- Departamento de Farmacobiología, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Mexico City, Mexico
Diverse neuropsychiatric disorders present dysfunctional memory and no effective treatment exits for them; likely as result of the absence of neural markers associated to memory. Neurotransmitter systems and signaling pathways have been implicated in memory and dysfunctional memory; however, their role is poorly understood. Hence, neural markers and cerebral functions and dysfunctions are revised. To our knowledge no previous systematic works have been published addressing these issues. The interactions among behavioral tasks, control groups and molecular changes and/or pharmacological effects are mentioned. Neurotransmitter receptors and signaling pathways, during normal and abnormally functioning memory with an emphasis on the behavioral aspects of memory are revised. With focus on serotonin, since as it is a well characterized neurotransmitter, with multiple pharmacological tools, and well characterized downstream signaling in mammals' species. 5-HT1A, 5-HT4, 5-HT5, 5-HT6, and 5-HT7 receptors as well as SERT (serotonin transporter) seem to be useful neural markers and/or therapeutic targets. Certainly, if the mentioned evidence is replicated, then the translatability from preclinical and clinical studies to neural changes might be confirmed. Hypothesis and theories might provide appropriate limits and perspectives of evidence.
Introduction
It should noted that while, memory formation and forgetting are functions of the brain (e.g., Fioravanti and Di Cesare, 1992; Wagner and Davachi, 2001; Wixted, 2004; Mansuy, 2005; Hardt et al., 2013; Hupbach, 2013; Callaghan et al., 2014; Li et al., 2015a); in contrast, diverse neuropsychiatric disorders present dysfunctional memory (Meyer-Lindenberg et al., 2012; Millan et al., 2012, 2014). AD is popular brain alteration presenting memory deficits and dementia and the leading cause of dementia, and a major public health priority; but dysfunctional memory is observed in other age-related neurodegenerative disorders, schizophrenia, post-traumatic stress disorder, strokes, etc. (Millan et al., 2014; Hashimoto, 2015). Certainly, no effective treatment for dysfunctional memory exists (e.g., Millan et al., 2012, 2014; Sun et al., 2015); likely due to the absence of neural markers associated to memory. Hence, memory, amnesia, forgetting (e.g., Tellez et al., 2012b) and AD (e.g., McConathy and Sheline, 2015; Muenchhoff et al., 2015; also Scarr et al., 2015) as well as mild cognitive impairment (MCI) (Eshkoor et al., 2015) require neural markers.
Certainly, AD is a very complex neuropsychiatric disorder, where memory becomes progressively dysfunctional (e.g., Solodkin and van Hoesen, 1997; Rodríguez et al., 2012) resulting in amnesia and dementia. In contrast, forgetting is unintentional process characterized as a failure to remember information or a rather strategic function of the brain that helps to reduce interference in the processing or retrieval of relevant information (Ludowiq et al., 2010). Likewise, forgetting as a physiological phenomenon occurs all the time (see McGaugh, 2013; see also Davis, 2010; Berry et al., 2012; Hardt et al., 2013; Kaku et al., 2013; Li and Richardson, 2013; Papenberg et al., 2013). However, the pharmacological and neuroanatomical bases of forgetting or memory have been little explored and as diverse neuropsychiatric disorders present dysfunctional memory, we are aiming potential neural markers.
For instance, phrasing neural markers and brain functions in PubMed (May 7, 21 and 29 or June 2, 2015) yield 318 or 319 (including 50 review papers) publications. Hence, herein, aiming clues about mapping neural markers link to cerebral functions and dysfunctions. Mainly memory formation, dysfunctional memory, and as forgetting, which has been little explored respect to neural markers. In spite of promissory findings, to our knowledge, no previous systematic works have been published addressing these issues. It should be noted that of the revised papers, several are rich in backgrounds and perspectives.
Examples illustrating the interaction among behavioral tasks (Box 1), control groups and molecular changes and/or pharmacological effects are mentioned in the following lines. Importantly, behavioral parameters, drug-treatment and cognitive processes interact in mammals (see below) and invertebrate species (e.g., Chen et al., 2014). Particularly the role of serotonin in memory: interactions with neurotransmitters and downstream signaling might be useful (e.g., Seyedabadi et al., 2014; Eskenazi et al., 2015). Although the focus herein are adult mammal animals; notwithstanding, important recent advances in invertebrate species, include Monje et al. (2013) reporting that flotillin-1 is an evolutionary-conserved memory-related protein up-regulated in implicit and explicit learning paradigms; thus, translational approach—from invertebrates to rodents—led to the identification of flotillin-1 as an evolutionary-conserved memory-related protein.
Box 1. Factors responsible for inconsistencies among laboratories.
Certainly, a number of factors might be produce similar results or be responsible for some inconsistencies among laboratories studying memory; which are complex and multi focal; which should provide an analytic framework offering key clues. Indeed, analysis of memory should include behavioral tasks, type of memory, the dynamic hierarchy of neural markers and brain areas involved in memory formation (e.g., Euston et al., 2012; Eskenazi et al., 2015) vs. no training, amnesia, anti-amnesic effects or forgetting (e.g., see below). Likewise, the species and the nature of behavioral task (e.g., appetitively or aversively motivated), curves of behavioral acquisition (i.e., multi-trial or two trials task) or patterns of behavioral responses (progressive vs. all or none response), cognitive demand (easy or difficult task), timing of drug administration (pre-training, post-training or pretest) and kind of drug (e.g., agonist or antagonist), protocols of training and testing together with neurobiological markers (e.g., Duewer et al., 1995; Patton, 1995) accompanying mnemonic processes deserve attention. Among the behavioral memory tasks available (e.g., Peele and Vincent, 1989; Myhrer, 2003; Lynch, 2004); importantly, the implementation of new instruments for measuring memory in behavioral tasks assists in gaining deeper insight into learning and memory processes (e.g., Cook et al., 2004; Walker et al., 2011; Markou et al., 2013; Leger et al., 2014; Wolf et al., 2014).
Actually, serotonin has pharmacological tools and well characterized downstream signaling in mammals' species (e.g., Marin et al., 2012; Borroto-Escuela et al., 2015; McCorvy and Roth, 2015); then serotonin and other neural markers are used for studying cerebral functions and dysfunctions (e.g., Tomie et al., 2003; Wellman et al., 2007; Cavallaro, 2008; Marcos et al., 2008; Da Silva Costa-Aze et al., 2012; Ménard and Quirion, 2012; Reichel et al., 2012; Rodríguez et al., 2012; Woods et al., 2012; Haahr et al., 2013; Alabdali et al., 2014; Freret et al., 2014; Kitamura et al., 2014; Kondo et al., 2014; Lecoutey et al., 2014; Leger et al., 2014; Seyedabadi et al., 2014; Leiser et al., 2015; Suzuki and Lucas, 2015; Westrich et al., 2015; Zilles et al., 2015). Evidence is organized according with 5-HT markers (i.e., receptors and transporter) but markers of other neurotransmission systems are included. Importantly, using well-established 5-HT neural markers (Blenau and Baumann, 2015; Lau et al., 2015; Müller and Homberg, 2015) might provide insights about known and novel markers and therapeutic targets. Müller and Homberg (2015) are providing an excellent analysis regarding 5-HT markers, drug use and addiction.
Memory Tasks and Molecular Changes
Memory Decline across Aging
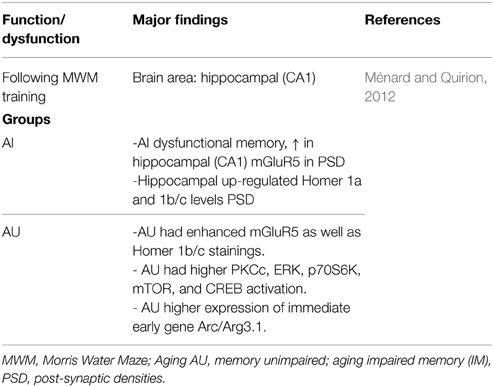
Ménard and Quirion (2012) using the Morris Water Maze (MWM) task, distinguish aged rats in two groups—memory-impaired (AI) and memory-unimpaired (AU) relative to 6-months old adult animals. Dysfunctional memory was associated to increased metabotropic glutamate receptors 5 (mGluR5) in hippocampal post-synaptic densities (PSD) (Table 1); Ménard and Quirion (2012) conclude that in successful cognitive aging (i.e., AU animals) present a critical role for mGluR5, Homer 1 proteins and downstream signaling pathways. Certainly, in terms of signaling respect to cognition-enhancing drug targets, insights are emerging (e.g., Seyedabadi et al., 2014; Gyurko et al., 2015; Ménard et al., 2015; Sun et al., 2015).
Autism: Neuro-inflammation and Neurotransmission Impairment
Although, Alabdali et al. (2014) did find that serotonin or dopamine in platelet-free plasma not correlated with social and cognitive dysfunction. It should be noted that serotonin has multiple markers (see below). And, several neurochemical parameters might show sensitivity and specificity; thus contributing to earlier and more accurate diagnosis of dysfunctional memory in disease such autism, AD, and the identification of effective treatments (e.g., Sheline et al., 2014a,b; Strac et al., 2015).
5-HT Systems
As already mentioned, serotonin (5-hydroxytryptamine, 5-HT) is one of the neurotransmitter well characterized in mammal species (e.g., Hoyer et al., 1994; Saulin et al., 2012; Borroto-Escuela et al., 2015; McCorvy and Roth, 2015), it has multiple neural markers, including receptors (i.e., 5-HT1A/1B/1D, 5-HT2A/2B/2C, 5-HT3, 5-HT4, 5-HT5, 5-HT6, and 5-HT7 receptors) and transporter (named SERT) as well as volume transmission. These 5-HT markers are present in brain areas involved in memory (e.g., Buhot et al., 2003a,b; Puig and Gulledge, 2011; Rodríguez et al., 2012; Barlow et al., 2015; Leiser et al., 2015), sentence compression (Zilles et al., 2015) and drug addiction (Müller and Homberg, 2015).
Serotonergic Gene Regulation during Learning and Memory
In an elegant work, Cavallaro (2008) using DNA microarrays analyzed hippocampal 5-HT receptors in two behavioral memory tasks and different times (Table 2); observing differential expressions in 12 receptors (Htr1a, Htr1b, Htr1d, Htr1f, Htr2a, Htr2c, Htr3a, Htr4, Htr5a, Htr5b, Htr6, and Htr7). At least Htr2c, Htr3a and Htr6 receptors had significant changes relative to swimming control animals and water maze trained animals. Htr2c expression was reduced at 1 h and increased at 24 h following training. Htr3a-mRNA was increased at 24 h, whereas Htr6 was decreased at 6 h; as observed in autoshaping (see below). In passive avoidance task, three 5-HT receptors showed changes in expression respect to naive and trained animals (i.e., conditioned animals, CA). Indeed, the expression of Htr3a was increased, whereas those of Htr1b and Htr4 were decreased. Certainly, expression of 5-HT receptors were also observed in control groups subjected to physical activity and mild stress (naive vs. swimming controls in the water maze; naive vs. CSTA and USTA in passive avoidance); notwithstanding, memory consolidation produced different magnitudes (e.g., Htr2c in the water maze) often opposite trends than in control animals (e.g., Htr3a in both water maze and passive avoidance). Producing cumulative patterns of gene expression, associated to time and 5-HT subtype receptor (see Cavallaro, 2008). Importantly, apparently water maze memory requires slight 5-HT7 receptor expression within 1-h; and passive avoidance memory involves expression of 5-HT1A−1F, 5-HT2A, and 5-HT5A receptors. Of course, remaining to determine if the suppression of the other 5-HT receptors is necessary. Certainly, the molecular requirements differ between water maze and passive avoidance.
Notably, Zaldivar and Krichmar (2013) observe in behaviorally naïve (i.e., untrained) animals, neurotransmitters changing including 5-HT receptors expression in areas regarded to neuromodulation or memory (amygdala); revealing connectivity and receptor localization, and patterns of expression among neurotransmission systems, receptors and brain areas.
5-HT1A Receptor
Although 5-HT1A receptor may serve as a biomarker for cognitive functioning and target for treatment of cognitive impairment; notwithstanding hitherto evidence remains sparse and inconsistent (Borg, 2008; Borg et al., 2009). Certainly, the situation is changing; e.g., Yoshimi et al. (2014) report that brexpiprazole, presents serotonin-dopamine activity, and 5-HT1A receptor partial agonism, attenuates phencyclidine-induced cognitive deficits; an effect blocked by the selective 5-HT1A receptor antagonist WAY-100,635 (which alone has no effect). Yoshimi et al. (2014) conclude that brexpiprazole could ameliorate cognitive deficits in schizophrenia and other neuropsychiatric diseases. Contrasting findings exist regarding the 5-HT1A partial agonists (e.g., buspirone), which alone impair memory in normal subjects (Meneses, 1999) but some of them (e.g., tandospirone) might be useful in the treatment of schizophrenia pathophysiology (Sumiyoshi et al., 2008). And, as tandospirone (e.g., Baba et al., 2015) also has anti-amnesic effects or facilitate performance in difficult memory tasks; hence, 5-HT1A partial agonists might be useful in the treatment of dysfunctional memory.
Certainly, while if 5-HT1A receptor agonists, partial agonists, or antagonists might be used for memory alterations (e.g., Meneses and Perez-Garcia, 2007; Pittalà et al., 2015); functional selectivity or biased agonism is revealing important insights regarding 5-HT1A and 5-HT3A receptors (e.g., Vardy and Kenakin, 2014; McCorvy and Roth, 2015). For instance, van Goethem et al. (2015) study “biased,” 5-HT1A receptor agonists in a novel object pattern separation task (relative to episodic memory); showing that by preferentially activating post-synaptic 5-HT1A heteroreceptors, or raphe-nuclei autoreceptors are potential novel molecular targets for improving memory. Likewise, Stroth et al. (2015) report that arylpiperazine ligands of 5-HT1A receptor preferentially affect cAMP signaling vs. β-arrestin-2 recruitment; proposing the development of signaling pathway-selective drugs targeting this receptor.
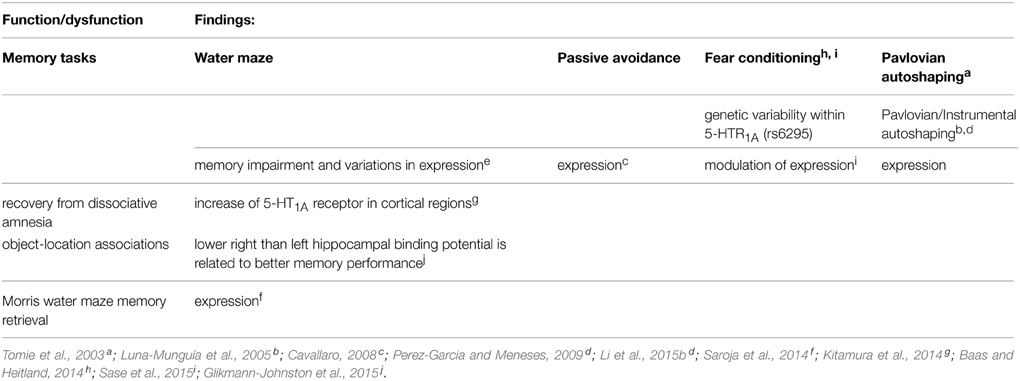
Notably, recovery from dissociative amnesia increases cortical 5-HT1A receptor (Kitamura et al., 2014; Table 3). Likewise, memory in autoshaping task (see Box 2) also increases 5-HT1A receptor expression in 14 brain areas, but decrements in 7 and no changes in 12 (Table 3); suggesting that upregulated, down-regulated, and “silence” 5-HT1A receptor in brain areas form part of neural circuits engaged in memory formation; thus demonstrating a high degree of specificity and memory mapping.
Box 2. Autoshaping tasks.
Autoshaping memory tasks have been focus by several research groups (e.g., Brown and Jenkins, 1968; Myer and Hull, 1974; Atnip, 1977; Oscos et al., 1988; Bussey et al., 1997, 2013; Lindner et al., 2003; Vanover et al., 2004; Ballaz et al., 2007; Rodriguez et al., 2008; Walker and Foley, 2010; Walker et al., 2011; Tomie et al., 2012; Krynetskiy et al., 2013; Markou et al., 2013; Gallistel et al., 2014; Holland et al., 2014; Lesaint et al., 2014; Talpos et al., 2014; Eskenazi et al., 2015; Talpos and Shoaib, 2015; in several animal species (e.g., Wasserman, 1981) including humans (Wilcove and Miller, 1974; Pithers, 1985). According with Holland et al. (2014), “autoshaping” or “sign-tracking” phenomenon has recently attracted considerable attention as a platform for studying individual differences in impulsivity, drug sensitization, and other traits associated with vulnerability to drug addiction. Autoshaping has been also used for detecting effects induced by memory, amnesia, drugs, genetic variations, aging and neural markers (e.g., Tomie et al., 2003, 2012; Vanover et al., 2004; Rodriguez et al., 2008; Fitzpatrick et al., 2013; Markou et al., 2013; Talpos et al., 2014). Notably, autoshaping is an associative automatized learning task (see below), and during memory consolidation of Pavlovian/instrumental autoshaping learning task, dentate gyrus, hippocampal CA1, basolateral amygdaloid nucleus and prefrontal cortex are require (see below). It should be noted that an important innovation, and growingly popular method of assessing cognitive functions is the automated touchscreen platform (e.g., Abela et al., 2013; Talpos et al., 2014; Delotterie et al., 2015), used for diverse cognitive tasks, comparable those in employ in human subjects (Horner et al., 2013), including autoshaping (e.g., Gallistel et al., 2014; Talpos et al., 2014; Silverman et al., 2015).
