- 1Princess Máxima Center for Pediatric Oncology, Utrecht, Netherlands
- 2Department of Pediatric Rehabilitation, Wilhelmina Children’s Hospital, University Medical Center Utrecht, Utrecht, Netherlands
- 3Department of Orthopedic Surgery and Sports Medicine, Amsterdam Movement Sciences, Cancer Center Amsterdam, Amsterdam University Medical Centers, University of Amsterdam, Amsterdam, Netherlands
- 4Department of Orthopedic Surgery, Leiden University Medical Center, Leiden, Netherlands
- 5Department of Orthopedic Surgery, Radboud University Medical Center, Nijmegen, Netherlands
- 6Division of Imaging and Oncology, University Medical Center Utrecht, Utrecht University, Utrecht, Netherlands
Introduction: Bone sarcoma patients face intensive treatment, including life-changing local therapy, which impacts both short- and long-term functioning. Moreover, bone sarcoma survivors experience the highest burden of adverse events of all childhood cancer survivors. To address these issues, we set up a structured multidisciplinary outpatient follow-up clinic for patients who completed treatment and integrated this clinic into the standard of care. This study protocol describes the methodology of a cross-sectional study that aims to systematically report the functional outcomes, adverse events, psychosocial outcomes and health-related quality of life of the cohort seen at this clinic.
Methods and analysis: Participants are recruited at the multidisciplinary follow-up clinic and their consent is obtained. Standard of care clinical assessments serve as the primary data source for this study. Furthermore, additional research assessments are performed to further expand our knowledge. Assessments are structured by standardized assessment sets that we developed based on literature review and joint national expertise in bone sarcoma care. The sets comply with international guidelines such as the World Health Organization's International Classification of Functioning, disability and health, and include a combination of patient-reported, clinician-reported and performance-based outcome measures for comprehensive representation of outcomes.
Discussion: This study will generate valuable knowledge on the functional outcomes, adverse events, psychosocial outcomes and quality of life of a national cohort of pediatric bone sarcoma patients in follow-up care. By aligning additional research assessments with standardized patient care, a comprehensive range of outcomes will be obtained while minimizing the patient's burden. Moreover, this protocol may serve as a template for clinics and research internationally, allowing for the merging of standardized outcome data in such rare disease. This will facilitate the optimization of current patient care and inform the important shared decision-making process for local treatment in future patients.
1 Introduction
Osteosarcoma and Ewing sarcoma are the most common malignant bone tumors in children with approximately 35 new diagnoses per year in the Netherlands (1). Treatment consists of neoadjuvant chemotherapy, local therapy and adjuvant chemotherapy. Local therapy is primarily surgical, though Ewing sarcoma patients may also receive radiotherapy based on tumor characteristics or as a standalone treatment in specific cases. Surgical options consist of limb-sparing surgery, including tumor resection followed by reconstruction using allografts, autografts, and/or prostheses, and ablative surgery (i.e., amputation or rotationplasty). The choice of surgery depends on oncological considerations, such as involvement of surrounding tissues and presence of metastases, as well as patient preferences. Given the complexity of treatment, a multidisciplinary approach is crucial for optimal care. Oncologists, orthopedic surgeons, pathologists, radiologists, rehabilitation physicians, physical therapists, and psychologists all play critical roles and must collaborate closely to maximize treatment and rehabilitation outcomes.
Despite this, the intensive treatment regimen of bone sarcoma patients causes them to experience the highest burden of adverse events among pediatric cancer survivors (2). This is largely due to the life-changing local therapy, which impacts both short- and long-term outcomes across multiple domains, i.e., functional outcomes, psychosocial outcomes, and/or overall health-related quality of life (HR-QOL) (3). Consequently, follow-up care for osteosarcoma and Ewing sarcoma patients is multifaceted and complex. In the recent past, our national comprehensive childhood cancer center, and the individual academic centers before the centralization of care, lacked a cohesive multidisciplinary approach to follow-up. This resulted in fragmented care. Typically, patients would visit a pediatric oncologist or late effects specialist regularly and, depending on the type of surgery, occasionally visit an orthopedic surgeon. Additional healthcare professionals, such as a physical therapist, rehabilitation physician and/or psychologist, were called upon indication.
To enhance the quality of follow-up care, integrate multidisciplinary expertise in our center, and streamline policy coordination, we set up a multidisciplinary outpatient follow-up clinic for bone sarcoma patients after completion of antitumor treatment. Through extensive research of literature supplemented with the available national expertise in bone sarcoma care, we identified the domains requiring evaluation during the clinic. As a result, the evaluated outcomes include important aspects of functional outcomes, adverse events, body image issues, emotional distress, social functioning, and overall HR-QOL. To assess these outcomes, we use a combination of patient-reported outcome measures (PROMs), clinician-reported outcome measures (ClinROMs) and performance-based outcome measures (PerBOMs). Standard of care investigations that are part of routine follow-up, as prescribed by international collaborative studies on osteosarcoma and Ewing sarcoma, are integrated according to the respective (inter)national bone sarcoma and late effects guidelines (4–6).
Following the domains requiring evaluation, the clinic includes carousel consultations with a pediatric oncologist or late effects specialist, orthopedic surgeon, rehabilitation physician, physical therapist, and psychologist. Since most follow-up patients were anticipated to benefit from screening by a physical therapist for mobility, strength, and functional movement—while not universally requiring the specialized adjustments provided by occupational therapists—physical therapists were included in the standard team. Evaluation of arm-hand function, typically performed by an occupational therapist, was incorporated into the physical therapist's role when necessary. Similarly, radiation oncologists were not included in the standard team, as the majority of patients were estimated to have not received radiation. Instead, radiotherapy-related late effects were monitored by the pediatric oncologist and orthopedic surgeon. In cases of specific questions or concerns, occupational therapists, radiation oncologists, or other relevant professionals were consulted or referred to as needed. Additionally, given the overlap in physical assessment, physical therapists and rehabilitation physicians conducted joint consultations.
Each multidisciplinary clinic is preceded by a preclinic team meeting among the healthcare professionals in the morning and followed by a postclinic team meeting in the late afternoon, all occurring on the same day. These meetings guarantee dedicated moments for the exchange of findings and interdisciplinary consultation and coordination. This, in turn, promotes effective collaboration and optimal coordinated patient care including streamlined referrals when indicated.
The implementation of this clinic as part of our standard of care, with its comprehensive and structured information gathering in a multidisciplinary format, presents us with an opportunity to systematically acquire valuable insights into the outcomes of a national cohort of bone sarcoma patients during follow-up. Therefore, in this study we aim to systematically evaluate functional outcomes, adverse events, psychosocial outcomes, and HR-QOL in patients treated for pediatric bone sarcoma and seen at the multidisciplinary follow-up clinic. Additionally, we will evaluate the added value of the multidisciplinary and structured care.
The findings of this study will be used to optimize follow-up care for bone sarcoma patients and survivors by identifying common challenges after treatment and refining the multidisciplinary approach to better address their needs. In addition, these insights will enhance the shared decision-making process among newly diagnosed patients, parents, and healthcare professionals by providing more comprehensive outcome data to guide discussions on local therapy options and long-term expectations.
2 Methods and analysis
2.1 Study design
This is a cross-sectional nationwide study conducted at the Princess Máxima Center for pediatric oncology. Since June 2018, all childhood cancer care in the Netherlands has been centralized at the Princess Máxima Center, the national comprehensive childhood cancer center. Prior to this centralization, pediatric oncology care was provided at one of six university medical centers across the country. The Princess Maxima Center treats all children and adolescents (<18 years) with cancer in the Netherlands. Additionally, the center provides lifelong follow-up and late effects care, ensuring continuity of specialized care after childhood cancer well into adulthood.
