- Department of Psychology and Collaborative Neuroscience Program, University of Guelph, Guelph, ON, Canada
Over the years, animal studies have revealed a role for the endocannabinoid system in the regulation of multiple aspects of opiate addiction. The current review provides an overview of this literature in regards to opiate withdrawal. The opiate withdrawal syndrome, hypothesized to act as a negative reinforcer in mediating continued drug use, can be characterized by the emergence of spontaneous or precipitated aversive somatic and affective states following the termination of drug use. The behaviors measured to quantify somatic opiate withdrawal and the paradigms employed to assess affective opiate withdrawal (e.g., conditioned place aversion) in both acutely and chronically dependent animals are discussed in relation to the ability of the endocannabinoid system to modulate these behaviors. Additionally, the brain regions mediating somatic and affective opiate withdrawal are elucidated with respect to their modulation by the endocannabinoid system. Ultimately, a review of these findings reveals dissociations between the brain regions mediating somatic and affective opiate withdrawal, and the ability of cannabinoid type 1 (CB1) receptor agonism/antagonism to interfere with opiate withdrawal within different brain sub regions.
Introduction
Opiate addiction is a chronic distressing brain disorder for which there are limited successful treatments that do not rely on the administration of synthetic opiate analogs (Stotts et al., 2009). One potential non-opioidergic therapeutic target is the endocannabinoid system. A review of the preclinical animal literature suggests that the endocannabinoid system is necessary for the development of opiate dependence and could prove to be useful in the treatment of opiate withdrawal.
Cannabinoids On Opiate Withdrawal
In animals, as in humans, opioid dependence is produced through chronic opiate exposure, or even, following the single administration of a high dose of an opiate, a state termed acute opiate dependence (Heishman et al., 1989; June et al., 1995). Once dependent, withdrawal can be induced either spontaneously through drug abstinence, or precipitated through the administration of an opiate antagonist such as naloxone. In each instance, dependent animals will display numerous somatic and behavioral symptoms that are characteristic of the withdrawal state experienced by human opiate addicts (Jaffe, 1990). The ability of different pharmacological manipulations to alter the severity or presence of these symptoms can be used as a measure of their potential in the treatment of opiate detoxification.
Somatic Withdrawal
In animals, the intensity of somatic withdrawal is quantified by scoring the presence or severity of several physical signs for 10–30 min immediately following precipitated withdrawal, or every 6–9 h for several days following spontaneous withdrawal (Maldonado et al., 1996). To facilitate the quantification of the withdrawal syndrome, Gellert and Holtzman (1978) developed a weighted scale consisting of graded symptoms including percentage of weight loss, number of escape jumps, number of wet dog shakes, number of abdominal constrictions, and checked signs including diarrhea, facial fasciculations/teeth chattering, swallowing, salivation, chromodacryorrhea, ptosis, abnormal posture, erection/ejaculation/genital grooming, and irritability. In assessing the ability of pharmacological cannabinoid manipulations to alleviate the intensity of somatic withdrawal, most studies use a variation of this scale to determine whether individual signs or a global rating of withdrawal has significantly decreased.
A review of the literature reveals that activation of the cannabinoid system acutely prior to withdrawal, or chronic inhibition of the system during the development of dependence, is most effective in reducing withdrawal severity (see Table 1 for specific agonists and antagonists and original references). In the majority of cases, acute treatment with a cannabinoid agonist (in particular agonists of the CB1 receptor) immediately prior to the induction of withdrawal in highly dependent animals (whether precipitated or spontaneous) is able to attenuate several aspects of the syndrome. Notably, a reduction in the incidence of escape jumps, paw tremors, weight loss, and diarrhea were most commonly reported in rodents (see Table 1 for original references). Exceptions to the reductions in withdrawal occurred only when the agonist (Δ9THC) was administered chronically during the development of morphine dependence (Cichewicz and Welch, 2003; Gerak and France, 2016), when rats were made acutely dependent on morphine prior to withdrawal (Hine et al., 1975b), or when the cannabinoid tested, cannabidiol, was not a CB1 receptor agonist (Hine et al., 1975d,e; Chesher and Jackson, 1985).
