
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 09 September 2013
Sec. Neuropharmacology
Volume 4 - 2013 | https://doi.org/10.3389/fphar.2013.00110
This article is part of the Research Topic New frontiers in the neuropsychopharmacology of mental illness View all 21 articles
Our knowledge of the pathophysiology of affect dysregulation has progressively increased, but the pharmacological treatments remain inadequate. Here, we summarize the current literature on deficits in somatostatin, an inhibitory modulatory neuropeptide, in major depression and other neurological disorders that also include mood disturbances. We focus on direct evidence in the human postmortem brain, and review rodent genetic and pharmacological studies probing the role of the somatostatin system in relation to mood. We also briefly go over pharmacological developments targeting the somatostatin system in peripheral organs and discuss the challenges of targeting the brain somatostatin system. Finally, the fact that somatostatin deficits are frequently observed across neurological disorders suggests a selective cellular vulnerability of somatostatin-expressing neurons. Potential cell intrinsic factors mediating those changes are discussed, including nitric oxide induced oxidative stress, mitochondrial dysfunction, high inflammatory response, high demand for neurotrophic environment, and overall aging processes. Together, based on the co-localization of somatostatin with gamma-aminobutyric acid (GABA), its presence in dendritic-targeting GABA neuron subtypes, and its temporal-specific function, we discuss the possibility that deficits in somatostatin play a central role in cortical local inhibitory circuit deficits leading to abnormal corticolimbic network activity and clinical mood symptoms across neurological disorders.
Mood disturbances are commonly observed in many neurological disorders. The chronic, recurrent and long duration of mood disturbances not only place an enormous emotional and financial burden on patients, but also on their families and society. Nearly 10% of all primary care office visits are depression-related (Stafford et al., 2000), but only 30% of patients with mood disturbances achieve remission with initial treatment (Trivedi et al., 2006). Somatostatin is a peptide expressed in multiple organs. In the brain, somatostatin (also known as somatotrophin release inhibiting factor and often abbreviated as SST, SRIF, or SOM) acts as a modulatory and inhibitory neuropeptide that is co-localized with gamma-aminobutyric acid (GABA), and that is involved in regulating multiple aspects of physiological and behavioral stress responses, including inhibition of hypothalamic hormone release, amygdala central nucleus output, and cortical local circuit integration of sensory input. Research advances over the past three decades suggest a critical role for somatostatin in the pathophysiology of mood disorders, and potential new therapeutic strategies. Several recent reviews have summarized the role of the somatostatin system, including in receptor subtypes (Patel, 1999; Csaba and Dournaud, 2001), pharmacological developments (Neggers and van der Lely, 2009), and during normal and pathological aging (Patel, 1999; Viollet et al., 2008; Martel et al., 2012). This article highlights current findings on the functional roles of somatostatin in local neuronal circuits, and reviews somatostatin deficits across neurological disorders, including neuropsychiatric disorders [e.g., major depressive disorder (MDD), bipolar disorder, schizophrenia], and neurodegenerative disorders (e.g., Parkinson’s, Alzheimer’s, and Huntington’s diseases; Table 1). This raises interesting questions, including first; whether the somatostatin deficits observed in neurological disorders represent common, distinct, or partly overlapping mechanisms of symptoms across disorders and, second, what may be the causes and biological mechanisms underlying the selective neuronal vulnerability of somatostatin-expressing neurons. In addition, we review somatostatin findings associated with affect regulation at the genetic, cellular, and pharmacological levels in animal studies. So far, these findings suggest that somatostatin deficits across different brain systems and diseases may play a central role in the affective symptom dimension rather than non-specific signals in neurological disorders (Figure 1). As somatostatin itself is not an ideal drug target, including for antidepressant effect, we suggest that further studies characterizing the intrinsic properties and biological vulnerabilities of somatostatin-expressing neurons, may identify novel targets with implications for understanding the function of local cell circuits and brain regions underlying affective symptoms across several neurological disorders.
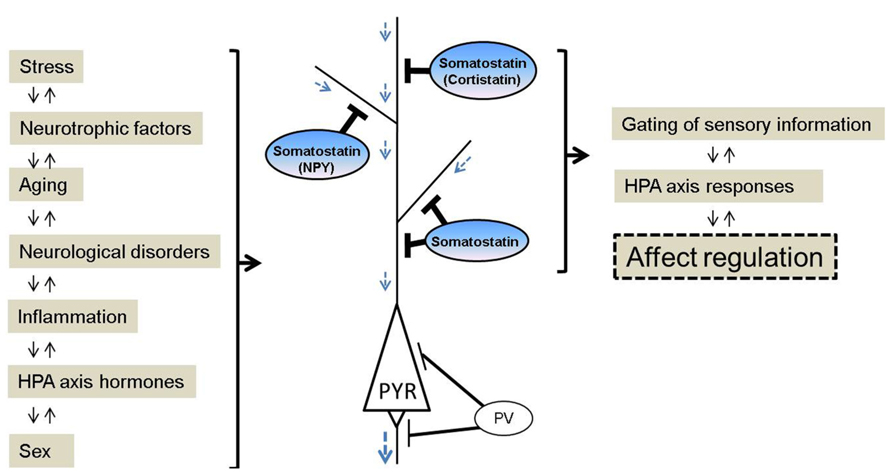
FIGURE 1. Schematic of somatostatin signaling, pathological regulators and biological functions relevant to affect regulation. Somatostatin pathway activity is responsive to (left panel), and regulates (right panel), several biological events, and molecular and cellular properties that have been linked to mood disturbances. Somatostatin and somatostatin-expressing interneurons are key conduits for regulating incoming information and pyramidal cell function. In contrast, other GABA neurons subtypes targeting the perisomatic pyramidal cell compartment are mostly not affected in major depression. NPY, neuropeptide Y; PYR, pyramidal neuron; PV, parvalbumin.
Patients with major depressive disorder (MDD) show decreased somatostatin levels in the cerebrospinal fluid (CSF; Agren and Lundqvist, 1984; Molchan et al., 1991; Kling et al., 1993), and transiently decreased CSF somatostatin which normalize with recovery in MDD (Rubinow et al., 1985; Post et al., 1988). Evidence for low levels of CSF somatostatin was found to correlate significantly with elevated urinary cortisol in MDD patients (Molchan et al., 1993). This is consistent with the altered hypothalamic-pituitary-adrenal (HPA) axis function described in some depressed patients (Holsboer, 2000). The route and characterization, however, from CSF somatostatin to MDD pathophysiology is not direct, potentially due to a paucity of information on factors regulating CSF somatostatin, and to inconclusive somatostatin/HPA axis studies in MDD patients. Hence, despite these early findings, interest in somatostatin in mood disorders has declined over time.
Human post-mortem studies from our group have described region-specific somatostatin deficits in MDD patients, including a down-regulation of somatostatin gene expression in the dorsolateral prefrontal cortex (dlPFC), subgenual anterior cingulate cortex (sgACC), and amygdala (Sibille et al., 2011; Tripp et al., 2011, 2012; Guilloux et al., 2012). In addition, two peptides co-localized with somatostatin, neuropeptide Y and cortistatin, are both significantly down-regulated in MDD patients (Tripp et al., 2011, 2012).These three neuropeptides (somatostatin, neuropeptide Y, and cortistatin) are markers of GABAergic neurons that specifically target the dendritic compartment of pyramidal cells (de Lecea et al., 1997; Viollet et al., 2008), and that are essential in gating incoming sensory information (Figure 1). Other types of GABAergic cell markers, such as parvalbumin and cholecystokinin, are mostly not affected by MDD (although see Tripp et al., 2012). Interestingly, these somatostatin deficits were systematically more robust in female subjects across cohorts and regions (Sibille et al., 2011; Tripp et al., 2011, 2012; Guilloux et al., 2012), consistent with the female heightened vulnerability to develop MDD, and suggesting that low somatostatin may represent a molecular correlate of sexual dimorphism in vulnerability to affect dysregulation. Notably, these findings are also consistent with earlier postmortem studies showing reduced calbindin-positive cell numbers in MDD (Rajkowska et al., 2007; Maciag et al., 2010), as somatostatin is mostly expressed in a subgroup of calbindin-positive cells (reviewed in Viollet et al., 2008). Converging evidence from down-regulation of somatostatin co-localized GABA markers in MDD across multiple human post-mortem studies suggests that this particular GABA subpopulation in the forebrain is selectively vulnerable, among other subtypes of GABA neurons. Furthermore, these local cell circuit-based findings introduce a new role for somatostatin in depression, which is distinct from its previously investigated role in the regulation of the HPA axis (Rubinow et al., 1983; Molchan et al., 1993; Weckbecker et al. 2003).
Schizophrenia is a neuropsychiatric disorder characterized by positive (e.g., hallucination), negative symptoms (e.g., emotional blunting, apathy) and cognitive symptoms. Somatostatin deficits in schizophrenia are demonstrated by a reduction of CSF somatostatin (Bissette et al., 1986; Reinikainen et al., 1990), decreased somatostatin gene expression in the dlPFC (Morris et al., 2008; Guillozet-Bongaarts et al., 2013), and decreased number and density of somatostatin-expressing neurons in the hippocampus (Konradi et al., 2011a), caudal entorhinal cortex and parasubiculum (Wang et al., 2011). Changes in somatostatin are also identified in bipolar disorder, which is clinically characterized by fluctuating mood. Studies in subjects with bipolar disorder indicate decreases in somatostatin cellular density in the caudal entorhinal cortex and parasubiculum (Wang et al., 2011), number of somatostatin-expressing neurons in the hippocampus (Konradi et al., 2011b), somatostatin gene expression in the dlPFC (trend level; Sibille et al., 2011) and hippocampus (Konradi et al., 2011b). In addition, patients with bipolar disorder show elevated CSF somatostatin during manic states (Sharma et al., 1995).
Alzheimer’s disease is a neurodegenerative disease with neuropsychiatric symptoms (Bungener et al., 1996). Decreased CSF somatostatin (Bissette et al., 1986; Tamminga et al., 1987) and decreased somatostatin immune-reactivity across cortical and subcortical regions is reported in subjects with Alzheimer’s disease, including temporal cortex, frontal cortex, and hippocampus (Davies et al., 1980; Rossor et al., 1980; Davies and Terry, 1981; Candy et al., 1985; Dournaud et al., 1994). Depression is a common comorbid symptom in Parkinson’s disease and predicts greater disability at any assessment point (Aarsland et al., 1999). Decreased CSF somatostatin, decreased somatostatin immuno-reactivity, and binding sites are also observed in the temporal cortex and frontal cortex of patients with Parkinson’s disease (Beal et al., 1986; Epelbaum et al., 1988). Notably, reduced CSF somatostatin in Parkinson’s disease appears to be irreversibly present at the onset of symptoms (Dupont et al., 1982).
