- 1Departments of Psychology and Biobehavioral Sciences, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 2Institute for Implementation Science, University Texas Health Science Center at Houston, Houston, TX, United States
- 3Departments of Occupational Therapy, Neurology, and Pediatrics, Washington University in St. Louis, St. Louis, MO, United States
- 4Departments of Global Pediatrics, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 5Departments of Hematology, St. Jude Children’s Research Hospital, Memphis, TN, United States
Introduction: Sickle cell disease (SCD) is a monogenic blood disorder characterized by neurodevelopmental delays. Most children with SCD do not receive developmental services due in part to disparities in care access. To inform the design of a developmental intervention for children with SCD, we evaluated factors that influence access to developmental services.
Methods: Interview data were collected from educational and medical providers (n = 15) and caregivers (n = 15) of children aged 4–6 years with SCD at a single center and the surrounding area. Caregivers completed questionnaires about their child's background/medical history, caregiver depression (PROMIS SF v1.0-8a), and caregiver knowledge of early development (Knowledge of Infant Development Inventory). A convergent design was used to integrate the qualitative and quantitative data.
Results: We identified three themes as factors that influence caregivers' access to developmental services: quality of medical and educational experiences, caregiver knowledge and beliefs about SCD and development, and caregiver preferences for developmental services. Most caregivers denied barriers to obtaining developmental services for their child, whereas providers acknowledged numerous barriers for families. Caregivers and providers shared that a positive caregiver-provider relationship facilitates access. Caregivers reported that there was limited attention to SCD within the hospital system and broader society. Caregivers displayed limited knowledge of early development, and providers identified these knowledge gaps as a barrier to utilizing developmental services. Caregivers expressed a strong interest in SCD education and community building.
Conclusions: Our mixed method analysis identified barriers and facilitators to developmental services for children with SCD.
1 Introduction
Sickle cell disease (SCD) is a monogenic blood disorder that impacts nearly all systems of the body (1). Approximately 100,000 individuals are estimated to have SCD in the United States, the majority of whom are African American (2). The brain is particularly vulnerable to the effects of SCD due to poor oxygen delivery, resulting in cerebrovascular insults (3–5) and accelerated white matter loss (6). Early and progressive neurocognitive deficits are often seen in patients diagnosed with SCD, negatively impacting academic performance (7, 8).
Individuals living with SCD have less access to comprehensive care compared to individuals with other chronic diseases due to a lack of SCD-trained providers and health insurance barriers (9). These disparities translate to low rates of preventive care, such as immunizations (10) and transcranial doppler screening for stroke prevention (11). Disparities in healthcare access extend to developmental services that may remediate developmental delays in young children with SCD, and better prepare them for academic success. The academic consequences are dire, as children with SCD fall below normative expectations on achievement assessments and have a high rate of grade retention (12). Developmental services include speech, occupational, physical and other early intervention therapies, as well as classroom-based interventions. Because neurocognitive deficits become more apparent with age (13, 14) and early intervention tends to be more effective (15, 16), developmental services for SCD should use a preventive approach to alter the developmental trajectory rather than a corrective approach after functional impairment occurs (17, 18). However, despite the benefits of early interventions, most children with SCD do not receive developmental services to address delays (19).
Many families with a child with SCD experience a double burden as members of a historically marginalized group coping with chronic illness (20). Although some facilitators and barriers are known to impact medical care for SCD families, no studies have provided a comprehensive view from both caregivers and providers of determinants affecting utilization of developmental services. Reported facilitators to medical care include convenience of combined appointments (i.e., having subspecialists knowledgeable about SCD present together) and the ability to communicate with knowledgeable staff about SCD concerns (21). Reported barriers to medical care include perceived discrimination, the distance from and transportation to SCD centers, financial strain, missing work, children missing school, and lack of provider knowledge and comfort (21, 22). Previous studies offer insight into barriers into medical care, but do not provide insight into barriers to developmental care, which differs from medical care in a variety of ways, not limited to treatment location, referral process, and provider training. There is a significant need to implement developmental interventions for children with SCD that are both feasible and acceptable as prior intervention trials have struggled with engagement and adherence (23, 24). A better understanding of caregivers' access to and willingness to obtain developmental services could help providers tailor services to the SCD population.