Autoshaping learning tasks involve classical and instrumental conditioning (i.e., stimulus-stimulus and stimulus-responding conditioning). It should be mentioned that long-lasting memories are most efficiently formed by multiple training sessions separated by appropriately timed intervals. Autoshaping meets this criterion and it allows modeling of behavioral situations requiring integration of information obtained from sign- and goal-tracking settings; representing memory of self-taught settings (Meneses, 2013, 2014). Certainly, autoshaping tasks (Pavlovian or instrumental; and Pavlovian/instrumental may produce initial modest and/or variable levels of conditioned responses (CR). Importantly, memory formation, amnesia and forgetting in Pavlovian/instrumental paradigms are accompanied by changes in neural markers, including 5-HT, glutamate, dopamine, and GABA transporters expression levels (Tomie et al., 2003; Tellez et al., 2012a,b), 5-HT receptor expression and cAMP production (Meneses, 2013). Certainly, forgetting as therapeutic targets for dysfunctional memory it has been little explored. As above mentioned, similar results, including pharmacological and neurobiological changes to those reported in autoshaping have been described in other memory behavioral tasks (for review see King et al., 2008; Marcos et al., 2008; Da Silva Costa-Aze et al., 2012; Reichel et al., 2012; Woods et al., 2012; Haahr et al., 2013; Freret et al., 2014; Nasehi et al., 2014b; Seyedabadi et al., 2014; Subramaniyan et al., 2014; Wilkinson et al., 2014; Delotterie et al., 2015; Sase et al., 2015; Westrich et al., 2015).
Behavioral parameters during STM and LTM
In addition to measuring CR in autoshaping, head-pokes (HP) during each training/testing session and head-pokes during CS (HP-CS) have recorded. These parameters provide information about exploration activity (HP) and food- intake motivation (Tellez et al., 2012a). For instance, as CR becomes progressive, HP-CS provides information on the association of CS-US and CR-US.
Maximum level of CR
It should be noted that as animals present different levels of CR, these values are normalized and the maximal CR level attained for each rat at 48 h is considered as 100% of performance. This value is then used to calculate the proportion or percentage of CR observed at 1.5, 24, and 216 h and the data of 1.5 h and 24 h are used as illustration; and multiple comparisons, including memory, forgetting, time vs. treatments for all behavioral parameters (Meneses and Tellez, 2015).
Memory, amnesia and forgetting and neural transporters analysis
As already mentioned autoshaping procedures produce variable levels of CR and a number of laboratories have been using autoshaping. It should be noted that, reproducibility among studies is important and expected that to vary (e.g., Marcus, 2014).
Importantly, Glikmann-Johnston et al. (2015) report that hippocampal human asymmetry in 5-HT1A receptor expression (using [18F] MPPF binding), accompanies memory for object-location associations; lower right than left hippocampal binding potential is related to better memory performance (Table 2). Aubert et al. (2013) also report that the dual 5-HT1A/7 receptor agonist 8-OH-DPAT increased transcription of adenylate cyclase 1 in the hippocampus (CA1), suggesting that memory function could play a role in altered pairmate interaction dynamics; and these changes might be caused by 8-OH-DPAT-induced up- or down-regulation of 5-HT1A and 5-HT7 receptor in the medial prefrontal cortex and in the hippocampus (CA1), respectively; and according with Aubert et al. (2013); and such as hypothesis is supported by rodent studies that implicate 5-HT7 function in contextual learning and memory consolidation.
On the other hand, genetic variability within 5-HT1A receptor (rs6295) is associated with contextual fear independent (Table 3) (Baas and Heitland, 2014). Likewise, Weber et al. (2015) report that conditional inactivation of the GLUA1-encoding Gria1 gene selectively in 5-HT neurons of adult mice (i.e., Gria1 5-HT-/- mice) exhibited a distinct anxiety phenotype but showed no alterations in locomotion, depression-like behavior, or learning and memory. Importantly, contextual fear task increases hippocampal AMPA-, GluN1- and 5-HT1A− containing receptor complexes (Sase et al., 2015) (Table 3). In addition, Saroja et al. (2014) studied spatial memory retrieval and hippocampal monoamine receptor (MAR) complexes (including 5-HT1A and 5-HT7 receptors, and dopamine D1 and D2 receptors and colocalizations) in mice of 3–12 and 18 months. D1, D2, and 5-HT7 containing receptor complex levels were decreasing with age while 5-HT1A receptor-containing complex was increased. In addition, the time spent in the target quadrant (i.e., memory retrieval) correlated with D1, 5-HT7, and 5-HT1A receptors complex expression. Saroja et al. (2014) conclude that individual monoamine receptors are linked to spatial memory retrieval and are modulated by age. This same group (Subramaniyan et al., 2015) reports that the receptor complex levels containing hippocampal GluN1 and GluN2A of NMDARs, GluA1 and GluA2 of AMPA receptors, nAch7 and the D1A dopamine receptors were elevated during spatial learning, whilst levels of GluA3 and 5-HT1A receptor containing complexes were reduced. Thus, supporting that 5-HT1A receptor is useful neurobiological marker of memory.
Pavlovian Autoshaping: 5-HT1A and 5-HT2 Receptors (Binding Sites)
Interestingly, Tomie et al. (2003), studied the effects of experience with Pavlovian autoshaping procedures (Box 2) on lever-press conditioned response (CR) performance and 3H-8-OH-DPAT-labeled binding of 5-HT1A and probably 5-HT7 (it should be noted that this drug has affinity for 5-HT7, see below; Table 3); as well as 125I-LSD-labeled binding of 5-HT2A receptors were evaluated in four groups of rats. The groups (Paired High CR and Paired Low CR) received Pavlovian autoshaping procedures wherein the presentation of a lever (conditioned stimulus, CS) was followed by the response-independent presentation of food (unconditioned stimulus, US). Group Paired High CR showed more rapid CR acquisition and higher asymptotic levels of lever-press autoshaping CR performance relative to Group Low CR. Group Omission received autoshaping with an omission contingency, such that performing the lever-press autoshaping CR resulted in the cancelation the food US, while Group Random received presentations of lever CS and food US randomly with respect to one another. Though Groups Omission and Random did not differ in lever-press autoshaping CR performance, Group Omission showed significantly lower levels of 5-HT1A binding in post-synaptic areas (frontal cortex, septum, caudate putamen), as well as significantly higher plasma corticosterone levels than Group Random. In addition, Group Random showed higher levels of 5-HT1A binding in pre-synaptic somatodendritic autoreceptors on dorsal raphe nucleus relative to the other three groups. Autoradiographic analysis of 5-HT2A receptor binding revealed no significant differences between Groups Paired High CR and Paired Low CR or between Groups Omission and Random in any brain regions. Notably, although extensive Pavlovian autoshaping training (Tomie et al., 2003) failed to produce any correlation between 5-HT1A or 5-HT2A receptor expression and CR; however, regardless the number of CR, Tomie et al. (2003) demonstrated correlation between both receptors expression and paired CS–US presentations. These data are also indicating that the neuroanatomical, neurochemical, and behavioral basis of Pavlovian and Pavlovian/Instrumental Autoshaping (P/I-A) are different (see Box 2). Although the latter could be considered as an instance of system processing styles (i.e., S-S, S-R, and stimulus-reinforcer [S-Rf] learning; see White and McDonald, 2002); nevertheless, the association of CR and 5-HT markers (Tomie et al., 2003) is replicated (Pérez-García et al., 2006; Pérez-García and Meneses, 2008). Notably, similar associations are observed in the Morris Water Maze and passive avoidance tasks (Cavallaro, 2008). Hence, the evidence supports the notion that 5-HT1A receptor provides diverse neurobiological markers, pharmacological and genetic tools that have been used to investigate a variety of functions and dysfunctions (for references Meneses and Liy-Salmeron, 2012). Likewise, 5-HT1A receptor also is therapeutic target, it seems to be useful for detecting functional and dysfunctional memory, and co-expression with other neurotransmission systems and serotonergic receptors.
5-HT1B/1D Receptor
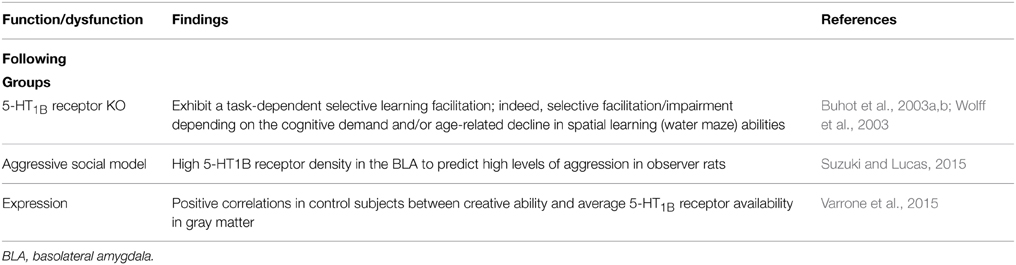
The Buhot et al. (2003a,b; Wolff et al., 2003) seminal work (see also Drago et al., 2010) showed that 5-HT1B receptor knockout mice exhibit a task-dependent selective learning facilitation; depending on the cognitive demand and/or age-related decline of spatial learning abilities (Table 4). In addition, pharmacological evidence indicates a possible involvement of hippocampal CA1 5-HT1B/1D and 5-HT2A/2B/2C receptors in harmaline-induced amnesia (Nasehi et al., 2014a). And 5-HT1B receptor activation disrupts delayed alternation (DAL) performance in mice (Woehrle et al., 2013) and chronic fluoxetine pretreatment blocks 5-HT1B receptor- induced deficits; suggesting a 5-HT1B receptor modulation in orbitofrontal-dependent DAL. The 5-HT1B-induced DAL deficits may provide a model for obsessive compulsive disorder (OCD; Woehrle et al., 2013). The above evidence is consistent with the possibility that 5-HT1B receptor inverse agonists might be useful for reversing memory deficits (e.g., Meneses, 2001; Meneses and Tellez, 2015). Importantly, 5-HT1B/1D receptor expression in the frontal cortex is correlated to memory impairment (Garcia-Alloza et al., 2004). Certainly, Drago et al. (2010) highlight that 5-HT1B receptor is a candidate modulator of the mnemonic and motivationally related symptoms in psychiatric illnesses. Moreover, positive correlations exist between creative ability and 5-HT1B receptor expression in gray matter of control subjects; as well as in Parkinson disease (PD) patients between depression and creative ability (Varrone et al., 2015); importantly, PD patients have poor semantic memory and creative ability (Varrone et al., 2015).
Neurobiological Mechanisms in the Observational Learning of Aggression
Suzuki and Lucas (2015) report that chronic passive exposure to aggression modifies expression of D2 receptor in the nucleus accumbens core (AcbC) and shell (AcbSh), and 5-HT1B receptor in the medial (MeA), basomedial (BMA), and basolateral (BLA) amygdala. And increased aggressive behavior reduced D2 receptor in bilateral AcbSh. Likewise, regardless of exposure aggression length 5-HT1B receptor was augmented in bilateral BLA. Finally, low D2 receptor expression in the AcbSh significantly interacted with high 5-HT1B receptor density in the BLA, predicting high levels of aggression in observer animals (Table 4). Suzuki and Lucas (2015) conclude that the dopamine-serotonin or AcbSh-BLA interactions; may be risk factors for aggression in observers chronically witness aggressive interactions (Suzuki and Lucas, 2015). Clearly, 5-HT1B receptor expression was useful in detecting learning and memory of aggression.
5-HT2A/2B/2C Receptors
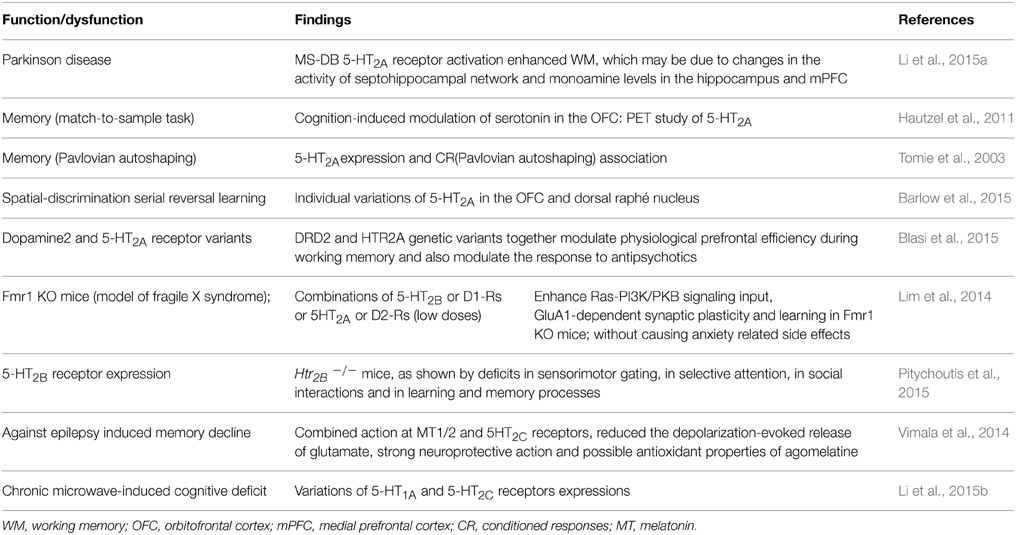
Li et al. (2015a) report that 5-HT2A receptor is highly expressed in the medial septum-diagonal band of Broca complex (MS-DB), especially in parvalbumin (PV)-positive neurons linked to hippocampal theta rhythm (involved in normal and dysfunctional memory of PD). The medial forebrain bundle (MFB) lesions impaired working memory, hippocampal theta, decreased firing rate and density of MS-DB PV-positive neurons, rhythm, and DA levels in septohippocampal system and medial prefrontal cortex (mPFC). Intra-MS-DB injection of the 5-HT2A receptor agonist 4-Bromo-3,6-dimethoxybenzocyclobuten-1-yl) methylamine hydrobromide (TCB-2) enhanced working memory, producing the opposite effects in control and lesioned and shortening TCB-effects; implicating dysfunctional 5-HT2A receptor. Li et al. (2015a) conclude that unilateral lesions of the MFB induced working memory deficit, and activation of MS-DB 5-HT2A receptor enhanced working memory, and involve monoamine levels in the hippocampus and mPFC. In addition, in a controlled cross-over PET study using a delayed match-to-sample task and the 5-HT2A receptor antagonist [18F] altanserin, Hautzel et al. (2011) report a cognition-induced modulation of serotonin in the orbitofrontal cortex (OFC). Importantly, Tomie et al. (2003) demonstrated an association between 5-HT2A receptor expression and memory formation in Pavlovian autoshaping task. In addition, individual differences in impulsive action and 5-HT2A receptor cortical variations have been noted (Fink et al., 2015). Also, D2 and 5-HT2A receptors present genetic variants and modulate physiological prefrontal cortex efficiency during working memory and response to antipsychotics (Blasi et al., 2015). Moreover, although an association between 5-HT2A receptor polymorphism (his452tyr) and memory performances in AD has been proposed; no differences in verbal memory were identified by Guglielmi et al. (2015).
Importantly, Barlow et al. (2015) report markers of serotonergic function in the orbitofrontal cortex and dorsal raphé nucleus predicting individual variation in spatial-discrimination serial reversal learning. These authors conclude that rats in the upper quintile of the distribution of perseverative responses during repeated S-R reversals have significantly reduced levels of the 5-HT metabolite, 5-hydroxy-indoleacetic acid, in the OFC. Additionally, 5-HT2A receptor expression in the OFC of mid- and high-quintile rats was significantly reduced compared with rats in the low-quintile group. These perturbations were accompanied by an increase in the expression of monoamine oxidase-A (MAO-A) and MAO-B in the lateral OFC and by a decrease in the expression of MAO-A, MAO-B, and tryptophan hydroxylase in the dorsal raphé nucleus of highly perseverative rats. Barlow et al. (2015) found no evidence of significant differences in markers of DA and 5-HT function in the DMS or MAO expression in the ventral tegmental area of low- vs. high-perseverative rats; indicating that diminished serotonergic tone (probably, at least via 5-HT2A receptor) in the OFC may be an endophenotype that predisposes to behavioral inflexibility and other forms of compulsive behavior (Barlow et al., 2015).
Moreover, Lim et al. (2014) investigated mechanisms of action of psychoactive drugs that modestly benefit the cognitive performance in fragile X patients (the most common form of inherited mental retardation); reporting that compounds activating 5HT2B receptor (5HT2B) or dopamine (DA) subtype 1-like receptors (D1-Rs) and/or those inhibiting 5HT2A or D2 receptors moderately enhance Ras-PI3K/PKB signaling input, GluA1-dependent synaptic plasticity, and learning in Fmr1 knockout mice (Lim et al., 2014). Unexpectedly, combinations of these 5-HT and DA compounds at low doses synergistically stimulate Ras-PI3K/PKB signal transduction and GluA1-dependent synaptic plasticity and remarkably restore normal learning in Fmr1 knockout mice without causing anxiety-related side effects. Lim et al. (2014) suggest that properly dosed and combined psychoactive drugs may effectively treat the cognitive impairment associated with fragile X syndrome. In addition, Htr2B−/− mice show deficits in sensorimotor gating, selective attention, social interactions as well as in learning and memory (i.e., fear conditioning and novel object recognition: STM and LTM) (Pitychoutis et al., 2015).
