- 1Faculty of Medicine, Nursing and Health Sciences, School of Psychological Sciences, Monash University, Melbourne, VIC, Australia
- 2Department of Neuropsychology, Austin Health, Melbourne, VIC, Australia
- 3Faculty of Medicine, Dentistry and Health Sciences, Melbourne School of Psychological Sciences, The University of Melbourne, Melbourne, VIC, Australia
- 4Centre for Advanced Imaging, The University of Queensland, Brisbane, QLD, Australia
Spatial cognition is fundamental for survival in the topographically complex environments inhabited by humans and other animals. The hippocampus, which has a central role in spatial cognition, is characterized by high concentration of serotonin (5-hydroxytryptamine; 5-HT) receptor binding sites, particularly of the 1A receptor (5-HT1A) subtype. This review highlights converging evidence for the role of hippocampal 5-HT1A receptors in spatial learning and memory. We consider studies showing that activation or blockade of the 5-HT1A receptors using agonists or antagonists, respectively, lead to changes in spatial learning and memory. For example, pharmacological manipulation to induce 5-HT release, or to block 5-HT uptake, have indicated that increased extracellular 5-HT concentrations maintain or improve memory performance. In contrast, reduced levels of 5-HT have been shown to impair spatial memory. Furthermore, the lack of 5-HT1A receptor subtype in single gene knockout mice is specifically associated with spatial memory impairments. These findings, along with evidence from recent cognitive imaging studies using positron emission tomography (PET) with 5-HT1A receptor ligands, and studies of individual genetic variance in 5-HT1A receptor availability, strongly suggests that 5-HT, mediated by the 5-HT1A receptor subtype, plays a key role in spatial learning and memory.
Introduction
The idea that serotonin (5-hydroxytryptamine; 5-HT) is involved in learning and memory has gained traction in recent years, after having first been suggested in the 1980s (Altman and Normile, 1988). Early pharmacological studies mostly implicated spatial memory. More recent studies involving advanced methodologies such as neurotransmitter positron emission tomography (PET) and knockout mouse models have continued to link serotonin to spatial memory.
Spatial memory includes the ability to learn the topographical configuration of environments, to locate objects, to recall previously encountered locations, and to navigate within environments. Many day-to-day activities performed by animals and humans depend on spatial memory. Knowing where one is, where food and water resources are, and how to get to safety are examples of effective use of spatial memories that are essential for animal survival. Humans depend on their ability to remember the locations of objects in the environment on a daily basis, ranging from retrieving a mobile phone from a purse to making one's way to work and back home (McNamara, 2013).
At a clinical level, the study of spatial memory is of particular significance to several neurological disorders such as dementia of the Alzheimer's type where impairments in spatial cognition are a central feature. In addition, spatial memory, and particularly the ability to process and remember spatial descriptions of environments, has been linked to certain types of learning disabilities in children (Mammarella et al., 2014).
Functional neuroimaging studies show that spatial memory is largely mediated by mesial temporal areas (for example, Maguire et al., 1996b, 1997, 1998a,b; Burgess et al., 2001; Hartley et al., 2003), and within these areas, the hippocampus is a key structure for spatial memory. These regions are characterized by high concentration of the 5-HT1A receptor binding sites.
Involvement of the 5-HT1A receptor in cognition is undisputed. This receptor subtype has been suggested as a therapeutic target and neural marker of memory deficits (Meneses, 1999; Meneses and Perez-Garcia, 2007; Thomas, 2015). In this review, we argue that the 5-HT1A receptor plays a key role in spatial learning and memory, and we present evidence to support this proposition. We first consider the correspondence between the neuroanatomy of spatial memory and the 5-HT1A receptor distribution. We then review studies using various experimental methods that have illustrated the role of 5-HT1A receptors in spatial learning and memory.
Neuroanatomy of Spatial Learning and Memory
Research on spatial memory has consistently implicated a hippocampal brain network consisting of the hippocampus proper, the parahippocampal cortices, fornix, parietal cortex, anterior thalamic nuclei, frontal cortex, and the striatum. The critical role of the hippocampal system in spatial learning and memory was first highlighted by Brenda Milner's early observations of “heightened” spatial memory deficits following temporal lobe excision for the relief of epileptic seizures (Milner, 1958, p. 251). Evidence for the importance of the hippocampus system has continued to accumulate, including very recent findings using single-neuron recording in human entorhinal cortex during virtual navigation (Miller et al., 2015). In terms of possible brain mechanisms underlying spatial learning and memory, findings have indicated that the rat hippocampus contains “place cells,” and these cells exhibit location-specific activity (O'Keefe and Dostrovsky, 1971; O'Keefe and Speakman, 1987). This discovery led to the hypothesis that the hippocampus stores a cognitive map of the spatial layout of the environment (O'Keefe and Nadel, 1978). More than three decades later, in 2005, “grid cells” were found in the rat's entorhinal cortex, which is the chief gateway into the hippocampus (Hafting et al., 2005). Grid cells generate a coordinate system that allows exact positioning and pathfinding. Together with other cells in the entorhinal cortex that recognize the direction of the head of the animal and the border of the environment (“head-direction cells”; Taube, 1998), grid cells form networks with place cells in the hippocampus. Overall this circuitry constitutes a comprehensive positioning system, an inner global positioning system, or GPS, in the brain.
In addition to these cell recording studies, lesions and stimulation of the hippocampus in non-human primate (Parkinson et al., 1988; Angeli et al., 1993) and rodents (Morris et al., 1982; Buhot et al., 1991) were shown to impair spatial learning and memory. Similarly, in humans, medial temporal lesions, especially on the right side, have been shown to impair recall of spatial location of objects (Smith and Milner, 1981, 1989; Pigott and Milner, 1993; Bohbot et al., 1998; Smith et al., 2011), increase spatial memory errors (using the None-Box Maze, Abrahams et al., 1997, 1999), and impair performances on virtual reality topographical memory tasks (Spiers et al., 2001b).
More precise links between particular spatial memory functions and regions within the hippocampal network have been established in some studies. For example, early studies indicated lateralization of hippocampal involvement in memory, with the right medial temporal lobe predominantly associated with visuospatial recall (for example, Milner, 1965; Smith and Milner, 1981, 1989; Pigott and Milner, 1993; Abrahams et al., 1997; Maguire et al., 1997; Gleissner et al., 1998; Lv et al., 2014), and the left medial temporal lobe with verbal material recall (for example, Saling et al., 1993; Hermann et al., 1997; Martin et al., 2002; Lillywhite et al., 2007). In keeping with this idea, a patient with Pick's disease involving the left temporal lobe showed a complete dissociation between topographical memory and verbal memory (Maguire and Cipolotti, 1998), although more recent findings (for example, Maguire et al., 1996a,b; Grön et al., 2000; Spiers et al., 2001a; Astur et al., 2002; Glikmann-Johnston et al., 2008; Cánovas et al., 2011) support involvement of both the left and right medial temporal lobes in spatial learning and memory.