Prior to full integration into standard care, the multidisciplinary follow-up clinic was tested with two patients to assess the feasibility of assessments and logistics. Since no challenges were encountered, the clinic was subsequently incorporated into standard follow-up care. From that point forward, all patients attending the multidisciplinary follow-up clinic have been screened for eligibility to participate in this study. Recruitment started November 18th, 2021, and the cohort is expected to be concluded within four years.
2.2 Participants
For inclusion a subject must meet all of the following criteria: (1) patient/survivor of osteosarcoma or Ewing(-like) sarcoma at least two years after diagnosis, (2) under 18 years of age at diagnosis, (3) date of diagnosis from 2003, (4) completed antitumor treatment without evidence of disease, (5) had a tumor located in the upper extremity (including the shoulder girdle), lower extremity or pelvis (including the sacrum), and (6) received local therapy with surgery and/or radiotherapy. Patients who do not meet all criteria, patients with a relapse or on palliative treatment, and patients with medical conditions unrelated to the local therapy of the primary tumor that severely limit physical activities are excluded from the study.
2.3 Sample size
Based on the national incidence of osteosarcoma and Ewing sarcoma in the pelvis and extremities in patients under 18 years (approximately 25 patients per year) and a survival rate of around 65%, we estimated that 16 new patients per year would reach follow-up without evidence of disease two years after diagnosis (calculation supported by data from the Dutch Childhood Oncology Group national Childhood Cancer Registry and Long-Term Effects After Childhood Cancer Registry). Given the expected inclusion period, covering patients diagnosed between 2003 and mid-2023, we estimated a total of 330 eligible patients in the Netherlands. At the time of centralization of childhood oncology care to the Princess Máxima Center in 2018, 150 patients were not transferred from the UMCs to the Princess Máxima Center for follow-up and late effects care (out of approximately 280 in total). This was primarily because two of the four national orthopedic oncology UMCs retained follow-up care for their cohorts. As a result, we anticipated inviting approximately 180 patients to the clinic. Considering a non-participation rate of 20%, we estimated that around 145 patients would be included in the study: 20 with tumors in the upper extremity, 20 with pelvic tumors, and 105 with lower extremity tumors.
2.4 Informed consent
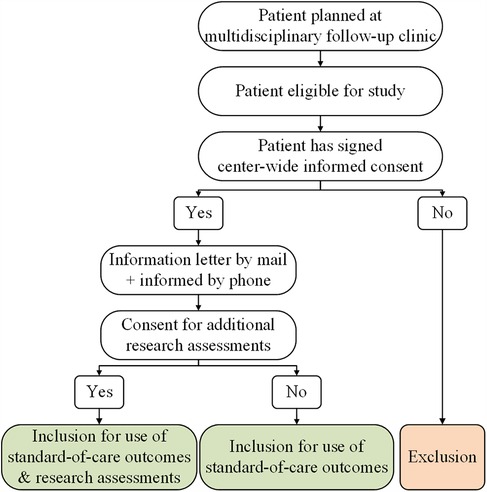
Our hospital has implemented a center-wide informed consent policy that covers the usage of all clinical data collected throughout a patient's journey. In accordance with this written consent, outcomes derived from the standard care provided at our multidisciplinary follow-up clinic can be used for the study if the patient is considered eligible. In addition, eligible patients are presented with the opportunity to participate in a small set of additional research assessments. Since these assessments go beyond the scope of the clinic's standard care, separate consent is required. Hence, patients are requested to provide written informed consent specifically for the additional research assessments conducted as part of this study (Figure 1). The incorporation of additional research assessments within the setup of the multidisciplinary follow-up clinic is illustrated in Figure 2.
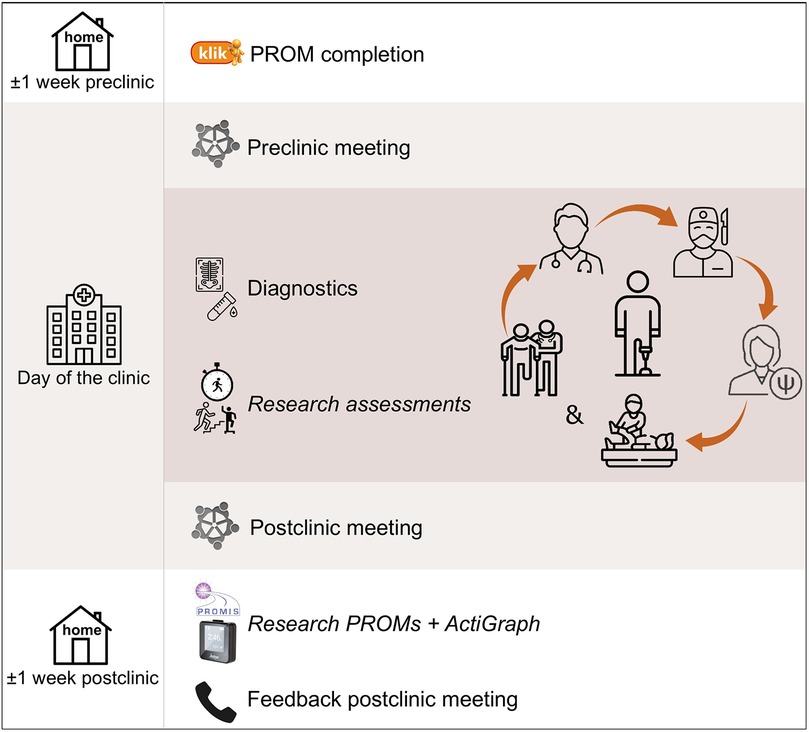
Figure 2. Setup of multidisciplinary clinic including incorporation of research assessments in italics.
2.5 Outcomes
The primary outcome of the study measures functional outcomes as classified by the World Health Organization's (WHO) International Classification of Functioning, disability and health (ICF) (see “conceptual frameworks” below) (7). Secondary outcomes include adverse events, psychosocial outcomes and HR-QOL. Furthermore, we will evaluate the multidisciplinary follow-up clinic by assessing: (1) patient satisfaction with the received care and (2) the output of care, defined as the action points initiated as a direct outcome of the clinic.
2.6 Conceptual frameworks
Two conceptual frameworks were selected to support the structured evaluation of the outcomes. For the functional outcomes we used the WHO's ICF (7). According to this framework, functioning is classified into three levels. “Body functions and structures” addresses the anatomical parts and physiological functions of body systems. “Activities” refers to the ability to execute tasks, and “Participation” describes to which extend a person can carry out their normal role functioning (Figure 3). Even though this framework was designed to map a patient's overall functioning, including e.g., physical, psychosocial and personal aspects, it is particularly useful in understanding and systematically describing functional outcomes.
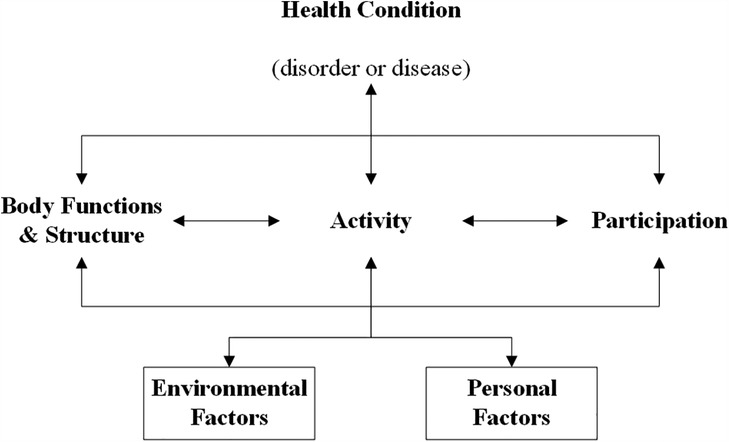
Figure 3. International classification of functioning, disability and health. Original work by “World Health Organization (7). License: CC BY-NC-SA 3.0 IGO”.
