
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 07 March 2025
Sec. Pediatric Cardiology
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1562782
This article is part of the Research Topic Surgical and Non-Surgical Intervention of Congenital Heart Disease Management in Developing and Developed Countries View all 9 articles
 Yanyun Huang1,2,†
Yanyun Huang1,2,† Yuting Chen1,†
Yuting Chen1,† Danyan Su1,2
Danyan Su1,2 Suyuan Qin1,2
Suyuan Qin1,2 Cheng Chen1,2
Cheng Chen1,2 Dongli Liu1,2
Dongli Liu1,2 Bingbing Ye1,2
Bingbing Ye1,2 Yuqin Huang1,2
Yuqin Huang1,2 Piaoliu Yuan1,2
Piaoliu Yuan1,2 Yusheng Pang1,2*
Yusheng Pang1,2*
Background: Transcatheter closure is now the preferred treatment for congenital heart disease complicated with pulmonary arterial hypertension (CHD-PAH), but its long-term effects are not well understood. We aimed to assess the safety, effectiveness, and outcome of this procedure in children with CHD-PAH.
Methods: We included 210 children with CHD-PAH at our hospital from 2012 to 2021 and collected their general, laboratory, echocardiographic, and hemodynamic data for analysis. A logistic regression analysis identified risk factors for persistent postclosure PAH (PP-PAH).
Results: Among the 210 patients, 84.29% had mild PAH, 8.57% had moderate PAH, and 7.14% had severe PAH. The device was successfully implanted in 98.10% of patients. Early adverse events occurred in 12.14% (n = 25) of patients, with residual shunts and arrhythmia being the most common complications, each affecting 2.91% (n = 6) of patients. Most complications were minor and temporary, except for two cases of residual shunt—one required surgical repair, and one case of complete left bundle branch block led to occluder removal. Postintervention, pulmonary arterial pressure (PAP) decreased significantly, and cardiomegaly resolved. PP-PAH was detected in 13 patients (6.31%). Preoperative pulmonary arterial systolic pressure [odds ratio [OR] = 1.033, 95% confidence interval [CI] = 1.005–1.061, P = 0.019] and right ventricular diameter (OR = 1.111, 95% CI = 1.039–1.187, P = 0.002) were found to be risk factors for PP-PAH.
Conclusion: Transcatheter closure is effective and safe for children with correctable CHD-PAH. Preoperative pulmonary arterial systolic pressure and right ventricular diameter are risk factors for PP-PAH.
Congenital heart disease (CHD), recognized as the most prevalent congenital anomaly, manifests in approximately 8 out of every 1,000 newborns (1). Despite advancements in diagnostic and therapeutic approaches, approximately 5%–10% of patients with CHD may develop pulmonary arterial hypertension (PAH) if the condition remains uncorrected, leading to significant morbidity and mortality (2). Persistent systemic-to-pulmonary shunting causes overflow in the pulmonary circulation, ultimately leading to irreversible remodeling of the pulmonary vasculature (3). Data suggest that PAH can be reversed by early closure of the congenital defect prior to the onset of vascular remodeling (4). Once pulmonary vascular remodeling becomes irreversible, defect closure is associated with accelerated disease progression (5). This renders decisions regarding the closure of the defect particularly challenging.
In current guidelines, pulmonary vascular resistance (PVR) and the ratio of pulmonary to systemic circulation (Qp/Qs) are considered the most critical reference indices for determining the indication for defect closure. The 2020 European Society of Cardiology (ESC) Guidelines suggest shunt closure for patients with PVR ≤ 5 wood units and Qp/Qs > 1.5 (6). The 2019 European Pediatric Pulmonary Vascular Disease Network (EPPVDN) recommends that a PVR index (PVRI) < 6 wood units/m2 is an indication for shunt closure, whereas a PVRI > 8 wood units/m2 is a contraindication (7). Unfortunately, these cutoff values rely largely on expert opinions and lack prospective data support. Despite a Qp/Qs > 1.5, some patients still develop or experience worsening PAH after defect correction. Consequently, decisions regarding shunt closure should be based on a comprehensive evaluation of all the clinical information, rather than relying solely on hemodynamic data. This conclusion highlights the need for follow-up and assessment of CHD-PAH patients after defect closure. Several reports have suggested that defect closure in patients with CHD-PAH may be linked to favorable outcomes (8–12). Conversely, other studies have indicated that PAH may continue to progress despite closure of the defect (13, 14). However, most prior studies involved patent ductus arteriosus (PDA) or atrial septal defect (ASD) patients and had relatively small sample sizes. Some of these studies were performed in adults. Little has been published focusing specifically on the outcomes of children with CHD-PAH after correction.
Percutaneous transcatheter closure of CHD-PAH is now the preferred treatment at many centers because of its minimal invasiveness, quick recovery, ability to monitor pulmonary arterial pressure (PAP), and the advatange of trial occlusion. However, little is known about its outcomes. In the present study, we retrospectively evaluated the safety, effectiveness, and outcomes of transcatheter closure in children with CHD-PAH.