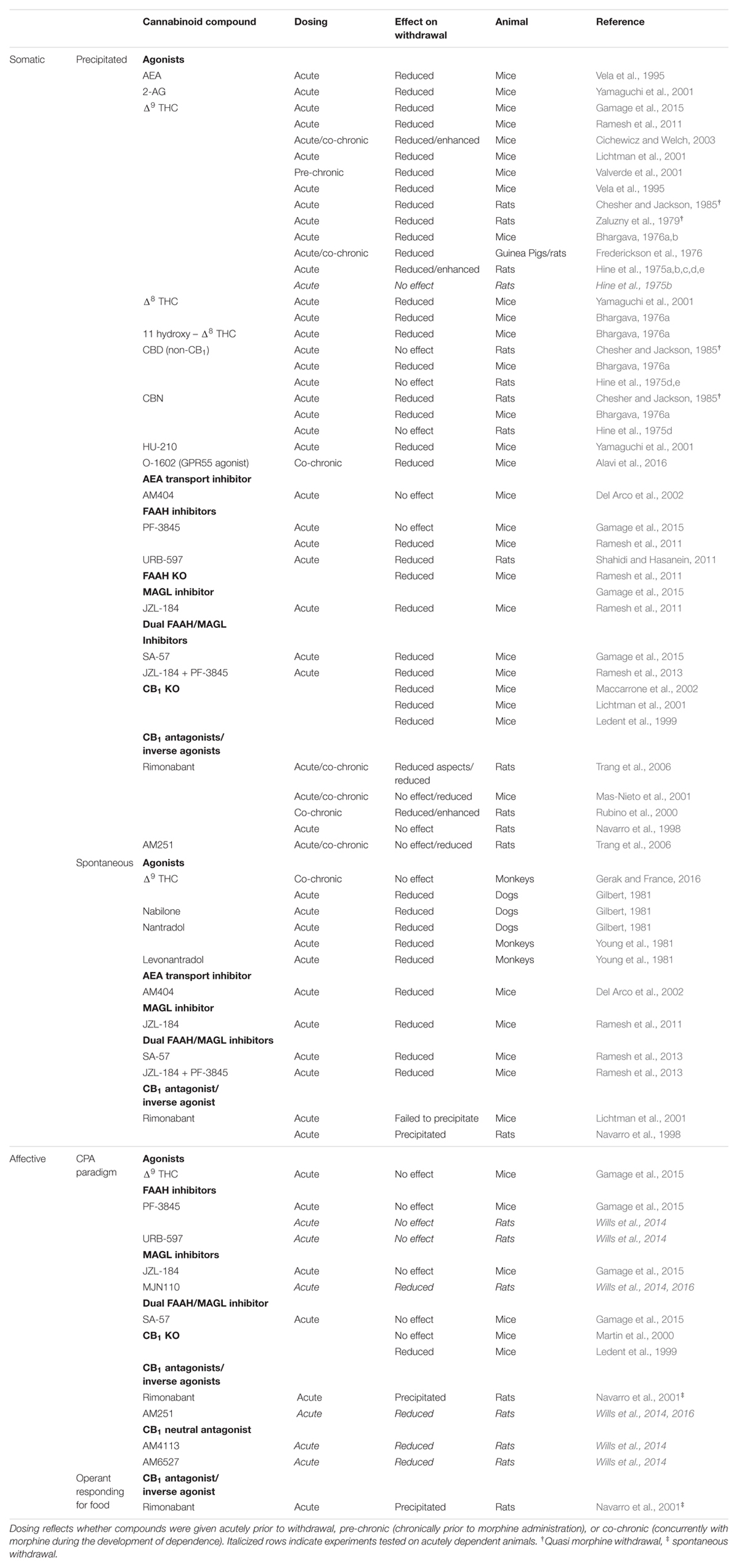
TABLE 1. Effect of different cannabinoid compounds on spontaneous or precipitated somatic and affective opioid withdrawal.
Somewhat contradictory, CB1 receptor knock-out (KO) mice show reduced somatic opioid withdrawal and cannabinoid antagonists (rimonabant and AM251) interfere with somatic opioid withdrawal (see Table 1). However, contrary to the cannabinoid agonists, antagonists were most effective in reducing withdrawal when delivered chronically (not acutely) during the development of opiate dependence. Indeed, when given chronically prior to precipitating withdrawal with naloxone, rimonabant and AM251 reliably reduced withdrawal severity (Rubino et al., 2000; Mas-Nieto et al., 2001; Trang et al., 2006). In contrast, when delivered acutely, antagonists were either without effect (Navarro et al., 1998; Mas-Nieto et al., 2001; Trang et al., 2006), or their ability to attenuate withdrawal was markedly reduced (Trang et al., 2006). In a case of spontaneous withdrawal (Navarro et al., 1998), acute rimonabant treatment was even found to precipitate withdrawal in morphine-dependent rats, however, this finding was not replicated when tested in mice (Lichtman et al., 2001). Ultimately, these findings, along with the results from the agonist studies, suggest that the endocannabinoid system plays an important role in the development of somatic opioid dependence and activation of the system during withdrawal, or chronic blockade during opioid dependence, can mitigate some of its adverse effects.
Unfortunately, CB1 receptor (CB1R) agonists and inverse agonists/antagonists are known to produce undesirable side effects (e.g., psychoactivity, depression) which limit their therapeutic potential (Moreira et al., 2009). Therefore, it is fortunate that non-psychoactive treatments (including Fatty Acid Amide Hydrolase [FAAH] and monoacylglycerol lipase [MAGL] inhibitors) which act to enhance endogenous cannabinoid tone have also been effective in alleviating somatic withdrawal (see Table 1 for specific agents and original references). While inhibition of the MAGL enzyme (which elevates endogenous 2-arachidonoyl glycerol [2-AG]) produced the most robust effects (Ramesh et al., 2011; Gamage et al., 2015), inhibitors or KO mice of the catabolic FAAH enzyme (which elevates endogenous anandamide [AEA]) reduced a subset of withdrawal symptoms in most cases (Ramesh et al., 2011; Shahidi and Hasanein, 2011), whereas the AEA transport inhibitor, AM404, was without effect (Del Arco et al., 2002). However, unlike FAAH inhibitors, MAGL inhibitors have been found to produce cannabimimetic side effects (e.g., hypomotility, hyperreflexia; Long et al., 2009) and can lead to the development of dependence and tolerance with repeated administration (Schlosburg et al., 2010). In light of this, Ramesh et al. (2013) and Gamage et al. (2015) tested the combinations of low doses of FAAH and MAGL inhibitors, or dual FAAH/MAGL inhibitors, for their effectiveness in reducing withdrawal maximally without additional side effects. Indeed, this combination of catabolic enzyme inhibitors proved to be highly effective in reducing withdrawal (including jumping, paw flutters, head shakes, diarrhea, and weight loss) but was absent of adverse effects. Consequently, when considering pharmacological interventions that may aid in the treatment of somatic aspects of opiate withdrawal, dual FAAH/MAGL inhibition (at low doses) is most promising.
