- 1 Division of Pharmacology, Faculty of Health Sciences, University of Stellenbosch, Cape Town, South Africa
- 2 Division of Medical Microbiology, Faculty of Health Sciences, University of Stellenbosch, Cape Town, South Africa
- 3 Synexa Life Sciences, Montague Gardens, Cape Town, South Africa
Despite the lack of sufficient information on the safety of herbal products, their use as alternative and/or complementary medicine is globally popular. There is also an increasing interest in medicinal herbs as precursor for pharmacological actives. Of serious concern is the concurrent consumption of herbal products and conventional drugs. Herb–drug interaction (HDI) is the single most important clinical consequence of this practice. Using a structured assessment procedure, the evidence of HDI presents with varying degree of clinical significance. While the potential for HDI for a number of herbal products is inferred from non-human studies, certain HDIs are well established through human studies and documented case reports. Various mechanisms of pharmacokinetic HDI have been identified and include the alteration in the gastrointestinal functions with consequent effects on drug absorption; induction and inhibition of metabolic enzymes and transport proteins; and alteration of renal excretion of drugs and their metabolites. Due to the intrinsic pharmacologic properties of phytochemicals, pharmacodynamic HDIs are also known to occur. The effects could be synergistic, additive, and/or antagonistic. Poor reporting on the part of patients and the inability to promptly identify HDI by health providers are identified as major factors limiting the extensive compilation of clinically relevant HDIs. A general overview and the significance of pharmacokinetic and pharmacodynamic HDI are provided, detailing basic mechanism, and nature of evidence available. An increased level of awareness of HDI is necessary among health professionals and drug discovery scientists. With the increasing number of plant-sourced pharmacological actives, the potential for HDI should always be assessed in the non-clinical safety assessment phase of drug development process. More clinically relevant research is also required in this area as current information on HDI is insufficient for clinical applications.
Introduction
There is increasing consumptions of medicinal herbs and herbal products globally, cutting across social and racial classes, as it is observed both in developing and developed countries (Cheng et al., 2002; Bodeker, 2007; Mitra, 2007). Medicinal plants were the major agents for primary health care for many centuries before the advent of modern medicine (Sheeja et al., 2006). Their use however declined in most developed western countries during the last century’s industrialization and urbanization (Ogbonnia et al., 2008). In the past two decades however a new resurgence in medicinal plants consumption was observed. According to the WHO, about 70% of the world population currently uses medicinal herbs as complementary or alternative medicine (Wills et al., 2000). It is estimated that over 40% of the adult American population consume herbal products for one medical reason or the other (Tachjian et al., 2010). A recent study involving 2055 patients in the US also reveals that the consumption pattern of traditional medications has no significant gender or social difference (Kessler et al., 2001). Consumption rate has also been particularly exponential in Canada (Calixto, 2000), Australia (Bensoussan et al., 2004), as well as Europe where the highest sales of herbal products have been reported in Germany and France (Capasso et al., 2003). In Africa, there is continuous addition to the list of medicinal herbs while consumption rate is also increasing. Between 60 and 85% native Africans use herbal medicine usually in combination (Van Wyk et al., 2009).
The indications for herbal remedies are diverse as they are employed in the treatment of a wide range of diseases (Ernst, 2005). Studies have shown that 67% of women use herbs for perimenopausal symptoms, 45% use it in pregnancy, and more than 45% parents give herbal medications to their children for various medical conditions (Ernst, 2004). Regulations in most countries do not require the demonstration of therapeutic efficacy, safety, or quality on the part of herbal remedies as most of them are promoted as natural and harmless (Homsy et al., 2004; Routledge, 2008). It is pertinent however, that herbs are not free from side effects as some have been shown to be toxic (Déciga-Campos et al., 2007; Patel et al., 2011). Recent study has shown habitual pattern of concomitant consumption of herbal and prescription medication. Kaufman et al. (2002) reported that 14–16% of American adult population consume herbal supplements often concomitantly with prescribed medications. Also, 49.4% of Israeli consumers of herbal remedies use them with prescription drugs (Giveon et al., 2004). This is significant bearing in mind that less than 40% of patients disclose their herbal supplement usage to their health care providers coupled with the fact that many physicians are unaware of the potential risks of herb–drug interactions (HDI; Klepser et al., 2000).
HDI is one of the most important clinical concerns in the concomitant consumption of herbs and prescription drugs. The necessity of polypharmacy in the management of most diseases further increases the risk of HDI in patients. The ability of intestinal and hepatic CYP to metabolize numerous structurally unrelated compounds, apart from being responsible for the poor oral bioavailability of numerous drugs is responsible for the large number of documented drug–drug and drug–food interactions (Quintieri et al., 2008). This is more so, considering that oral drug delivery is the most employed in the management of most disease conditions in which case, drug interaction alters both bioavailability and pharmacokinetic disposition of the drug. This alteration and the resulting poor control of plasma drug concentrations would particularly be of concern for drugs that have a narrow therapeutic window or a precipitous dose–effect profile (Aungst, 2000; Perucca, 2006). The risk of pharmacokinetic drug interaction poses two major extremity challenges – pharmacotoxicity and treatment failure. The former can result from the inhibition of the metabolic enzymes responsible for the metabolism and clearance of the drugs while the latter may be the consequence of enzymatic induction leading to faster drug metabolism. This is in addition to the intrinsic pharmacodynamic actions of the herbal products themselves which may include potentiating, additive, antagonism, or neutralization effects.
Until recently, HDI was often unsuspected by physicians for several reasons. Most trained physicians lack adequate knowledge on herbal drugs and their potentials for drug interactions (Clement et al., 2005; Ozcakir et al., 2007; Fakeye and Onyemadu, 2008); herbal products also vary considerably in compositions depending on the source and package (Liang et al., 2004; Sousa et al., 2011); most patients do not consider it necessary to disclose their herbal consumptions to physicians who themselves hardly inquire such (Cassidy, 2003; Howell et al., 2006; Chao et al., 2008; Kennedy et al., 2008). Further challenges with herbal medications include scientific misidentification, product contamination and adulteration, mislabeling, active ingredient instability, variability in collection procedures, and failure of disclosure on the part of patients (Boullata and Nace, 2000). A fairly recent systematic review by Izzo and Ernst (2009) on the interactions between medicinal herbs and prescribed medications provide some more details on these.
Herbal products are made of complex mixture of pharmacologically active phytochemicals (Mok and Chau, 2006), most of which are secondary metabolites generated through the shikimate, acetate–malonate, and acetate–mevalonate pathways. These constituents include phenolics (such as tannins, lignins, quinolones, and salicylates), phenolic glycosides (such as flavonoids, cyanogens, and glucosinolates), terpenoids (such as sesquiterpenes, steroids, carotenoids, saponins, and iridoids), alkaloids, peptides, polysaccharides (such as gums and mucilages), resins, and essential oils which often contain some of the aforementioned classes of phytochemicals (Wills et al., 2000; Wang et al., 2008). This complexity increases the risk of clinical drug interactions.
Aim, Search Strategy, and Selection Criteria
The current review was therefore aimed at providing an overview of known and recently reported HDI with interest in the evidence available and the mechanism thereof. The review was systematically conducted by searching the databases of MEDLINE, PUBMED, EMBASE, and COCHRAINE libraries for original researches, and case reports on HDI using the following search terms or combinations thereof: “drug–herb,” “herb–drug,” “interaction,” “cytochrome P450,” “plant,” “extract,” “medicinal,” “concomitant administration,” “herbal and orthodox medicines.” Relevant search terms were employed to accommodate the various individual medicinal herbs employed in Africa, America, Asia, Europe, and Australia. The reported interactions and their mechanisms, with orthodox medications were searched and collated. Searches were not limited by date or place of publications but to publications available in English language.
Results
Clinical Presentation of Herb–Drug Interactions
Clinical presentations of HDI vary widely depending on the herbs and the drugs concerned. Typical clinical presentation of HDI include the potentiation of the effects of oral corticosteroids in the presence of liquorice (Glycyrrhiza glabra; Liao et al., 2010); potentiation of warfarin effects with resultant bleeding in the presence of garlic (Allium sativum; Borrelli et al., 2007), dong quai (Angelica sinensis; Nutescu et al., 2006), or danshen (Salvia miltiorrhiza; Chan, 2001); decreased blood levels of nevirapine, amitriptyline, nifedipine, statins, digoxin, theophylline, cyclosporine, midazolam, and steroids in patients concurrently consuming St John’s wort (SJW; Hypericum perforatum; De Maat et al., 2001; Henderson et al., 2002; Johne et al., 2002; Mannel, 2004; Borrelli and Izzo, 2009), decreased oral bioavailability of prednisolone in the presence of the Chinese herbal product xiao-chai-hu tang (sho-saiko-to; Fugh-Berman, 2000); ginseng (Panax ginseng)-induced mania in patients on antidepressants (Engelberg et al., 2001); production of extrapyramidal effects as a result of the combination of neuroleptic drugs with betel nut (Areca catechu; Huang et al., 2003; Coppola and Mondola, 2012); increased blood pressure induced by tricyclic antidepressant-yohimbe (Pausinystalia yohimbe) combination (Tam et al., 2001), increased phenytoin clearance and frequent seizures when combined with Ayurvedic syrup shankhapushpi (Patsalos and Perucca, 2003), among other clinical manifestations. These clinical presentations depend on the mechanism of HDI.
Evidence-Based HDI Studies and Clinical Relevance
Herb–drug interactions have been reported through various study techniques. While these reports usually give evidence of potential interactions, the level of evidence varies often failing to predict the magnitude or clinical significance of such HDI. Apart from the specific limitations attributable to study methods employed, major draw-back in deducting relevant conclusions from reported HDI include misidentification and poor characterization of specimen, presence and nature of adulterants (some of which may be allergens), variations in study methodologies including extraction procedures, source location of herbs involved, seasonal variation in the phytochemical composition of herbal materials, under-reporting and genetic factors involved in drug absorption, metabolism, and dynamics. Table 1 provides some limitations of the study methods.
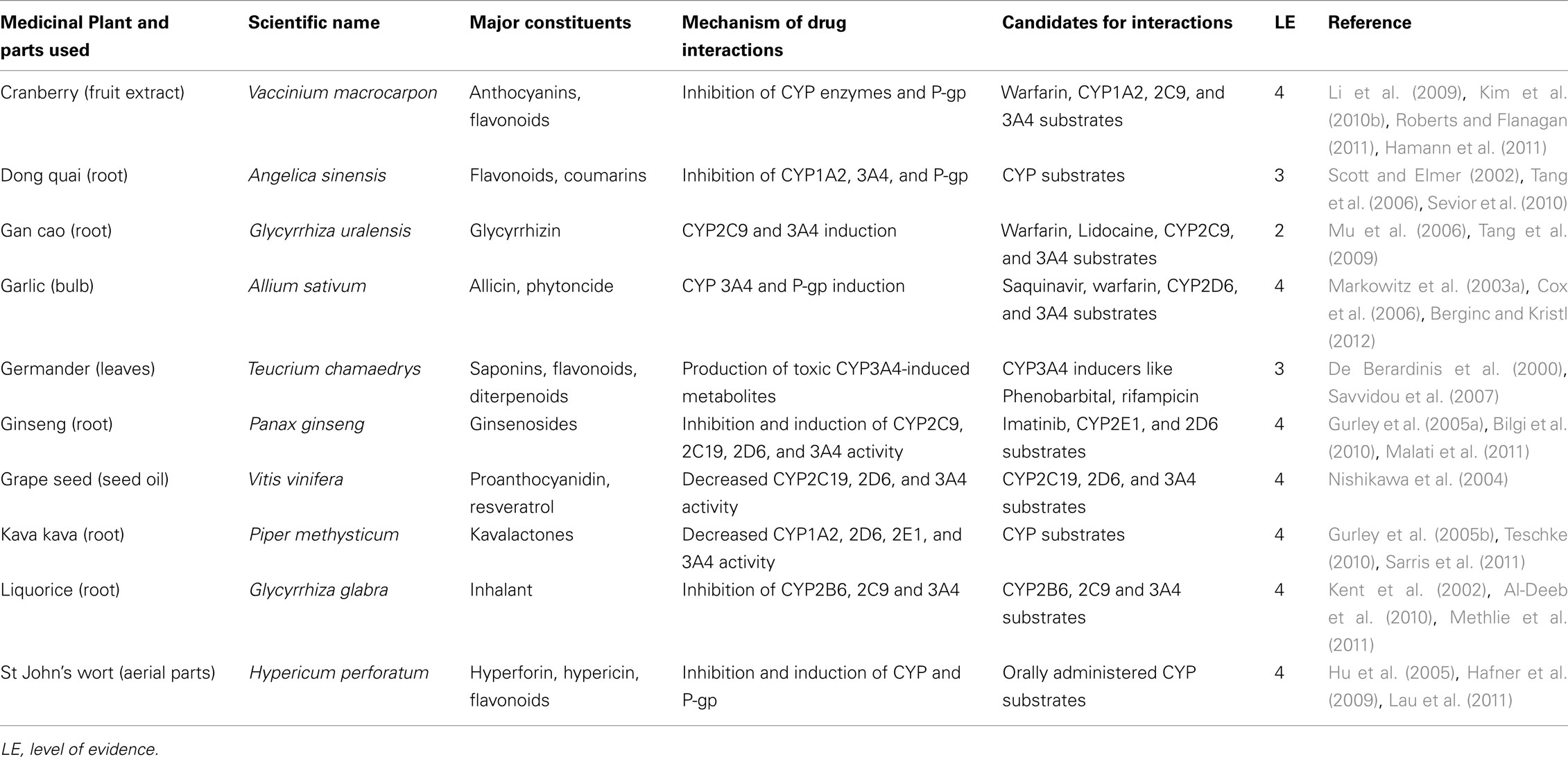
Recently, structured assessment procedures are emerging in an attempt to provide levels of evidence for drug interactions. In addition to evidence of interaction, such assessment take into consideration clinical relevance of the potential adverse event resulting from the interaction, the modification- and patient-specific risk factors, and disease conditions for which the interaction is important. Van Roon et al. (2005) developed a system of hierarchical evidence-based structured assessment procedure of drug–drug interaction. This can be applicable to HDI. This method particularly allows the extraction of HDIs that have been well established and those that are merely inferred from certain phytochemical characteristics. A modified form of this method as presented in Table 2 is applied in this paper to provide the nature and level of evidence for the HDIs mentioned.
