
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
HYPOTHESIS AND THEORY article
Front. Pharmacol., 25 November 2014
Sec. Neuropharmacology
Volume 5 - 2014 | https://doi.org/10.3389/fphar.2014.00256
This article is part of the Research TopicNeuronal and glial structural plasticity induced by drugs of abuseView all 10 articles
GABAA receptors are the main fast inhibitory neurotransmitter receptors in the mammalian brain, and targets for many clinically important drugs widely used in the treatment of anxiety disorders, insomnia and in anesthesia. Nonetheless, there are significant risks associated with the long-term use of these drugs particularly related to development of tolerance and addiction. Addictive mechanisms of GABAA receptor drugs are poorly known, but recent findings suggest that those drugs may induce aberrant neuroadaptations in the brain reward circuitry. Recently, benzodiazepines, acting on synaptic GABAA receptors, and modulators of extrasynaptic GABAA receptors (THIP and neurosteroids) have been found to induce plasticity in the ventral tegmental area (VTA) dopamine neurons and their main target projections. Furthermore, depending whether synaptic or extrasynaptic GABAA receptor populations are activated, the behavioral outcome of repeated administration seems to correlate with rewarding or aversive behavioral responses, respectively. The VTA dopamine neurons project to forebrain centers such as the nucleus accumbens and medial prefrontal cortex, and receive afferent projections from these brain regions and especially from the extended amygdala and lateral habenula, forming the major part of the reward and aversion circuitry. Both synaptic and extrasynaptic GABAA drugs inhibit the VTA GABAergic interneurons, thus activating the VTA DA neurons by disinhibition and this way inducing glutamatergic synaptic plasticity. However, the GABAA drugs failed to alter synaptic spine numbers as studied from Golgi-Cox-stained VTA dendrites. Since the GABAergic drugs are known to depress the brain metabolism and gene expression, their likely way of inducing neuroplasticity in mature neurons is by disinhibiting the principal neurons, which remains to be rigorously tested for a number of clinically important anxiolytics, sedatives and anesthetics in different parts of the circuitry.
GABAA receptor agonists generally depress brain activity. Benzodiazepines (BZs) and other GABAmimetic drugs depress gene expression in the brain, including the neuroplasticity-related genes such as brain-derived neurotrophic factor (BDNF), nerve growth factor (NGF), and Fos-genes (Zafra et al., 1991; Huopaniemi et al., 2004). BZs and volatile anesthetic isoflurane also have been reported to reduce long-term potentiation (LTP) in several brain regions (Higashima et al., 1998; Kulisch et al., 2011; Piao et al., 2013). Moreover, BZs and general anesthetics are able to impair neurogenesis in both young and adult animals (Wu and Castren, 2009; Erasso et al., 2013; Thal et al., 2014). All these facts suggest that GABAA receptor agonists have little potential for neuroplasticity outside the critical periods of brain development, when the role of GABAergic interneurons is obligatory (Hensch and Stryker, 2004). On the other hand, by suppressing the local inhibitory regulation, GABAA receptors on GABAergic interneurons can indirectly cause activation of principal neuron populations, a network effect called disinhibition. For example, GABAergic neurons in the lateral part of the central nucleus of amygdala are excited by low acute doses of BZs through disinhibition and this is associated with anxiolytic effects (Beck and Fibiger, 1995; Salminen et al., 1996; Panhelainen and Korpi, 2012). Furthermore, GABAA receptor agonists can activate the dopaminergic (DAergic) neurons in the ventral tegmental area (VTA) by disinhibition (Heikkinen et al., 2009; Tan et al., 2010; Vashchinkina et al., 2012).
In this review we will discuss recent advances in understanding how GABAA receptor modulators affect both GABAergic and glutamatergic synapses and also how their acute or repeated treatments can modulate plasticity of the reward system. Given the widespread application of GABAA receptor drugs, understanding more fully how they modulate brain reward system may help to develop new strategies for designing novel compounds to overcome their therapeutic limitations.
Benzodiazepines, inhalational and intravenous anesthetics, barbiturates, neurosteroids, and other GABAmimetic drugs – all share the interaction with the GABAA receptor and facilitation of receptor function to produce strong pharmacological and behavioral actions (Sieghart, 1995; Korpi et al., 2002). They act on distinct sites on the GABAA receptor and increase membrane anion (Cl- and bicarbonate) conductance, thereby in most cases inducing hyperpolarization, which has an inhibitory effect on the firing of the postsynaptic neurons.
The GABAA receptors belong to the “cys-loop” superfamily of ligand-gated ion channels (Alexander et al., 2013). They exist as heteropentameric structures, commonly composed of two α subunits, two β subunits, and one γ or δ subunit (McKernan and Whiting, 1996). The BZ-sensitive GABAA receptors contain either α1, α2, α3, and/or α5 subunits and as a result of containing the γ2 subunit, they are preferentially located at synaptic sites (with the exception of α5), whereas the α4 or α6 subunit-containing GABAA receptors are highly sensitive to neurosteroids, GABAmimetic drugs such as muscimol and THIP (gaboxadol; 4,5,6,7-tetrahydroisoxazolol[4,5-c]pyridine-3-ol), general anesthetics such as isoflurane and etomidate. Since they often possess the δ subunit instead of γ2, they are located at peri- or extrasynaptic sites (Olsen and Sieghart, 2009).
GABAA receptor modulators are effective in the wide range of indications (anxiety disorders, panic, insomnia, muscle spams, seizure control in epilepsy and alcohol withdrawal, sedation of aggressive patients, calming down anxious patients before operations, and induction of anesthesia) by acting on distinct GABAA receptor subtypes which demonstrate a unique heterogeneity in terms of function, kinetics, pharmacological profile, and distribution in brain (Uusi-Oukari and Korpi, 2010). Combination of pharmacological and genetic approaches has revealed that 1) α1 subunit-containing GABAA receptors mainly mediate the sedative and addictive effects of BZs, 2) α2 or α3 subunit-containing receptors mediate the anxiolytic and muscle-relaxant effects, and 3) α5 subunit-containing receptors mediate the memory-impairing effects of BZs (Crestani et al., 1999; Rudolph and Mohler, 2004). Furthermore, there is growing evidence that hippocampal extrasynaptic α5 subunit-containing receptors contribute to amnestic effects by general anesthetics (Grasshoff et al., 2005), while the stimulation of δ subunit-containing receptors mediate anxiolytic, anticonvulsive, anesthetic, and aversive effects of neurosteroids in mice (Mihalek et al., 1999; Vashchinkina et al., 2014).
Despite the usefulness of GABAA receptor modulators, their use may lead to side-effects that limit their efficacy. General anesthetics may induce postoperative cognitive dysfunction for weeks or months in elderly patients (Canet et al., 2003; Newman et al., 2007), and their use in prolonged operations at neonatal period, although necessary, have raised serious thoughts about possible neuronal damage and long-term cognitive effects as seen in preclinical models (Jevtovic-Todorovic et al., 2013). Beside the short-term undesirable effects of BZs such as dizziness and motor impairment, their long-term effects include disruption of sleep architecture, confusion and memory impairment, tolerance and dependence (Licata and Rowlett, 2008). Furthermore, chronic increased exposure to neurosteroids appears to accelerate development of Alzheimer’s disease symptoms in various mouse models (Bengtsson et al., 2012, 2013). Thus, the use of these drugs causes persistent neuroadaptation in the brain that also may contribute to adverse effects.
Addiction is increasingly seen as a disease of aberrant neuroadaptation in the brain reward system (Volkow and Baler, 2014). The VTA dopamine neurons are considered as an essential hub at least for the early phases of addiction. VTA has reciprocal connections with many forebrain centers such as the nucleus accumbens (NAc, ventral striatum), dorsal striatum, the medial prefrontal cortex (mPFC), the extended amygdala (particularly including the basolateral (BLA) and central nuclei of amygdala and the bed nuclei of stria terminalis) and lateral habenula, which circuitries form the major part of reward and aversion pathways.
The risk of developing addiction is one of the main challenges restricting the clinical use of BZs and other GABAA modulators (O’Brien, 2005). Addiction-related behaviors can also be studied in experimental animal models. Drugs of abuse activate the reward system and produce reinforcing effects in drug self-administration, in potentiation of the intracranial electrical self-stimulation reward, and in drug-induced learning and conditioning. Various non-selective BZ agonists, such as diazepam and midazolam, are self-administered (Tan et al., 2010). They are rewarding in conditioned place preference paradigm (Spyraki et al., 1985) and able to increase the rate of responding for and to decrease the threshold of the intracranial self-stimulation reward (Straub et al., 2010). The α1 subunit-preferring agonist zolpidem is self-administered in baboons (Ator, 2002), but produces no place preference in rats (Meririnne et al., 1999), showing thus somewhat unclear effects. This might be due to zolpidem’s strong dose-dependent sedative effect. The α2 subunit-containing GABAA receptors in the NAc were necessary for the midazolam preference in a test giving the mice a choice between sucrose solution and sucrose/midazolam solution (Engin et al., 2014). The endogenous neurosteroid GABAA agonist allopregnanolone displays variable effects in animal models of addiction. It can maintain oral self-administration, although not in operant setting (Sinnott et al., 2002). It decreases the threshold of the intracranial self-stimulation reward (Fish et al., 2014), and when injected intraperitoneally (ip), it has been shown to induce either rewarding (Finn et al., 1997) or aversive conditioning effects (Beauchamp et al., 2000). Propofol is rewarding in conditioned place preference (Pain et al., 1996), while isoflurane and GABAA β2/3 subunit-selective etomidate have not been tested so far.
In attempts to further localize the reward-related effects of GABAA modulators in brain circuitry, intracerebral drug infusions have been used. GABAA agonist muscimol and antagonist bicuculline show interesting effects as they both are reinforcing and rewarding in drug-naïve animals when infused into the VTA (Ikemoto et al., 1997, 1998; Laviolette and van der Kooy, 2001). These results have been explained as effects arising from two reward systems, one being DAergic and the other non-DAergic. Muscimol is believed to inhibit VTA GABA neurons and thus disinhibit VTA DA neurons leading to DA antagonist-sensitive reward, while bicuculline also acts on VTA GABA neurons but activates them to target a poorly defined non-DAergic reward pathway insensitive to DA receptor antagonists (Laviolette and van der Kooy, 2001). It remains to be studied, e.g., whether bicuculline acts on VTA GABA neurons projecting to NAc cholinergic interneurons, thereby potentiating appetitive associative learning (Brown et al., 2012). These intracerebral infusion studies should be replicated by regionally and neuronally more specific methods such as chemogenetics or optogenetics to avoid possible confounding infusion-site diffusion to other regions and to various neuronal populations. Furthermore, the brain circuitry for reward and aversion goes well beyond the VTA, stressing the importance of systemic drug experiments for translational relevance.
