
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 04 April 2011
Sec. Neuropharmacology
volume 2 - 2011 | https://doi.org/10.3389/fphar.2011.00018
This article is part of the Research Topic Ethanol and GABAA receptors - New Insights View all 7 articles
 Sangeetha V. Iyer1
Sangeetha V. Iyer1 Rodrigo A. Benavides2
Rodrigo A. Benavides2 Dev Chandra1,2
Dev Chandra1,2 James M. Cook3
James M. Cook3 Sundari Rallapalli3
Sundari Rallapalli3 Harry L. June4
Harry L. June4 Gregg E. Homanics1,2*
Gregg E. Homanics1,2*
Alcohol (ethanol) is widely consumed for its desirable effects but unfortunately has strong addiction potential. Some imidazobenzodiazepines such as Ro15-4513 are able to antagonize many ethanol-induced behaviors. Controversial biochemical and pharmacological evidence suggest that the effects of these ethanol antagonists and ethanol are mediated specifically via overlapping binding sites on α4/δ-containing GABAA-Rs. To investigate the requirement of α4-containing GABAA-Rs in the mechanism of action of Ro15-4513 on behavior, wildtype (WT) and α4 knockout (KO) mice were compared for antagonism of ethanol-induced motor incoordination and hypnosis. Motor effects of ethanol were tested in two different fixed speed rotarod assays. In the first experiment, mice were injected with 2.0 g/kg ethanol followed 5 min later by 10 mg/kg Ro15-4513 (or vehicle) and tested on a rotarod at 8 rpm. In the second experiment, mice received a single injection of 1.5 g/kg ethanol ± 3 mg/kg Ro15-4513 and were tested on a rotarod at 12 rpm. In both experiments, the robust Ro15-4513 antagonism of ethanol-induced motor ataxia that was observed in WT mice was absent in KO mice. A loss of righting reflex (LORR) assay was used to test Ro15-4513 (20 mg/kg) antagonism of ethanol (3.5 g/kg)-induced hypnosis. An effect of sex was observed on the LORR assay, so males and females were analyzed separately. In male mice, Ro15-4513 markedly reduced ethanol-induced LORR in WT controls, but α4 KO mice were insensitive to this effect of Ro15-4513. In contrast, female KO mice did not differ from WT controls in the antagonistic effects of Ro15-4513 on ethanol-induced LORR. We conclude that Ro15-4513 requires α4-containing receptors for antagonism of ethanol-induced LORR (in males) and motor ataxia.
The molecular mechanisms by which alcohol (ethanol) exerts its effects on the brain largely remain an enigma. Understanding the mechanisms of action of this widely used drug is essential for the rational design of therapeutic interventions to combat alcohol use disorders. Although ethanol is consumed for its many pleasurable effects, the adverse effects of ethanol consumption are staggering. Alcohol use disorders in the United States alone annually are responsible for 105,000 deaths (McGinnis and Foege, 1999) and cost society at least $185 billion. The personal costs to those affected are incalculable.
γ-Aminobutyric acid (GABA) type A receptors (GABAA-Rs) have long been implicated as key molecular targets of ethanol because drugs that are known to act via GABAA-Rs (i.e., benzodiazepines, barbiturates) produce behavioral effects that are similar to ethanol intoxication (e.g., Hu et al., 1987). Tolerance to these GABAergic agents also produces cross tolerance to ethanol (e.g., Khanna et al., 1998). Furthermore, a few select imidazobenzodiazepines (which specifically target GABAA-Rs) are effective ethanol antagonists (e.g., Suzdak et al., 1986). However, despite overwhelming evidence that GABAA-Rs are primary targets of ethanol action, progress toward elucidation of the specific GABAA-R isoforms that mediate ethanol and ethanol antagonist action has been challenging and plagued by controversy (Lovinger and Homanics, 2007). Definitive elucidation of the targets of ethanol and ethanol antagonists has the potential to dramatically impact the search for effective treatments for alcohol abuse and alcoholism.
The development of specific ethanol antagonists has been the focus of much research since the discovery of Ro15-4513 – an imidazobenzodiazepine drug synthesized by Hoffmann-La Roche & Co. Ro15-4513 acts as an ethanol antagonist both in biochemical assays (Kolata, 1986; Suzdak et al., 1986) and behavioral studies (Lister and Nutt, 1988; Suzdak et al., 1988; Samson et al., 1989; Becker and Hale, 1991). Behaviorally, Ro15-4513 antagonizes the sedative, motor impairing, anxiolytic, amnestic, and anticonvulsant effects of ethanol, but not the hypothermic or lethal effects (For reviews, see Wallner et al., 2006a; Wallner and Olsen, 2008). Ro15-4513 has minimal intrinsic effects at doses that antagonize ethanol action (Bonetti et al., 1988; Glowa et al., 1988). In spite of Ro15-4513’s clear behavioral effects, the molecular mechanism of action of Ro15-4513 has not been conclusively established. With the recent discovery of GABAA-Rs (α4/6β2/3δ) that are highly sensitive to sobriety impairing concentrations of ethanol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003), interest in the molecular mechanism of action of Ro15-4513 has been revived.