The evidence outlined in this review provides only a glimpse of the potential full range of somatostatin deficits across neurological disorders, as multiple other brain regions and disease categories await further characterization (Table 1). Taken together, the cumulative evidence demonstrates that somatostatin deficits are common neurochemical and molecular features in individuals with neurological disorders, regardless of their categorical diagnosis. While somatostatin studies of cell number and gene expression in human postmortem brains suggest a specific alteration of somatostatin-positive neurons across neurological disorders, it is possible that changes and dys-synchronization of additional components of local neuronal circuits contribute to a common symptom dimension, which we speculate includes low affect and mood dysregulation. Hence, this review is not comprehensive, but rather, highlights the recent findings in brain somatostatin signaling and the potential role of somatostatin deficits in affect dysregulation for integrating categorical models of mood symptoms into a dimensional model across neurological disorders.
Somatostatin is a modulatory neuropeptide that synergizes with GABA-mediated inhibition, and that specifically targets the distal dendritic compartment of pyramidal neurons in cortical local circuits (Kawaguchi and Kubota, 1997; Gentet et al., 2012). Somatostatin inhibits release of numerous hormones from the hypothalamus, including corticotrophin releasing hormone (CRH; Wang et al., 1987; Patel, 1999). The somatostatin gene product is composed of 14 or 28 amino-acid residues. Both forms of somatostatin, somatostatin-14 and somatostatin-28, are generated by tissue-specific post-translational processing of the 116 amino-acid pre-pro-somatostatin peptide (Warren and Shields, 1984; Tostivint et al., 2008). Somatostatin-14 is predominantly produced in the central nervous system (CNS) but also in many peripheral organs (Epelbaum, 1986). Somatostatin-28 is mainly synthesized along the gastrointestinal tract (Fitz-Patrick and Patel, 1981). The 5′-upstream sequence of the somatostatin gene contains cyclic-AMP response element (CRE; Montminy et al., 1986), making its expression activity-dependent. Thus, somatostatin expression is preferentially altered by various stressors, such as seizures (Vezzani and Hoyer, 1999; Tallent and Qiu, 2008) and electrical foot shock (Ponomarev et al., 2010). Moreover, mice with conditional homozygous and constitutive heterozygous brain-derived neurotrophic factor (Bdnf) knockout or disruption of exon IV-expressing Bdnf transcripts show decreased somatostatin gene expression (Glorioso et al., 2006; Martinowich et al., 2011; Guilloux et al., 2012), demonstrating that somatostatin expression depends on Bdnf signaling. However, the molecular mechanisms by which this neurotrophic factor controls somatostatin and somatostatin-expressing neurons are still unknown.
Somatostatin, cortistatin and their receptors are closely intertwined systems (de Lecea et al., 1996, 1997; reviewed in Spier and de Lecea, 2000; de Lecea, 2008). Sharing high structural homology with somatostatin, cortistatin binds to all somatostatin receptor subtypes and is known to be regulated by exon IV-expressing Bdnf transcripts (Martinowich et al., 2011). However, distinct from somatostatin, cortistatin binds to additional receptors (e.g., growth hormone secretagogue receptor 1a and Mas-related gene X2 receptor) (Robas et al., 2003; Siehler et al., 2008) and has different physiological properties (e.g., activation of cation selective currents not responsive to somatostatin; Spier and de Lecea, 2000), suggesting that somatostatin and cortistatin may both contribute to affect regulation in an integrated, yet differential mode. The intracellular pathway of somatostatin signaling coupled to all five somatostatin receptors subtypes (Sst1-5) is through the activation of inhibitory G protein (Gi) and the following inhibition of adenylyl cyclase, leading to reduction of cAMP levels, activation of phosphotyrosine phosphatases, and modulation of mitogen-activated protein kinases and phospholipase C (Koch and Schonbrunn, 1984; Koch et al., 1988).
Sst1-5 present different patterns of coexpression in the brain (Kluxen et al., 1992; Moller et al., 2003; reviewed in Martel et al., 2012). Sst1 is found in retina, basal ganglia and hypothalamus, Sst2 is highly abundant in several telencephalic structures (neocortex, hippocampus, and amygdala), Sst3 immunoreactivity has only been described in neuronal cilia (Schulz et al., 2000), Sst4 is expressed in olfactory bulb, cerebral cortex and CA1 region of the hippocampus (Schreff et al., 2000), and expression of Sst5 has been detected in cerebral cortex, hippocampus, amygdala, preoptic area, and hypothalamus (Stroh et al., 1999; Strowski et al., 2003; Olias et al., 2004). Interestingly, when co-expressed in the same cells, Sst5 influences Sst2 internalization and trafficking and modulates cellular desensitization to the effects of somatostatin-14 (Sharif et al., 2007), suggesting that the precise actions of somatostatin depend on the specific interaction of the Sst1-5 receptors expressed locally in each brain region.
The relatively high degree of amino acid conservation across species indicates that somatostatin-related genes have been highly constrained during evolution (Patel, 1999; Olias et al., 2004). Accordingly, there are currently very few reports linking somatostatin gene polymorphisms with neurological disorders. A primate-specific single nucleotide polymorphism (SNP) in the human somatostatin gene [C/T polymorphism (rs4988514)] is associated with increased risk in Alzheimer’s disease progression and additive effect with the APOE epsilon4 allele(Vepsalainen et al., 2007; Xue et al., 2009), although this was not confirmed in larger genome-wide association studies (GWAS) (Hollingworth et al., 2011; Guerreiro et al., 2013). Leu48Met and Pro335Leu SNPs in the SST5 gene are of potential significance to patients with bipolar disorder (Nyegaard et al., 2002), but no associations of SST5 SNPs are found in patients with autism (Lauritsen et al., 2003). The paucity of associations with somatostatin gene variants is surprising and may reflect either strong negative selection against genetic variations in this gene, or alternatively, dilution of signal due to heterogeneity of DSM-IV-based cohorts in genetic association studies. So, dimensional phenotypes, as defined by clusters of mood symptoms, which are closer to gene functions may have implications for future genetic studies of somatostatin and other genes.
Gamma-aminobutyric acid (GABA) neurons are a diverse group of inhibitory cells which co-release neuropeptides in order to support a fine-tuning of neuronal signaling and architecture. The local inhibitory circuits provide spatiotemporal control of information processing through at least 20 subtypes of cortical GABA neurons, which are based on their expression of different calcium binding proteins and neuropeptides, localization, targeting, and differential electrophysiological properties. Recent detailed reviews on GABA neuron subpopulations have been published (Csaba and Dournaud, 2001; Di Cristo et al., 2004; Markram et al., 2004; Tan et al., 2008; Fishell and Rudy, 2011; Gentet et al., 2012; DeFelipe et al., 2013; Le Magueresse and Monyer, 2013). Approximately 20–30% of GABA neurons in the mouse somatosensory cortex express somatostatin (Lee et al., 2010; Rudy et al., 2011), and 40–50% of GABA neurons contain parvalbumin without overlapping with somatostatin in the frontal cortex, primary somatosensory cortex and visual cortex of mouse (Gonchar et al., 2007; Xu et al., 2010) and the visual cortex of rat (Gonchar and Burkhalter, 1997).
Recent reports focusing on the patterns of cortical neuronal connectivity show that somatostatin-expressing interneurons mediate the firing of pyramidal neurons with a fine level of specificity among cortical layers. Integrating optogenetic and electrophysiology approaches, mouse somatostatin-expressing interneurons in layer 2/3 of the somatosensory cortex provide a tonic inhibition to the distal dendrites of excitatory pyramidal neurons by sharpening selectivity during periods of quiet wakefulness, which may contribute to synchronized firing in cortical networks and sensorimotor integration (Gentet et al., 2012). Interestingly, in mouse somatosensory cortex, somatostatin-expressing interneurons show a spatially precise connectivity with pyramidal neurons through direct targeting in layers 2/3 or indirectly through inhibition of local parvalbumin interneurons in layer 4 (Xu et al., 2013). Moreover, in layers 2/3 of the mouse prefrontal cortex, somatostatin-expressing interneurons compartmentalizes inhibitions of calcium signaling to spine heads, not shafts, suggesting that dendrite-targeting inhibition through somatostatin-expressing interneurons may contribute to downstream cellular processes such as synaptic plasticity (Chiu et al., 2013). In mouse visual cortex, somatostatin-expressing interneurons are found to mediate response levels of specific subsets of pyramidal neurons whereas parvalbumin-expressing neurons alter response gain (Wilson et al., 2012). Parvalbumin-expressing neurons receive excitatory input from the thalamus and make strong synapses on the soma and axons of their target cells (Kawaguchi and Kubota, 1997) to control spike timing of the output neurons. In contrast, somatostatin-expressing neurons mostly do not receive input from thalamus (Beierlein et al., 2003; Cruikshank et al., 2010) and are instead activated through feed-forward mechanisms by activated pyramidal neurons. Somatostatin-expressing interneurons preferentially target distal dendrites of pyramidal neurons in layer 2/3 to modulate the processing of incoming sensory information before it is integrated at the soma level (Di Cristo et al., 2004; Markram et al., 2004; Tan et al., 2008; Murayama et al., 2009; Xu et al., 2013). Hence, the distinct GABAergic and prototypical inhibitory populations, expressing either parvalbumin or somatostatin, shape the spatiotemporal control of multiple post-synaptic potentials in cortical local circuits, and provide a framework to investigate the role of inhibitory circuits in physiology and pathology.