Growing evidence indicates that neuroadaptation (plasticity) in the brain appears in both glutamatergic and GABAergic synapses (for review, see Castillo et al., 2011; Luscher and Malenka, 2011; Kullmann et al., 2012). Synaptic connections are highly plastic and constantly modified by environmental factors and learning tasks. It should be noted that generation of new glutamatergic and GABAergic synapses proceeds under distinct mechanisms with different factors regulating the processes (described below).
The major sites of contact for glutamatergic presynaptic terminals are dendritic spines (Gray, 1959; Dailey and Smith, 1996; Hering and Sheng, 2001), and dynamic changes in the morphology of dendritic spines have been associated with changes in synaptic strength (Segal, 2005). Spine morphology is subject to rapid alteration by patterns of neuronal activity and by activation of postsynaptic glutamate receptors (Lang et al., 2004; Matsuzaki et al., 2004). Thus, synaptic efficacy can be regulated by multiple mechanisms.
Conversely, the predominant sites of contact for GABAergic presynaptic terminals are not located on spines, but directly on dendritic shafts (Freund and Buzsaki, 1996; Somogyi et al., 1998). However, GABAergic system also interacts with synapses on spines. Recent work has indicated that the activity of GABAA receptors affects spine maturation (Heinen et al., 2003; Jacob et al., 2009). In rat hippocampal cultures, reduced GABAA receptor endocytosis (and increased activity) is associated with reduced spine maturation and reduced levels of postsynaptic density protein-95 (PSD-95; Jacob et al., 2009), and in the visual cortex of adult GABAA receptor α1 subunit knockout mice (resulting in compensatory increased inhibition), the spine density is reduced with a corresponding reduction in PSD-95 expression (Heinen et al., 2003), results of both studies being in agreement with GABAA receptor activation reducing LTP (Higashima et al., 1998). Another study (Shen et al., 2010) showed that development-dependent expression of α4 subunit-containing GABAA receptors in dendritic spines of CA1 hippocampal neurons plays a role in LTP induction. Notably, activation of these receptors reduces depolarization that is needed to remove Mg2+ block of NMDA receptors, thus leading to reduced LTP. Furthermore, a small population of GABAergic interneurons that express somatostatin makes synapses targeting directly on dendritic spine heads in the mouse mPFC (Chiu et al., 2013), being able to control local postsynaptic Ca2+ fluxes within the spines. All these different mechanisms indicate that the GABAA receptor activity may regulate rapid dynamics and long-term structural events in glutamate synapses.
The strength of GABAergic synapses is determined by the size of releasable pool of presynaptic GABA and number and/or diversity of GABAA receptors sitting on postsynaptic membrane, which, in turn, is largely determined by receptor trafficking to and from the plasma membrane, including receptor insertion, lateral diffusion within membrane, removal, recycling and degradation (Vithlani et al., 2011). Interestingly, high-resolution two-photon imaging in organotypic hippocampal cultures has revealed that new GABAergic synapses are formed by the appearance of new boutons at pre-existing axon-dendrite crossings (Wierenga et al., 2008). Hence, in that model, plasticity in GABAergic synapses depends on the number of available axon-dendrite crossings, which makes it more restricted than plasticity sites in glutamatergic connections. However, it is not known yet whether these in vitro results can be generalized to all GABAergic synapses, and, therefore, further in vivo studies are needed.
In addition to fast modulation of channel gating, both BZs and neurosteroids have longer lasting effects by controlling the number and subtypes of GABAA receptors on the plasma membrane (Vithlani et al., 2011; Deeb et al., 2012; Abramian et al., 2014). Notably, treatment of hippocampal cultures with BZ agonist flurazepam modulates GABAA receptor trafficking by promoting selective degradation of α2 subunit-containing GABAA receptors after their removal from the postsynaptic membrane, leading to a reduction in synapse size and number, and finally to depression of synaptic inhibition (Jacob et al., 2012). The development of tolerance to the sedative effects of diazepam is associated with a decrease in [3H]L-655,708 binding to the hippocampal dentate gyrus α5 subunit-containing receptors (dependent on α1 subunit-containing receptors), an effect which was extended by the absence of tolerance in α5 subunit point-mutant mice (van Rijnsoever et al., 2004). Treatment of C. elegans with the GABA-site agonist muscimol also results in selective removal of GABAA receptors from synapses (Davis et al., 2010). In contrast, neurosteroids can selectively enhance the trafficking of extrasynaptic GABAA receptors by insertion of new α4 subunit-containing GABAA receptors into the membrane, resulting in an enhancement of tonic inhibition in mice (Abramian et al., 2014). Thus, the neuronal adaptations to GABAA drugs via modulation of receptor trafficking produce long-lasting changes in the efficacy of GABAergic inhibition. However, it should be noted that the effects of GABAA ligands on receptor subunits are very much dependent on the experimental model, and the effects seen in cell culture models have often been difficult to reproduce in vivo (Uusi-Oukari and Korpi, 2010).
The most obvious impediment to understanding neuroadaptation induced by GABAA receptor modulators is the diversity of their target neurons. In fact, each neuron controlled by inhibitory terminals expresses its unique combination of GABAA receptors (Luddens et al., 1995; Olsen and Sieghart, 2009). A further obstacle to the studying of neuronal and structural drug-induced plasticity is made by the fact that interneurons, as a major target for GABAA receptor modulators, themselves are innervated by both glutamatergic and GABAergic synapses. In addition, endogenous GABAA receptor modulators, such as neurosteroids, whose levels constantly fluctuate, e.g., during stress, menstrual cycle and development, can directly affect memory and learning processing (Maguire et al., 2005; Shen et al., 2010), as well as influence the action of other GABAA receptor modulators.
Potassium-chloride co-transporter 2 (KCC2) is expressed in neurons to create the driving force for chloride ions to travel into the cell through the GABAA receptor anion channel, which then leads to hyperpolarizing GABA actions (Rivera et al., 1999). Independently of its Cl- transport function KCC2 has also gained attention due to its structural role in both glutamatergic and GABAergic synapses (Li et al., 2007; Horn et al., 2010; Sun et al., 2013). In glutamatergic synapses, KCC2 located in the neck and head of dendritic spines binds to actin cytoskeleton via the linker protein 4.1 N (Li et al., 2007). While the exact molecular mechanisms still remain elusive, the interaction of KCC2 with the cytoskeleton is crucial for the maturation of spines and for the stability of AMPA receptor clusters (Li et al., 2007; Gauvain et al., 2011). In contrast to glutamatergic synapses, GABAergic synapses are usually located directly on the dendritic shaft (Freund and Buzsaki, 1996; Somogyi et al., 1998). KCC2 expression is regulated by a cell adhesion molecule neuroligin-2 which is mostly localized at GABAergic synapses. Knockdown of neuroligin-2 down-regulates expression of KCC2 and reduces GABAergic synaptogenesis, and interestingly, by affecting the KCC2 levels it also down-regulates the number of glutamatergic synapses (Sun et al., 2013).
Although there is no direct evidence yet on drug-induced structural changes through KCC2-dependent mechanisms, several studies demonstrated changes in expression levels of KCC2. Chronic treatment with the BZ agonist zolpidem up-regulated the KCC2 expression in mouse limbic forebrain (Shibasaki et al., 2013). Also the neurosteroid allopregnanolone transiently modifies KCC2 expression and protein levels during brain maturation in male rats (Modol et al., 2014). However, treatment with general anesthetics, midazolam, propofol, and ketamine, does not alter the expression of KCC2 in rats during the first two postnatal weeks when developmental maturation of KCC2 expression is going on (Lacoh et al., 2013). Future studies should be directed to clarify whether KCC2-mediated mechanisms play a role in neuronal plasticity induced by GABAergic drugs (Kang et al., 2006), and which particular isoforms of KCC2 play role in structural changes, since a recent study suggests that KCC2a and KCC2b isoforms have different brain regional distributions and likely different roles in neuronal functions (Markkanen et al., 2014).
The VTA has been widely studied given its fundamental role in motivation and reward (for review, see Luscher and Malenka, 2011). VTA DA neurons project mainly to the NAc and mPFC and less extensively to the hippocampus and amygdala. They receive glutamatergic inputs from many brain regions, including the mPFC, lateral hypothalamus, lateral habenula and hippocampus, and in addition to local inhibitory control from the VTA GABAergic interneurons, GABAergic inputs to DA neurons arise from the NAc, ventral pallidum, nuclei of the extended amygdala, and rostromedial tegmental nucleus (Jhou et al., 2009; Omelchenko and Sesack, 2009; Watabe-Uchida et al., 2012).
Glutamatergic transmission in the VTA is critical to the reinforcing effects of drugs of abuse: suppressing the glutamatergic transmission in the VTA attenuates cocaine and heroin reward (Xi and Stein, 2002; You et al., 2007) and prevents the reinstatement of cocaine- or heroin seeking (Bossert et al., 2004; Sun et al., 2005). Glutamatergic synapses on VTA DA neurons can undergo both NMDAR-dependent LTP and NMDAR-independent long-term depression (LTD; Jones et al., 2000; Thomas and Malenka, 2003). Synaptic plasticity in the mesolimbic DA system was early on hypothesized to play a role in the process of drug reinforcement and addiction. During the last decade, evidence from electrophysiological studies have accumulated showing that in addition to acute activation of VTA DA neurons, drugs of abuse also induce long-lasting plasticity in the synapses of these neurons. Several classical drugs of abuse such as cocaine, amphetamine, morphine, nicotine, and ethanol share the ability to induce an NMDAR-dependent LTP at glutamatergic synapses of VTA DA neurons via insertion of new GluA2 subunit-lacking AMPARs (Ungless et al., 2001; Saal et al., 2003; Luscher and Malenka, 2011).
In addition to the potentiation of glutamatergic transmission, different addictive drugs such as morphine, nicotine, cocaine, and ethanol have been found to impair GABAergic transmission in the VTA DA neurons by preventing the LTP of GABAergic synapses (LTPGABA; Nugent et al., 2007; Guan and Ye, 2010). In the VTA, LTPGABA is triggered by NMDA receptor activation at glutamate synapses and requires nitric oxide-cGMP signaling (Nugent et al., 2007; Nugent and Kauer, 2008). Blockade of LTPGABA could additionally increase release of DA by silencing local GABA neurons (Liu et al., 2000; Nugent and Kauer, 2008; Niehaus et al., 2010). Which particular GABAergic inputs are involved in LTPGABA and whether the GABAA receptor modulators induce LTPGABA have not been investigated so far.