Regarding 5-HT2C receptor, Vimala et al. (2014) highlight that epilepsy affects negatively cognitive function, producing depression, anxiety, etc. Mentioning among other issues that agomelatine is a novel antidepressant acting as melatonin MT1 and MT2 receptor agonist and 5-HT2C receptor antagonist; producing reduction in the depolarization-evoked release of glutamate, strong neuroprotective action and possible antioxidant effects (Vimala et al., 2014); producing hippocampal neuronal cell survival and neurogenesis, neuroprotective effect in hippocampus and frontal cortex and the antioxidant potential may contribute to the protective action of agomelatine against epilepsy induced memory decline (Vimala et al., 2014). In addition, Walker and Foley (2010) report that administration of the 5-HT2C inverse agonist mianserin impaired autoshaped operant response on day 2 than any other agent tested. In addition, decreasing the length of the acquisition session to 1-h augmented the difficulty of the autoshaping task further modulating the consolidation effects produced by the 5-HT2C ligands (Walker and Foley, 2010). Moreover, Li et al. (2015b) report that repeat exposition to 2.856 GHz microwaves (averaging 5–30 mW/cm2) affects spatial learning and memory function, morphology structure of the hippocampus, electroencephalogram (EEG) and neurotransmitter content (amino acid and monoamine); including expression of 5-HT1A 2A, and 2C receptors. Li et al. (2015b) demonstrated that chronic exposure to microwave could induce dose-dependent deficit of spatial learning and memory and inhibition of brain electrical activity, the degeneration of hippocampus neurons, and the disturbance of neurotransmitters; including hippocampal and cortical expression of 5-HT1A and 5-HT2C receptors.
Importantly, 5-HT2A/2B/2C receptors are useful detecting learning and memory changes and drug effects. Aloyo et al. (2009) remind us of inverse agonism at 5-HT2A and 5-HT2C receptors.
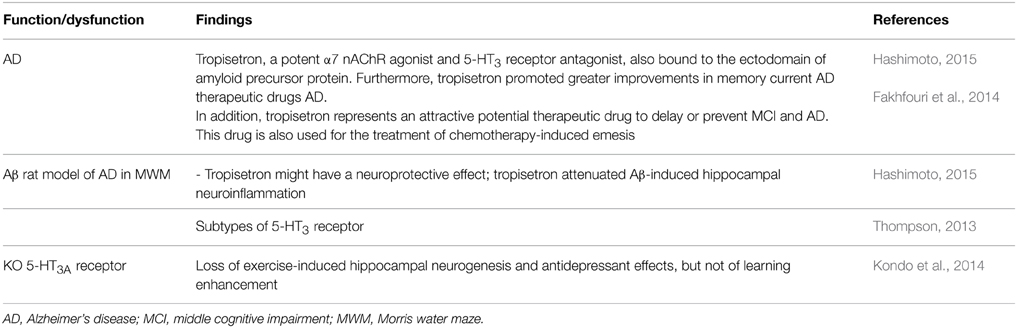
5-HT3 Receptor
5-HT3 receptor antagonists (e.g., tropisetron, ondansetron) have a long dated antiamnesic effects, including attenuation of age-associated memory impairment (e.g., Costall and Naylor, 1992; see also Shimizu et al., 2013). Recent evidence, from preclinical studies suggests that the interaction between amyloid-β peptides (Aβ) and the α7 nicotinic acetylcholine receptor (α7 nAChR) (Hashimoto, 2015) (Table 5). And tropisetron is also a α7 nAChR agonist and 5-HT3 receptor antagonist; binding to amyloid precursor protein and enhancing memory in AD patients (Table 5). Importantly, 5-HT3 receptor antagonists have been useful in treatments such as chemotherapy-induced emesis to neuroprotection (Fakhfouri et al., 2014; Hashimoto, 2015). Certainly, subtypes of 5-HT3 receptor exist (Thompson, 2013); and their mechanisms are complex. For instance, Kozuska et al. (2014) deal with the multiple salt bridges in the intracellular domain of the 5HT3A receptor and these interactions increase the overall rigidity of the receptor, stabilize its low conducting state and affect the ligand cooperativity; suggesting that the allosteric effects of these regions on the receptor may be involved in a possible “reverse” allosteric modulation of 5HT3 receptor. In addition, it should be noted that agonist- and antagonist-induced up-regulation of surface 5-HT3A receptor (Morton et al., 2015).
Moreover, Kondo et al. (2014) studied 5-HT3A receptor subunit-deficient (htr3a-/-) mice revealing loss of exercise-induced hippocampal neurogenesis and antidepressant effects, but not of learning enhancement (Table 5). Kondo et al. (2014) conclude that the 5-HT3 receptor is the critical target of 5-HT action in the brain following exercise, being indispensable for hippocampal neurogenesis and antidepressant effects induced by exercise.
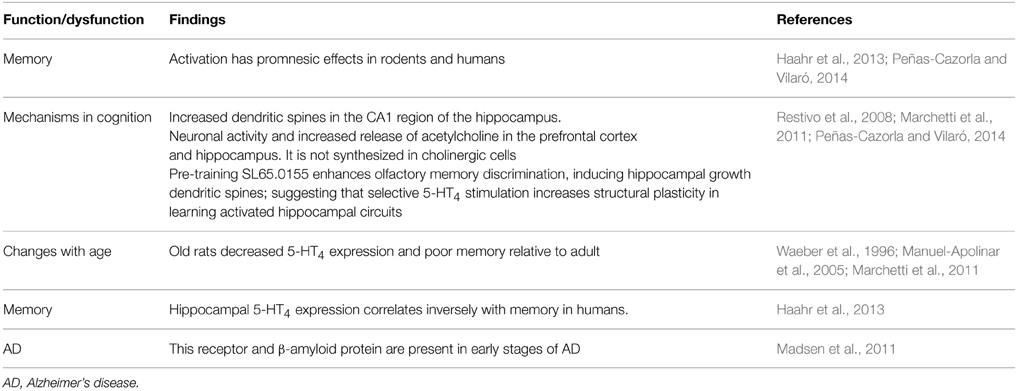
5-HT4 receptor
It should be noted that earlier evidence indicated that 5-HT4 receptor decreased in AD (see Eglen et al., 1995). Activation of 5-HT4 receptor has pro-cognitive effects on memory tasks (e.g., Bockaert et al., 2011; Peñas-Cazorla and Vilaró, 2014; Ramirez et al., 2014; Claeysen et al., 2015). Notably, Madsen et al. (2011) observe cerebral 5-HT4 receptor up-regulation starts at a preclinical stage of dementia and it continues while dementia is still at a mild stage and these authors speculate that this upregulation may be a compensatory effect of decreased levels of interstitial 5-HT, increase acetylcholine release or to counteract Aβ accumulation and improved cognitive function. Hippocampal 5-HT4 receptor expression correlates inversely with human memory (Haarh et al., 2013; Table 6). Also, old rats have decreased 5-HT4 receptor expression and poor memory relative to adult (Table 6).
In addition, evidence suggests that serotonergic activity, via 5-HT4 receptors in hippocampal, striatum, and cortical areas, mediates memory function and provides further evidence for a complex and regionally specific regulation over 5-HT receptor expression during memory formation (Manuel-Apolinar et al., 2005).
Segu et al. (2010) report adaptive changes in cholinergic systems, which may circumvent the absence of 5-HT4 receptor to maintain long-term memory under baseline conditions. In contrast, despite of adaptive mechanisms, the absence of 5-HT4 receptor aggravates scopolamine-induced memory impairments. The mechanisms whereby 5-HT4 receptor mediates a tonic influence on ChAT activity and muscarinic receptors remain to be determined (Segu et al., 2010). Restivo et al. (2008) highlight that pharmacological modulation of synaptic efficacy is a prominent target in the identification of promnesic compounds and that pre-training administration of the 5-HT4 receptor partial agonist SL65.0155 enhances olfactory discrimination and potentiates learning-induced dendritic spine growth in the mouse hippocampus; without affecting spine density in the pseudo-trained mice and, by itself, it does not promote spine growth. Likewise, the 5-HT4 receptor antagonist RS39604 prior to SL65.0155 prevents both improved memory and additional formation of spines; thus confirming the 5-HT4 receptor specificity of the observed effects (Restivo et al., 2008); and these authors conclude that 5-HT4 receptor stimulation selectively increases experience-dependent structural plasticity in learning-activated hippocampal circuits.
Marchetti et al. (2011) have also highlighted that in developing rats as well as in rats ranging from 3 to 9 months of age, significant modifications of 5-HT4 receptor expression have been observed (for references see Marchetti et al., 2011). These same authors propone that the poor memory formation observed in aged rats (Marchetti et al., 2011). And corresponding decreases in 5-HT4 receptor expression in brain areas (e.g., hippocampus, amygdala, etc.) involved in memory formation, could explain improved memory, dendritic spines (Restivo et al., 2008), neuronal excitability and release of the neurotransmitter acetylcholine (Ach) (see Segu et al., 2010; Marchetti et al., 2011; Peñas-Cazorla and Vilaró, 2014). Clearly, 5-HT4 receptor is useful neural marker of dysfunctional and memory formation as well as therapeutic target. Moreover, studying 5-HT expression during memory formation is giving new fresh insights (e.g., Haahr et al., 2013). Importantly, Haahr et al. (2013) report that hippocampal 5-HT4 receptor expression correlates inversely with human memory performance.
5-HT5
As mentioned above, Cavallaro (2008) reported that passive avoidance memory involves expression of several 5-HT receptors, including 5-HT5A. 5-HT5 receptor occurs in brain areas implicated in learning and memory. Post-training administration of the 5-HT5A receptor antagonist SB-6995516 decreased CR during short-term (STM; 1.5-h; at 0.1 mg/kg) and long-term memory (LTM; 24-h; at 3.0 mg/kg). Moreover, considering that there are no selective 5-HT5A receptor agonists, next, diverse doses of the serotonin precursor l-tryptophan were studied during STM and LTM, showing that l-tryptophan (5–100 mg/kg) facilitated performance, particularly at 50 mg/kg. In interactions experiments, l-tryptophan (50 mg/kg) attenuated the impairment effect induced by SB-699551 (either 0.3 or 3.0 mg/kg) (Gonzalez et al., 2013). All together this evidence suggests that the blockade of 5-HT5A receptor appear to be able to impair STM and LTM (24 h) in autoshaping task, while its stimulation might facilitate it. Of course further investigation is necessary, meanly with selective 5-HT5A compounds (Gonzalez et al., 2013). Interestingly, Yamazaki et al. (2014, 2015) reported that a 5-HT5A receptor antagonist ameliorates positive symptoms and cognitive impairment in animal models of schizophrenia and in aged rats and induced-amnesia. An analogous case is observed regarding 5-HT1A partial agonists (see above).
Returning to 5-HT5 receptor, Karimi et al. (2013) report that it has long been known that hippocampal spatial memory and the ability to navigate through space are sexually dimorphic traits among mammals, and numerous studies have shown that these traits can be altered by means of sex hormone manipulation. Male and female rat pups were injected with estradiol and testosterone respectively, at early stage of their lives to examine the effect of sex hormone manipulation on mRNA expression of Slc9a4, Nr3c2, Htr5b, and Mas1; among other results, these authors report that expressions of these genes are strongly influenced by sex hormones in both the frontal cortex and hippocampus, especially in male hippocampus, in which expression of all genes were up-regulated. Htr5b was the gene that was affected only in the males (Karimi et al., 2013). Hence, considering the pharmacological evidence mentioned above, probably learning and memory might be affected in these animals.
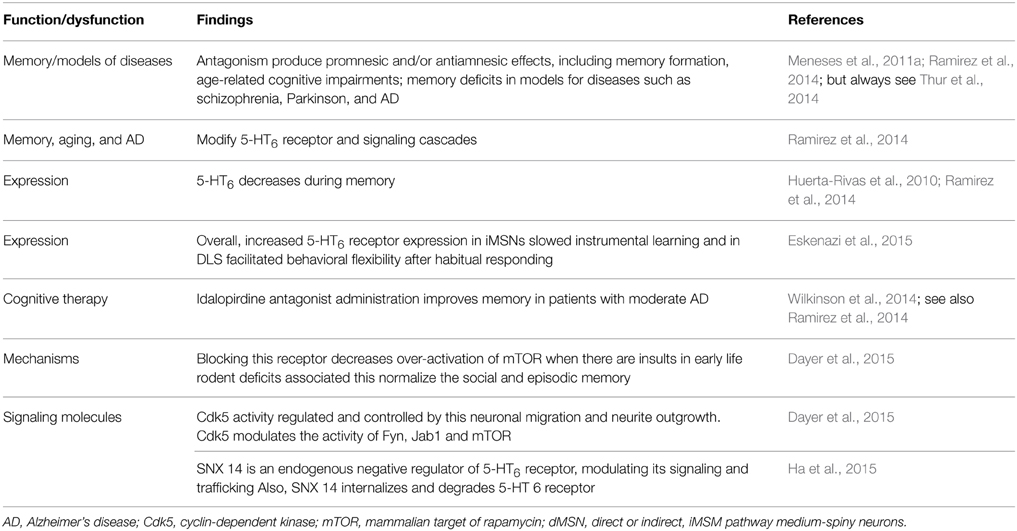
5-HT6 receptor
Diverse 5-HT6 receptor antagonists produce promnesic and/or antiamnesic effects in conditions, such as memory formation, age-related cognitive impairments; memory deficits in models of diseases such as schizophrenia, PD and AD (e.g., King et al., 2008; Claeysen et al., 2015). However, not all papers report promnesic and/antiamnesic effects of 5-HT6 receptor antagonists (e.g., Thur et al., 2014) (Table 7); probably related to timing, drug and memory task used. Memory, aging, and AD modify 5-HT6 receptors and signaling cascades; and 5-HT6 drugs modulate memory, which is accompanied with neural changes. Indeed, in an elegant work Eskenazi et al. (2015) manipulated selectively overexpression of 5-HT6 receptor in either direct or indirect pathway striatal medium-spiny neurons (dMSN and iMSN, respectively), revealing that increased 5-HT6 receptor expression in iMSNs delays instrumental learning and in DLS facilitates behavioral flexibility after habitual responding. It should be noted that 5-HT6 receptor expression decreases during memory (e.g., Huerta-Rivas et al., 2010; Ramirez et al., 2014). In addition, de Bruin and Kruse (2015) suggest that cognition could be improved by 5-HT6 receptor antagonists, by increasing the number of NCAM PSA-immunoreactive neurons in the dendate gyrus, inhibit mTOR and Fyn-tyrosine kinase and interact with DARPP-32.
Notably, 5-HT6 receptor antagonists are, among, serotonergic therapies for cognitive symptoms in AD (e.g., Ramirez et al., 2014). Indeed, Wilkinson et al. (2014) report safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate AD. In addition, 5-HT6 receptor is providing new insights about plasticity (Dayer et al., 2015). For example, at early stages of neuronal development, expression of 5-HT6 receptor constitutively regulates the activity of the cyclin-dependent kinase (Cdk) 5 and, through this mechanism, controls cellular processes involved in circuit formation (e.g., neuronal migration, neurite outgrowth). In addition, 5-HT6 receptor modulates developmental targets, including Fyn, Jab1, and mammalian target of rapamycin (mTOR). In therapeutic terms such as blockade of pathological over-activation of the mTOR pathway induced by early life insults in rodents and normalizes the associated social and episodic memory deficits. It should be noted that 5-HT6 receptor and Cdk5; and the latter mediates neuronal differentiation (e.g., hippocampus, striatum) in an agonist-independent manner (Seo and Tsai, 2014). In addition, Ha et al. (2015) report that 5-HT6 receptor directly interacts with SNX14 (protein-coupled receptors/regulators of G protein signaling), which regulates internalization; degradation of 5-HT6 receptor and cAMP production. This finding might be related to the evidence that 5-HT6 receptor agonists and antagonists modulate cAMP production and improve memory formation (e.g., Meneses et al., 2011c). We do not know yet why 5-HT6 receptor agonists and antagonists (e.g., Woods et al., 2012) may facilitate memory or may reverse amnesia in some memory tasks. However, 5-HT6 receptor inverse agonist might be useful (e.g., Hostetler et al., 2014; but see also Benhamú et al., 2014).
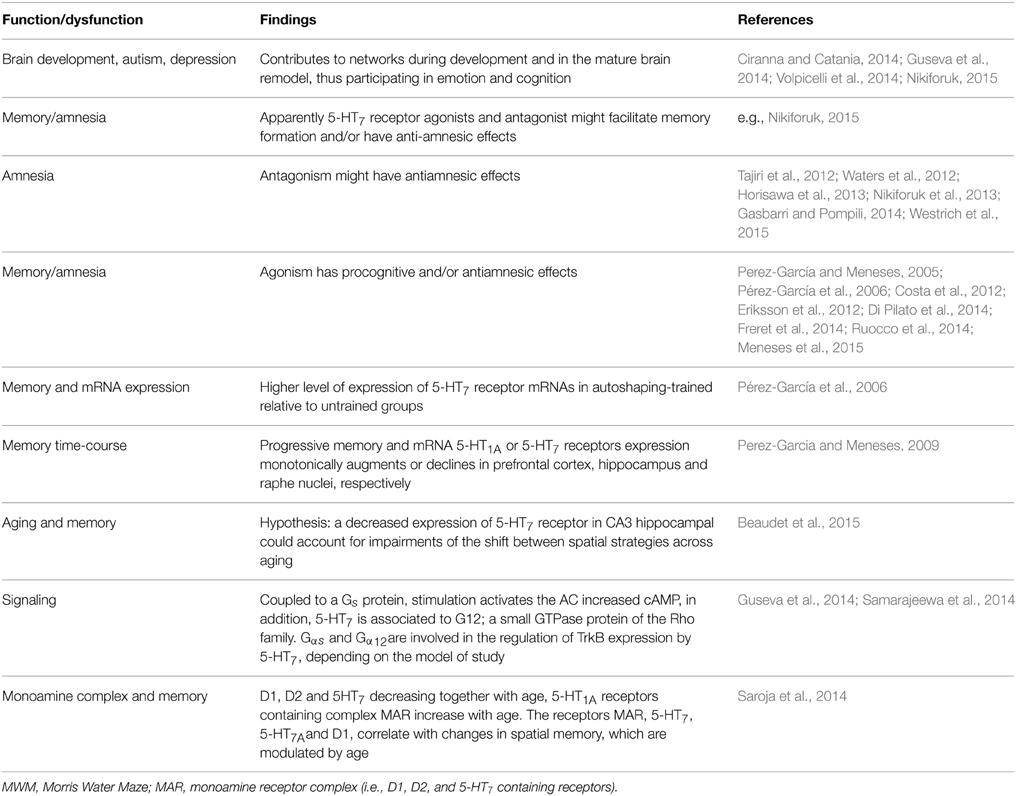
5-HT7 Receptor
Recently Nikiforuk (2015) is providing perspectives of 5-HT7 receptor in the search for treatments for CNS disorders: including normal and dysfunctional serotonin-induced phase shifting of the circadian rhythm control of memory as well as locomotor and exploratory activity, anxiety, depression; and Guseva et al. (2014) about molecular mechanisms responsible for the 5-HT7 receptor-mediated signaling. Gasbarri and Pompili (2014) noted that 5-HT7 receptor antagonism might have antiamnesic effects (see also Horisawa et al., 2013). Gasbarri et al. (2008) suggested that 5-HT7 receptor blockade had procognitive effect, when the learning task implicated a high degree of difficulty. Others report that 5-HT7 receptor agonists facilitate memory and have antiamnesic effects (Table 8); remaining clarifying why of the paradoxical effects.