This investigation uses the Health Equity Implementation Framework (25) (HEIF) to identify determinants of access to developmental services specific to the SCD community. Unlike previous studies on facilitators and barriers that impact care (21, 22), this study incorporates both caregiver and provider perspectives. We used a convergent mixed methods design (26) to better understand what factors impact access to and preferences for developmental services. The current study has three aims: (1) identify barriers and facilitators to developmental treatment faced by caregivers of children ages 4–6 with SCD; (2) understand which factors inform decisions about medical, developmental, and educational interventions for caregivers of young children with SCD; and (3) identify caregiver preferences that will increase developmental treatment utilization for young children with SCD.
2 Materials and methods
2.1 Participants and procedures
Medical and educational providers and caregivers of children with SCD ages 4–6 were recruited from the Memphis area. Snowball sampling was used to find providers familiar with the unique developmental needs of young children with SCD, starting with providers at St. Jude Children's Research Hospital and a local early childhood education provider. Providers at these organizations recommended colleagues with experience providing or connecting families to developmental services, and/or experience working with families of young children with SCD. Caregivers were recruited through the Sickle Cell Clinical Research and Intervention Program (SCCRIP), a longitudinal cohort study of patients with an SCD diagnosis (27). Caregivers were eligible if they spoke English and had a child enrolled in the cohort study between the ages of 4 and 6 years with any SCD genotype.
This was a descriptive cross-sectional study. We used a convergent mixed methods design, involving parallel collection of both quantitative and qualitative data. This approach was used as both quantitative and qualitative domains of interest were identified a priori, consistent with the HEIF framework (25). All HEIF domains were examined qualitatively and quantitatively either through frequency counts or formal questionnaires. Certain themes arose from interviews that were not captured quantitatively, and interpretation was primarily based on qualitative data collected. Interviews with providers were conducted over a virtual video platform or over the phone and were audio recorded. All caregiver interviews were conducted over the phone and audio recorded. Audio recording of interviews was granted by participants during the informed consent process. Interviews were transcribed verbatim using a third-party service and checked for accuracy by two study team members. Caregivers also completed four questionnaires: two descriptive questionnaires about their child's background and medical history, a questionnaire about caregiver depression, and a questionnaire testing the caregiver's knowledge of early development. The procedures of this study were approved by the Institutional Review Board at St. Jude Children's Research Hospital.
2.2 Qualitative interviews
Two semi-structured interview guides, one for caregivers and one for providers, were developed based on the HEIF used by Woodward et al. (25) to study barriers and facilitators to treatment for the hepatitis C virus. The HEIF integrates healthcare disparities and implementation science concepts into a framework that considers barriers and facilitators to intervention implementation at the following levels: recipients (patients and providers), clinical encounters, innovation/characteristics of treatment, and the context of the health care system. This framework allows for the identification of disparities in access to care and racially disparate decisions by providers (28). Caregivers were asked about potential barriers and facilitators to developmental services for their child with SCD. Caregivers with children who had not received developmental services were asked what they would expect from a developmental service based on their experience with their child's medical treatment or education. Providers were asked the same questions and answered based on their knowledge of developmental services and their interactions with caregivers. Additional questions for caregivers were included to learn about their knowledge of SCD and development and their preferences for a proposed intervention (see Supplementary File 1 and Supplementary File 2 for interview guides).
2.3 Quantitative measures
2.3.1 Depression
The Patient Reported Outcomes Measurement Information System (PROMIS) is a collection of item banks made by the National Institute of Health and includes a depression questionnaire about negative mood, decrease in positive affect, information processing deficits, negative views of the self, and negative social cognition. The PROMIS SF v1.0 - Depression 8a, an 8-item depression short form, is highly correlated to the full depression bank (r = 0.96) (29). Items have five response options (Never, Rarely, Sometimes, Often, and Always), and response pattern scoring is used to calculate a raw score that is transformed into a T-score with a general population mean of 50 and standard deviation (SD) of 10. A T-score above 60 indicates moderate depression, and a T-score above 70 indicates severe depression (30). The general population used to norm the T-scores (n = 11,796) is similar in gender, age, race/ethnicity, and education to the 2010 U.S. census (31).