Since the positive modulators of GABAA receptor benzodiazepine site have well-known abuse potential and act as positive and/or negative (Panlilio et al., 2005) reinforcers in different animal models of addiction, their effects on synaptic plasticity in VTA DA neurons have been recently studied. Indeed, diazepam, and zolpidem, similarly to the other drugs of abuse, were shown to induce plasticity in the glutamatergic synapses contacting VTA DA neurons (Heikkinen et al., 2009). Particularly, BZs induced an LTP that was prevented by co-administration of the BZ antagonist flumazenil and by the NMDA receptor antagonist dizocilpine (MK-801; Heikkinen et al., 2009). BZ-induced LTP in VTA DA neurons was associated with insertion of new GluA2-lacking AMPA receptors, and intra-VTA local network was sufficient for the LTP induction via inhibition of VTA GABAergic interneurons (Tan et al., 2010). Furthermore, Tan et al. (2010) examined a mutant mouse with BZ-insensitive α1 subunits, and found that in these mice midazolam was not able to inhibit the firing of VTA GABAergic interneurons, to disinhibit the DA neurons, to induce plasticity at glutamatergic synapses or to support drug-reinforcement behavior. This suggests that the BZ-induced disinhibition of DA neurons is a key mechanism involved in BZ-induced plasticity in VTA DA neurons as well as in BZ reinforcement.
There is a need for new anxiolytic/sedative drugs with no abuse potential. One approach has been to target a GABAergic system separate from the benzodiazepine-sensitive GABAA receptors, i.e., the extrasynaptic GABAA receptors containing δ-subunit (Olsen and Sieghart, 2009). However, δ-subunit is expressed along the reward pathway in the VTA, NAc, mPFC, and hippocampus (Pirker et al., 2000; Hortnagl et al., 2013). THIP and muscimol, which act on GABAA receptor agonist sites with preferential activation of the high-affinity extrasynaptic receptors (Chandra et al., 2010), have been shown to increase firing rates of DA neurons (Waszczak and Walters, 1980). Thus, it was necessary to study the effects of modulators of the extrasynaptic GABAA system on plasticity in reward pathway and the possible reinforcing potential of these drugs.
A single dose of THIP and another extrasynaptic GABAA receptor modulator neurosteroid ganaxolone dose-dependently induced similar AMPA receptor-mediated LTP in VTA DA neurons via the primary action of increased tonic inhibition of VTA GABAergic interneurons (Vashchinkina et al., 2012, 2014). Importantly, both THIP- and ganaxolone-induced plasticity lasted at least for six days, while BZs cause this effect for three days only (Heikkinen et al., 2009). The effects of THIP and ganaxolone were absent in δ-GABAA receptor knockout mice, and both treatments enhanced AMPA current rectification, indicating reduced targeting of GluA2 subunits.
Surprisingly, despite of aforementioned similar effects on neuroadaptations in VTA DA neurons, modulators of synaptic, and extrasynaptic GABAA receptors induce distinct behavior in the drug self-administration and place conditioning paradigms which have been previously associated with activity of VTA DA neurons (for review, see Luscher and Malenka, 2011). While activation of the synaptic α1 subunit-containing GABAA receptors in the VTA by oral midazolam is reinforcing in the self-administration, the activation of extrasynaptic receptors by ip THIP or ganaxolone leads to avoidance behavior as seen in conditioned place aversion, and THIP is not self-administered either by mice or baboons (Tan et al., 2010; Vashchinkina et al., 2012, 2014). These aversive effects were abolished in GABAA receptor δ subunit-deficient mice (Vashchinkina et al., 2014), suggesting a specific role of this receptor population in the VTA. These behavioral findings support the hypothesis that activation of synaptic and extrasynaptic GABAA receptors is rewarding and aversive, respectively. This may depend on the primary brain areas targeted, as illustrated by intracerebral injections of muscimol (see below) that also preferentially targets extrasynaptic receptors (Chandra et al., 2010). By using place-conditioning system, muscimol infusion into the NAc shell provoked conditioned place preference when infused anteriorly and conditioned aversion when infused posteriorly (Reynolds and Berridge, 2002). Intra-BLA infusions of muscimol and bicuculline had no effect on reward (Zarrindast et al., 2004; Macedo et al., 2006).
Furthermore, it is now becoming clear that aversive drugs or experiences can acutely activate certain DA neurons in the VTA and also induce a long-lasting potentiation at their glutamatergic synapses. This was actually reported already in the early paper of Saal et al. (2003) where the authors showed that in mice a 5-min swimming stress at 6°C water bath induces similar plasticity in the VTA DA neurons as the classical drugs of abuse.
DA neurons are divergent in many respects, e.g., in their electrophysiological features, vulnerability to neurodegeneration and regulation by neuropeptides (Korotkova et al., 2004; Lammel et al., 2011). In particular, Lammel et al. (2011) have shown that DA neurons in the mouse VTA are organized into anatomical and electrophysiological subpopulations inside the DAergic nuclei depending on their projection terminal fields. However, in rats, the VTA neurons might be more heterogeneously organized (Margolis et al., 2006, 2012). The inhibitory control from VTA GABA interneurons is an important regulator of VTA DA neuron activity and the following behavioral outcome. Aversive stimuli have been shown to increase the firing of VTA GABA neurons (Creed et al., 2014). However, while GABA neurons in the VTA seem to quite faithfully respond to aversion by excitation, the responses in VTA DA neurons are more heterogeneous: activation, no response or inhibition have been observed in monkeys (Matsumoto and Hikosaka, 2009). Rewarding or aversive stimuli might modulate the activity of DA neurons differently depending on the brain area to which these neurons project. In mice, a cocaine experience selectively affected DA cells projecting to the NAc medial shell, while an aversive stimulus influenced DA cells projecting to the PFC, and the DA neurons projecting to the NAc lateral shell were modified by both rewarding and aversive stimuli, suggesting that the mesocorticolimbic DA system is comprised of anatomically distinct circuits, modified by different motivational relevance (Lammel et al., 2011). In anesthetized rats, foot shock inhibited DA neurons in the dorsal VTA, whereas the DA neurons in the ventral VTA became phasically excited (Brischoux et al., 2009). In mice, the majority of the VTA DA neurons decreased firing under fearful events, but a small group of DA neurons were activated (Wang and Tsien, 2011). Another study reported that a similar number of DA neurons were activated, inhibited or unaltered by tail pinch, and it also showed that in mice with an impaired NMDA receptor-mediated control of DA neurons the DAergic activation in response to an aversive stimulus was attenuated, leading to impaired aversive conditioning (Zweifel et al., 2011). These findings suggest that increases in DA signaling can be evoked by stimuli with motivational relevance to either rewarding or aversive direction, and they point toward putative multiple populations of VTA DA neurons with different afferent and efferent connections.
Mouse DA neurons with pronounced hyperpolarization-activated cation current Ih-current are found in the lateral VTA and they project to the lateral NAc shell, while the DA neurons of the medial posterior VTA project to the mPFC and medial NAc shell, and have no or very small Ih-currents (Lammel et al., 2011). It should be noted that the study revealing BZ-induced glutamatergic plasticity in VTA DA neurons used a large Ih-current as a marker for DA neurons (Heikkinen et al., 2009). Thus, mostly a subpopulation of VTA DA neurons that projected to the lateral NAc shell was studied. In the studies with THIP and ganaxolone, a genetically modified mouse line with a fluorescent protein marker expressed in tyrosine hydroxylase-positive neurons was used (Vashchinkina et al., 2012, 2014). This allowed recording also from the DA neurons in more medial areas of the VTA with small or no Ih-currents and projecting to the mPFC. It is possible that BZs are positively reinforcing due to mainly activating and modifying the DA neurons detecting reward whereas the drugs activating the extrasynaptic GABAA receptors lead to conditioned aversion because they activate and induce LTP in the DA neurons involved in negative motivation. This hypothesis remains to be carefully tested, although the post-study examination of the recording sites for DA neuron plasticity by THIP failed to indicate any anatomical localization within the VTA (Vashchinkina et al., 2012).
Both BZs and extrasynaptic GABAA modulators produce strong inhibition of the VTA GABA interneurons (Heikkinen et al., 2009; Tan et al., 2010; Vashchinkina et al., 2012, 2014). Thus, a similar disinhibitory mechanism is believed to induce the activation and persistent modulation of VTA DA neurons by both classes of GABAA drugs, which in turn suggests that they might be targeting different populations of VTA GABA interneurons enriched with synaptic and/or extrasynaptic GABAA receptors. Interestingly, a recent study shows that the volatile solvent toluene, that also is a positive modulator of GABAA receptors, induces LTP at the glutamatergic synapses of VTA DA neurons that project to NAc core and shell, whereas it failed to affect the synapses of mPFC-projecting neurons (Beckley et al., 2013). Importantly, toluene is abused by humans, and in rodent models of addiction it increases firing of VTA DA neurons and DA release in the NAc and exhibits positive reinforcement (Lee et al., 2006; Riegel et al., 2007; Lubman et al., 2008). Effects of toluene on GABA interneurons have not been studied so far.
In summary, the possible VTA heterogeneity of both the principal DA neurons and the GABA interneurons and projection neurons in responding to and mediating stimuli and affecting behaviors is an important subject for future research. This information would be needed to further develop rational treatment ideas for addiction.
Midbrain (VTA and substantia nigra) and striatal (NAc and the dorsomedial and dorsolateral striatae) components of the basal ganglia and mesolimbic pathway are connected in a spiraling manner so that ventral striatal regions project to medial parts of the midbrain DAergic area, which subsequently sends projections to more dorsal striatal regions which in turn project to the more lateral midbrain and so on (Haber et al., 2000). An appealing emerging hypothesis is that the initial drug-induced plasticity in the DAergic midbrain and subsequently in the ventral striatum would recruit more and more dorsal striatal regions during chronic drug use and reinforce the connectivity within these spiral projections, thus leading to compulsive drug-seeking manifesting at the late stages of addiction (Grueter et al., 2012). The dorsomedial and the dorsolateral parts of striatum regulate goal-directed and stimulus–response habitual movements, respectively (Yin and Knowlton, 2006; Redgrave et al., 2010). The majority of the neurons of ventral and dorsal striatum are projecting medium spiny neurons (MSNs), whose activity depends to a great extent on excitatory inputs from cortical and limbic regions (Sesack and Grace, 2010). Plasticity at excitatory synapses of the striatum would thus change the output of striatal circuits, i.e., the motivated as well as compulsive behaviors. Addiction seems to involve exceptionally intense drug experience-driven synaptic and structural plasticity at different levels of the mesolimbic DA system (Robinson and Kolb, 1999).