The study was conducted at the First Affiliated Hospital of Guangxi Medical University, enrolling CHD-PAH patients who had transcatheter closures between January 2012 and December 2021. PAH is diagnosed when the mean pulmonary arterial pressure (mPAP) is >20 mmHg via cardiac catheterization (15). The patient inclusion criteria were as follows: (1) meeting the PAH diagnostic criteria; (2) having simple CHD, including PDA, ASD, ventricular septal defect (VSD), or their combination; (3) a PVRI < 6 wood units/m2; (4)age < 18 years; and (5) availability of complete and traceable medical records. Patients with other causes of PAH were excluded from the study. The patients were categorized into three groups according to the mPAP levels: mild (20–40 mmHg), moderate (41–55 mmHg), and severe (>55 mmHg). After the intervention, PAH was evaluated by transthoracic echocardiography (TTE) during follow-up. PAH was identified by a pulmonary arterial systolic pressure (PASP) exceeding 35 mmHg and categorized as mild (35–50 mmHg), moderate (50–70 mmHg), or severe (>70 mmHg) (16–18). Persistent postclosure PAH (PP-PAH) was defined as a PASP that remained abnormal at 6 months postprocedure and persisted until the final follow-up (12). The Ethics Committee of the First Affiliated Hospital of Guangxi Medical University granted ethical approval (No. 2021; KY-E-156).
The procedure was performed under general anesthesia. The VSD and PDA were accessed via the right femoral vein and artery, and ASD was accessed via the right femoral vein only. Heparin (100 IU/kg) was given intravenously postfemoral cannulation. All patients underwent right and left cardiac catheterization for hemodynamic assessment. Blood gas analysis was conducted at each site. Qp/Qs and PVR were calculated using the estimated oxygen consumption. Angiography and intraoperative TTE were used to determine the location and size of the defect, which guided the occluder selection. In patients with a ratio of pulmonary arterial pressure to aortic pressure (Pp/Ps) > 0.8, we attempted transcatheter closure to assess reversibility. The following criteria were used (9): (1) a ≥ 20% reduction in mPAP or PASP; (2) stable aortic pressure; and (3) no worsening of symptoms such as dyspnea, irritability, pallor, chest pain, or heart rate drop. We monitored patients for at least 20 min during defect occlusion. The device was released if all criteria were met; otherwise, the occluder was retracted.
We gathered general information such as sex, age, body mass index, and main symptoms and signs from the medical records. Laboratory data, including levels of troponin I, electrolytes, cystatin, creatine kinase-MB, uric acid, and brain natriuretic peptide (BNP), were collected within 24 h following admission. Upon admission, each patient received TTE, and echocardiographic parameters, including the PASP, left atrial dimension (LAD), left ventricular end-diastolic dimension (LVEDD), pulmonary artery dimension (PAD), aortic dimension (AOD), left ventricular ejection fraction (LVEF), and right ventricular dimension (RVD), were documented. The x-ray data, including the cardiothoracic ratio and eminence of the PA segment, were also recorded. The hemodynamic data from cardiac catheterization before intervention were recorded.
Patients received regular follow-up exams, including physical exams, electrocardiograms, x-rays, and TTE, at 24 h; 1, 3, 6, and 12 months; and then annually. The patients' records were thoroughly reviewed, and a phone survey was attempted for those lacking documented follow-up data.
Data analysis was conducted using SPSS (version 26.0 for Windows, SPSS, Inc., Chicago, Illinois) and R (R version 4.2.1) software. The normality test was conducted using the Shapiro–Wilk test. Quantitative variables are shown as the mean ± standard deviation for normally distributed data or as the median with the interquartile range for skewed data. The Student's t test or analysis of variance was employed for quantitative variables with a normal distribution, whereas the Mann‒Whitney U-test or Kruskal‒Wallis H test was used otherwise. Categorical variables are presented as frequencies (percentages), and comparisons between groups were performed using Fisher's exact test or the chi-square test. Logistic regression analysis was conducted to assess the risk factors for PP-PAH. Factors with a P value less than 0.1 in the univariate analysis were incorporated into the multivariate logistic regression model. A P value less than 0.05 was regarded as statistically significant.
The study enrolled 210 children with CHD-PAH, including 87 males and 123 females. In children with PAH, the prevalence rates were 84.29% for mild cases (n = 177), 8.57% for moderate cases (n = 18), and 7.14% for severe cases (n = 15). Most moderate-to-severe PAH patients occurred in the PDA group, whereas all VSD patients had mild PAH. Ninety-two patients (43.81%) experienced recurrent respiratory infections, and eighty-two (39.05%) experienced growth retardation, with 28 (13.33%) experiencing shortness of breath and 21 experiencing fatigue (10.00%). Heart murmur was found in 97.14% of the patients, with 10.48% of the patients having an augmented P2. The patients had a mean mPAP of 30.05 ± 12.28 mmHg, a Pp/Ps ratio of 0.46 ± 0.19, and a median PVRI of 2.04 (1.44–3.21) wood units/m². While in the severe PAH group. Compared with those with mild PAH, patients with moderate and severe PAH presented an increased cardiothoracic ratio, LAD, LVEDD, PAD, and Pp/Ps (P < 0.05), but there were no significant differences across the groups in AOD, RVD, LVEF, mRAP, Qp/Qs, or PVRI. At baseline, 7 patients (3.33%) were receiving at least one pulmonary vasodilator, including 5 patients who were receiving bosentan and 2 who were receiving multiple agents (1 bosentan l + iloprost and 1 bosentan + sildenafil). Table 1 summarizes the baseline characteristics of the patients.