Mice mutant for somatostatin were created by deleting the coding region of the pre-pro-somatostatin (the last ten codons of the first exon; Zeyda et al., 2001). Somatostatin knockout (KO; SstKO) mice show intact motor coordination and motor learning, but have a significant impairment in motor learning as demands of motor coordination are increased. Overall, a detailed analysis demonstrated that SstKO mice are healthy, fertile, and show no overt behavioral phenotypes, including anxiety-like behavior in the open-field and fear conditioning tests. Notably, SstKO mice display high basal plasma levels of corticosterone and growth hormone (Zeyda et al., 2001), confirming a somatostatin-mediated inhibition of HPA axis function. Similarly, mice lacking individual Sst1-5 receptors have been tested in numerous biological fields. Of these, Sst2 emerged as the primary receptor of interest (Zeyda and Hochgeschwender, 2008), and Sst2KO mice display increased anxiety-like behavior in the elevated plus maze and open field, increased immobility in the forced swim test, decreased locomotion coupled with an increase of pituitary adrenocorticotropic hormone release instead of growth hormone (Viollet et al., 2000). In line with the observed changes in Sst2KO mice, acute predator stress in rats led to up-regulated Sst2 gene expression in the amygdala and cingulate cortex, shown correlated with Fos expression in the amygdala (Nanda et al., 2008). As the product of a different gene, cortistatin shares a high structural and functional similarity with somatostatin-14 (de Lecea et al., 1996, 1997). Notably, compared with the weak inhibitory effects of somatostatin on the basal release of CRH from rat hypothalamus and hippocampus, cortistatin exhibits strong inhibition of the expression and release of basal CRH (Tringali et al., 2012). These findings suggest that Sst2 may regulate affective phenotypes and HPA axis responses both through somatostatin and cortistatin. Given the limitations of human studies, SstKO mice provide an opportunity to explore the causal role of somatostatin in affect dysregulation and the underlying neural mechanisms. Such insights, however, will require systematic behavioral characterization with fine spatial and temporal resolution by including female cohorts and region-specific manipulation at different developmental stages. Based on the published studies to date, it is still unclear whether these mutants recapitulate behavioral features of mood disorders. Knowing the effects of somatostatin signaling on neuroendocrine regulation, future studies need to assess the molecular and cellular systems that somatostatin mutations converge upon, and where the exact neural circuits are affected. Moreover, combining genetic and environmental factors in animal models is critical to enhance the accuracy of disease modeling and translational efforts. For example, acute or chronic exposure to stress or to stress hormones may capture how such etiological factors determine the vulnerability to external insults, in contrast to baseline behavioral testing. In addition, mood disorder-related sex differences are observed in community-based epidemiological studies, where the factor of seeking treatment is removed (Kornstein et al., 2000; Festinger et al., 2008; Leach et al., 2008) and findings of low somatostatin in the amygdala appear more robust in postmortem studies of female MDD subjects (Tripp et al., 2012), suggesting that gender/sex may represent a biological predisposing factor, or at least a moderating factor, in the intrinsic vulnerability of the somatostatin system.
Although many mood disorders emerge during adolescence (Paus et al., 2008), behavioral abnormalities including affect dysregulation are often heritable and apparent before diagnostic criteria are met (McGuffin et al., 2003; Geller et al., 2006). It is unclear when somatostatin deficits occur and potentially begin to contribute to the formation of affective symptoms. Tracking somatostatin system using new anatomic techniques with refined cellular definition, from Brainbow (Livet et al., 2007) to CLARITY (Chung et al., 2013) and SeeDB (Ke et al., 2013), across different developmental stages may help identify age-dependent neural architecture and disease mechanisms related to somatostatin function.
As native somatostatin peptides have a very short half-life time (approximate 1–3 min; Sheppard et al., 1979), long-acting and highly potent somatostatin analogues are currently available for the treatment of acromegaly and neuroendocrine tumors, including octreotide (long-acting; LAR-OCT; Bauer et al., 1982) and Lanreotide (slow release or autogel; Bevan, 2005; Molitch, 2008). Compared to somatostatin, pharmacological tools of the five somatostatin receptor subtypes have lagged behind, partly due to the lack of high-affinity antagonists.
In addition, several novel somatostatin therapy models are available: (1) Universal somatostatin (Schmid and Schoeffter, 2004): a somatostatin molecular analog with high binding affinity to all or most human somatostatin receptors. An example is SOM230, which interacts with Sst1,2,3,5 and particularly potent at Sst5 compared with LAR-OCT; (2) Chimeric somatostatin/dopamine molecule (Saveanu et al., 2002; Pivonello et al., 2005): a somatostatin and dopamine hybrid agonist, based on reports that dopamine and somatostatin receptors can hetero-oligomerize to enhance functional responses (Rocheville et al., 2000). An example is BIM-23A760, which accelerates the suppression of growth hormone and adrenocorticotropic hormone by the interaction with Sst2 and Drd2 simultaneously; (3) Chimeric-somatostatin vaccinations (Haffer, 2012): a fusion protein expressing chloramphenicol acetyl transferase protein and somatostatin. Two somatostatin vaccinations, JH17 and JH18, can effectively reduce weight gain and reduce final body weight percentage of normal, non-obese mice and mice with diet-induced obesity via the intra-peritoneal route; (4) Non-peptide antagonists, such as SRA880 (Sst1 selective), ACQ090 (Sst3 selective) and Sst4 selective β peptide agonists (Rivier et al., 2003; Hoyer et al., 2004). Despite this extensive list, the practical use of somatostatin in the brain is hampered by the multiple effects of the peptide, by the need for small molecules targeting specific, high affinity receptors on the target cells in specific brain regions, and by the need for feasible routes of administration that lead to fast delivery into the brain.
The potential for using somatostatin analogues as treatment in the CNS is emerging for treatment of epilepsy (Vezzani and Hoyer, 1999; Tallent and Qiu, 2008), pain (Mollenholt et al., 1994; Taura et al., 1994) and headaches (Sicuteri et al., 1984; Kapicioglu et al., 1997); potential use for treatment of mood disorders is suggested by reversal of emotion-like behaviors in rodent models. Several pharmacological studies support a role of somatostatin in affect regulation. Intra-ventricular administration of somatostatin in rats produces anxiolytic- and antidepressant-like behaviors in the elevated plus-maze and forced swim tests, and a neurophysiological signature of anxiolytic drugs (e.g., reduction of theta frequency and theta frequency curve slope; Engin et al., 2008). Mice with intra-amygdalar and intra-septal microinfusions of somatostatin-14 and somatostatin-28 display reduced anxiety-like behavior in the elevated plus-maze and shock-probe tests (Yeung et al., 2011). Moreover, anxiolytic effects in the elevated plus-maze test are described after intra-cerebroventricular infusions of a selective Sst2 receptor agonist, but not after infusions of the other four receptor agonists; antidepressant-like effects in the forced swim test are observed following infusions of either Sst2 or Sst3 agonists (Engin and Treit, 2009). Another agent to enhance somatostatin functioning, SRA880 (an antagonist of auto-receptor Sst1), synergizes with imipramine in causing antidepressant-like effects in the tail suspension test and increases Bdnf mRNA expression in the mouse cerebral cortex (Nilsson et al., 2012).
Significant efforts have been directed toward the characterization of the downstream targets of antidepressant treatment, with a focus on somatostatin. A recent study demonstrates that chronic imipramine treatment increases somatostatin expression in mouse hypothalamus (Nilsson et al., 2012). However, there is inconsistency regarding the effect of chronic citalopram treatment on somatostatin levels in rats (Kakigi et al., 1992; Prosperini et al., 1997; Pallis et al., 2006, 2009). Repeated administration of imipramine, maprotiline, mianserin, carbamazepine or zotepine has no effect on somatostatin levels in various brain regions of rats (Weiss et al., 1987; Kakigi et al., 1992). While some somatostatin receptors seem to exert anxiolytic or antidepressant-like effects, there is no direct evidence supporting somatostatin receptors as downstream targets of current antidepressants. Together, these findings suggest that somatostatin levels are mostly unchanged by antidepressants. It is unclear whether somatostatin, GABA, or GABA functioning in somatostatin-expressing interneurons may be the real mediators or antidepressant targets. Future studies are needed to determine the involvement of somatostatin receptors and associated intracellular signaling pathways in the therapeutic effects of antidepressants, or whether somatostatin effects are independent of current antidepressant modalities.
It is possible that low somatostatin in diseases acts as a biomarker for deregulated function of somatostatin-expressing neurons. As such, it is essential to identify upstream factors responsible for the dysfunction of somatostatin-expressing interneurons in neurological disorders. We speculate that intrinsic cellular properties in somatostatin-expressing neurons may determine their selective vulnerability to various insults. Pathways underlying this high vulnerability may include high intrinsic oxidative stress related to mitochondria, high sensitivity to inflammation, high dependence on neurotrophic environment, and cellular developmental and aging processes. These canonical pathways might provide novel cell-based perspectives in the treatment of affected somatostatin-expressing cells across neurological disorders.
Oxidative stress produced by mitochondria during respiration is a common pathogenic mechanism implicated in neurological disorders (Sorce and Krause, 2009; Stefanescu and Ciobica, 2012). Depressed states in mood disorders are associated with decreased brain energy generation (Baxter et al., 1985, 1989). Mitochondrial dysfunction together with the oxidative stress accumulation has been proposed to synergistically contribute to the neuro-endangerment processes underlying depression (Gardner et al., 2003; Burnett et al., 2005) and neurodegenerative diseases (Lin and Beal, 2006; Mancuso et al., 2007; Petrozzi et al., 2007). Similarly, high baseline oxidative stress could be an intrinsic characteristic of vulnerable neuronal populations. Notably, neuronal nitric oxide synthase (nNOS) and NADPH diaphorase (NADPHd), two enzymes that produce reactive oxidative species, are extensively and almost exclusively co-localized with somatostatin and neuropeptide Y (Dun et al., 1994; Figueredo-Cardenas et al., 1996; Jaglin et al., 2012), hence providing a neurochemical basis for high susceptibility of somatostatin-expressing neurons to generate oxidative stress in response to pathophysiological insults.