GABAA receptor modulators are abused, but to date there are no data available whether they are able to induce changes in neuronal spine density in the reward system. Earlier work by Sarti et al. (2007) demonstrated a correlation of cocaine-induced plasticity of glutamatergic synapses with an increase in spine density of the rat VTA neurons. In that work, the traditional Golgi-Cox impregnation was used to study dendritic spines and cell morphology was used to identify and subtype VTA DA neurons. Since GABAA receptor modulators also induce similar glutamate receptor neuroplasticity in VTA DA neurons, we were puzzled with a question whether diazepam and/or THIP alter morphology and spine density. At 24 h after single injections of the doses of diazepam and THIP, which induce LTP in the VTA (Heikkinen et al., 2009; Vashchinkina et al., 2012), the brains of mice were subjected to Golgi staining and spine counting (Figure 1). In addition to the VTA, we analyzed the hippocampal CA1 and CA3 regions, since BZs are known to induce persistent synaptic plasticity particularly in the pyramidal neurons of the CA1 and CA3 regions (Table 1).
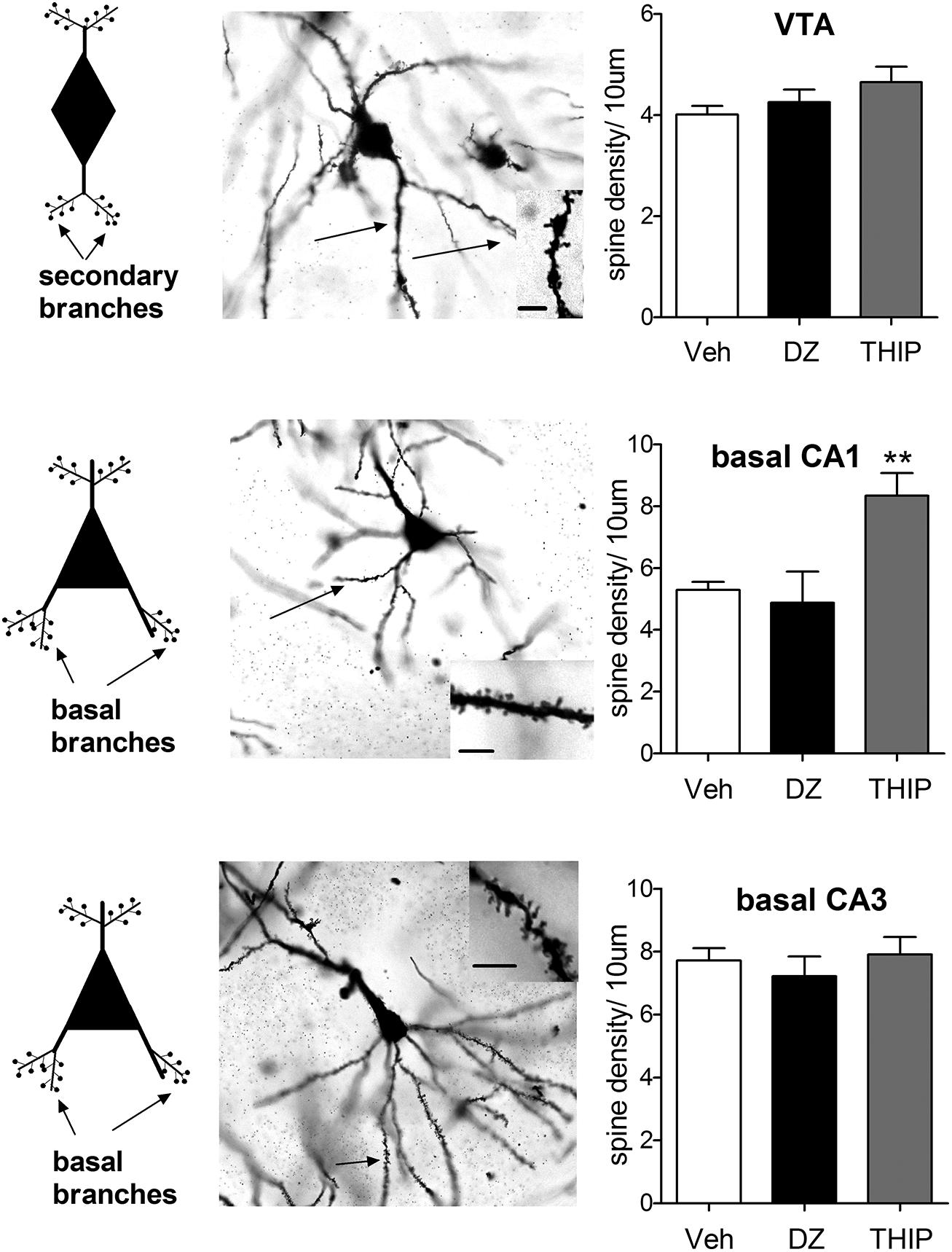
FIGURE 1. Pyramidal cells of hippocampal CA1 region exhibit distinct morphological responses to diazepam and THIP treatment. THIP increased the spine density on the basal secondary dendrites of CA1 pyramidal cells (F2,15 = 9.69, p < 0.01), but not in CA3 or VTA (p > 0.05). In contrast, diazepam (DZ) did not show any changes in spine density of the basal nor apical dendritic spines in these areas (p > 0.05). On the left, schematic neurons depict the spines either secondary or basal dendritic branches that were assessed here. In the middle, representative images of Golgi-stained neurons on horizontal sections. Insets are dendrites showing spines. Scale bar is 10 μm. On the right, plots showing the spine densities 24 h after a single injection of DZ or THIP. Mean ± SEM for n = 10, 5, and 5 mice vehicle (Veh), DZ, and THIP groups, respectively. **p < 0.01, (one-way ANOVA followed by Bonferroni post hoc test). C57BL/6J mice (21–26 days old) were decapitated at 24 h after treatment with DZ (5 mg/kg, ip), THIP (6 mg/kg, ip) or vehicle (Heikkinen et al., 2009; Vashchinkina et al., 2012; All animal procedures were approved by the Southern Finland Provincial Government). Brains were rapidly dissected and immediately placed into Golgi-Cox fixative and processed using the FD Rapid GolgiStain Kit (FD Neuro Technologies). Impregnated brains were then cut in serial horizontal 80-μm-thick sections using a cryostat (Leica Microsystems, Nussloch, Germany) and stained using the kit protocol. For each brain area, spine density per 10 μm was assessed in five cells. Notably, in VTA cells spines were determined on secondary dendrites, in CA1 and CA3 pyramidal cells on basal dendritic branches, using the 100× objective of Zeiss Axioplan microscope (Carl Zeiss MicroImaging GmbH, NA = 1.3, Ph3, DIC, oil), and later analyzed by National Institutes of Health ImageJ software (http://rsb.info.nih.gov/ij). Spine counting was performed blind concerning the treatments. Only protrusions that show continuity with the dendritic shaft were considered as spines.
We found that basal spine density counts in both hippocampal and VTA neurons are in line with the results of (Sarti et al., 2007). Treatment with THIP increased the spine density on the basal secondary dendrites of CA1 pyramidal cells (Figure 1), but not in CA3 or VTA. In contrast, diazepam treatment did not show any changes in spine density of the basal dendritic spines in these areas, nor in the apical ones (data not shown). This is generally consistent with a previous study, in which acute and chronic treatment with BZs was not associated with up-regulation of BDNF or c-Fos protein levels in the hippocampus (Licata et al., 2013). Since Sarti et al. (2007) found clear increase in VTA spines after neuroplasticity-inducing dose of cocaine, our results suggest different mechanisms or different VTA DA neuron populations might have been involved in the effects of various GABAA ligands. Additional studies using retrograde neurotracers to clarify the targets and novel immunofluorescent co-staining methods for precise phenotyping of the neurons (Spiga et al., 2011) are needed to resolve the affected VTA neurons. In spite of the comparable VTA neuroplasticity-inducing doses used, diazepam and THIP produced distinct effects on spine remodeling. These results show that the acute effects of GABA-drugs are not consistently accompanied by changes in spine densities of the dendrites in hippocampal or VTA neurons.
Repeated exposure to cocaine has been shown to increase the number of silent synapses and the density of dendritic spines in NAc shell (Robinson and Kolb, 1999; Huang et al., 2009; Dobi et al., 2011; Kim et al., 2011). Also dorsal striatum MSNs exhibit an increased dendritic spine density following chronic cocaine exposure (Ren et al., 2010). Months after repeated exposure to methamphetamine the spine density increased in MSNs of the dorsolateral striatum, a structure that supports habitual behaviors but decreased in dorsomedial striatum which is important in goal-directed movements (Jedynak et al., 2007). A primate study of chronic ethanol drinking reported increased spine density in the putamen (the primate analog of dorsolateral striatum in rodents) as well as enhanced glutamatergic transmission and increased intrinsic excitability of MSNs in this area (Cuzon Carlson et al., 2011). This pattern of structural plasticity in the dorsal striatum supports the concept that during the progression of addiction the behavior is driven toward habitual drug taking and seeking.
Withdrawal from chronic treatment with addictive drugs leads to hypofunction of VTA DA neurons. For example, withdrawal from cannabinoids or morphine profoundly affects the morphological characteristics of VTA DA neurons and spine density of MSNs of the NAc shell (Spiga et al., 2003, 2010). Whether chronic treatment with GABAA receptor modulators alters morphology of VTA DA neurons or NAc and dorsal striatal MSNs, with respect to addiction and/or withdrawal, remains to be studied.
Broad distribution of GABAA receptors throughout the brain suggests more widespread neuroplasticity effects of GABAA receptor modulators in other brain regions such as the NAc, mPFC, hippocampus, and amygdala (Licata and Rowlett, 2008). In fact, several reports showed blockade of hippocampal LTP after acute in vitro effects of BZs and isoflurane (summarized in Table 1). This is consistent with the known effect of cognitive dysfunction induced by general anesthetics, and memory impairment by BZs in humans (Canet et al., 2003; Newman et al., 2007). On the other hand, withdrawal symptoms after repeated administration of these drugs are associated with synthesis of new glutamatergic receptors and potentiation of LTP in the hippocampus and cortex (Table 1). Thus, the glutamatergic receptors appear to be regulated differently depending on the specific phase of the drug effect.
GABAergic neurotransmission is known to participate in neuronal plasticity processes during the critical periods in development, and drugs acting on GABAA receptors, such as BZs, have been considered more as blunting neuroplasticity than inducing it. However, recent experiments on the midbrain dopamine systems have revealed that GABAA drugs acting on different receptor subtypes induce persistent neuroplasticity in glutamate receptors of the VTA DA neurons, but so far there is little evidence in support of widespread structural changes caused by these drugs. The main mechanism of how the GABAA drugs induce plasticity involves disinhibition via primary inhibition of GABAergic interneurons. It is likely that more dynamic methods, such as in vivo microscopy on dendritic spines, will be needed to better understand the structural neuroplasticity/neurotoxicity effects of general anesthetics, BZs and other types of anxiolytics and sedatives. Based on the present data, the selection of an anesthetic agent will be important for these future experiments!