2.3.2 Knowledge of development
The Knowledge of Infant Development Inventory (KIDI) is a 58-item questionnaire that assesses parent knowledge of infant norms and milestones, principles of development, parenting strategies, and health and safety (32). Item responses include Agree, Disagree, and Not sure for 39 questions, and Agree, Younger, Older, or Not sure for 19 questions that make a declarative statement like “Babies say their first real word at 6 months.” Two response scores were calculated (Attempted and Accuracy). The KIDI's internal consistency across a diverse sample from 10 studies (N = 820) is acceptable (α = 0.88) (32). A study by Hamilton and Orme (33) found that the KIDI had high convergent validity with the Knowledge of Child Development Inventory (r = 0.68) and the Parent Opinion Question (r = 0.51).
2.3.3 Demographic and medical information
A demographics questionnaire and a medical history questionnaire were used to gather background information about the sampled caregivers. The demographics questionnaire asked about caregiver characteristics including education, marital status, income, health care coverage, race, and ethnicity. The medical history questionnaire asked about the child's SCD-related complications and treatment, prenatal and birth history, and developmental and educational history.
2.4 Qualitative analysis
A qualitative content analysis was performed on the interview data to identify descriptive themes. After the data were transcribed and assessed for quality, each transcript was coded by two independent coders (MT and EM) using Delve Tool software (34). Two coders used a deductive approach for questions derived from the HEIF (25) and questions about caregiver preferences on the proposed interventions. First, a codebook was created that aligned with the barrier and facilitator domains of the HEIF and questions specific to medical experiences and preferences. In addition to the a priori codes, other codes for recurring topics discussed throughout the interviews were inductively derived, including “Caregiver decision making” and “Beliefs about learning and academics.” The two coders met during and after their independent coding processes to discuss their findings and resolve coding discrepancies.
After the codes for each transcript were agreed upon by the two coders, the data were visually reorganized by code in a categorization matrix (35). The categorization matrix is an arrangement of summarized participant responses with a row for each participant and a column for each code. The matrix enabled condensing of the data (i.e., whether a barrier was or was not endorsed, and whether a facilitator was identified). The data were further abstracted from the matrix as the coders analyzed the code columns to write an interpretive summary of responses. Responses were also analyzed by row to identify patterns within individual responses. Analysis of the interpretive summaries allowed for the re-contextualizing of data into descriptive themes that described the experiences, beliefs, and preferences of providers and caregivers (36). To further analyze within each theme for subthemes, responses were transformed into frequencies of endorsed barriers and facilitators as detailed below.
2.5 Quantitative data analysis
Responses to barrier questions derived from the HEIF were quantified by dichotomizing responses that did or did not endorse a specific barrier and analyzed for frequencies. Responses to the inductively-derived codes of “Medical knowledge of SCD,” “Knowledge of SCD and development,” “Sources of knowledge,” “Perceptions of society,” and “Decision making” were quantified for the frequency of topics discussed within each code. For the PROMIS, descriptive statistics were calculated for caregiver responses. For the KIDI, an accuracy score was calculated from the ratio of the total correct of the total attempted items per participant. Mean and SD were used to analyze the accuracy and over/underestimate scores from the KIDI. For the medical and demographic questionnaires, frequencies were used to describe the categorical data and mean and SD were used to analyze the continuous data.
2.6 Data integration
A convergent design was used in this mixed methods analysis (26), beginning with the independent analysis of qualitative and quantitative data, followed by the integration of the two data sets to identify ways in which the two methods of caregiver reporting confirm, contradict, or expand on caregiver experiences. The transformation of interview responses into frequencies informed the construction of descriptive themes. A basic joint display (37) report transformed interview data, interview quotes, and survey data, while also showing the results in the context of the HEIF domains.