Brain-derived neurotrophic factor (BDNF) and its receptor neurotrophic tyrosine kinase receptor type 2 (TrkB) have been implicated in mood disorders (Guilloux et al., 2012; Tripp et al., 2012). BDNF-TrkB signaling is one of the key mediators for maintaining normal somatostatin gene expression (Glorioso et al., 2006; Martinowich et al., 2011). Progressively impairing BDNF-TrkB signaling in patients with mood disturbances may directly impact the biology of somatostatin-expressing neurons, resulting in somatostatin deficits. In addition, Bdnf-TrkB signaling itself is vulnerable to increased inflammation (Goshen et al., 2008; Koo and Duman, 2008; Song and Wang, 2011) and high glucocorticoids insults (Hodes et al., 2012). Mild oxidative stress inhibits tyrosine phosphatases activity (Barrett et al., 2005), potentially leading to impaired TrkB downstream signaling. Cortistatin and neuropeptide Y expression partly overlaps with the somatostatin neuron population in rodents (Figueredo-Cardenas et al., 1996; de Lecea et al., 1997; Xu et al., 2010). Comparing the profile of gene changes between subjects with MDD and mice with genetically-altered Bdnf signaling suggest that the reduced somatostatin, neuropeptide Y and cortistatin are partly downstream from a combination of reduced constitutive and activity-dependent Bdnf signaling (Guilloux et al., 2012). In contrast, markers for other GABA neuron subtypes targeting the perisomatic area region cell body and axon initial segment of pyramidal neurons (i.e., cholecystokinin and calretinin), appear to be independent of BDNF signaling and unaffected in MDD patients (Guilloux et al., 2012; Tripp et al., 2012). Hence, it is possible that the somatostatin-specific cellular function and vulnerability are partly mediated by BDNF-TrkB signaling during both physiological and pathological processes of affect regulation.
Inflammation has been implicated as a contributing factor in the onset and progression of many neurological disorders (Di Filippo et al., 2008). Mood disturbances are associated with an activated inflammatory response system (Padmos et al., 2008; Miller et al., 2009), including increased levels of peripheral interleukins and tumor necrosis factor-alpha in MDD patients (Kaestner et al., 2005; Howren et al., 2009; Dowlati et al., 2010; Maes, 2011). Inflammatory illnesses are associated with more depressive episodes (Celik et al., 2010; Maes et al., 2012), suggesting that prior depression may sensitize inflammatory responses. Patients treated with inflammatory cytokines, such as interferon-α, are at greater risk of developing depressive episodes (Castera et al., 2006; Lotrich et al., 2007). Somatostatin released from sensory nerves and somatostatin receptors on peripheral blood mononuclear cells play a crucial role in anti-inflammation through inhibition of pro-inflammatory peptide release (Szolcsanyi et al., 1998; Kurnatowska and Pawlikowski, 2000; Helyes et al., 2004). Rats with chronic inflammation induced by lipopolysaccharide show decreased hippocampal somatostatin expression (Gavilan et al., 2007). It is possible that there is crosstalk among peripheral inflammation, somatostatin function, and central effects of somatostatin-expressing neurons. Hence, decreasing somatostatin expression due to cellular impairment in the progress of neurological diseases may further enhance inflammation in a vicious cycle, leading to exacerbated cellular vulnerability of somatostatin-expressing neurons.
Aging is associated with a considerable increase in an activated, pro-inflammatory state (Wei et al., 1992; Bruunsgaard and Pedersen, 2003), a decline in circulating levels of Bdnf (Erickson et al., 2010), and increased oxidative damage (Sohal and Weindruch, 1996). Somatostatin expression is significantly decreased with age in human cortical regions, but parvalbumin expression is not altered by age (Erraji-Benchekroun et al., 2005; Glorioso et al., 2011). Similarly, the number of hippocampal somatostatin-expressing interneurons decreases in aged rats, but the number of parvalbumin-expressing neurons remains the same (Vela et al., 2003). Somatostatin and IL-1β mRNA expression are negatively correlated in aged hippocampus of rats (Gavilan et al., 2007). Comparing the effects of aging on somatostatin expression in the sgACC, an accelerated reduction is found in patients with MDD compared to normal aging subjects (Tripp et al., 2012), suggesting a pattern resulting in an early aging phenomenon which we have speculated may be synergistically induced by normal age-related changes and depression-related pathological change (Douillard-Guilloux et al., 2013).
Here we have focused on somatostatin, a GABA marker, down-regulated in MDD, schizophrenia, bipolar disorder, and neurodegenerative diseases. Exploring cross-disease molecular (somatostatin) and cellular (somatostatin-expressing interneurons) pathological findings suggests a dimensional pathological phenotype that is specific to the somatostatin gene/cell biological entity rather than to categorical brain disorders. Based on these results we speculate that common risk factors affecting somatostatin and somatostatin-expressing neurons may impact information processing in the cortical local circuits (Figure 1). Clarifying the role of somatostatin and its regulation of GABA inhibition in affect regulation could provide new strategies for predicting, delaying, and treating neurological diseases with mood disturbances. A number of questions remain. For example, are the prevalent somatostatin deficits seen in multiple diseases reflected in a common symptom dimension, such as low mood, across neurological diseases? What are the critical events that determine the vulnerability of somatostatin-expressing neurons? And what are the pathogenic mechanisms that mediate the observed disease-related molecular and cellular phenotypes? One possibility is that inflammation, oxidative stress, aging, and reduced neurotrophic support may all converge to affect somatostatin-expressing neurons. Targeting these pathways may exert neuro-protective effects on somatostatin-expressing neurons, as a potential therapeutic approach with implications for several neuropsychiatric disorders and neurodegenerative diseases.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
This work was supported by National Institute of Mental Health (NIMH-MH093723, MH084060 and MH077159). We thank Beverly French for careful review of the manuscript.
Aarsland, D., Larsen, J. P., Lim, N. G., Janvin, C., Karlsen, K., Tandberg, E., et al. (1999). Range of neuropsychiatric disturbances in patients with Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 67, 492–496. doi: 10.1136/jnnp.67.4.492
Agren, H., and Lundqvist, G. (1984). Low levels of somatostatin in human CSF mark depressive episodes. Psychoneuroendocrinology 9, 233–248. doi: 10.1016/0306-4530(84)90003-9
Barrett, D. M., Black, S. M., Todor, H., Schmidt-Ullrich, R. K., Dawson, K. S., and Mikkelsen, R. B. (2005). Inhibition of protein-tyrosine phosphatases by mild oxidative stresses is dependent on S-nitrosylation. J. Biol. Chem. 280, 14453–14461. doi: 10.1074/jbc.M411523200
Bauer, W., Briner, U., Doepfner, W., Haller, R., Huguenin, R., Marbach. P., et al. (1982). SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 31, 1133–1140. doi: 10.1016/0024-3205(82)90087-X
Baxter, L. R. Jr., Phelps, M. E., Mazziotta, J. C., Schwartz, J. M., Gerner, R. H., Selin, C. E. et al. (1985). Cerebral metabolic rates for glucose in mood disorders. Studies with positron emission tomography and fluorodeoxyglucose F 18. Arch. Gen. Psychiatry 42, 441–447. doi: 10.1001/archpsyc.1985.01790280019002
Baxter, L. R. Jr., Schwartz, J. M., Phelps, M. E., Mazziotta, J. C., Guze, B. H., Selin, C. E. et al. (1989). Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch. Gen. Psychiatry 46, 243–250. doi: 10.1001/archpsyc.1989.01810030049007
Beal, M. F., Mazurek, M. F., and Martin, J. B. (1986). Somatostatin immunoreactivity is reduced in Parkinson’s disease dementia with Alzheimer’s changes. Brain Res. 397, 386–388. doi: 10.1016/0006-8993(86)90644-X
Beierlein, M., Gibson, J. R., and Connors, B. W. (2003). Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J. Neurophysiol. 90, 2987–3000. doi: 10.1152/jn.00283.2003
Bevan, J. S. (2005). The antitumoral effects of somatostatin analog therapy in acromegaly. J. Clin. Endocrinol. Metab. 90, 1856–1863. doi: 10.1210/jc.2004-1093
Bissette, G., Widerlov, E., Walleus, H., Karlsson, I., Eklund, K., Forsman, A., et al. (1986). Alterations in cerebrospinal fluid concentrations of somatostatinlike immunoreactivity in neuropsychiatric disorders. Arch. Gen. Psychiatry 43, 1148–1151. doi: 10.1001/archpsyc.1986.01800120034008
Bruunsgaard, H., and Pedersen, B. K. (2003). Age-related inflammatory cytokines and disease. Immunol. Allergy Clin. North Am. 23, 15–39. doi: 10.1016/S0889-8561(02)00056-5
Bungener, C., Jouvent, R., and Derouesne, C. (1996). Affective disturbances in Alzheimer’s disease. J. Am. Geriatr. Soc. 44, 1066–1071.
Burnett, B. B., Gardner, A., and Boles, R. G. (2005). Mitochondrial inheritance in depression, dysmotility and migraine? J. Affect Disord. 88, 109–116. doi: 10.1016/j.jad.2005.05.009
Candy, J. M., Gascoigne, A. D., Biggins, J. A., Smith, A. I., Perry, R. H., Perry, E. K., et al. (1985). Somatostatin immunoreactivity in cortical and some subcortical regions in Alzheimer’s disease. J. Neurol. Sci. 71, 315–323. doi: 10.1016/0022-510X(85)90070-X
Castera, L., Constant, A., Henry, C., Champbenoit, P., Bernard, P. H., De Ledinghen, V., et al. (2006). Impact on adherence and sustained virological response of psychiatric side effects during peginterferon and ribavirin therapy for chronic hepatitis C. Aliment. Pharmacol. Ther. 24, 1223–1230. doi: 10.1111/j.1365-2036.2006.03107.x
Celik, C., Erdem, M., Cayci, T., Ozdemir, B., Ozgur Akgul, E., Kurt, Y. G., et al. (2010). The association between serum levels of neopterin and number of depressive episodes of major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 34, 372–375. doi: 10.1016/j.pnpbp.2010.01.002
Chiu, C. Q., Lur, G., Morse, T. M., Carnevale, N. T., Ellis-Davies, G. C., and Higley, M. J. (2013). Compartmentalization of GABAergic inhibition by dendritic spines. Science 340, 759–762. doi: 10.1126/science.1234274
Chung, K., Wallace, J., Kim, S. Y., Kalyanasundaram, S., Andalman, A. S., Davidson, T. J., et al. (2013). Structural and molecular interrogation of intact biological systems. Nature 497, 332–337. doi: 10.1038/nature12107
Cruikshank, S. J., Urabe, H., Nurmikko, A. V., and Connors, B. W. (2010). Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron 65, 230–245. doi: 10.1016/j.neuron.2009.12.025
Csaba, Z., and Dournaud, P. (2001). Cellular biology of somatostatin receptors. Neuropeptides 35, 1–23. doi: 10.1054/npep.2001.0848
Davies, P., Katzman, R., and Terry, R. D. (1980). Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementa. Nature 288, 279–280. doi: 10.1038/288279a0
Davies, P., and Terry, R. D. (1981). Cortical somatostatin-like immunoreactivity in cases of Alzheimer’s disease and senile dementia of the Alzheimer type. Neurobiol. Aging 2, 9–14. doi: 10.1016/0197-4580(81)90053-1
DeFelipe, J., Lopez-Cruz, P. L., Benavides-Piccione, R., Bielza, C., Larranaga, P., Anderson, S., et al. (2013). New insights into the classification and nomenclature of cortical GABAergic interneurons. Nat. Rev. Neurosci. 14, 202–216. doi: 10.1038/nrn3444
de Lecea, L. (2008). Cortistatin – functions in the central nervous system. Mol. Cell. Endocrinol. 286, 88–95. doi: 10.1016/j.mce.2007.12.014
de Lecea, L., Criado, J. R., Prospero-Garcia, O., Gautvik, K. M., Schweitzer, P., Danielson, P. E., et al. (1996). A cortical neuropeptide with neuronal depressant and sleep-modulating properties. Nature 381, 242–245. doi: 10.1038/381242a0
de Lecea, L., del Rio, J. A., Criado, J. R., Alcantara, S., Morales, M., Danielson, P. E., et al. (1997). Cortistatin is expressed in a distinct subset of cortical interneurons. J. Neurosci. 17, 5868–5880.