Elena Vashchinkina, Anne Panhelainen, Teemu Aitta-aho, and Esa R. Korpi conceived and designed the experiments, Elena Vashchinkina performed the experiments and data analyses, and Elena Vashchinkina, Anne Panhelainen, Teemu Aitta-aho, and Esa R. Korpi wrote the paper.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The work was supported by the Academy of Finland (Esa R. Korpi), the Sigrid Juselius Foundation (Esa R. Korpi, Teemu Aitta-aho), the Finnish Foundation for Alcohol Studies (Elena Vashchinkina, Anne Panhelainen, Teemu Aitta-aho), the Orion-Farmos Research Foundation (Esa R. Korpi, Teemu Aitta-aho), the Jane and Aatos Erkko Foundation (Anne Panhelainen, Esa R. Korpi), and the Finnish Cultural Foundation (Anne Panhelainen).
Abramian, A. M., Comenencia-Ortiz, E., Modgil, A., Vien, T. N., Nakamura, Y., Moore, Y. E.,et al. (2014). Neurosteroids promote phosphorylation and membrane insertion of extrasynaptic GABAA receptors. Proc. Natl. Acad. Sci. U.S.A. 111, 7132–7137. doi: 10.1073/pnas.1403285111
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Alexander, S. P., Benson, H. E., Faccenda, E., Pawson, A. J., Sharman, J. L., Spedding, M.,et al. (2013). The concise guide to pharmacology 2013/14: ligand-gated ion channels. Br. J. Pharmacol. 170, 1582–1606. doi: 10.1111/bph.12446
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ator, N. A. (2002). Relation between discriminative and reinforcing effects of midazolam, pentobarbital, chlordiazepoxide, zolpidem, and imidazenil in baboons. Psychopharmacology 163, 477–487. doi: 10.1007/s00213-002-1076-4
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Beauchamp, M. H., Ormerod, B. K., Jhamandas, K., Boegman, R. J., and Beninger, R. J. (2000). Neurosteroids and reward: allopregnanolone produces a conditioned place aversion in rats. Pharmacol. Biochem. Behav. 67, 29–35. doi: 10.1016/S0091-3057(00)00299-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Beck, C. H., and Fibiger, H. C. (1995). Conditioned fear-induced changes in behavior and in the expression of the immediate early gene c-fos: with and without diazepam pretreatment. J. Neurosci. 15, 709–720.
Beckley, J. T., Evins, C. E., Fedarovich, H., Gilstrap, M. J., and Woodward, J. J. (2013). Medial prefrontal cortex inversely regulates toluene-induced changes in markers of synaptic plasticity of mesolimbic dopamine neurons. J. Neurosci. 33, 804–813. doi: 10.1523/JNEUROSCI.3729-12.2013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Bengtsson, S. K., Johansson, M., Backstrom, T., Nitsch, R. M., and Wang, M. (2013). Brief but chronic increase in allopregnanolone cause accelerated AD pathology differently in two mouse models. Curr. Alzheimer Res. 10, 38–47.
Bengtsson, S. K., Johansson, M., Backstrom, T., and Wang, M. (2012). Chronic allopregnanolone treatment accelerates Alzheimer’s disease development in AbetaPP(Swe)PSEN1(DeltaE9) mice. J. Alzheimers Dis. 31, 71–84.
Bossert, J. M., Liu, S. Y., Lu, L., and Shaham, Y. (2004). A role of ventral tegmental area glutamate in contextual cue-induced relapse to heroin seeking. J. Neurosci. 24, 10726–10730. doi: 10.1523/JNEUROSCI.3207-04.2004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brischoux, F., Chakraborty, S., Brierley, D. I., and Ungless, M. A. (2009). Phasic excitation of dopamine neurons in ventral VTA by noxious stimuli. Proc. Natl. Acad. Sci. U.S.A. 106, 4894–4899. doi: 10.1073/pnas.0811507106
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Brown, M. T., Tan, K. R., O’Connor, E. C., Nikonenko, I., Muller, D., and Luscher, C. (2012). Ventral tegmental area GABA projections pause accumbal cholinergic interneurons to enhance associative learning. Nature 492, 452–456. doi: 10.1038/nature11657
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Canet, J., Raeder, J., Rasmussen, L. S., Enlund, M., Kuipers, H. M., Hanning, C. D.,et al. (2003). Cognitive dysfunction after minor surgery in the elderly. Acta Anaesthesiol. Scand. 47, 1204–1210. doi: 10.1046/j.1399-6576.2003.00238.x
Castillo, P. E., Chiu, C. Q., and Carroll, R. C. (2011). Long-term plasticity at inhibitory synapses. Curr. Opin. Neurobiol. 21, 328–338. doi: 10.1016/j.conb.2011.01.006
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chandra, D., Halonen, L. M., Linden, A. M., Procaccini, C., Hellsten, K., Homanics, G. E.,et al. (2010). Prototypic GABA(A) receptor agonist muscimol acts preferentially through forebrain high-affinity binding sites. Neuropsychopharmacology 35, 999–1007. doi: 10.1038/npp.2009.203
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Chiu, C. Q., Lur, G., Morse, T. M., Carnevale, N. T., Ellis-Davies, G. C., and Higley, M. J. (2013). Compartmentalization of GABAergic inhibition by dendritic spines. Science 340, 759–762. doi: 10.1126/science.1234274
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Creed, M. C., Ntamati, N. R., and Tan, K. R. (2014). VTA GABA neurons modulate specific learning behaviors through the control of dopamine and cholinergic systems. Front. Behav. Neurosci. 8:8. doi: 10.3389/fnbeh.2014.00008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Crestani, F., Lorez, M., Baer, K., Essrich, C., Benke, D., Laurent, J. P.,et al. (1999). Decreased GABAA-receptor clustering results in enhanced anxiety and a bias for threat cues. Nat. Neurosci. 2, 833–839. doi: 10.1038/12207
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Cuzon Carlson, V. C., Seabold, G. K., Helms, C. M., Garg, N., Odagiri, M., Rau, A. R.,et al. (2011). Synaptic and morphological neuroadaptations in the putamen associated with long-term, relapsing alcohol drinking in primates. Neuropsychopharmacology 36, 2513–2528. doi: 10.1038/npp.2011.140
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dailey, M. E., and Smith, S. J. (1996). The dynamics of dendritic structure in developing hippocampal slices. J. Neurosci. 16, 2983–2994.
Davis, K. M., Sturt, B. L., Friedmann, A. J., Richmond, J. E., Bessereau, J. L., Grant, B. D.,et al. (2010). Regulated lysosomal trafficking as a mechanism for regulating GABAA receptor abundance at synapses in Caenorhabditis elegans. Mol. Cell Neurosci. 44, 307–317. doi: 10.1016/j.mcn.2010.04.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Deeb, T. Z., Maguire, J., and Moss, S. J. (2012). Possible alterations in GABAA receptor signaling that underlie benzodiazepine-resistant seizures. Epilepsia 53(Suppl. 9), 79–88. doi: 10.1111/epi.12037
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
del Cerro, S., Jung, M., and Lynch, G. (1992). Benzodiazepines block long-term potentiation in slices of hippocampus and piriform cortex. Neuroscience 49, 1–6. doi: 10.1016/0306-4522(92)90071-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Dobi, A., Seabold, G. K., Christensen, C. H., Bock, R., and Alvarez, V. A. (2011). Cocaine-induced plasticity in the nucleus accumbens is cell specific and develops without prolonged withdrawal. J. Neurosci. 31, 1895–1904. doi: 10.1523/JNEUROSCI.5375-10.2011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Engin, E., Bakhurin, K. I., Smith, K. S., Hines, R. M., Reynolds, L. M., Tang, W.,et al. (2014). Neural basis of benzodiazepine reward: requirement for alpha2 containing GABAA receptors in the nucleus accumbens. Neuropsychopharmacology 39, 1805–1815. doi: 10.1038/npp.2014.41
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Erasso, D. M., Camporesi, E. M., Mangar, D., and Saporta, S. (2013). Effects of isoflurane or propofol on postnatal hippocampal neurogenesis in young and aged rats. Brain Res. 1530, 1–12. doi: 10.1016/j.brainres.2013.07.035
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Finn, D. A., Phillips, T. J., Okorn, D. M., Chester, J. A., and Cunningham, C. L. (1997). Rewarding effect of the neuroactive steroid 3 alpha-hydroxy-5 alpha-pregnan-20-one in mice. Pharmacol. Biochem. Behav. 56, 261–264. doi: 10.1016/S0091-3057(96)00218-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Fish, E. W., Whitman, B. J., Diberto, J. F., Robinson, J. E., Morrow, A. L., and Malanga, C. J. (2014). Effects of the neuroactive steroid allopregnanolone on intracranial self-stimulation in C57BL/6J Mice. Psychopharmacology 231, 3415–3423. doi: 10.1007/s00213-014-3600-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Freund, T. F., and Buzsaki, G. (1996). Interneurons of the hippocampus. Hippocampus 6, 347–470. doi: 10.1002/(SICI)1098-1063 (1996)6:4<347::AID-HIPO1>3.0.CO;2-I
Gauvain, G., Chamma, I., Chevy, Q., Cabezas, C., Irinopoulou, T., Bodrug, N.,et al. (2011). The neuronal K-Cl cotransporter KCC2 influences postsynaptic AMPA receptor content and lateral diffusion in dendritic spines. Proc. Natl. Acad. Sci. U.S.A. 108, 15474–15479. doi: 10.1073/pnas.1107893108
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Grasshoff, C., Rudolph, U., and Antkowiak, B. (2005). Molecular and systemic mechanisms of general anaesthesia: the ‘multi-site and multiple mechanisms’ concept. Curr. Opin. Anaesthesiol. 18, 386–391. doi: 10.1097/01.aco.0000174961.90135.dc
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Gray, E. G. (1959). Electron microscopy of synaptic contacts on dendrite spines of the cerebral cortex. Nature 183, 1592–1593. doi: 10.1038/1831592a0
Grueter, B. A., Rothwell, P. E., and Malenka, R. C. (2012). Integrating synaptic plasticity and striatal circuit function in addiction. Curr. Opin. Neurobiol. 22, 545–551. doi: 10.1016/j.conb.2011.09.009
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Guan, Y. Z., and Ye, J. H. (2010). Ethanol blocks long-term potentiation of GABAergic synapses in the ventral tegmental area involving mu-opioid receptors. Neuropsychopharmacology 35, 1841–1849. doi: 10.1038/npp.2010.51
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Haber, S. N., Fudge, J. L., and McFarland, N. R. (2000). Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J. Neurosci. 20, 2369–2382.