Since the ICF describes the various components of psychosocial outcomes and HR-QOL less detailed, we used the framework reported by Anthony et al. to systematically classify and assess these outcomes (8). In this review, items of quality of life measures used in pediatric oncology are analyzed and categorized into major domains, subdomains and identifying concepts, leading to a structured overview of the aspects of HR-QOL in this population.
2.7 Measurement of outcomes
Data on study outcomes are derived from: (1) a structured evaluation of standard-of-care assessments at the multidisciplinary follow-up clinic, and (2) additional research assessments.
Assessments already in use in follow-up care or deemed essential but inconsistently assessed or documented, were standardized into a core measurement set to ensure uniformity across patients. Beyond these standard-of-care assessments, we included research-specific evaluations expected to deepen understanding and potentially guide future clinical practice.
2.7.1 Standard of care measurement set
The standard of care assessments conducted at the multidisciplinary follow-up clinic comprise a spectrum of measures that collectively cover the domains important to patients with bone sarcoma. The following aspects were considered in the selection process: suitability for the age of the bone sarcoma population, availability of textual measurements in Dutch (for PROMs), psychometric properties, availability of norm values (if applicable), adequate coverage of relevant domains with minimal overlap, and acceptable patient burden in terms of question content and time investment. Patients are requested to complete the PROMs at home via the KLIK (Quality of Life in Clinical Practice) PROM online portal prior to the clinic visit (9). ClinROMs and PerBOMs are conducted during the clinic itself.
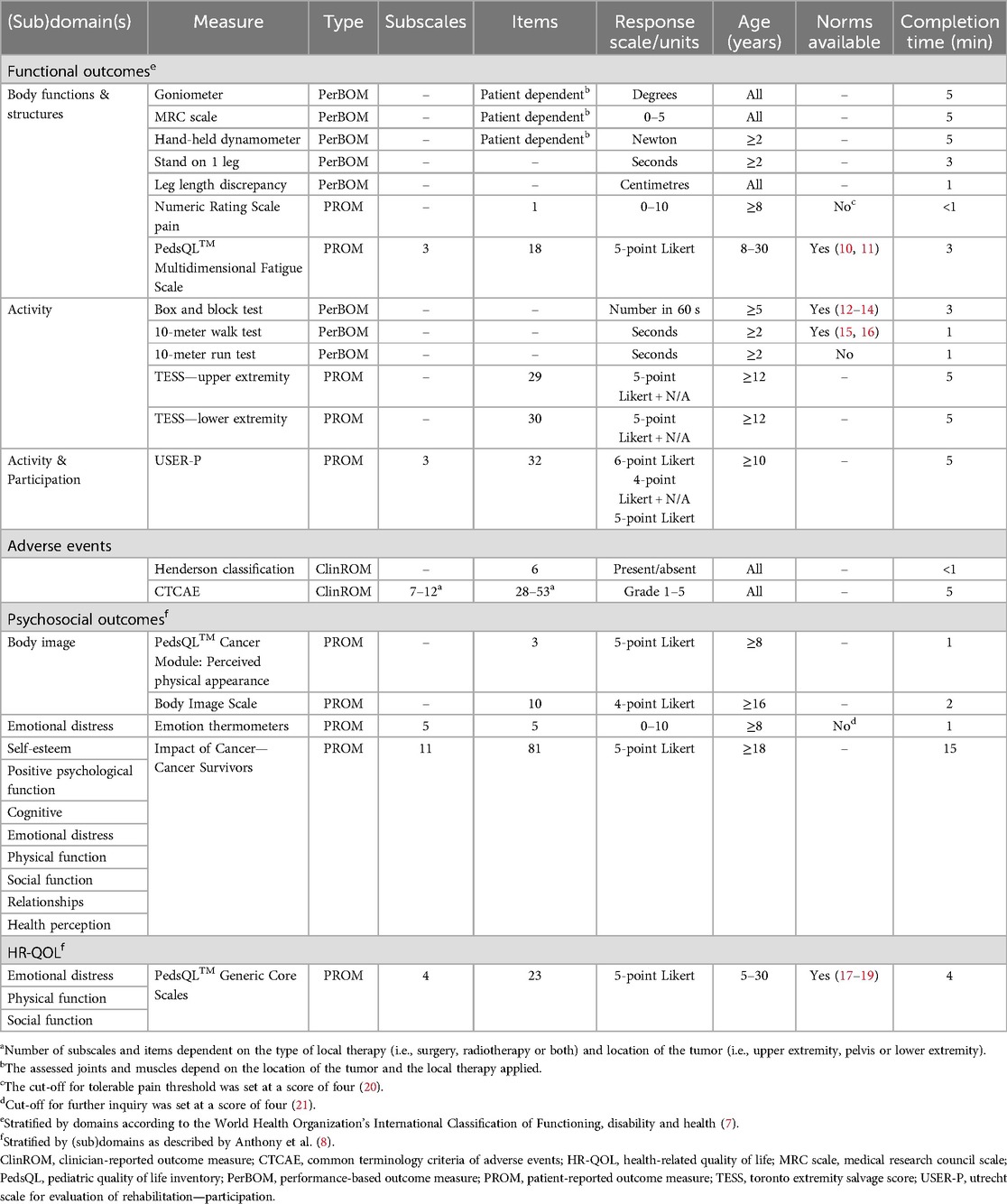
A brief description of the included standard of care assessments per outcome is described below. The detailed properties of the assessments, along with their corresponding domains as described by the ICF (for functional outcomes) and by Anthony et al. (for psychosocial outcomes), are presented in Table 1 (7, 8).
2.7.1.1 Functional outcomes
A standardized physical assessment stratified by affected body part and/or type of surgery, is completed by the physical therapist and rehabilitation physician (see Supplementary Data Sheets S1 and S2). Joints and muscles are structurally examined including range of motion, muscle power, and functional and timed testing.
The box and block test is included for patients with upper extremity tumors to assess lateral movement of the arm in the frontal space, which is crucial in daily life (12, 13). For this test, a patient is asked to transfer as many blocks as possible using one hand from one side of the box, over a 15-centimeter high partition, to the other side of the box within 60 s. The test is performed independently for each hand.
Standing on one leg and the 10-meter walk and 10-meter run tests are performed in patients with pelvic or lower extremity tumors to assess balance, walking and running, which play a crucial role in daily functioning (22).
Patients rate their pain intensity over the past week using a numeric rating scale, where 0 represents no pain and 10 the worst imaginable pain (23).
General fatigue, sleep/rest, and cognitive fatigue are evaluated by the Pediatric Quality of Life Inventory Multidimensional Fatigue Scale (PedsQL™ MFS) self-report (24).
The Toronto Extremity Salvage Score (TESS) assesses the ability to perform daily life activities. This PROM includes separate versions for upper and pelvic/lower extremity tumors (25, 26).
The Utrecht Scale for Evaluation of Rehabilitation—Participation (USER-P) explores the frequency of daily activities, experienced limitations, and patient satisfaction with functioning (27, 28).
2.7.1.2 Collection of adverse events
The Common Terminology Criteria for Adverse Events (CTCAE) is used to report the adverse events resulting from local therapy (surgery and/or radiotherapy) that are encountered at the follow-up clinic (29). This system comprises a large number of events graded on five levels from mild to death. To address the most relevant events in our outpatient clinic population, a selection of CTCAE terms was made stratified by tumor location and type of local therapy (see Supplementary Data Sheet S3). Completion of CTCAE items is distributed based on expertise among the pediatric oncologist/late effects specialist, orthopedic surgeon, and rehabilitation specialist. For example, the orthopedic surgeon assesses musculoskeletal and connective tissue disorders such as decreased joint range of motion, osteonecrosis, and muscle weakness. The rehabilitation specialist evaluates pain, gait abnormalities, and lymphedema, while the pediatric oncologist/late effects specialist assesses gastrointestinal symptoms following radiotherapy.