Affective Withdrawal
In animals, affective opioid withdrawal can be measured using a number of motivational paradigms including the conditioned place aversion (CPA), intracranial self-stimulation, and operant responding for food (Maldonado et al., 1996). In evaluating the role of the endocannabinoid system in affective opioid withdrawal, the CPA paradigm has been most commonly employed. This paradigm typically involves pairing naloxone-precipitated morphine withdrawal (in acutely or chronically dependent animals) with a specific environmental context, such that, upon re-exposure to this context in a drug-free state, animals will preferentially avoid the withdrawal paired context versus a context that was previously paired with a placebo saline injection (Sanchis-Segura and Spanagel, 2006). This avoidance of the withdrawal context is used as a measure of the intensity of the aversive affect experienced during opioid withdrawal, and can be used to assess the potential of pharmacological treatments to reduce affective withdrawal. Indeed, pharmacological treatments that are currently used in the treatment of opioid withdrawal (e.g., buprenorphine) are effective in reducing the establishment of avoidance behavior in this paradigm (Stinus et al., 2005). Also employed is the operant responding for food paradigm (Navarro et al., 2001). In this paradigm, a pharmacological treatment is deemed effective in reducing affective withdrawal if it is able to suppress a withdrawal-induced reduction in operant responding for food (Maldonado et al., 1996).
A review of the literature reveals a more complicated role for the endocannabinoid system in its ability to alleviate affective opioid withdrawal than was observed for somatic withdrawal (see Table 1); granted the studies conducted thus far are fairly limited. Indeed, as with somatic withdrawal, both activation and inhibition of the cannabinoid system has been found effective in reducing affective opioid withdrawal, but with greater inconsistencies. Contrary to somatic withdrawal, the cannabinoid agonist, Δ9THC, and the FAAH enzyme inhibitors, PF3845 and URB-597, tested were unable to modify the establishment of a naloxone-precipitated CPA (Wills et al., 2014; Gamage et al., 2015). However, while activation of the cannabinoid system via exogenous cannabinoid administration or endogenous elevation of AEA proved to have little efficacy, inhibition of MAGL activity and concomitant elevation of 2-AG showed mixed (Wills et al., 2014, 2016; Gamage et al., 2015) but more promising results. Given that these discrepant findings were obtained by different laboratories using different procedures, compounds (JZL-184 vs. MJN110), and species (mice vs. rats), more research into the potential of MAGL inhibition in reducing affective opioid withdrawal will be required in order to elucidate its role. Furthermore, while the only dual FAAH/MAGL inhibitor (SA-57) investigated was unable to modify a naloxone-precipitated CPA (Gamage et al., 2015), additional research into the ability of such compounds to reduce affective withdrawal should continue owing to their effectiveness in reducing somatic withdrawal with minor adverse effects (noted in previous section).
Although the deleterious reputation of cannabinoid antagonists/inverse agonists may limit their therapeutic potential (Bergman et al., 2008), blockade of the endocannabinoid system may be effective for reducing affective opioid withdrawal. Indeed, acute CB1R antagonism (with both AM251, and the neutral CB1 antagonists, AM4113, and AM6527) was effective in preventing the establishment of a naloxone-precipitated CPA (Wills et al., 2014, 2016). This is in contrast to the ability of acute CB1 antagonism to reliably reduce somatic withdrawal, but could be attributed to the fact that the CPA paradigm may provide a more sensitive measure of opioid withdrawal (Azar et al., 2003). Of special consideration is that both antagonists (which have been found to possess inverse agonist activity) and neutral antagonists (void of intrinsic activity; Sink et al., 2008) were effective in preventing the establishment of the CPA. This is particularly important since these ‘neutral’ antagonists have also been found to lack the adverse qualities attributed to typical cannabinoid antagonists (Bergman et al., 2008). While the only evaluations of chronic inhibition of the endocannabinoid system (CB1 KO mice) yielded mixed results (Ledent et al., 1999; Martin et al., 2000), chronic antagonism of the endocannabinoid system during the development of dependence using ‘neutral’ antagonists should be investigated given that acute treatments have been reported to precipitate both spontaneous affective and somatic withdrawal (Navarro et al., 1998, 2001).
Neurobiological Correlates and Potential Mechanisms of Interaction
Although the exact mechanisms through which the endocan nabinoid system is able to modulate opioid withdrawal remain to be elucidated, several theories have been proposed. Briefly, it has been hypothesized that cannabinoid and opioids interact through (1) reciprocal endogenous neurotransmitter/peptide release, (2) common signal-transduction pathways, and (3) receptor heterodimerization (see Parolaro et al., 2010; Scavone et al., 2013, for a review). An overview of these mechanisms in the brain regions most attributed to somatic and affective withdrawal is discussed.
Somatic Withdrawal
The neuroanatomical substrates most sensitive to the appearance of somatic opioid withdrawal include the periaqueductal gray (PAG) and locus coeruleus (LC; see Maldonado et al., 1996, for a review). Indeed, microinjection of methylnaloxonium, a hydrophilic opiate antagonist, into these regions was most sensitive in eliciting the opiate withdrawal syndrome, with active symptoms such as jumping being particularly prominent (Maldonado et al., 1992). In revisiting the withdrawal symptoms most commonly attenuated by cannabinoid modulation (e.g., jumping and paw tremors), it becomes apparent that these neuroanatomical substrates may represent regions through which cannabinoids interact with the opioid system to attenuate somatic withdrawal.
Much evidence indicates the PAG as a locus for cannabinoid–opioid interactions. Indeed, anatomical immunolabeling has co-localized the CB1R and μ-opioid receptor (MOR) within this region. Furthermore, 8% of immunoreactive MOR PAG neurons received immunoreactive CB1R appositions, indicating a role for presynaptic cannabinoid modulation (Wilson-Poe et al., 2012). In addition, sub-chronic CB1R agonist treatment with THC or AM356 was reported to increase proenkephalin mRNA levels in the PAG (Manzanares et al., 1998). A reciprocal effect following repeated morphine treatment is also suggested given the ability of the CB1R antagonist AM251 to increase the frequency of spontaneous miniature inhibitory postsynaptic currents (IPSCs) in morphine, but not saline treated animals, suggesting an elevation of endocannabinoid tone (Wilson-Poe et al., 2014). Chronic (up to 7 days) CB1R agonist treatment has also been reported to up-regulate MOR density (Viganò et al., 2005), with tolerance developing with prolonged (14 day) treatment (Corchero et al., 2004). Finally, chronic THC pre-opiate exposure or acute rimonabant treatment has been found to induce or attenuate Fos immunoreactivity in the PAG from acute heroin or morphine administration, respectively (Singh et al., 2004, 2005).