Haseneder, R., Kratzer, S., von Meyer, L., Eder, M., Kochs, E., and Rammes, G. (2009). Isoflurane and sevoflurane dose-dependently impair hippocampal long-term potentiation. Eur. J. Pharmacol. 623, 47–51. doi: 10.1016/j.ejphar.2009.09.022
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Heikkinen, A. E., Moykkynen, T. P., and Korpi, E. R. (2009). Long-lasting modulation of glutamatergic transmission in VTA dopamine neurons after a single dose of benzodiazepine agonists. Neuropsychopharmacology 34, 290–298. doi: 10.1038/npp.2008.89
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Heinen, K., Baker, R. E., Spijker, S., Rosahl, T., van Pelt, J., and Brussaard, A. B. (2003). Impaired dendritic spine maturation in GABAA receptor alpha1 subunit knock out mice. Neuroscience 122, 699–705. doi: 10.1016/S0306-4522(03)00477-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hensch, T. K., and Stryker, M. P. (2004). Columnar architecture sculpted by GABA circuits in developing cat visual cortex. Science 303, 1678–1681. doi: 10.1126/science.1091031
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hering, H., and Sheng, M. (2001). Dendritic spines: structure, dynamics and regulation. Nat. Rev. Neurosci. 2, 880–888. doi: 10.1038/35104061
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Higashima, M., Kinoshita, H., and Koshino, Y. (1998). Differences in the effects of zolpidem and diazepam on recurrent inhibition and long-term potentiation in rat hippocampal slices. Neurosci. Lett. 3, 77–80. doi: 10.1016/S0304-3940(98)00178-5
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Horn, Z., Ringstedt, T., Blaesse, P., Kaila, K., and Herlenius, E. (2010). Premature expression of KCC2 in embryonic mice perturbs neural development by an ion transport-independent mechanism. Eur. J. Neurosci. 31, 2142–2155. doi: 10.1111/j.1460-9568.2010.07258.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Hortnagl, H., Tasan, R. O., Wieselthaler, A., Kirchmair, E., Sieghart, W., and Sperk, G. (2013). Patterns of mRNA and protein expression for 12 GABAA receptor subunits in the mouse brain. Neuroscience 236, 345–372. doi: 10.1016/j.neuroscience.2013.01.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Huang, Y. H., Lin, Y., Mu, P., Lee, B. R., Brown, T. E., Wayman, G.,et al. (2009). In vivo cocaine experience generates silent synapses. Neuron 63, 40–47. doi: 10.1016/j.neuron.2009.06.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Huopaniemi, L., Keist, R., Randolph, A., Certa, U., and Rudolph, U. (2004). Diazepam-induced adaptive plasticity revealed by alpha1 GABAA receptor-specific expression profiling. J. Neurochem. 88, 1059–1067. doi: 10.1046/j.1471-4159.2003.02216.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ikemoto, S., Murphy, J. M., and McBride, W. J. (1997). Self-infusion of GABAA antagonists directly into the ventral tegmental area and adjacent regions. Behav. Neurosci. 111, 369–380. doi: 10.1037/0735-7044.111.2.369
Ikemoto, S., Murphy, J. M., and McBride, W. J. (1998). Regional differences within the rat ventral tegmental area for muscimol self-infusions. Pharmacol. Biochem. Behav. 61, 87–92. doi: 10.1016/S0091-3057(98)00086-0
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Izzo, E., Auta, J., Impagnatiello, F., Pesold, C., Guidotti, A., and Costa, E. (2001). Glutamic acid decarboxylase and glutamate receptor changes during tolerance and dependence to benzodiazepines. Proc. Natl. Acad. Sci. U.S.A. 98, 3483–3488. doi: 10.1073/pnas.051628698
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jacob, T. C., Michels, G., Silayeva, L., Haydon, J., Succol, F., and Moss, S. J. (2012). Benzodiazepine treatment induces subtype-specific changes in GABAA receptor trafficking and decreases synaptic inhibition. Proc. Natl. Acad. Sci. U.S.A. 109, 18595–18600. doi: 10.1073/pnas.1204994109
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jacob, T. C., Wan, Q., Vithlani, M., Saliba, R. S., Succol, F., Pangalos, M. N.,et al. (2009). GABAA receptor membrane trafficking regulates spine maturity. Proc. Natl. Acad. Sci. U.S.A. 106, 12500–12505. doi: 10.1073/pnas.0903943106
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jedynak, J. P., Uslaner, J. M., Esteban, J. A., and Robinson, T. E. (2007). Methamphetamine-induced structural plasticity in the dorsal striatum. Eur. J. Neurosci. 25, 847–853. doi: 10.1111/j.1460-9568.2007.05316.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jevtovic-Todorovic, V., Absalom, A. R., Blomgren, K., Brambrink, A., Crosby, G., Culley, D. J.,et al. (2013). Anaesthetic neurotoxicity and neuroplasticity: an expert group report and statement based on the BJA Salzburg Seminar. Br. J. Anaesth. 111, 143–151. doi: 10.1093/bja/aet177
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jhou, T. C., Geisler, S., Marinelli, M., Degarmo, B. A., and Zahm, D. S. (2009). The mesopontine rostromedial tegmental nucleus: a structure targeted by the lateral habenula that projects to the ventral tegmental area of Tsai and substantia nigra compacta. J. Comp. Neurol. 513, 566–596. doi: 10.1002/cne.21891
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Jones, S., Kornblum, J. L., and Kauer, J. A. (2000). Amphetamine blocks long-term synaptic depression in the ventral tegmental area. J. Neurosci. 20, 5575–5580.
Kang, T. C., Kim, D. S., Kim, J. E., Kwak, S. E., Yoo, K. Y., Hwang, I. K.,et al. (2006). Altered expression of K+ -Cl- cotransporters affects fast paired-pulse inhibition during GABA receptor activation in the gerbil hippocampus. Brain Res. 1072, 8–14. doi: 10.1016/j.brainres.2005.12.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kim, S., Shin, J. K., Yoon, H. S., and Kim, J. H. (2011). Blockade of ERK phosphorylation in the nucleus accumbens inhibits the expression of cocaine-induced behavioral sensitization in rats. Korean J. Physiol. Pharmacol. 15, 389–395. doi: 10.4196/kjpp.2011.15.6.389
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Korotkova, T. M., Ponomarenko, A. A., Brown, R. E., and Haas, H. L. (2004). Functional diversity of ventral midbrain dopamine and GABAergic neurons. Mol. Neurobiol. 29, 243–259. doi: 10.1385/MN:29:3:243
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Korpi, E. R., Grunder, G., and Luddens, H. (2002). Drug interactions at GABAA receptors. Prog. Neurobiol. 67, 113–159. doi: 10.1016/S0301-0082(02)00013-8
Kulisch, C., Eckers, N., and Albrecht, D. (2011). Method of euthanasia affects amygdala plasticity in horizontal brain slices from mice. J. Neurosci. Methods 201, 340–345. doi: 10.1016/j.jneumeth.2011.08.022
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Kullmann, D. M., Moreau, A. W., Bakiri, Y., and Nicholson, E. (2012). Plasticity of inhibition. Neuron 75, 951–962. doi: 10.1016/j.neuron.2012.07.030
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lacoh, C. M., Bodogan, T., Kaila, K., Fiumelli, H., and Vutskits, L. (2013). General anaesthetics do not impair developmental expression of the KCC2 potassium-chloride cotransporter in neonatal rats during the brain growth spurt. Br. J. Anaesth. 110(Suppl. 1), i10–i18. doi: 10.1093/bja/aet063
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lammel, S., Ion, D. I., Roeper, J., and Malenka, R. C. (2011). Projection-specific modulation of dopamine neuron synapses by aversive and rewarding stimuli. Neuron 70, 855–862. doi: 10.1016/j.neuron.2011.03.025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lang, C., Barco, A., Zablow, L., Kandel, E. R., Siegelbaum, S. A., and Zakharenko, S. S. (2004). Transient expansion of synaptically connected dendritic spines upon induction of hippocampal long-term potentiation. Proc. Natl. Acad. Sci. U.S.A. 101, 16665–16670. doi: 10.1073/pnas.0407581101
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Laviolette, S. R., and van der Kooy, D. (2001). GABAA receptors in the ventral tegmental area control bidirectional reward signaling between dopaminergic and non-dopaminergic neural motivational systems. Eur. J. Neurosci. 13, 1009–1015. doi: 10.1046/j.1460-9568.2001.01458.x
Lee, D. E., Gerasimov, M. R., Schiffer, W. K., and Gifford, A. N. (2006). Concentration-dependent conditioned place preference to inhaled toluene vapors in rats. Drug Alcohol Depend. 85, 87–90. doi: 10.1016/j.drugalcdep.2006.03.013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Li, H., Khirug, S., Cai, C., Ludwig, A., Blaesse, P., Kolikova, J.,et al. (2007). KCC2 interacts with the dendritic cytoskeleton to promote spine development. Neuron 56, 1019–1033. doi: 10.1016/j.neuron.2007.10.039
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Licata, S. C., and Rowlett, J. K. (2008). Abuse and dependence liability of benzodiazepine-type drugs: GABAA receptor modulation and beyond. Pharmacol. Biochem. Behav. 90, 74–89. doi: 10.1016/j.pbb.2008.01.001
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Licata, S. C., Shinday, N. M., Huizenga, M. N., Darnell, S. B., Sangrey, G. R., Rudolph, U.,et al. (2013). Alterations in brain-derived neurotrophic factor in the mouse hippocampus following acute but not repeated benzodiazepine treatment. PLoS ONE 8:e84806. doi: 10.1371/journal.pone.0084806
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Liu, F., Wan, Q., Pristupa, Z. B., Yu, X. M., Wang, Y. T., and Niznik, H. B. (2000). Direct protein-protein coupling enables cross-talk between dopamine D5 and γ-aminobutyric acid a receptors. Nature 403, 274–280. doi: 10.1038/35001232
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Lubman, D. I., Yucel, M., and Lawrence, A. J. (2008). Inhalant abuse among adolescents: neurobiological considerations. Br. J. Pharmacol. 154, 316–326. doi: 10.1038/bjp.2008.76
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Luddens, H., Korpi, E. R., and Seeburg, P. H. (1995). GABAA/benzodiazepine receptor heterogeneity: neurophysiological implications. Neuropharmacology 34, 245–254. doi: 10.1016/0028-3908(94)00158-O
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Luscher, C., and Malenka, R. C. (2011). Drug-evoked synaptic plasticity in addiction: from molecular changes to circuit remodeling. Neuron 69, 650–663. doi: 10.1016/j.neuron.2011.01.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Macedo, C. E., Martinez, R. C., and Brandao, M. L. (2006). Conditioned and unconditioned fear organized in the inferior colliculus are differentially sensitive to injections of muscimol into the basolateral nucleus of the amygdala. Behav. Neurosci. 120, 625–631. doi: 10.1037/0735-7044.120.3.625