Failure modes of reconstructions after limb-sparing surgery are recorded by the orthopedic surgeon using the Henderson classification (30). Two versions of this classification are available: one for failure of endoprostheses and another for biological reconstructions.
2.7.1.3 Psychosocial outcomes and HR-QOL
Based on literature review supplemented with psychosocial expertise in our center, the selection of measures for psychosocial outcomes and HR-QOL was focused on coverage of the (sub)domains “emotional distress”, “body image”, “social function” and overall HR-QOL (8).
The Emotion Thermometers tool measures emotional distress in the past week by asking the patient to rate “Distress”, “Depression”, “Anxiety”, and “Anger” on a visual analogue scale, where zero represents none and ten represents extreme (21, 31). A fifth thermometer assesses the “Need for help”, ranging from being able to manage by yourself to being desperately in need of help.
Because of the impact of local therapy on patients' physical appearance, body image is considered crucial in counselling and evaluating patients' well-being. Body image is measured in all patients by a subscale of the Pediatric Quality of Life Inventory Cancer Module (PedsQL™ CM) self-report called “Perceived physical appearance” (24). For patients 16 years and older, this evaluation is supplemented by the Body Image Scale (BIS); a short PROM originally developed for cancer survivors (32).
The Impact Of Cancer–Cancer Survivors (IOC-CS) addresses the impact of childhood cancer on adult life (33). This PROM encompasses a comprehensive assessment of diverse facets of long-term survivorship.
The Pediatric Quality of Life Inventory Generic Core Scales (PedsQL™ GCS) self-report examines overall HR-QOL by including physical functioning, emotional functioning, social functioning and school/work functioning (34). It allows for longitudinal assessment by providing distinct self-report forms for different age groups.
2.7.2 Research measurement set
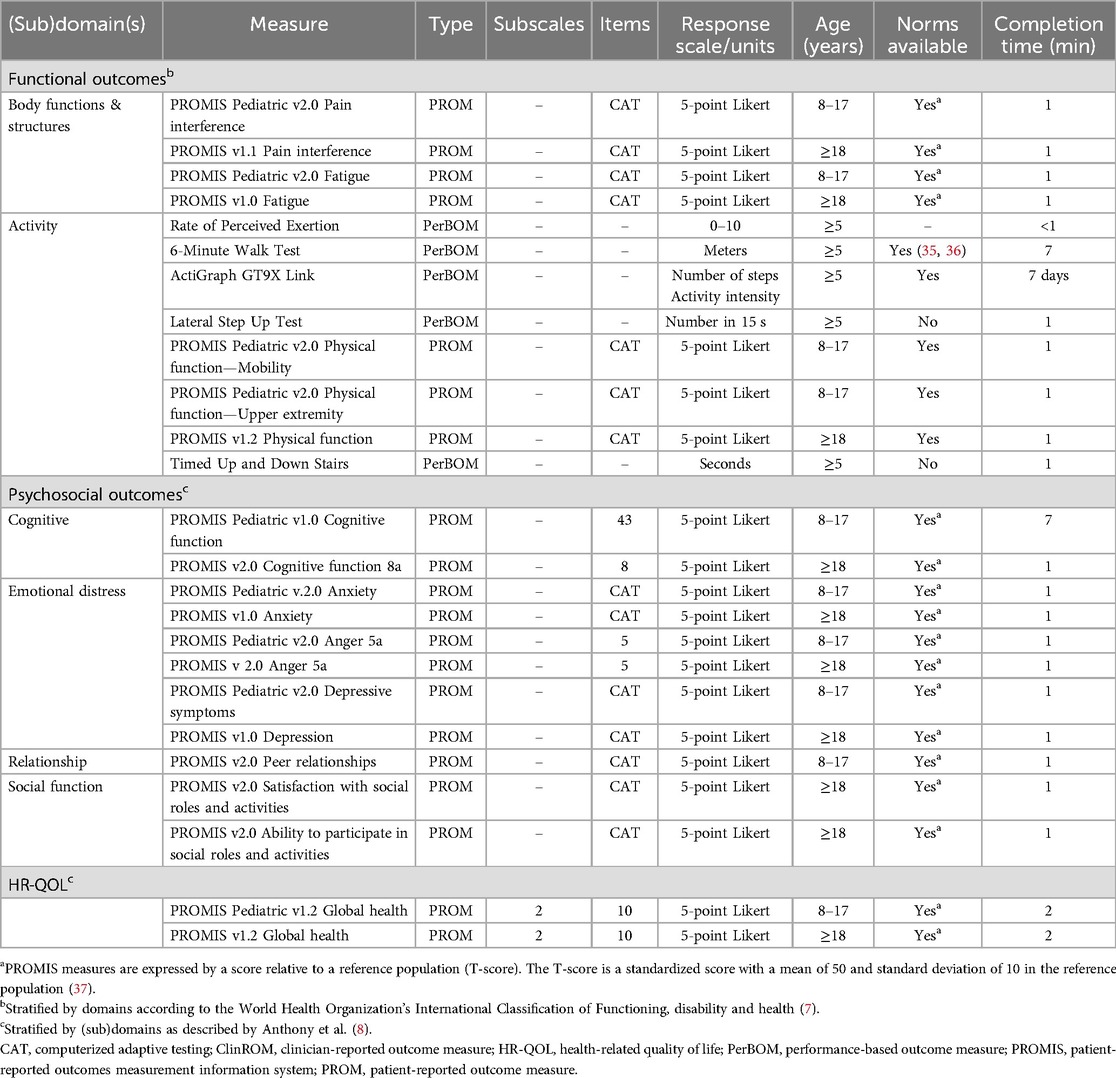
The measures described below are performed in patients who consent for the additional research measurements of the study. Detailed properties of the assessments are listed in Table 2.
2.7.2.1 Functional outcomes
The 6-Minute Walk Test (6-MWT) is employed to expand the assessment of walking. Patients walk as far as possible within a six-minute timeframe on a flat surface of at least 20 meters in length (38).
After the 6-MWT, the rate of perceived exertion (RPE) is recorded by asking the patient to rate the effort required to perform the 6-MWT on a scale from one to ten with higher numbers representing increasing intensity levels (39).
The Timed Up and Down Stairs (TUDS) is executed to further deepen the assessment of daily life activities by recording the time needed to ascend and descend one flight of stairs (40).
Functional strength of the legs is assessed by the Lateral Step Up Test (LSUT). To start this test, the patient places the leg being tested on a 15-centimeter high step, while the other leg remains on the floor. The patient is then instructed to fully extend the hip and knee of the tested leg, followed by flexion of the hip and knee until the contralateral foot touches the floor again. The final score is the number of movements within 15 seconds (41).
The ActiGraph GT9X Link (ActiGraph, LLC, Pensacola, FL) is used to objectively measure physical activity. This is a small device worn around the hip that measures step counts, acceleration (and therefore intensity of an activity) and posture. Patients are asked to wear the ActiGraph at home for seven days during day time. For adequate interpretation of results, patients are instructed to keep a brief diary noting the time periods when the ActiGraph was not worn.
The 6-MWT, RPE, TUDS and LSUT are exclusively conducted in patients in follow-up for a pelvic or lower extremity tumor. The ActiGraph is worn by all patients.