Notably, Saroja et al. (2014), highlight that although evidence about monoamine receptor (MAR) biochemistry and pharmacology in aging exists, work on MAR complexes rather than subunits is limited; in consequence, MAR complexes in hippocampi of three different age groups (3–12 and 18 months) in mice and to link MAR changes to spatial memory retrieval in the water maze were determine (Table 8). MAR complexes were separated in order to show the pattern of dopamine and 5-HT1A and 5-HT7 receptors and colocalizations (Saroja et al., 2014). For instance, D1-D2 and 5-HT7 receptors containing receptor complex levels decreased with age while 5-HT1A receptor-containing complex was increasing. D1, 5-HT7, and 5-HT1A receptor complex correlated with good retrieval memory in the water maze; hence, individual monoamine receptors are linked to spatial memory and are modulated by age. However, Beaudet et al. (2015) mention that changes in the level of transcription of the 5-HT7 receptor mRNA did not account for the age-related difference observed at the protein level, at least in hippocampal CA3 region; besides, 5-HT7 receptor might also be putatively subjected, across aging, to modifications in their affinity or to changes in their coupling to G-proteins or other signaling pathways. Notably, Beaudet et al. (2015) suggest that a decreased expression of 5-HT7 receptor in CA3 hippocampal could account for impairments of the shift between spatial strategies across aging (Table 8).
Moreover, when the time-course (0–120 h) of autoshaped CR is progressive; then mRNA 5-HT1A or 5-HT7 receptors expression is monotonically augmented or decreased in prefrontal cortex, hippocampus and raphe nuclei, respectively (Perez-Garcia and Meneses, 2009). Hence, 5-HT1A and 5-HT7 receptors expression might be regulated by the level of memory formation and to be brain areas dependent. Moreover, the cyclic adenosine monophosphate (cAMP) is a second messenger and a central component of intracellular signaling pathways that regulate a wide range of biological functions, including memory (e.g., Kandel, 2001). And progressive time-course of memory formation in an autoshaping learning task (Pérez-García and Meneses, 2008); shows that ex-vivo cAMP production from trained and over-trained groups compared to untrained ones, the former group had the highest levels of cAMP and the latter rats showed increased production but less relative to trained rats. Importantly these changes varied according with normal memory or amnesia and brain areas; hence cAMP production is important in the signaling case in mammalian memory formation (Pérez-García and Meneses, 2008).
The above findings should be considered in the context that apparently 5-HT7 receptor agonists and antagonist (e.g., Nikiforuk, 2015) might facilitate memory formation and/or have anti-amnesic effects. Other interesting recent finding is that according with Rojas et al. (2014) serotonin regulates neurite outgrowth through 5-HT1A and 5-HT7 receptors in cultured hippocampal neurons. Certainly, De Filippis et al. (2015) highlight that promnesic effects of the 5-HT7 receptor agonist LP-211 treatment strongly depend on the basal level of performance. Notably, Ruocco et al. (2014) report that 5-HT7 receptor stimulation improves selective spatial attention and produces permanent changes in several neural markers, including expression of glutamatergic receptors and dopamine transporter (DAT).
Very importantly, 5-HT7 receptor can form heterodimers with 5-HT1A receptors both in-vitro and in-vivo (see Guseva et al., 2014) and according with these authors, from the functional point of view, heterodimerization decreases Gi-protein coupling of 5-HT1A receptor and attenuates receptor-mediate deactivation of G-protein-gated potassium (GIRK) channels, without substantial changes in the coupling of 5-HT7 receptor to the Gs-protein. Moreover, heterodimerization significantly facilitated internalization of 5-HT1A receptor, while internalization kinetics of 5-HT7 receptor was decelerated upon heterodimerization (see Guseva et al., 2014).
Factors Responsible for Inconsistencies among Laboratories
Neural Transporters, Memory, Forgetting and Drugs
Notwithstanding neurotransmission systems are related to memory formation, amnesia and/or therapeutic targets for memory alterations, the role of transporters γ-aminobutyric acid (GABA, GAT1), glutamate (neuronal glutamate transporter excitatory amino acid carrier; EACC1), dopamine (DAT) and serotonin (SERT) is poorly understood. Emerging evidence indicates that memory formation (short- and long-term memory; STM and LTM, respectively) in a Pavlovian/instrumental autoshaping (see Box 1) is associated to up-regulation of prefrontal cortex GAT1 and EAAC1, striatal SERT, DAT and EACC1; while, hippocampal EACC1, GAT1, and SERT are down-regulated (Tellez et al., 2012a,b; Table 9; Figure 1). Moreover, pharmacological analysis shows that methamphetamine (METH)- induced amnesia down-regulated SERT, DAT, EACC1, and GAT1 in hippocampus and the GAT1 in striatum; no-changes are observed in prefrontal cortex. Fluoxetine (antidepressant, 5-HT uptake inhibitor) improved memory consolidation (particularly LTM), which is associated to DAT, GAT1 (prefrontal cortex) up-regulation, but GAT1 (striatum) and SERT (hippocampus) down-regulation. Fluoxetine plus METH prevented amnesia, which was associated to DAT, EACC1 and GAT1 (prefrontal cortex), SERT and DAT (hippocampus) and EACC1 or DAT (striatal) up-regulation.
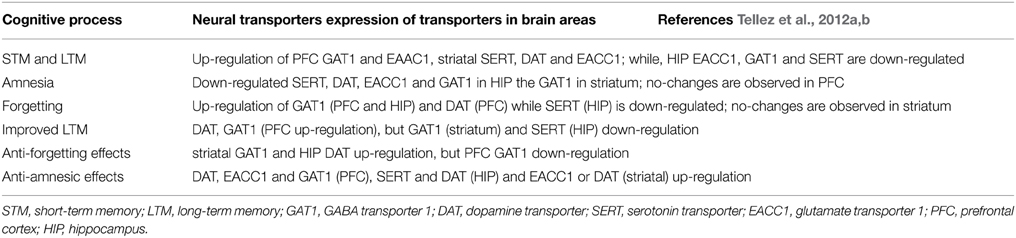
Table 9. Neural transporters during STM and LTM, amnesia (methamphetamine), forgetting, (fluoxetine) improved LTM, (fluoxetine) anti-forgetting effects and anti-amnesic (fluoxetine plus methamphetamine) effects.
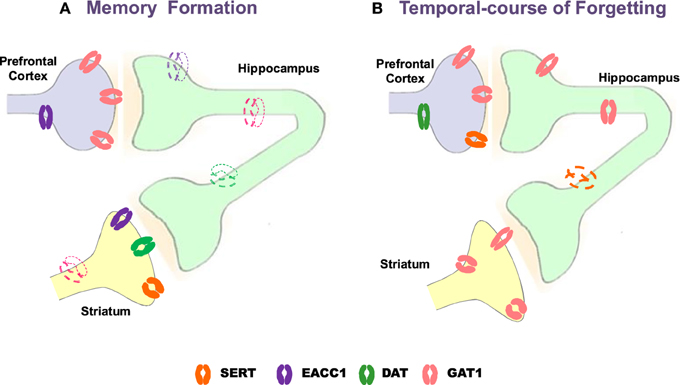
Figure 1. Schematic representation of changes with Western blot analysis of neural transporters in prefrontal cortex, hippocampus and striatum during memory formation and temporal-course of forgetting. Strong color refers to up-regulation, slight color refers to down-regulation. GAT1, GABA transporter 1; EAAC1, neuronal glutamate transporter excitatory amino acid carrier-1; DAT, dopamine transporter SERT, serotonin transporter (modified from Tellez et al., 2010, 2012a,b).
Memory Formation/Forgetting and SERT Expression
Forgetting in Pavlovian/instrumental autoshaping is associated to up-regulation of GAT1 (PFC and HIP) and DAT (PFC) while SERT (HIP) is down-regulated; no-changes are observed in striatum (Table 9). Methamphetamine alone not affected forgetting but up-regulates hippocampal DAT and EACC, prefrontal cortex DAT and striatal GAT1 or EACC1. Fluoxetine alone prevents forgetting, which is associated to striatal GAT1 and hippocampal DAT up-regulation, but prefrontal cortex GAT1 down-regulation. Fluoxetine plus METH prevent forgetting, which is associated to hippocampal DAT, prefrontal cortex SERT and striatal GAT1, DAT, or SERT up-regulation, but prefrontal cortex GAT1 down-regulation. Together these results show that forgetting provokes primarily hippocampal and prefrontal cortex transporters changes; it represents a cognitive process hardly modifiable and its prevention could causes different transporters expression patterns. Notably, together the results suggest that: (1) memory formation, amnesia and anti-amnesic effects are associated to specific patterns of transporters expression; (2) STM and LTM, forgetting and anti-forgetting effects are associated to specific patters of transporters expression and brain areas; (3) amnesia and forgetting affect different brain areas and produce differential patters of transporter expression. Hence, in pharmacological and neuroanatomical terms, amnesia and forgetting differ.
Neural Transporters and Brain Functions and Dysfunctions
It should be noted that neural transporters regulate intra-synaptic levels of neurotransmitter, which allows a global picture of synapses. Moreover, diverse evidence indicates that memory formation, forgetting, amnesia, and/or anti-amnesic effects can also be modulated by changes in the expression of neurotransmitter transporters (e.g., Schmitt and Hiemke, 2002; Chen et al., 2011; Reichel et al., 2012; Yang et al., 2013). Hence, a brief overview of evidence involving GAT1, EAAT1, SERT, and DAT as well other neurobiological markers regarding memory and other cerebral functions is include.
GAT 1
Attention deficit/hyperactivity disorder (ADHD) is featured by hyperactivity, impaired sustained attention, impulsivity, and usually varying degrees of dysfunctional learning and memory (see also Meneses et al., 2011b) and motor incoordination (Yang et al., 2013). Importantly, Yang et al. (2013) report that GAT1 gene knockout (KO) mouse (GAT1−/−) is hyperactive and exhibit impaired memory performance (Morris water maze). KO GAT1 mice have low levels of attentional focusing and increased impulsivity; the hyperactivity in these KO mice is reduced by both methylphenidate and amphetamine; Yang et al. (2013) suggest that GAT1 KO mouse is a new animal model for ADHD studying and GAT1 may be a new target to treat ADHD. Schmitt and Hiemke (2002) note that GABA is cleaved from the synaptic cleft by uptake (see Hu and Quick, 2008), via specific transporters and inhibition of such transporters increases the effectiveness of physiologically released GABA. Increased GABAergic neurotransmission has an impact on learning and memory. Indeed, tiagabine, a GABA-transporter inhibitor, impaired learning (Morris water-maze) and retrieval (only at the probe trial; Schmitt and Hiemke, 2002). But, Sałat et al. (2015) note that tiagabine slightly decreased memory but did not augment that induced by scopolamine. According with Shi et al. (2012), homozygous GAT1(−/−) mice exhibit impaired hippocampus-dependent learning and memory; and they evaluated the impact of endogenous reduced GABA reuptake on cognitive behaviors. Learning and memory of heterozygous GAT1(+/−) mice was determined in passive avoidance and Morris water maze; showing that GAT1(+/−) mice displayed increased learning and memory, decreased anxiety-like behaviors, and highest synaptic plasticity relative to wild-type and homozygous GAT1(−/−) mice; and authors conclude that a moderate reduction in GAT1 activity is associated to learning and memory facilitation (Shi et al., 2012); which is consistent, in part, with GAT1 reduced and increased expression in autoshaping amnesia, forgetting and improved memory as well as anti-amnesic and anti-forgetting effects (see Table 10). In addition, Pang et al. (2011) testing the GABAergic immunotoxin; GAT1-saporin (GAT1-SAP), report no alterations in spatial reference memory. But GAT1-SAP impaired the platform location in a delayed match to position test (changing daily the platform location). In the active avoidance task, intraseptal GAT1-SAP impaired extinction but not acquisition (Pang et al., 2011). In contrast, GAT1-Saporin into the medial septum/vertical limb of the diagonal band (MS/VDB) spared mnemonic function and use of environmental cues; however, self-movement cue processing was compromised (Köppen et al., 2013).
EAAT1
According with Chen et al. (2011), an imbalance of neurotransmitters (e.g., glutamate, acetylcholine, dopamine, and serotonin) has been proposed as the neurobiological basis of behavioral symptoms of AD, hence they are hypothesizing that altered reuptake of neurotransmitters by vesicular glutamate transporters (VGLUTs), excitatory amino acid transporters (EAATs), the vesicular acetylcholine transporter (VAChT), SERT or DAT. Examining protein and mRNA levels of these transporters in post-mortem prefrontal cortex from patients and matched non-AD controls, Chen et al. (2011) found that protein and mRNA levels of VGLUTs, EAAT1-3, VAChT, and SERT are reduced in AD, without changing DAT (Table 11). Chen et al. (2011) conclude that the reduced VAChT expression could contribute to cholinergic deficit in AD and altered neurotransmitter transporters could contribute to the pathophysiology of AD; which are potential targets for therapy (Chen et al., 2011).
Likewise, Kindlundh-Högberg et al. (2010) investigated the effect of intermittent 3,4-methylenedioxy-metamphetamine (MDMA; ecstasy) administration upon gene-transcript expression of the glutamate transporters (EAAT1, EAAT2-1, EAAT2-2), glutamate receptor subunits of AMPA (GluR1, GluR2, GluR3), glutamate receptor subunits of NMDA (NR1, NR2A, and NR2B), and metabotropic glutamate receptors (mGluR1, mGluR2, mGluR3, mGluR5); showing increased cortical expression of GluR2, mGluR1, mGluR5, NR1, NR2A, NR2B, EAAT1, and EAAT2-2 (Kindlundh-Högberg et al., 2010). In the caudate putamen, mRNA levels of GluR3, NR2A, and NR2B receptor subunits are increased; in contrast, GluR1 is reduced in the hippocampus but in the hypothalamus GluR1, GluR3, mGluR1, and mGluR3 expression is increased (Kindlundh-Högberg et al., 2010; see also Carmona et al., 2009); concluding that repeated MDMA administration is associated with changes in gene-transcript expressions of glutamatergic NMDA and AMPA receptor subunits, metabotropic receptors and transporters in brain areas mediating learning and memory (Kindlundh-Högberg et al., 2010). In addition, decreased expression of vesicular glutamate transporter 1 (VGLUT1+/−) respect to wild-type (WT) mice occur with chronic mild stress (CMS)-induced, affecting several functions and impairing recognition memory. In addition, Heo et al. (2012) detect hippocampal glutamate transporter 1 (GLT-1) complex expression during training and memory in the Multiple T-maze.
SERT
Reichel et al. (2012) report that control rats spent more time interacting with the objects in the changed locations. In contrast, contingent or non-contingent methamphetamine (meth) disrupted object-in-place (OIP) task performance as seen by similar amounts of time spent with all objects, regardless of location. While only acute meth binge produced signs of neurotoxicity, both meth regimens decreased SERT in the perirhinal cortex and hippocampus. Only meth self-administration resulted in a selective decrease in NET. Meth-induced changes in SERT function in the OIP circuitry may underlie memory deficits independently of overt neurotoxic effects (Reichel et al., 2012). It should be noted that SERT is reduced in AD (Chen et al., 2011; Claeysen et al., 2015).