The cortices adjacent to the hippocampus, which provide the hippocampus with its main source of direct cortical input and output, have also been implicated in spatial learning and memory. For example, some studies indicated bilateral involvement of the parahippocampal gyri (Aguirre et al., 1996, 1998; Aguirre and D'Esposito, 1997; Epstein and Kanwisher, 1998; Mellet et al., 2000; Zeidman et al., 2012), whereas other studies indicate unilateral, predominantly right-sided involvement (Habib and Sirigu, 1987; Owen et al., 1996; Bohbot et al., 2000; Ploner et al., 2000). In terms of other regions of the hippocampal formation, in non-human primates, cells in the entorhinal cortex are active during the performance of a variation of the delayed matching to sample task (memory for objects) and the delayed matching to place task (memory for place) (Suzuki et al., 1997). Location-specific activity of neurons has also been recorded within the rat entorhinal cortex (Quirk et al., 1992). Furthermore, lesions to the entorhinal cortex in rats have been shown to result in deficits in acquisition and retention of the Eight-Arm Radial Maze and the Morris Water Maze (Cho and Jaffard, 1995; Nagahara et al., 1995; Davis et al., 2001; Devi et al., 2003). In humans, entorhinal stimulation applied during learning the locations of landmarks enhanced subsequent memory for these locations (Suthana et al., 2012). In a single-neuron recording study, entorhinal cortex neurons activated at multiple related areas of a virtual environment (Miller et al., 2015). Combined lesions of entorhinal and perirhinal cortices impaired rats' performance in spatial memory tasks (Otto et al., 1997; Kaut and Bunsey, 2001). In contrast, perirhinal lesions alone yielded inconsistent results, with some studies showing impaired performance in certain tests of spatial memory (Wiig and Bilkey, 1994a,b; Liu and Bilkey, 1998a,b,c, 1999, 2001), while in others spatial memory was spared (Glenn and Mumby, 1998; Bussey et al., 1999, 2001; Machin et al., 2002; Ramos, 2002, 2013; Moran and Dalrymple-Alford, 2003). Thus, involvement of the perirhinal cortex in spatial learning and memory may be related to the specific memory paradigm employed.
In the following section, we provide an overview of 5-HT synthesis, electrophysiology, and receptor distribution to illustrate the concordance between 5-HT receptor distribution and brain areas involved in spatial memory, focusing on the hippocampus (see Figure 1). Subsequently, we review the evidence that 5-HT, mediated by the 5-HT1A receptor, is involved in the modulation of spatial learning and memory.
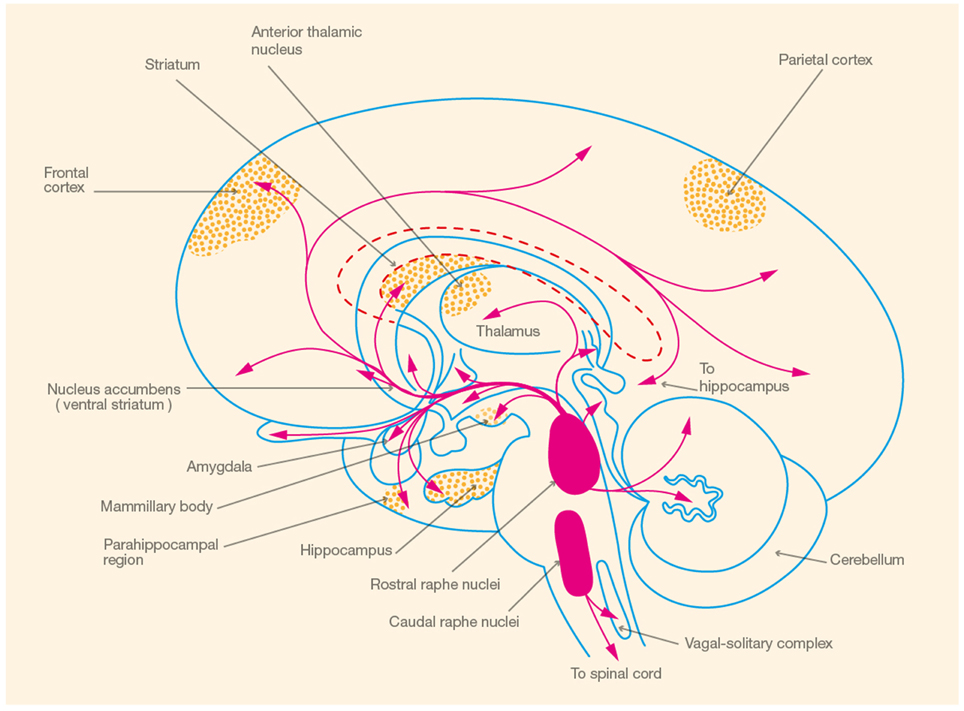
Figure 1. Schematic illustration of brain areas involved in spatial memory ( ) and the corresponding serotonergic pathways (
) and the corresponding serotonergic pathways ( ) (Adapted from Heimer, 1994, p. 227).
) (Adapted from Heimer, 1994, p. 227).
Serotonin (5-Hydroxytryptamine; 5-HT) and the 5-HT1A Receptor
Neurons that synthesize 5-HT are clustered in several nuclei along the midline of the brainstem, the most prominent of which are the raphe nuclei. Axons of these neurons innervate almost all regions of the central nervous system (CNS) and thus affect a great variety of behaviors, such as sleep/wake cycle, food intake, sexual behavior, emotional state, and cognitive processes, particularly learning and memory (Frazer and Hensler, 1994). 5-HT is synthesized from the amino acid tryptophan. The initial step in synthesis is the conversion of tryptophan to 5-hydroxytryptophan (5-HTP) by the enzyme tryptophan hydroxylase. Aromatic amino acid decarboxylase (AACD) then converts 5-HTP to 5-HT. 5-HT release occurs via exocytosis and is Ca2+-dependent. After 5-HT release, the actions of 5-HT in the synapse are terminated by 5-HT transporters, located on the plasma membrane of serotonergic neurons, which reuptake 5-HT back into the serotonergic neurons. 5-HT catabolism occurs by monamine oxidase A (MAO-A) (Frazer and Hensler, 1994; Adell et al., 2002).
Seven types of 5-HT receptors have been identified, termed 5-HT1-7, and among these are 14 distinct receptor subtypes. Each 5-HT receptor subtype has unique structural and pharmacological characteristics and a distinct distribution in the CNS. Of special interest is the 5-HT1A receptor, which is highly concentrated within the hippocampal system. 5-HT1A receptors are mainly concentrated in the limbic system, particularly the hippocampus (dentate gyrus and CA1), lateral septum, and amygdala, in cingulate and entorhinal cortices, and in the dorsal and median raphe nuclei, many of the regions implicated in spatial learning and memory. In contrast, only low concentrations are present in the striatum, substantia nigra, and the cerebellum (Barnes and Sharp, 1999; Lanfumey and Hamon, 2000). Autoradiography and immunohistochemical methods show that 5-HT1A receptors are located post-synaptically, as well as on the serotonergic neurons themselves in the raphe nuclei where they act as somatodendritic autoreceptors (Verge et al., 1985, 1986; Hoyer et al., 1986; Pazos et al., 1987; Zifa and Fillon, 1992; Hall et al., 1997; Lanfumey and Hamon, 2000). At the cellular level, 5-HT1A receptors reside on hippocampal pyramidal and granule cells (Lanfumey and Hamon, 2000). The highest density of these receptors are found in the granular layer (Hall et al., 1997).
In both hippocampus and dorsal raphe regions, 5-HT1A receptor activation results in neuronal hyperpolarization through the interaction with G-protein and the opening of K+ channels (Hamon et al., 1990; Frazer and Hensler, 1994; Lanfumey and Hamon, 2000). Since 5-HT1A receptors are located pre- and post-synaptically, endogenous 5-HT and/or 5-HT1A receptor agonists have different effects. 5-HT1A somatodentritic autoreceptors modulate synaptic transmission. When activated via endogenous 5-HT and/or 5-HT1A receptor agonists, they inhibit the serotonergic neuron on which they reside, and reduce 5-HT release. In contrast, at post-synaptic receptors such as occur in the hippocampus, 5-HT1A agonists facilitate 5-HT neurotransmission (Lanfumey and Hamon, 2000). Brain areas that are critical for spatial learning and memory, such as those that are part of the hippocampal formation, harbor the post-synaptic 5-HT1A receptors.
