, R. G. (1993). The involvement of kupffer cells in carbon tetrachloride toxicity. Toxicol. Appl. Pharm. 119, 275–279. doi: 10.1006/taap.1993.1069
Ellman, G. L. (1959). Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82, 70–77. doi: 10.1016/0003-9861(59)90090-6
Fu, W., Chen, J., Cai, Y., Lei, Y., Chen, L., Pei, L., et al. (2010). Antioxidant, free radical scavenging, anti-inflammatory and hepatoprotective potential of the extract from Parathelypteris nipponica. J. Ethnopharmacol. 130, 521–528. doi: 10.1016/j.jep.2010.05.039
Habib, N. C., Serra-Barcellona, C., Honoré, S. M., Genta, S. B., and Sánchez, S. S. (2015). Yacon roots (Smallanthus sonchifolius) improve oxidative stress in diabetic rats. Pharm. Biol. 53, 1183–1193. doi: 10.3109/13880209.2014.970285
He, J., Huang, B., Ban, X., Tian, J., Zhu, L., and Wang, Y. (2012). In vitro and in vivo antioxidant activity of the ethanolic extract from Meconopsis quintuplinervia. J. Ethnopharmacol. 141, 104–110. doi: 10.1016/j.jep.2012.02.006
Hic, P., and Balic, J. (2012). Effect of sample dilution on estimated values of antioxidant capacity by photochemiluminiscence method. Acta Univ. Agric. Silvic. Mendel. Brun. 8, 67–72. doi: 10.11118/actaun201260080067
Huang, B., Ban, X., He, J. S., Tong, J., Tian, J., and Wang, Y. (2010). Hepatoprotective and antioxidant activity of ethanolic extracts of edible lotus (Nelumbo nucifera Gaertn.) leaves. Food Chem. 120, 873–878. doi: 10.1016/j.foodchem.2009.11.020
Ishiyama, H., Sato, M., Matsumura, K., Sento, M., Keiki, O., and Hobara, T. (1995). Proliferation of hepatocytes and attenuation from carbon tetrachloride hepatotoxicity by gadolinium chloride in rats. Pharmacol. Toxicol. 77, 293–298. doi: 10.1111/j.1600-0773.1995.tb01030.x
Kalita, H., Boruah, D. C., Deori, M., Hazarika, A., Sarma, R., Kumari, S., et al. (2016). Antidiabetic and antilipidemic effect of Musa balbisiana root extract: a potent agent for glucose homeostasis in streptozotocin-induced diabetic rat. Front. Pharmacol. 7:102. doi: 10.3389/fphar.2016.00102
Kalita, S., Devi, B., Kandimalla, R., Sharma, K. K., Sharma, A., Kalita, K., et al. (2015). Chloramphenicol encapsulated in poly-𝜀-caprolactone-pluronic composite: nanoparticles for treatment of MRSA-infected burn wounds. Int. J. Nanomedicine 10, 2971–2984. doi: 10.2147/IJN.S75023
Kandimalla, R., Dash, S., Kalita, S., Choudhury, B., Malampati, S., Kalita, K., et al. (2016b). Bioactive guided fractions of Annona reticulata L. bark: protection against liver toxicity and inflammation through inhibiting oxidative stress and proinflammatory cytokines. Front. Pharmacol. 7:168. doi: 10.3389/fphar.2016.00168
Kandimalla, R., Kalita, S., Choudhury, B., Dash, S., Kalita, K., and Kotoky, J. (2016c). Chemical composition and anti-candidiasis mediated wound healing property of Cymbopogon nardus essential oil on chronic diabetic wounds. Front. Pharmacol. 7:198. doi: 10.3389/fphar.2016.00198
Kandimalla, R., Kalita, S., Choudhury, B., Devi, D., Kalita, D., Kalita, K., et al. (2016a). Fiber from ramie plant (Boehmeria nivea): a novel suture biomaterial. Mater. Sci. Eng. C Mater. Biol. Appl. 62, 816–822. doi: 10.1016/j.msec.2016.02.040
Khadri, A., Neffati, M., Smiti, S., Falé, P., Lino, A. R. L., Serralheiro, M. L. M., et al. (2010). Antioxidant, anti-acetylcholinesterase and antimicrobial activities of Cymbopogon schoenanthus L. Spreng (lemon grass) from Tunisia. LWT-Food Sci. Technol. 43, 331–336. doi: 10.1016/j.lwt.2009.08.004
Kong, F. L., Zhang, M. W., Kuang, R. B., Yu, S. J., Chi, J. W., and Wei, Z. C. (2010). Antioxidant activities of different fractions of polysaccharide purified from pulp tissue of litchi (Litchi chinensis Sonn). Carbohydr. Polym. 81, 612–616. doi: 10.1016/j.carbpol.2010.03.021
Liang, D., Zhou, Q., Gong, W., Wang, Y., Nie, Z., He, H., et al. (2011). Studies on the antioxidant and hepatoprotective activities of polysaccharides from Talinum triangulare. J. Ethnopharmacol. 136, 316–321. doi: 10.1016/j.jep.2011.04.047
Maheswari, C., Maryammal, R., and Venkatanarayanan, R. (2008). Hepatoprotective activity of “Orthosiphon stamineus” on liver damage caused by paracetamol in rats. Jordan J. Biol. Sci. 1, 105–108.