Parrott (2013) highlights that decreased SERT (hippocampus, parietal cortex, and prefrontal cortex expression) in abstinent Ecstasy/MDMA users is associated to dysfunctional declarative and prospective memory. Even the children of mothers who take Ecstasy/MDMA during pregnancy have psychomotor impairments (Parrott, 2013). In addition, Thomasius et al. (2006) report reduced SERT expression, which might be a transient effect of heavy ecstasy use, since it partially recovered as the users reduced their MDMA use; though this parameter may not necessarily be a valid indicator of the number or integrity of serotonergic neurons. Importantly, ex-ecstasy users' verbal memory show no sign of improvement even after over 2.5 years of abstinence and thus may represent persistent functional consequences of MDMA neurotoxicity; alternative causes such as pre-existing group differences cannot be excluded (Thomasius et al., 2006). In addition, AD and drugs of abuse like d-methamphetamine (METH) or MDMA have been associated to decrements in the SERT expression and memory deficits; thus supporting the notion that the SERT plays a key role in both normal and pathological states (e.g., Line et al., 2014). Particularly, the s allele of the polymorphic regulatory region of the SERT or 5-HTT gene promoter is associated with reduced 5-HTT expression and vulnerability to psychiatric disorders, including anxiety and depression. Moreover, the l allele increases 5-HTT expression and is generally considered protective (Line et al., 2014). However, Line et al. (2014) suggest that 5-HTT over-expression results in a reduced sensitivity to both positive and negative reinforcers, and produces some maladaptive effects, supporting recent suggestions that l allele homozygosity may be a potential risk factor for disabling psychiatric traits (Line et al., 2014). In contrast, increased 5-HTT expression reduces negative cognitive bias for stimuli with uncertain outcomes (McHugh et al., 2015). And Brigman et al. (2010) report that fluoxetine-treated C57BL/6J mice made fewer errors than controls during the early phase of learning reversal when perseverative behavior is relatively high and 5-HTT null mice made fewer errors than controls in completing the reversal task (Table 12). And these authors suggest that inactivating 5-HTT improves reversal learning, which is relevant for the pathophysiology and treatment of neuropsychiatric disorders characterized by executive dysfunction (Brigman et al., 2010) and possibly post-traumatic stress disorder.
Certainly, SERT is providing useful information as neural marker and therapeutic target. For instance, Wallace et al. (2014) report that vortioxetine, a novel, multimodal-acting antidepressant, is a 5-HT3, 5-HT7, and 5-HT1D receptor antagonist, a 5-HT1B receptor partial agonist, a 5-HT1A receptor agonist, and inhibits the 5-HT transporter. This drug changes the expression of multiple genes involved in neuronal plasticity by antidepressant treatment, which is associated with improved cognitive function and a reduction in depression-like behavior in middle-aged mice (Li et al., 2015c).
Hence, the SERT expression seems to be a reliable neural marker related to memory mechanisms, its alterations and potential treatment (Meneses, 2013). Resulting crucial determining the pharmacological, neural and molecular mechanisms associated to these changes and therapeutic targets. For instance, Sheline et al. (2014a) report that serotonin signaling suppresses generation of amyloid-β (Aβ) in-vitro and in animal models of AD and healthy individuals. In fact, in an aged transgenic AD mouse model the antidepressant citalopram (5-HT uptake inhibitor) in dose-dependent manner decreased Aβ in cerebrospinal fluid, suggesting AD prevention trials (Sheline et al., 2014a,b).
DAT
According with Mereu et al. (2013), modafinil (MOD) and its R-enantiomer (R-MOD) are used for narcolepsy and sleep disorders; and also employed, off-label used as cognitive enhancers in individuals with mental disorders, including substance abusers that demonstrate impaired cognitive function. Their mechanisms of action include inhibition of dopamine (DA) reuptake via the DAT in diverse brain areas (Mereu et al., 2013; Table 13). Importantly, memantine (MEM), a dual antagonist of NMDA and alpha7 receptors, is neuroprotector against MDMA in rats, and it also prevents MDMA effect on SERT functionality and METH effect on DAT (Escubedo et al., 2009). Moreover, Söderqvist et al. (2012) have noted that dopamine plays an important role not only in dysfunctional working memory (WM) but also for improving it, including variation in DAT1, improving WM and fluid intelligence in preschool-age children following cognitive training; concluding with the role of dopamine in determining cognitive plasticity (Söderqvist et al., 2012). Ruocco et al. (2014) report that 5-HT7 receptor stimulation (low doses) was associated to among other findings reduced horizontal activity and (at higher dose) increased selective spatial attention, the DAT levels were decreased (low dose), and modulated expression of NMDA receptors.
It should be noted that, before the perspective of the absence of effective treatments for dysfunctional memory and regardless the mechanisms; environmental interventions and exercise (physical and cognitive) seem offer feasible approaches (e.g., Mora, 2013; Mo et al., 2015).
Conclusions
Of course if the above findings are replicated over time, across countries and in different experimental settings, they might provide insights about serotonin and other neurotransmission systems presenting convergent changes in diverse neural markers and signaling; thus, allowing the study of different brain functions and dysfunctions, including memory. Hence, diverse approaches might support the translatability of using neural markers and cerebral functions and dysfunctions (e.g., memory formation, AD, MCI). Likewise, hypothesis and theories (e.g., Borroto-Escuela et al., 2015) might provide appropriate limits and perspectives of the diversity of evidence. Certainly, at least, 5-HT1A, 5-HT4, 5-HT5, 5-HT6, and 5-HT7 receptors as well as SERT seem to be useful as neural markers and therapeutic targets.
Conflict of Interest Statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abela, A. R., Dougherty, S. D., Fagen, E. D., Hill, C. J. R., and Chudasama, Y. (2013). Inhibitory control deficits in rats with ventral hippocampal lesions. Cereb. Cortex 23, 1396–1409. doi: 10.1093/cercor/bhs121
Alabdali, A., Al-Ayadhi, L., and El-Ansary, A. (2014). Association of social and cognitive impairment and biomarkers in autism spectrum disorders. J. Neuroinflammation 11:4. doi: 10.1186/1742-2094-11-4
Aloyo, V. J., Berg, K. A., Spampinato, U., Clarke, W. P., and Harvey, J. A. (2009). Current status of inverse agonism at serotonin2A (5-HT2A) and 5-HT2C receptors. Pharmacol. Ther. 121, 160–173. doi: 10.1016/j.pharmthera.2008.10.010
Atnip, G. W. (1977). Stimulus- and response-reinforcer contingencies in autoshaping, operant, classical, and omission training procedures in rats. J. Exp. Anal. Behav. 28, 59–69. doi: 10.1901/jeab.1977.28-59
Aubert, Y., Allers, K. A., Sommer, B., de Kloet, E. R., Abbott, D. H., and Datson, N. A. (2013). Brain region-specific transcriptomic markers of serotonin-1A receptor agonist action mediating sexual rejection and aggression in female marmoset monkeys. J. Sex Med. 10, 1461–1475. doi: 10.1111/jsm.12131
Baas, J. M., and Heitland, I. (2014). The impact of cue learning, trait anxiety and genetic variation in the serotonin 1A receptor on contextual fear. Int. J. Psychophysiol. doi: 10.1016/j.ijpsycho.2014.10.016. [Epub ahead of print].
Baba, S., Murai, T., Nakako, T., Enomoto, T., Ono, M., Shimizu, I., et al. (2015). The serotonin 5-HT1A receptor agonist tandospirone improves executive function in common marmosets. Behav. Brain Res. 287, 120–126. doi: 10.1016/j.bbr.2015.03.025
Ballaz, S. J., Akil, H., and Watson, S. J. (2007). The 5-HT7 receptor: role in novel object discrimination and relation to novelty-seeking behavior. Neuroscience 149, 192–202. doi: 10.1016/j.neuroscience.2007.07.043
Barlow, R. L., Alsiö, J., Jupp, B., Rabinovich, R., Shrestha, S., Roberts, A. C., et al. (2015). Markers of serotonergic function in the orbitofrontal cortex and dorsal raphé nucleus predict individual variation in spatial-discrimination serial reversal learning. Neuropsychopharmacology 40, 1619–1630. doi: 10.1038/npp.2014.335
Beaudet, G., Bouet, V., Jozet-Alves, C., Schumann-Bard, P., Dauphin, F., Paizanis, E., et al. (2015). Spatial memory deficit across aging: current insights of the role of 5-HT7 receptors. Front. Behav. Neurosci. 8:448. doi: 10.3389/fnbeh.2014.00448
Benhamú, B., Martín-Fontecha, M., Vázquez-Villa, H., Pardo, L., and López-Rodríguez, M. L. (2014). Serotonin 5-HT6 receptor antagonists for the treatment of cognitive deficiency in Alzheimer's disease. J. Med. Chem. 57, 7160–7181. doi: 10.1021/jm5003952
Berry, J. A., Cervantes-Sandoval, I., Nicholas, E. P., and Davis, R. L. (2012). Dopamine is required for learning and forgetting in Drosophila. Neuron 74, 530–542. doi: 10.1016/j.neuron.2012.04.007
Blasi, G., Selvaggi, P., Fazio, L., Antonucci, L. A., Taurisano, P., Masellis, R., et al. (2015). Variation in Dopamine D2 and Serotonin 5-HT2A receptor genes is associated with working memory processing and response to treatment with antipsychotics. Neuropsychopharmacology 40, 1600–1608. doi: 10.1038/npp.2015.5
Blenau, W., and Baumann, A. (eds.). (2015). Serotonin Receptor Technologies, in Neuromethods, Vol. 95. New York, NY: Springer Science+Business Media.
Bockaert, J., Claeysen, S., Compan, V., and Dumuis, A. (2011). 5-HT4 receptors, a place in the sun: act two. Curr. Opin. Pharmacol. 11, 87–93. doi: 10.1016/j.coph.2011.01.012
Borg, J. (2008). Molecular imaging of the 5-HT1A receptor in relation to human cognition. Behav. Brain Res. 195, 103–111. doi: 10.1016/j.bbr.2008.06.011
Borg, J., Henningsson, S., Saijo, T., Inoue, M., Bah, J., Westberg, L., et al. (2009). Serotonin transporter genotype is associated with cognitive performance but not regional 5-HT1A receptor binding in humans. Int. J. Neuropsychopharmacol. 12, 783–792. doi: 10.1017/S1461145708009759
Borroto-Escuela, D. O., Agnati, L. F., Bechter, K., Jansson, A., Tarakanov, A. O., and Fuxe, K. (2015). The role of transmitter diffusion and flow versus extracellular vesicles in volume transmission in the brain neural-glial networks. Philos. Trans. R. Soc. Lond. B Biol. Sci. 370:20140183. doi: 10.1098/rstb.2014.0183
Brigman, J. L., Mathur, P., Harvey-White, J., Izquierdo, A., Saksida, L. M., Bussey, T. J., et al. (2010). Pharmacological or genetic inactivation of the serotonin transporter improves reversal learning in mice. Cereb. Cortex 20, 1955–1963. doi: 10.1093/cercor/bhp266
Brown, P. L., and Jenkins, H. M. (1968). Auto-shaping of the pigeon's key-peck. J. Exp. Anal. Behav. 11, 1–8. doi: 10.1901/jeab.1968.11-1
Buhot, M. C., Wolff, M., Benhassine, N., Costet, P., Hen, R., and Segu, L. (2003a). Spatial learning in the 5-HT1B receptor knockout mouse: selective facilitation/impairment depending on the cognitive demand. Learn. Mem. 10, 466–477.
Buhot, M. C., Wolff, M., Savova, M., Malleret, G., Hen, R., and Segu, L. (2003b). Protective effect of 5-HT1B receptor gene deletion on the age-related decline in spatial learning abilities in mice. Behav. Brain Res. 142, 135–142. doi: 10.1016/S0166-4328(02)00400-X
Bussey, T. J., Barch, D. M., and Baxter, M. G. (2013). Testing long-term memory in animal models of schizophrenia: suggestions from CNTRICS. Neurosci. Biobehav. Rev. 37, 2141–2148. doi: 10.1016/j.neubiorev.2013.06.005
Bussey, T. J., Everitt, B. J., and Robbins, T. W. (1997). Dissociable effects of cingulate and medial frontal cortex lesions on stimulus-reward learning using a novel Pavlovian autoshaping procedure for the rat: implications for the neurobiology of emotion. Behav. Neurosci. 111, 908–919. doi: 10.1037/0735-7044.111.5.908
Callaghan, B. L., Li, S., and Richardson, R. (2014). The elusive engram: what can infantile amnesia tell us about memory? Trends Neurosci. 37, 47–53. doi: 10.1016/j.tins.2013.10.007
Carmona, M., Muraib, K., Wanga, L., Roberts, A., and Pasquale, E. (2009). Glial ephrin-A3 regulates hippocampal dendritic spine morphology and glutamate transport. Proc. Natl. Acad. Sci. U.S.A. 106, 12524–12529. doi: 10.1073/pnas.0903328106
Cavallaro, S. (2008). Genomic analysis of serotonin receptors in learning and memory. Behav. Brain Res. 195, 2–6. doi: 10.1016/j.bbr.2007.12.003
Chen, K. H., Reese, E. A., Kim, H. W., Rapoport, S. I., and Rao, J. S. (2011). Disturbed neurotransmitter transporter expression in Alzheimer's disease brain. J. Alzheimers Dis. 26, 755–766. doi: 10.3233/JAD-2011-110002
Chen, S., Cai, D., Pearce, K., Sun, P. Y., Roberts, A. C., and Glanzman, D. L. (2014). Reinstatement of long-term memory following erasure of its behavioral and synaptic expression in Aplysia. Elife 3:e03896. doi: 10.7554/eLife.03896
Ciranna, L., and Catania, M. V. (2014). 5-HT7 receptors as modulators of neuronal excitability, synaptic transmission and plasticity: physiological role and possible implications in autism spectrum disorders. Front. Cell. Neurosci. 8:250. doi: 10.3389/fncel.2014.00250
Claeysen, S., Bockaert, J., and Giannoni, P. (2015). Serotonin: a new hope in Alzheimer's disease? ACS Chem. Neurosci. doi: 10.1021/acschemneuro.5b00135. [Epub ahead of print].
Cook, R. G., Geller, A. I., Zhang, G. R., and Gowda, R. (2004). Touch screen-enhanced visual learning in rats. Behav. Res. Meth. Instrum. 36, 101–106. doi: 10.3758/BF03195555
Costa, L., Spatuzza, M., D'Antoni, S., Bonaccorso, C. M., Trovato, C., Musumeci, S. A., et al. (2012). Activation of 5-HT7 serotonin receptors reverses metabotropic glutamate receptor-mediated synaptic plasticity in wild-type and Fmr1 knockout mice, a model of Fragile X syndrome. Biol. Psychiatry 72, 924–933. doi: 10.1016/j.biopsych.2012.06.008
Costall, B., and Naylor, R. J. (1992). Astra award lecture. The psychopharmacology of 5-HT3 receptors. Pharmacol. Toxicol. 71, 401–415. doi: 10.1111/j.1600-0773.1992.tb00570.x
Dayer, A. G., Jacobshagen, M., Chaumont-Dubel, S., and Marin, P. (2015). 5-HT6 receptor: a new player controlling the development of neural circuits. ACS Chem. Neurosci. doi: 10.1021/cn500326z. [Epub ahead of print].
Da Silva Costa-Aze, V., Quiedeville, A., Boulouard, M., and Dauphin, F. (2012). 5-HT6 receptor blockade differentially affects scopolamine-induced deficits of working memory, recognition memory and aversive learning in mice. Psychopharmacology (Berl). 222, 99–115. doi: 10.1007/s00213-011-2627-3
de Bruin, N. M., and Kruse, C. G. (2015). 5-HT6 receptor antagonists: potential efficacy for the treatment of cognitive impairment in Schizophrenia. Curr. Pharm. Des. [Epub ahead of print].
De Filippis, B., Chiodi, V., Adriani, W., Lacivita, E., Mallozzi, C., Leopoldo, M., et al. (2015). Long-1 lasting beneficial effects of central serotonin receptor 7 stimulation in female mice modeling Rett syndrome. Front. Behav. Neurosci. 9:86. doi: 10.3389/fnbeh.2015.00086
Delotterie, D. F., Mathis, C., Cassel, J. C., Rosenbrock, H., Dorner-Ciossek, C., and Marti, A. (2015). Touchscreen tasks in mice to demonstrate differences between hippocampal and striatal functions. Neurobiol. Learn. Mem. 120, 16–27. doi: 10.1016/j.nlm.2015.02.007
Di Pilato, P., Niso, M., Adriani, W., Romano, E., Travaglini, D., Berardi, F., et al. (2014). Selective agonists for serotonin 7 (5-HT7) receptor and their applications in preclinical models: an overview. Rev. Neurosci. 25, 401–415. doi: 10.1515/revneuro-2014-0009
Drago, A., Alboni, S., Brunello, N., De Ronchi, D., and Serretti, A. (2010). HTR1B as a risk profile maker in psychiatric disorders: a review through motivation and memory. Eur. J. Clin. Pharmacol. 66, 5–27. doi: 10.1007/s00228-009-0724-6
Duewer, D., Currie, L., Reeder, D., Leigh, S., Liu, H., and Mudd, L. (1995). Interlaboratory comparison of autoradiographic DNA profiling measurements. 2. Measurement uncertainty and its propagation. Anal. Chem. 67, 1220–1231. doi: 10.1021/ac00103a013
Eglen, R. M., Wong, E. H., Dumuis, A., and Bockaert, J. (1995). Central 5-HT4 receptors. TIPS 16, 391–398.
Eriksson, T. M., Holst, S., Stan, T. L., Hager, T., Sjögren, B., Ogren, S. Ö., et al. (2012). 5-HT1Aand 5-HT7 receptor crosstalk in the regulation of emotional memory: implications for effects of selective serotonin reuptake inhibitors. Neuropharmacology 63, 1150–1160. doi: 10.1016/j.neuropharm.2012.06.061
Escubedo, E., Camarasa, J., Chipana, C., García-Ratés, S., and Pubill, D. (2009). Involvement of nicotinic receptors in methamphetamine- and MDMA-induced neurotoxicity: pharmacological implications. Int. Rev. Neurobiol. 88, 121–166. doi: 10.1016/S0074-7742(09)88006-9
Eshkoor, S. A., Hamid, T. A., Mun, C. Y., and Ng, C. K. (2015). Document mild cognitive impairment and its management in older people. Clin. Interv. Aging 10, 687–693. doi: 10.2147/CIA.S73922
Eskenazi, D., Brodsky, M., and Neumaier, J. F. (2015). Deconstructing 5-HT6 receptor effects on striatal circuit function. Neuroscience 299, 97–106. doi: 10.1016/j.neuroscience.2015.04.046
Euston, D. R., Gruber, A. J., and McNaughton, B. L. (2012). The role of medial prefrontal cortex in memory and decision making. Neuron 76, 1057–1070. doi: 10.1016/j.neuron.2012.12.002
Fakhfouri, G., Mousavizadeh, K., Mehr, S. E., Dehpour, A. R., Zirak, M. R., Ghia, J. E., et al. (2014). From chemotherapy-induced emesis to neuroprotection: therapeutic opportunities for 5-HT3 receptor antagonists. Mol. Neurobiol. doi: 10.1007/s12035-014-8957-5. [Epub ahead of print].