Mechanisms of Herb–Drug Interactions
The overlapping substrate specificity in the biotransformational pathways of the physiologic systems is seen as the major reason for drug–drug, food–drug, and HDI (Marchetti et al., 2007). The ability of different chemical moieties to interact with receptor sites and alter physiological environment can explain pharmacodynamic drug interactions while pharmacokinetic interactions arise from altered absorption, interference in distribution pattern as well as changes and competition in the metabolic and excretory pathways (Izzo, 2005). The major underlying mechanism of pharmacokinetic HDI, like drug–drug interaction, is either the induction or inhibition of intestinal and hepatic metabolic enzymes particularly the CYP enzyme family. Additionally, similar effect on drug transporters and efflux proteins particularly the p-glycoproteins in the intestines is responsible in most other cases (Meijerman et al., 2006; Nowack, 2008; Farkas et al., 2010). The pre-systemic activity of CYP and efflux proteins often influence oral bioavailability, thus the modulating activity of co-administered herbal products has been shown to result in pronounced reduction or increase in the blood levels of the affected drugs (Brown et al., 2008).
Potential for in vivo drug interactions are often inferred from in vitro studies with liver enzymes. The correlation of in vitro results with in vivo behavior has yielded reliable results in certain cases in terms of in vivo predictability although the extent of clinical significant is poorly inferable (Rostami-Hodjegan and Tucker, 2007; Iwamoto et al., 2008; Xu et al., 2009; Umehara and Camenisch, 2011). Thus most of the well established HDIs, as will be seen in subsequent sections, were initially demonstrated through in vitro studies.
The interaction of herbal products with hepatic enzymes can also result in pharmacodynamic effects (van den Bout-van den Beukel et al., 2008; Nivitabishekam et al., 2009; Asdaq and Inamdar, 2010; Dasgupta et al., 2010; Kim et al., 2010a.) Specific liver injury inducible by phytochemical agents includes elevation in transaminases (Zhu et al., 2004; Saleem et al., 2010), acute and chronic hepatitis (Stedman, 2002; Pierard et al., 2009), liver failure (Durazo et al., 2004), veno-occlusive disorders (DeLeve et al., 2002), liver cirrhosis (Lewis et al., 2006), fibrosis (Chitturi and Farrell, 2000), cholestasis (Chitturi and Farrell, 2008), zonal or diffusive hepatic necrosis (Savvidou et al., 2007), and steatosis (Wang et al., 2009). Mechanism of liver injury may include bioactivation of CYP, oxidative stress, mitochondrial injury, and apoptosis (Cullen, 2005).
Induction and inhibition of metabolic enzymes
The CYP superfamily is generally involved in oxidative, peroxidative, and reductive biotransformation of xenobiotics and endogenous compounds (Nebert and Russell, 2002; Hiratsuka, 2011). It is conventionally divided into families and subfamilies based on nucleotide sequence homology (Fasinu et al., 2012). There is a high degree of substrate specificity among the various families. CYP belonging to the families 1, 2, and 3 are principally involved in xenobiotic metabolism while others play a major role in the formation and elimination of endogenous compounds such as hormones, bile acids, and fatty acids (Norlin and Wikvall, 2007; Amacher, 2010). The most important CYP subfamilies responsible for drug metabolism in humans are 1A2, 2A6, 2C9, 2C19, 2D6, 2E1, 3A4, and 3A5 (Ono et al., 1996; Wang and Chou, 2010).
CYP1A1 and 1A2 are the two major members of the human CYP1A subfamily. CYP 1A1 is mainly expressed in extra-hepatic tissues such as the kidney, the intestines, and the lungs while CYP1A2 constitutes about 15% of total hepatic CYP (Martignoni et al., 2006). CYP2B6 is involved in drug metabolism while most other members of the CYP2B subfamily play less significant metabolic roles (Pavek and Dvorak, 2008). The subfamily 2C is the second most abundant CYP after 3A representing over 20% of the total CYP present in the human liver. It comprises three active members: 2C8, 2C9, and 2C19 all of which are also involved in the metabolism of some endogenous compounds including retinol and retinoic acid (Lewis, 2004). Few clinically relevant drugs including paracetamol, chlorzoxazone, and enflurane are metabolized by CYP2E1, the most active of the 2E subfamily (Leclercq et al., 2000). CYP3A subfamily constitutes over 40% of the total CYP in the human body (although the levels may vary 40-fold among individuals) with CYP3A4 being the most abundant of all isoforms highly expressed in the liver and the intestines and participates in the metabolism of about half of drugs in use today (Ferguson and Tyndale, 2011; Singh et al., 2011). The specificity and selectivity of substrates and inhibitors for these enzymes are particularly useful in pharmacokinetic and toxicological studies.
Induction is the increase in intestinal and hepatic enzyme activity as a result of increased mRNA transcription leading to protein levels higher than normal physiologic values. When this happens, there is a corresponding increase in the rate of drug metabolism affecting both the oral bioavailability and the systemic disposition. In the formulation and dosage design of oral medications, allowance is often made for pre-systemic metabolism in order to achieve predictable systemic bioavailability. A disruption in this balance can result in significant changes in blood concentrations of the drugs. Certain herbal products have been shown to be capable of inducing CYP. Concomitant administration of enzyme-inducing herbal products and prescription drugs can therefore result in sub-therapeutic plasma levels of the latter with therapeutic failure as a possible clinical consequence.
Apart from enzyme induction, herbal products can also inhibit enzyme activities. The inhibition of CYP and other metabolic enzymes is usually competitive with instantaneous and inhibitor concentration-dependent effects (Zhang and Wong, 2005). Most inhibitors are also substrates of CYP (Zhou, 2008). This phenomenon alters pharmacokinetic profiles of xenobiotics significantly. As a result of the suppression of the anticipated pre-systemic intestinal and hepatic metabolism, unusually high plasma levels of xenobiotics are observed. Toxic manifestation could be the ultimate effect of this observation. An equally clinically important consequence of enzyme inhibition is drug accumulation due to subdued hepatic clearance. These effects will be of particular concerns in drugs with narrow therapeutic window or steep dose–response profiles.
St John’s wort is one of the most widely used herbal antidepressants (Lawvere and Mahoney, 2005; Høyland, 2011). It is a potent inducer of CYP3A4 and depending on the dose, duration and route of administration, it may induce or inhibit other CYP isozymes and P-gp (Roby et al., 2000; Markowitz et al., 2003b; Tannergren et al., 2004; Madabushi et al., 2006). Studies from case reports indicate that, due to its inducing effects on CYP3A4, it significantly reduces the plasma levels of CYP3A4 substrates including cyclosporine, simvastatin, indinavir, warfarin, amitriptyline, tacrolimus, oxycodone, and nevirapine (Henderson et al., 2002; Johne et al., 2002; Nieminen et al., 2010; Vlachojannis et al., 2011). It has also been reported that the alteration in the blood serum concentration of cyclosporine due to SJW has led to organ rejection in patients (Ernst, 2002; Murakami et al., 2006). Reports of breakthrough bleeding and unplanned pregnancies due to interaction between SJW and oral contraceptives have also been documented (Hu et al., 2005). The group of drugs with the highest potential for clinically significant pharmacokinetic drug interaction with SJW is the antidepressants as SJW itself is consumed by patients with depression. Its concomitant use with SSRI like sertraline and paroxetine has been reported to result in symptoms of central serotonergic syndrome (Barbenel et al., 2000; Dannawi, 2002; Spinella and Eaton, 2002; Birmes et al., 2003; Bonetto et al., 2007). It has also been said to increase the incidence of hypoglycemia in patients on tolbutamide without apparent alteration in the pharmacokinetic profile of tolbutamide (Mannel, 2004). It also inhibits the production of SN-38, an active metabolite of irinotecan, in cancer patients.
Amitriptyline is a substrate to both CYP3A4 and intestinal P-gp. The risk of therapeutic failure is thus high due to induction of CYP3A4-dependent metabolism activities resulting in poor oral bioavailability. In a study by Johne et al. (2002), a 21% decrease in the area under the plasma concentration–time curve of amitriptyline was observed in 12 depressed patients who were concomitantly administered with extracts of SJW and amitriptyline for 2 weeks.
Other CYP and P-gp substrates whose pharmacokinetic profile have been reportedly altered by SJW include anticoagulants like phenprocoumon and warfarin; antihistamines like fexofenadine; antiretroviral drugs including protease inhibitors and reverse transcriptase inhibitors; hypoglycemic agents such as tolbutamide; immunosuppressants like cyclosporine, tacrolimus, and mycophenolic acid; anticonvulsants such as carbamazepine; anti-cancer like irinotecan; bronchodilators like theophylline; antitussive like dextromethorphan; cardiovascular drugs like statins, digoxin, and dihydropyridine calcium channel blockers; oral contraceptives; opiates like methadone and loperamide; and benzodiazepines including alprazolam and midazolam (Greeson et al., 2001; Di et al., 2008; Hojo et al., 2011). Following a single dose administration of 300 mg standardized extracts of SJW containing 5% hyperforin in humans, a maximum plasma concentration of 0.17–0.5 μM hyperforin yielding a [I]/Ki > 0.22, in vivo extrapolation suggests a high possibility of in vivo pharmacokinetic drug interaction (Agrosi et al., 2000). Bray et al. (2002) confirmed through animal studies that SJW modulates various CYP enzymes. Dresser et al. (2007) demonstrated that SJW is capable of inducing CYP3A4 in healthy subjects through the observation of increased urinary clearance of midazolam. Thus animal and human studies further confirm SJW as containing both inhibitory and inducing constituents on various CYP isozymes. These effects may depend on dosage and duration of administration, and may also be species- and tissue-specific. While the individual phytochemical constituents of SJW have elicited varying effects on the metabolic activity of the CYP isozymes, whole extracts and major constituents especially hyperforin have been reported to inhibit the metabolic activities of CYP1A2, 2C9, 2C19, 2D6, and 3A4 via in vitro studies and in vivo studies (Lee et al., 2006; Madabushi et al., 2006; Hokkanen et al., 2011).
Ginkgo biloba have been reported to induce CYP 2C19-dependent omeprazole metabolism in healthy human subjects (Yin et al., 2004). Piscitelli et al. (2002) in a garlic–saquinavir interaction study reported 51% decrease in saquinavir oral bioavailability caused by the presence of garlic and attributable to garlic-induced CYP3A4 induction. Its effects on the warfarin pharmacokinetic has also been reported in animal models (Taki et al., 2012).
Although grapefruit juice is not consumed for medicinal purposes, the discovery of the inhibitory activity of its flavonoid contents on CYP has led to further researches in medicinal herbs which have revealed HDI potentials in flavonoid-containing herbal remedies (Choi and Burm, 2006; Palombo, 2006; Paine et al., 2008; Quintieri et al., 2008; Alvarez et al., 2010). A related CYP inhibitor is rotenone. By interfering with the electron transfer of the heme iron, rotenone, a naturally occurring phytochemical found in several plants such as the jicama vine plant is known to inhibit CYP activity (Sanderson et al., 2004). Resveratrol, a natural polymer, and tryptophan, an amino acid have been documented as potent CYP inhibitors (Rannug et al., 2006). Some herbal medications and their phytochemical constituents capable of interacting with CYP are presented in Table 3. A more detailed involvement of CYP in HDI is detailed in some recently published reviews (Delgoda and Westlake, 2004; Pal and Mitra, 2006; Cordia and Steenkamp, 2011; Liu et al., 2011).
Phase II metabolic enzymes including uridine diphosphoglucuronosyl transferase (UGT), N-acetyl transferase (NAT), glutathione S-transferase (GST), and sulfotransferase (ST) catalyze the attachment of polar and ionizable groups to phase I metabolites aiding their elimination. While cytochrome P450-mediated HDI have been extensively investigated in various studies, the effects of herbal extracts on phase II enzymes have not been adequately studied. However, there is sufficient evidence in literature to suggest the potentials of phase II enzymes to induce clinically significant HDI.
In a study carried out in rat models by Sheweita et al. (2002), extracts of hypoglycemic herbs, Cymbopogon proximus, Zygophyllum coccineum, and Lupinus albus reduced the activity of GST and GSH. Curcumin, from Curcuma longa, an herbal antioxidant with anti-inflammatory and antitumor properties increased the activity of GST and quinone reductase in the ddY mice liver (Iqbal et al., 2003). Valerian, an herbal sleeping aid has also demonstrated the potential of inducing HDI through the inhibition of UGT. Up to 87% of inhibition of UGT activity by valerian extract was reported in an in vitro study utilizing estradiol and morphine as probe substrate (Alkharfy and Frye, 2007). Kampo, a traditional Japanese medicine made of a mixture of several medicinal herbs has shown inhibitory effects on some phase II enzymes. In an in vitro study by Nakagawa et al. (2009), nine out of 51components of kampo medicine elicited more than 50% inhibition of UGT2B7-mediated morphine 3-glucuronidation. In the same study, extracts of kanzo (Glycyrrhizae radix), daio (Rhei rhizoma), and keihi (Cinnamomi cortex) elicited more than 80% inhibition of morphine AZT glucuronidation. This result agrees with Katoh et al. (2009) who carried out similar studies on rhei, keihi, and ogon (Scutellariae radix).
Apart from the well-known effects on Ginkgo biloba on CYP enzymes as illustrated earlier, its extracts have demonstrated potent inhibition of mycophenolic acid glucuronidation investigated in human liver and intestinal microsomes (Mohamed and Frye, 2010).
In a study to investigate the influence of 18 herbal remedies on the activity of human recombinant sulfotransferase 1A3 employing dopamine and ritodrine as substrates, extracts of grape seed, milk thistle, gymnema, SJW, ginkgo leaf, banaba, rafuma, and peanut seed coat showed potent inhibition with IC50 values lower than putative gastrointestinal concentration (Nagai et al., 2009). Similarly, Mohamed and Frye (2011b) reported the inhibition of UGT1A4 by green tea derived epigallocatechin gallate; UGT 1A6 and UGT1A9 by milk thistle; UGT 1A6 by saw palmetto; and UGT 1A9 by cranberry. A recent publication presents evidence of potential HDI mediated by UGT (Mohamed and Frye, 2011a).