While the investigation of cannabinoid–opioid interaction in the LC is less extensive than in the PAG, a recent anatomical study confirmed the co-existence of MOR and CB1R immunoreactivity in somatodendritic compartments of catecholaminergic neurons. Additionally, as in the PAG, immunoreactive CB1R axon terminals formed synaptic contacts with MOR dendrites (Scavone et al., 2010). Ultimately, these findings suggest a role for cannabinoid–opioid interactions in the PAG and LC via common signal transduction mechanisms, or potential receptor heterodimerization given their close proximity and reports of this occurrence (Schoffelmeer et al., 2006). Additionally, as discussed above, interactions in endogenous neurotransmitter release are also evident in the PAG. These mechanisms of interaction correspond to the general ability of CB1R agonism or chronic CB1R antagonism to reduce symptoms of somatic opioid withdrawal.
Affective Withdrawal
The brain structures most sensitive to the motivating or affective component of opioid withdrawal include the nucleus accumbens (NAc) and the amygdala (see Maldonado et al., 1996, for a review). Indeed, microinjection of hydrophilic opiate antagonists into these regions was most sensitive in producing the establishment of a CPA (Stinus et al., 1990), and suppressing operant responding in morphine dependent rats (Koob et al., 1989). An evaluation of cannabinoid–opioid interactions within these regions also reveals these sites to be crucial in the ability of the cannabinoid system to modulate affective opioid withdrawal.
There is substantial research supporting cannabinoid–opioid interactions within the NAc. As in the PAG and LC, immunolabeling has revealed evidence for CB1R and MOR localization on the same neurons in the NAc shell and core, as well as expression on synaptically linked neurons (Pickel et al., 2004). Furthermore, CB1R and MOR allosterically interact in this region causing non-additive and synergistic effects on glutamate and GABA release, respectively. These effects were reversed by their respective antagonists, but antagonist co-administration blocked these antagonistic effects, suggesting the potential for G-protein coupled heterodimeric receptor complexes (Schoffelmeer et al., 2006). Consistent with these findings, reports also suggest CB1R and MOR synergy on cAMP/PKA signaling that is mediated through βγ dimers (Yao et al., 2006). Chronic opioid and cannabinoid administration has also been found to alter cannabinoid and opioid stimulated G-protein receptor coupling, respectively; though while cannabinoids enhance MOR binding (Viganò et al., 2005), opioids have produced more mixed effects on CB1R binding (Viganò et al., 2003, 2005; Fattore et al., 2007). Reports of changes in receptor densities have also been noted, with chronic opioid administration increasing CB1R density (Gonzalez et al., 2002), mRNA and protein levels (Ren et al., 2009), and cannabinoid agonist treatment increasing MOR density (Corchero et al., 2004; Fattore et al., 2007; Molaei et al., 2016). However, while cannabinoid agonists seem to consistently increase MOR density, the effects of CB1R gene deletion have been less consistent in reducing expressivity (Urigüen et al., 2005; Lane et al., 2010). Cannabinoid–opioid interactions on neuronal activation and dopamine release have also been reported in the NAc. Indeed, cannabinoid agonists increase while antagonists decrease Fos expression induced by acute morphine (Singh et al., 2004, 2005), and CB1R KO mice report decreased morphine-induced dopamine release (Mascia et al., 1999). Finally, cross-talk on endogenous neurotransmitter and peptide release has also been described, with increased AEA and decreased 2-AG content following chronic opioid administration, and increased Met-enkephalin immunoreactivity (Valverde et al., 2001), proenkephalin mRNA (Manzanares et al., 1998), and β-endorphin levels (Solinas et al., 2004) following acute or chronic cannabinoid (THC or CP-55,940) administration. These findings are consistent with the ability of cannabinoid agonists to decrease affective opioid withdrawal and provide evidence for interactions within the NAc at the neurotransmitter, receptor and signal transduction level.
A second region of interest attributed to mediating affective opioid withdrawal is the amygdala. Although there is less research investigating cannabinoid–opioid interactions within this region in comparison to the NAc, effects on receptor density and functionality have been noted. Specifically, acute and repeated cannabinoid agonist (THC or WIN 55,212-2) administration produced increases in MOR density and G-protein receptor coupling in the amygdala (Corchero et al., 2004; Fattore et al., 2007). Similar findings were also reported on the effects of chronic opioid administration on CB1R density and receptor coupling when considering the amygdala as a whole (Fattore et al., 2007), however, decreases in CB1R density have been described when analyzing the basolateral amygdala (BLA) individually (Gonzalez et al., 2002). In addition to receptor interactions, alterations in Fos immunoreactivity (Fos IR; a marker of neuronal activation) have also been noted. Indeed, acute treatment of the cannabinoid antagonist, rimonabant, attenuated acute morphine-induced Fos immunoreactivity in the amygdala as a whole (Singh et al., 2004), while investigations of the central nucleus of the amygdala (CeA) specifically revealed decreases in acute heroin-induced Fos IR following chronic THC pre-treatment (Singh et al., 2005), and additive effects on naloxone-induced Fos IR following acute THC exposure (Allen et al., 2003). Although the above studies do not make any direct comparisons between the BLA and CeA on cannabinoid–opioid interactions, a recent experiment suggests a functional double dissociation between these regions in the ability of the cannabinoid system to modulate affective opioid withdrawal. Indeed, cannabinoid agonism (via inhibition of MAGL activity with MJN110) within the BLA, while cannabinoid antagonism (AM251) within the CeA, is able to interfere with the establishment of a naloxone-precipitated CPA in acutely morphine dependent rats (Wills et al., 2016). Though the mechanisms mediating the dissociation between these regions were not investigated, this finding suggests potential differences in cannabinoid–opioid modulation within different sub-regions of the amygdala.