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maguire, J. L., Stell, B. M., Rafizadeh, M., and Mody, I. (2005). Ovarian cycle-linked changes in GABAA receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat. Neurosci. 8, 797–804. doi: 10.1038/nn1469
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Margolis, E. B., Lock, H., Hjelmstad, G. O., and Fields, H. L. (2006). The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J. Physiol. 577, 907–924. doi: 10.1113/jphysiol.2006.117069
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Margolis, E. B., Toy, B., Himmels, P., Morales, M., and Fields, H. L. (2012). Identification of rat ventral tegmental area GABAergic neurons. PLoS ONE 7:e42365. doi: 10.1371/journal.pone.0042365
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Markkanen, M., Karhunen, T., Llano, O., Ludwig, A., Rivera, C., Uvarov, P.,et al. (2014). Distribution of neuronal KCC2a and KCC2b isoforms in mouse CNS. J. Comp. Neurol. 522, 1897–1914. doi: 10.1002/cne.23510
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Matsumoto, M., and Hikosaka, O. (2009). Two types of dopamine neuron distinctly convey positive and negative motivational signals. Nature 459, 837–841. doi: 10.1038/nature08028
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Matsuzaki, M., Honkura, N., Ellis-Davies, G. C., and Kasai, H. (2004). Structural basis of long-term potentiation in single dendritic spines. Nature 429, 761–766. doi: 10.1038/nature02617
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Maubach, K. A., Martin, K., Choudhury, H. I., and Seabrook, G. R. (2004). Triazolam suppresses the induction of hippocampal long-term potentiation. Neuroreport 15, 1145–1149. doi: 10.1097/00001756-200405190-00013
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
McKernan, R. M., and Whiting, P. J. (1996). Which GABAA-receptor subtypes really occur in the brain? Trends Neurosci. 19, 139–143. doi: 10.1016/S0166-2236(96)80023-3
Meririnne, E., Kankaanpaa, A., Lillsunde, P., and Seppala, T. (1999). The effects of diazepam and zolpidem on cocaine- and amphetamine-induced place preference. Pharmacol. Biochem. Behav. 62, 159–164. doi: 10.1016/S0091-3057(98)00139-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mihalek, R. M., Banerjee, P. K., Korpi, E. R., Quinlan, J. J., Firestone, L. L., Mi, Z. P.,et al. (1999). Attenuated sensitivity to neuroactive steroids in γ-aminobutyrate type a receptor δ subunit knockout mice. Proc. Natl. Acad. Sci. U.S.A. 96, 12905–12910. doi: 10.1073/pnas.96.22.12905
Modol, L., Casas, C., Llido, A., Navarro, X., Pallares, M., and Darbra, S. (2014). Neonatal allopregnanolone or finasteride administration modifies hippocampal K Cl co-transporter expression during early development in male rats. J. Steroid Biochem. Mol. Biol. 143C, 343–347. doi: 10.1016/j.jsbmb.2014.05.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Mori, K., Togashi, H., Kojima, T., Matsumoto, M., Ohashi, S., Ueno, K.,et al. (2001). Different effects of anxiolytic agents, diazepam and 5-HT(1A) agonist tandospirone, on hippocampal long-term potentiation in vivo. Pharmacol. Biochem. Behav. 69, 367–372. doi: 10.1016/S0091-3057(01)00546-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Newman, S., Stygall, J., Hirani, S., Shaefi, S., and Maze, M. (2007). Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology 106, 572–590. doi: 10.1097/00000542-200703000-00023
Niehaus, J. L., Murali, M., and Kauer, J. A. (2010). Drugs of abuse and stress impair LTP at inhibitory synapses in the ventral tegmental area. Eur. J. Neurosci. 32, 108–117. doi: 10.1111/j.1460-9568.2010.07256.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nugent, F. S., and Kauer, J. A. (2008). LTP of GABAergic synapses in the ventral tegmental area and beyond. J. Physiol. 586, 1487–1493. doi: 10.1113/jphysiol.2007.148098
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Nugent, F. S., Penick, E. C., and Kauer, J. A. (2007). Opioids block long-term potentiation of inhibitory synapses. Nature 446, 1086–1090. doi: 10.1038/nature05726
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
O’Brien, C. P. (2005). Benzodiazepine use, abuse, and dependence. J. Clin. Psychiatry 66(Suppl. 2), 28–33.
Olsen, R. W., and Sieghart, W. (2009). GABAA receptors: subtypes provide diversity of function and pharmacology. Neuropharmacology 56, 141–148. doi: 10.1016/j.neuropharm.2008.07.045
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Omelchenko, N., and Sesack, S. R. (2009). Ultrastructural analysis of local collaterals of rat ventral tegmental area neurons: GABA phenotype and synapses onto dopamine and GABA cells. Synapse 63, 895–906. doi: 10.1002/syn.20668
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pain, L., Oberling, P., Sandner, G., and Di Scala, G. (1996). Effect of propofol on affective state as assessed by place conditioning paradigm in rats. Anesthesiology 85, 121–128. doi: 10.1097/00000542-199607000-00017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Panhelainen, A. E., and Korpi, E. R. (2012). Evidence for a role of inhibition of orexinergic neurons in the anxiolytic and sedative effects of diazepam: a c-Fos study. Pharmacol. Biochem. Behav. 101, 115–124. doi: 10.1016/j.pbb.2011.12.011
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Panlilio, L. V., Thorndike, E. B., and Schindler, C. W. (2005). Lorazepam reinstates punishment-suppressed remifentanil self-administration in rats. Psychopharmacology 179, 374–382. doi: 10.1007/s00213-004-2040-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Piao, M. H., Liu, Y., Wang, Y. S., Qiu, J. P., and Feng, C. S. (2013). Volatile anesthetic isoflurane inhibits LTP induction of hippocampal CA1 neurons through α4β2 nAChR subtype-mediated mechanisms. Ann. Fr. Anesth. Reanim. 32, e135–e141. doi: 10.1016/j.annfar.2013.05.012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Pirker, S., Schwarzer, C., Wieselthaler, A., Sieghart, W., and Sperk, G. (2000). GABAA receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101, 815–850. doi: 10.1016/S0306-4522(00)00442-5
Rammes, G., Starker, L. K., Haseneder, R., Berkmann, J., Plack, A., Zieglgansberger, W.,et al. (2009). Isoflurane anaesthesia reversibly improves cognitive function and long-term potentiation (LTP) via an up-regulation in NMDA receptor 2B subunit expression. Neuropharmacology 56, 626–636. doi: 10.1016/j.neuropharm.2008.11.002
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Redgrave, P., Coizet, V., Comoli, E., McHaffie, J. G., Leriche, M., Vautrelle, N.,et al. (2010). Interactions between the midbrain superior colliculus and the basal ganglia. Front. Neuroanat. 4:132. doi: 10.3389/fnana.2010.00132
Ren, Z., Sun, W. L., Jiao, H., Zhang, D., Kong, H., Wang, X.,et al. (2010). Dopamine D1 and N-methyl-D-aspartate receptors and extracellular signal-regulated kinase mediate neuronal morphological changes induced by repeated cocaine administration. Neuroscience 168, 48–60. doi: 10.1016/j.neuroscience.2010.03.034
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Reynolds, S. M., and Berridge, K. C. (2002). Positive and negative motivation in nucleus accumbens shell: bivalent rostrocaudal gradients for GABA-elicited eating, taste “liking“/”disliking” reactions, place preference/avoidance, and fear. J. Neurosci. 22, 7308–7320.
Riegel, A. C., Zapata, A., Shippenberg, T. S., and French, E. D. (2007). The abused inhalant toluene increases dopamine release in the nucleus accumbens by directly stimulating ventral tegmental area neurons. Neuropsychopharmacology 32, 1558–1569. doi: 10.1038/sj.npp.1301273
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Rivera, C., Voipio, J., Payne, J. A., Ruusuvuori, E., Lahtinen, H., Lamsa, K.,et al. (1999). The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397, 251–255. doi: 10.1038/16697
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Robinson, T. E., and Kolb, B. (1999). Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur. J. Neurosci. 11, 1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x
Rudolph, U., and Mohler, H. (2004). Analysis of GABAA receptor function and dissection of the pharmacology of benzodiazepines and general anesthetics through mouse genetics. Annu. Rev. Pharmacol. Toxicol. 44, 475–498. doi: 10.1146/annurev.pharmtox.44.101802.121429
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Saal, D., Dong, Y., Bonci, A., and Malenka, R. C. (2003). Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron 37, 577–582. doi: 10.1016/S0896-6273(03)00021-7
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Salminen, O., Lahtinen, S., and Ahtee, L. (1996). Expression of Fos protein in various rat brain areas following acute nicotine and diazepam. Pharmacol. Biochem. Behav. 54, 241–248. doi: 10.1016/0091-3057(95)02132-9
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sarti, F., Borgland, S. L., Kharazia, V. N., and Bonci, A. (2007). Acute cocaine exposure alters spine density and long-term potentiation in the ventral tegmental area. Eur. J. Neurosci. 26, 749–756. doi: 10.1111/j.1460-9568.2007.05689.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Segal, M. (2005). Dendritic spines and long-term plasticity. Nat. Rev. Neurosci. 6, 277–284. doi: 10.1038/nrn1649
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sesack, S. R., and Grace, A. A. (2010). Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology 35, 27–47. doi: 10.1038/npp.2009.93
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shen, H., Sabaliauskas, N., Sherpa, A., Fenton, A. A., Stelzer, A., Aoki, C.,et al. (2010). A critical role for α4βδ GABAA receptors in shaping learning deficits at puberty in mice. Science 327, 1515–1518. doi: 10.1126/science.1184245
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Shibasaki, M., Masukawa, D., Ishii, K., Yamagishi, Y., Mori, T., and Suzuki, T. (2013). Involvement of the K+-Cl- co-transporter KCC2 in the sensitization to morphine-induced hyperlocomotion under chronic treatment with zolpidem in the mesolimbic system. J. Neurochem. 125, 747–755. doi: 10.1111/jnc.12258
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sieghart, W. (1995). Structure and pharmacology of γ-aminobutyric acid receptor subtypes. Pharmacol. Rev. 47, 181–234.