5-HT1A and Spatial Learning and Memory
Evidence to support a role for the 5-HT1A receptor in spatial learning and memory comes from a variety of experimental methods, including mouse “knockout” models, direct receptor activation and blockade, neurotransmitter PET imaging, genetic studies, and manipulation of 5-HT concentrations. We organize this review according to the primary experimental method used. Studies cited here are summarized in Table 1.
Knockout Mouse Models
Studies using genetically modified animals, particularly those of single gene deletions in knockout mice, provide the strongest evidence for the role of the 5-HT1A receptor in learning and memory (see Bert et al., 2008 for a review of learning and memory in 5-HT1A-receptor mutant mice). Sarnyai et al. (2000) assessed 5-HT1A-deficient mice on hippocampal-related spatial learning and memory tasks, the Morris Water Maze and the “Y” shape Maze. Their results showed that lack of 5-HT1A receptors is specifically associated with spatial learning and memory impairments. Wolff et al. (2004) demonstrated similar impairments in learning and retention of the Morris Water Maze in young-adult 5-HT1A knockout mice, but not in aged 5-HT1A knockout mice. The authors suggested that the reduced effect of the mutation in aged animals possibly reflects the lower efficacy of autoreceptors due to aging and/or a prevalence of hippocampal heteroreceptors.
5-HT1A Receptor Stimulation
5-HT1A agonists and antagonists modulate 5-HT neurotransmission and have been shown to directly alter spatial learning performance. Typically, antagonists have been found to impair spatial memory, whereas agonists are found to ameliorate the antagonist-induced spatial deficits, or allowed normal performance. For example, in a study by Micheau and Van Marrewijk (1999), intra-peritoneal administration of the 5-HT1A receptor agonist 8-hydroxy-2-(di-n-propylamino) tetraline (8-OH-DPAT) improved acquisition of a spatial discrimination task in an 8-arm radial maze. An intra-septal infusion of 8-OH-DPAT produced the same pattern of findings, although the improvement was less pronounced. Bertrand et al. (2000) showed contradictory findings, however, reporting that intra-septal infusion of 8-OH-DPAT impaired spatial learning. Administration of 8-OH-DPAT into the rat dorsal raphe had no effect on Delayed Non-Matching to Position (spatial working memory) task performance at any dose. In comparison, administration of the same compound into the median raphe improved performance accuracy. When 8-OH-DPAT was administered into the dorsal hippocampus, however, it produced a small impairment in performance (Warburton et al., 1997). 8-OH-DPAT also impaired performance on a water maze task (Carli et al., 1995) and on the eight-arm radial maze (Egashira et al., 2006). These findings demonstrate different effect for pre- and post-synaptic 5-HT1A receptor stimulation on spatial learning and memory tasks.
Additional evidence for the role of 5-HT1A receptor agonists in spatial memory comes from animal models of traumatic brain injury (TBI). In this model, animals are subjected to controlled cortical lesion to mimic TBI, and then memory is examined at different time points following injury and after administration of 5-HT1A agonists. These studies showed that TBI-induced spatial memory deficits are attenuated by treatment with the 5-HT1A receptor agonist buspirone (Olsen et al., 2012) and 8-OH-DPAT (Cheng et al., 2008). Furthermore, a combined therapeutic regimen of buspirone and environmental enrichment was found to be more effective than either alone in enhancing spatial learning in brain injured pediatric rats (Monaco et al., 2014).
Imaging Serotonergic Neurotransmission
Because the 5-HT1A receptor plays an important role in a range of physiological processes and in the pathophysiology of a variety of psychiatric and neurodegenerative disorders, synthesis of 5-HT1A receptor agents has been carried out primarily for their therapeutic potential. In recent years, more than 20 compounds have been labeled with carbon-11, fluorine-18, or iodine-123 for imaging and quantification of the 5-HT1A receptor with PET and SPECT (for review see Passchier and Van Waarde, 2001). The most successful radioligands thus far are [carbonyl-11C] WAY-100635 (WAY), [carbonyl-11C]desmethyl-WAY 100635 (DWAY), 2′-methoxyphenyl-(N-2′-pyridinyl)-p-[18F]fluoro-benzamidoethylpiperazine ([18F]MPPF), and [11C]robalzotan (NAD-299) (Passchier and Van Waarde, 2001). To the best of our knowledge, the only study that examined 5-HT1A receptor density and spatial learning and memory (i.e., object-location, navigation, and floor plan drawing) in humans using the PET ligand [18F]MPPF was recently published by our group (Glikmann-Johnston et al., 2015). In this study, healthy participants performed spatial virtual environment tasks during PET scanning. We found an association between hippocampal asymmetry in [18F]MPPF binding and performance on the object-location task. A lower binding potential in the right vs. the left hippocampus was related to better memory performance. This finding indicates that reduced right vs. left hippocampal 5-HT1A receptor availability enhances object-place associative memory. Although not within the scope of this review, it is important to note that Theodore et al. (2012) used similar experimental methodology in verbal memory using the 18FCWAY PET ligand. In their study, reduced left hippocampal 5-HT1A receptor binding in temporal lobe epilepsy (TLE) patients was related to delayed auditory verbal memory impairment, independent of the side of the epileptic focus. More cognitive serotonergic imaging studies are needed to build up the evidence for the role of 5-HT1A receptor in fundamental components of human spatial memory.
Genetic Variance in 5-HT1A Receptor Availability
Congenital differences in 5-HT1A receptor availability were found to be related to spatial memory, specifically length variations in the serotonin-transporter-gene-linked polymorphic region (5-HTTLPR). 5-HTTLPR is a 44-base pair insertion/deletion functional polymorphism in the promotor region of the serotonin transporter (5-HTT) gene (Lesch et al., 1996). This polymorphism produces two common alleles designated long (L) and short (S), and was found to affect 5-HT1A receptor availability (David et al., 2005). Human (Roiser et al., 2006, 2007) and primate (Jedema et al., 2010) carriers of S allele demonstrated superior performance compared to L carriers on a variety of cognitive tasks, including hippocampal-dependent visual memory tasks (a computerized version of the Block Design subtest of the Wechsler Adult Intelligence Test and the CANTAB Pattern Recognition Memory and Delayed Match to Sample).
Manipulations of 5-HT Levels
Pharmacological alterations of 5-HT concentrations, by altering either 5-HT release or reuptake, have been shown to influence spatial memory. Overall, increased extracellular 5-HT concentrations maintain or improve memory performance, and reduced levels of the neurotransmitter impair spatial memory. Changes in 5-HT release are thought to indirectly stimulate post-synaptic 5-HT1A receptors, which reside on areas important to spatial learning and memory, thereby affecting memory function (Lesch et al., 1996; Kuypers and Ramaekers, 2005). Support for this hypothesis is found in a study by du Jardin et al. (2014) with the use of parachlorophenylalanine (PCPA). This compound inhibits tryptophan hydroxylase, and thus reduces 5-HT synthesis. In their study, PCPA induced 5-HT depletion in rats and caused memory deficits on object recognition and Y-maze spontaneous alternation tests. The selective 5-HT1A receptor agonist flesinoxan significantly occupied 5-HT1A receptors and restored PCPA-induced memory deficits in both tests. Although other agents had similar effects on spatial memory function (e.g., 3,4-methylenedioxymethamphetamine/MDMA: Fox et al., 2000; Skelton et al., 2006; Vorhees et al., 2007; Fisk et al., 2011; D-fenfluramine: Morford et al., 2002; methamphetamine: Vorhees et al., 1994, 2000, 2008; Schröder et al., 2003), studies to date did not involve the 5-HT1A receptor directly. Even though the 5-HT1A receptor is the most abundant in the hippocampus, it is not possible to exclude other receptor subtypes that 5-HT stimulate in this area (5-HT2A, 5-HT6, and 5-HT7), and that may have an effect on spatial memory.