Fink, L. H., Anastasio, N. C., Fox, R. G., Rice, K. C., Moeller, F. G., and Cunningham, K. A. (2015). Individual differences in impulsive action reflect variation in the cortical Serotonin 5-HT2A receptor system. Neuropsychopharmacology 40, 1957–1968. doi: 10.1038/npp.2015.46
Fioravanti, M., and Di Cesare, F. (1992). Forgetting curves in long-term memory: evidence for a multistage model of retention. Brain Cogn. 18, 116–124. doi: 10.1016/0278-2626(92)90073-U
Fitzpatrick, C. J., Gopalakrishnan, S., Cogan, E. S., Yager, L. M., Meyer, P. J., Lovic, V., et al. (2013). Variation in the form of Pavlovian conditioned approach behavior among outbred male Sprague-Dawley rats from different vendors and colonies: sign-tracking vs. goal-tracking. PLoS ONE 8:e75042. doi: 10.1371/journal.pone.0075042
Freret, T., Paizanis, E., Beaudet, G., Gusmao-Montaigne, A., Nee, G., Dauphin, F., et al. (2014). Modulation of 5-HT7 receptor: effect on object recognition performances in mice. Psychopharmacology 231, 393–400. doi: 10.1007/s00213-013-3247-x
Gallistel, C. R., Balci, F., Freestone, D., Kheifets, A., and King, A. (2014). Automated, quantitative cognitive/behavioral screening of mice: for genetics, pharmacology, animal cognition and undergraduate instruction. J. Vis. Exp. e51047. doi: 10.3791/51047
Garcia-Alloza, M., Hirst, W. D., Chen, C. P., Lasheras, B., Francis, P. T., and Ramírez, M. J. (2004). Differential involvement of 5-HT1B/1D and 5-HT6 receptors in cognitive and non-cognitive symptoms in Alzheimer's disease. Neuropsychopharmacology 29, 410–416. doi: 10.1038/sj.npp.1300330
Garcia-Garcia, A. L., Elizalde, N., Matrov, D., Harro, J., Wojcik, S. M., Venzala, E., et al. (2009). Increased vulnerability to depressive-like behavior of mice with decreased expression of VGLUT1. Biol. Psychiatry 66, 275–282. doi: 10.1016/j.biopsych.2009.02.027
Gasbarri, A., Cifariello, A., Pompili, A., and Meneses, A. (2008). Effect of 5-HT7 antagonist SB-269970 in the modulation of working and reference memory in the rat. Behav. Brain Res. 195, 164–170. doi: 10.1016/j.bbr.2007.12.020
Gasbarri, A., and Pompili, A. (2014). Serotonergic 5-HT7 receptors and cognition. Rev. Neurosci. 25, 311–323. doi: 10.1515/revneuro-2013-0066
Glikmann-Johnston, Y., Saling, M. M., Chen, J., O'Keefe, G., Gong, S., Tochon-Danguy, H., et al. (2015). Hippocampal 5-HT1A receptor binding is related to object-location memory in humans. Brain Struct. Funct. 220, 559–570. doi: 10.1007/s00429-013-0675-7
Gonzalez, R., Chávez-Pascacio, K., and Meneses, A. (2013). Role of 5-HT5A receptors in the consolidation of memory. Behav. Brain Res. 252, 246–251. doi: 10.1016/j.bbr.2013.05.051
Guglielmi, V., Bizzarro, A., Valenza, A., Lauria, A., Tiziano, F., Lomastro, R., et al. (2015). A Functional 5HT2A receptor polymorphism (HIS452TYR) and memory performances in Alzheimer's disease. Int. J. Neurosci. 22, 1–16. doi: 10.3109/00207454.2015.1045976
Guseva, D., Wirth, A., and Ponimaskin, E. (2014). Cellular mechanisms of the 5-HT7 receptor-mediated signaling. Front. Behav. Neurosci. 8:306. doi: 10.3389/fnbeh.2014.00306
Gyurko, M. D., Stetak, A., Soti, C., and Csermely, P. (2015). Multitarget network strategies to influence memory and forgetting: the ras/mapk pathway as a novel option. Mini Rev. Med. Chem. 15, 696–704. doi: 10.2174/1389557515666150219144336
Ha, C. M., Park, D., Kim, Y., Na, M., Panda, S., Won, S., et al. (2015). SNX14 is a bifunctional negative regulator for neuronal 5-HT6 receptor signaling. J. Cell Sci. 128, 1848–1861. doi: 10.1242/jcs.169581
Haahr, M. E., Fisher, P., Holst, K., Madsen, K., Jensen, C. G., Marner, L., et al. (2013). The 5-HT4 receptor levels in hippocampus correlates inversely with memory test performance in humans. Hum. Brain Mapp. 34, 3066–3074. doi: 10.1002/hbm.22123
Hardt, O., Nader, K., and Nadel, L. (2013). Decay happens: the role of active forgetting in memory. Trends Cogn. Sci. 17, 111–120. doi: 10.1016/j.tics.2013.01.001
Hashimoto, K. (2015). Tropisetron and its targets in Alzheimer's disease. Expert Opin. Ther. Targets 19, 1–5. doi: 10.1517/14728222.2014.983901
Hautzel, H., Müller, H. W., Herzog, H., and Grandt, R. (2011). Cognition-induced modulation of serotonin in the orbitofrontal cortex: a controlled cross-over PET study of a delayed match-to-sample task using the 5-HT2a receptor antagonist [18F] altanserin. Neuroimage 58, 905–911. doi: 10.1016/j.neuroimage.2011.06.009
Heo, S., Jung, G., Beuk, T., Höger, H., and Lubec, G. (2012). Hippocampal glutamate transporter 1 (GLT-1) complex levels are paralleling memory training in the multiple T-maze in C57BL/6J mice. Brain Struct. Funct. 217, 363–378. doi: 10.1007/s00429-011-0362-5
Holland, P. C., Asem, J. S., Galvin, C. P., Keeney, C. H., Hsu, M., Miller, A., et al. (2014). Blocking in autoshaped lever-pressing procedures with rats. Learn. Behav. 42, 1–21. doi: 10.3758/s13420-013-0120-z
Horisawa, T., Nishikawa, H., Toma, S., Ikeda, A., Horiguchi, M., Ono, M., et al. (2013). The role of 5-HT7 receptor antagonism in the amelioration of MK-801-induced learning and memory deficits by the novel atypical antipsychotic drug lurasidone. Behav. Brain Res. 244, 66–69. doi: 10.1016/j.bbr.2013.01.026
Horner, A. E., Heath, C. J., Hvoslef-Eide, M., Kent, B. A., Kim, C. H., Nilsson, S. R., et al. (2013). The touchscreen operant platform for testing learning and memory in rats and mice. Nat. Protoc. 8, 1961–1984. doi: 10.1038/nprot.2013.122
Hostetler, G., Dunn, D., McKenna, B. A., Kopec, K., and Chatterjee, S. (2014). In search of potent 5-HT6 receptor inverse agonists. Chem. Biol. Drug Design 83, 666–669. doi: 10.1111/cbdd.12279
Hoyer, D., Clarke, D. E., Fozard, J. R., Hartig, P. R., Martin, G. R., Mylecharane, E. J., et al. (1994). International union of pharmacology classification of receptors for 5-hydroxytryptamine (Serotonin). Pharmacol. Rev. 46, 157–203.
Hu, J., and Quick, M. (2008). Substrate-mediated regulation of γ-aminobutyric acid transporter 1 in rat brain. Neuropharmacology 54, 309–318. doi: 10.1016/j.neuropharm.2007.09.013
Huerta-Rivas, A., Pérez-García, G., González-Espinosa, C., and Meneses, A. (2010). Time-course of 5-HT6 receptor mRNA expression during memory consolidation and amnesia. Neurobiol. Learn. Mem. 93, 99–110. doi: 10.1016/j.nlm.2009.08.009
Hupbach, A. (2013). When forgetting preserves memory. Front. Psychol. 4:32. doi: 10.3389/fpsyg.2013.00032
Kaku, M., Yamada, K., and Ichitani, Y. (2013). Can rats control previously acquired spatial information? Evidence of “directed forgetting” phenomenon in delay-interposed radial maze behavior. Behav. Brain Res. 248, 1–6. doi: 10.1016/j.bbr.2013.03.030
Kandel, E. R. (2001). The molecular biology of memory storage: a dialogue between genes and synapses. Science 294, 1030–1038. doi: 10.1126/science.1067020
Karimi, B., Hafidzi, M. N., Panandam, J. M., and Fuzina, N. H. (2013). Comparison of effect of sex hormone manipulation during neonatal period, on mRNA expression of Slc9a4, Nr3c2, Htr5b and Mas1 in hippocampus and frontal cortex of male and female rats. J. Biol. Regul. Homeost. Agents 27, 869–874.
Kindlundh-Högberg, A. M., Blomqvist, A., Malki, R., and Schiöth, H. B. (2008). Extensive neuroadaptive changes in cortical gene-transcript expressions of the glutamate system in response to repeated intermittent MDMA administration in adolescent rats. BMC Neurosci. 9:39. doi: 10.1186/1471-2202-9-39
Kindlundh-Högberg, A. M., Pickering, C., Wicher, G., Hobér, D., Schiöth, H. B., and Fex Svenningsen, A. (2010). MDMA (Ecstasy) decreases the number of neurons and stem cells in embryonic cortical cultures. Cell. Mol. Neurobiol. 30, 13–21. doi: 10.1007/s10571-009-9426-y
King, M. V., Marsden, C. A., Fone, K. C. (2008). A role for the 5-HT1A, 5-HT4 and 5-HT6 receptors in learning and memory. Trends Pharmacol. Sci. 29, 482–492. doi: 10.1016/j.tips.2008.07.001
Kitamura, S., Yasuno, F., Inoue, M., Kosaka, J., Kiuchi, K., Matsuoka, K., et al. (2014). Increased binding of 5-HT1A receptors in a dissociative amnesic patient after the recovery process. Psychiatry Res. 224, 67–71. doi: 10.1016/j.pscychresns.2014.07.001
Kondo, M., Nakamura, Y., Ishida, Y., and Shimada, S. (2014). The 5-HT3 receptor is essential for exercise-induced hippocampal neurogenesis and antidepressant effects. Mol. Psychiatry doi: 10.1038/mp.2014.153. [Epub ahead of print].
Köppen, J. R., Winter, S. S., Stuebing, S. L., Cheatwood, J. L., and Wallace, D. G. (2013). Infusion of GAT1-saporin into the medial septum/vertical limb of the diagonal band disrupts self-movement cue processing and spares mnemonic function. Brain Struct. Funct. 218, 1099–1114. doi: 10.1007/s00429-012-0449-7
Kozuska, J. L., Paulsen, I. M., Belfield, W. J., Martin, I. L., Cole, D. J., Holt, A., et al. (2014). Impact of intracellular domain flexibility upon properties of activated human 5-HT3 receptors. Br. J. Pharmacol. 171, 1617–1628. doi: 10.1111/bph.12536
Krynetskiy, E., Krynetskaia, N., Rihawi, D., Wieczerzak, K., Ciummo, V., and Walker, E. (2013). Establishing a model for assessing DNA damage in murine brain cells as a molecular marker of chemotherapy-associated cognitive impairment. Life Sci. 93, 605–610. doi: 10.1016/j.lfs.2013.03.013
Lau, T., Proissl, V., Ziegler, J., and Schloss, P. (2015). Visualization of neurotransmitter uptake and release in serotonergic neurons. J. Neurosci. Methods 241, 10–17. doi: 10.1016/j.jneumeth.2014.12.009
Lecoutey, C., Hedou, D., Freret, T., Giannoni, P., Gaven, F., Since, M., et al. (2014). Design of donecopride, a dual serotonin subtype 4 receptor agonist/acetylcholinesterase inhibitor with potential interest for Alzheimer's disease treatment. Proc. Natl. Acad. Sci. U.S.A. 111, E3825–E3830. doi: 10.1073/pnas.1410315111
Leger, M., Paizanis, E., Dzahini, K., Quiedeville, A., Bouet, V., Cassel, J. C., et al. (2014). Environmental enrichment duration differentially affects behavior and neuroplasticity in adult mice. Cereb. Cortex. doi: 10.1093/cercor/bhu119. [Epub ahead of print].
Leiser, S. C., Li, Y., Pehrson, A. L., Dale, E., Smagin, G., and Sanchez, C. (2015). Serotonergic regulation of prefrontal cortical circuitries involved in cognitive processing: a review of individual 5-HT receptor mechanisms and concerted effects of 5-HT receptors exemplified by the multimodal antidepressant vortioxetine. ACS Chem. Neurosci. doi: 10.1021/cn500340j. [Epub ahead of print].
Lesaint, F., Sigaud, O., Flagel, S. B., Robinson, T. E., and Khamassi, M. (2014). Modelling individual differences in the form of Pavlovian conditioned approach responses: a dual learning systems approach with factored representations. PLoS Comput. Biol. 10:e1003466. doi: 10.1371/journal.pcbi.1003466
Li, H. J., Peng, R. Y., Wang, C. Z., Qiao, S. M., Yong, Z., Gao, Y. B., et al. (2015b). Alterations of cognitive function and 5-HT system in rats after long term microwave exposure. Physiol. Behav. 140, 236–246. doi: 10.1016/j.physbeh.2014.12.039
Li, L. B., Zhang, L., Sun, Y. N., Han, L. N., Wu, Z. H., Zhang, Q. J., et al. (2015a). Activation of serotonin2A receptors in the medial septum-diagonal band of Broca complex enhanced working memory in the hemiparkinsonian rats. Neuropharmacology 91, 23–33. doi: 10.1016/j.neuropharm.2014.11.025
Li, S., and Richardson, R. (2013). Traces of memory: reacquisition of fear following forgetting is NMDAr-independent. Learn. Mem. 20, 174–182. doi: 10.1101/lm.029504.112
Li, Y., Abdourahman, A., Tamm, J. A., Pehrson, A. L., Sánchez, C., and Gulinello, M. (2015c). Reversal of age-associated cognitive deficits is accompanied by increased plasticity-related gene expression after chronic antidepressant administration in middle-aged mice. Pharmacol. Biochem. Behav. 135, 70–82. doi: 10.1016/j.pbb.2015.05.013
Lim, C. S., Hoang, E. T., Viar, K. E., Stornetta, R. L., Scott, M. M., and Zhu, J. J. (2014). Pharmacological rescue of Ras signaling, GluA1-dependent synaptic plasticity, and learning deficits in a fragile X model. Genes Dev. 28, 273–289. doi: 10.1101/gad.232470.113
Lindner, M. D., Hodges, D. B. Jr., Hogan, J. B., Orie, A. F., Corsa, J. A., Barten, D. M., et al. (2003). An assessment of the effects of serotonin 6 (5-HT6) receptor antagonists in rodent models of learning. J. Pharmacol. Exp. Ther. 307, 682–691. doi: 10.1124/jpet.103.056002
Line, S. J., Barkus, C., Rawlings, N., Jennings, K., McHugh, S., Sharp, T., et al. (2014). Reduced sensitivity to both positive and negative reinforcement in mice over-expressing the 5-hydroxytryptamine transporter. Eur. J. Neurosci. 40, 3735–3745. doi: 10.1111/ejn.12744
Ludowiq, E., Möller, J., Bien, C., Münte, T., Elger, C., and Rosburg, T. (2010). Active suppression in the mediotemporal lobe during directed forgetting. Neurobiol. Learn. Mem. 93, 352–361. doi: 10.1016/j.nlm.2009.12.001
Luna-Munguía, H., Manuel-Apolinar, L., Rocha, L., and Meneses, A. (2005). 5-HT1A receptor expression during memory formation. Psychopharmacology (Berl). 181, 309–318. doi: 10.1007/s00213-005-2240-4
Lynch, M. A. (2004). Long term potentiation. Physiol. Rev. 84, 87–136. doi: 10.1152/physrev.00014.2003
Madsen, K., Neumann, W. J., Holst, K., Marner, L., Haahr, M. T., Lehel, S., et al. (2011). Cerebral serotonin 4 receptors and amyloid-β in early Alzheimer's disease. J. Alzheimers Dis. 26, 457–466. doi: 10.3233/JAD-2011-110056
Mansuy, I. M. (2005). Forgetting: theories and potential mechanisms. Med. Sci. 21, 83–88. doi: 10.1051/medsci/200521183
Manuel-Apolinar, L., Rocha, L., Pascoe, D., Castillo, E., Castillo, C., and Meneses, A. (2005). Modifications of 5-HT4 receptor expression in rat brain during memory consolidation. Brain Res. 1042, 73–81. doi: 10.1016/j.brainres.2005.02.020
Marchetti, E., Jacquet, M., Escoffier, G., Miglioratti, M., Dumuis, A., Bockaert, J., et al. (2011). Enhancement of reference memory in aged rats by specific activation of 5-HT4 receptors using an olfactory associative discrimination task. Brain Res. 1405, 49–56. doi: 10.1016/j.brainres.2011.06.020
Marcos, B., García-Alloza, M., Gil-Bea, F. J., Chuang, T. T., Francis, P. T., Chen, C. P., et al. (2008). Involvement of an altered 5-HT6 receptor functions in behavioral symptoms of Alzheimer's disease. J. Alzheimers Dis. 14, 43–50.