3 Results
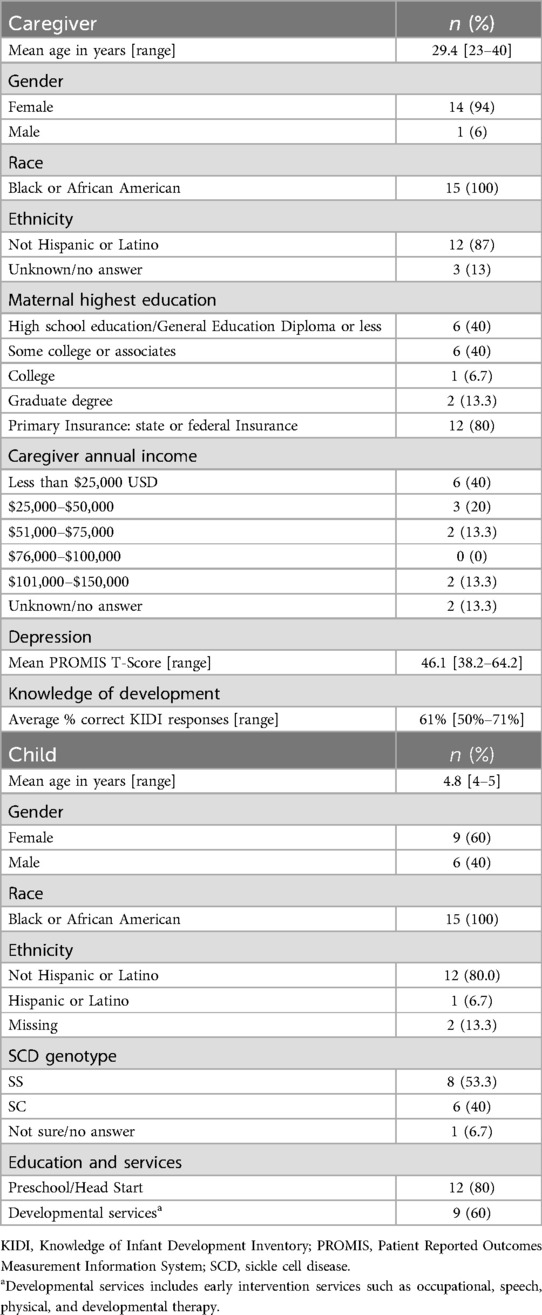
Fifteen providers with a range of 5–29 years of experience in the medical, education, and/or advocacy sectors participated in interviews. Roles of the providers included hospital-school coordinators who advocate for medical and educational needs (n = 4), hospital teachers (n = 2), school leadership from the hospital and the community (n = 4), early childhood health and disability specialists (n = 2), an adult SCD advocate, a hematologist, and a hematology administrator. Seventeen caregivers were enrolled and fifteen caregivers completed surveys and interviews (2 caregivers were lost to follow up). Caregivers ranged in age from 23 to 40 years (Mean = 29.4), were primarily female (94%), and all identified as Black or African American. Most caregivers had children who attended preschool (80%) and/or received a developmental service (60%). Characteristics of the caregivers and their children can be found in Table 1.
The study team identified three themes as factors that influence caregivers' access to developmental services: quality of medical and educational experiences, caregiver knowledge and beliefs about SCD and development, and caregiver preferences for developmental services (Figure 1).

Figure 1. Results of the thematic analysis. The themes represent factors that influence caregivers’ access to developmental services.
3.1 Theme 1: quality of medical and educational experiences
3.1.1 Providers reported that caregivers encounter significant barriers
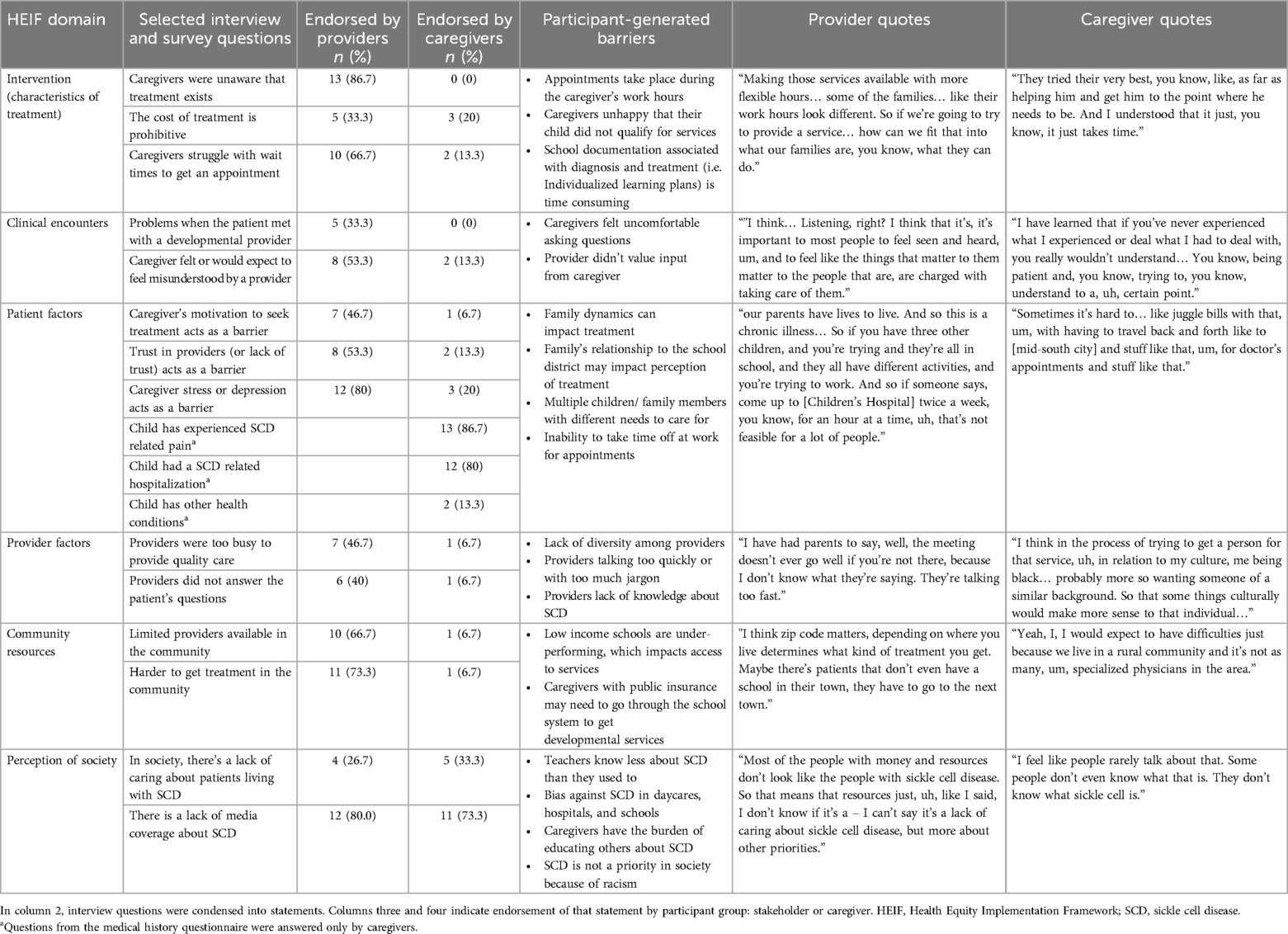