Conclusion
The findings reviewed here provide converging evidence in support of the hypothesis that 5-HT, mediated by the 5-HT1A receptor, plays a key role in hippocampal-dependent spatial memory in animals and humans. Strong evidence comes from knockout mouse models. These studies have shown that 5-HT1A receptor knockouts are specifically associated with deficits in performance on spatial memory tasks. A variety of agonists and antagonists active at the 5-HT1A receptor modulate 5-HT neurotransmission and induce a change in spatial learning. Blockade of the 5-HT1A receptor impairs spatial memory, while receptor activation ameliorates antagonist-induced spatial memory deficits. Another line of evidence emerges from studies that vary neurotransmitter levels pharmacologically. Typically, increased 5-HT extracellular concentrations maintain or improve memory performance, and reduction in neurotransmitter levels impairs spatial memory.
Recent advances in human neurotransmitter research methods allow for more direct quantification of 5-HT1A receptor availability during spatial learning and memory. Initial results from neuroimaging studies with the use of neurotransmitter PET indicate the contribution of endogenous serotonin release or 5-HT1A receptor density to spatial memory, particularly to the ability to recall the location of objects in the environment (Glikmann-Johnston et al., 2015). The mapping of the human genome provides further evidence at the individual person level for the association between 5-HT1A receptor density and spatial memory.
Theories of hippocampal involvement in spatial memory include: (a) the cognitive map theory of O'Keefe and Nadel (1978); (b) the theory proposed by Olton and colleagues (Olton et al., 1979; Olton and Paras, 1979), in which the hippocampus is crucial for working memory; and, (c) the theory that attributes a binding mechanism to the hippocampus to form spatial memories such as object location (for example, Chalfonte et al., 1996; Eichenbaum et al., 1996). The evidence reviewed in this paper involving 5-HT, particularly the 1A receptor subtype, and spatial memory is further supported by the well-established notion of the involvement of the hippocampus in spatial memory function.
A substantial number of studies have examined the role of 5-HT in spatial learning and memory and have demonstrated, particularly in animals, a strong relation between 5-HT and spatial memory. Yet several significant questions remain. We suggest that additional research is needed to clarify the relationship between 5-HT1A receptor modulation and specific aspects of spatial memory, including object location and spatial frames of reference, allocentric vs. egocentric representations, and navigation and episodic memory within a topographical framework (Burgess et al., 2002; Burgess, 2008). Also, research is needed into how the serotonergic system interacts with other major neurotransmitter systems, including the acetylcholineric system, to modulate spatial memory.
For patients with damage to the temporal lobes due to progressive pathology such as Alzheimer's disease, impairments of spatial memory are often the first symptoms reported. The idea that hippocampal 5-HT1A receptor plays a key role in spatial learning and memory may be informative for early intervention strategies, and for improving patient outcomes in diseases affecting the temporal lobes.
Author Contributions
YG-J, MS, DR, and JS wrote the article, reviewed the article, and approved the final version for publication.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abrahams, S., Morris, R. G., Polkey, C. E., Jarosz, J. M., Cox, T. C. S., Graves, M., et al. (1999). Hippocampal involvement in spatial and working memory: a structural MRI analysis of patients with unilateral mesial temporal lobe sclerosis. Brain Cogn. 41, 39–65. doi: 10.1006/brcg.1999.1095
Abrahams, S., Pickering, A., Polkey, C. E., and Morris, R. G. (1997). Spatial memory deficits in patients with unilateral damage to the right hippocampal formation. Neuropyschologia 35, 11–24. doi: 10.1016/S0028-3932(96)00051-6
Adell, A., Celada, P., Abellán, M. T., and Artigas, F. (2002). Origin and functional role of the extracellular serotonin in the midbrain raphe nuclei. Brain Res. Rev. 39, 154–180. doi: 10.1016/S0165-0173(02)00182-0
Aguirre, G. K., and D'Esposito, M. (1997). Environmental knowledge is subserved by separable dorsal/ventral neural areas. J. Neurosci. 17, 2512–2518.
Aguirre, G. K., Detre, J. A., Alsop, D. C., and D'Esposito, M. (1996). The parahippocampus subserves topographical learning in man. Cereb. Cortex 6, 823–829. doi: 10.1093/cercor/6.6.823
Aguirre, G. K., Zarahn, E., and D'Esposito, M. (1998). Neural components of topographical representation. Proc. Natl. Acad. Sci. U.S.A. 95, 839–846. doi: 10.1073/pnas.95.3.839
Altman, H. J., and Normile, H. J. (1988). What is the nature of the role of the serotonergic nervous system in learning and memory: prospects for development of an effective treatment strategy for senile dementia. Neurobiol. Aging 9, 627–638. doi: 10.1016/S0197-4580(88)80124-6
Angeli, S. J., Murray, E. A., and Mishkin, M. (1993). Hippocampectomized monkeys can remember one place but not two. Neuropsychologia 31, 1021–1030. doi: 10.1016/0028-3932(93)90030-4
Astur, R. S., Taylor, L. B., Mamelak, A. N., Philpott, L., and Sutherland, R. J. (2002). Humans with hippocampus damage display severe spatial memory impairments in a virtual Morris water task. Behav. Brain Res. 132, 77–84. doi: 10.1016/S0166-4328(01)00399-0
Barnes, N. M., and Sharp, T. (1999). A review of central 5-HT receptors and their function. Neuropharmacology 38, 1083–1152. doi: 10.1016/S0028-3908(99)00010-6
Bert, B., Fink, H., Rothe, J., Walstab, J., and Bönisch, H. (2008). Learning and memory in 5-HT(1A)-receptor mutant mice. Behav. Brain Res. 195, 78–85. doi: 10.1016/j.bbr.2008.02.028
Bertrand, F., Lehmann, O., Lazarus, C., Jeltsch, H., and Cassel, J. C. (2000). Intraseptal infusions of 8-OH-DPAT in the rat impairs water-maze performances: effects on memory or anxiety? Neurosci. Lett. 279, 45–48. doi: 10.1016/s0304-3940(99)00948-9
Bohbot, V. D., Allen, J. J. B., and Nadel, L. (2000). Memory deficits characterized by patterns of lesions to the hippocampus and parahippocampal cortex. Ann. N.Y. Acad. Sci. 911, 355–368. doi: 10.1111/j.1749-6632.2000.tb06737.x
Bohbot, V. D., Kalina, M., Stepankova, K., Spackova, N., Petrides, M., and Nadel, L. (1998). Spatial memory deficits in patients with lesions to the right hippocampus and to the right parahippocampal cortex. Neuropsychologia 36, 1217–1238. doi: 10.1016/S0028-3932(97)00161-9
Buhot, M. C., Chapuis, N., Scardigli, P., and Herrmann, T. (1991). Spatial problem-solving in a wheel-shaped maze: quantitative and qualitative analyses of the behavioural changes following damage to the hippocampus in the rat. Behav. Brain Res. 44, 67–79. doi: 10.1016/S0166-4328(05)80240-2
Burgess, N. (2008). Spatial cognition and the brain. Ann. N.Y. Acad. Sci. 1124, 77–97. doi: 10.1196/annals.1440.002
Burgess, N., Maguire, E. A., and O'Keefe, J. (2002). The human hippocampus and spatial and episodic memory. Neuron 35, 625–641. doi: 10.1016/S0896-6273(02)00830-9
Burgess, N., Maguire, E. A., Spiers, H. J., and O'Keefe, J. (2001). A temporoparietal and prefrontal network for retrieving the spatial context of lifelike events. Neuroimage 14, 439–453. doi: 10.1006/nimg.2001.0806
Bussey, T. J., Dias, R., Amin, E., Muir, J. L., and Aggleton, J. P. (2001). Perirhinal cortex and place-object conditional learning in the rat. Behav. Neurosci. 115, 776–785. doi: 10.1037/0735-7044.115.4.776
Bussey, T. J., Muir, J. L., and Aggleton, J. P. (1999). Functionally dissociating aspects of event memory: the effects of combined perirhinal and postrhinal cortex lesions on object and place memory in the rat. J. Neurosci. 19, 495–502.