Marcus, E. (2014). Credibility and reproducibility. Cell 159, 965–966. doi: 10.1016/j.cell.2014.11.016
Marin, P., Becamel, C., Dumuis, A., and Bockaert, J. (2012). 5-HT receptor-associated protein networks: new targets for drug discovery in psychiatric disorders? J. Curr. Drug Targets 13, 28–52. doi: 10.2174/138945012798868498
Markou, A., Salamone, J. D., Bussey, T. J., Mar, A. C., Brunner, D., Gilmour, G., et al. (2013). Measuring reinforcement learning and motivation constructs in experimental animals: relevance to the negative symptoms of schizophrenia. Neurosci. Biobehav. Rev. 37, 2149–2165. doi: 10.1016/j.neubiorev.2013.08.007
McConathy, J., and Sheline, Y. I. (2015). Imaging biomarkers associated with cognitive decline: a review. Biol. Psychiatry 77, 685–692. doi: 10.1016/j.biopsych.2014.08.024
McCorvy, J. D., and Roth, B. L. (2015). Structure and function of serotonin G protein-coupled receptors. Pharmacol. Ther. 150, 129–142. doi: 10.1016/j.pharmthera.2015.01.009
McGaugh, J. L. (2013). Making lasting memories: remembering the significant. Proc. Natl. Acad. Sci. U.S.A. 110, 10402–10407. doi: 10.1073/pnas.1301209110
McHugh, S. B., Barkus, C., Lima, J., Glover, L. R., Sharp, T., and Bannerman, D. M. (2015). SERT and uncertainty: serotonin transporter expression influences information processing biases for ambiguous aversive cues in mice. Genes Brain Behav. 14, 330–336. doi: 10.1111/gbb.12215
Ménard, C., Gaudreau, P., and Quirion, R. (2015). “Signaling pathways relevant to cognition-enhancing drug targets,” in Cognitive Enhancement, Handbook of Experimental Pharmacology, eds K. M. Kantak and J. G. Wettstein (Heidelberg; New York; Dordrecht; London: Springer Cham), 59–98.
Ménard, C., and Quirion, R. (2012). Successful cognitive aging in rats: a role for mGluR5 glutamate receptors, Homer 1 proteins and downstream signaling pathways. PLoS ONE 7:e28666. doi: 10.1371/journal.pone.0028666
Meneses, A. (1999). 5-HT system and cognition. Neurosci. Biobehav. Rev. 23, 1111–1125. doi: 10.1016/S0149-7634(99)00067-6
Meneses, A. (2001). Could the 5-HT1B receptor inverse agonism affect learning consolidation? Neurosci. Biobehav. Rev. 25, 193–201. doi: 10.1016/S0149-7634(01)00007-0
Meneses, A. (2013). 5-HT systems: emergent targets for memory formation and memory alterations. Rev. Neurosci. 24, 629–664. doi: 10.1515/revneuro-2013-0026
Meneses, A. (2014). “Neurotransmitters and memory: cholinergic, glutamatergic, gabaergic, dopaminergic, serotonergic, signaling, and memory,” in Identification of Neural Markers Accompanying Memory, ed A. Meneses (San Diego, CA: Elsevier), 5–45.
Meneses, A., and Liy-Salmeron, G. (2012). Serotonin and emotion, learning and memory. Rev. Neurosci. 23, 543–553. doi: 10.1515/revneuro-2012-0060
Meneses, A., and Perez-Garcia, G. (2007). 5-HT1A receptors and memory. Neurosci. Biobehav. Rev. 31, 705–727. doi: 10.1016/j.neubiorev.2007.02.001
Meneses, A., Perez-Garcia, G., Liy-Salmeron, G., Ponce-López, T., Lacivita, E., and Leopoldo, M. (2015). 5-HT7 receptor activation: procognitive and antiamnesic effects. Psychopharmacology (Berl). 232, 595–603. doi: 10.1007/s00213-014-3693-0
Meneses, A., Pérez-García, G., Ponce-Lopez, T., and Castillo, C. (2011c). 5-HT6 receptor memory and amnesia: behavioral pharmacology–learning and memory processes. Int. Rev. Neurobiol. 96, 27–47. doi: 10.1016/B978-0-12-385902-0.00002-4
Meneses, A., Perez-Garcia, G., Ponce-Lopez, T., Tellez, R., and Castillo, C. (2011b). Serotonin transporter and memory. Neuropharmacology 61, 355–363. doi: 10.1016/j.neuropharm.2011.01.018
Meneses, A., Perez-Garcia, G., Ponce-Lopez, T., Tellez, R., Gallegos-Cari, A., and Castillo, C. (2011a). Spontaneously hypertensive rat (SHR) as an animal model for ADHD: a short overview. Rev. Neurosci. 22, 365–371. doi: 10.1515/rns.2011.024
Meneses, A., and Tellez, R. (2015). “Autoshaping memory formation and retention loss: are serotonin and other neurotransmitter transporters involved?” in Serotonin Receptor Technologies, Neuromethods, Vol. 95, eds W. Blenau and A. Baumann (New York, NY: Springer Science+Business Media), 125–149.
Mereu, M., Bonci, A., Newman, A. H., and Tanda, G. (2013). The neurobiology of modafinil as an enhancer of cognitive performance and a potential treatment for substance use disorders. Psychopharmacology (Berl). 229, 415–434. doi: 10.1007/s00213-013-3232-4
Meyer-Lindenberg, A., Murphy, D., Rolls, E., Saletu, B., Spedding, M., Sweeney, J., et al. (2012). Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 1, 141–168. doi: 10.1038/nrd3628
Millan, M. J., Agid, Y., Brune, M., Bullmore, E. T., Carter, C. S., Clayton, N. S., et al. (2012). Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat. Rev. Drug. Discov. 11, 141–168. doi: 10.1038/nrd3628
Millan, M. J., Agid, Y., Brüne, M., Bullmore, E. T., Carter, C. S., Clayton, N. S., et al. (2014). The clinical use of cerebrospinal fluid biomarker testing for Alzheimer's disease diagnosis: a consensus paper from the Alzheimer's biomarkers standardization initiative. Alzheimers Dement. 10, 808–817. doi: 10.1016/j.jalz.2014.03.003
Mo, C., Hannan, A. J., and Renoir, T. (2015). Environmental factors as modulators of neurodegeneration: insights from gene-environment interactions in Huntington's disease. Neurosci. Biobehav. Rev. 52, 178–192. doi: 10.1016/j.neubiorev.2015.03.003
Monje, F. J., Divisch, I., Demit, M., Lubec, G., and Pollak, D. D. (2013). Flotillin-1 is an evolutionary-conserved memory-related protein up-regulated in implicit and explicit learning paradigms. Ann. Med. 45, 301–307. doi: 10.3109/07853890.2013.770637
Mora, F. (2013). Successful brain aging: plasticity, environmental enrichment, and lifestyle. Dialogues Clin. Neurosci. 15, 45–52.
Morton, R. A., Baptista-Hon, D. T., Hales, T. G., and Lovinger, D. M. (2015). Agonist- and antagonist-induced up-regulation of surface 5-HT3A receptors. Br. J. Pharmacol. doi: 10.1111/bph.13197. [Epub ahead of print].
Muenchhoff, J., Poljak, A., Song, F., Raftery, M., Brodaty, H., Duncan, M., et al. (2015). Plasma protein profiling of mild cognitive impairment and Alzheimer's disease across two independent cohorts. J. Alzheimers Dis. 43, 1355–1373.
Müller, C. P., and Homberg, J. R. (2015). The role of serotonin in drug use and addiction. Behav. Brain Res. 277, 146–192. doi: 10.1016/j.bbr.2014.04.007
Myer, J. S., and Hull, J. H. (1974). Autoshaping and instrumental learning in the rat. J. Comp. Physiol. Psychol. 86, 724–729. doi: 10.1037/h0036165
Myhrer, T. (2003). Neurotransmitter systems involved in learning and memory in the rat: a meta-analysis based on studies of four behavioral tasks. Brain Res. Rev. 41, 268–287. doi: 10.1016/S0165-0173(02)00268-0
Nasehi, M., Jamshidi-Mehr, M., Khakpai, F., and Zarrindast, M. R. (2014a). Possible involvement of CA1 5-HT1B/1D and 5-HT2A/2B/2C receptors in harmaline-induced amnesia. Pharmacol. Biochem. Behav. 125, 70–77. doi: 10.1016/j.pbb.2014.08.007
Nasehi, M., Tabatabaie, M., Khakpai, F., and Zarrindast, M. (2014b). The effects of CA1 5HT4 receptors in MK801-induced amnesia and hyperlocomotion. Neurosci. Lett. 587C, 73–78. doi: 10.1016/j.neulet.2014.12.019
Nikiforuk, A. (2015). Targeting the Serotonin 5-HT7 receptor in the search for treatments for CNS disorders: rationale and progress to date. CNS Drugs 29, 265–275. doi: 10.1007/s40263-015-0236-0
Nikiforuk, A., Kos, T., Fijał, K., Hołuj, M., Rafa, D., and Popik, P. (2013). Effects of the selective 5-HT7 receptor antagonist SB-269970 and amisulpride on ketamine-induced schizophrenia-like deficits in rats. PLoS ONE 8:e66695. doi: 10.1371/journal.pone.0066695
Oscos, A., Martinez, J. L. Jr., and McGaugh, J. L. (1988). Effects of post-training d-amphetamine on acquisition of an appetitive autoshaped lever press response in rats. Psychopharmacology (Berl) 95, 132–134. doi: 10.1007/BF00212781
Pang, K. C., Jiao, X., Sinha, S., Beck, K. D., and Servatius, R. J. (2011). Damage of GABAergic neurons in the medial septum impairs spatial working memory and extinction of active avoidance: effects on proactive interference. Hippocampus 21, 835–846. doi: 10.1002/hipo.20799
Papenberg, G., Bäckman, L., Nagel, I. E., Nietfeld, W., Schröder, J., Bertram, L., et al. (2013). Dopaminergic gene polymorphisms affect long-term forgetting in old age: further support for the magnification hypothesis. J. Cogn. Neurosci. 25, 571–579. doi: 10.1162/jocn_a_00359
Parrott, A. C. (2013). MDMA, serotonergic neurotoxicity, and the diverse functional deficits of recreational ‘Ecstasy’ users. Neurosci. Biobehav. Rev. 37, 1466–1484. doi: 10.1016/j.neubiorev.2013.04.016
Patton, W. (1995). Biologist's perspective on analytical imaging systems as applied to protein gel electrophoresis. J. Chromatogr. A 698, 55–87. doi: 10.1016/0021-9673(94)00987-K
Peele, D. B., and Vincent, A. (1989). Strategies for assessing learning and memory, 1978–1987: a comparison of behavioral toxicology, psychopharmacology, and neurobiology. Neurosci. Biobehav. Rev. 13, 317–322. doi: 10.1016/S0149-7634(89)80068-5
Peñas-Cazorla, R., and Vilaró, M. T. (2014). Serotonin 5-HT4 receptors and forebrain cholinergic system: receptor expression in identified cell populations. Brain Struct. Funct. doi: 10.1007/s00429-014-0864-z. [Epub ahead of print].
Pérez-García, G., González-Espinosa, C., and Meneses, A. (2006). An mRNA expression analysis of stimulation and blockade of 5-HT7 receptors during memory consolidation. Behav. Brain Res. 169, 83–92. doi: 10.1016/j.bbr.2005.12.013
Perez-Garcia, G., and Meneses, A. (2009). Memory time-course: mRNA 5-HT1A and 5-HT7 receptors. Behav. Brain Res. 202, 102–113. doi: 10.1016/j.bbr.2009.03.027
Pérez-García, G., and Meneses, A. (2008). Ex-vivo study of 5-HT1A and 5-HT7 receptor agonists and antagonists on cAMP accumulation during memory formation and amnesia. Behav. Brain Res. 195, 139–146. doi: 10.1016/j.bbr.2008.07.033
Perez-García, G. S., and Meneses, A. (2005). Effects of the potential 5-HT7 receptor agonist AS 19 in an autoshaping learning task. Behav. Brain Res. 163, 136–140. doi: 10.1016/j.bbr.2005.04.014
Pithers, R. T. (1985). The roles of event contingencies and reinforcement in human autoshaping and omission responding. Learn. Motiv. 16, 210–237. doi: 10.1016/0023-9690(85)90013-X
Pittalà, V., Siracusa, M. A., Salerno, L., Romeo, G., Modica, M. N., Madjid, N., et al. (2015). Analysis of mechanisms for memory enhancement using novel and potent 5-HT1A receptor ligands. Eur. J. Neuropsychopharmacol. doi: 10.1016/j.euroneuro.2015.04.017. [Epub ahead of print].
Pitychoutis, P. M., Belmer, A., Moutkine, I., Adrien, J., and Maroteaux, L. (2015). Mice lacking the serotonin Htr2B receptor gene present an antipsychotic-sensitive schizophrenic-like phenotype. Neuropsychopharmacology. doi: 10.1038/npp.2015.126. [Epub ahead of print].
Puig, M. V., and Gulledge, A. T. (2011). Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol. Neurobiol. 44, 449–464. doi: 10.1007/s12035-011-8214-0
Ramirez, M. J., Lai, M. K., Tordera, R. M., and Francis, P. T. (2014). Serotonergic therapies for cognitive symptoms in Alzheimer's disease: rationale and current status. Drugs 74, 729–736. doi: 10.1007/s40265-014-0217-5
Reichel, C. M., Ramsey, L. A., Schwendt, M., McGinty, J. F., and See, R. E. (2012). Methamphetamine-induced changes in the object recognition memory circuit. Neuropharmacology 62, 1119–1126. doi: 10.1016/j.neuropharm.2011.11.003
Restivo, L., Roman, F., Dumuis, A., Bockaert, J., Marchetti, E., and Ammassari-Teule, M. (2008). The promnesic effect of G-protein-coupled 5-HT4 receptors activation is mediated by a potentiation of learning-induced spine growth in the mouse hippocampus. Neuropsychopharmacology 33, 2427–2434. doi: 10.1038/sj.npp.1301644
Rodríguez, J. J., Noristani, H. N., and Verkhratsky, A. (2012). The serotonergic system in ageing and Alzheimer's disease. Prog. Neurobiol. 99, 15–41. doi: 10.1016/j.pneurobio.2012.06.010
Rodriguez, J. S., Boctor, S. Y., Phelix, C. F., and Martinez, J. L. Jr. (2008). Differences in performance between Sprague-Dawley and Fischer 344 rats in positive reinforcement tasks. Pharmacol. Biochem. Behav. 89, 17–22. doi: 10.1016/j.pbb.2007.10.017
Rojas, P. S., Neira, D., Muñoz, M., Lavandero, S., and Fiedler, J. L. (2014). Serotonin (5-HT) regulates neurite outgrowth through 5-HT1A and 5-HT7 receptors in cultured hippocampal neurons. J. Neurosci. Res. 92, 1000–1009. doi: 10.1002/jnr.23390
Ruocco, L. A., Treno, C., Gironi Carnevale, U. A., Arra, C., Boatto, G., Nieddu, M., et al. (2014). Prepuberal stimulation of 5-HT7-R by LP-211 in a rat model of hyper-activity and attention-deficit: permanent effects on attention, brain amino acids and synaptic markers in the fronto-striatal interface. PLoS ONE 9:e83003. doi: 10.1371/journal.pone.0083003
Sałat, K., Podkowa, A., Mogilski, S., Zarêba, P., Kulig, K., Sałat, R., et al. (2015). The effect of GABA transporter 1 (GAT1) inhibitor, tiagabine, on scopolamine-induced memory impairments in mice. Pharmacol. Rep. doi: 10.1016/j.pharep.2015.04.018
Samarajeewa, A., Goldemann, L., Vasefi, M. S., Ahmed, N., Gondora, N., Khanderia, C., et al. (2014). 5-HT7 receptor activation promotes an increase in TrkB receptor expression and phosphorylation. Front. Behav. Neurosci. 8:391. doi: 10.3389/fnbeh.2014.00391
Saroja, S. R., Kim, E. J., Shanmugasundaram, B., Höger, H., and Lubec, G. (2014). Hippocampal monoamine receptor complex levels linked to spatial memory decline in the aging C57BL/6J. Behav. Brain Res. 264, 1–8. doi: 10.1016/j.bbr.2014.01.042
Sase, S., Stork, O., Lubec, G., and Li, L. (2015). Contextual fear conditioning modulates hippocampal AMPA-, GluN1- and serotonin receptor 5-HT1A-containing receptor complexes. Behav. Brain Res. 278C, 44–54. doi: 10.1016/j.bbr.2014.09.035
Saulin, A. I., Savli, M., and Lanzenberger, R. (2012). Serotonin and molecular neuroimaging in humans using PET. Amino Acids 42, 2039–2057. doi: 10.1007/s00726-011-1078-9
Scarr, E., Millan, M. J., Bahn, S., Bertolino, A., Turck, C. W., Kapur, S., et al. (2015). Biomarkers for psychiatry: the journey from fantasy to fact, a report of the 2013 CINP Think Tank. Int. J. Neuropsychopharmacol. doi: 10.1093/ijnp/pyv042. [Epub ahead of print].