Certain phytochemicals including coumarin, limettin, auraptene, angelicin, bergamottin, imperatorin, and isopimpinellin have also been reported to be capable of inducing hepatic GST activities (Kleiner et al., 2008). While the clinical significance of these findings are yet to be determined, it is noteworthy that phase II metabolic enzymes may play significant roles in HDIs.
Inhibition and induction of transport and efflux proteins
The ATP-binding cassette (ABC) family of drug transporters plays significant roles in the absorption, distribution, and elimination of drugs. P-gp, the most studied member of this family is a 170-kDa plasma glycoprotein encoded by the human MDRI gene. It is constitutively expressed in a number of body tissues and concentrated on the apical epithelial surfaces of the bile canaliculi of the liver, the proximal tubules of the kidneys, the pancreatic ductal cells, the columnar mucosal cells of the small intestine, colon, and the adrenal glands (Marzolini et al., 2004; Degorter et al., 2012). It is actively involved in drug absorption and elimination from the intestines the liver, kidneys, and the brain. Specifically these proteins are involved in the processes of hepatobiliary, direct intestinal, and urinary excretion of drugs and their metabolites (Szakács et al., 2008). Thus, the modulation of P-gp, or competitive affinity as substrates for its binding sites by co-administered herbs presents a potential for alteration in the pharmacokinetic profile of the drug.
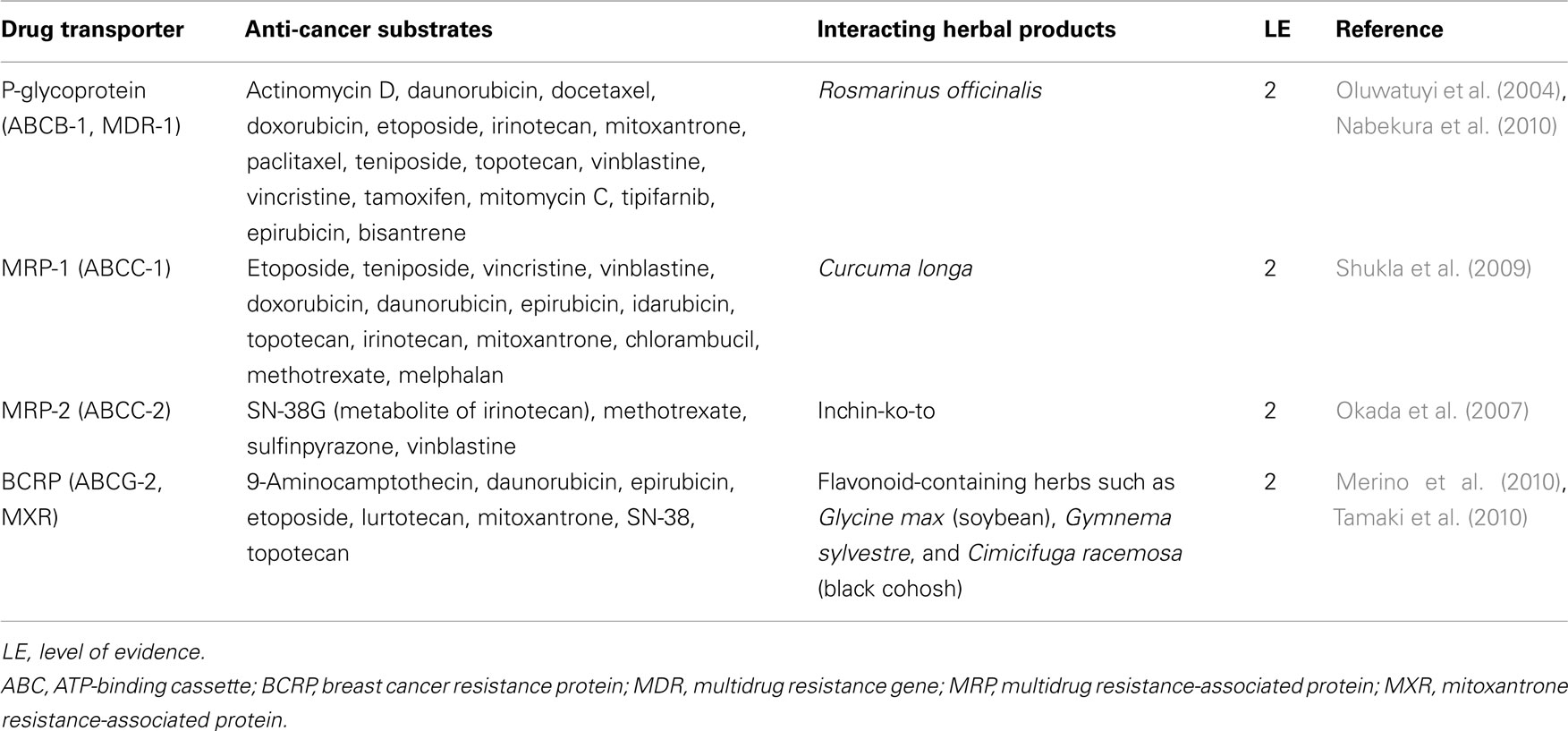
Pharmacokinetic interaction occurs when herbal drugs inhibit or decrease the normal activity level of drug transporters through a competitive or non-competitive mechanism. Interactions can also occur through the induction of transport proteins via the increase of the mRNA of the relevant protein. Studies have identified a number of clinically important P-gp inhibitors including phytochemicals – flavonoids, furanocoumarins, reserpine, quinidine, yohimbine, vincristine, vinblastine among others (Krishna and Mayer, 2001; Zhou et al., 2004; Patanasethanont et al., 2007; Iwanaga et al., 2010; Eichhorn and Efferth, 2011; Yu et al., 2011). Borrel et al. (1994) reported that mobile ionophores such as valinomycin, nonactin, nigericin, monensin, calcimycin, and lasalocid inhibit the efflux of anthracycline by P-gp whereas channel-forming ionophores such as gramicidin do not (Larsen et al., 2000). A number of herbal products which interact with CYP also have similar effects on transport proteins (Table 3). The transport proteins are actively involved in the pharmacokinetics of anti-cancer drugs and account for one of the well-known mechanisms of multiple resistance of cancerous cells to chemotherapeutic agents (Bebawy and Sze, 2008; Bosch, 2008; He et al., 2011). The influence of some herbs on transport proteins is presented in Table 4. Clinically relevant interactions between herbal medicine and chemotherapeutic agents are detailed in a recent review by Yap et al. (2010).
Alteration of gastrointestinal functions
Besides their influence on the intestinal metabolic enzymes and efflux proteins, herbal medications can alter the absorption of concomitantly administered medicines through a number of mechanisms. Changes in the gastrointestinal pH and other biochemical factors can alter dissolution properties and the absorption of pH-dependent drugs such as ketoconazole and itraconazole. Complexation and chelation, leading to the formation of insoluble complexes and competition at the sites of absorption especially with site-specific formulations can greatly affect the absorption of medicines. Anthranoid-containing plants – cassia (Cassia senna), Cascara (Rhamnus purshiana), rhubarb (Rheum officinale), and soluble fibers including guar gum and psyllium can decrease drug absorption by decreasing GI transit time. They are known to increase GIT motility. On concomitant use with prescribed medication, significant alteration in the absorption of the latter has been reported due to decreased GI transit time (Fugh-Berman, 2000).
Izzo et al. (1997) demonstrated that anthranoids could be harmful to the gut epithelium by inhibiting Na+/K+ ATPase and increasing the activity of nitric oxide synthase. This significantly increased intestinal transit due to the alteration in the intestinal water and salt absorption and the subsequent fluid accumulation. In a study conducted by Munday and Munday (1999), a garlic-derived compound was shown to increase the tissue activities of quinone reductase and glutathione transferase in the gastrointestinal tract of the rat. In view of their roles in metabolism, both enzymes are considered chemoprotective especially from chemical carcinogens. In addition to CYP and P-gp mediated mechanisms, the well-known ginseng-induced pharmacokinetic HDI may also be due to its gastrointestinal effects especially its inhibitory effects on gastric secretion (Suzuki et al., 1991). The potential of rhein and danthron to increase the absorption of furosemide, a poorly water-soluble drug, has been demonstrated through in vitro studies (Laitinen et al., 2007). In a study carried out on mice, a Chinese herbal plant, Polygonum paleaceum, showed the potential to depress the motility of the gastrointestinal tract, inhibit defecation reflex and delay gastric emptying (Zhang, 2002). A similar study demonstrated the inhibitory effects of two Chinese traditional herbal prescriptions, Fructus aurantii immaturus and Radix paeoniae alba on gastrointestinal movement (Fang et al., 2009).
The absorption of drugs such as phenoxymethylpenicillin, metformin, glibenclamide, and lovastatin may be reduced by high-fiber herbal products through the sequestration of bile acids (Colalto, 2010). Mochiki et al. (2010) reported the ability of Kampo, a traditional Japanese medicine, to stimulate elevated intestinal blood flow, and to induce increased secretion of gastrointestinal hormones including motilin, vasoactive intestinal peptide, and calcitonin gene-related peptide. Similarly, another traditional Japanese medicine has been shown to increase the intestinal secretion of ghrelin, a hunger-related hormone, leading to delayed gastric emptying (Tokita et al., 2007; Kawahara et al., 2009; Hattori, 2010; Matsumura et al., 2010). Also, Qi et al. (2007) demonstrated the capability of Da-Cheng-Qi-Tang, a traditional Chinese herbal formula, to increase plasma motilin, enhance gastrointestinal motility, improve gastric dysrhythmia, and reduce gastroparesis after abdominal surgery. These effects have the potential of reducing the intestinal transit time of concurrently administered drug, with the risk of reduced absorption.
Alteration in renal elimination
This involves herbal products capable of interacting with renal functions, leading to altered renal elimination of drugs. Such interaction can result from the inhibition of tubular secretion, tubular reabsorption, or interference with glomerular filtration (Isnard et al., 2004). In addition to this group of herbal products are those products consumed as diuretics. The mechanism of herbal diuresis is complex and non-uniform. Certain herbs increase the glomerular filtration rate but do not stimulate electrolyte secretion while some others act as direct tubular irritants (Crosby et al., 2001; Al-Ali et al., 2003). Some herbs capable of interacting with renal functions and drug elimination are presented in Table 5.
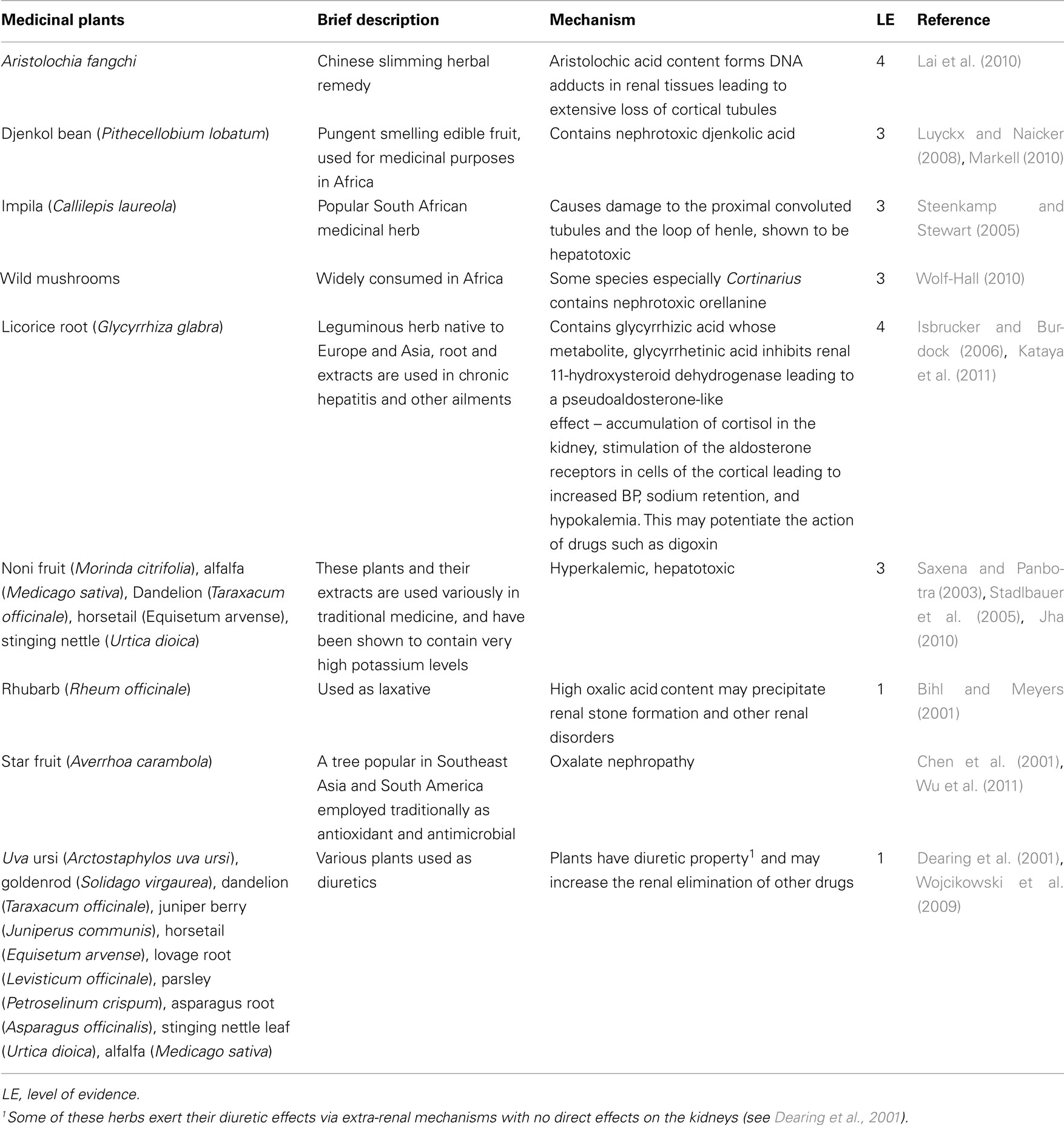
Table 5. Some herbal remedies capable of interacting with other drugs via alteration in renal functions.