Cánovas, R., León, I., Serrano, P., Roldán, M. D., and Cimadevilla, J. M. (2011). Spatial navigation impairment in patients with refractory temporal lobe epilepsy: evidence from a new virtual reality-based task. Epilepsy Behav. 22, 364–369. doi: 10.1016/j.yebeh.2011.07.021
Carli, M., Luschi, R., Garofalo, P., and Samanin, R. (1995). 8-OH-DPAT impairs spatial but not visual learning in a water maze by stimulating 5-HT1A receptors in the hippocampus. Behav. Brain Res. 67, 67–74. doi: 10.1016/0166-4328(94)00105-O
Chalfonte, B. L., Verfaellie, M., Johnson, M. K., and Reiss, L. (1996). Spatial location memory in amnesia: binding item and location information under incidental and intentional encoding conditions. Memory 4, 591–614. doi: 10.1080/741940998
Cheng, J. P., Hoffman, A. N., Zafonte, R. D., and Kline, A. E. (2008). A delayed and chronic treatment regimen with the 5-HT1A receptor agonist 8-OH-DPAT after cortical impact injury facilitates motor recovery and acquisition of spatial learning. Behav. Brain Res. 194, 79–85. doi: 10.1016/j.bbr.2008.06.025
Cho, Y. H., and Jaffard, R. (1995). Spatial location learning in mice with ibotenate lesions of entorhinal cortex or subiculum. Neurobiol. Learn. Mem. 64, 285–290. doi: 10.1006/nlme.1995.0011
David, S. P., Venkatesha Murthy, N., Rabiner, E. A., Munafo, M. R., Johnstone, E. C., Jacob, R., et al. (2005). A functional genetic variation of the serotonin (5-HT) transporter affects 5-HT1A receptor binding in humans. J. Neurosci. 25, 2586–2590. doi: 10.1523/JNEUROSCI.3769-04.2005
Davis, A. E., Gimenez, A. M., and Therrien, B. (2001). Effects of entorhinal cortex lesions on sensory integration and spatial learning. Nurs. Res. 50, 77–85. doi: 10.1097/00006199-200103000-00003
Devi, L., Diwakar, L., Raju, T. R., and Kutty, B. M. (2003). Selective neurodegeneration of hippocampus and entorhinal cortex correlates with spatial learning impairments in rats with bilateral ibotenate lesions of ventral subiculum. Brain Res. 960, 9–15. doi: 10.1016/S0006-8993(02)03699-5
du Jardin, K. G., Jensen, J. B., Sanchez, C., and Pehrson, A. L. (2014). Vortioxetine dose-dependently reverses 5-HT depletion-induced deficits in spatial working and object recognition memory: a potential role for 5-HT1A receptor agonism and 5-HT3 receptor antagonism. Eur. Neuropsychopharmacol. 24, 160–171. doi: 10.1016/j.euroneuro.2013.07.001
Egashira, N., Yano, A., Ishigami, N., Mishima, K., Iwasaki, K., Fujioka, M., et al. (2006). Investigation of mechanisms mediating 8-OH-DPAT-induced impairment of spatial memory: involvement of 5-HT1A receptors in the dorsal hippocampus in rats. Brain Res. 1069, 54–62. doi: 10.1016/j.brainres.2005.10.103
Eichenbaum, H., Schoebaum, G., Young, B., and Bunsey, M. (1996). Functional organization of the hippocampal memory system. Proc. Natl. Acad. Sci. U.S.A. 93, 13500–13507. doi: 10.1073/pnas.93.24.13500
Epstein, R., and Kanwisher, N. (1998). A cortical representation of the local visual environment. Nature 392, 598–601. doi: 10.1038/33402
Fisk, J. E., Montgomery, C., and Hadjiefthyvoulou, F. (2011). Visuospatial working memory impairment in current and previous ecstasy/polydrug users. Hum. Psychopharmacol. 26, 313–321. doi: 10.1002/hup.1207
Fox, H. C., Parrott, A. C., and Turner, J. J. D. (2000). Heavy MDMA (“ecstasy”) users: selective performance deficits on the Cambridge Neuropsychological Test Automated Battery (CANTAB). Int. J. Neuropsychopharmacol. 3, S325. doi: 10.1017/S1461145700009998
Frazer, A., and Hensler, J. G. (1994). “Serotonin,” in Basic Neurochemistry, 5th Edn., eds G. J. Siegel, B. W. Agranoff, R. W. Albers, and P. B. Molinoff (New York, NY: Raven Press), 283–308.
Gleissner, U., Helmstaedter, C., and Elger, C. E. (1998). Right hippocampal contribution to visual memory: a presurgical and postsurgical study in patients with temporal lobe epilepsy. J. Neurol. Neurosurg. Psychiatr. 65, 665–669. doi: 10.1136/jnnp.65.5.665
Glenn, M. J., and Mumby, D. G. (1998). Place memory is intact in rats with perirhinal cortex lesions. Behav. Neurosci. 112, 1353–1365. doi: 10.1037/0735-7044.112.6.1353
Glikmann-Johnston, Y., Saling, M. M., Chen, J., Cooper, K. A., Beare, R. J., and Reutens, D. C. (2008). Structural and functional correlates of unilateral mesial temporal lobe spatial memory impairment. Brain 131, 3006–3018. doi: 10.1093/brain/awn213
Glikmann-Johnston, Y., Saling, M. M., Chen, J., O'Keefe, G., Gong, S., Tochon-Danguy, H., et al. (2015). Hippocampal 5-HT receptor binding is related to object-location memory in humans. Brain Struct. Funct. 220, 559–570. doi: 10.1007/s00429-013-0675-7
Grön, G., Wunderlich, A. P., Spitzer, M., Tomczak, R., and Riepe, M. W. (2000). Brain activation during human navigation: gender-different neural networks as substrate of performance. Nat. Neurosci. 3, 404–408. doi: 10.1038/73980
Habib, M., and Sirigu, A. (1987). Pure topographical disorientation: a definition and anatomical basis. Cortex 23, 73–85. doi: 10.1016/S0010-9452(87)80020-5
Hafting, T., Fyhn, M., Molden, S., Moser, M. B., and Moser, E. I. (2005). Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806. doi: 10.1038/nature03721
Hall, H., Lundkvist, C., Halldin, C., Farde, L., Pike, V. W., McCarron, J. A., et al. (1997). Autoradiographic localization of 5-HT1A receptors in the post-mortem human brain using [3H]WAY-100635 and [11C]WAY-100635. Brain Res. 745, 96–108. doi: 10.1016/S0006-8993(96)01131-6
Hamon, M., Gozlan, H., El Mestikawy, S., Emerit, M. B., Bolanos, F., and Schechter, L. (1990). The central 5-HT1A receptors: pharmacological, biochemical, functional, and regulatory properties. Ann. N.Y. Acad. Sci. 600, 114–131. doi: 10.1111/j.1749-6632.1990.tb16877.x
Hartley, T., Maguire, E. A., Spiers, H. J., and Burgess, N. (2003). The well-worn route and the path less traveled: distinct neural bases of route following and wayfinding in humans. Neuron 37, 877–888. doi: 10.1016/S0896-6273(03)00095-3
Heimer, L. (1994). The Human Brain and Spinal Cord: Functional Neuroanatomy and Dissection Guide. New York, NY: Springer-Verlag.