Conclusion
A review of the literature reveals that acute pharmacological activation or chronic inhibition of the cannabinoid system may interfere with opioid withdrawal. In particular, dual FAAH/MAGL enzyme inhibitors or neutral CB1R antagonists are most promising, though further investigations are necessary. In the central nervous system, cannabinoid–opioid interactions are abundant and present within neuroanatomical regions important in mediating opioid withdrawal including the amygdala, the NAc, the PAG, and the LC. However, while interactions have been described, more research on the mechanisms through which endocannabinoid modulation is able to attenuate opioid withdrawal will be required in order to gain a more cohesive understanding of its therapeutic potential.
Author Contributions
KW wrote the article; LP edited the article.
Funding
This research was funded by NSERC Grant #92057 to LP and Scholarship to KW.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Alavi, M. S., Hosseinzadeh, H., Shamsizadeh, A., and Roohbakhsh, A. (2016). The effect of O-1602, an atypical cannabinoid, on morphine-induced conditioned place preference and physical dependence. Pharmacol. Rep. 68, 592–597. doi: 10.1016/j.pharep.2015.12.009
Allen, K. V., McGregor, I. S., Hunt, G. E., Singh, M. E., and Mallet, P. E. (2003). Regional differences in naloxone modulation of Δ9-THC induced Fos expression in rat brain. Neuropharmacology 44, 264–272. doi: 10.1016/S0028-3908(02)00364-7
Azar, M. R., Jones, B. C., and Schulteis, G. (2003). Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology 170, 42–50. doi: 10.1007/s00213-003-1514-y
Bergman, J., Delatte, M. S., Paronis, C. A., Vemuri, K., Thakur, G. A., and Makriyannis, A. (2008). Some effects of CB1 antagonists with inverse agonist and neutral biochemical properties. Physiol. Behav. 93, 666–670. doi: 10.1016/j.physbeh.2007.11.007
Bhargava, H. N. (1976a). Effect of some cannabinoids on naloxone-precipitated abstinence in morphine-dependent mice. Psychopharmacology 49, 267–270. doi: 10.1007/BF00426828
Bhargava, H. N. (1976b). Inhibition of naloxone-induced withdrawal in morphine dependent mice by l-trans-Δ9-tetrahydrocannabinol. Eur. J. Pharmacol. 36, 259–262. doi: 10.1016/0014-2999(76)90283-1
Chesher, G. B., and Jackson, D. M. (1985). The quasi-morphine withdrawal syndrome: effect of cannabinol, cannabidiol and tetrahydrocannabinol. Pharmacol. Biochem. Behav. 23, 13–15. doi: 10.1016/0091-3057(85)90122-4
Cichewicz, D. L., and Welch, S. P. (2003). Modulation of oral morphine antinociceptive tolerance and naloxone-precipitated withdrawal signs by oral Delta 9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 305, 812–817. doi: 10.1124/jpet.102.046870
Corchero, J., Oliva, J. M., García-Lecumberri, C., Martin, S., Ambrosio, E., and Manzanares, J. (2004). Repeated administration with Δ9-tetrahydrocannabinol regulates μ-opioid receptor density in the rat brain. J. Psychopharmacol. 18, 54–58. doi: 10.1177/0269881104040237
Del Arco, I., Navarro, M., Bilbao, A., Ferrer, B., Piomelli, D., and Rodríguez De Fonseca, F. (2002). Attenuation of spontaneous opiate withdrawal in mice by the anandamide transport inhibitor AM404. Eur. J. Pharmacol. 454, 103–104. doi: 10.1016/S0014-2999(02)02483-4
Fattore, L., Viganò, D., Fadda, P., Rubino, T., Fratta, W., and Parolaro, D. (2007). Bidirectional regulation of mu-opioid and CB1-cannabinoid receptor in rats self-administering heroin or WIN 55,212-2. Eur. J. Neurosci. 25, 2191–2200. doi: 10.1111/j.1460-9568.2007.05470.x
Frederickson, R. C., Hewes, C. R., and Aiken, J. W. (1976). Correlation between the in vivo and an in vitro expression of opiate withdrawal precipitated by naloxone: their antagonism by l-(-)-delta9-tetrahydrocannabinol. J. Pharmacol. Exp. Ther. 199, 375–384.
Gamage, T. F., Ignatowska-Jankowska, B. M., Muldoon, P. P., Cravatt, B. F., Damaj, M. I., and Lichtman, A. H. (2015). Differential effects of endocannabinoid catabolic inhibitors on morphine withdrawal in mice. Drug Alcohol Depend. 146, 7–16. doi: 10.1016/j.drugalcdep.2014.11.015
Gellert, V. F., and Holtzman, S. G. (1978). Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J. Pharmacol. Exp. Ther. 205, 536–546.
Gerak, L. R., and France, C. P. (2016). Combined treatment with morphine and 9-tetrahydrocannabinol in rhesus monkeys: antinociceptive tolerance and withdrawal. J. Pharmacol. Exp. Ther. 357, 357–366. doi: 10.1124/jpet.115.231381
Gilbert, P. E. (1981). A comparison of THC, nantradol, nabilone, and morphine in the chronic spinal dog. J. Clin. Pharmacol. 21(8–9 Suppl.), 311S–319S. doi: 10.1002/j.1552-4604.1981.tb02609.x
Gonzalez, S., Fernandez, J., and Parolaro, D. (2002). Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB 1 receptor binding and mRNA levels. Drug Alcohol Depend. 66, 77–84. doi: 10.1016/S0376-8716(01)00186-7
Heishman, S. J., Stitzer, M. L., Bigelow, G. E., and Liebson, I. A. (1989). Acute opioid physical dependence in postaddict humans: naloxone dose effects after brief morphine exposure. J. Pharmacol. Exp. Ther. 248, 127–134.