Pharmacodynamic synergy, addition, and antagonism
Herb–drug interaction can occur through the synergistic or additive actions of herbal products with conventional medications as a result of affinities for common receptor sites (Ma et al., 2009). This can precipitate pharmacodynamic toxicity or antagonistic effects (Table 6). Like most other herbs, SJW contains complex mixture of phytochemicals including phenylpropanes, naphthodanthrones, acylphloroglucinols, flavonoids, flavanol glycosides, and biflavones. Hyperforin is known to inhibit the reuptake of neurotransmitters (dopamine, serotonin, noradrenalin) and is believed to be the bioactive responsible for the antidepressant activity of SJW.
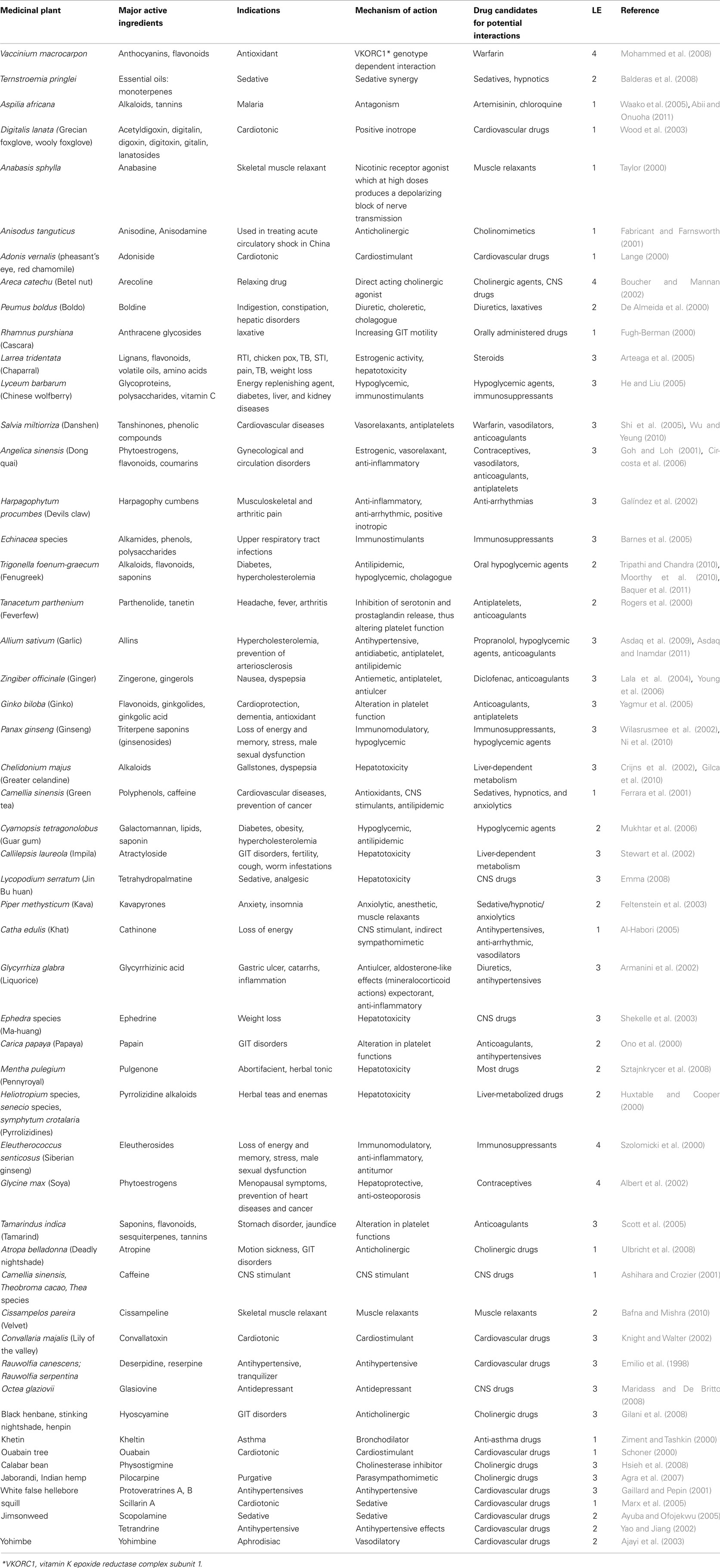
Table 6. Some examples of pharmacodynamic interactions between herbal products and conventional drugs.
Conclusion
Concomitant use of herbs and conventional drugs may present with untoward events. Evidence available in literature indicates various mechanisms through which this can occur. By interacting with conventional medication, herbal remedies may precipitate manifestations of toxicity or in the other extreme, therapeutic failure. A good knowledge of the potential of commonly consumed herbal medicines to interact with prescription medicines, irrespective of the nature of evidence available, will equip health professionals in their practice. Apart from those demonstrated in significant number of human subjects, not all reported HDIs are clinically significant. As such, more clinically relevant research in this area is necessary. This review provides information on commonly used herbs and their potentials for HDI within the levels of evidence currently available.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors will like to acknowledge the support of HOPE Kapstadt-Stiftung (HOPE Cape Town) and the Stellenbosch University Rural Medical Education Partnership Initiative (SURMEPI) for providing funds for this study.
References
Abii, T. A., and Onuoha, E. N. (2011). The chemical constituents of the leaf of Aspilia africana as a scientific backing to its tradomedical potentials. Agric. J. 6, 28–30.
Agra, M. F., De Freitas, P. F., and Barbosa-Filho, J. M. (2007). Synopsis of the plants known as medicinal and poisonous in Northeast Brazil. Rev. Bras. Farmacogn. 17, 114–140.
Agrosi, M., Mischatti, S., Harrasser, P. C., and Savio, D. (2000). Oral bioavailability of active principles from herbal products in humans: a study on Hypericum perforatum extracts using the soft gelatin capsule technology. Phytomedicine 7, 455–462.
Ajayi, A. A., Newaz, M., Hercule, H., Saleh, M., Bode, C. O., and Oyekan, A. O. (2003). Endothelin-like action of Pausinystalia yohimbe aqueous extract on vascular and renal regional hemodynamics in Sprague Dawley rats. Methods Find. Exp. Clin. Pharmacol. 25, 817–822.
Al-Ali, M., Wahbi, S., Twaij, H., and Al-Badr, A. (2003). Tribulus terrestris: preliminary study of its diuretic and contractile effects and comparison with Zea mays. J. Ethnopharmacol. 85, 257–260.
Albert, A., Altabre, C., Baró, F., Buendía, E., Cabero, A., Cancelo, M. J., Castelo-Branco, C., Chantre, P., Duran, M., Haya, J., Imbert, P., Julía, D., Lanchares, J. L., Llaneza, P., Manubens, M., Miñano, A., Quereda, F., Ribes, C., and Vázquez, F. (2002). Efficacy and safety of a phytoestrogen preparation derived from Glycine max (L.) Merr in climacteric symptomatology: a multicentric, open, prospective and non-randomized trial. Phytomedicine 9, 85–92.
Al-Deeb, I. D., Arafat, T. A., and Irshaid, Y. M. (2010). The effect of Licorice drink on the systemic exposure of Verapamil in rabbits. Drug Metab. Lett. 4, 173–179.
Al-Habori, M. (2005). The potential adverse effects of habitual use of Catha edulis (khat). Expert Opin. Drug Saf. 4, 1145–1154.
Alkharfy, K. M., and Frye, R. F. (2007). Effect of valerian, valerian/hops extracts, and valerenic acid on glucuronidation in vitro. Xenobiotica 37, 113–123.
Alvarez, A. I., Real, R., Perez, M., Mendoza, G., Prieto, J. G., and Merino, G. (2010). Modulation of the activity of ABC transporters (P-glycoprotein, MRP2, BCRP) by flavonoids and drug response. J. Pharm. Sci. 99, 598–617.
Amacher, D. E. (2010). The effects of cytochrome P450 induction by xenobiotics on endobiotic metabolism in pre-clinical safety studies. Toxicol. Mech. Methods 20, 159–166.
Armanini, D., Fiore, C., Mattarello, M. J., Bielenberg, J., and Palermo, M. (2002). History of the endocrine effects of Licorice. Exp. Clin. Endocrinol. Diabetes 110, 257–261.
Arteaga, I., Andrade-Cetto, A., and Cárdenas, R. (2005). Larrea tridentata (Creosote bush), an abundant plant of Mexican and US-American deserts and its metabolite nordihydroguaiaretic acid. J. Ethnopharmacol. 98, 231–239.
Asdaq, S. M., and Inamdar, M. N. (2010). Pharmacodynamic interaction of captopril with garlic in isoproterenol-induced myocardial damage in rat. Phytother. Res. 24, 720–725.
Asdaq, S. M., and Inamdar, M. N. (2011). Pharmacodynamic and pharmacokinetic interactions of propranolol with garlic (Allium sativum) in rats. Evid. Based Complement. Alternat. Med. 2011, 824042.
Asdaq, S. M., Inamdar, M. N., and Asad, M. (2009). Effect of conventional antihypertensive drugs on hypolipidemic action of garlic in rats. Indian J. Exp. Biol. 47, 176–181.
Ashihara, H., and Crozier, A. (2001). Caffeine: a well-known but little mentioned compound in plant science. Trends Plant Sci. 6, 407–413.
Ayuba, V. O., and Ofojekwu, P. C. (2005). “Effects of extracts of dried seeds of toloache, Datura innoxia as anaesthesia on the African catfish Clarias gariepinus fingerlings,” in 19th Annual Conference of the Fisheries Society of Nigeria. Available at: http://aquaticcommons.org/3986/1/25.pdf [accessed November 03, 2011].
Bafna, A., and Mishra, S. (2010). Antioxidant and immunomodulatory activity of the alkaloidal fraction of Cissampelos pareira linn. Sci. Pharm. 78, 21–31.
Balderas, J. L., Reza, V., Ugalde, M., Guzmán, L., Serrano, M. I., Aguilar, A., and Navarrete, A. (2008). Pharmacodynamic interaction of the sedative effects of Ternstroemia pringlei (Rose) Standl. with six central nervous system depressant drugs in mice. J. Ethnopharmacol. 119, 47–52.
Baquer, N. Z., Kumar, P., Taha, A., Kale, R. K., Cowsik, S. M., and McLean, P. (2011). Metabolic and molecular action of Trigonella foenum-graecum (fenugreek) and trace metals in experimental diabetic tissues. J. Biosci. 36, 383–396.
Barbenel, D. M., Yusufi, B., O’Shea, D., and Bench, C. J. (2000). Mania in a patient receiving testosterone replacement postorchidectomy taking St John’s wort and sertraline. J. Psychopharmacol. 14, 84–86.
Barnes, J., Anderson, L. A., Gibbons, S., and Phillipson, J. D. (2005). Echinacea species (Echinacea angustifolia (DC.) Hell, Echinacea pallida (Nutt.) Nutt., Echinacea purpurea (L.) Moench: a review of their chemistry, pharmacology and clinical properties. J. Pharm. Pharmacol. 57, 929–954.
Bebawy, M., and Sze, D. M. (2008). Targeting P-glycoprotein for effective oral anti-cancer chemotherapeutics. Curr. Cancer Drug Targets 8, 47–52.
Bensoussan, A., Myers, S. P., Wu, S. M., and O’Connor, K. (2004). Naturopathic and Western herbal medicine practice in Australia – a workforce survey. Complement. Ther. Med. 12, 17–27.
Berginc, K., and Kristl, A. (2012). The effect of garlic supplements and phytochemicals on the ADMET properties of drugs. Expert Opin. Drug Metab. Toxicol. 8, 295–310.
Bihl, G., and Meyers, A. (2001). Recurrent renal stone disease – advances in pathogenesis and clinical management. Lancet 358, 651–656.
Bilgi, N., Bell, K., Ananthakrishnan, A. N., and Atallah, E. (2010). Imatinib and Panax ginseng: a potential interaction resulting in liver toxicity. Ann. Pharmacother. 44, 926–928.
Birmes, P., Coppin, D., Schmitt, L., and Lauque, D. (2003). Serotonin syndrome: a brief review. CMAJ 168, 1439–1442.
Bodeker, G. (2007). Traditional health systems: policy, biodiversity, and global interdependence. J. Altern. Complement. Med. 1, 231–243.
Bonetto, N., Santelli, L., Battistin, L., and Cagnin, A. (2007). Serotonin syndrome and rhabdomyolysis induced by concomitant use of triptans, fluoxetine and hypericum. Cephalalgia 27, 1421–1423.
Borrel, M. N., Pereira, E., Fiallo, M., and Garnier-Suillerot, A. (1994). Mobile ionophores are a novel class of P-glycoprotein inhibitors. The effects of ionophores on 4′-O-tetrahydropyranyl-adriamycin incorporation in K562 drug-resistant cells. Eur. J. Biochem. 223, 125–133.
Borrelli, F., Capasso, R., and Izzo, A. A. (2007). Garlic (Allium sativum L.): adverse effects and drug interactions in humans. Mol. Nutr. Food Res. 51, 1386–1397.
Borrelli, F., and Izzo, A. A. (2009). Herb-drug interactions with St John’s wort (Hypericum perforatum): an update on clinical observations. AAPS J. 11, 710–727.
Bosch, T. M. (2008). Pharmacogenomics of drug-metabolizing enzymes and drug transporters in chemotherapy. Methods Mol. Biol. 448, 63–76.
Boucher, B. J., and Mannan, N. (2002). Metabolic effects of the consumption of Areca catechu. Addict. Biol. 7, 103–110.
Boullata, J. I., and Nace, A. M. (2000). Safety issues with herbal medicine. Pharmacotherapy 20, 257–269.
Bray, B. J., Perry, N. B., Menkes, D. B., and Rosengren, R. J. (2002). St John’s wort extract induces CYP3A and CYP2E1 in the Swiss Webster mouse. Toxicol. Sci. 66, 27–33.
Brown, L., Heyneke, O., Brown, D., van Wyk, J. P., and Hamman, J. H. (2008). Impact of traditional medicinal plant extracts on antiretroviral drug absorption. J. Ethnopharmacol. 119, 588–592.
Calixto, J. B. (2000). Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents). Braz. J. Med. Biol. Res. 33, 179–189.