Hermann, B. P., Seidenberg, M., Schoenfeld, J., and Davies, K. (1997). Neuropsychological characteristics of the syndrome of mesial temporal lobe epilepsy. Arch. Neurol. 54, 369–376. doi: 10.1001/archneur.1997.00550160019010
Hoyer, D., Pazos, A., Probst, A., and Palacios, J. M. (1986). Serotonin receptors in the human brain - I. characterization and autoradiographic localization of 5-HT1A recognition sites. apparent absence of 5-HT1B recognition sites. Brain Res. 376, 85–96. doi: 10.1016/0006-8993(86)90902-9
Jedema, H. P., Gianaros, P. J., Geer, P. J., Kerr, D. D., Liu, S., Higley, J. D., et al. (2010). Cognitive impact of genetic variation of the serotonin transporter in primates is associated with differences in brain morphology rather than serotonin neurotransmission. Mol. Psychiatry 15, 512–522. doi: 10.1038/mp.2009.90
Kaut, K. P., and Bunsey, M. D. (2001). The effects of lesions to the rat hippocampus or rhinal cortex on olfactory and spatial memory: retrograde and anterograde findings. Cogn. Affect. Behav. Neurosci. 1, 270–286. doi: 10.3758/CABN.1.3.270
Kuypers, K. P., and Ramaekers, J. G. (2005). Transient memory impairment after acute dose of 75mg 3.4-Methylene-dioxymethamphetamine. J. Psychopharmacol. 19, 633–639. doi: 10.1177/0269881105056670
Lanfumey, L., and Hamon, M. (2000). Central 5-HT1A receptors: regional distribution and functional characteristics. Nucl. Med. Biol. 27, 429–435. doi: 10.1016/S0969-8051(00)00107-4
Lesch, K. P., Bengel, D., Heils, A., Sabol, S. Z., Greenberg, B. D., Petri, S., et al. (1996). Association of anxiety-related traits with a polymorphism in the serotonin transporter gene regulatory region. Science 274, 1527–1531. doi: 10.1126/science.274.5292.1527
Lillywhite, L. M., Saling, M. M., Briellmann, R. S., Weintrob, D. L., Pell, G. S., and Jackson, G. D. (2007). Differential contributions of the hippocampus and rhinal cortices to verbal memory in epilepsy. Epilepsy Behav. 10, 553–559. doi: 10.1016/j.yebeh.2007.03.002
Liu, P., and Bilkey, D. K. (1998a). Excitotoxic lesions centered on perirhinal cortex produce delay-dependent deficits in a test of spatial memory. Behav. Neurosci. 112, 512–524.
Liu, P., and Bilkey, D. K. (1998b). Lesions of perirhinal cortex produce spatial memory deficits in the radial maze. Hippocampus 8, 114–121.
Liu, P., and Bilkey, D. K. (1998c). Perirhinal cortex contributions to performance in the morris water maze. Behav. Neurosci. 112, 304–315.
Liu, P., and Bilkey, D. K. (1999). The effect of excitotoxic lesions centered on the perirhinal cortex in two versions of the radial arm maze task. Behav. Neurosci. 113, 672–682. doi: 10.1037/0735-7044.113.4.672
Liu, P., and Bilkey, D. K. (2001). The effect of excitotoxic lesions centered on the hippocampus or perirhinal cortex in object recognition and spatial memory tasks. Behav. Neurosci. 115, 94–111. doi: 10.1037/0735-7044.115.1.94
Lv, Z. X., Huang, D. H., Ye, W., Chen, Z. R., Huang, W. L., and Zheng, J. O. (2014). Alteration of functional connectivity within visuospatial working memory-related brain network in patients with right temporal lobe epilepsy: a resting-state fMRI study. Epilepsy Behav. 35, 64–71. doi: 10.1016/j.yebeh.2014.04.001
Machin, P., Vann, S. D., Muir, J. L., and Aggleton, J. P. (2002). Neurotoxic lesions of the rat perirhinal cortex fail to disrupt the acquisition or performance of tests of allocentric spatial memory. Behav. Neurosci. 116, 232–240. doi: 10.1037/0735-7044.116.2.232
Maguire, E. A., Burgess, N., Donnett, J. G., Frackowiak, R. S. J., Frith, C. D., and O'Keefe, J. (1998a). Knowing where and getting there: a human navigation network. Science 280, 291–294.
Maguire, E. A., Burke, T., Phillips, J., and Staunton, H. (1996a). Topographical disorientation following unilateral temporal lobe lesions in humans. Neuropsychologia 34, 993–1001.
Maguire, E. A., and Cipolotti, L. (1998). Selective sparing of topographical memory. J. Neurol. Neurosurg. Psychiatr. 65, 903–909. doi: 10.1136/jnnp.65.6.903
Maguire, E. A., Frackowiak, R. S. J., and Frith, C. D. (1996b). Learning to find your way: a role for the human hippocampal formation. Proc. Biol. Sci. 263, 1745–1750.
Maguire, E. A., Frackowiak, R. S. J., and Frith, C. D. (1997). Recalling routes around London: activation of the right hippocampus in taxi drivers. J. Neurosci. 17, 7103–7110.
Maguire, E. A., Frith, C. D., Burgess, N., Donnett, J. G., and O'Keefe, J. (1998b). Knowing where things are: parahippocampal involvement in encoding object location in virtual large-scale space. J. Cogn. Neurosci. 10, 61–76.
Mammarella, I. C., Meneghetti, C., Pazzaglia, F., and Cornoldi, C. (2014). Memory and comprehension deficits in spatial descriptions of children with non-verbal and reading disabilities. Front. Psychol. 5:1534. doi: 10.3389/fpsyg.2014.01534
Martin, R. C., Kretzmer, T., Palmer, C., Sawrie, S., Knowlton, R., Faught, E., et al. (2002). Risk to verbal memory following anterior temporal lobectomy in patients with severe left-sided hippocampal sclerosis. Arch. Neurol. 59, 1895–1901. doi: 10.1001/archneur.59.12.1895
McNamara, T. P. (2013). “Spatial memory: properties and organization,” in Handbook of Spatial Cognition, eds D. Waller and L. Nadel (Washington, DC: American Psychological Association), 173–190.