Among the 210 patients, 206 had successful interventions. The occlusion success rate could reach 98.10%. In one of the four cases in which closure failed, the patient was a 5.9-year-old girl with a 16 mm VSD. The procedure failed because of the large size of the VSD and insufficient aortic valve rim, and the patient was transferred for surgery repair. The second patient was a 4.6-year-old girl with an 11 mm VSD. The occluder was not well formed after being released. Finally, the intervention was stopped. The third patient was a 5.4-year-old boy with an 11 mm VSD. The device was malpositioned immediately after release, and a surgical emergency operation was performed to remove the occluder. During follow-up, the VSD tended to close, with a diameter of 2 mm at the last follow-up. The last patient was a 5.3-year-old girl with a 25 mm ASD who was treated with a 26 mm occluder. Third-degree atrioventricular block occurred during the closure process, and the plugging was suspended. After sinus rhythm was restored, a 24 mm occluder was selected for repeated occlusion, but it was not successful because the occluder was too small. Nine patients underwent trial occlusion, resulting in a decrease in mPAP from 55.44 to 31.89 mmHg and in PASP from 77.56 to 49.11 mmHg (P < 0.001), without affecting systemic blood pressure.
Early adverse events occurred in 12.14% (n = 25) of the patients. A residual shunt was detected in 2.91% (n = 6) of the patients, with most being small and temporary, persisting in only 2 patients. One patient required surgical repair at the 2-year follow-up due to an increased shunt volume, whereas another had a residual shunt at 18 months but showed no clinical symptoms. Arrhythmia occurred in 2.91% (n = 6) of the patients, including 2 left anterior branch blocks, 1 third-degree atrioventricular block (III°AVB), 1 II°AVB, 1 supraventricular tachycardia, and 1 atrial premature beat. Most patients normalized during follow-up, except for one patient who developed a complete left bundle branch block and II° AVB from a left anterior branch block. The patient experienced syncope once during follow-up, and since the parent declined pacemaker treatment, the occluder was removed and surgical repair performed. One patient experienced a temporary drop in heart rate and blood oxygen during the procedure, which improved after atropine administration. One patient experienced bleeding and required a blood transfusion. Other complications included 4 cases of stress fever, 4 side effects from intravenous anesthesia, 1 case of hemolysis, 1 case of myocardial injury, and 1 case of femoral fistula, all of which improved before discharge. A summary is provided in Table 2.
After the intervention, all 25 patients received pulmonary vasodilators: 15 were on monotherapy (13 with bosentan, 2 with beraprost sodium) and 10 were on combination therapy (8 with bosentan + beraprost sodium, 1 with bosentan + iloprost, 1 with bosentan + sildenafil). During a follow-up of 1.25 to 10.50 years, no deaths occurred, and the occluding devices remained stable and properly shaped. No cases of infective endocarditis, heart failure, thromboembolism, new arrhythmias, or device-related atrioventricular valve regurgitation were reported.
PAH was monitored by TTE postoperatively. Correlation analysis revealed a positive relationship between PASP measurements obtained via TTE and heart catheterization (r = 0.566, P < 0.001; Figure 1). The PAP decreased significantly after the intervention and stabilized over time (Figure 2). Thirty-four patients had PAH 24 h postintervention; 15 remained affected at 3 months, and 13 remained affected at the final follow-up. Out of 206 patients who underwent transcatheter closure, 13 developed PP-PAH, 32 were lost to follow-up, and 161 had normal pulmonary pressure. Among 15 with severe PAH, 3 developed PP-PAH, 3 were lost to follow-up, and the rest normalized. Figure 3 illustrates the trends in PAH before and after the intervention. Significant decreases in LAD, RVD, PAD, LVEF, PASP, and the cardiothoracic ratio were observed at the 1-month, 3-month, 6-month, 1-year, and 2-year follow-ups (P < 0.05, Table 3). The AOD and LVEDD significantly changed at 1 year but not at 2 years (P > 0.05).

Figure 1. Correlation analysis between pulmonary arterial systolic pressure measured by echocardiography vs. cardiac catheterization.