Marklund, S., and Marklund, G. (1974). Involvement of superoxide anion radical in the autoxidation of pyrogallol and convenient assay for superoxide dismutase. Eur. J. Biochem. 47, 469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x
Moreno, M. I. N., Isla, M. I., and Sampietro, A. R. (2000). Comparison of the free radical scavenging activity of propolis from several regions of Argentina. J. Ethnopharmacol. 71, 109–114. doi: 10.1016/S0378-8741(99)00189-0
Muriel, P., Alba, N., Perez-Alvarez, V. M., Shibayama, M., and Tsutsumi, V. K. (2001). Kupffer cells inhibition prevents hepatic lipid peroxidation and damage induced by carbon tetrachloride. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 130, 219–226.
Oyaizu, M. (1986). Studies on products of browning reaction Antioxidtive activities of browning reaction products prepared from glucoseamine. Jpn. J. Nutr. Diet. 44, 307–315. doi: 10.5264/eiyogakuzashi.44.307
Ozer, J., Ratner, M., Shaw, M., Bailey, W., and Schomaker, S. (2008). The current state of serum biomarkers of hepatotoxicity. J. Toxicol. 245, 194–205. doi: 10.1016/j.tox.2007.11.021
Patel, R. G., Pathak, N. L., Rathod, J. D., Patel, L. B., and Bhatt, N. M. (2011). Phytopharmacological properties of randia dumetorum as a potential medicinal tree: an overview. J. Appl. Pharm. Sci. 10, 24–26.
Sabir, S. M., and Rocha, J. B. (2008). Antioxidant and hepatoprotective activity of aqueous extract of Solanum fastigiatum (false Jurubeba) against paracetamol-induced liver damage in mice. J. Ethnopharmacol. 120, 226–232. doi: 10.1016/j.jep.2008.08.017
Saeed, M. M., and Farzaneh, N. (2010). Antioxidant activity of some edible plants of the Turkmen Sahra region in northern Iran. Food Chem. 119, 1637–1642. doi: 10.1016/j.foodchem.2009.09.057
Sarwar, R., Farooq, U., Khan, A., Naz, S., Khan, S., Khan, A., et al. (2015). Evaluation of antioxidant, free radical scavenging, and antimicrobial activity of Quercus incana Roxb. Front. Pharmacol. 6:277. doi: 10.3389/fphar.2015.00277
Shakya, A. K., Sharma, N., Saxena, M., Shrivastava, S., and Shukla, S. (2012). Evaluation of the antioxidant and hepatoprotective effect of Majoon-e-Dabeed-ul-ward against carbon tetrachloride induced liver injury. Exp. Toxicol. Pathol. 64, 767–773. doi: 10.1016/j.etp.2011.01.014
Tu, C., Gao, D., Li, X.-F., Li, C. Y., Li, R. S., Zhao, Y. L., et al. (2015). Inflammatory stress potentiates emodin-induced liver injury in rats. Front. Pharmacol. 6:233. doi: 10.3389/fphar.2015.00233
Vuda, M., D’Souza, R., Upadhya, S., Kumar, V., Rao, N., Kumar, V., et al. (2012). Hepatoprotective and antioxidant activity of aqueous extract of Hybanthus enneaspermus against CCl4-induced liver injury in rats. Exp. Toxicol. Pathol. 64, 855–859. doi: 10.1016/j.etp.2011.03.006
Keywords: Randia dumetorum, antioxidant, liver protection, inflammation, cytokines
Citation: Kandimalla R, Kalita S, Saikia B, Choudhury B, Singh YP, Kalita K, Dash S and Kotoky J (2016) Antioxidant and Hepatoprotective Potentiality of Randia dumetorum Lam. Leaf and Bark via Inhibition of Oxidative Stress and Inflammatory Cytokines. Front. Pharmacol. 7:205. doi: 10.3389/fphar.2016.00205
Received: 17 December 2015; Accepted: 27 June 2016;
Published: 14 July 2016.
Edited by:
Michael Heinrich, University of London, UKReviewed by:
Yun K. Tam, Sinoveda Canada Inc., CanadaXiao Yu Tian, Chinese University of Hong Kong, China
Copyright © 2016 Kandimalla, Kalita, Saikia, Choudhury, Singh, Kalita, Dash and Kotoky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jibon Kotoky, amtvdG9reUBnbWFpbC5jb20= Raghuram Kandimalla, cmFnaHVyYW0ucGhhcm1hQGdtYWlsLmNvbQ==
 Raghuram Kandimalla
Raghuram Kandimalla Sanjeeb Kalita
Sanjeeb Kalita Bikas Saikia
Bikas Saikia Bhaswati Choudhury
Bhaswati Choudhury Yogendra P. Singh
Yogendra P. Singh Kasturi Kalita2
Kasturi Kalita2 Jibon Kotoky
Jibon Kotoky