GABAA-R isoforms composed of α4/6β2/3δ are mired in controversy regarding their role in the mechanisms of action of both ethanol and the imidazobenzodiazepine Ro15-4513. While numerous studies have presented persuasive evidence that these receptor subtypes are very sensitive to potentiation by low concentrations of ethanol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003; Wei et al., 2004; Hanchar et al., 2005; Fleming et al., 2007; Mody et al., 2007; Olsen et al., 2007; Santhakumar et al., 2007), others have failed to observe this pharmacology (Borghese et al., 2006; Storustovu and Ebert, 2006; Yamashita et al., 2006; Casagrande et al., 2007). Similarly, it has been difficult to reach a consensus on the pharmacology of Ro15-4513 at these receptors. Until recently, it was generally accepted that the γ2 subunit was required for benzodiazepine binding. Remarkably, α4/α6βδ containing receptors were recently shown to bind Ro15-4513 with low nanomolar affinity (Hanchar et al., 2006) and Ro15-4513 was able to reverse the effects of ethanol on these receptors (Wallner et al., 2006b). Competitive binding studies revealed that flumazenil and β-carboline derivatives also displaced bound Ro15-4513 from α4δ-containing receptors (Hanchar et al., 2006). Furthermore, Ro15-4513 binding was also dose dependently inhibited by ethanol (Hanchar et al., 2006). The conclusion of these studies was that a common binding pocket exists on α4/α6βδ GABAA-Rs that mediates ethanol action and Ro15-4513 antagonism of ethanol action (Hanchar et al., 2006; Wallner et al., 2006b). However, despite the convincing nature of these studies, results of other studies have failed to support such a mechanism. Korpi et al. (2007) failed to detect high affinity Ro15-4513 binding to recombinant α4/α6δ containing receptors or to cerebellar sections containing native α6δ receptors. Mehta et al. (2007) failed to observe ethanol inhibition of Ro15-4513 binding to δ-containing GABAA-Rs from rat brain. Thus, the evidence that ethanol and Ro15-4513 compete for a binding site on α4/6δ GABAA-Rs is equivocal at present.
In summary, it is firmly established that imidazobenzodiazepines such as Ro15-4513 antagonize some of the behavioral effects of ethanol, but the mechanism of action of these drugs is highly controversial. Results of several electrophysiologic and pharmacologic binding studies suggest that ethanol and these ethanol antagonists compete for a common binding pocket on α4/α6δ containing GABAA-Rs (Hanchar et al., 2006; Wallner et al., 2006b). However, to date no studies investigating involvement of α4 receptors in Ro15-4513 antagonsim of ethanol action have examined this on the behavioral level. Therefore, to test the hypothesis that α4-containing receptors are required for Ro15-4513 antagonism of ethanol-induced behaviors, α4 wildtype (WT), and knockout (KO) mice (Chandra et al., 2006) were compared on ethanol-induced behavioral assays that are known to be antagonized by Ro15-4513. We previously demonstrated that moderate to high dose ethanol-induced behavioral responses were normal in the KO mice (Chandra et al., 2008) and at the cellular level, tonic current potentiation by Ro15-4513 was reduced in dentate gyrus granule neurons of α4 KO mice (Liang et al., 2008).
Homozygous α4 KO and WT littermate mice of the F3–4 generations were derived from mixed background (C57BL/6J and Strain 129S1/X1) heterozygous parents as described (Chandra et al., 2006). Mice were group housed, kept on an alternating 12 h light–dark cycle and allowed access to food and water ad libitum. Mice were genotyped by Southern Blot analysis of tail DNA (Chandra et al., 2006). All animals were between 8 and 16 weeks of age at the time of behavioral testing. All experiments were approved by the Institutional Animal Care and Use Committee at the University of Pittsburgh and were conducted in compliance with the Guide for the Care and Use of Laboratory Animals. Mice were tested for antagonism of ethanol induced behaviors by the imidazobenzodiazepine Ro15-4513 as described below. Control experiments with Ro15-4513 alone were not conducted.
Two separate experiments were performed using fixed speed rotarod assays. Ro15-4513 was a generous gift from Roche Biomedical Laboratories Inc. (Burlington, NC, USA). Ethanol used in all experiments was 200 proof absolute ethanol, ACS/USP grade obtained from Pharmco (Brookfield, CT, USA).
For the first rotarod experiment, mice were injected with ethanol (2 g/kg) 5 min prior to injection with Ro15-4513 (10 mg/kg) or vehicle (0.3% Tween-80 in saline). Ro15-4513 was freshly prepared in 0.3% Tween-80 in saline at 1 mg/ml on the morning of the experiment in BD Falcon round bottomed tubes. A uniform suspension was achieved by sonicating (Fisher Scientific Sonic Dismembrator Model 100) on ice. Test animals were transferred in their cages to a behavioral testing room ∼24 h before the experiment. Mice were weighed and subjected to training trials on a fixed speed rotarod (Ugo Basile 7650, Varese, Italy) at 8 rpm. Mice were given at least four training trials to achieve a performance of 100 s on the rotarod. Training trials were administered 15 min apart. No differences were observed between genotypes in training to baseline performance on these or previously published (Chandra et al., 2010) experiments. Following training, mice were housed overnight in the testing room. On the test day, mice were re-tested at least twice on the rotarod to ensure compliance with criteria. Mice that did not achieve the criterion of 100 s on two consecutive trials were excluded from the experiment (n = 1 WT and 1 KO). Following this, ethanol and Ro15-4513 injections were administered in a volume of 0.01 ml/g body weight. Mice were tested for rotarod performance at 15, 30, 45, and 60 min following injection.