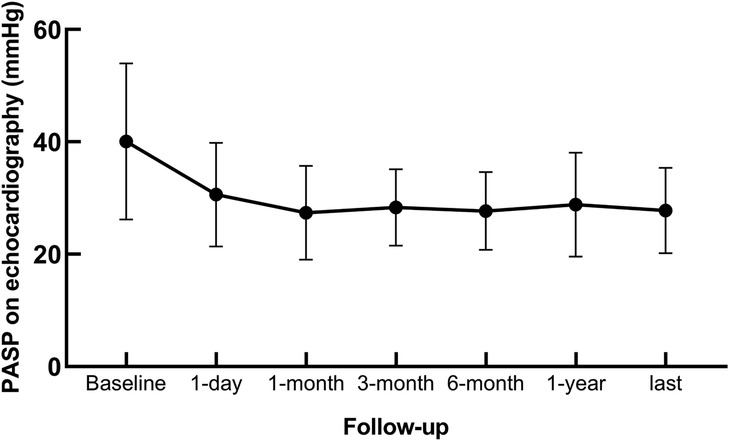
Figure 2. Changes in pulmonary arterial systolic pressure in patients with congenital heart disease complicated with pulmonary arterial hypertension.
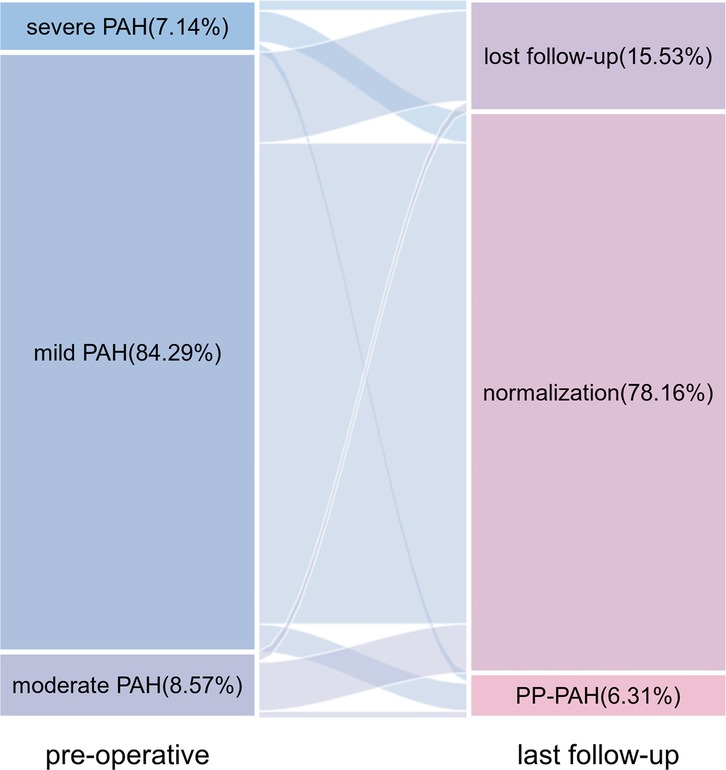
Figure 3. Sankey diagram of changes in pulmonary arterial hypertension from baseline to the last follow-up.
Univariate analysis indicated that RVD was linked to PP-PAH [odds ratio [OR] = 1.096, 95% confidence interval [CI] = 1.029–1.168, P = 0.004]. Stepwise selection was used for further analysis. Multivariate analysis revealed that an increase in the PASP of 1 mmHg increased the risk of PP-PAH by 3.3% (OR = 1.033, 95% CI = 1.005–1.061; P = 0.019). Additionally, for each 1 millimeter increase in RVD, the risk of PP-PAH increased by 1.1-fold (OR = 1.111, 95% CI = 1.039–1.187, P = 0.002) (Table 4). To assess the probability of PP-PAH across different baseline PASP levels, patients were stratified into two groups: PASP ≥ 50 mmHg and PASP < 50 mmHg. The incidence of PP-PAH was significantly higher in the PASP ≥ 50 mmHg group compared to the PASP < 50 mmHg group (21.74% vs. 5.30%, P < 0.05, Figure 4).
In the present study, the clinical characteristics and outcomes of children with CHD-PAH who underwent percutaneous defect closure were assessed at a tertiary medical center in Guangxi, China. The main finding was that transcatheter closure was effective and yielded positive results in selected patients with CHD-PAH. Although PASP generally improved after closure, some patients still experienced PAH after defect correction, which was linked to the baseline PASP and RVD.
Transcatheter closure for CHD has been shown to be an effective therapy for the wide spectrum of CHD, with advancements in techniques leading to a high success rate. This study revealed a 98.10% success rate for interventional blockade in CHD-PAH patients, which is consistent with the literature (11, 19–22). At 24 h postprocedure, PAH had completely regressed in 83.50% of patients. Over time, PAP gradually normalized; however, approximately 6.31% of the patients still presented with PAH at the last follow-up, which was regarded as PP-PAH. Comparative studies related to this topic are limited. Several studies have reported an estimated prevalence of PAH postclosure ranging from 13%–25%, which is higher than that obtained in our study (8, 12, 23). This discrepancy may be attributed to variations in the inclusion criteria. In our study, the majority of participants presented with mild PAH, whereas other studies primarily included individuals with moderate to severe PAH. Additionally, the number of heart chambers and the diameters of the pulmonary artery and aorta significantly decreased and normalized, which is consistent with previous studies (11, 24). Therefore, transcatheter closure of CHD-PAH has a definite effect.