When asked about specific barriers to developmental care, most providers endorsed at least some of the barriers within each HEIF domain (Table 2). The barriers most frequently endorsed were the patient's housing or living situation (n = 14), caregivers' unawareness that treatment exists (n = 13), lack of media attention around treatment (n = 12), caregiver stress or depression (n = 12), and lack of transportation (n = 12). Providers also shared experiences of caregivers not understanding a developmental diagnosis or treatment and/or not believing a treatment was necessary.
Some providers further stated that specific barriers are related to systemic issues in hospitals and schools. One such issue is miscommunication between providers and caregivers about why a child needs services or how services can be helpful. This issue arises particularly when providers speak too quickly or use jargon. One health services specialist working in early childhood education explained how she helps caregivers ask questions:
“We actually have forms that we give to parents that, that ask doctors specific questions.. we have found that some parents do not know what to ask. I’ve actually had parents – I say, ‘Well, when you get there, this is my cell number. Call me and hand the phone to the doctor.’” (Provider 16)
Another barrier expressed primarily by education providers is caregiver mistrust of the medical or educational system. Educators described conversations with caregivers who associate early intervention with the stigma around special education. One educator explained that caregivers who received special education services before schools adopted inclusion models may avoid developmental services out of fear that their child may be labeled or ostracized.