Capasso, F., Gaginella, T. S., Grandolini, G., and Izzo, A. A. (2003). Phytotherapy: A Quick Reference to Herbal Medicine. Berlin: Spriger-Verlag.
Cassidy, A. (2003). Are herbal remedies and dietary supplements safe and effective for breast cancer patients? Breast Cancer Res. 5, 300–302.
Chan, T. Y. (2001). Interaction between warfarin and danshen (Salvia miltiorrhiza). Ann. Pharmacother. 35, 501–504.
Chao, M. T., Wade, C., and Kronenberg, F. (2008). Disclosure of complementary and alternative medicine to conventional medical providers: variation by race/ethnicity and type of CAM. J. Natl. Med. Assoc. 100, 1341–1349.
Chen, C. L., Fang, H. C., Chou, K. J., Wang, J. S., and Chung, H. M. (2001). Acute oxalate nephropathy after ingestion of star fruit. Am. J. Kidney Dis. 37, 418–422.
Cheng, B., Hung, C. T., and Chiu, W. (2002). Herbal medicine and anaesthesia. Hong Kong Med. J. 8, 123–130.
Chitturi, S., and Farrell, G. C. (2000). Herbal hepatotoxicity: an expanding but poorly defined problem. J. Gastroenterol. Hepatol. 15, 1093–1099.
Chitturi, S., and Farrell, G. C. (2008). Hepatotoxic slimming aids and other herbal hepatotoxins. J. Gastroenterol. Hepatol. 23, 366–373.
Choi, J. S., and Burm, J. P. (2006). Enhanced nimodipine bioavailability after oral administration of nimodipine with morin, a flavonoid, in rabbits. Arch. Pharm. Res. 29, 333–338.
Circosta, C., Pasquale, R. D., Palumbo, D. R., Samperi, S., and Occhiuto, F. (2006). Estrogenic activity of standardized extract of Angelica sinensis. Phytother. Res. 20, 665–669.
Clement, Y. N., Williams, A. F., Khan, K., Bernard, T., Bhola, S., Fortuné, M., Medupe, O., Nagee, K., and Seaforth, C. E. (2005). A gap between acceptance and knowledge of herbal remedies by physicians: the need for educational intervention. BMC Complement. Altern. Med. 5, 20. doi:10.1186/1472-6882-5-20
Colalto, C. (2010). Herbal interactions on absorption of drugs: mechanisms of action and clinical risk assessment. Pharmacol. Res. 62, 207–227.
Coppola, M., and Mondola, R. (2012). Potential action of betel alkaloids on positive and negative symptoms of schizophrenia: a review. Nord. J. Psychiatry 66, 73–78.
Cordia, W., and Steenkamp, V. (2011). Drug interactions in African herbal remedies. Drug Metabol. Drug Interact. 26, 53–63.
Cox, M. C., Low, J., Lee, J., Walshe, J., Denduluri, N., Berman, A., Permenter, M. G., Petros, W. P., Price, D. K., Figg, W. D., Sparreboom, A., and Swain, S. M. (2006). Influence of garlic (Allium sativum) on the pharmacokinetics of docetaxel. Clin. Cancer Res. 12, 4636–4640.
Crijns, A. P., se Smet, P. A., van den Heuvel, M., Schot, B. W., and Haagsma, E. B. (2002). Acute hepatitis after use of a herbal preparation with greater celandine (Chelidonium majus). Ned. Tijdschr. Geneeskd. 19, 124–128.
Crosby, E. C., Dolan, R. L., Benson, J. E., Luetkemeier, M. J., Barton, R. G., and Askew, E. W. (2001). Herbal diuretic induced dehydration and resting metabolic rate. Med. Sci. Sports Exerc. 33, S163.
Dannawi, M. (2002). Possible serotonin syndrome after combination of buspirone and St John’s wort. J. Psychopharmacol. (Oxford) 16, 401.
Dasgupta, A., Kidd, L., Poindexter, B. J., and Bick, R. J. (2010). Interference of hawthorn on serum digoxin measurements by immunoassays and pharmacodynamic interaction with digoxin. Arch. Pathol. Lab. Med. 134, 1188–1192.
De Almeida, E. R., Melo, A. M., and Xavier, H. (2000). Toxicological evaluation of the hydro-alcohol extract of the dry leaves of Peumus boldus and boldine in rats. Phytother. Res. 14, 99–102.
De Berardinis, V., Moulis, C., Maurice, M., Beaune, P., Pessayre, D., Pompon, D., and Loeper, J. (2000). Human microsomal epoxide hydrolase is the target of germander-induced autoantibodies on the surface of human hepatocytes. Mol. Pharmacol. 3, 542–551.
De Maat, M. M., Hoetelmans, R. M. W., Mathôt, R. A., van Gorp, E. C., Meenhorst, P. L., Mulder, J. W., and Beijnen, J. H. (2001). Drug interactions between St John’s wort and nevirapine. AIDS 15, 420–421.
Dearing, D. M., Mangione, A. M., and Karasov, W. H. (2001). Plant secondary compounds as diuretics: an overlooked consequence. Am. Zool. 41, 890–901.
Déciga-Campos, M., Rivero-Cruz, I., Arriaga-Alba, M., Castañeda-Corral, G., Angeles-López, G. E., Navarrete, A., and Mata, R. (2007). Acute toxicity and mutagenic activity of Mexican plants used in traditional medicine. J. Ethnopharmacol. 110, 334–342.
Degorter, M. K., Xia, C. Q., Yang, J. J., and Kim, R. B. (2012). Drug transporters in drug efficacy and toxicity. Annu. Rev. Pharmacol. Toxicol. 52, 249–273.
DeLeve, L. D., Schulman, H. M., and MacDonald, G. B. (2002). Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin. Liver Dis. 22, 27–42.
Delgoda, R., and Westlake, A. C. G. (2004). Herbal interactions involving cytochrome P450 enzymes: a mini review. Toxicol. Rev. 23, 239–249.
Di, Y. M., Li, C. G., Xue, C. C., and Zhou, S. F. (2008). Clinical drugs that interact with St. John’s wort and implication in drug development. Curr. Pharm. Des. 14, 1723–1742.
Dresser, G. K., Schwarz, U. I., Wilkinson, G. R., and Kim, R. B. (2007). Coordinate induction of both cytochrome P4503A and MDR1 by St John’s wort in healthy subjects. Clin. Pharmacol. Ther. 73, 41–50.
Durazo, F. A., Lassman, C., Han, S. H., Saab, S., Lee, N. P., Kawano, M., Saggi, B., Gordon, S., Farmer, D. G., Yersiz, H., Goldstein, R. L., Ghobrial, M., and Busuttil, R. W. (2004). Fulminant liver failure due to usnic acid for weight loss. Am. J. Gastroenterol. 5, 950–952.
Eichhorn, T., and Efferth, T. (2011). Pglycoprotein and its inhibition in tumors by phytochemicals derived from Chinese herbal medicine. J. Ethnopharmacol. PMID: 21859398. [Epub ahead of print].
Emilio, L., Ghisalberti, E. L., Pennacchio, M., and Alexander, E. (1998). Survey of secondary plant metabolites with cardiovascular activity. Pharm. Biol. 36, 237–279.
Emma, C. (2008). Lycopodium similiaplex-induced acute hepatitis: a case report. Eur. J. Gastroenterol. Hepatol. 20, 469–471.
Engelberg, D., McCutcheon, A., and Wiseman, S. (2001). A case of ginseng-induced mania. J. Clin. Psychopharmacol. 21, 535–537.
Ernst, E. (2002). St John’s wort supplements endanger the success of organ transplantation. Arch. Surg. 137, 316–319.
Ernst, E. (2005). The efficacy of herbal medicine – an overview. Fundam. Clin. Pharmacol. 19, 405–409.
Fabricant, D. S., and Farnsworth, N. R. (2001). The value of plants used in traditional medicine for drug discovery. Environ. Health Perspect. 109, 69–75.
Fakeye, T. O., and Onyemadu, O. (2008). Evaluation of knowledge base of hospital pharmacists and physicians on herbal medicines in Southwestern Nigeria. Pharm. Pract. 6, 88–92.
Fang, Y. S., Shan, D. M., Liu, J. W., Xu, W., Li, C. L., Wu, H. Z., and Ji, G. (2009). Effect of constituents from fructus Aurantii immaturus and radix Paeoniae alba on gastrointestinal movement. Planta Med. 75, 24–31.
Farkas, D., Shader, R. I., von Moltke, L. L., and Greenblatt, D. J. (2010). “Mechanisms and consequences of drug–drug interactions,” in Pharmaceutical Sciences Encyclopedia: Drug Discovery, Development, and Manufacturing (John Wiley & Sons, Inc.). doi: 10.1002/9780470571224.pse055
Fasinu, P., Bouic, P. J., and Rosenkranz, B. (2012). Liver-based in vitro technologies for drug biotransformation studies – a review. Curr. Drug Metab. 13, 215–224.
Feltenstein, M. W., Lambdin, L. C., Ganzera, M., Ranjith, H., Dharmaratne, W., Nanayakkara, N. P., Khan, I. A., and Sufka, K. J. (2003). Anxiolytic properties of piper methysticum extract samples and fractions in the chick social–separation–stress procedure. Phytother. Res. 17, 210–216.
Ferguson, C. S., and Tyndale, R. F. (2011). Cytochrome P450 enzymes in the brain: emerging evidence of biological significance. Trends Pharmacol. Sci. 32, 708–714.
Ferrara, L., Montesano, D., and Senatore, A. (2001). The distribution of minerals and flavonoids in the tea plant Camellia sinensis. Farmaco 56, 397–401.
Gaillard, Y., and Pepin, G. (2001). Case report: LC–EI-MS determination of veratridine and cevadine in two fatal cases of Veratrum album poisoning. J. Anal. Toxicol. 25, 481–485.
Galíndez, J. S., Lanza, A. M. D., and Matellano, L. F. (2002). Biologically active substances from the genus Scrophularia. Pharm. Biol. 40, 45–59.
Gilani, A. H., Khan, A. U., Raoof, M., Ghayur, M. N., Siddiqui, B. S., Vohra, W., and Begum, S. (2008). Gastrointestinal, selective airways and urinary bladder relaxant effects of Hyoscyamus niger are mediated through dual blockade of muscarinic receptors and Ca2+ channels. Fundam. Clin. Pharmacol. 22, 87–99.
Gilca, M., Gaman, L., Panait, E., Stoian, I., and Atanasiu, V. (2010). Chelidonium majus – an integrative review: traditional knowledge versus modern findings. Forsch. Komplementmed. 17, 241–248.
Giveon, S. M., Liberman, N., Klang, S., and Kahan, E. (2004). Are people who use ‘natural drugs’ aware of their potentially harmful side effects and reporting to family physician? Patient Educ. Couns. 53, 5–11.
Goh, S. Y., and Loh, K. C. (2001). Gynaecomastia and the herbal tonic “dong quai.” Singapore Med. J. 42, 115–116.
Greeson, J. M., Sanford, B., and Monti, D. A. (2001). St. John’s wort (Hypericum perforatum): a review of the current pharmacological, toxicological, and clinical literature. Psychopharmacology (Berl.) 153, 402–414.
Gurley, B. J., Gardner, S. F., Hubbard, M. A., Williams, D. K., Gentry, W. B., Cui, Y., and Ang, C. Y. (2005a). Clinical assessment of botanical supplementation on cytochrome P450 phenotypes in the elderly: St John’s wort, garlic oil, Panax ginseng, and Ginkgo biloba. Drugs Aging 22, 525–539.
Gurley, B. J., Gardner, S. F., Hubbard, M. A., Williams, D. K., Gentry, W. B., Khan, I. A., and Shah, A. (2005b). In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1 and 3A4/5 phenotypes. Clin. Pharmacol. Ther. 77, 415–426.
Hafner, V., Jäger, M., Matthée, A. K., Ding, R., Burhenne, J., Haefeli, W. E., and Mikus, G. (2009). Effect of simultaneous induction and inhibition of CYP3A by St John’s wort and ritonavir on CYP3A activity. Clin. Pharmacol. Ther. 87, 191–196.
Hamann, G. L., Campbell, J. D., and George, C. M. (2011). Warfarin-cranberry juice interaction. Ann. Pharmacother. 45, e17.
He, L., and Liu, P. (2005). Effect of Lycium barbarum polysaccharides on oxidative stress of diabetic nephropathy in type 2 diabetic rats. Chinese J. Hosp. Pharm. 12, 34–40.
He, S. M., Li, R., Kanwar, J. R., and Zhou, S. F. (2011). Structural and functional properties of human multidrug resistance protein 1 (MRP1/ABCC1). Curr. Med. Chem. 18, 439–481.
Henderson, L., Yue, Q. Y., Bergquist, C., Gerden, B., and Arlett, P. (2002). St John’s wort (Hypericum perforatum): drug interactions and clinical outcomes. Br. J. Clin. Pharmacol. 54, 349–356.
Hiratsuka, M. (2011). In vitro assessment of the allelic variants of cytochrome P450. Drug Metab. Pharmacokinet. [Epub online ahead of print].
Hojo, Y., Echizenya, M., Ohkubo, T., and Shimizu, T. (2011). Drug interaction between St John’s wort and zolpidem in healthy subjects. J. Clin. Pharm. Ther. 36, 711–715.
Hokkanen, J., Tolonen, A., Mattila, S., and Turpeinen, M. (2011). Metabolism of hyperforin, the active constituent of St. John’s wort, in human liver microsomes. Eur. J. Pharm. Sci. 42, 273–284.
Homsy, J., King, R., Tenywa, J., Kyeyune, P., Opio, A., and Balaba, D. (2004). Defining minimum standards of practice for incorporating African traditional medicine into HIV/AIDS prevention, care, and support: a regional initiative in eastern and southern Africa. J. Altern. Complement. Med. 10, 905–910.
Howell, L., Kochhar, K., Saywell, R. Jr., Zollinger, T., Koehler, J., Mandzuk, C., Sutton, B., Sevilla-Martir, J., and Allen, D. (2006). Use of herbal remedies by Hispanic patients: do they inform their physician? J. Am. Board Fam. Med. 19, 566–578.
Høyland, H. K. (2011). Use of Saint John’s wort against mild depression. Tidsskr. Nor. Laegeforen. 131, 837–839.