The presence of a residual shunt is the key indicator for evaluating the success of transcatheter closure in CHD patients. This study revealed that the incidence of immediate residual shunt was 2.91%, which is lower than previously reported rates (11, 20, 25, 26). Most shunts were small and resolved in 4 patients within 1 year, although 1 patient had a persistent shunt at 18 months without symptoms, and another patient required surgery at 2 years owing to an increased shunt volume. Residual shunts often result from incorrect occluder sizing, so selecting the appropriate occluder type and location is essential to ensure complete closure without impacting aortic and atrioventricular valve functions.
Arrhythmia, which affected 2.91% of the patients in the present study, is a common complication of transcatheter closure, which is consistent with previous cardiac catheterization studies in CHD (27). There were two cases of left anterior branch block, one case each of third-degree and second-degree atrioventricular block, one case of supraventricular tachycardia, and one atrial premature beat. Most patients normalized during follow-up, except for one patient who progressed to complete left bundle branch block and II° AVB from a left anterior branch block. The issue was resolved after removing the occluder and performing surgical repair. Postoperative arrhythmia may result from temporary inflammation or edema at the device site, direct compression trauma, or scar formation in the conduction tissue (28). Steroid administration has been shown to have a positive effect on postoperative arrhythmias (20, 28). To prevent arrhythmia, prolonged intracardiac procedures should be avoided, and an occluder that is slightly larger (by 1–2 mm) than the defect should be used to prevent tissue compression and edema.
Complications resulting from femoral artery access in cardiac catheterization may include bleeding, hematoma, and arteriovenous fistula. In this study, one patient developed an arteriovenous fistula, and another needed blood transfusion due to bleeding. Other adverse events included stress fever, anesthesia side effects, hemolysis, myocardial injury, and temporary heart rate drops, all of which improved before discharge. Postclosure, no infective endocarditis, heart failure, thromboembolism, occluder issues, or deaths occurred during follow-up. Transcatheter closure for CHD-PAH has few complications and favorable safety.
PAH is a common complication of CHD, particularly in patients with uncorrected systemic-to-pulmonary shunts (29). Increased pulmonary blood flow is considered a crucial trigger for pulmonary vascular remodeling, leading to irreversible changes in the pulmonary vasculature (30). Timely correction of the shunt may lead to full disease regression, but this potential is lost after a certain point (3). Closure beyond the reversible phase may increase the mortality risk and worsen the prognosis compared with patients with uncorrected CHD-PAH (5). Approximately 10% of children with CHD and PAH are considered ineligible for shunt closure (3). These findings highlight the importance of differentiating reversible from irreversible PAH. Currently, Qp/Qs < 1.5 and PVRI > 8 wood units/m2 are regarded as contraindications for shunt closure (6, 7). However, these values were derived using the oximetric shunt formula, which relies on measurements from collected arterial and venous blood gases and is susceptible to calculation errors. The current guidelines recommend the use of a multiparametric approach that considers all the available information, rather than relying solely on hemodynamic data, in decisions regarding shunt closure (31). In this study, none of the patients had cyanosis or symptoms related to heart failure. Echocardiography revealed a left-to-right shunt in blood flow. The median PVRI for all patients was 2.04 wood units/m2, and 2.20 wood units/m2 in the severe PAH subgroup. Notably, all patients had a PVRI below 6 wood units/m2, aligning with international guidelines for defect closure. Among 33 patients with moderate-to-severe PAH, 9 underwent trial occlusion, achieving a ≥20% reduction in mPAP and/or PASP without affecting systemic blood pressure, thus meeting the closure criteria. Although the patients were assessed as correctable in the present study; 13 still had persistent PAH after closure. PAH can occur much later than 1 to 10 years after intervention (5). A prior study revealed that the cumulative incidence of PH after closure increased over time even in patients with mild defects (32). These alarming results support the monitoring of all CHD-PAH patients after shunt closure, even those with mild PAH.
This study revealed that preoperative PASP and RVD are risk factors for PP-PAH. Sadiq et al. (19) reported that a PASP with oxygen ≤75 mmHg is associated with the regression of PAH. The substantial increase in PAP before intervention indicates that the recovery from PAH-related vascular changes is slow, even after shunt interruption. Some patients may also experience left heart insufficiency before or after surgery due to the increased load. Consequently, it takes a considerable amount of time for PAP to normalize postsurgery. To the best of our knowledge, this is the first study to identify RVD as a risk factor for persistent PAH after intervention. Previous studies have shown that RVD is associated with adverse clinical outcomes (33, 34). RV pressure overload leads to RV dilation, impairing RV function and increasing tricuspid valve regurgitation, which in turn worsens RV dilation (33). These findings necessitate additional research with larger sample sizes and extended follow-up due to the relatively small PP-PAH sample size.