The second rotarod experiment was similar to the first experiment with the following exceptions. Ro15-4513 (3 mg/kg) and ethanol (1.5 g/kg) were administered as a single i.p. injection in a volume of 0.01 ml/g body weight. Control treated animals received ethanol alone. Ro15-4513 was prepared directly in ethanol (1.57 mg/ml) by heating in a water bath with temperature not exceeding 65°C and intermittent vortexing. This stock solution was prepared on the day of the experiment in BD Falcon round bottomed tubes and was diluted with saline to a final concentration of 0.3 mg/ml Ro15-4513 in 15% ethanol immediately prior to injection. This method of preparation was adopted to enhance the bioavailability of Ro15-4513 (personal communication with Dr. Martin Wallner, UCLA). The chemical identity of Ro15-4513 was not confirmed following the solubilization procedure. To make the task sufficiently difficult for these doses, the mice were tested on a fixed speed rotarod at 12 rpm. All training and testing procedures were conducted similar to the first experiment. One WT animal did not perform to criteria on the test day and was therefore excluded.
Mice were injected i.p. with Ro15-4513 (20 mg/kg; 0.01 ml/g BW) or vehicle 15 min prior to i.p. injection with ethanol (3.5 g/kg; 0.02 ml/g BW). Ro15-4513 was prepared in 0.3% Tween-80 in saline as described in the first rotarod experiment. Upon losing their righting reflex, mice were placed in a supine position on V-shaped troughs. Mice were considered to have regained their righting reflex if they were successful in righting themselves three consecutive times within 30 s. A heat lamp was used during the course of the experiment to ensure normothermia.
All results are presented as mean ± SEM. For all experiments, data were analyzed for an effect of sex. Except where noted below, no effect of sex was observed and males and females were combined for further analysis. For the rotarod experiments, two different statistical analyses were conducted. First, performance on the rotarod at each timepoint was compared by two-way ANOVA followed by Fisher’s post hoc test where appropriate. Second, area under curves (AUC) were calculated for each genotype-treatment combination. AUCs were calculated as the area between baseline performance (100 s) and the performance curve of each genotype over the 60-min period. AUCs were compared by two-way ANOVA followed by Fisher’s post hoc test where appropriate. The loss of righting reflex (LORR) experiments were analyzed by two-way ANOVA and Fisher’s post hoc tests.
The ability of Ro15-4513 to antagonize ethanol-induced motor ataxia was initially tested on a fixed speed rotarod (8 rpm) following injection with 2 g/kg ethanol 5 min before injection with 10 mg/kg Ro15-4513 or vehicle. Rotarod performance and AUCs are shown in Figures 1A,B, respectively. Comparison of performance on the rotarod at the 15-min time point by two-way ANOVA revealed an effect of treatment (F1,41 = 5, p < 0.05), an interaction of treatment with genotype (F1,41 = 6, p < 0.05), and a trend for an effect of genotype alone (F1,41 = 3, p = 0.077). Post hoc analysis comparing the effect of Ro15-4513 vs vehicle within each genotype at the 15-min time point revealed that Ro15-4513 antagonized the effect of ethanol in WT mice (p = 0.01), but not in KO mice. Comparison of the performance on the rotarod at the 30-, 45-, and 60-min time points by two-way ANOVA did not reveal an effect of treatment, genotype, or interaction. These data were also analyzed using a more conservative statistical approach by comparing the AUCs. An overall ANOVA conducted on the AUCs indicated that the effect of genotype, treatment, and the interaction of genotype with treatment were not significant.