2.7.2.2 Psychosocial outcomes and HR-QOL
The Patient-Reported Outcomes Measurement Information System (PROMIS) was developed by the National Institutes of Health (NIH) (42, 43). It contains a great variety of item banks for patient-reported measurements of physical, mental, and social health in children and adults. The item banks selected to evaluate problems within our bone sarcoma population are: global health, pain interference, fatigue, physical function, anxiety, anger, depressive symptoms/depression, cognitive functioning, peer relationships (children only), ability to participate in social roles and activities (adults only) and satisfaction with social roles and activities (adults only). When available, computerized adaptive testing (CAT) is used to reduce the number of items needed to complete the item bank. In the Netherlands and Belgium, the Dutch-Flemish PROMIS was created for the translation of the NIH item banks (44, 45). PROMIS is expected to dominate the field of questionnaires in the near future because of increasing use in research and expected rapid integration in patient care.
2.7.3 Evaluation of multidisciplinary care
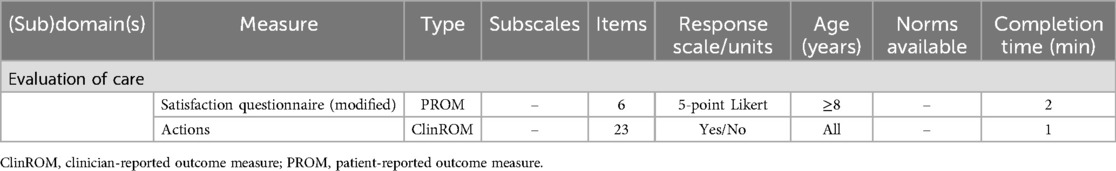
Patient satisfaction with the multidisciplinary follow-up care is assessed via a modified version of the satisfaction questionnaire developed by Blaauwbroek et al. (see Supplementary Data Sheet S4, Table 3) (46).
To record the output of care, a predefined action list is utilized, which systematically tracks the output of the clinic for each patient (see Supplementary Data Sheet S5, Table 3). Relevant action points per healthcare professional can be marked, provided that they are in response to a problem or question identified during the clinic. Actions resulting from standard information provision are not noted, since these do not indicate the clinic's added value. Examples of recorded actions include “redirection of primary care physical therapy”, “psychoeducation”, “referral to a primary care psychologist”, and “tailored advice or information provision”. Any changes in the policy of an individual healthcare professional or the establishment of a new policy based on collective consultation are also documented in the action list.
In line with the study protocol, we conducted an interim analysis of patient satisfaction after the first 60 patients were included. We expected at least 37 out of 60 patients (lower limit of the exact 95% confidence interval: 50%), meaning at least 50%, to be satisfied with the multidisciplinary clinic for it to continue. Otherwise, revisions would need to be made based on patients' feedback where necessary. Patient satisfaction was notably high, with 98% of patients reporting satisfaction with the care provided during the multidisciplinary clinic. As a result, the clinic was continued as planned.
2.8 Clinical data collection
Data are collected in Castor EDC, a web-based electronic data capture platform for clinical research (47). At inclusion, patients are registered in Castor via a record ID. A separate subject identification list is kept password-protected by the study coordinators. The data collected include patient characteristics such as age and sex, oncological diagnosis, treatment, and follow-up details including ClinROMs and PerBOMs completed at the clinic visit. PROMs are presented to patients via the KLIK (Quality of Life in Clinical Practice) PROM portal, a web-based platform designed for completing PROM assessments that is widely used in our hospital (9). The KLIK PROM portal enables patients and/or parents to complete assessments on HR-QOL, symptoms and psychosocial and physical functioning at home prior to scheduled outpatient consultations. The results are presented to the healthcare professionals through an ePROfile that is integrated with the patient's electronic health record. This ePROfile allows healthcare professionals to monitor and screen for potential issues and discuss the results with the patient and/or parent during consultation (48). To facilitate secure delivery of pseudonymized data from the KLIK PROM portal at study closure, the subject's record ID is entered into his/her account by the study coordinators upon enrolment.
2.9 Privacy
Access to the Castor database is secured by login credentials and is restricted to the principal investigator (JM), two study coordinators (LT and HiV), two supervising researchers (LH and WB), and a data manager. The data only contain pseudonymized data and are entered into the database by the study coordinators. As per hospital guidelines, data will be stored for 15 years after study closure. During the inclusion period of the study, outcomes in the KLIK PROM portal can be accessed solely by treating healthcare professionals. At study closure, a pseudonymized export file including all the data will be generated by the KLIK PROM team based on the record IDs and informed consent forms of the subjects.
2.10 Quality of data collection
The KLIK PROM portal requires subjects to complete all items before a PROM can be finished, preventing missing values. One of the study coordinators (LT) reviews the scoring of the adverse events following every clinic to ensure consistency and verify item completion. Inconsistencies or missing items are addressed by returning them to the healthcare professional for amendment.
2.11 Data analysis and general statistical considerations
Descriptive statistics will be provided for the entire group and relevant subgroups. Categorical variables will be presented as frequencies (n) and percentages (%). Continuous variables will be plotted graphically to assess distribution and determine the most appropriate method for data presentation and analysis. Means and standard deviations will be reported if variables are normally distributed. Medians and IQR's will be described otherwise. General considerations of the statistical analysis are described below. Detailed hypotheses and associated statistical analysis methods will be presented in subsequent papers stemming from this study.
The relationships between the outcomes, type of local therapy, and patient- and cancer-related factors will be explored using ANOVA, Bland-Altman analysis, or correlation coefficients, depending on the hypothesis and type of variable. If the data permits, a multiple regression analysis will be performed. Patient satisfaction and output of care will be expressed by descriptive statistics. If norm data are available, means will be compared using two sample t-tests, ANOVA or nonparametric alternatives depending on the variable. If raw norm data is available, scores will be compared by regression analysis if assumptions can be met.
Imputation will be applied to missing data for patient and tumor characteristics. The method of imputation will depend on the percentage of missing data (49). The handling of missing values for the outcome measures will be decided upon at data analysis and will be described in the subsequent papers. To account for multiple testing, the Benjamini-Hochberg procedure will be applied to mitigate the risk of false discovery.
Statistical analyses will be performed using R (50).
2.12 Ethical considerations
The Medical Research Ethics Committee of the University Medical Center Utrecht (Utrecht, the Netherlands) confirmed that the Medical Research Involving Human Subjects Act does not apply to this study.
In accordance with Dutch ethical regulations, informed consent must be obtained from both parents or the guardian for patients aged 15 years and younger. For patients from the age of 12, informed consent from the patient is also required. Patients aged 16 years and older are entitled to give their own consent.
3 Discussion
Involvement of a multidisciplinary team is essential in the care of patients with musculoskeletal sarcoma and is generally well-arranged for patients on-treatment (51). However, once patients transition to follow-up care, guidelines and logistics for maintaining this comprehensive approach are often less well established. Given the complexity and impact of local therapy and the duration and intensity of chemotherapy, it is important to continue multidisciplinary care for bone sarcoma patients after completion of primary treatment. Comprehensive and collaborative care by specialized healthcare professionals is needed to effectively identify and manage adverse events, rehabilitation challenges, and difficulties in the patients' daily life. To address this, a multidisciplinary follow-up clinic was established at our national hospital, providing a structured and systematic evaluation of bone sarcoma patients after local therapy across a wide range of outcomes. This approach enhances follow-up care for individual bone sarcoma patients, while simultaneously enabling the study of outcomes from a unique nationwide pediatric bone sarcoma cohort.