Di Cristo, G., Wu, C., Chattopadhyaya, B., Ango, F., Knott, G., Welker, E., et al. (2004). Subcellular domain-restricted GABAergic innervation in primary visual cortex in the absence of sensory and thalamic inputs. Nat. Neurosci. 7, 1184–1186. doi: 10.1038/nn1334
Di Filippo, M., Sarchielli, P., Picconi, B., and Calabresi, P. (2008). Neuroinflammation and synaptic plasticity: theoretical basis for a novel, immune-centred, therapeutic approach to neurological disorders. Trends Pharmacol. Sci. 29, 402–412. doi: 10.1016/j.tips.2008.06.005
Douillard-Guilloux, G., Guilloux, J. P., Lewis, D. A., and Sibille, E. (2013). Anticipated brain molecular aging in major depression. Am. J. Geriatr. Psychiatry 21, 450–460. doi: 10.1016/j.jagp.2013.01.040
Dournaud, P., Cervera-Pierot, P., Hirsch, E., Javoy-Agid, F., Kordon, C., Agid, Y., et al. (1994). Somatostatin messenger RNA-containing neurons in Alzheimer’s disease: an in situ hybridization study in hippocampus, parahippocampal cortex and frontal cortex. Neuroscience 61, 755–764. doi: 10.1016/0306-4522(94)90399-9
Dowlati, Y., Herrmann, N., Swardfager, W., Liu, H., Sham, L., Reim, E. K., et al. (2010). A meta-analysis of cytokines in major depression. Biol. Psychiatry 67, 446–457. doi: 10.1016/j.biopsych.2009.09.033
Dun, N. J., Dun, S. L., Wong, R. K., and Forstermann, U. (1994). Colocalization of nitric oxide synthase and somatostatin immunoreactivity in rat dentate hilar neurons. Proc. Natl. Acad. Sci. U.S.A. 91, 2955–2959. doi: 10.1073/pnas.91.8.2955
Dupont, E., Christensen, S. E., Hansen, A. P., de Fine Olivarius, B., and Orskov, H. (1982). Low cerebrospinal fluid somatostatin in Parkinson disease: an irreversible abnormality. Neurology 32, 312–314. doi: 10.1212/WNL.32.3.312
Engin, E., Stellbrink, J., Treit, D., and Dickson, C. T. (2008). Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: behavioral and neurophysiological evidence. Neuroscience 157, 666–676. doi: 10.1016/j.neuroscience.2008.09.037
Engin, E., and Treit, D. (2009). Anxiolytic and antidepressant actions of somatostatin: the role of sst2 and sst3 receptors. Psychopharmacology (Berl.) 206, 281–289. doi: 10.1007/s00213-009-1605-5
Epelbaum, J. (1986). Somatostatin in the central nervous system: physiology and pathological modifications. Prog. Neurobiol. 27, 63–100. doi: 10.1016/0301-0082(86)90012-2
Epelbaum, J., Javoy-Agid, F., Enjalbert, A., Krantic, S., Kordon, C., and Agid, Y. (1988). Somatostatin concentrations and binding sites in human frontal cortex are differentially affected in Parkinson’s disease associated dementia and in progressive supranuclear palsy. J. Neurol. Sci. 87, 167–174. doi: 10.1016/0022-510X(88)90242-0
Erickson, K. I., Prakash, R. S., Voss, M. W., Chaddock, L., Heo, S., McLaren, M., et al. (2010). Brain-derived neurotrophic factor is associated with age-related decline in hippocampal volume. J. Neurosci. 30, 5368–5375. doi: 10.1523/JNEUROSCI.6251-09.2010
Erraji-Benchekroun, L., Underwood, M. D., Arango, V., Galfalvy, H., Pavlidis, P., Smyrniotopoulos, P., et al. (2005). Molecular aging in human prefrontal cortex is selective and continuous throughout adult life. Biol. Psychiatry 57, 549–558. doi: 10.1016/j.biopsych.2004.10.034
Festinger, D. S., Marlowe, D. B., Dugosh, K. L., Croft, J. R., and Arabia, P. L. (2008). Higher magnitude cash payments improve research follow-up rates without increasing drug use or perceived coercion. Drug Alcohol Depend. 96, 128–135. doi: 10.1016/j.drugalcdep.2008.02.007
Figueredo-Cardenas, G., Morello, M., Sancesario, G., Bernardi, G., and Reiner, A. (1996). Colocalization of somatostatin, neuropeptide Y, neuronal nitric oxide synthase and NADPH-diaphorase in striatal interneurons in rats. Brain Res. 735, 317–324. doi: 10.1016/0006-8993(96)00801-3
Fishell, G., and Rudy, B. (2011). Mechanisms of inhibition within the telencephalon: “where the wild things are”. Annu. Rev. Neurosci. 34, 535–567. doi: 10.1146/annurev-neuro-061010-113717
Fitz-Patrick, D., and Patel, Y. C. (1981). Evidence for somatostatin precursors in human stomach, placenta, and amniotic fluid. J. Clin. Endocrinol. Metab. 53, 372–376. doi: 10.1210/jcem-53-2-372
Gardner, A., Johansson, A., Wibom, R., Nennesmo, I., von Dobeln, U., Hagenfeldt, L., et al. (2003). Alterations of mitochondrial function and correlations with personality traits in selected major depressive disorder patients. J. Affect. Disord. 76, 55–68. doi: 10.1016/S0165-0327(02)00067-8
Gavilan, M. P., Revilla, E., Pintado, C., Castano, A., Vizuete, M. L., Moreno-González, I., et al. (2007). Molecular and cellular characterization of the age-related neuroinflammatory processes occurring in normal rat hippocampus: potential relation with the loss of somatostatin GABAergic neurons. J. Neurochem. 103, 984–996. doi: 10.1111/j.1471-4159.2007.04787.x
Geller, B., Tillman, R., Bolhofner, K., Zimerman, B., Strauss, N. A., and Kaufmann, P. (2006). Controlled, blindly rated, direct-interview family study of a prepubertal and early-adolescent bipolar I disorder phenotype: morbid risk, age at onset, and comorbidity. Arch. Gen. Psychiatry 63, 1130–1138. doi: 10.1001/archpsyc.63.10.1130
Gentet, L. J., Kremer, Y., Taniguchi, H., Huang, Z. J., Staiger, J. F., and Petersen, C. C. (2012). Unique functional properties of somatostatin-expressing GABAergic neurons in mouse barrel cortex. Nat. Neurosci. 15, 607–612. doi: 10.1038/nn.3051
Glorioso, C., Oh, S., Douillard, G. G., and Sibille, E. (2011). Brain molecular aging, promotion of neurological disease and modulation by sirtuin 5 longevity gene polymorphism. Neurobiol. Dis. 41, 279–290. doi: 10.1016/j.nbd.2010.09.016
Glorioso, C., Sabatini, M., Unger, T., Hashimoto, T., Monteggia, L. M., Lewis, D. A., et al. (2006). Specificity and timing of neocortical transcriptome changes in response to BDNF gene ablation during embryogenesis or adulthood. Mol. Psychiatry 11, 633–648. doi: 10.1038/sj.mp.4001835
Gonchar, Y., and Burkhalter, A. (1997). Three distinct families of GABAergic neurons in rat visual cortex. Cereb. Cortex 7, 347–358. doi: 10.1093/cercor/7.4.347
Gonchar, Y., Wang, Q., and Burkhalter, A. (2007). Multiple distinct subtypes of GABAergic neurons in mouse visual cortex identified by triple immunostaining. Front. Neuroanat. 1:3. doi: 10.3389/neuro.05.003.2007
Goshen, I., Kreisel, T., Ben-Menachem-Zidon, O., Licht, T., Weidenfeld, J., Ben-Hur, T., et al. (2008). Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Mol. Psychiatry 13, 717–728. doi: 10.1038/sj.mp.4002055
Guerreiro, R., Wojtas, A., Bras, J., Carrasquillo, M., Rogaeva, E., Majounie, E., et al. (2013). TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 368, 117–127. doi: 10.1056/NEJMoa1211851
Guilloux, J. P., Douillard-Guilloux, G., Kota, R., Wang, X., Gardier, A. M., Martinowich, K., et al. (2012). Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol. Psychiatry 17, 1130–1142. doi: 10.1038/mp.2011.113
Guillozet-Bongaarts, A. L., Hyde, T. M., Dalley, R. A., Hawrylycz, M. J., Henry, A., Hof, P. R., et al. (2013). Altered gene expression in the dorsolateral prefrontal cortex of individuals with schizophrenia. Mol. Psychiatry. doi: 10.1038/mp.2013.30 [Epub ahead of print].