Target therapies had shown favorable functional and haemodynamic results in patients with PAH (31). Compared with other group 1 PAH subgroups, there is limited evidence on the use of PAH-approved medications in patients with CHD-PAH. Experts recommended PAH therapies for: (1) lowering PVR for safe repair, (2) post-repair treatment, (3) improving quality of life in patients with Eisenmenger syndrome, and (4) reducing PAP and PVR in patients with pulmonary hypertensive vascular disease (35). Currently, evidence-based treatment data for PAH with systemic-to-pumonary remain relatively limited. Treatment decisions often rely more on expert clinical experience. In this study, 25 patients with moderate to severe PAH were treated with pulmonary vasodilators. Seven were treated before intervention due to a PASP over 70 mmHg measured by TTE, while 18 received therapy post-intervention based on mPAP from cardiac catheterization. At the last follow-up, 4 out of 25 patients still had PAH, while the rest returned to normal. No drug-related adverse effects were noted. Rong et al. (11) suggested that patients with moderate to severe PAH require early targeted drug interventions post-surgery. However, the impact of PAH therapies on patients with systemic-to-pulmonary shunts is not well understood. More extensive multicenter studies are needed to confirm the efficacy and safety of these treatments for this group.
Heart catheterization is the gold standard for PAH diagnosis, but its invasive nature makes it unsuitable for repeated assessments (36). Only a few patients receive repeated cardiac catheterization after the procedure (11, 12). The TTE estimates PASP by measuring peak tricuspid regurgitation velocity, offering an alternative for assessing PAH after closure. Multiple studies have demonstrated good consistency between PASP estimated by TEE and values obtained through cardiac catheterization (9, 11, 37). In our study, the PASP estimated by TTE correlated well with cardiac catheterization measurements, indicating that TTE was a reliable method for assessing PAH postintervention.
There were several limitations in this study. First, selection bias was inevitable because the study was retrospective. Second, the PP-PAH sample size was relatively small. Therefore, the findings should be considered preliminary. Larger samples and extended follow-up are necessary for further validation of these findings. Third, TTE assessment rather than catheterization was selected to measure the PASP after intervention.
This study revealed that transcatheter closure is an effective and safe therapeutic option for children with correctable CHD-PAH. Preoperative PASP and RVD are risk factors for PP-PAH. Long-term follow-up is essential for all CHD-PAH patients after shunt closure.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by The Ethics Committee of the First Affiliated Hospital of Guangxi Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
YH: Formal analysis, Software, Validation, Writing – original draft. YC: Formal analysis, Conceptualization, Data curation, Methodology, Writing – review & editing. DS: Formal analysis, Software, Visualization, Writing – review & editing. SQ: Formal analysis, Software, Visualization, Writing – review & editing. CC: Data curation, Writing – review & editing. DL: Data curation, Writing – review & editing. BY: Formal Analysis, Software, Validation, Writing – review & editing. YH: Data curation, Writing – review & editing. PY: Data curation, Writing – review & editing. YP: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was funded by a grant from the National Natural Science Foundation of China (No. 82260022) and Guangxi Clinical Research Center for Pediatric Disease (No. AD22035219).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. van der Linde D, Konings EE, Slager MA, Witsenburg M, Helbing WA, Takkenberg JJ, et al. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol. (2011) 58(21):2241–7. doi: 10.1016/j.jacc.2011.08.025
2. Jiang X, Jing ZC. Epidemiology of pulmonary arterial hypertension. Curr Hypertens Rep. (2013) 15(6):638–49. doi: 10.1007/s11906-013-0397-5
3. van der Feen DE, Bartelds B, de Boer RA, Berger RMF. Pulmonary arterial hypertension in congenital heart disease: translational opportunities to study the reversibility of pulmonary vascular disease. Eur Heart J. (2017) 38(26):2034–41. doi: 10.1093/eurheartj/ehx034