There is a growing body of literature assessing outcomes in bone sarcoma patients; however, defining clear benchmarks remains challenging due to the heterogeneity of the population and the predominance of small and/or outdated cohorts (3, 52–55). Existing studies often focus on short-term complications or chemotherapy-related events but lack a comprehensive evaluation of the permanent local therapy-related adverse events (2, 56–58). Functional outcomes are frequently assessed with limited scope, without fully covering the domains outlined by the ICF (53, 54, 59, 60). Additionally, studies comparing functional outcomes across different surgical approaches show variability in findings (61–63). This variability is also observed for psychosocial outcomes and quality of life, underscoring the need for further exploration (3).
Our study, which evaluates a large nationwide cohort of bone sarcoma patients, will significantly contribute to the existing knowledge base by providing a more comprehensive understanding of local therapy-related outcomes in follow-up. By identifying prevalent challenges, comparing surgical approaches, and exploring associated factors, we aim to enhance patient counseling, optimize intervention strategies, and take the first steps toward defining clearer benchmarks. For newly diagnosed patients, these findings will also inform the decision support models used in shared-decision making for local therapy options, and help guide postoperative care and rehabilitation to improve long-term patient outcomes.
3.1 Strengths and limitations
By adhering to international frameworks and incorporating the extensive expertise of our bone sarcoma experts along with literature review, this study relies on a comprehensive assessment of all the domains relevant to this patient population. Importantly, we have minimized the patient's assessment burden by aligning the study of outcomes with standardized patient care. Moreover, this systematic and comprehensive protocol creates a framework for international comparison and collaboration which is crucial for increasing our knowledge of the outcomes of bone sarcoma patients.
This study also has several limitations. First, the cross-sectional design hinders the evaluation of changes in outcomes over time. However, since the standardized assessment measures are part of our standard of care, we will collect longitudinal data in the future that could be used in a subsequent study. Second, while all childhood oncology care in the Netherlands was centralized at the Princess Máxima Center in 2018, not all patients in follow-up were transferred from the UMCs. Since this selection was based on the treating UMC rather than patient or tumor characteristics, we do not expect this to have impact on the representativeness of our cohort compared to the national pediatric bone sarcoma population. Third, despite our focus on patients with bone sarcoma of the extremities and pelvis (including 75% of bone sarcoma patients), we expect that subcohort analysis will remain limited to the most frequent bone sarcoma locations and interventions. However, by sharing our study protocol with the international community, we expect that merging internationally generated data will facilitate more detailed subcohort analysis in the near future. Finally, while this study focuses on the most prevalent bone sarcoma sites, i.e., the extremities and pelvis, we recognize the importance of multidisciplinary follow-up for patients with bone sarcomas at other locations. These patients face distinct challenges, requiring specialized assessments and healthcare professionals, making their inclusion in the current standardized clinic suboptimal. We are working toward expanding this approach to ensure comparable multidisciplinary care for all bone sarcoma patients, specifically designed for each tumor site, including head and neck and trunk.
3.2 Dissemination
The data will be published according to the methodology described above. After study closure, the database and subject identification list will be managed by the trial and data center of our hospital. Any future requests for the use of study data will be assessed by the trial and data center.
The study coordinators will report the results of the study as soon as possible after finalization of the data analysis through multiple publications. We will adhere to the guidelines of the publishing journal for determination of the authorships.
4 Conclusion
In this study, we systematically assess functional outcomes, adverse events, psychosocial outcomes, and health-related quality of life in a nationwide cohort of pediatric bone sarcoma patients in follow-up. The integrated multidisciplinary approach ensures optimal evaluation of key outcomes for these patients, while enabling transdisciplinary advice and personalized care strategies. By aligning research with standardized multidisciplinary care, we minimize the burden of additional research measurements while studying a comprehensive range of outcomes that will significantly contribute to the current knowledge base.
By sharing our study protocol, we aim to provide a framework for international collaboration generating valuable data on outcomes in this rare patient group to optimize their follow-up care. This data will also inform decision support models that enhance the shared decision-making process for patients facing life-changing local therapy, guide postoperative care and rehabilitation, and improve long-term care for future survivors.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The requirement of ethical approval was waived by Medical Research Ethics Committee Utrecht for the studies involving humans because the Medical Research Involving Human Subjects Act (WMO) does not apply to the study. Therefore, an official approval of this study by the MREC Utrecht is not required under the WMO. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
LT: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. LH: Conceptualization, Methodology, Writing – review & editing. WB: Conceptualization, Investigation, Methodology, Writing – review & editing. IO: Investigation, Methodology, Writing – review & editing. CR: Investigation, Writing – review & editing. HiV: Investigation, Project administration, Writing – review & editing. LB: Methodology, Writing – review & editing. JV: Methodology, Writing – review & editing. ML: Methodology, Writing – review & editing. RS-L: Methodology, Writing – review & editing. AV: Writing – review & editing, Methodology. HM-S: Formal analysis, Writing – review & editing. AP: Writing – review & editing. HeV: Writing – review & editing. MK: Writing – review & editing. LK: Methodology, Writing – review & editing. SW: Investigation, Methodology, Writing – review & editing. HaV: Formal analysis, Writing – review & editing. JB: Investigation, Methodology, Writing – review & editing. MV: Investigation, Methodology, Writing – review & editing. MG: Conceptualization, Methodology, Writing – review & editing. HS: Conceptualization, Investigation, Methodology, Writing – review & editing. JM: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1534153/full#supplementary-material
References
1. Dutch Childhood Oncology Group Registry. Available at: https://www.skion.nl/info/4208/kinderoncologie-in-cijfers/4209/skion-basisregistratie (Accessed September 29, 2023).
2. Geenen MM, Cardous-Ubbink MC, Kremer LCM, Van Den Bos C, Van Der Pal HJH, Heinen RC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. J Am Med Assoc. (2007) 297:2705–15. doi: 10.1001/jama.297.24.2705
3. Stokke J, Sung L, Gupta A, Lindberg A, Rosenberg AR. Systematic review and meta-analysis of objective and subjective quality of life among pediatric, adolescent, and young adult bone tumor survivors. Pediatr Blood Cancer. (2015) 62:1616–29. doi: 10.1002/pbc.25514
4. Whelan JS, Bielack SS, Marina N, Smeland S, Jovic G, Hook JM, et al. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment. Ann Oncol. (2015) 26:407–14. doi: 10.1093/annonc/mdu526
5. Anderton J, Moroz V, Marec-Bérard P, Gaspar N, Laurence V, Martín-Broto J, et al. International randomised controlled trial for the treatment of newly diagnosed EWING sarcoma family of tumours—EURO EWING 2012 protocol. Trials. (2020) 21:1–9. doi: 10.1186/s13063-019-4026-8
6. de Bont JM, van der Pal HJH, Koopman MMW, Kremer LCM, Loonen JJ, Louwerens M, et al. Follow-up na kinderkanker, meer dan 5 jaar na diagnose. Update 2023. (2023). Available at: https://www.skion.nl/ondersteunende-behandelingen-kwaliteit-van-leven (Accessed February 24, 2025).
7. World Health Organization. International Classification of Functioning, Disability and Health. Geneva: ICF (2001). Available at: https://iris.who.int/handle/10665/42407 (Accessed August 12, 2024).