Mellet, E., Bricogne, S., Tzourio-Mazoyer, N., Ghaëm, O., Petit, L., Zago, L., et al. (2000). Neural correlates of topographic mental exploration: the impact of route versus survey perspective learning. Neuroimage 12, 588–600. doi: 10.1006/nimg.2000.0648
Meneses, A. (1999). 5-HT system and cognition. Neurosci. Biobehav. Rev. 23, 1111–1125. doi: 10.1016/S0149-7634(99)00067-6
Meneses, A., and Perez-Garcia, G. (2007). 5-HT(1A) receptors and memory. Neurosci. Biobehav. Rev. 31, 705–727. doi: 10.1016/j.neubiorev.2007.02.001
Micheau, J., and Van Marrewijk, B. (1999). Stimulation of 5-HT1A receptors by systemic or medial septum injection induces anxiogenic-like effects and facilitates acquisition of a spatial discrimination task in mice. Prog. Neuropsychopharmacol. Biol. Psychiatry 23, 1113–1133. doi: 10.1016/S0278-5846(99)00057-3
Miller, J. F., Fried, I., Suthana, N., and Jacobs, J. (2015). Repeating spatial activations in human entorhinal cortex. Curr. Biol. 25, 1080–1085. doi: 10.1016/j.cub.2015.02.045
Milner, B. (1958). “Psychological defects produced by temporal lobe excision,” in The Brain and Human Behavior: Proceedings of the Association for Research in Nervous and Mental Disease, eds H. C. Solomon, S. Cobb, and W. Penfield (Baltimore, MD: The Williams & Wilkins Company).
Milner, B. (1965). Visually-guided maze learning in man: effects of bilateral hippocampal, bilateral frontal, and unilateral cerebral lesions. Neuropsychologia 3, 317–338. doi: 10.1016/0028-3932(65)90005-9
Monaco, C. M., Gebhardt, K. M., Chlebowski, S. M., Shaw, K. E., Cheng, J. P., Henchir, J. J., et al. (2014). A combined therapeutic regimen of buspirone and environmental enrichment is more efficacious than either alone in enhancing spatial learning in brain-injured pediatric rats. J. Neurotrauma 31, 1934–1941. doi: 10.1089/neu.2014.3541
Moran, J. P., and Dalrymple-Alford, J. C. (2003). Perirhinal cortex and anterior thalamic lesions: coparative effects on learning and memory. Behav. Neurosci. 117, 1326–1341. doi: 10.1037/0735-7044.117.6.1326
Morford, L. L., Inman-Wood, S. L., Gudelsky, G. A., Williams, M. T., and Vorhees, C. V. (2002). Impaired spatial and sequential learning in rats treated neonatally with D-fenfluramine. Eur. J. Neurosci. 16, 491–500. doi: 10.1046/j.1460-9568.2002.02100.x
Morris, R. G. M., Garrud, P., Rawlins, J. N. P., and O'Keefe, J. (1982). Place navigation impaired in rats with hippocampal lesions. Nature 297, 681–683. doi: 10.1038/297681a0
Nagahara, A. H., Otto, T., and Gallagher, M. (1995). Entorhinal-perirhinal lesions impair performance of rats on two versions of place learning in the Morris water maze. Behav. Neurosci. 109, 3–9. doi: 10.1037/0735-7044.109.1.3
O'Keefe, J., and Dostrovsky, J. (1971). The hippocampus as a spatial map. priliminary evidence from unit activity in the freely-moving rat. Brain Res. 34, 171–175. doi: 10.1016/0006-8993(71)90358-1
O'Keefe, J., and Nadel, L. (1978). The Hippocampus as a Cognitive Map. London: Oxford University Press.
O'Keefe, J., and Speakman, A. (1987). Single unit activity in the rat hippocampus during a spatial memory task. Exp. Brain Res. 68, 1–27. doi: 10.1007/BF00255230
Olsen, A. S., Sozda, C. N., Cheng, J. P., Hoffman, A. N., and Kline, A. E. (2012). Traumatic brain injury-induced cognitive and histological deficits are attenuated by delayed and chronic treatment with the 5-HT1A-receptor agonist buspirone. J. Neurotrauma 29, 1898–1907. doi: 10.1089/neu.2012.2358
Olton, D. S., Becker, J. T., and Handelsmann, G. E. (1979). Hippocampus, space, and memory. Behav. Brain Sci. 2, 313–365. doi: 10.1017/S0140525X00062713
Olton, D. S., and Paras, B. C. (1979). Spatial memory and hippocampal function. Neuropsychologia 17, 669–682. doi: 10.1016/0028-3932(79)90042-3
Otto, T., Wolf, D., and Walsh, T. J. (1997). Combined lesions of perirhinal and entorhinal cortex impair rats' performance in two versions of the spatially guided radial-arm maze. Neurobiol. Learn. Mem. 68, 21–31. doi: 10.1006/nlme.1997.3778
Owen, A. M., Milner, B., Petrides, M., and Evans, E. C. (1996). A specific role for the right parahippocampal gyrus in the retrieval of objects-location: a positron emission tomography study. J. Cogn. Neurosci. 8, 588–602. doi: 10.1162/jocn.1996.8.6.588
Parkinson, J. K., Murray, E. A., and Mishkin, M. (1988). A selective mnemonic role for the hippocampus in monkeys: memory for the location of objects. J. Neurosci. 8, 4159–4167.
Passchier, J., and Van Waarde, A. (2001). Visualisation of serotonin-1A (5-HT1A) receptors in the central nervous system. Eur. J. Nucl. Med. 28, 113–129. doi: 10.1007/s002590000394
Pazos, A., Probst, A., and Palacios, J. M. (1987). Serotonin receptors in the human brain - III. Autoradiographic mapping of serotonin-1 receptors. Neuroscience 21, 97–122. doi: 10.1016/0306-4522(87)90326-5
Pigott, S., and Milner, B. (1993). Memory for different aspects of complex visual scenes after unilateral temporal- or frontal- lobe resection. Neuropsychologia 31, 1–15. doi: 10.1016/0028-3932(93)90076-C
Ploner, C. J., Gaymard, B. M., Rivaud-Péchoux, S., Baulac, M., Clémenceau, S., Samson, S., et al. (2000). Lesions affecting the parahippocampal cortex yield spatial memory deficits in humans. Cereb. Cortex 10, 1211–1216. doi: 10.1093/cercor/10.12.1211
Quirk, G. J., Muller, R. U., Kubie, J. L., and Ranck, J. B. Jr. (1992). The positional firing properties of medial entorhinal neurons: description and comparison with hippocampal place cells. J. Neurosci. 12, 1945–1963.