Hsieh, M. J., Yen, Z. S., Chen, S. C., and Fang, C. C. (2008). Acute cholinergic syndrome following ingestion of contaminated herbal extract. Emerg. Med. J. 25, 781–782.
Hu, Z., Yang, X., Ho, P. C., Chan, S. Y., Heng, P. W., Chan, E., Duan, W., Koh, H. L., and Zhou, S. (2005). Herb-drug interactions: a literature review. Drugs 65, 1239–1282.
Huang, Z., Xiao, B., Wang, X., Li, Y., and Deng, H. (2003). Betel nut indulgence as a cause of epilepsy. Seizure 12, 406–408.
Huxtable, R. J., and Cooper, R. A. (2000). “Pyrrolizidine alkaloids: physicochemical correlates of metabolism and toxicity,” in Natural and Selected Synthetic Toxins, eds A. T. Tu, and W. Gaffield (Washington: American Chemical Society), 100–117.
Iqbal, M., Sharma, S. D., Okazaki, Y., Fujisawa, M., and Okada, S. (2003). Dietary supplementation of curcumin enhances antioxidant and phase II metabolizing enzymes in ddY male mice: possible role in protection against chemical carcinogenesis and toxicity. Pharmacol. Toxicol. 92, 33–38.
Isbrucker, R. A., and Burdock, G. A. (2006). Risk and safety assessment on the consumption of Licorice root (Glycyrrhiza sp.), its extract and powder as a food ingredient, with emphasis on the pharmacology and toxicology of glycyrrhizin. Regul. Toxicol. Pharmacol. 46, 167–192.
Isnard, B. C., Deray, G., Baumelou, A., Le Quintrec, M., and Vanherweghem, J. L. (2004). Herbs and the kidney. Am. J. Kidney Dis. 44, 1–11.
Iwamoto, M., Kassahun, K., Troyer, M. D., Hanley, W. D., Lu, P., Rhoton, A., Petry, A. S., Ghosh, K., Mangin, E., DeNoia, E. P., Wenning, L. A., Stone, J. A., Gottesdiener, K. M., and Wagner, J. A. (2008). Lack of a pharmacokinetic effect of raltegravir on midazolam: in vitro/in vivo correlation. J. Clin. Pharmacol. 48, 209–214.
Iwanaga, K., Hayashi, M., Hamahata, Y., Miyazaki, M., Shibano, M., Taniguchi, M., Baba, K., and Kakemi, M. (2010). Furanocoumarin derivatives in Kampo extract medicines inhibit cytochrome P450 3A4 and P-glycoprotein. Drug Metab. Dispos. 38, 1286–1294.
Izzo, A. A. (2005). Herb–drug interactions: an overview of the clinical evidence. Fundam. Clin. Pharmacol. 19, 1–16.
Izzo, A. A., and Ernst, E. (2009). Interactions between herbal medicines and prescribed drugs: an updated systematic review. Drugs 69, 1777–1798.
Izzo, A. I., Sautebin, L., Rombola, L., and Capasso, F. (1997). The role of constitutive nitric oxide synthase in senna- and cascara-induced diarrhoea in the rat. Eur. J. Pharmacol. 323, 93–97.
Johne, A., Schmider, J., Brockmöller, J., Stadelmann, A. M., Störmer, E., Bauer, S., Scholler, G., Langheinrich, M., and Roots, I. (2002). Decreased plasma levels of amitriptyline and its metabolites on comedication with an extract from St. John’s wort (Hypericum perforatum). J. Clin. Psychopharmacol. 22, 46–54.
Kataya, H. H., Hamza, A. A., Ramadan, G. A., and Khasawneh, M. A. (2011). Effect of licorice extract on the complications of diabetes nephropathy in rats. Drug Chem. Toxicol. 34, 101–108.
Katoh, M., Yoshioka, Y., Nakagawa, N., and Yokoi, T. (2009). Effects of Japanese herbal medicine, Kampo, on human UGT1A1 activity. Drug Metab. Pharmacokinet. 24, 226–234.
Kaufman, D., Kelly, J., Rosenberg, L., Anderson, T. E., and Mitchell, A. A. (2002). Recent patterns of medication use in the ambulatory adult population of the United States. JAMA 287, 37–44.
Kawahara, H., Mitani, Y., Nomura, M., Nose, K., Yoneda, A., Hasegawa, T., Kubota, A., and Fukuzawa, M. (2009). Impact of rikkunshito, an herbal medicine, on delayed gastric emptying in profoundly handicapped patients. Pediatr. Surg. Int. 25, 987–990.
Kennedy, J., Wang, C., and Wu, C. (2008). Patient disclosure about herb and supplement use among adults in the US. Evid. Based Complement. Alternat. Med. 5, 451–456.
Kent, U. M., Aviram, M., Rosenblat, M., and Hollenberg, P. F. (2002). The Licorice root derived isoflavan glabridin inhibits the activities of human cytochrome P450S 3A4, 2B6, and 2C9. Drug Metab. Dispos. 30, 709–715.
Kessler, R. C., Davis, R. B., Foster, D. F., Van Rompay, M. I., Walters, E. E., Wilkey, S. A., Kaptchuk, T. J., and Eisenberg, D. M. (2001). Long-term trends in the use of complementary and alternative medical therapies in the United States. Ann. Intern. Med. 135, 262–268.
Kim, B. H., Kim, K. P., Lim, K. S., Kim, J. R., Yoon, S. H., Cho, J. Y., Lee, Y. O., Lee, K. H., Jang, I. J., Shin, S. G., and Yu, K. S. (2010a). Influence of Ginkgo biloba extract on the pharmacodynamic effects and pharmacokinetic properties of ticlopidine: an open-label, randomized, two-period, two-treatment, two-sequence, single-dose crossover study in healthy Korean male volunteers. Clin. Ther. 32, 380–390.
Kim, E., Sy-Cordero, A., Graf, T. N., Brantley, S. J., Paine, M. F., and Oberlies, N. H. (2010b). Isolation and identification of intestinal CYP3A inhibitors from cranberry (Vaccinium macrocarpon) using human intestinal microsomes. Planta Med. 77, 265–270.
Kleiner, H. E., Xia, X., Sonoda, J., Zhang, J., Pontius, E., Abey, J., Evans, R. M., Moore, D. D., and DiGiovanni, J. (2008). Effects of naturally occurring coumarins on hepatic drug-metabolizing enzymes in mice. Toxicol. Appl. Pharmacol. 232, 337–350.
Klepser, T. B., Doucette, W. R., and Horton, M. R. (2000). Assessment of patients’ perceptions and beliefs regarding herbal therapies. Pharmacotherapy 20, 83–87.
Knight, A. P., and Walter, R. G. (2002). “Plants affecting the cardiovascular system,” in A Guide to Plant Poisoning of Animals in North America, eds A. P. Knight, and R. G. Walter Available at: http://www.ivis.org/special_books/Knight/chap2/ivis.pdf [accessed November 05 2011].
Krishna, R., and Mayer, L. D. (2001). Modulation of P-glycoprotein (PGP) mediated multidrug resistance (MDR) using chemosensitizers: recent advances in the design of selective MDR modulators. Curr. Med. Chem. Anticancer Agents 1, 163–174.
Lai, M. N., Wang, S. M., Chen, P. C., Chen, Y. Y., and Wang, J. D. (2010). Population-based case-control study of Chinese herbal products containing aristolochic acid and urinary tract cancer risk. J. Natl. Cancer Inst. 102, 179–186.
Laitinen, L., Takala, E., Vuorela, H., Vuorela, P., Kaukonen, A. M., and Marvola, M. (2007). Anthranoid laxative influence the absorption of poorly permeable drugs in human intestinal cell culture model (Caco-2). Eur. J. Pharm. Biopharm. 66, 135–145.
Lala, L. G., D’Mello, P. M., and Naik, S. R. (2004). Pharmacokinetic and pharmacodynamic studies on interaction of “Trikatu” with diclofenac sodium. J. Ethnopharmacol. 91, 277–280.
Lange, D. (2000). Conservation and Sustainable use of Adonis Vernalis, a Medicinal Plant in International Trade. Bonn: Federal Agency for Nature Conservation, 88.
Larsen, A. K., Escargueil, A. E., and Skladanowski, A. (2000). Resistance mechanisms associated with altered intracellular distribution of anticancer agents. Pharmacol. Ther. 85, 217–229.
Lau, W. C., Welch, T. D., Shields, T., Rubenfire, M., Tantry, U. S., and Gurbel, P. A. (2011). The effect of St John’s wort on the pharmacodynamic response of clopidogrel in hyporesponsive volunteers and patients: increased platelet inhibition by enhancement of CYP3A4 metabolic activity. J. Cardiovasc. Pharmacol. 57, 86–93.
Leclercq, I. A., Farrell, G. C., Field, J., Bell, D. R., Gonzalez, F. J., and Robertson, G. R. (2000). CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J. Clin. Invest. 105, 1067–1075.
Lee, J. Y., Duke, R. K., Tran, V. H., Hook, J. M., and Duke, C. C. (2006). Hyperforin and its analogues inhibit CYP3A4 enzyme activity. Phytochemistry 67, 2550–6250.
Lewis, J. H., Ahmed, M., Shobassy, A., and Palese, C. (2006). Drug-induced liver disease. Curr. Opin. Gastroenterol. 22, 223–233.
Li, M., Andrew, M. A., Wang, J., Salinger, D. H., Vicini, P., Grady, R. W., Phillips, B., Shen, D. D., and Anderson, G. D. (2009). Effects of cranberry juice on pharmacokinetics of beta-lactam antibiotics following oral administration. Antimicrob. Agents Chemother. 53, 2725–2732.
Liang, Y., Xie, P., and Chan, K. (2004). Quality control of herbal medicines. J. Chromatogr. 812, 53–70.
Liao, H., Ma, T., Li, Y., Chen, J. T., and Chang, Y. S. (2010). Concurrent use of corticosteroids with Licorice-containing TCM preparations in Taiwan: a National Health Insurance Database Study. J. Altern. Complement. Med. 16, 539–544.
Liu, C. X., Yi, X. L., Si, D. Y., Xiao, X. F., He, X., and Li, Y. Z. (2011). Herb-drug interactions involving drug metabolizing enzymes and transporters. Curr. Drug Metab. 12, 835–849.
Luyckx, V. A., and Naicker, S. (2008). Acute kidney injury associated with the use of traditional medicines. Nat. Clin. Pract. Nephrol. 4, 664–671.
Ma, X. H., Zheng, C. J., Han, L. Y., Xie, B., Jia, J., Cao, Z. W., Li, Y. X., and Chen, Y. Z. (2009). Synergistic therapeutic actions of herbal ingredients and their mechanisms from molecular interaction and network perspectives. Drug Discov. Today 14, 579–588.
Madabushi, R., Frank, B., Drewelow, B., Derendorf, H., and Butterweck, V. (2006). Hyperforin in St. John’s wort drug interactions. Eur. J. Clin. Pharmacol. 62, 225–233.
Malati, C. Y., Robertson, S. M., Hunt, J. D., Chairez, C., Alfaro, R. M., Kovacs, J. A., and Penzak, S. R. (2011). Influence of Panax ginseng on cytochrome P450 (CYP)3A and P-glycoprotein (P-gp) activity in healthy participants. J. Clin. Pharmacol. doi:10.1177/0091270011407194. [Epub ahead of print].
Mannel, M. (2004). Drug interactions with St John’s wort: mechanisms and clinical implications. Drug Saf. 27, 773–797.
Marchetti, S., Mazzanti, R., and Beijnen, J. H. (2007). Concise review: clinical relevance of drug–drug and herb–drug interactions mediated by the ABC transporter ABCB1 (MDR1, P-glycoprotein). Oncologist 12, 927–941.
Maridass, M., and De Britto, A. J. (2008). Origins of plant derived medicines. Ethnobotanical Leaflets 12, 373–387.
Markell, M. S. (2010). “Herbal remedies and the patient with chronic kidney disease,” in Herbal Supplements: Efficacy, Toxicity, Interactions with Western Drugs, and Effects on Clinical Laboratory Tests. doi:10.1002/9780470910108.ch6
Markowitz, J. S., DeVane, C. L., Chavin, K. D., Taylor, R. M., Ruan, Y., and Donovan, J. L. (2003a). Effects of garlic (Allium sativun L) supplementation on cytochrome P450 2D6 and 3A4 activity in healthy volunteers. Clin. Pharmacol. Ther. 74, 170–177.
Markowitz, J. S., Donovan, J. L., DeVane, C. L., Taylor, R. M., Ruan, Y., Wang, J. S., and Chavin, K. D. (2003b). Effect of St John’s wort on drug metabolism by induction of cytochrome P450 3A4 Enzyme. JAMA 290, 1500–1504.
Martignoni, M., Groothuis, G. M. M., and de Kanter, R. (2006). Species differences between mouse, rat, dog, monkey and human CYP-mediated drug metabolism, inhibition and induction. Expert Opin. Drug Metab. Toxicol. 2, 875–894.
Marx, J., Pretorius, E., Espag, W. J., and Bester, M. J. (2005). Urginea sanguinea: medicinal wonder or death in disguise? Environ. Toxicol. Pharmacol. 20, 26–34.
Marzolini, C., Paus, E., Buclin, T., and Kim, R. B. (2004). Polymorphisms in human MDR1 (P-glycoprotein): recent advances and clinical relevance. Clin. Pharmacol. Ther. 75, 13–33.
Matsumura, T., Arai, M., Yonemitsu, Y., Maruoka, D., Tanaka, T., Suzuki, T., Yoshikawa, M., Imazeki, F., and Yokosuka, O. (2010). The traditional Japanese medicine rikkunshito increases the plasma level of ghrelin in humans and mice. J. Gastroenterol. 45, 300–307.
Meijerman, I., Beijnen, J. H., and Schellens, J. H. M. (2006). Herb-drug interactions in oncology: focus on mechanisms of induction. Oncologist 11, 742–752.
Merino, G., Perez, M., Real, R., Egidom, E., Prieto, J. G., and Alvarez, A. I. (2010). In vivo inhibition of BCRP/ABCG2 mediated transport of nitrofurantoin by the isoflavones genistein and daidzein: a comparative study in BCRP1 mice. Pharm. Res. 27, 2098–2105.