8. Anthony SJ, Selkirk E, Sung L, Klaassen RJ, Dix D, Scheinemann K, et al. Considering quality of life for children with cancer: a systematic review of patient-reported outcome measures and the development of a conceptual model. Qual Life Res. (2014) 23:771–89. doi: 10.1007/s11136-013-0482-x
9. Schepers SA, Sint Nicolaas SM, Haverman L, Wensing M, Schouten van Meeteren AYN, Veening MA, et al. Real-world implementation of electronic patient-reported outcomes in outpatient pediatric cancer care. Psychooncology. (2017) 26:951–9. doi: 10.1002/pon.4242
10. Gordijn SM, Cremers EMP, Kaspers GJL, Gemke RJBJ. Fatigue in children: reliability and validity of the Dutch PedsQLTM multidimensional fatigue scale. Qual Life Res. (2011) 20:1103–8. doi: 10.1007/s11136-010-9836-9
11. Haverman L, Limperg PF, van Oers HA, van Rossum MAJ, Maurice-Stam H, Grootenhuis MA. Psychometric properties and Dutch norm data of the PedsQL multidimensional fatigue scale for young adults. Qual Life Res. (2014) 23:2841–7. doi: 10.1007/s11136-014-0734-4
12. Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the box and block test of manual dexterity. Am J Occup Ther. (1985) 39:386–91. doi: 10.5014/ajot.39.6.386
13. Mathiowetz V, Federman S, Wiemer D. Box and block test of manual dexterity: norms for 6–19 year olds. Can J Occup Ther. (1985) 52:241–5. doi: 10.1177/000841748505200505
14. Jongbloed-Pereboom M, Nijhuis-van der Sanden MWG, Steenbergen B. Norm scores of the box and block test for children ages 3–10 years. Am J Occup Ther. (2013) 67:312–8. doi: 10.5014/ajot.2013.006643
15. Bohannon RW, Williams Andrews A. Normal walking speed: a descriptive meta-analysis. Physiotherapy. (2011) 97:182–9. doi: 10.1016/j.physio.2010.12.004
16. Cadieux JM, Pyhala SL, Johnson JV. Pediatric walking speed normal reference values in a local population. Pediatr Phys Ther. (2023) 35:314–20. doi: 10.1097/PEP.0000000000001015
17. Schepers SA, van Oers HA, Maurice-Stam H, Huisman J, Verhaak CM, Grootenhuis MA, et al. Health related quality of life in Dutch infants, toddlers, and young children. Health Qual Life Outcomes. (2017) 15:81. doi: 10.1186/s12955-017-0654-4
18. Engelen V, Haentjens MM, Detmar SB, Koopman HM, Grootenhuis MA. Health related quality of life of Dutch children: psychometric properties of the PedsQL in The Netherlands. BMC Pediatr. (2009) 9:68. doi: 10.1186/1471-2431-9-68
19. Limperg PF, Haverman L, van Oers HA, van Rossum MAJ, Maurice-Stam H, Grootenhuis MA. Health related quality of life in Dutch young adults: psychometric properties of the PedsQL generic core scales young adult version. Health Qual Life Outcomes. (2014) 12:9. doi: 10.1186/1477-7525-12-9
20. Gerbershagen HJ, Rothaug J, Kalkman CJ, Meissner W. Determination of moderate-to-severe postoperative pain on the numeric rating scale: a cut-off point analysis applying four different methods. Br J Anaesth. (2011) 107:619–26. doi: 10.1093/bja/aer195
21. Mitchell AJ, Baker-Glenn EA, Granger L, Symonds P. Can the distress thermometer be improved by additional mood domains? Part I. Initial validation of the emotion thermometers tool. Psychooncology. (2010) 19:125–33. doi: 10.1002/pon.1523
22. Collen FM, Wade DT, Bradshaw CM. Mobility after stroke: reliability of measures of impairment and disability. Int Disabil Stud. (1990) 12:6–9. doi: 10.3109/03790799009166594
23. Hays RD, Bjorner JB, Revicki DA, Spritzer KL, Cella D. Development of physical and mental health summary scores from the patient-reported outcomes measurement information system (PROMIS) global items. Qual Life Res. (2009) 18:873–80. doi: 10.1007/s11136-009-9496-9
24. Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL™ in pediatric cancer: reliability and validity of the pediatric quality of life inventory™ generic core scales, multidimensional fatigue scale, and cancer module. Cancer. (2002) 94:2090–106. doi: 10.1002/cncr.10428
25. Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS. Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res. (1996) 5:508–16. doi: 10.1007/BF00540024
26. Willeumier JJ, Van Der Wal CWPG, Van Der Wal RJP, Dijkstra PDS, Vliet Vlieland TPM, Van De Sande MAJ. Cross-cultural adaptation, translation, and validation of the Toronto extremity salvage score for extremity bone and soft tissue tumor patients in Netherlands. Sarcoma. (2017) 2017:1–6. doi: 10.1155/2017/6197525
27. Post MWM, van de Port IGL, Kap B, van Berlekom SHB. Development and validation of the Utrecht scale for evaluation of clinical rehabilitation (USER). Clin Rehabil. (2009) 23:909–17. doi: 10.1177/0269215509341524
28. Zee CH, Kap A, Mishre RR, Schouten EJ, Post MWM. Responsiveness of four participation measures to changes during and after outpatient rehabilitation. J Rehabil Med. (2011) 43:1003–9. doi: 10.2340/16501977-0879
29. US Department of Health and Human Services. National Institutes of Health. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. (2017). Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50 (Accessed September 29, 2023)).
30. Henderson ER, O’Connor MI, Ruggieri P, Windhager R, Funovics PT, Gibbons CL, et al. Classification of failure of limb salvage after reconstructive surgery for bone tumours: a modified system including biological and expandable reconstructions. Bone Joint J. (2014) 96B:1436–40. doi: 10.1302/0301-620X.96B11.34747
31. Mitchell AJ, Baker-Glenn EA, Park B, Granger L, Symonds P. Can the distress thermometer be improved by additional mood domains? Part II. What is the optimal combination of emotion thermometers? Psychooncology. (2010) 19:134–40. doi: 10.1002/pon.1557
32. Hopwood P, Fletcher I, Lee A, Al Ghazal S. A body image scale for use with cancer patients. Eur J Cancer. (2001) 37:189–97. doi: 10.1016/S0959-8049(00)00353-1
33. Zebrack BJ, Donohue JE, Gurney JG, Chesler MA, Bhatia S, Landier W. Psychometric evaluation of the impact of cancer (IOC-CS) scale for young adult survivors of childhood cancer. Qual Life Res. (2010) 19:207–18. doi: 10.1007/s11136-009-9576-x
34. Varni JW, Seid M, Kurtin PS. PedsQL™ 4.0: reliability and validity of the pediatric quality of life inventory™ version 4.0 generic core scales in healthy and patient populations. Med Care. (2001) 39:800–12. doi: 10.1097/00005650-200108000-00006
35. Mylius CF, Paap D, Takken T. Reference value for the 6-minute walk test in children and adolescents: a systematic review. Expert Rev Respir Med. (2016) 10:1335–52. doi: 10.1080/17476348.2016.1258305
36. Gibbons WJ, Fruchter N, Sloan S, Levy RD. Reference values for a multiple repetition 6-minute walk test in healthy adults older than 20 years. J Cardiopulm Rehabil. (2001) 21:87–93. doi: 10.1097/00008483-200103000-00005
37. Reeve BB, Hays RD, Bjorner JB, Cook KF, Crane PK, Teresi JA, et al. Psychometric evaluation and calibration of health-related quality of life item banks: plans for the patient-reported outcomes measurement information system (PROMIS). Med Care. (2007) 45:22–31. doi: 10.1097/01.mlr.0000250483.85507.04
38. Butland R, Pang J, Gross E, Woodcock A, Geddes D. Two-, six-, and 12-minute walking tests in respiratory disease. Br Med J. (1982) 284:1607–8. doi: 10.1136/bmj.284.6329.1607
39. Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. (1970) 2:92–8. doi: 10.2340/1650197719702239298
40. Zaino CA, Marchese VG, Westcott SL. Timed up and down stairs test: preliminary reliability and validity of a new measure of functional mobility. Pediatr Phys Ther. (2004) 16:90–8. doi: 10.1097/01.PEP.0000127564.08922.6A
41. Worrell TW, Borchert B, Erner K, Fritz J, Leerar P. Effect of a lateral step-up exercise protocol on quadriceps and lower extremity performance. J Orthop Sports Phys Ther. (1993) 18:646–53. doi: 10.2519/jospt.1993.18.6.646
42. Cella D, Yount S, Rothrock N, Gershon R, Cook K, Reeve B, et al. The patient-reported outcomes measurement information system (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. (2007) 45:S3–S11. doi: 10.1097/01.mlr.0000258615.42478.55