Haffer, K. N. (2012). Effects of novel vaccines on weight loss in diet-induced-obese (DIO) mice. J. Anim. Sci. Biotechnol. 3, 21. doi: 10.1186/2049-1891-3-21
Helyes, Z., Szabo, A., Nemeth, J., Jakab, B., Pinter, E., Banvolgyi, A., et al. (2004). Antiinflammatory and analgesic effects of somatostatin released from capsaicin-sensitive sensory nerve terminals in a Freund’s adjuvant-induced chronic arthritis model in the rat. Arthritis Rheum. 50, 1677–1685. doi: 10.1002/art.20184
Hodes, G. E., Brookshire, B. R., Hill-Smith, T. E., Teegarden, S. L., Berton, O., and Lucki, I. (2012). Strain differences in the effects of chronic corticosterone exposure in the hippocampus. Neuroscience 222, 269–280. doi: 10.1016/j.neuroscience.2012.06.017
Hollingworth, P., Harold, D., Sims, R., Gerrish, A., Lambert, J. C., Carrasquillo, M. M., et al. (2011). Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet. 43, 429–435. doi: 10.1038/ng.803
Holsboer, F. (2000). The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23, 477–501. doi: 10.1016/S0893-133X(00)00159-7
Howren, M. B., Lamkin, D. M., and Suls, J. (2009). Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom. Med. 71, 171–186. doi: 10.1097/PSY.0b013e3181907c1b
Hoyer, D., Nunn, C., Hannon, J., Schoeffter, P., Feuerbach, D., Schuepbach, E., et al. (2004). SRA880, in vitro characterization of the first non-peptide somatostatin sst(1) receptor antagonist. Neurosci. Lett. 361, 132–135. doi: 10.1016/j.neulet.2004.02.017
Jaglin, X. H., Hjerling-Leffler, J., Fishell, G., and Batista-Brito, R. (2012). The origin of neocortical nitric oxide synthase-expressing inhibitory neurons. Front. Neural Circuits 6:44. doi: 10.3389/fncir.2012.00044
Kaestner, F., Hettich, M., Peters, M., Sibrowski, W., Hetzel, G., Ponath, G., et al. (2005). Different activation patterns of proinflammatory cytokines in melancholic and non-melancholic major depression are associated with HPA axis activity. J. Affect. Disord. 87, 305–311. doi: 10.1016/j.jad.2005.03.012
Kakigi, T., Maeda, K., Kaneda, H., and Chihara, K. (1992). Repeated administration of antidepressant drugs reduces regional somatostatin concentrations in rat brain. J. Affect. Disord. 25, 215–220. doi: 10.1016/0165-0327(92)90078-K
Kapicioglu, S., Gokce, E., Kapicioglu, Z., and Ovali, E. (1997). Treatment of migraine attacks with a long-acting somatostatin analogue (octreotide, SMS 201–995). Cephalalgia 17, 27–30. doi: 10.1046/j.1468-2982.1997.1701027.x
Kawaguchi, Y., and Kubota, Y. (1997). GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb. Cortex 7, 476–486. doi: 10.1093/cercor/7.6.476
Ke, M. T., Fujimoto, S., and Imai, T. (2013). SeeDB: a simple and morphology-preserving optical clearing agent for neuronal circuit reconstruction. Nat. Neurosci. 16, 1154–1161. doi: 10.1038/nn.3447
Kling, M. A., Rubinow, D. R., Doran, A. R., Roy, A., Davis, C. L., Calabrese, J. R., et al. (1993). Cerebrospinal fluid immunoreactive somatostatin concentrations in patients with Cushing’s disease and major depression: relationship to indices of corticotropin-releasing hormone and cortisol secretion. Neuroendocrinology 57, 79–88. doi: 10.1159/000126345
Kluxen, F. W., Bruns, C., and Lubbert, H. (1992). Expression cloning of a rat brain somatostatin receptor cDNA. Proc. Natl. Acad. Sci. U.S.A. 89, 4618–4622. doi: 10.1073/pnas.89.10.4618
Koch, B. D., Blalock, J. B., and Schonbrunn, A. (1988). Characterization of the cyclic AMP-independent actions of somatostatin in GH cells. I. An increase in potassium conductance is responsible for both the hyperpolarization and the decrease in intracellular free calcium produced by somatostatin. J. Biol. Chem. 263, 216–225.
Koch, B. D., and Schonbrunn, A. (1984). The somatostatin receptor is directly coupled to adenylate cyclase in GH4C1 pituitary cell membranes. Endocrinology 114, 1784–1790. doi: 10.1210/endo-114-5-1784
Konradi, C., Yang, C. K., Zimmerman, E. I., Lohmann, K. M., Gresch, P., Pantazopoulos, H., et al. (2011a). Hippocampal interneurons are abnormal in schizophrenia. Schizophr. Res. 131, 165–173. doi: 10.1016/j.schres.2011.06.007
Konradi, C., Zimmerman, E. I., Yang, C. K., Lohmann, K. M., Gresch, P., Pantazopoulos, H., et al. (2011b). Hippocampal interneurons in bipolar disorder. Arch. Gen. Psychiatry 68, 340–350. doi: 10.1001/archgenpsychiatry.2010.175
Koo, J. W., and Duman, R. S. (2008). IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. U.S.A. 105, 751–756. doi: 10.1073/pnas.0708092105
Kornstein, S. G., Schatzberg, A. F., Thase, M. E., Yonkers, K. A., McCullough, J. P., Keitner, G. I., et al. (2000). Gender differences in chronic major and double depression. J. Affect. Disord. 60, 1–11. doi: 10.1016/S0165-0327(99)00158-5
Kurnatowska, I., and Pawlikowski, M. (2000). Effect of somatostatin analog-octreotide on the adjuvant arthritis in rat. Neuro Endocrinol. Lett. 21, 121–126.
Lauritsen, M. B., Nyegaard, M., Betancur, C., Colineaux, C., Josiassen, T. L., Kruse, T. A., et al. (2003). Analysis of transmission of novel polymorphisms in the somatostatin receptor 5 (SSTR5) gene in patients with autism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 121B,100–104. doi: 10.1002/ajmg.b.20050
Leach, L. S., Christensen, H., Mackinnon, A. J., Windsor, T. D., and Butterworth, P. (2008). Gender differences in depression and anxiety across the adult lifespan: the role of psychosocial mediators. Soc. Psychiatry Psychiatr. Epidemiol. 43, 983–998. doi: 10.1007/s00127-008-0388-z
Lee, S., Hjerling-Leffler, J., Zagha, E., Fishell, G., and Rudy, B. (2010). The largest group of superficial neocortical GABAergic interneurons expresses ionotropic serotonin receptors. J. Neurosci. 30, 16796–16808. doi: 10.1523/JNEUROSCI.1869-10.2010
Le Magueresse, C., and Monyer, H. (2013). GABAergic interneurons shape the functional maturation of the cortex. Neuron 77, 388–405. doi: 10.1016/j.neuron.2013.01.011
Lin, M. T., and Beal, M. F. (2006). Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795. doi: 10.1038/nature05292
Livet, J., Weissman, T. A., Kang, H., Draft, R. W., Lu, J., Bennis, R. A., et al. (2007). Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature 450, 56–62. doi: 10.1038/nature06293
Lotrich, F. E., Rabinovitz, M., Gironda, P., and Pollock, B. G. (2007). Depression following pegylated interferon-alpha: characteristics and vulnerability. J. Psychosom. Res. 63, 131–135. doi: 10.1016/j.jpsychores.2007.05.013
Maciag, D., Hughes, J., O’Dwyer, G., Pride, Y., Stockmeier, C. A., Sanacora, G., et al. (2010). Reduced density of calbindin immunoreactive GABAergic neurons in the occipital cortex in major depression: relevance to neuroimaging studies. Biol. Psychiatry 67, 465–470. doi: 10.1016/j.biopsych.2009.10.027
Maes, M. (2011). Depression is an inflammatory disease, but cell-mediated immune activation is the key component of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 664–675. doi: 10.1016/j.pnpbp.2010.06.014
Maes, M., Mihaylova, I., Kubera, M., and Ringel, K. (2012). Activation of cell-mediated immunity in depression: association with inflammation, melancholia, clinical staging and the fatigue and somatic symptom cluster of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 36, 169–175. doi: 10.1016/j.pnpbp.2011.09.006
Mancuso, C., Scapagini, G., Curro, D., Giuffrida Stella, A. M., De Marco, C., Butterfield, D. A., et al. (2007). Mitochondrial dysfunction, free radical generation and cellular stress response in neurodegenerative disorders. Front. Biosci. 12:1107–1123. doi: 10.2741/2130
Markram, H., Toledo-Rodriguez, M., Wang, Y., Gupta, A., Silberberg, G., and Wu, C. (2004). Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5, 793–807. doi: 10.1038/nrn1519
Martel, G., Dutar, P., Epelbaum, J., and Viollet, C. (2012). Somatostatinergic systems: an update on brain functions in normal and pathological aging. Front. Endocrinol. (Lausanne) 3:154. doi: 10.3389/fendo.2012.00154
Martinowich, K., Schloesser, R. J., Jimenez, D. V., Weinberger, D. R., and Lu, B. (2011). Activity-dependent brain-derived neurotrophic factor expression regulates cortistatin-interneurons and sleep behavior. Mol. Brain 4, 11. doi: 10.1186/1756-6606-4-11
McGuffin, P., Rijsdijk, F., Andrew, M., Sham, P., Katz, R., and Cardno, A. (2003). The heritability of bipolar affective disorder and the genetic relationship to unipolar depression. Arch. Gen. Psychiatry 60, 497–502. doi: 10.1001/archpsyc.60.5.497
Miller, A. H., Maletic, V., and Raison, C. L. (2009). Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 65, 732–741. doi: 10.1016/j.biopsych.2008.11.029