Ramos, J. M. (2013). Differential contribution of hippocampus, perirhinal cortex and postrhinal cortex to allocentric spatial memory in the radial maze. Behav. Brain Res. 247, 59–64. doi: 10.1016/j.bbr.2013.03.017
Ramos, J. M. J. (2002). The perirhinal cortex and long-term spatial memory in rats. Brain Res. 947, 294–298. doi: 10.1016/S0006-8993(02)03044-5
Roiser, J. P., Müller, U., Clark, L., and Sahakian, B. J. (2007). The effects of acute tryptophan depletion and serotonin transporter polymorphism on emotional processing in memory and attention. Int. J. Neuropsychopharmacol. 10, 449–461. doi: 10.1017/S146114570600705X
Roiser, J. P., Rogers, R. D., Cook, L. J., and Sahakian, B. J. (2006). The effect of polymorphism at the serotonin transporter gene on decision-making, memory and executive function in ecstasy users and controls. Psychopharmacology (Berl) 188, 213–227. doi: 10.1007/s00213-006-0495-z
Saling, M. M., Berkovic, S. F., O'shea, M. F., Kalnins, R. M., Darby, D. G., and Bladin, P. F. (1993). Lateralization of verbal memory and unilateral hippocampal sclerosis: evidence of task-specific effects. J. Clin. Exp. Neuropsychol. 15, 608–618. doi: 10.1080/01688639308402582
Sarnyai, Z., Sibille, E. L., Pavildes, C., Fenster, R. J., McEwen, B. S., and Toth, M. (2000). Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin 1A receptors. Proc. Natl. Acad. Sci. U.S.A. 97, 14731–14736. doi: 10.1073/pnas.97.26.14731
Schröder, N., O'Dell, S. J., and Marshall, J. F. (2003). Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse 49, 89–96. doi: 10.1002/syn.10210
Skelton, M. R., Williams, M. T., and Vorhees, C. V. (2006). Treatment with MDMA from P11-20 disrupts spatial learning and path integration learning in adolescent rats but only spatial learning in older rats. Psychopharmacology (Berl) 189, 307–318. doi: 10.1007/s00213-006-0563-4
Smith, M. L., Bigel, M., and Miller, L. A. (2011). Visual paired-associate learning: in search of material-specific effects in adult patients who have undergone temporal lobectomy. Epilepsy Behav. 20, 326–330. doi: 10.1016/j.yebeh.2010.11.019
Smith, M. L., and Milner, B. (1981). The role of the right hippocampus in the recall of spatial location. Neuropsychologia 19, 781–793. doi: 10.1016/0028-3932(81)90090-7
Smith, M. L., and Milner, B. (1989). Right hippocampal impairment in the recall of spatial location: encoding deficit or rapid forgetting? Neuropsychologia 27, 71–81.
Spiers, H. J., Burgess, N., Hartley, T., Vargha-Khadem, F., and O'Keefe, J. (2001a). Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus 11, 715–725. doi: 10.1002/hipo.1087
Spiers, H. J., Burgess, N., Maguire, E. A., Baxendale, S. A., Hartley, T., Thompson, P. J., et al. (2001b). Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain 124, 2476–2489. doi: 10.1093/brain/124.12.2476
Suthana, N., Haneef, Z., Stern, J., Mukamel, R., Behnke, E., Knowlton, B., et al. (2012). Memory enhancement and deep-brain stimulation of the entorhinal area. N. Engl. J. Med. 366, 502–510. doi: 10.1056/NEJMoa1107212
Suzuki, W. A., Miller, E. K., and Desimone, R. (1997). Object and place memory in the macaque entorhinal cortex. J. Neurophysiol. 78, 1062–1081.
Taube, J. S. (1998). Head direction cells and the neurophysiological basis for a sense of direction. Prog. Neurobiol. 55, 225–256. doi: 10.1016/S0301-0082(98)00004-5
Theodore, W. H., Wiggs, E. A., Martinez, A. R., Dustin, I. H., Khan, O. I., Apple, S., et al. (2012). Serotonin 1A receptors, depression, and memory in temporal lobe epilepsy. Epilepsia 53, 129–133. doi: 10.1111/j.1528-1167.2011.03309.x
Thomas, S. A. (2015). Neuromodulatory signaling in hippocampus-dependent memory retrieval. Hippocampus 25, 415–431. doi: 10.1002/hipo.22394
Verge, D., Daval, G., Marcinkiewicz, M., Patey, A., El Mestikawy, S., Gozlan, H., et al. (1986). Quantitative autoradiography of multiple 5-HT1 receptor subtypes in the brain of control or 5,7-dihydroxytryptamine-treated rats. J. Neurosci. 6, 3473–3482.
Verge, D., Daval, G., Patey, A., Gozlan, H., El Mestikawy, S., and Hamon, M. (1985). Presynaptic 5-HT autoreceptors on serotonergic cell bodies and/or dendrites but not terminals are of the 5-HT1A subtype. Eur. J. Pharmacol. 113, 463–464. doi: 10.1016/0014-2999(85)90099-8
Vorhees, C. V., Ahrens, K. G., Acuff-Smith, K. D., Schilling, M. A., and Fisher, J. E. (1994). Methamphetamine exposure during early postnatal development in rats: I. Acoustic startle augmentation and spatial learning deficits. Psychopharmacology (Berl) 114, 392–401. doi: 10.1007/BF02249328
Vorhees, C. V., Herring, N. R., Schaefer, T. L., Grace, C. E., Skelton, M. R., Johnson, H. L., et al. (2008). Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int. J. Dev. Neurosci. 26, 599–610. doi: 10.1016/j.ijdevneu.2008.04.002
Vorhees, C. V., Inman-Wood, S. L., Morford, L. L., Broening, H. W., Fukumura, M., and Moran, M. S. (2000). Adult learning deficits after neonatal exposure to D-methamphetamine: selective effects on spatial navigation and memory. J. Neurosci. 20, 4732–4739.
Vorhees, C. V., Schaefer, T. L., and Williams, M. T. (2007). Developmental effects of +/-3,4-methylenedioxymethamphetamine on spatial versus path integration learning: effects of dose distribution. Synapse 61, 488–499. doi: 10.1002/syn.20379
Warburton, E. C., Harrison, A. A., Robbins, T. W., and Everitt, B. J. (1997). Contrasting effects of systemic and intracerebral infusions of the 5-HT1A receptor agonist 8-OH-DPAT on spatial short-term working memory in rats. Behav. Brain Res. 84, 247–258. doi: 10.1016/S0166-4328(96)00154-4
Wiig, K. A., and Bilkey, D. K. (1994a). The effects of perirhinal cortical lesions on spatial reference memory in the rat. Behav. Brain Res. 63, 101–109.
Wiig, K. A., and Bilkey, D. K. (1994b). Perirhinal cortex lesions in rats disrupt performance in a spatial DNMS task. Neuroreport 5, 1405–1408.
Wolff, M., Costet, P., Gross, C., Hen, R., Segu, L., and Buhot, M. C. (2004). Age-dependent effects of serotonin-1A receptor gene deletion in spatial learning abilities in mice. Brain Res. Mol. Brain Res. 130, 39–48. doi: 10.1016/j.molbrainres.2004.07.012
Zeidman, P., Mullally, S. L., Schwarzkopf, D. S., and Maguire, E. A. (2012). Exploring the parahippocampal cortex response to high and low spatial frequency spaces. Neuroreport 23, 503–507. doi: 10.1097/WNR.0b013e328353766a
Keywords: serotonin, 5-HT1A receptor, hippocampus, spatial cognition, memory
Citation: Glikmann-Johnston Y, Saling MM, Reutens DC and Stout JC (2015) Hippocampal 5-HT1A Receptor and Spatial Learning and Memory. Front. Pharmacol. 6:289. doi: 10.3389/fphar.2015.00289
Received: 03 September 2015; Accepted: 19 November 2015;
Published: 10 December 2015.
Edited by:
Alfredo Meneses, Center for Research and Advanced Studies of the National Polytechnic Institute, MexicoReviewed by:
Santiago J. Ballaz, University of Navarra, SpainAgnieszka Nikiforuk, Institute of Pharmacology of the Polish Academy of Sciences, Poland
Antonella Gasbarri, University of l'Aquila, Italy
Copyright © 2015 Glikmann-Johnston, Saling, Reutens and Stout. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yifat Glikmann-Johnston, eWlmYXQuZ2xpa21hbm4tam9obnN0b25AbW9uYXNoLmVkdQ==
 Yifat Glikmann-Johnston
Yifat Glikmann-Johnston Michael M. Saling
Michael M. Saling David C. Reutens
David C. Reutens Julie C. Stout
Julie C. Stout