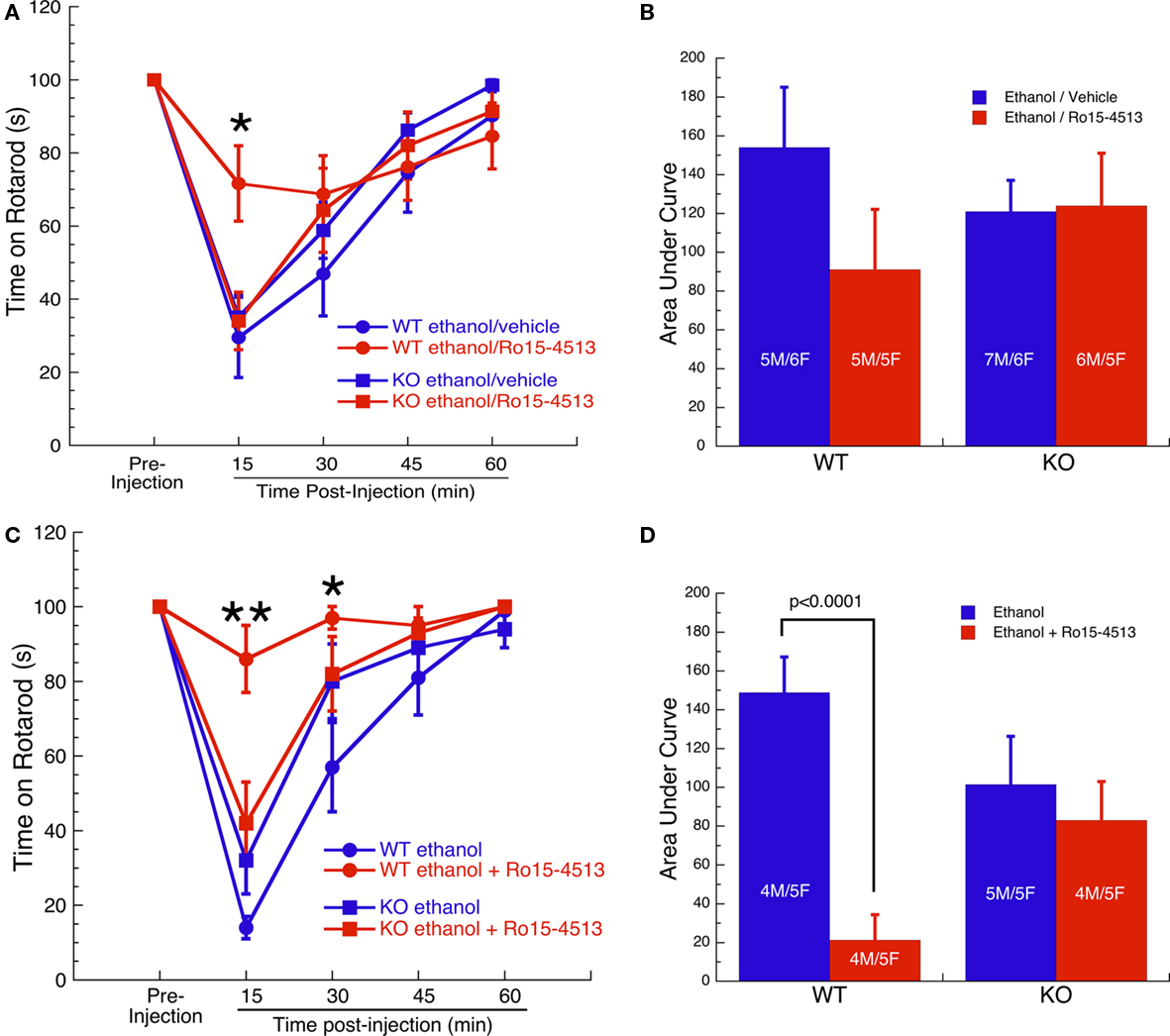
Figure 1. Ro15-4513 antagonism of ethanol-induced motor ataxia. (A) Performance of WT and GABAA-Rα4 subunit KO mice on fixed speed (8 rpm) rotarod following pretreatment with 2 g/kg ethanol 5 min prior to treatment with 10 mg/kg Ro15-4513 or vehicle.*p ≤ 0.01 – WT ethanol/vehicle vs WT ethanol/Ro15-4513. (B) AUCs of response curves in (A). Effect of genotype, treatment, and the interaction were not significant. (C) Performance on fixed speed (12 rpm) rotarod following treatment with 1.5 g/kg ethanol ±3 mg/kg Ro15-4513. *p < 0.005, **p < 0.0001 – WT ethanol vs WT ethanol + Ro15-4513. (D) AUCs of response curves in (C). WT and KO mice did not differ following injection of ethanol alone. Ro15-4513 antagonized ethanol-induced motor ataxia in WT, but not in KO mice. All values are expressed as mean ± SEM.
A second test of Ro15-4513 antagonism of ethanol-induced motor ataxia was conducted using a slightly different experimental protocol. The ability of Ro15-4513 to antagonize ethanol-induced motor ataxia was tested on a fixed speed rotarod (12 rpm) assay following a single injection of either 1.5 g/kg ethanol alone or the same dose of ethanol plus 3 mg/kg Ro15-4513. This was done to increase the bioavailability of Ro15-4513. Because a lower dose of ethanol was used, the speed of the rotarod had to be increased to make the task sufficiently difficult. Rotarod performance is shown in Figure 1C. Two-way ANOVA on the 15-min time point revealed a significant effect of treatment (F1,33 = 23, p < 0.0001), treatment by genotype interaction (F1,33 = 13, p ≤ 0.001), but no effect of genotype. Post hoc analysis of the 15-min time point within each genotype revealed that Ro15-4513 antagonized the effects of ethanol in WT mice (p < 0.0001) but not in KO mice. Two-way ANOVA on the 30-min time point revealed a significant effect of treatment (F1,33 = 5, p < 0.05), treatment by genotype interaction (F1,33 = 4, p ≤ 0.05), but no effect of genotype. Post hoc analysis of the 30-min time point within each genotype revealed that Ro15-4513 antagonized the effects of ethanol in WT mice (p < 0.01) but not in KO mice. Comparison of the performance on the rotarod at the 45- and 60-min time points by two-way ANOVA did not reveal an effect of treatment, genotype, or interaction. To compare groups using a more conservative statistical approach, area under the response curves were calculated (Figure 1D). ANOVA conducted on AUC’s revealed a main effect of treatment (F1,33 = 14, p < 0.001) and an interaction of treatment with genotype (F1,33 = 8, p < 0.01). The effect of genotype alone was not significant. Fisher’s post hoc tests revealed that WT and KO mice did not differ in their response to ethanol alone. Ro15-4513 reduced the amount of ethanol-induced motor ataxia in WT (p < 0.0001) but not in KO mice. Thus, Ro15-4513 robustly antagonized ethanol-induced motor ataxia in WT mice, but Ro15-4513 was without effect in GABAA-R α4 subunit KO mice.