Simon, W., Hapfelmeier, G., Kochs, E., Zieglgansberger, W., and Rammes, G. (2001). Isoflurane blocks synaptic plasticity in the mouse hippocampus. Anesthesiology 94, 1058–1065. doi: 10.1097/00000542-200106000-00021
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sinnott, R. S., Mark, G. P., and Finn, D. A. (2002). Reinforcing effects of the neurosteroid allopregnanolone in rats. Pharmacol. Biochem. Behav. 72, 923–929. doi: 10.1016/S0091-3057(02)00776-1
Somogyi, P., Tamas, G., Lujan, R., and Buhl, E. H. (1998). Salient features of synaptic organisation in the cerebral cortex. Brain Res. Brain Res. Rev. 26, 113–135. doi: 10.1016/S0165-0173(97)00061-1
Song, J., Shen, G., Greenfield, L. J. Jr., and Tietz, E. I. (2007). Benzodiazepine withdrawal-induced glutamatergic plasticity involves up-regulation of GluR1-containing α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors in Hippocampal CA1 neurons. J. Pharmacol. Exp. Ther. 322, 569–581. doi: 10.1124/jpet.107.121798
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Spiga, S., Acquas, E., Puddu, M. C., Mulas, G., Lintas, A., and Diana, M. (2011). Simultaneous Golgi-Cox and immunofluorescence using confocal microscopy. Brain Struct. Funct. 216, 171–182. doi: 10.1007/s00429-011-0312-2
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Spiga, S., Lintas, A., Migliore, M., and Diana, M. (2010). Altered architecture and functional consequences of the mesolimbic dopamine system in cannabis dependence. Addict. Biol. 15, 266–276. doi: 10.1111/j.1369-1600.2010.00218.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Spiga, S., Serra, G. P., Puddu, M. C., Foddai, M., and Diana, M. (2003). Morphine withdrawal-induced abnormalities in the VTA: confocal laser scanning microscopy. Eur. J. Neurosci. 17, 605–612. doi: 10.1046/j.1460-9568.2003.02435.x
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Spyraki, C., Kazandjian, A., and Varonos, D. (1985). Diazepam-induced place preference conditioning: appetitive and antiaversive properties. Psychopharmacology 87, 225–232. doi: 10.1007/BF00431813
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Straub, C. J., Carlezon, W. A. Jr., and Rudolph, U. (2010). Diazepam and cocaine potentiate brain stimulation reward in C57BL/6J mice. Behav. Brain Res. 206, 17–20. doi: 10.1016/j.bbr.2009.08.025
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sun, C., Zhang, L., and Chen, G. (2013). An unexpected role of neuroligin-2 in regulating KCC2 and GABA functional switch. Mol. Brain 6, 23. doi: 10.1186/1756-6606-6-23
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Sun, W., Akins, C. K., Mattingly, A. E., and Rebec, G. V. (2005). Ionotropic glutamate receptors in the ventral tegmental area regulate cocaine-seeking behavior in rats. Neuropsychopharmacology 30, 2073–2081. doi: 10.1038/sj.npp.1300744
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Tan, K. R., Brown, M., Labouebe, G., Yvon, C., Creton, C., Fritschy, J. M.,et al. (2010). Neural bases for addictive properties of benzodiazepines. Nature 463, 769–774. doi: 10.1038/nature08758
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Thal, S. C., Timaru-Kast, R., Wilde, F., Merk, P., Johnson, F., Frauenknecht, K.,et al. (2014). Propofol impairs neurogenesis and neurologic recovery and increases mortality rate in adult rats after traumatic brain injury. Crit. Care Med. 42, 129–141. doi: 10.1097/CCM.0b013e3182a639fd
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Thomas, M. J., and Malenka, R. C. (2003). Synaptic plasticity in the mesolimbic dopamine system. Philos. Trans. R. Soc. Lond. B Biol. Sci. 358, 815–819. doi: 10.1098/rstb.2002.1236
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Ungless, M. A., Whistler, J. L., Malenka, R. C., and Bonci, A. (2001). Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature 411, 583–587. doi: 10.1038/35079077
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Uusi-Oukari, M., and Korpi, E. R. (2010). Regulation of GABAA receptor subunit expression by pharmacological agents. Pharmacol. Rev. 62, 97–135. doi: 10.1124/pr.109.002063
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
van Rijnsoever, C., Tauber, M., Choulli, M. K., Keist, R., Rudolph, U., Mohler, H.,et al. (2004). Requirement of α5- GABAA receptors for the development of tolerance to the sedative action of diazepam in mice. J. Neurosci. 24, 6785–6790. doi: 10.1523/JNEUROSCI.1067-04.2004
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Van Sickle, B. J., Xiang, K., and Tietz, E. I. (2004). Transient plasticity of hippocampal CA1 neuron glutamate receptors contributes to benzodiazepine withdrawal-anxiety. Neuropsychopharmacology 29, 1994–2006. doi: 10.1038/sj.npp.1300531
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vashchinkina, E., Manner, A. K., Vekovischeva, O., den Hollander, B., Uusi-Oukari, M., Aitta-Aho, T.,et al. (2014). Neurosteroid agonist at GABAA receptor induces persistent neuroplasticity in VTA dopamine neurons. Neuropsychopharmacology 39, 727–737. doi: 10.1038/npp.2013.258
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vashchinkina, E., Panhelainen, A., Vekovischeva, O. Y., Aitta-aho, T., Ebert, B., Ator, N. A.,et al. (2012). GABA site agonist gaboxadol induces addiction-predicting persistent changes in ventral tegmental area dopamine neurons but is not rewarding in mice or baboons. J. Neurosci. 32, 5310–5320. doi: 10.1523/JNEUROSCI.4697-11.2012
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Vithlani, M., Terunuma, M., and Moss, S. J. (2011). The dynamic modulation of GABAA receptor trafficking and its role in regulating the plasticity of inhibitory synapses. Physiol. Rev. 91, 1009–1022. doi: 10.1152/physrev.00015.2010
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Volkow, N. D., and Baler, R. D. (2014). Addiction science: uncovering neurobiological complexity. Neuropharmacology 76(Pt B), 235–249. doi: 10.1016/j.neuropharm.2013.05.007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wang, D. V., and Tsien, J. Z. (2011). Convergent processing of both positive and negative motivational signals by the VTA dopamine neuronal populations. PLoS ONE 6:e17047. doi: 10.1371/journal.pone.0017047
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Waszczak, B. L., and Walters, J. R. (1980). Intravenous GABA agonist administration stimulates firing of A10 dopaminergic neurons. Eur. J. Pharmacol. 66, 141–144. doi: 10.1016/0014-2999(80)90308-8
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Watabe-Uchida, M., Zhu, L., Ogawa, S. K., Vamanrao, A., and Uchida, N. (2012). Whole-brain mapping of direct inputs to midbrain dopamine neurons. Neuron 74, 858–873. doi: 10.1016/j.neuron.2012.03.017
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wierenga, C. J., Becker, N., and Bonhoeffer, T. (2008). GABAergic synapses are formed without the involvement of dendritic protrusions. Nat. Neurosci. 11, 1044–1052. doi: 10.1038/nn.2180
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Wu, X., and Castren, E. (2009). Co-treatment with diazepam prevents the effects of fluoxetine on the proliferation and survival of hippocampal dentate granule cells. Biol. Psychiatry 66, 5–8. doi: 10.1016/j.biopsych.2009.01.023
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Xi, Z. X., and Stein, E. A. (2002). Blockade of ionotropic glutamatergic transmission in the ventral tegmental area reduces heroin reinforcement in rat. Psychopharmacology 164, 144–150. doi: 10.1007/s00213-002-1190-3
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Xiang, K., and Tietz, E. I. (2007). Benzodiazepine-induced hippocampal CA1 neuron α-amino-3-hydroxy-5-methylisoxasole-4-propionic acid (AMPA) receptor plasticity linked to severity of withdrawal anxiety: differential role of voltage-gated calcium channels and N-methyl-D-aspartic acid receptors. Behav. Pharmacol. 18, 447–460. doi: 10.1097/FBP.0b013e3282d28f2b
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Xu, J. Y., and Sastry, B. R. (2005). Benzodiazepine involvement in LTP of the GABAergic IPSC in rat hippocampal CA1 neurons. Brain Res. 1062, 134–143. doi: 10.1016/j.brainres.2005.09.008
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Yin, H. H., and Knowlton, B. J. (2006). The role of the basal ganglia in habit formation. Nat. Rev. Neurosci. 7, 464–476. doi: 10.1038/nrn1919
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
You, Z. B., Wang, B., Zitzman, D., Azari, S., and Wise, R. A. (2007). A role for conditioned ventral tegmental glutamate release in cocaine seeking. J. Neurosci. 27, 10546–10555. doi: 10.1523/JNEUROSCI.2967-07.2007
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zafra, F., Castren, E., Thoenen, H., and Lindholm, D. (1991). Interplay between glutamate and γ-aminobutyric acid transmitter systems in the physiological regulation of brain-derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc. Natl. Acad. Sci. U.S.A. 88, 10037–10041. doi: 10.1073/pnas.88.22.10037
Zarrindast, M. R., Ahmadi, S., Haeri-Rohani, A., Rezayof, A., Jafari, M. R., and Jafari-Sabet, M. (2004). GABAA receptors in the basolateral amygdala are involved in mediating morphine reward. Brain Res. 1006, 49–58. doi: 10.1016/j.brainres.2003.12.048
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zschenderlein, C., Gebhardt, C., von Bohlen Und Halbach, O., Kulisch, C., and Albrecht, D. (2011). Capsaicin-induced changes in LTP in the lateral amygdala are mediated by TRPV1. PLoS ONE 6:e16116. doi: 10.1371/journal.pone.0016116
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Zweifel, L. S., Fadok, J. P., Argilli, E., Garelick, M. G., Jones, G. L., Dickerson, T. M.,et al. (2011). Activation of dopamine neurons is critical for aversive conditioning and prevention of generalized anxiety. Nat. Neurosci. 14, 620–626. doi: 10.1038/nn.2808
Pubmed Abstract | Pubmed Full Text | CrossRef Full Text | Google Scholar
Keywords: GABAA receptor, benzodiazepines, THIP, neurosteroids, dopamine neurons, neuroadaptation, dendritic spines
Citation: Vashchinkina E, Panhelainen A, Aitta-aho T and Korpi ER (2014) GABAA receptor drugs and neuronal plasticity in reward and aversion: focus on the ventral tegmental area. Front. Pharmacol. 5:256. doi: 10.3389/fphar.2014.00256
Received: 27 August 2014; Accepted: 03 November 2014;
Published online: 25 November 2014.
Edited by:
Justin Gass, Medical University of South Carolina, USAReviewed by:
Enrico Sanna, University of Cagliari, ItalyCopyright © 2014 Vashchinkina, Panhelainen, Aitta-aho and Korpi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Esa R. Korpi, Department of Pharmacology, Institute of Biomedicine, University of Helsinki, P.O. Box 63, Helsinki FIN-00014, Finland e-mail:ZXNhLmtvcnBpQGhlbHNpbmtpLmZp
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.