Schmitt, U., and Hiemke, C. (2002). Tiagabine, a gamma-amino-butyric acid transporter inhibitor impairs spatial learning of rats in the Morris water-maze. Behav. Brain Res. 133, 391–394. doi: 10.1016/S0166-4328(02)00008-6
Segu, L., Lecomte, M. J., Wolff, M., Santamaria, J., Hen, R., Dumuis, A., et al. (2010). Hyperfunction of muscarinic receptor maintains long-term memory in 5-HT4 receptor knock-out mice. PLoS ONE 5:e9529. doi: 10.1371/journal.pone.0009529
Seo, J., and Tsai, L. H. (2014). Neuronal differentiation: 5-HT6R can do it alone. Nat. Chem. Biol. 10, 488–489. doi: 10.1038/nchembio.1557
Seyedabadi, M., Fakhfouri, G., Ramezani, V., Mehr, S. E., and Rahimian, R. (2014). The role of serotonin in memory: interactions with neurotransmitters and downstream signaling. Exp. Brain Res. 232, 723–738. doi: 10.1007/s00221-013-3818-4
Sheline, Y. I., West, T., Yarasheski, K., Jasielec, M. S., Hettinger, J. C., Tripoli, D. L., et al. (2014b). Reply to comment on “An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice.” Sci. Transl. Med. 6, 268lr4. doi: 10.1126/scitranslmed.3010609
Sheline, Y. I., West, T., Yarasheski, K., Swarm, R., Jasielec, M. S., Fisher, J. R., et al. (2014a). An antidepressant decreases CSF Aβ production in healthy individuals and in transgenic AD mice. Sci. Transl. Med. 6, 236re4. doi: 10.1126/scitranslmed.3008169
Shi, J., Cai, Y., Liu, G., Gong, N., Liu, Z., Xu, T., et al. (2012). Enhanced learning and memory in GAT1 heterozygous mice. Acta Biochim. Biophys. Sin. 44, 359–356. doi: 10.1093/abbs/gms005
Shimizu, S., Mizuguchi, Y., and Ohno, Y. (2013). Improving the treatment of schizophrenia: role of 5-HT receptors in modulating cognitive and extrapyramidal motor functions. CNS Neurol. Disord. Drug Targets 12, 861–869. doi: 10.2174/18715273113129990088
Silverman, J. L., Gastrell, P. T., Karras, M. N., Solomon, J., and Crawley, J. N. (2015). Cognitive abilities on transitive inference using a novel touchscreen technology for mice. Cereb. Cortex 25, 1133–1142. doi: 10.1093/cercor/bht293
Söderqvist, S., Bergman Nutley, S., Peyrard-Janvid, M., Matsson, H., Humphreys, K., Kere, J., et al. (2012). Dopamine, working memory, and training induced plasticity: implications for developmental research. Dev. Psychol. 48, 836–843. doi: 10.1037/a0026179
Solodkin, A., and van Hoesen, G. (1997). “Neuropathology and functional anatomy of Alzheimer's disease,” in Pharmacological Treatment of Alzheimer's Disease, eds J. Brioni and M. Decker (New York, NY: Wiley-Liss), 151–177.
Strac, D. S., Muck-Seler, D., and Pivac, N. (2015). Neurotransmitter measures in the cerebrospinal fluid of patients with Alzheimer's disease: a review. Psychiatr. Danub. 27, 14–24.
Stroth, N., Niso, M., Colabufo, N. A., Perrone, R., Svenningsson, P., Lacivita, E., et al. (2015). Arylpiperazine agonists of the Serotonin 5-HT1A receptor preferentially activate cAMP signaling versus. recruitment of β-Arrestin-2. Bioorganic Med. Chem. doi: 10.1016/j.bmc.2015.05.042
Subramaniyan, S., Hajali, V., Scherf, T., Sase, S. J., Sialana, F. J., Gröger, M., et al. (2015). Hippocampal receptor complexes paralleling LTP reinforcement in the spatial memory holeboard test in the rat. Behav. Brain Res. 283C, 162–174. doi: 10.1016/j.bbr.2015.01.036
Subramaniyan, S., Heo, S., Patil, S., Li, L., Hoger, H., Pollak, A., et al. (2014). A hippocampal nicotinic acetylcholine alpha 7-containing receptor complex is linked to memory retrieval in the multiple-T-maze in C57BL/6j mice. Behav. Brain Res. 270, 137–145. doi: 10.1016/j.bbr.2014.05.012
Sumiyoshi, T., Bubenikova-Valesova, V., Horacek, J., and Bert, B. (2008). Serotonin1A receptors in the pathophysiology of schizophrenia: development of novel cognition-enhancing therapeutics. Adv. Ther. 25, 1037–1056. doi: 10.1007/s12325-008-0102-2
Sun, M. K., Nelson, T. J., and Alkon, D. L. (2015). Towards universal therapeutics for memory disorders. Trends Pharmacol. Sci. 36, 384–394. doi: 10.1016/j.tips.2015.04.004
Suzuki, H. I., and Lucas, L. R. (2015). Neurochemical correlates of accumbal dopamine D2 and amygdaloid 5-HT 1B receptor densities on observational learning of aggression. Cogn. Affect. Behav. Neurosci. 15, 460–474. doi: 10.3758/s13415-015-0337-8
Tajiri, M., Hayata-Takano, A., Seiriki, K., Ogata, K., Hazama, K., Shintani, N., et al. (2012). Serotonin 5-HT7 receptor blockade reverses behavioral abnormalities in PACAP-deficient mice and receptor activation promotes neurite extension in primary embryonic hippocampal neurons: therapeutic implications for psychiatric disorders. J. Mol. Neurosci. 48, 473–481. doi: 10.1007/s12031-012-9861-y
Talpos, J. C., Aerts, N., Fellini, L., and Steckler, T. (2014). A touch-screen based paired-associates learning (PAL) task for the rat may provide a translatable pharmacological model of human cognitive impairment. Pharmacol. Biochem. Behav. 122, 97–106. doi: 10.1016/j.pbb.2014.03.014
Talpos, J., and Shoaib, M. (2015). Executive function. Handb. Exp. Pharmacol. 228, 191–213. doi: 10.1007/978-3-319-16522-6_6
Tellez, R., Gómez-Viquez, L., Liy-Salmeron, G., and Meneses, A. (2012b). GABA, glutamate, dopamine and serotonin transporters expression on forgetting. Neurobiol. Learn. Mem. 98, 66–77. doi: 10.1016/j.nlm.2012.05.001
Tellez, R., Gómez-Víquez, L., and Meneses, A. (2012a). GABA, glutamate, dopamine and serotonin transporters expression on memory formation and amnesia. Neurobiol. Learn. Mem. 97, 189–201. doi: 10.1016/j.nlm.2011.12.002
Tellez, R., Rocha, L., Castillo, C., and Meneses, A. (2010). Autoradiographic study of serotonin transporter during memory formation. Behav. Brain Res. 12, 12–26. doi: 10.1016/j.bbr.2010.03.015
Thomasius, R., Zapletalova, P., Petersen, K., Buchert, R., Andresen, B., Wartberg, L., et al. (2006). Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective. J. Psychopharmacol. 20, 211–225. doi: 10.1177/0269881106059486
Thompson, A. J. (2013). Recent developments in 5-HT3 receptor pharmacology. Trends Pharmacol. Sci. 34, 100–109. doi: 10.1016/j.tips.2012.12.002
Thur, K. E., Nelson, A. J., and Cassaday, H. J. (2014). Ro 04-6790-induced cognitive enhancement: no effect in trace conditioning and novel object recognition procedures in adult male Wistar rats. Pharmacol. Biochem. Behav. 127, 42–48. doi: 10.1016/j.pbb.2014.10.006
Tomie, A., Di Poce, J., Aguado, A., Janes, A., Benjamin, D., and Pohorecky, L. (2003). Effects of autoshaping procedures on 3H-8-OH-DPAT-labeled 5-HT1a binding and 125I-LSD-labeled 5-HT2a binding in rat brain. Brain Res. 975, 167–178. doi: 10.1016/S0006-8993(03)02631-3
Tomie, A., Lincks, M., Nadarajah, S. D., Pohorecky, L. A., and Yu, L. (2012). Pairings of lever and food induce Pavlovian conditioned approach of sign-tracking and goal-tracking in C57BL/6 mice. Behav. Brain Res. 226, 571–578. doi: 10.1016/j.bbr.2011.10.021
van Goethem, N. P., Schreiber, R., Newman-Tancredi, A., Varney, M., and Prickaerts, J. (2015). Divergent effects of the “biased,” 5-HT1A receptor agonists F15599 and F13714 in a novel object pattern separation task. Br. J. Pharmacol. 172, 2532–2543. doi: 10.1111/bph.13071
Vanover, K. E., Harvey, S. C., Son, T., Bradley, S. R., Kold, H., Makhay, M., et al. (2004). Pharmacological characterization of AC-90179 [2-(4-methoxyphenyl)-N-(4-methyl-benzyl)-N-(1-methyl-piperidin-4-yl)-acetamide hydrochloride]: a selective serotonin 2A receptor inverse agonist. J. Pharmacol. Exp. Ther. 310, 943–951. doi: 10.1124/jpet.104.066688
Vardy, E., and Kenakin, T. (2014). The tail wags the dog: possible mechanism for reverse allosteric control of ligand-activated channels. Br. J. Pharmacol. 171, 1614–1616. doi: 10.1111/bph.12550
Varrone, A., Svenningsson, P., Marklund, P., Fatouros-Bergman, H., Forsberg, A., Halldin, C., et al. (2015). 5-HT1B receptor imaging and cognition: a positron emission tomography study in control subjects and Parkinson's disease patients. Synapse 69, 365–374. doi: 10.1002/syn.21823
Vimala, P. V., Bhutada, P. S., and Patel, F. R. (2014). Therapeutic potential of agomelatine in epilepsy and epileptic complications. Med. Hypotheses 82, 105–110. doi: 10.1016/j.mehy.2013.11.017
Volpicelli, F., Speranza, L., di Porzio, U., Crispino, M., and Perrone-Capano, C. (2014). The serotonin receptor 7 and the structural plasticity of brain circuits. Front. Behav. Neurosci. 8:318. doi: 10.3389/fnbeh.2014.00318
Waeber, C., Sebben, M., Bockaert, J., and Dumuis, A. (1996). Regional distribution and ontogeny of 5-HT4 binding sites in rat brain. Behav. Brain Res. 73, 259–262. doi: 10.1016/0166-4328(96)00108-8
Wagner, A., and Davachi, L. (2001). Cognitive neuroscience: forgetting of things past. Curr. Biol. 11, R964–R967. doi: 10.1016/S0960-9822(01)00575-9
Walker, E. A., and Foley, J. J. (2010). Acquisition session length modulates consolidation effects produced by 5-HT2C ligands in a mouse autoshaping-operant procedure. Behav. Pharmacol. 21, 83–89. doi: 10.1097/FBP.0b013e328337bde7
Walker, E. A., Foley, J. J., Clark-Vetri, R., and Raffa, R. B. (2011). Effects of repeated administration of chemotherapeutic agent tamoxifen, methotrexate, and 5-fluorouracil on the acquisition and retention of a learned response in mice. Psychopharmacology (Berl). 217, 539–548. doi: 10.1007/s00213-011-2310-8
Wallace, A., Pehrson, A. L., Sánchez, C., and Morilak, D. A. (2014). Vortioxetine restores reversal learning impaired by 5-HT depletion or chronic intermittent cold stress in rats. Int. J. Neuropsychopharmacol. 17, 1695–1706. doi: 10.1017/S1461145714000571
Wasserman, E. A. (1981). “Response evocation in autoshaping: contribution of cognitive and comparative_evolutionary analysis to an understanding of directed action,” in Autoshaping and Conditioning Theory, eds C. M. Locurto, H. S. Terrace, and J. Gibbon (New York, NY: Academic Press), 21–54.
Waters, K. A., Stean, T. O., Hammond, B., Virley, D. J., Upton, N., Kew, J. N., et al. (2012). Effects of the selective 5-HT7 receptor antagonist SB-269970 in animal models of psychosis and cognition. Behav. Brain Res. 228, 211–218. doi: 10.1016/j.bbr.2011.12.009
Weber, T., Vogt, M. A., Gartside, S. E., Berger, S. M., Lujan, R., Lau, T., et al. (2015). Adult AMPA GLUA1 receptor subunit loss in 5-HT neurons results in a specific anxiety-phenotype with evidence for dysregulation of 5-HT neuronal activity. Neuropsychopharmacology 40, 1471–1484. doi: 10.1038/npp.2014.332
Wellman, C. L., Izquierdo, A., Garrett, J. E., Martin, K. P., Carroll, J., Millstein, R., et al. (2007). Impaired stress-coping and fear extinction and abnormal corticolimbic morphology in serotonin transporter knock-out mice. J. Neurosci. 27, 684–691. doi: 10.1523/JNEUROSCI.4595-06.2007
Westrich, L., Haddjeri, N., Dkhissi-Benyahya, O., and Sanchez, C. (2015). Involvement of 5-HT7 receptors in vortioxetine's modulation of circadian rhythms and episodic memory in rodents. Neuropharmacology 89, 382–390. doi: 10.1016/j.neuropharm.2014.10.015
White, N. M., and McDonald, R. J. (2002). Multiple parallel memory systems in the brain of the rat. Neurobiol. Learn. Mem. 77, 125–184.
Wilcove, W. G., and Miller, J. C. (1974). CS-UCS presentations and a lever: human autoshaping. J. Exp. Psychol. 103, 868–877. doi: 10.1037/h0037388
Wilkinson, D., Windfeld, K., and Colding-Jørgensen, E. (2014). Safety and efficacy of idalopirdine, a 5-HT6 receptor antagonist, in patients with moderate Alzheimer's disease (LADDER): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 13, 1092–1099. doi: 10.1016/S1474-4422(14)70198-X
Wixted, J. T. (2004). The psychology and neuroscience of forgetting. Annu. Rev. Psychol. 55, 235–269. doi: 10.1146/annurev.psych.55.090902.141555
Woehrle, N. S., Klenotich, S. J., Jamnia, N., Ho, E. V., and Dulawa, S. C. (2013). Effects of chronic fluoxetine treatment on serotonin 1B receptor-induced deficits in delayed alternation. Psychopharmacology (Berl). 227, 545–551. doi: 10.1007/s00213-013-2985-0
Wolf, J. E., Urbano, C. M., Ruprecht, C. M., and Leising, K. J. (2014). Need to train your rat? There is an App for that: a touchscreen behavioral evaluation system. Behav. Res. Methods 46, 206–214. doi: 10.3758/s13428-013-0366-6
Wolff, M., Savova, M., Malleret, G., Hen, R., Segu, L., and Buhot, M. C. (2003). Serotonin 1B knockout mice exhibit a task-dependent selective learning facilitation. Neurosci. Lett. 338, 1–4. doi: 10.1016/S0304-3940(02)01339-3
Woods, S., Clarke, N., Layfield, R., and Fone, K. (2012). 5-HT6 receptor agonists and antagonists enhance learning and memory in a conditioned emotion response paradigm by modulation of cholinergic and glutamatergic mechanisms. Br. J. Pharmacol. 167, 436–449. doi: 10.1111/j.1476-5381.2012.02022.x
Yamazaki, M., Harada, K., Yamamoto, N., Yarimizu, J., Okabe, M., Shimada, T., et al. (2014). ASP5736, a novel 5-HT5A receptor antagonist, ameliorates positive symptoms and cognitive impairment in animal models of schizophrenia. Eur. Neuropsychopharmacol. 24, 1698–1708. doi: 10.1016/j.euroneuro.2014.07.009
Yamazaki, M., Okabe, M., Yamamoto, N., Yarimizu, J., and Harada, K. (2015). Novel 5-HT5A receptor antagonists ameliorate scopolamine-induced working memory deficit in mice and reference memory impairment in aged rats. J. Pharmacol. Sci. 127, 362–369. doi: 10.1016/j.jphs.2015.02.006
Yang, P., Cai, G., Cai, Y., Fei, J., and Liu, G. (2013). Gamma aminobutyric acid transporter subtype 1 gene knockout mice: a new model for attention deficit/hyperactivity disorder. Acta Biochim. Biophys. Sin. 45, 578–585. doi: 10.1093/abbs/gmt043
Yoshimi, N., Fujita, Y., Ohgi, Y., Futamura, T., Kikuchi, T., and Hashimoto, K. (2014). Effects of brexpiprazole, a novel serotonin-dopamine activity modulator, on phencyclidine-induced cognitive deficits in mice: a role for serotonin 5-HT1A receptors. Pharmacol. Biochem. Behav. 124, 245–249. doi: 10.1016/j.pbb.2014.06.008
Zaldivar, A., and Krichmar, J. L. (2013). Interactions between the neuromodulatory systems and the amygdala: exploratory survey using the allen mouse brain atlas. Brain Struct. Funct. 218, 1513–1530. doi: 10.1007/s00429-012-0473-7
Keywords: memory, drugs, neural markers
Citation: Meneses A (2015) Serotonin, neural markers, and memory. Front. Pharmacol. 6:143. doi: 10.3389/fphar.2015.00143
Received: 17 April 2015; Accepted: 29 June 2015;
Published: 21 July 2015.
Edited by:
Andrew D. Powell, University of Birmingham, UKReviewed by:
Gillian Grafton, University of Birmingham, UKThomas Freret, University of Caen, France
Copyright © 2015 Meneses. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alfredo Meneses, Departamento de Farmacobiología, Centro de Investigación y de Estudios Avanzados del Instituto Politécnico Nacional, Tenorios 235, Granjas Coapa, Mexico City 14330, Mexico,YW1lbmVzZXNAbXNuLmNvbQ==
 Alfredo Meneses
Alfredo Meneses