Methlie, P., Husebye, E., Hustad, S. S., Lien, E. A., and Løvås, K. (2011). Grapefruit juice and liquorice increase cortisol availability in patients with Addison’s disease. Eur. J. Endocrinol. 165, 761–769.
Mitra, A. (2007). Anti-diabetic uses of some common herbs in tribal belts of Midnapur (West) district of Bengal. EthnoMed 1, 37–45.
Mochiki, E., Yanai, M., Ohno, T., and Kuwano, H. (2010). The effect of traditional Japanese medicine (Kampo) on gastrointestinal function. Surg. Today 40, 1105–1111.
Mohamed, M. E., and Frye, R. F. (2010). Inhibition of intestinal and hepatic glucuronidation of mycophenolic acid by Ginkgo biloba extract and flavonoids. Drug Metab. Dispos. 38, 270–275.
Mohamed, M. E., and Frye, R. F. (2011a). Effects of herbal supplements on drug glucuronidation. Review of clinical, animal, and in vitro studies. Planta Med. 77, 311–321.
Mohamed, M. E., and Frye, R. F. (2011b). Inhibitory effects of commonly used herbal extracts on UDP-glucuronosyltransferase 1A4, 1A6, and 1A9 enzyme activities. Drug Metab. Dispos. 39, 1522–1528.
Mohammed, A. M. I., Jiang, X., Williams, K. M., Day, R. O., Roufogalis, B. D., Liauw, W. S., Xu, H., and McLachlan, A. J. (2008). Pharmacodynamic interaction of warfarin with cranberry but not with garlic in healthy subjects. Br. J. Pharmacol. 154, 1691–1700.
Mok, D. K. W., and Chau, F. (2006). Chemical information of Chinese medicines: a challenge to chemist. Chemometrics Intell. Lab. Syst. 82, 210–217.
Moorthy, R., Prabhu, K. M., and Murthy, P. S. (2010). Mechanism of anti-diabetic action, efficacy and safety profile of GII purified from fenugreek (Trigonella foenum-graceum Linn.) seeds in diabetic animals. Indian J. Exp. Biol. 48, 1119–1122.
Mu, Y., Zhang, J., Zhang, S., Zhou, H. H., Toma, D., Ren, S., Huang, L., Yaramus, M., Baum, A., Venkataramanan, R., and Xie, W. (2006). Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. J. Pharmacol. Exp. Ther. 316, 1369–1377.
Mukhtar, H. M., Ansari, S. H., Bhat, Z. A., and Naved, T. (2006). Antihyperglycemic activity of Cyamopsis tetragonoloba. Beans on blood glucose levels in alloxan-induced diabetic rats. Pharm. Biol. 44, 10–13.
Munday, R., and Munday, C. M. (1999). Low doses of diallyl disulfide, a compound derived from garlic, increase tissue activities of quinone reductase and glutathione transferase in the gastrointestinal tract of the rat. Nutr. Cancer 34, 42–48.
Murakami, Y., Tanaka, T., Murakami, H., Tsujimoto, M., Ohtani, H., and Sawada, Y. (2006). Pharmacokinetic modelling of the interaction between St John’s wort and ciclosporin A. Br. J. Clin. Pharmacol. 61, 671–676.
Nabekura, T., Yamaki, T., Hiroi, T., Ueno, K., and Kitagawa, S. (2010). Inhibition of anticancer drug efflux transporter P-glycoprotein by rosemary phytochemicals. Pharmacol. Res. 61, 259–263.
Nagai, M., Fukamachi, T., Tsujimoto, M., Ogura, K., Hiratsuka, A., Ohtani, H., Hori, S., and Sawada, Y. (2009). Inhibitory effects of herbal extracts on the activity of human sulfotransferase isoform sulfotransferase 1A3 (SULT1A3). Biol. Pharm. Bull. 32, 105–159.
Nakagawa, N., Katoh, M., Yoshioka, Y., Nakajima, M., and Yokoi, T. (2009). Inhibitory effects of Kampo medicine on human UGT2B7 activity. Drug Metab. Pharmacokinet. 24, 490–499.
Nebert, D. W., and Russell, D. W. (2002). Clinical importance of the cytochrome P450. Lancet 360, 1155–1162.
Ni, W., Zhang, X., Wang, B., Chen, Y., Han, H., Fan, Y., Zhou, Y., and Tai, G. (2010). Antitumor activities and immunomodulatory effects of ginseng neutral polysaccharides in combination with 5-fluorouracil. J. Med. Food 13, 270–277.
Nieminen, T. H., Hagelberg, N. M., Saari, T. I., Neuvonen, M., Laine, K., Neuvonen, P. J., and Olkkola, K. T. (2010). St John’s wort greatly reduces the concentrations of oral oxycodone. Eur. J. Pain 14, 854–859.
Nishikawa, M., Ariyoshi, N., Kotani, A., Ishii, I., Nakamura, H., Nakasa, H., Ida, M., Nakamura, H., Kimura, N., Kimura, M., Hasegawa, A., Kusu, F., Ohmori, S., Nakazawa, K., and Kitada, M. (2004). Effects of continuous ingestion of green tea or grape seed extracts on the pharmacokinetics of midazolam. Drug Metab. Pharmacokinet. 19, 280–289.
Nivitabishekam, S. N., Asad, M., and Prasad, V. S. (2009). Pharmacodynamic interaction of Momordica charantia with rosiglitazone in rats. Chem. Biol. Interact. 177, 247–253.
Norlin, M., and Wikvall, K. (2007). Enzymes in the conversion of cholesterol into bile acids. Curr. Mol. Med. 7, 199–218.
Nowack, R. (2008). Review article: cytochrome P450 enzyme, and transport protein mediated herb-drug interactions in renal transplant patients: grapefruit juice, St John’s wort – and beyond! Nephrology (Carlton) 13, 337–347.
Nutescu, E. A., Shapiro, N. L., Ibrahim, S., and West, P. (2006). Warfarin and its interactions with foods, herbs and other dietary supplements. Expert Opin. Drug Saf. 5, 433–451.
Ogbonnia, S., Adekunle, A. A., Bosa, M. K., and Enwuru, V. N. (2008). Evaluation of acute and subacute toxicity of Alstonia congensis Engler (Apocynaceae) bark and Xylopia aethiopica (Dunal) A. Rich (Annonaceae) fruits mixtures used in the treatment of diabetes. Afr. J. Biotechnol. 7, 701–705.
Okada, K., Shoda, J., Kano, M., Suzuki, S., Ohtake, N., Yamamoto, M., Takahashi, H., Utsunomiya, H., Oda, K., Sato, K., Watanabe, A., Ishii, T., Itoh, K., Yamamoto, M., Yokoi, T., Yoshizato, K., Sugiyama, Y., and Suzuki, H. (2007). Inchinkoto, a herbal medicine and its ingredients dually exert Mrp2/MRP2-mediated choleresis and Nrf2-mediated antioxidative action in rat liver. Am. J. Physiol. 292, G1450–G1463.
Oluwatuyi, M., Kaatz, G. W., and Gibbons, S. (2004). Antibacterial and resistance modifying activity of Rosmarinus officinalis. Phytochemistry 65, 3249–3254.
Ono, A. E., Owo, O. I., Itam, E. H., and Konya, R. S. (2000). Blood pressure depression by the fruit juice of Carica papaya (L.) in renal and DOCA-induced hypertension in the rat. Phytother. Res. 14, 235–239.
Ono, S., Hatanaka, T., Hotta, H., Satoh, T., Gonzalez, F. J., and Tsutsui, M. (1996). Specificity of substrate and inhibitor probes for cytochrome P450s: evaluation of in vitro metabolism using cDNA-expressed human P450 and human liver microsomes. Xenobiotica 26, 681–693.
Ozcakir, A., Sadikoglu, G., Bayram, N., Mazicioglu, M. M., Bilgel, N., and Beyhan, I. (2007). Turkish general practitioners and complementary/alternative medicine. J. Altern. Complement. Med. 13, 1007–1010.
Paine, M. F., Widmer, W. W., Pusek, S. N., Beavers, K. L., Criss, A. B., Snyder, J., and Watkins, P. B. (2008). Further characterization of a furanocoumarin-free grapefruit juice on drug disposition: studies with cyclosporine. Am. J. Clin. Nutr. 87, 863–871.
Pal, D., and Mitra, A. K. (2006). MDR- and CYP3A4-mediated drug–herbal interactions. Life Sci. 78, 2131–2145.
Palombo, E. A. (2006). Phytochemicals from traditional medicinal plants used in the treatment of diarrhoea: modes of action and effects on intestinal function. Phytother. Res. 20, 717–724.
Patanasethanont, D., Nagai, J., Yumoto, R., Murakami, T., Sutthanut, K., Sripanidkulchai, B. O., Yenjai, C., and Takano, M. (2007). Effects of Kaempferia parviflora extracts and their flavone constituents on P-glycoprotein function. J. Pharm. Sci. 96, 223–233.
Patel, M., Bessong, P., and Liu, H. (2011). Traditional medicines, HIV, and related infections: workshop 2C. Adv. Dent. Res. 23, 159–164.
Patsalos, P., and Perucca, E. (2003). Clinically important drug interactions in epilepsy: interactions between antiepileptic drugs and other drugs. Lancet 2, 4734–4781.
Pavek, P., and Dvorak, Z. (2008). Xenobiotic-induced transcriptional regulation of xenobiotic metabolizing enzymes of the cytochrome P450 superfamily in human extrahepatic tissues. Curr. Drug Metab. 9, 129–143.
Perucca, E. (2006). Clinically relevant drug interactions with antiepileptic drugs. Br. J. Clin. Pharmacol. 61, 246–255.
Pierard, S., Coche, J. C., Lanthier, P., Dekoninck, X., Lanthier, N., Rahier, J., and Geubel, A. P. (2009). Severe hepatitis associated with the use of black cohosh: a report of two cases and an advice for caution. Eur. J. Gastroenterol. Hepatol. 21, 941–945.
Piscitelli, S. C., Burstein, A. H., Welden, N., Gallicano, K. D., and Falloon, J. (2002). The effect of garlic supplements on the pharmacokinetics of saquinavir. Clin. Infect. Dis. 34, 234–238.
Qi, Q. H., Wang, J., Liang, G. G., and Wu, X. Z. (2007). Da-Cheng-Qi-Tang promotes the recovery of gastrointestinal motility after abdominal surgery in humans. Dig. Dis. Sci. 52, 1562–1570.
Quintieri, L., Palatini, P., Nassi, A., Ruzza, P., and Floreani, M. (2008). Flavonoids diosmetin and luteolin inhibit midazolam metabolism by human liver microsomes and recombinant CYP3A4 and CYP3A5 enzymes. Biochem. Pharmacol. 75, 1426–1437.
Rannug, U., Agurell, E., Rannug, A., and Cederberg, H. (2006). Certain tryptophan photoproducts are inhibitors of cytochrome P450-dependent mutagenicity. Environ. Mol. Mutagen. 20, 289–296.
Roberts, D., and Flanagan, P. (2011). Case report: cranberry juice and warfarin. Home Healthc. Nurse 29, 92–97.
Roby, C. A., Anderson, G., Kantor, E., Dryer, D. A., and Burstein, A. H. (2000). St John’s wort: effect on CYP3A4 activity. Clin. Pharmacol. Ther. 67, 451–457.
Rogers, K. L., Grice, I. D., and Griffiths, L. R. (2000). Inhibition of platelet aggregation and 5-HT release by extracts of Australian plants used traditionally as headache treatments. Eur. J. Pharm. Sci. 9, 355–363.
Rostami-Hodjegan, A., and Tucker, G. T. (2007). Simulation and prediction of in vivo drug metabolism in human populations from in vitro data. Nat. Rev. Drug Discov. 6, 140–148.
Routledge, P. A. (2008). The European herbal medicines directive: could it have saved the lives of Romeo and Juliet? Drug Saf. 31, 416–418.
Saleem, T. S. M., Chetty, C. M., Ramkanth, S., Rajan, V. S. T., Kumar, K. M., and Gauthaman, K. (2010). Hepatoprotective herbs – a review. Int. J. Res. Pharm. Sci. 1, 1–5.
Sanderson, J. T., Hordijk, J., Denison, M. S., Springsteel, M. F., Nantz, M. H., and van den Berg, M. (2004). Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cell. Toxicol. Sci. 82, 70–79.
Sarris, J., LaPorte, E., and Schweitzer, I. (2011). Kava: a comprehensive review of efficacy, safety, and psychopharmacology. Aust. N. Z. J. Psychiatry 45, 27–35.
Savvidou, S., Goulis, J., Giavazis, I., Patsiaoura, K., Hytiroglou, P., and Arvanitakis, C. (2007). Herb-induced hepatitis by Teucrium polium L.: report of two cases and review of the literature. Eur. J. Gastroenterol. Hepatol. 19, 507–511.
Saxena, A. K., and Panbotra, B. R. (2003). Herbal remedies: renal tragedies. Swiss Med. Wkly. 133, 188–189.
Schoner, W. (2000). Ouabain, a new steroid hormone of adrenal gland and hypothalamus. Exp. Clin. Endocrinol. Diabetes. 108, 449–454.
Scott, G. N., and Elmer, G. W. (2002). Update on natural product – drug interactions. Am. J. Health Syst. Pharm. 59, 339–347.
Scott, M., Dinehart, S. M., and Henry, L. (2005). Dietary supplements: altered coagulation and effects on bruising. Dermatol. Surg. 31, 819–836.
Sevior, D. K., Hokkanen, J., Tolonen, A., Abass, K., Tursas, L., Pelkonen, O., and Ahokas, J. T. (2010). Rapid screening of commercially available herbal products for the inhibition of major human hepatic cytochrome P450 enzymes using the N-in-one cocktail. Xenobiotica 40, 2452–2454.
Sheeja, K., Shihab, P. K., and Kuttan, G. (2006). Antioxidant and anti-inflammatory activities of the plant Andrographis paniculata Nees. Immunopharmacol. Immunotoxicol. 28, 129–140.
Shekelle, P. G., Hardy, M. L., Morton, S. C., Maglione, M., Mojica, W. A., Suttorp, M. J., Rhodes, S. L., Jungvig, L., and Gagné, J. (2003). Efficacy and safety of ephedra and ephedrine for weight loss and athletic performance. JAMA 289, 1537–1545.