4. Lévy M, Maurey C, Celermajer DS, Vouhé PR, Danel C, Bonnet D, et al. Impaired apoptosis of pulmonary endothelial cells is associated with intimal proliferation and irreversibility of pulmonary hypertension in congenital heart disease. J Am Coll Cardiol. (2007) 49(7):803–10. doi: 10.1016/j.jacc.2006.09.049
5. Manes A, Palazzini M, Leci E, Bacchi Reggiani ML, Branzi A, Galiè N. Current era survival of patients with pulmonary arterial hypertension associated with congenital heart disease: a comparison between clinical subgroups. Eur Heart J. (2014) 35(11):716–24. doi: 10.1093/eurheartj/eht072
6. Baumgartner H, De Backer J, Babu-Narayan SV, Budts W, Chessa M, Diller GP, et al. 2020 ESC guidelines for the management of adult congenital heart disease. Eur Heart J. (2021) 42(6):563–645. doi: 10.1093/eurheartj/ehaa554
7. Hansmann G, Koestenberger M, Alastalo TP, Apitz C, Austin ED, Bonnet D, et al. 2019 Updated consensus statement on the diagnosis and treatment of pediatric pulmonary hypertension: the European pediatric pulmonary vascular disease network (EPPVDN), endorsed by AEPC, ESPR and ISHLT. J Heart Lung Transplant. (2019) 38(9):879–901. doi: 10.1016/j.healun.2019.06.022
8. Seol JH, Jung SY, Lee HB, Kim AY, Kim EH, Min IK, et al. Outcomes in patients with pulmonary arterial hypertension underwent transcatheter closure of an atrial septal defect. J Clin Med. (2023) 12(7):2540. doi: 10.3390/jcm12072540
9. Huang ZW, Fan ZX, Sun JT, Li WM, Gao YQ, Quan YH, et al. The short- and medium-term results of transcatheter closure of atrial septal defect with severe pulmonary arterial hypertension. Heart Vessels. (2012) 27(6):603–9. doi: 10.1007/s00380-011-0187-4
10. Salavitabar A, Krishnan US, Turner ME, Vincent JA, Torres AJ, Crystal MA. Safety and outcomes of transcatheter closure of patent ductus arteriosus in children with pulmonary artery hypertension. Tex Heart Inst J. (2020) 47(4):250–7. doi: 10.14503/thij-19-6982
11. Rong X, Ye Q, Wang Q, Wang J, Zhu Q, Chen Y, et al. Post-interventional evaluation and follow-up in children with patent ductus arteriosus complicated with moderate to severe pulmonary arterial hypertension: a retrospective study. Front Cardiovasc Med. (2021) 8:693414. doi: 10.3389/fcvm.2021.693414
12. Zhang DZ, Zhu XY, Lv B, Cui CS, Han XM, Sheng XT, et al. Trial occlusion to assess the risk of persistent pulmonary arterial hypertension after closure of a large patent ductus arteriosus in adolescents and adults with elevated pulmonary artery pressure. Circ Cardiovasc Interv. (2014) 7(4):473–81. doi: 10.1161/circinterventions.113.001135
13. O'Donnell C, Ruygrok PN, Whyte K, Wilson NJ. Progressive pulmonary hypertension post atrial septal defect device closure-early symptomatic improvement may not predict outcome. Heart Lung Circ. (2010) 19(12):713–6. doi: 10.1016/j.hlc.2010.08.010
14. Lammers AE, Bauer LJ, Diller GP, Helm PC, Abdul-Khaliq H, Bauer UMM, et al. Pulmonary hypertension after shunt closure in patients with simple congenital heart defects. Int J Cardiol. (2020) 308:28–32. doi: 10.1016/j.ijcard.2019.12.070
15. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. (2019) 53(1):1801913. doi: 10.1183/13993003.01913-2018
16. Barst RJ, McGoon M, Torbicki A, Sitbon O, Krowka MJ, Olschewski H, et al. Diagnosis and differential assessment of pulmonary arterial hypertension. J Am Coll Cardiol. (2004) 43(12):40s–7. doi: 10.1016/j.jacc.2004.02.032
17. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the joint task force for the diagnosis and treatment of pulmonary hypertension of the European society of cardiology (ESC) and the European respiratory society (ERS): endorsed by: association for European paediatric and congenital cardiology (AEPC), international society for heart and lung transplantation (ISHLT). Eur Heart J. (2016) 37(1):67–119. doi: 10.1093/eurheartj/ehv317
18. Faqih SA, Noto-Kadou-Kaza B, Abouamrane LM, Mtiou N, El Khayat S, Zamd M, et al. Pulmonary hypertension: prevalence and risk factors. Int J Cardiol Heart Vasc. (2016) 11:87–9. doi: 10.1016/j.ijcha.2016.05.012
19. Sadiq M, Rehman AU, Hyder N, Qureshi AU, Kazmi T, Qureshi SA. Intermediate- and long-term follow-up of device closure of patent arterial duct with severe pulmonary hypertension: factors predicting outcome. Cardiol Young. (2017) 27(1):26–36. doi: 10.1017/s1047951115002772
20. Weryński P, Skorek P, Wójcik A, Rudek-Budzyńska A, Dziewulska A, Rudziński A. Recent achievements in transcatheter closure of ventricular septal defects: a systematic review of literature and a meta-analysis. Kardiol Pol. (2021) 79(2):161–9. doi: 10.33963/kp.15708