Antagonism of ethanol (3.5 g/kg)-induced LORR by Ro15-4513 (20 mg/kg) yielded an overall effect of gender (F1,74 = 16, p < 0.0001). Hence, males and females were analyzed separately. In males (Figure 2A), a two-way ANOVA revealed a main effect of treatment (F1,36 = 4, p ≤ 0.05), an interaction of genotype with treatment (F1,36 = 9, p < 0.01), but not a main effect of genotype. Post hoc analysis revealed no differences in duration of LORR between WT and KO mice that received ethanol alone. Ro15-4513 reduced the duration of ethanol-induced LORR in WT mice (p < 0.0005) compared to ethanol alone. In contrast, Ro15-4513 had no effect the duration of ethanol-induced LORR in KO mice. Thus, male α4 KO mice are resistant to Ro15-4513 antagonism of ethanol-induced LORR.
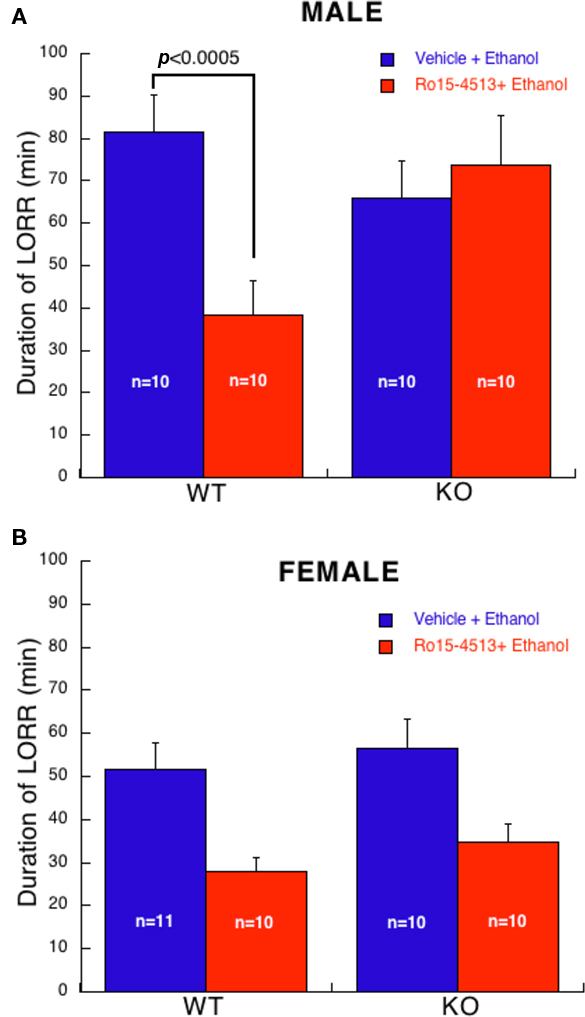
Figure 2. Antagonism of ethanol (3. 5 g/kg)-induced LORR by Ro15-4513 (20 mg/kg). (A) In male mice, Ro15-4513 antagonized the duration of ethanol-induced LORR in WT, but not in KO. (B) In female mice, Ro15-4513 antagonized ethanol-induced LORR (p < 0.0001) irrespective of genotype. All values are expressed as mean ± SEM.
Analysis of female mice (Figure 2B) revealed a main effect of treatment (F1,37 = 18, p < 0.001) but no effect of genotype or interaction of genotype with treatment. Note, one female KO mouse treated with Ro15-4513 + ethanol whose duration of LORR was greater than 2 SD from the group mean was excluded from this analysis. Thus, in female mice, Ro15-4513 reduced the duration of ethanol-induced LORR but this was not influenced by genotype.
The primary goal of this study was to test the hypothesis that α4-containing GABAA-Rs are required in vivo for the mechanism by which the imidazobenzodiazepine Ro15-4513 antagonizes the behavioral signs of ethanol intoxication. Previous publications provided pharmacologic and electrophysiologic evidence for (Hanchar et al., 2006; Wallner et al., 2006b) and against (Cook et al., 2005; Korpi et al., 2007; Mehta et al., 2007) this hypothesis, but none of the prior studies addressed the role of α4-containing GABAA-Rs at the behavioral level. The results of the present study revealed that α4-containing receptors are required for the ethanol antagonistic effects of Ro15-4513 on behavior. In addition, consistent with previous observations (Chandra et al., 2008), we confirmed that the behavioral effects of ethanol alone were comparable between WT and α4 KO mice.
We first tested the effects of Ro15-4513 on ethanol-induced motor ataxia. Previous studies established that ethanol-induced motor incoordination is robustly antagonized by Ro15-4513 (Hoffman et al., 1987; Stinchcomb et al., 1989; Dar, 1995). We previously reported that tonic current potentiation by Ro15-4513 is ablated in α4 KO hippocampal dentate granule neurons (Liang et al., 2008). Administration of Ro15-4513 reduced ethanol-induced motor ataxia and improved performance in WT mice but not in KO mice. Thus, α4 KO mice were completely insensitive to Ro15-4513 reversal of ethanol-induced motor incoordination. In contrast, WT mice showed varying degrees of reversal that may have been dependent upon dose and/or method of preparation/administration of the drug. These results are most consistent with the hypothesis that Ro15-4513 functions through α4-containing GABAA-Rs to antagonize ethanol-induced motor ataxia.
We also tested Ro15-4513 reversal of ethanol-induced hypnosis using the LORR assay. Previous studies have demonstrated that Ro15-4513 was able to partially reverse this high dose ethanol effect (Harris et al., 1995; Homanics et al., 1997; Mihalek et al., 2001). The duration of ethanol-induced LORR was reduced by ∼52% in WT males by Ro15-4513. In stark contrast, Ro15-4513 had no effect on the duration of ethanol-induced LORR in KO males. This is also consistent with the lack of effect of Ro15-4513 on tonic current potentiation in α4 KO neurons compared to WT (Liang et al., 2008). Results with female mice revealed that Ro15-4513 antagonized ethanol-induced LORR, but this was not influenced by genotype. Thus, males and females respond differently to this effect of Ro15-4513. Thus, we conclude that in male mice, Ro15-4513 functions through α4-containing GABAA-Rs to antagonize ethanol-induced hypnosis.