Sheweita, S. A., Newairy, A. A., Mansour, H. A., and Yousef, M. I. (2002). Effect of some hypoglycemic herbs on the activity of phase I and II drug-metabolizing enzymes in alloxan-induced diabetic rats. Toxicology 174, 131–139.
Shi, Z., He, J., Yao, T., Chang, W., and Zhao, M. (2005). Simultaneous determination of cryptotanshinone, tanshinone I and tanshinone IIA in traditional Chinese medicinal preparations containing Radix Salvia miltiorrhiza by HPLC. J. Pharm. Biomed. Anal. 37, 481–486.
Shukla, S., Zaher, H., Hartz, A., Bauer, B., Ware, J. A., and Ambudkar, S. V. (2009). Curcumin inhibits the activity of ABCG2/BCRP1, a multidrug resistance-linked ABC drug transporter in mice. Pharm. Res. 26, 480–487.
Singh, D., Kashyap, A., Pandey, R. V., and Saini, K. S. (2011). Novel advances in cytochrome P450 research. Drug Discov. Today 16, 793–799.
Sousa, S. A., Pascoa, H., Conceição, E. C., Alves, S. F., Diniz, D. G. A., Paula, J. R., and Bara, M. T. F. (2011). Dissolution test of herbal medicines containing Paullinia cupana: validation of methods for quantification and assessment of dissolution. Braz. J. Pharm. Sci. 47, 269–277.
Spinella, M., and Eaton, L. A. (2002). Hypomania induced by herbal and pharmaceutical psychotropic medicines following mild traumatic brain injury. Brain Inj. 16, 359–367.
Stadlbauer, V., Fickert, P., Lackner, C., Schmerlaib, J., Krisper, P., Trauner, M., and Stauber, R. E. (2005). Hepatotoxicity of NONI juice: report of two cases. World J. Gastroenterol. 11, 4758–6470.
Steenkamp, V., and Stewart, M. (2005). Nephrotoxicity associated with exposure to plant toxins, with particular reference to Africa. Ther. Drug Monit. 27, 270–277.
Stewart, M. J., Steenkamp, V., van der Merwe, S., Zuckerman, M., and Crowther, N. J. (2002). The cytotoxic effects of a traditional Zulu remedy, irnpila (Callilepis laureola). Hum. Exp. Toxicol. 21, 643–647.
Suzuki, Y., Ito, Y., Konno, C., and Furuya, T. (1991). Effects of tissue cultured ginseng on gastric secretion and pepsin activity. Yakugaku Zasshi 111, 770–774.
Szakács, G., Váradi, A., Özvegy-Laczka, C., and Sarkadi, B. (2008). The role of ABC transporters in drug absorption, distribution, metabolism, excretion and toxicity (ADME–Tox). Drug Discov. Today 13, 379–393.
Szolomicki, S., Samochowiec, L., Wójcicki, J., and Drozdzik, M. (2000). The influence of active components of Eleutherococcus senticosus on cellular defence and physical fitness in man. Phytother. Res. 14, 30–35.
Sztajnkrycer, M. D., Otten, E. J., Bond, G. R., Lindsell, C. J., and Goetz, R. J. (2008). Mitigation of pennyroyal oil hepatotoxicity in the mouse. Acad. Emerg. Med. 10, 1024–1028.
Tachjian, A., Maria, V., and Jahangir, A. (2010). Use of herbal products and potential interactions in patients with cardiovascular diseases. J. Am. Coll. Cardiol. 9, 515–525.
Taki, Y., Yokotani, K., Yamada, S., Shinozuka, K., Kubota, Y., Watanabe, Y., and Umegaki, K. (2012). Ginkgo biloba extract attenuates warfarin-mediated anticoagulation through induction of hepatic cytochrome P450 enzymes by bilobalide in mice. Phytomedicine 19, 177–182.
Tam, S. W., Worcel, M., and Wyllie, M. (2001). Yohimbine – a clinical review. Pharmacol. Ther. 91, 215–243.
Tamaki, H., Satoh, H., Hori, S., Ohtani, H., and Sawada, Y. (2010). Inhibitory effects of herbal extracts on breast cancer resistance protein (BCRP) and structure-inhibitory potency relationship of isoflavonoids. Drug Metab. Pharmacokinet. 25, 170–179.
Tang, J., Song, X., Zhu, M., and Zhang, J. (2009). Study on the pharmacokinetic drug-drug interaction potential of Glycyrrhiza uralensis, a traditional Chinese medicine, with lidocaine in rats. Phytother. Res. 23, 603–607.
Tang, J. C., Zhang, J. N., Wu, Y. T., and Li, Z. X. (2006). Effect of the water extract and ethanol extract from traditional Chinese medicines Angelica sinensis (Oliv.) Diels, Ligusticum chuanxiong Hort and Rheum palmatum L. on rat liver cytochrome P450 activity. Phytother. Res. 20, 1046–1051.
Tannergren, C., Engman, H., Knutson, L., Hedeland, M., Bondesson, U., and Lennernäs, H. (2004). St John’s wort decreases the bioavailability of R- and S-verapamil through induction of the first-pass metabolism. Clin. Pharmacol. Ther. 75, 298–309.
Taylor, L. (2000). Plants Based Drugs and Medicines. Available at: http://www.rain-tree.com/plantdrugs.htm [accessed November 03, 2011].
Teschke, R. (2010). Kava hepatotoxicity: pathogenetic aspects and prospective considerations. Liver Int. 30, 1270–1279.
Tokita, Y., Yuzurihara, M., Sakaguchi, M., Satoh, K., and Kase, Y. (2007). The pharmacological effects of Daikenchuto, a traditional herbal medicine, on delayed gastrointestinal transit in rat postoperative ileus. J. Pharmacol. Sci. 104, 303–310.
Tripathi, U. N., and Chandra, D. (2010). Anti-hyperglycemic and anti-oxidative effect of aqueous extract of Momordica charantia pulp and Trigonella foenum graecum seed in alloxan-induced diabetic rats. Indian J. Biochem. Biophys. 47, 227–233.
Ulbricht, C., Chao, W., Costa, D., Rusie-Seamon, E., Weissner, W., and Woods, J. (2008). Clinical evidence of herb-drug interactions: a systematic review by the natural standard research collaboration. Curr. Drug Metab. 9, 1063–1120.
Umehara, K. I., and Camenisch, G. (2011). Novel in vitro-in vivo extrapolation (IVIVE) method to predict hepatic organ clearance in rat. Pharm. Res. 29, 603–617.
van den Bout-van den Beukel, C. J., Hamza, O. J., Moshi, M. J., Matee, M. I., Mikx, F., Burger, D. M., Koopmans, P. P., Verweij, P. E., Schoonen, W. G., and van der Ven, A. J. (2008). Evaluation of cytotoxic, genotoxic and CYP450 enzymatic competition effects of Tanzanian plant extracts traditionally used for treatment of fungal infections. Basic Clin. Pharmacol. Toxicol. 102, 515–526.
Van Roon, E. N., Flikweert, S., le Comte, M., Langendijk, P. N., Kwee-Zuiderwijk, W. J., Smits, P., and Brouwers, J. R. (2005). Clinical relevance of drug-drug interactions – a structured assessment procedure. Drug Saf. 28, 1131–1139.
Van Wyk, B., Van Oudtshoorn, B., and Gericke, N. (2009). Medicinal Plants of South Africa, 2nd Edn. Pretoria: Briza Publications, 336.
Vlachojannis, J., Cameron, M., and Chrubasik, S. (2011). Drug interactions with St. John’s wort products. Pharmacol. Res. 63, 254–256.
Waako, P. J., Smith, P., and Folb, P. I. (2005). In vitro interactions of Aspilia africana (Pers.) C. D. Adams, a traditional antimalarial medicinal plant, with artemisinin against Plasmodium falciparum. J. Ethnopharmacol. 102, 262–268.
Wang, J. F., and Chou, K. C. (2010). Molecular modeling of cytochrome P450 and drug metabolism. Curr. Drug Metab. 11, 342–346.
Wang, J. F., Wei, D. Q., and Chou, K. C. (2008). Drug candidates from traditional Chinese medicines. Curr. Top. Med. Chem. 8, 1656–6165.
Wang, Y., Shi, B., Cheng, Y., Xu, J., Jiang, C. F., and Xie, W. F. (2009). Drug-induced liver disease: an 8-year study of patients from one gastroenterological department. J. Dig. Dis. 10, 195–200.
Wilasrusmee, C., Kittur, S., Siddiqui, J., Bruch, D., Wilasrusmee, S., and Kittur, D. S. (2002). In vitro mmunomodulatory effects of ten commonly used herbs on murine lymphocytes. J. Altern. Complement. Med. 8, 467–475.
Wills, R. B. H., Bone, K., and Morgan, M. (2000). Herbal products: active constituents, modes of action and quality control. Nutr. Res. Rev. 13, 47–77.
Wojcikowski, K., Wohlmuth, H., Johnson, D. W., Rolfe, M., and Gobe, G. (2009). An in vitro investigation of herbs traditionally used for kidney and urinary system disorders: potential therapeutic and toxic effects. Nephrology (Carlton) 14, 70–79.
Wolf-Hall, C. (2010). “Fungal and mushroom toxins,” in Pathogens and Toxins in Food: Challenges and Interventions, eds V. K. Juneja, and J. N. Sofos (Washington, DC: ASM Press), 275–285.
Wood, M. J., Stewart, R. L., Merry, H., Johnstone, D. E., and Cox, J. L. (2003). Use of complementary and alternative medical therapies in patients with cardiovascular disease. Am. Heart J. 145, 806–812.
Wu, C. L., Chiu, P. F., Yang, Y., Wen, Y. K., Chiu, C. C., and Chang, C. C. (2011). Sustained low-efficiency daily diafiltration with hemoperfusion as a therapy for severe star fruit intoxication: a report of two cases. Ren. Fail. 33, 837–841.
Wu, W. W., and Yeung, J. H. (2010). Inhibition of warfarin hydroxylation by major tanshinones of Danshen (Salvia miltiorrhiza) in the rat in vitro and in vivo. Phytomedicine 17, 219–226.
Xu, L., Chen, Y., Pan, Y., Skiles, G. L., and Shou, M. (2009). Prediction of human drug-drug interactions from time-dependent inactivation of CYP3A4 in primary hepatocytes using a population-based simulator. Drug Metab. Dispos. 37, 2330–2339.
Yagmur, E., Piatkowski, A., Groger, A., Pallua, N., Gressner, A. M., and Kiefer, P. (2005). Bleeding complication under Gingko biloba medication. Am. J. Hematol. 79, 343–344.
Yao, W. X., and Jiang, M. X. (2002). Effects of tetrandrine on cardiovascular electrophysiologic properties. Acta Pharmacol. Sin. 23, 1069–1074.
Yap, K. Y., See, C. S., and Chan, A. (2010). Clinically-relevant chemotherapy interactions with complementary and alternative medicines in patients with cancer. Recent Pat. Food Nutr. Agric. 2, 12–55.
Yin, O. Q. P., Tomlinson, B., and Waye, M. M. Y. (2004). Pharmacogenetics and herb-drug interactions: experience with Ginkgo biloba and omeprazole. Pharmacogenetics 14, 841–850.
Young, H. Y., Liao, J. C., Chang, Y. S., Luo, Y. L., Lu, M. C., and Peng, W. H. (2006). Synergistic effect of ginger and nifedipine on human platelet aggregation: a study in hypertensive patients and normal volunteers. Am. J. Chin. Med. 34, 545–551.
Yu, C. P., Wu, P. P., Hou, Y. C., Lin, S. P., Tsai, S. Y., Chen, C. T., and Chao, P. D. (2011). Quercetin and rutin reduced the bioavailability of cyclosporine from Neoral, an immunosuppressant, through activating P-glycoprotein and CYP 3A4. J. Agric. Food Chem. 59, 4644–4648.
Zhang, X. (2002). Effect on gastrointestinal functions of Polygonum paleaceum. Zhong Yao Cai 25, 192–193.
Zhang, Z., and Wong, Y. N. (2005). Enzyme kinetics for clinically relevant CYP inhibition. Curr. Drug Metab. 6, 241–257.
Zhou, S., Lim, L. Y., and Chowbay, B. (2004). Herbal modulation of P-glycoprotein. Drug Metab. Rev. 36, 57–104.
Zhou, S. F. (2008). Drugs behave as substrates, inhibitors and inducers of human cytochrome P450 3A4. Curr. Drug Metab. 9, 310–322.
Zhu, B., Sun, Y., Yun, X., Han, S., Piao, M. L., Murata, Y., and Tada, M. (2004). Resistance imparted by traditional Chinese medicines to the acute change in glutamic pyruvic transaminase, alkaline phosphatase and creatine kinase activities in rat blood caused by blood. Biosci. Biotechnol. Biochem. 68, 1160–1163.
Keywords: Herb–drug interaction, traditional medicine, phytochemicals, transport proteins, cytochrome P450
Citation: Fasinu PS, Bouic PJ and Rosenkranz B (2012) An overview of the evidence and mechanisms of herb–drug interactions. Front. Pharmacol. 3:69. doi: 10.3389/fphar.2012.00069
Received: 20 December 2011; Accepted: 05 April 2012;
Published online: 30 April 2012.
Edited by:
Javed S. Shaikh, Cardiff Research Consortium: A CAPITA Group Plc Company, IndiaReviewed by:
Sirajudheen Anwar, University of Messina, ItalyDomenico Criscuolo, Genovax, Italy
Roger Verbeeck, Université Catholique de Louvain, Belgium
Copyright: © 2012 Fasinu, Bouic and Rosenkranz. This is an open-access article distributed under the terms of the Creative Commons Attribution Non Commercial License, which permits non-commercial use, distribution, and reproduction in other forums, provided the original authors and source are credited.
*Correspondence: Bernd Rosenkranz, Division of Pharmacology, Department of Medicine, University of Stellenbosch, PO Box 19063, Tygerberg, Cape Town 7505, South Africa. e-mail:cm9zZW5rcmFuekBzdW4uYWMuemE=

 Patrick J. Bouic2,3
Patrick J. Bouic2,3