21. Li H, Shi Y, Zhang S, Ren Y, Rong X, Wang Z, et al. Short- and medium-term follow-up of transcatheter closure of perimembranous ventricular septal defects. BMC Cardiovasc Disord. (2019) 19(1):222. doi: 10.1186/s12872-019-1188-y
22. Mandal KD, Su D, Pang Y. Long-term outcome of transcatheter device closure of perimembranous ventricular septal defects. Front Pediatr. (2018) 6:128. doi: 10.3389/fped.2018.00128
23. Arafuri N, Murni IK, Idris NS, Uiterwaal C, Savitri AI, Nugroho S, et al. Survival of left-to-right shunt repair in children with pulmonary arterial hypertension at a tertiary hospital in a low-to-middle-income country. Glob Heart. (2021) 16(1):25. doi: 10.5334/gh.831
24. García-Montes JA, Camacho-Castro A, Sandoval-Jones JP, Buendía-Hernández A, Calderón-Colmenero J, Patiño-Bahena E, et al. Closure of large patent ductus arteriosus using the amplatzer septal occluder. Cardiol Young. (2015) 25(3):491–5. doi: 10.1017/s1047951114000183
25. Pass RH, Hijazi Z, Hsu DT, Lewis V, Hellenbrand WE. Multicenter USA amplatzer patent ductus arteriosus occlusion device trial: initial and one-year results. J Am Coll Cardiol. (2004) 44(3):513–9. doi: 10.1016/j.jacc.2004.03.074
26. Wang J, Zuo J, Yu S, Yi D, Yang X, Zhu X, et al. Effectiveness and safety of transcatheter closure of perimembranous ventricular septal defects in adults. Am J Cardiol. (2016) 117(6):980–7. doi: 10.1016/j.amjcard.2015.12.036
27. Brida M, Diller GP, Nashat H, Barracano R, Kempny A, Uebing A, et al. Cardiac catheter intervention complexity and safety outcomes in adult congenital heart disease. Heart. (2020) 106(18):1432–7. doi: 10.1136/heartjnl-2019-316148
28. Butera G, Carminati M, Chessa M, Piazza L, Micheletti A, Negura DG, et al. Transcatheter closure of perimembranous ventricular septal defects: early and long-term results. J Am Coll Cardiol. (2007) 50(12):1189–95. doi: 10.1016/j.jacc.2007.03.068
29. Myers PO, Tissot C, Beghetti M. Assessment of operability of patients with pulmonary arterial hypertension associated with congenital heart disease. Circ J. (2014) 78(1):4–11. doi: 10.1253/circj.cj-13-1263
30. van der Feen DE, Bartelds B, de Boer RA, Berger RMF. Assessment of reversibility in pulmonary arterial hypertension and congenital heart disease. Heart. (2019) 105(4):276–82. doi: 10.1136/heartjnl-2018-314025
31. Humbert M, Kovacs G, Hoeper MM, Badagliacca R, Berger RMF, Brida M, et al. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J. (2023) 61(1):2200879. doi: 10.1183/13993003.00879-2022
32. van Riel AC, Blok IM, Zwinderman AH, Wajon EM, Sadee AS, Bakker-de Boo M, et al. Lifetime risk of pulmonary hypertension for all patients after shunt closure. J Am Coll Cardiol. (2015) 66(9):1084–6. doi: 10.1016/j.jacc.2015.06.1318
33. Jone PN, Hinzman J, Wagner BD, Ivy DD, Younoszai A. Right ventricular to left ventricular diameter ratio at end-systole in evaluating outcomes in children with pulmonary hypertension. J Am Soc Echocardiogr. (2014) 27(2):172–8. doi: 10.1016/j.echo.2013.10.014
34. Ghio S, Pazzano AS, Klersy C, Scelsi L, Raineri C, Camporotondo R, et al. Clinical and prognostic relevance of echocardiographic evaluation of right ventricular geometry in patients with idiopathic pulmonary arterial hypertension. Am J Cardiol. (2011) 107(4):628–32. doi: 10.1016/j.amjcard.2010.10.027
35. Jone PN, Ivy DD, Hauck A, Karamlou T, Truong U, Coleman RD, et al. Pulmonary hypertension in congenital heart disease: a scientific statement from the American heart association. Circ Heart Fail. (2023) 16(7):e00080. doi: 10.1161/hhf.0000000000000080
36. D'Alto M, Dimopoulos K, Coghlan JG, Kovacs G, Rosenkranz S, Naeije R. Right heart catheterization for the diagnosis of pulmonary hypertension: controversies and practical issues. Heart Fail Clin. (2018) 14(3):467–77. doi: 10.1016/j.hfc.2018.03.011
Keywords: transcatheter closure, pulmonary arterial hypertension, congenital heart disease, children, follow-up
Citation: Huang Y, Chen Y, Su D, Qin S, Chen C, Liu D, Ye B, Huang Y, Yuan P and Pang Y (2025) Follow-up of transcatheter closure of congenital heart disease complicated with pulmonary arterial hypertension in children. Front. Pediatr. 13:1562782. doi: 10.3389/fped.2025.1562782
Received: 18 January 2025; Accepted: 24 February 2025;
Published: 7 March 2025.
Edited by:
Inga Voges, University Medical Center Schleswig-Holstein, GermanyReviewed by:
Maria Joao Baptista, Centro Hospitalar Universitário de São João (CHUSJ), PortugalCopyright: © 2025 Huang, Chen, Su, Qin, Chen, Liu, Ye, Huang, Yuan and Pang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yusheng Pang, cGFuZ3l1c2hAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.