GABAA-Rs containing α4 do not appear to be universal targets for all ethanol-induced behaviors that are sensitive to antagonism by Ro15-4513. It has been reported that Ro15-4513 antagonism of operant responding for ethanol is mediated in part by α5 containing GABAA-Rs (Stephens et al., 2005). It would be of obvious interest to test α4 KO mice for this effect of Ro15-4513 to determine if α4 also contributes to this behavioral effect. However, we have not done so to date.
If ethanol and Ro15-4513 compete for a common binding site on α4/δ receptors that is responsible for the behavioral effects of these drugs as proposed (Hanchar et al., 2006; Wallner et al., 2006b), why are α4 KO mice resistant to Ro15-4513 but not ethanol alone (Chandra et al., 2008)? Ethanol most certainly exerts its behavioral effects via multiple targets in addition to α4/δ GABAA-Rs, including many non-GABAA-R targets. Therefore, it is conceivable that the targets of ethanol that remain in the KO masked the absence of α4. Alternatively, global KO of α4may have resulted in developmental alterations that compensated for the ethanol response at α4 GABAA-Rs. In contrast, imidazobenzodiazepines like Ro15-4513 exert their effects exclusively via GABAA-Rs. This potentially reduces the possibility of masking or compensating for the absence of α4 GABAA-Rs. In direct support of this suggestion, we have observed that in dentate gyrus following KO of α4 and ablation of ethanol potentiation of extrasynaptic tonic currents, synaptic responses to ethanol are markedly increased (Liang et al., 2008). In contrast, following KO of α4 and ablation of Ro15-4513 potentiation of extrasynaptic tonic currents, synaptic responses to Ro15-4513 are unaltered (Liang et al., 2008).While it is unlikely that the hippocampus is the primary brain region underlying ethanol-induced motor impairment, it is likely that the compensation observed in hippocampus also takes place in other brain regions (Liang et al., 2008).
While the results in this report demonstrate a clear requirement for α4-containing GABAA-R for Ro15-4513 antagonism of ethanol-induced motor incoordination and LORR, this study alone does not reveal the α4 subunit partners that are responsible, i.e., we cannot distinguish between α4β×δ and α4β×γ2 combinations. We previously reported that tonic current potentiation by Ro15-4513 was ablated in α4 KO hippocampal dentate granule neurons (Liang et al., 2008). Presumably, this Ro15-4513 sensitive tonic current that was ablated was normally composed of α4βγ2-containing GABAA-Rs since they are markedly potentiated by Ro15-4513 whereas α4δ-containing receptors are not (Wallner et al., 2006b). Even though α4δ receptors are not potentiated by Ro15-4513, they still bind this compound with high affinity (Hanchar et al., 2006) and it has been proposed that this binding to α4δ receptors antagonizes the effects of ethanol (Hanchar et al., 2006; Wallner et al., 2006b). However, our previously reported results that Ro15-4513 antagonism of ethanol-induced LORR was normal in δ subunit KO mice (Mihalek et al., 2001) argue against this. Thus, for ethanol-induced LORR, it appears that δ is not required for antagonism by Ro15-4513. This conclusion is in agreement with the recent report demonstrating that Ro15-4513 antagonizes ethanol-induced sedation via γ2 GABAA-Rs (Linden et al., 2011). The results of the present report together with the studies of Mihalek et al. (2001) and Linden et al. (2011) implicate α4β×γ2 GABAA-Rs as critical targets for Ro15-4513 antagonism of ethanol action, at least for the specific ethanol-induced behaviors that were tested in these reports.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The authors would like to thank Carolyn Ferguson and David Werner for expert technical assistance, and Martin Wallner for suggestions regarding preparation of Ro15-4513 solutions. This work was supported by NIH grants AA10422, AA13004, and DE14184.
Becker, H. C., and Hale, R. L. (1991). RO15-4513 antagonizes the anxiolytic effects of ethanol in a nonshock conflict task at doses devoid of anxiogenic activity. Pharmacol. Biochem. Behav. 39, 803–807.
Bonetti, E. P., Burkard, W. P., Gabl, M., Hunkeler, W., Lorez, H. P., Martin, J. R., Moehler, H., Osterrieder, W., Pieri, L., Polc, P., Richards, J. G., Schaffner, R., Scherschlicht, R., Schoch, P., and Haefely, W. E. (1988). Ro 15-4513: partial inverse agonism at the BZR and interaction with ethanol. Pharmacol. Biochem. Behav. 31, 733–749.
Borghese, C. M., Storustovu, S., Ebert, B., Herd, M. B., Belelli, D., Lambert, J. J., Marshall, G., Wafford, K. A., and Harris, R. A. (2006). The δ subunit of γ-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J. Pharmacol. Exp. Ther. 316, 1360–1368.
Casagrande, S., Cupello, A., Pellistri, F., and Robello, M. (2007). Only high concentrations of ethanol affect GABAA receptors of rat cerebellum granule cells in culture. Neurosci. Lett. 414, 273–276.