Hine, B., Friedman, E., Torrelio, M., and Gershon, S. (1975a). Morphine-dependent rats: blockade of precipitated abstinence by tetrahydrocannabinol. Science 187, 443–445. doi: 10.1126/science.1167428
Hine, B., Friedman, E., Torrelio, M., and Gershon, S. (1975b). Tetrahydrocannabinol-attenuated abstinence and induced rotation in morphine-dependent rats: possible involvement of dopamine. Neuropharmacology 14, 607–610. doi: 10.1016/0028-3908(75)90128-8
Hine, B., Torrelio, M., and Gershon, S. (1975c). Attenuation of precipitated abstinence in methadone-dependent rats by delta 9-THC. Psychopharmacol. Commun. 1, 275–283.
Hine, B., Torrelio, M., and Gershon, S. (1975d). Differential effect of cannabinol and cannabidiol on THC-induced responses during abstinence in morphine-dependent rats. Res. Commun. Chem. Pathol. Pharmacol. 12, 185–188.
Hine, B., Torrelio, M., and Gershon, S. (1975e). Interactions between cannabidiol and Δ9-THC during abstinence in morphine-dependent rats. Life Sci. 17, 851–857. doi: 10.1016/0024-3205(75)90435-X
Jaffe, J. H. (1990). “Drug addiction and drug abuse,” in Goodman and Gilman’s the Pharmacological Basis of Therapeutics, ed. L. L. Brunton (New York, NY: McGraw-Hill Education), 522–573.
June, H. L., Stitzer, M. L., and Cone, E. (1995). Acute physical dependence: time course and relation to human plasma morphine concentrations. Clin. Pharmacol. Ther. 57. 270–280. doi: 10.1016/0009-9236(95)90152-3
Koob, G. F., Wall, T. L., and Bloom, F. E. (1989). Nucleus accumbens as a substrate for the aversive stimulus effects of opiate withdrawal. Psychopharmacology 98, 530–534. doi: 10.1007/BF00441954
Lane, D. A., Chan, J., Lupica, C. R., and Pickel, V. M. (2010). Cannabinoid-1 receptor gene deletion has a compartment-specific affect on the dendritic and axonal availability of μ-opioid receptors and on dopamine axons in the mouse nucleus accumbens. Synapse 64, 886–897. doi: 10.1002/syn.20807
Ledent, C., Valverde, O., Cossu, G., Petitet, F., Aubert, J. F., Beslot, F., et al. (1999). Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science 283, 401–404. doi: 10.1126/science.283.5400.401
Lichtman, A. H., Sheikh, S. M., Loh, H. H., and Martin, B. R. (2001). Opioid and cannabinoid modulation of precipitated withdrawal in delta(9)-tetrahydrocannabinol and morphine-dependent mice. J. Pharmacol. Exp. Ther. 298, 1007–1014.
Long, J. Z., Li, W., Booker, L., Burston, J. J., Kinsey, S. G., Schlosburg, J. E., et al. (2009). Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol. 5, 37–44. doi: 10.1038/nchembio.129
Maccarrone, M., Valverde, O., Barbaccia, M. L., Castañé, A., Maldonado, R., Ledent, C., et al. (2002). Age-related changes of anandamide metabolism in CB1 cannabinoid receptor knockout mice: correlation with behaviour. Eur. J. Neurosci. 15, 1178–1186. doi: 10.1046/j.1460-9568.2002.01957.x
Maldonado, R., Stinus, L., Gold, L. H., and Koob, G. F. (1992). Role of different brain structures in the expression of the physical morphine withdrawal syndrome. J. Pharmacol. Exp. Ther. 261, 669–677.
Maldonado, R., Stinus, L., and Koob, G. F. (1996). Neurobiological Mechanisms of Opiate Withdrawal. Austin, TX: R.G. Landes Company.
Manzanares, J., Corchero, J., Romero, J., Fernandez-Ruiz, J. J., Ramos, J. A., and Fuentes, J. A. (1998). Chronic administration of cannabinoids regulates proenkephalin mRNA levels in selected regions of the rat brain. Mol. Brain Res. 55, 126–132. doi: 10.1016/S0169-328X(97)00371-9
Martin, M., Ledent, C., Parmentier, M., Maldonado, R., and Valverde, O. (2000). Cocaine, but not morphine, induces conditioned place preference and sensitization to locomotor responses in CB1 knockout mice. Euro. J. Neurosci. 12, 4038–4046. doi: 10.1046/j.1460-9568.2000.00287.x
Mascia, M. S., Obinu, M. C., Ledent, C., Parmentier, M., Böhme, G. A., Imperato, A., et al. (1999). Lack of morphine-induced dopamine release in the nucleus accumbens of cannabinoid CB1 receptor knockout mice. Eur. J. Pharmacol. 383, R1–R2. doi: 10.1016/S0014-2999(99)00656-1
Mas-Nieto, M., Pommier, B., Tzavara, E. T., Caneparo, A., Le Fur, G., Roques, B. P., et al. (2001). Reduction of opioid dependence by the CB (1) antagonist SR141716A in mice: evaluation of the interest in pharmacotherapy of opioid addiction. Br. J. Pharmacol. 132, 1809–1816. doi: 10.1038/sj.bjp.0703990
Molaei, M., Fatahi, Z., Zaringhalam, J., and Haghparast, A. (2016). CB1 cannabinoid agonist (WIN55,212-2) within the basolateral amygdala induced sensitization to morphine and increased the level of μ-Opioid receptor and c-fos in the nucleus accumbens. J. Mol. Neurosci. 58, 446–455. doi: 10.1007/s12031-016-0716-9
Moreira, F. A., Grieb, M., and Lutz, B. (2009). Central side-effects of therapies based on CB1 cannabinoid receptor agonists and antagonists: focus on anxiety and depression. Best Pract. Res. Clin. Endocrinol. Metab. 23, 133–144. doi: 10.1016/j.beem.2008.09.003
Navarro, M., Carrera, M. R., Fratta, W., Valverde, O., Cossu, G., Fattore, L., et al. (2001). Functional interaction between opioid and cannabinoid receptors in drug self-administration. J. Neurosci. 21, 5344–5350.