43. HealthMeasures: PROMIS. Available at: http://www.healthmeasures.net/promis (Accessed September 29, 2023)).
44. Terwee CB, Roorda LD, de Vet HCW, Dekker J, Westhovens R, van Leeuwen J, et al. Dutch–Flemish translation of 17 item banks from the Patient-Reported Outcomes Measurement Information System (PROMIS). Qual Life Res. (2014) 23:1733–41. doi: 10.1007/s11136-013-0611-6
45. Haverman L, Grootenhuis MA, Raat H, van Rossum MAJ, van Dulmen-den Broeder E, Hoppenbrouwers K, et al. Dutch–Flemish translation of nine pediatric item banks from the Patient-Reported Outcomes Measurement Information System (PROMIS)®. Qual Life Res. (2016) 25:761–5. doi: 10.1007/s11136-015-0966-y
46. Blaauwbroek R, Tuinier W, Meyboom-de Jong B, Kamps WA, Postma A. Shared care by paediatric oncologists and family doctors for long-term follow-up of adult childhood cancer survivors: a pilot study. Lancet Oncol. (2008) 9:232–8. doi: 10.1016/S1470-2045(08)70034-2
47. Castor EDC. (2019). Available at: https://www.castoredc.com (Accessed September 29, 2023).
48. Haverman L, Van Oers HA, Van Muilekom MM, Grootenhuis MA. Options for the interpretation of and recommendations for acting on different PROMs in daily clinical practice using KLIK. Med Care. (2019) 57:S52–8. doi: 10.1097/MLR.0000000000001061
50. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2023).
51. Ruggieri P, Cerchiaro M, Angelini A. Multidisciplinary approach in patients with metastatic fractures and oligometastases. Injury. (2023) 54:268–70. doi: 10.1016/j.injury.2022.11.027
52. Marina NM, Liu Q, Donaldson SS, Sklar CA, Armstrong GT, Oeffinger KC, et al. Longitudinal follow-up of adult survivors of Ewing sarcoma: a report from the childhood cancer survivor study. Cancer. (2017) 123:2551–60. doi: 10.1002/cncr.30627
53. Bekkering WP, Vliet Vlieland TP, Fiocco M, Koopman HM, Schoones JW, Nelissen RG, et al. Quality of life, functional ability and physical activity after different surgical interventions for bone cancer of the leg: a systematic review. Surg Oncol. (2012) 21:e39–47. doi: 10.1016/j.suronc.2011.09.002
54. Mei J, Zhu XZ, Wang ZY, Cai XS. Functional outcomes and quality of life in patients with osteosarcoma treated with amputation versus limb-salvage surgery: a systematic review and meta-analysis. Arch Orthop Trauma Surg. (2014) 134:1507–16. doi: 10.1007/s00402-014-2086-5
55. Nagarajan R, Clohisy DR, Neglia JP, Yasui Y, Mitby PA, Sklar C, et al. Function and quality-of-life of survivors of pelvic and lower extremity osteosarcoma and ewing’s sarcoma: the childhood cancer survivor study. Br J Cancer. (2004) 91:1858–65. doi: 10.1038/sj.bjc.6602220
56. Groundland JS, Ambler SB, Houskamp DJ, Orriola JJ, Binitie OT, Letson GD. Surgical and functional outcomes after limb-preservation surgery for tumor in pediatric patients: a systematic review. JBJS Rev. (2016) 4:1–13. doi: 10.2106/JBJS.RVW.O.00013
57. Bishop MW, Ness KK, Li C, Liu W, Srivastava DK, Chemaitilly W, et al. Cumulative burden of chronic health conditions in adult survivors of osteosarcoma and ewing sarcoma: a report from the St. Jude lifetime cohort study. Cancer Epidemiol Biomarkers Prev. (2020) 29:1627–38. doi: 10.1158/1055-9965.EPI-20-0076
58. Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson M, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. (2006) 355:1572–82. doi: 10.1056/NEJMsa060185
59. Furtado S, Errington L, Godfrey A, Rochester L, Gerrand C. Objective clinical measurement of physical functioning after treatment for lower extremity sarcoma—a systematic review. Eur J Surg Oncol. (2017) 43:968–93. doi: 10.1016/j.ejso.2016.10.002
60. Pakulis PJ, Young NL, Davis AM. Evaluating physical function in an adolescent bone tumor population. Pediatr Blood Cancer. (2005) 45:635–43. doi: 10.1002/pbc.20383
61. Bekkering WP, Vliet Vlieland TPM, Koopman HM, Schaap GR, Schreuder HWB, Beishuizen A, et al. Functional ability and physical activity in children and young adults after limb-salvage or ablative surgery for lower extremity bone tumors. J Surg Oncol. (2011) 103:276–82. doi: 10.1002/jso.21828
62. Ginsberg JP, Rai SN, Carlson CA, Meadows AT, Hinds PS, Spearing EM, et al. A comparative analysis of functional outcomes in adolescents and young adults with lower-extremity bone sarcoma. Pediatr Blood Cancer. (2007) 49:964–9. doi: 10.1002/pbc.21018
Keywords: adverse events, bone sarcoma, functional outcome, orthopedic surgery, pediatric oncology, study protocol, quality of life
Citation: Tigelaar LG, Haveman LM, Bekkering WP, Oude Lansink ILB, Rohrich CD, Van der Hoek H, Beek LR, Van Dijk J, Langemeijer MEM, Slooff-Lentink RW, Van der Aa-Van Delden AM, Maurice-Stam H, Peek AML, Van der Pal HJH, Koopman MMW, Kremer LCM, Westerbos SJ, Van Tinteren H, Bramer JAM, Van de Sande MAJ, Grootenhuis MA, Schreuder HWB and Merks JHM (2025) A multidisciplinary and structured approach for comprehensive evaluation of functional outcomes, adverse events, psychosocial outcomes and health-related quality of life after local therapy for bone sarcoma in children: protocol for a cross-sectional study. Front. Pediatr. 13:1534153. doi: 10.3389/fped.2025.1534153
Received: 25 November 2024; Accepted: 20 March 2025;
Published: 15 April 2025.
Edited by:
Giuseppe Maria Milano, Bambino Gesù Children's Hospital (IRCCS), ItalyReviewed by:
Sebastian Dorin Asaftei, Ospedale Infantile "Regina Margherita", Citta della Salute e della Scienza, ItalyGerhard Hobusch, Medical University of Vienna, Austria
Copyright: © 2025 Tigelaar, Haveman, Bekkering, Oude Lansink, Rohrich, Van der Hoek, Beek, Van Dijk, Langemeijer, Slooff-Lentink, Van der Aa-Van Delden, Maurice-Stam, Peek, Van der Pal, Koopman, Kremer, Westerbos, Van Tinteren, Bramer, Van de Sande, Grootenhuis, Schreuder and Merks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johannes H. M. Merks, ai5oLm0ubWVya3NAcHJpbnNlc21heGltYWNlbnRydW0ubmw=
 Leonie G. Tigelaar
Leonie G. Tigelaar Lianne M. Haveman1
Lianne M. Haveman1 Heleen Maurice-Stam
Heleen Maurice-Stam Martha A. Grootenhuis
Martha A. Grootenhuis Johannes H. M. Merks
Johannes H. M. Merks