Chandra, D., Halonen, L. M., Linden, A. M., Procaccini, C., Hellsten, K., Homanics, G. E., and Korpi, E. R. (2010). Prototypic GABA(A) receptor agonist muscimol acts preferentially through forebrain high-affinity binding sites. Neuropsychopharmacology 35, 999–1007.
Chandra, D., Jia, F., Liang, J., Peng, Z., Suryanarayanan, A., Werner, D. F., Spigelman, I., Houser, C. R., Olsen, R. W., Harrison, N. L., and Homanics, G. E. (2006). GABAA receptor α4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc. Natl. Acad. Sci. U.S.A. 103, 15230–15235.
Chandra, D., Werner, D. F., Olsen, R. W., Harrison, N. L., and Homanics, G. E. (2008). Normal acute behavioral responses to moderate/high dose ethanol in GABAA receptor α4 subunit knockout mice. Alcohol. Clin. Exp. Res. 32, 10–18.
Cook, J. B., Foster, K. L., Eiler, W. J. II, McKay, P. F., Woods, J. II, Harvey, S. C., Garcia, M., Grey, C., McCane, S., Mason, D., Cummings, R., Li, X., Cook, J. M., and June, H. L. (2005). Selective GABAA alpha5 benzodiazepine inverse agonist antagonizes the neurobehavioral actions of alcohol. Alcohol. Clin. Exp. Res. 29, 1390–1401.
Dar, M. S. (1995). Antagonism by intracerebellar Ro15-4513 of acute ethanol-induced motor incoordination in mice. Pharmacol. Biochem. Behav. 52, 217–223.
Fleming, R. L., Wilson, W. A., and Swartzwelder, H. S. (2007). The magnitude and ethanol sensitivity of tonic GABAA receptor mediated inhibition in dentate gyrus changes from adolescence to adulthood. J. Neurophysiol. 97, 3806–3811.
Glowa, J. R., Crawley, J., Suzdak, P. D., and Paul, S. M. (1988). Ethanol and the GABA receptor complex: studies with the partial inverse benzodiazepine receptor agonist Ro 15-4513. Pharmacol. Biochem. Behav. 31, 767–772.
Hanchar, H. J., Chutsrinopkun, P., Meera, P., Supavilai, P., Sieghart, W., Wallner, M., and Olsen, R. W. (2006). Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15-4513 to α4/6β3δGABAA receptors. Proc. Natl. Acad. Sci. U.S.A. 103, 8546–8551.
Hanchar, H. J., Dodson, P. D., Olsen, R. W., Otis, T. S., and Wallner, M. (2005). Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat. Neurosci. 8, 339–345.
Harris, B. D., Moody, E. J., Gu, Z. Q., and Skolnick, P. (1995). Contribution of “diazepam-insensitive” GABAA receptors to the alcohol antagonist properties of Ro 15-4513 and related imidazobenzodiazepines. Pharmacol. Biochem. Behav. 52, 113–118.
Hoffman, P. L., Tabakoff, B., Szabo, G., Suzdak, P. D., and Paul, S. M. (1987). Effect of an imidazobenzodiazepine, Ro15-4513, on the incoordination and hypothermia produced by ethanol and pentobarbital. Life Sci. 41, 611–619.
Homanics, G. E., Ferguson, C., Quinlan, J. J., Daggett, J., Snyder, K., Lagenaur, C., Mi, Z. P., Wang, X. H., Grayson, D. R., and Firestone, L. L. (1997). Gene knockout of the α6 subunit of the γ-aminobutyric acid type A receptor: lack of effect on responses to ethanol, pentobarbital, and general anesthetics. Mol. Pharmacol. 51, 588–596.
Hu, W. Y., Reiffenstein, R. J., and Wong, L. (1987). Interaction between flurazepam and ethanol. Alcohol Drug Res. 7, 107–117.
Khanna, J. M., Kalant, H., Chau, A., and Shah, G. (1998). Rapid tolerance and crosstolerance to motor impairment effects of benzodiazepines, barbiturates, and ethanol. Pharmacol. Biochem. Behav. 59, 511–519.
Korpi, E. R., Debus, F., Linden, A.-M., Malecot, C., Leppa, E., Vekovischeva, O., Rabe, H., Bohme, I., Aller, M. I., Wisden, W., and Luddens, H. (2007). Does ethanol act preferentially via selected brain GABAA receptor subtypes? the current evidence is ambiguous. Alcohol 41, 163–176.
Liang, J., Suryanarayanan, A., Chandra, D., Homanics, G. E., Olsen, R. W., and Spigelman, I. (2008). Functional consequences of GABAA receptor α4 subunit deletion on synaptic and extrasynaptic currents in mouse dentate granule cells. Alcohol. Clin. Exp. Res. 32, 19–26.
Linden, A.-M., Schmitt, U., Leppa, E., Wulff, P., Wisden, W., Luddens, H., and Korpi, E. R. (2011). Ro 15-4513 antagonizes alcohol-induced sedation in mice through alpha-beta-gamma2-type GABAA receptors. Front. Neurosci. 5:3. doi: 10.3389/fnins.2011.00003
Lister, R. G., and Nutt, D. J. (1988). Ro15-4513 and its interaction with ethanol. Adv. Alcohol Subst. Abuse 7, 119–123.
Lovinger, D. M., and Homanics, G. E. (2007). Tonic for what ails us? high-affinity GABA(A) receptors and alcohol. Alcohol 41, 139–143.