Navarro, M., Chowen, C. A. J., Arco, I., Villanúa, M. A., Martin, Y., Roberts, A. J., et al. (1998). CB 1 cannabinoid receptor antagonist-induced opiate withdrawal in morphine-dependent rats. Neuropharmacology 9, 3397–3402.
Parolaro, D., Rubino, T., Vigano, D., Massi, P., Guidali, C., and Realini, N. (2010). Cellular mechanisms underlying the interaction between cannabinoid and opioid system. Curr. Drug Targets 11, 393–405. doi: 10.2174/138945010790980367
Pickel, V. M., Chan, J., Kash, T. L., Rodriguez, J. J., and MacKie, K. (2004). Compartment-specific localization of cannabinoid 1 (CB1) and mu-opioid receptors in rat nucleus accumbens. Neuroscience 127, 101–112. doi: 10.1016/j.neuroscience.2004.05.015
Ramesh, D., Gamage, T. F., Vanuytsel, T., Owens, R. A., Abdullah, R. A., Niphakis, M. J., et al. (2013). Dual inhibition of endocannabinoid catabolic enzymes produces enhanced antiwithdrawal effects in morphine-dependent mice. Neuropsychopharmacology 38, 1039–1049. doi: 10.1038/npp.2012.269
Ramesh, D., Ross, G. R., Schlosburg, J. E., Owens, R. A., Abdullah, R. A., Kinsey, S. G., et al. (2011). Blockade of endocannabinoid hydrolytic enzymes attenuates precipitated opioid withdrawal symptoms in mice. J. Pharmacol. Exp. Ther. 339, 173–185. doi: 10.1124/jpet.111.181370
Ren, Y., Whittard, J., Higuera-Matas, A., Morris, C. V., and Hurd, Y. L. (2009). Cannabidiol, a nonpsychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J. Neurosci. 29, 14764–14769. doi: 10.1523/JNEUROSCI.4291-09.2009
Rubino, T., Massi, P., Vigano, D., Fuzio, D., and Parolaro, D. (2000). Long-term treatment with SR141716A, the CB1 receptor antagonist, influences morphine withdrawal syndrom. Life Sci. 66, 2213–2219. doi: 10.1016/S0024-3205(00)00547-6
Sanchis-Segura, C., and Spanagel, R. (2006). Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol. 11, 2–38. doi: 10.1111/j.1369-1600.2006.00012.x
Scavone, J. L., Mackie, K., and Van Bockstaele, E. J. (2010). Characterization of cannabinoid-1 receptors in the locus coeruleus: relationship with mu-opioid receptors. Brain Res. 1312, 18–31. doi: 10.1016/j.brainres.2009.11.023
Scavone, J. L., Sterling, R. C., and Van Bockstaele, E. J. (2013). Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience 248, 637–654. doi: 10.1016/j.neuroscience.2013.04.034
Schlosburg, J. E., Blankman, J. L., Long, J. Z., Nomura, D. K., Pan, B., Kinsey, S. G., et al. (2010). Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat. Neurosci. 13, 1113–1119. doi: 10.1038/nn.2616
Schoffelmeer, A. N., Hogenboom, F., Wardeh, G., and De Vries, T. J. (2006). Interactions between CB1 cannabinoid and mu opioid receptors mediating inhibition of neurotransmitter release in rat nucleus accumbens core. Neuropharmacology 51, 773–781. doi: 10.1016/j.neuropharm.2006.05.019
Shahidi, S., and Hasanein, P. (2011). Behavioral effects of fatty acid amide hydrolase inhibition on morphine withdrawal symptoms. Brain Res. Bull. 86, 118–122. doi: 10.1016/j.brainresbull.2011.06.019
Singh, M. E., McGregor, I. S., and Mallet, P. E. (2005). Repeated exposure to Δ9-tetrahydrocannabinol alters heroin-induced locomotor sensitisation and Fos-immunoreactivity. Neuropharmacology 49, 1189–1200. doi: 10.1016/j.neuropharm.2005.07.008
Singh, M. E., Verty, A. N. A., Price, I., McGregor, I. S., and Mallet, P. E. (2004). Modulation of morphine-induced Fos-immunoreactivity by the cannabinoid receptor antagonist SR 141716. Neuropharmacology 47, 1157–1169. doi: 10.1016/j.neuropharm.2004.08.008
Sink, K. S., McLaughlin, P. J., Wood, J. A. T., Brown, C., Fan, P., Vemuri, V. K., et al. (2008). The novel cannabinoid CB1 receptor neutral antagonist AM4113 suppresses food intake and food-reinforced behavior but does not induce signs of nausea in rats. Neuropsychopharmacology 33, 946–955. doi: 10.1038/sj.npp.1301476
Solinas, M., Zangen, A., Thiriet, N., and Goldberg, S. R. (2004). β-Endorphin elevations in the ventral tegmental area regulate the discriminative effects of Δ-9-tetrahydrocannabinol. Eur. J. Neurosci. 19, 3183–3192. doi: 10.1111/j.0953-816X.2004.03420.x
Stinus, L., Cador, M., Zorrilla, E. P., and Koob, G. F. (2005). Buprenorphine and a CRF1 antagonist block the acquisition of opiate withdrawal-induced conditioned place aversion in rats. Neuropsychopharmacology 30, 90–98. doi: 10.1038/sj.npp.1300487
Stinus, L., Le Moal, M., and Koob, G. F. (1990). Nucleus accumbens and amygdala are possible substrates for the aversive stimulus effects of opiate withdrawal. Neuroscience 37, 767–773. doi: 10.1016/0306-4522(90)90106-E