Molchan, S. E., Hill, J. L., Martinez, R. A., Lawlor, B. A., Mellow, A. M., Rubinow, D. R., et al. (1993). CSF somatostatin in Alzheimer’s disease and major depression: relationship to hypothalamic-pituitary-adrenal axis and clinical measures. Psychoneuroendocrinology 18, 509–519. doi: 10.1016/0306-4530(93)90044-L
Molchan, S. E., Lawlor, B. A., Hill, J. L., Martinez, R. A., Davis, C. L., Mellow, A. M., et al. (1991). CSF monoamine metabolites and somatostatin in Alzheimer’s disease and major depression. Biol. Psychiatry 29, 1110–1118. doi: 10.1016/0006-3223(91)90253-I
Molitch, M. E. (2008). Lanreotide Autogel in the management of acromegaly. Drugs 68, 724. doi: 10.2165/00003495-200868050-00014
Mollenholt, P., Rawal, N., Gordh, T. Jr., and Olsson, Y. (1994). Intrathecal and epidural somatostatin for patients with cancer. Analgesic effects and postmortem neuropathologic investigations of spinal cord and nerve roots. Anesthesiology 81, 534–542. doi: 10.1097/00000542-199409000-00004
Moller, L. N., Stidsen, C. E., Hartmann, B., and Holst, J. J. (2003). Somatostatin receptors. Biochim. Biophys. Acta 1616, 1–84. doi: 10.1016/S0005-2736(03)00235-9
Montminy, M. R., Sevarino, K. A., Wagner, J. A., Mandel, G., and Goodman, R. H. (1986). Identification of a cyclic-AMP-responsive element within the rat somatostatin gene. Proc. Natl. Acad. Sci. U.S.A. 83, 6682–6686. doi: 10.1073/pnas.83.18.6682
Morris, H. M., Hashimoto, T., and Lewis, D. A. (2008). Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb. Cortex 18, 1575–1587. doi: 10.1093/cercor/bhm186
Murayama, M., Perez-Garci, E., Nevian, T., Bock, T., Senn, W., and Larkum, M. E. (2009). Dendritic encoding of sensory stimuli controlled by deep cortical interneurons. Nature 457, 1137–1141. doi: 10.1038/nature07663
Nanda, S. A., Qi, C., Roseboom, P. H., and Kalin, N. H. (2008). Predator stress induces behavioral inhibition and amygdala somatostatin receptor 2 gene expression. Genes Brain Behav. 7, 639–648. doi: 10.1111/j.1601-183X.2008.00401.x
Neggers, S. J., and van der Lely, A. J. (2009). Somatostatin analog and pegvisomant combination therapy for acromegaly. Nat. Rev. Endocrinol. 5, 546–552. doi: 10.1038/nrendo.2009.175
Nilsson, A., Stroth, N., Zhang, X., Qi, H., Falth, M., Skold, K., et al. (2012). Neuropeptidomics of mouse hypothalamus after imipramine treatment reveal somatostatin as a potential mediator of antidepressant effects. Neuropharmacology 62, 347–357. doi: 10.1016/j.neuropharm.2011.08.004
Nyegaard, M., Borglum, A. D., Bruun, T. G., Collier, D. A., Russ, C., Mors, O., et al. (2002). Novel polymorphisms in the somatostatin receptor 5 (SSTR5) gene associated with bipolar affective disorder. Mol. Psychiatry 7, 745–754. doi: 10.1038/sj.mp.4001049
Olias, G., Viollet, C., Kusserow, H., Epelbaum, J., and Meyerhof, W. (2004). Regulation and function of somatostatin receptors. J. Neurochem. 89, 1057–1091. doi: 10.1111/j.1471-4159.2004.02402.x
Padmos, R. C., Hillegers, M. H., Knijff, E. M., Vonk, R., Bouvy, A., Staal, F. J., et al. (2008). A discriminating messenger RNA signature for bipolar disorder formed by an aberrant expression of inflammatory genes in monocytes. Arch. Gen. Psychiatry 65, 395–407. doi: 10.1001/archpsyc.65.4.395
Pallis, E., Vasilaki, A., Fehlmann, D., Kastellakis, A., Hoyer, D., Spyraki, C., et al. (2009). Antidepressants influence somatostatin levels and receptor pharmacology in brain. Neuropsychopharmacology 34, 952–963. doi: 10.1038/npp.2008.133
Pallis, E. G., Spyraki, C., and Thermos, K. (2006). Chronic antidepressant treatment modulates the release of somatostatin in the rat nucleus accumbens. Neurosci. Lett. 395, 76–81. doi: 10.1016/j.neulet.2005.10.055
Patel, Y. C. (1999). Somatostatin and its receptor family. Front. Neuroendocrinol. 20:157–198. doi: 10.1006/frne.1999.0183
Paus, T., Keshavan, M., and Giedd, J. N. (2008). Why do many psychiatric disorders emerge during adolescence? Nat. Rev. Neurosci. 9, 947–957. doi: 10.1038/nrn2513
Petrozzi, L., Ricci, G., Giglioli, N. J., Siciliano, G., and Mancuso, M. (2007). Mitochondria and neurodegeneration. Biosci. Rep. 27, 87–104. doi: 10.1007/s10540-007-9038-z
Pivonello, R., Ferone, D., Lamberts, S. W., and Colao, A. (2005). Cabergoline plus lanreotide for ectopic Cushing’s syndrome. N. Engl. J. Med. 352, 2457–2458. doi: 10.1056/NEJM200506093522322
Ponomarev, I., Rau, V., Eger, E. I., Harris, R. A., and Fanselow, M. S. (2010). Amygdala transcriptome and cellular mechanisms underlying stress-enhanced fear learning in a rat model of posttraumatic stress disorder. Neuropsychopharmacology 35, 1402–1411. doi: 10.1038/npp.2010.10
Post, R. M., Rubinow, D. R., Kling, M. A., Berrettini, W., and Gold, P. W. (1988). Neuroactive substances in cerebrospinal fluid. Normal and pathological regulatory mechanisms. Ann. N. Y. Acad. Sci. 531, 15–28. doi: 10.1111/j.1749-6632.1988.tb31808.x
Prosperini, E., Rizzi, M., Fumagalli, F., Tarizzo, G., Samanin, R., and Bendotti, C. (1997). Acute and chronic treatments with citalopram lower somatostatin levels in rat brain striatum through different mechanisms. J. Neurochem. 69, 206–213. doi: 10.1046/j.1471-4159.1997.69010206.x
Rajkowska, G., O’Dwyer, G., Teleki, Z., Stockmeier, C. A., and Miguel-Hidalgo, J. J. (2007). GABAergic neurons immunoreactive for calcium binding proteins are reduced in the prefrontal cortex in major depression. Neuropsychopharmacology 32, 471–482. doi: 10.1038/sj.npp.1301234
Reinikainen, K. J., Koponen, H., Jolkkonen, J., and Riekkinen, P. J. (1990). Decreased somatostatin-like immunoreactivity in the cerebrospinal fluid of chronic schizophrenic patients with cognitive impairment. Psychiatry Res. 33, 307–312. doi: 10.1016/0165-1781(90)90047-9
Rivier, J., Erchegyi, J., Hoeger, C., Miller, C., Low, W., Wenger, S., et al. (2003). Novel sst(4)-selective somatostatin (SRIF) agonists. 1. Lead identification using a betide scan. J. Med. Chem. 46, 5579–5586. doi: 10.1021/jm030243c
Robas, N., Mead, E., and Fidock, M. (2003). MrgX2 is a high potency cortistatin receptor expressed in dorsal root ganglion. J. Biol. Chem. 278, 44400–44404. doi: 10.1074/jbc.M302456200
Rocheville, M., Lange, D. C., Kumar, U., Patel, S. C., Patel, R. C., and Patel, Y. C. (2000). Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science 288, 154–157. doi: 10.1126/science.288.5463.154
Rossor, M. N., Emson, P. C., Mountjoy, C. Q., Roth, M., and Iversen, L. L. (1980). Reduced amounts of immunoreactive somatostatin in the temporal cortex in senile dementia of Alzheimer type. Neurosci. Lett. 20, 373–377. doi: 10.1016/0304-3940(80)90177-9
Rubinow, D. R., Gold, P. W., Post, R. M., and Ballenger, J. C. (1985). CSF somatostatin in affective illness and normal volunteers. Prog. Neuropsychopharmacol. Biol. Psychiatry 9, 393–400. doi: 10.1016/0278-5846(85)90192-7
Rubinow, D. R., Gold, P. W., Post, R. M., Ballenger, J. C., Cowdry, R., Bollinger, J., et al. (1983). CSF somatostatin in affective illness. Arch. Gen. Psychiatry 40, 409–412. doi: 10.1001/archpsyc.1983.01790040063009
Rudy, B., Fishell, G., Lee, S., and Hjerling-Leffler, J. (2011). Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev. Neurobiol. 71, 45–61. doi: 10.1002/dneu.20853
Saveanu, A., Lavaque, E., Gunz, G., Barlier, A., Kim, S., Taylor, J. E., et al. (2002). Demonstration of enhanced potency of a chimeric somatostatin-dopamine molecule, BIM-23A387, in suppressing growth hormone and prolactin secretion from human pituitary somatotroph adenoma cells. J. Clin. Endocrinol. Metab. 87, 5545–5552. doi: 10.1210/jc.2002-020934
Schmid, H. A., and Schoeffter, P. (2004). Functional activity of the multiligand analog SOM230 at human recombinant somatostatin receptor subtypes supports its usefulness in neuroendocrine tumors. Neuroendocrinology 80(Suppl. 1), 47–50. doi: 10.1159/000080741
Schreff, M., Schulz, S., Handel, M., Keilhoff, G., Braun, H., Pereira, G., et al. (2000). Distribution, targeting, and internalization of the sst4 somatostatin receptor in rat brain. J. Neurosci. 20, 3785–3797.
Schulz, S., Handel, M., Schreff, M., Schmidt, H., and Hollt, V. (2000). Localization of five somatostatin receptors in the rat central nervous system using subtype-specific antibodies. J. Physiol. Paris 94, 259–264. doi: 10.1016/S0928-4257(00)00212-6
Sharif, N., Gendron, L., Wowchuk, J., Sarret, P., Mazella, J., Beaudet, A., et al. (2007). Coexpression of somatostatin receptor subtype 5 affects internalization and trafficking of somatostatin receptor subtype 2. Endocrinology 148, 2095–2105. doi: 10.1210/en.2006-1266
Sharma, R. P., Bissette, G., Janicak, P. G., Davis, J. M., and Nemeroff, C. B. (1995). Elevation of CSF somatostatin concentrations in mania. Am. J. Psychiatry 152, 1807–1809.