McGinnis, J. M., and Foege, W. H. (1999). Mortality and morbidity attributable to use of addictive substances in the United States. Proc. Assoc. Am. Physicians 111, 109–118.
Mehta, A. K., Marutha Ravindran, C. R., and Ticku, M. K. (2007). Low concentrations of ethanol do not affect radioligand binding to the δ-subunit-containing GABAA receptors in the rat brain. Brain Res. 1165, 15–20.
Mihalek, R. M., Bowers, B. J., Wehner, J. M., Kralic, J. E., VanDoren, M. J., Morrow, A. L., and Homanics, G. E. (2001). GABAa-receptor delta subunit knockout mice have multiple defects in behavioral responses to ethanol. Alcohol. Clin. Exp. Res. 25, 1708–1718.
Mody, I., Glykys, J., and Wei, W. (2007). A new meaning for “Gin & Tonic”: tonic inhibition as the target for ethanol action in the brain. Alcohol 41, 145–153.
Olsen, R. W., Hanchar, H. J., Meera, P., and Wallner, M. (2007). GABAA receptor subtypes: the “one glass of wine” receptors. Alcohol 41, 201–209.
Samson, H. H., Haraguchi, M., Tolliver, G. A., and Sadeghi, K. G. (1989). Antagonism of ethanol-reinforced behavior by the benzodiazepine inverse agonists Ro15-4513 and FG 7142: relation to sucrose reinforcement. Pharmacol. Biochem. Behav. 33, 601–608.
Santhakumar, V., Wallner, M., and Otis, T. S. (2007). Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol 41, 211–221.
Stephens, D. N., Pistovcakova, J., Worthing, L., Atack, J. R., and Dawson, G. R. (2005). Role of GABAA alpha5-containing receptors in ethanol reward: the effects of targeted gene deletion, and a selective inverse agonist. Eur. J. Pharmacol. 526, 240–250.
Stinchcomb, A., Bowers, B. J., and Wehner, J. M. (1989). The effects of ethanol and Ro 15-4513 on elevated plus-maze and rotarod performance in long-sleep and short-sleep mice. Alcohol 6, 369–376.
Storustovu, S. I., and Ebert, B. (2006). Pharmacological characterization of agonists at delta-containing GABAA receptors: functional selectivity for extrasynaptic receptors is dependent on the absence of gamma2. J. Pharmacol. Exp. Ther. 316, 1351–1359.
Sundstrom-Poromaa, I., Smith, D. H., Gong, Q. H., Sabado, T. N., Li, X., Light, A., Wiedmann, M., Williams, K., and Smith, S. S. (2002). Hormonally regulated α4β2δGABAA receptors are a target for alcohol. Nat. Neurosci. 5, 721–722.
Suzdak, P. D., Glowa, H. R., Crawley, J. N., Schwartz, R. D., Skolnick, P., and Paul, S. M. (1986). A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science 234, 1243–1247.
Suzdak, P. D., Paul, S. M., and Crawley, J. N. (1988). Effects of Ro15-4513 and other benzodiazepine receptor inverse agonists on alcohol-induced intoxication in the rat. J. Pharmacol. Exp. Ther. 245, 880–886.
Wallner, M., Hanchar, H. J., and Olsen, R. W. (2003). Ethanol enhances α4β3δ and α6β3δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc. Natl. Acad. Sci. U.S.A. 100, 15218–15223.
Wallner, M., Hanchar, H. J., and Olsen, R. W. (2006a). Low dose acute alcohol effects on GABAA receptor subtypes. Pharmacol. Ther. 112, 513–528.
Wallner, M., Hanchar, H. J., and Olsen, R. W. (2006b). Low-dose alcohol actions on α4β3δ GABAA receptors are reversed by the behavioral alcohol antagonist Ro15-4513. Proc. Natl. Acad. Sci. U.S.A. 103, 8540–8545.
Wallner, M., and Olsen, R. W. (2008). Physiology and pharmacology of alcohol: the imidazobenzodiazepine alcohol antagonist site on subtypes of GABAA receptors as an opportunity for drug development? Br. J. Pharmacol. 154, 288–298.
Wei, W., Faria, L. C., and Mody, I. (2004). Low ethanol concentrations selectively augment the tonic inhibition mediated by δ subunit-containing GABAA receptors in hippocampal neurons. J. Neurosci. 24, 8379–8382.
Keywords: alcohol, alcohol antagonist, alcohol receptor, benzodiazepine, extrasynaptic GABAA receptor, Ro15-4513
Citation: Iyer SV, Benavides RA, Chandra D, Cook JM, Rallapalli S, June HL and Homanics GE (2011) α4-Containing GABAA receptors are required for antagonism of ethanol-induced motor incoordination and hypnosis by the imidazobenzodiazepine Ro15-4513. Front. Pharmacol. 2:18. doi: 10.3389/fphar.2011.00018
Received: 04 November 2010;
Accepted: 21 March 2011;
Published online: 04 April 2011.
Edited by:
A. Leslie Morrow, University of North Carolina School of Medicine, USAReviewed by:
Esa R. Korpi, University of Helsinki, FinlandCopyright: © 2011 Iyer, Benavides, Chandra, Cook, Rallapalli, June and Homanics. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Gregg E. Homanics, Department of Anesthesiology, University of Pittsburgh, Room 6060 Biomedical Science Tower-3, 3501 Fifth Avenue, Pittsburgh, PA 15261, USA.e-mail:aG9tYW5pY3NnZUBhbmVzLnVwbWMuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.