Stotts, A. L., Dodrill, C. L., and Kosten, T. R. (2009). Opioid dependence treatment: options in pharmacotherapy. Expert Opin. Pharmacother. 10, 1727–1740. doi: 10.1517/14656560903037168
Trang, T., Ma, W., Chabot, J.-G., Quirion, R., and Jhamandas, K. (2006). Spinal modulation of calcitonin gene-related peptide by endocannabinoids in the development of opioid physical dependence. Pain 126, 256–271. doi: 10.1016/j.pain.2006.07.008
Urigüen, L., Berrendero, F., Ledent, C., Maldonado, R., and Manzanares, J. (2005). Kappa- and delta-opioid receptor functional activities are increased in the caudate putamen of cannabinoid CB1 receptor knockout mice. Eur. J. Neurosci. 22, 2106–2110. doi: 10.1111/j.1460-9568.2005.04372.x
Valverde, O., Noble, F., Beslot, F., Daugé, V., Fournié-Zaluski, M. C., and Roques, B. P. (2001). Δ9-tetrahydrocannabinol releases and facilitates the effects of endogenous enkephalins: reduction in morphine withdrawal syndrome without change in rewarding effect. Eur. J. Neurosci. 13, 1816–1824. doi: 10.1046/j.0953-816x.2001.01558.x
Vela, G., Ruiz-gayo, M., and Fuentes, J. A. (1995). Anandamide decreases naloxone-precipitated withdrawal signs in mice chronically treated with morphine. Neuropharmacology 34, 665–668. doi: 10.1016/0028-3908(95)00032-2
Viganò, D., Grazia Cascio, M., Rubino, T., Fezza, F., Vaccani, A., Di Marzo, V., et al. (2003). Chronic morphine modulates the contents of the endocannabinoid, 2-arachidonoyl glycerol, in rat brain. Neuropsychopharmacology 28, 1160–1167.
Viganò, D., Rubino, T., Vaccani, A., Bianchessi, S., Marmorato, P., Castiglioni, C., et al. (2005). Molecular mechanisms involved in the asymmetric interaction between cannabinoid and opioid systems. Psychopharmacology 182, 527–536. doi: 10.1007/s00213-005-0114-4
Wills, K. L., Petrie, G. N., Millett, G., Limebeer, C. L., Rock, E. M., Niphakis, M. J., et al. (2016). Double dissociation of monoacylglycerol lipase inhibition and CB1 antagonism in the central amygdala, basolateral amygdala, and the interoceptive insular cortex on the affective properties of acute naloxone-precipitated morphine withdrawal in rats. Neuropsychopharmacology 41, 1865–1873. doi: 10.1038/npp.2015.356
Wills, K. L., Vemuri, K., Kalmar, A., Lee, A., Limebeer, C. L., Makriyannis, A., et al. (2014). CB1 antagonism: interference with affective properties of acute naloxone-precipitated morphine withdrawal in rats. Psychopharmacology 231, 4291–4300. doi: 10.1007/s00213-014-3575-5
Wilson-Poe, A. R., Lau, B. K., and Vaughan, C. W. (2014). Repeated morphine treatment alters cannabinoid modulation of GABAergic synaptic transmission within the rat periaqueductal grey. Br. J. Pharmacol. 172, 681–690. doi: 10.1111/bph.12809
Wilson-Poe, A. R., Morgan, M. M., Aicher, S. A., and Hegarty, D. M. (2012). Distribution of CB1 cannabinoid receptors and their relationship with mu-opioid receptors in the rat periaqueductal gray. Neuroscience 213, 191–200. doi: 10.1016/j.neuroscience.2012.03.038
Yamaguchi, T., Hagiwara, Y., Tanaka, H., Sugiura, T., Waku, K., Shoyama, Y., et al. (2001). Endogenous cannabinoid, 2-arachidonoylglycerol, attenuates naloxone-precipitated withdrawal signs in morphine-dependent mice. Brain Res. 909, 121–126. doi: 10.1016/S0006-8993(01)02655-5
Yao, L., Mcfarland, K., Fan, P., Jiang, Z., and Ueda, T. (2006). Adenosine A2a blockade prevents synergy between μ-opiate and cannabinoid CB1 receptors and eliminates heroin-seeking behavior in addicted rats. Proc. Natl. Acad. Sci. U.S.A. 103, 7877–7882. doi: 10.1073/pnas.0602661103
Young, A. M., Katz, J. L., and Woods, J. H. (1981). Behavioral effects of levonantradol and nantradol in the rhesus monkey. J. Clin. Pharmacol. 21(8–9 Suppl.), 348S–360S. doi: 10.1002/j.1552-4604.1981.tb02614.x
Keywords: cannabinoid, opioid, withdrawal, animal, amygdala, nucleus accumbens, locus coeruleus, periaqueductal gray
Citation: Wills KL and Parker LA (2016) Effect of Pharmacological Modulation of the Endocannabinoid System on Opiate Withdrawal: A Review of the Preclinical Animal Literature. Front. Pharmacol. 7:187. doi: 10.3389/fphar.2016.00187
Received: 05 May 2016; Accepted: 13 June 2016;
Published: 28 June 2016.
Edited by:
Allyn C. Howlett, Wake Forest School of Medicine, USAReviewed by:
Avital Schurr, University of Louisville, USAJoseph F. Cheer, University of Maryland School of Medicine, USA
Copyright © 2016 Wills and Parker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Linda A. Parker, cGFya2VybEB1b2d1ZWxwaC5jYQ==
 Kiri L. Wills
Kiri L. Wills Linda A. Parker
Linda A. Parker