Sheppard, M., Shapiro, B., Pimstone, B., Kronheim, S., Berelowitz, M., and Gregory, M. (1979). Metabolic clearance and plasma half-disappearance time of exogenous somatostatin in man. J. Clin. Endocrinol. Metab. 48, 50–53. doi: 10.1210/jcem-48-1-50
Sibille, E., Morris, H. M., Kota, R. S., and Lewis, D. A. (2011). GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int. J. Neuropsychopharmacol. 14, 721–734. doi: 10.1017/S1461145710001616
Sicuteri, F., Geppetti, P., Marabini, S., and Lembeck, F. (1984). Pain relief by somatostatin in attacks of cluster headache. Pain 18, 359–365. doi: 10.1016/0304-3959(84)90048-4
Siehler, S., Nunn, C., Hannon, J., Feuerbach, D., and Hoyer, D. (2008). Pharmacological profile of somatostatin and cortistatin receptors. Mol. Cell. Endocrinol. 286, 26–34. doi: 10.1016/j.mce.2007.12.007
Sohal, R. S., and Weindruch, R. (1996). Oxidative stress, caloric restriction, and aging. Science 273, 59–63. doi: 10.1126/science.273.5271.59
Song, C., and Wang, H. (2011). Cytokines mediated inflammation and decreased neurogenesis in animal models of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 35, 760–768. doi: 10.1016/j.pnpbp.2010.06.020
Sorce, S., and Krause, K. H. (2009). NOX enzymes in the central nervous system: from signaling to disease. Antioxid. Redox. Signal. 11, 2481–2504. doi: 10.1089/ars.2009.2578
Spier, A. D., and de Lecea, L. (2000). Cortistatin: a member of the somatostatin neuropeptide family with distinct physiological functions. Brain Res. Brain Res. Rev. 33, 228–241. doi: 10.1016/S0165-0173(00)00031-X
Stafford, R. S., Ausiello, J. C., Misra, B., and Saglam, D. (2000). National patterns of depression treatment in primary care. Prim. Care Companion J. Clin. Psychiatry 2, 211–216. doi: 10.4088/PCC.v02n0603
Stefanescu, C., and Ciobica, A. (2012). The relevance of oxidative stress status in first episode and recurrent depression. J. Affect. Disord. 143, 34–38. doi: 10.1016/j.jad.2012.05.022
Stroh, T., Kreienkamp, H. J., and Beaudet, A. (1999). Immunohistochemical distribution of the somatostatin receptor subtype 5 in the adult rat brain: predominant expression in the basal forebrain. J. Comp. Neurol. 412, 69–82. doi: 10.1002/(SICI)1096-9861(19990913)412:1<69::AID-CNE5>3.0.CO;2-V
Strowski, M. Z., Kohler, M., Chen, H. Y., Trumbauer, M. E., Li, Z., Szalkowski, D., et al. (2003). Somatostatin receptor subtype 5 regulates insulin secretion and glucose homeostasis. Mol. Endocrinol. 17, 93–106. doi: 10.1210/me.2001-0035
Szolcsanyi, J., Helyes, Z., Oroszi, G., Nemeth, J., and Pinter, E. (1998). Release of somatostatin and its role in the mediation of the anti-inflammatory effect induced by antidromic stimulation of sensory fibres of rat sciatic nerve. Br. J. Pharmacol. 123, 936–942. doi: 10.1038/sj.bjp.0701685
Tallent, M. K., and Qiu, C. (2008). Somatostatin: an endogenous antiepileptic. Mol. Cell. Endocrinol. 286, 96–103. doi: 10.1016/j.mce.2007.12.004
Tamminga, C. A., Foster, N. L., Fedio, P., Bird, E. D., and Chase, T. N. (1987). Alzheimer’s disease: low cerebral somatostatin levels correlate with impaired cognitive function and cortical metabolism. Neurology 37, 161–165. doi: 10.1212/WNL.37.1.161
Tan, Z., Hu, H., Huang, Z. J., and Agmon, A. (2008). Robust but delayed thalamocortical activation of dendritic-targeting inhibitory interneurons. Proc. Natl. Acad. Sci. U.S.A. 105, 2187–2192. doi: 10.1073/pnas.0710628105
Taura, P., Planella, V., Balust, J., Beltran, J., Anglada, T., Carrero, E., et al. (1994). Epidural somatostatin as an analgesic in upper abdominal surgery: a double-blind study. Pain 59, 135–140. doi: 10.1016/0304-3959(94)90057-4
Tostivint, H., Lihrmann, I., and Vaudry, H. (2008). New insight into the molecular evolution of the somatostatin family. Mol. Cell. Endocrinol. 286, 5–17. doi: 10.1016/j.mce.2008.02.029
Tringali, G., Greco, M. C., Lisi, L., Pozzoli, G., and Navarra, P. (2012). Cortistatin modulates the expression and release of corticotrophin releasing hormone in rat brain. Comparison with somatostatin and octreotide. Peptides 34, 353–359. doi: 10.1016/j.peptides.2012.02.004
Tripp, A., Kota, R. S., Lewis, D. A., and Sibille, E. (2011). Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol. Dis. 42, 116–124. doi: 10.1016/j.nbd.2011.01.014
Tripp, A., Oh, H., Guilloux, J. P., Martinowich, K., Lewis, D. A., and Sibille, E. (2012). Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am. J. Psychiatry 169, 1194–1202. doi: 10.1176/appi.ajp.2012.12020248
Trivedi, M. H., Rush, A. J., Wisniewski, S. R., Nierenberg, A. A., Warden, D., Ritz, L., et al. (2006). Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry 163, 28–40. doi: 10.1176/appi.ajp.163.1.28
Vela, J., Gutierrez, A., Vitorica, J., and Ruano, D. (2003). Rat hippocampal GABAergic molecular markers are differentially affected by ageing. J. Neurochem. 85, 368–377. doi: 10.1046/j.1471-4159.2003.01681.x
Vepsalainen, S., Helisalmi, S., Koivisto, A. M., Tapaninen, T., Hiltunen, M., and Soininen, H. (2007). Somatostatin genetic variants modify the risk for Alzheimer’s disease among Finnish patients. J. Neurol. 254, 1504–1508. doi: 10.1007/s00415-007-0539-2
Vezzani, A., and Hoyer, D. (1999). Brain somatostatin: a candidate inhibitory role in seizures and epileptogenesis. Eur. J. Neurosci. 11, 3767–3776. doi: 10.1046/j.1460-9568.1999.00838.x
Viollet, C., Lepousez, G., Loudes, C., Videau, C., Simon, A., and Epelbaum, J. (2008). Somatostatinergic systems in brain: networks and functions. Mol. Cell. Endocrinol. 286, 75–87. doi: 10.1016/j.mce.2007.09.007
Viollet, C., Vaillend, C., Videau, C., Bluet-Pajot, M. T., Ungerer, A., L’Héritier, A., et al. (2000). Involvement of sst2 somatostatin receptor in locomotor, exploratory activity and emotional reactivity in mice. Eur. J. Neurosci. 12, 3761–3770. doi: 10.1046/j.1460-9568.2000.00249.x
Wang, A. Y., Lohmann, K. M., Yang, C. K., Zimmerman, E. I., Pantazopoulos, H., Herring, N., et al. (2011). Bipolar disorder type 1 and schizophrenia are accompanied by decreased density of parvalbumin- and somatostatin-positive interneurons in the parahippocampal region. Acta Neuropathol. 122, 615–626. doi: 10.1007/s00401-011-0881-4
Wang, X. M., Tresham, J. J., Coghlan, J. P., and Scoggins, B. A. (1987). Intracerebroventricular infusion of a cyclic hexapeptide analogue of somatostatin inhibits hemorrhage-induced ACTH release. Neuroendocrinology 45, 325–327. doi: 10.1159/000124747
Warren, T. G., and Shields, D. (1984). Expression of preprosomatostatin in heterologous cells: biosynthesis, posttranslational processing, and secretion of mature somatostatin. Cell 39, 547–555. doi: 10.1016/0092-8674(84)90461-6
Weckbecker, G., Lewis, I., Albert, R., Schmid, H. A., Hoyer, D., and Bruns, C. (2003). Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat. Rev. Drug Discov. 2, 999–1017. doi: 10.1038/nrd1255
Wei, J., Xu, H., Davies, J. L., and Hemmings, G. P. (1992). Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 51, 1953–1956. doi: 10.1016/0024-3205(92)90112-3
Weiss, S. R., Nguyen, T., Rubinow, D. R., Helke, C. J., Narang, P. K., Post, R. M., et al. (1987). Lack of effect of chronic carbamazepine on brain somatostatin in the rat. J. Neural. Transm. 68, 325–333. doi: 10.1007/BF02098507
Wilson, N. R., Runyan, C. A., Wang, F. L., and Sur, M. (2012). Division and subtraction by distinct cortical inhibitory networks in vivo. Nature 488, 343–348. doi: 10.1038/nature11347
Xu, H., Jeong, H. Y., Tremblay, R., and Rudy, B. (2013). Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron 77, 155–167. doi: 10.1016/j.neuron.2012.11.004
Xu, X., Roby, K. D., and Callaway, E. M. (2010). Immunochemical characterization of inhibitory mouse cortical neurons: three chemically distinct classes of inhibitory cells. J. Comp. Neurol. 518, 389–404. doi: 10.1002/cne.22229
Xue, S., Jia, L., and Jia, J. (2009). Association between somatostatin gene polymorphisms and sporadic Alzheimer’s disease in Chinese population. Neurosci. Lett. 465, 181–183. doi: 10.1016/j.neulet.2009.09.002
Yeung, M., Engin, E., and Treit, D. (2011). Anxiolytic-like effects of somatostatin isoforms SST 14 and SST 28 in two animal models (Rattus norvegicus) after intra-amygdalar and intra-septal microinfusions. Psychopharmacology (Berl.) 216, 557–567. doi: 10.1007/s00213-011-2248-x
Zeyda, T., Diehl, N., Paylor, R., Brennan, M. B., and Hochgeschwender, U. (2001). Impairment in motor learning of somatostatin null mutant mice. Brain Res. 906, 107–114. doi: 10.1016/S0006-8993(01)02563-X
Keywords: somatostatin, somatostatin-expressing interneurons, SST, SOM, SRIF, depression, mood disorders, GABA inhibition
Citation: Lin L-C and Sibille E (2013) Reduced brain somatostatin in mood disorders: a common pathophysiological substrate and drug target? Front. Pharmacol. 4:110. doi: 10.3389/fphar.2013.00110
Received: 25 May 2013; Accepted: 13 August 2013;
Published online: 09 September 2013.
Edited by:
Thibault Renoir, Florey Institute of Neuroscience and Mental Health, AustraliaReviewed by:
Jean-Philippe Guilloux,Université Paris Sud, FranceCopyright © 2013 Lin and Sibille. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Etienne Sibille, Department of Psychiatry, Center for Neuroscience, University of Pittsburgh, Bridgeside Point II, 450 Technology Drive, Suite 231, Pittsburgh, PA 15219, USA e-mail:c2liaWxsZWVsQHVwbWMuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.