Providers also discussed health and socioeconomic inequalities faced by caregivers of children with SCD and how such inequalities impact caregiver motivation, mental health, and trust in developmental providers. One provider explained how low caregiver wellbeing impacts access to services:
“The stress, you know, you have so many stress factors, living in the high crime area, living in a food desert, not having a car, uh, living in an abusive situation, not having enough money to buy food. Yeah. I mean, of course… your hierarchy is not about services for your child; it’s about everyday existence.” (Provider 12)
3.1.2 Caregivers endorsed few barriers but acknowledged the difficulty of balancing priorities
Unlike providers, most caregivers endorsed few, if any, of the HEIF barriers (Table 2). For example, most providers endorsed some or all of the barriers in the patient factor of the HEIF domain, but the only patient-factor barriers that were endorsed by more than one caregiver were distance from the hospital or clinic (n = 3), transportation (n = 3), trusting providers (n = 2) and experiences of stress or depression (n = 3).
To better contextualize caregiver responses to the patient-factor HEIF questions, we asked caregivers to complete a brief depression symptom inventory (PROMIS SF V.1- Depression 8a) Caregiver scores ranged from 38.2 to 64.2 with a mean of 46.1 (SD = 9.4), which is below the standardized T-score of 50 in the general population. Two caregivers (13.0%) scored in the clinical range for depressive symptoms. Another patient factor that may impact access to developmental services is the severity of the child's SCD symptoms. The majority of caregivers reported that their child had experienced SCD-related pain (n = 13, M = 86.7%) and that their child had been hospitalized for SCD complications before the age of 3 (n = 12, M = 80%).
Caregivers described other personal barriers that were not specifically asked about in the interview. The most discussed personal factors that interfered with their child's treatment were the caregivers' own medical conditions and the inability to miss work for appointments. One caregiver explained how their family manages medical appointments:
“Um, maybe my own like health issues and appointments, but I have been, so far everything has worked out as far as scheduling wise. Um, so if I have an appointment or an issue going on myself, then her dad would just step in. Um, it might cost him to take some time off work, but he would step in to make sure she gets her appointment.” (Caregiver 7)
This quote demonstrates a reality described by many caregivers – the problem solving required to prioritize their child's treatment. A caregiver explained how her efforts to balance her job with her child's medical treatment has led to negative internalizing:
“Having to lose so many jobs to take care of my child like it just makes you feel like nobody cares about your child but you. But you have to pay the bills so it is really depressing not being able to keep one.” (Caregiver 13)
Few characteristics of treatment were identified as problematic by caregivers who have obtained developmental services for their child (Table 2). All caregivers with experience with early intervention or another developmental service spoke positively about the treatment and providers. Positive comments included that early intervention was highly engaging to their child, that the providers were patient, that providers included them in activities, and that they observed improvement with their child.
Caregivers without experience with developmental services endorsed few concerns about the treatment itself or its accessibility, with two exceptions. Three caregivers expressed that they would expect the cost of the treatment to be a barrier to care, and two caregivers expected to feel misunderstood by a developmental provider. As one of them explained,
“I feel that providers don’t really take personal experiences into consideration when they’re offering treatments… like if you’re not just taking their advice or wanting to like give medicine that they’re suggesting for your child, I feel like they don’t really listen to your point of view.” (Caregiver 5)
3.1.3 A positive caregiver-provider relationship facilitates access
Both providers and caregivers spoke about how the relationship between caregivers and providers facilitates caregiver access to services. Caregivers explained their desire to be heard and understood by providers, and providers explained how trust increases the likelihood that caregivers will pursue treatment for their child. Caregivers stated that providers need to listen to caregivers, make them feel comfortable asking questions, and ask families for feedback. One caregiver described the positive communication with her provider as follows:
“…they always make us feel comfortable… I guess, the way they communicate with us. Um, they tell us everything. They sit down. That’s the number one thing … They make us feel like, you know, we’re just having a, a regular conversation and it’s not uncomfortable” (Caregiver 2)
Providers also discussed the need for strong communication between caregivers and providers, and some emphasized the need for providers to speak clearly and concisely. One provider explained that, ideally, the relationship between providers and caregivers is one of partnership:
“I can learn from you and you can learn from me, kind of shared, um, thought partner model would help caregivers, um, kind of let their guard down and be open to, um, services and listening to what’s going on and really asking the parent or caregiver their experience and… reminding them, you know, they're the expert with their child and building up their confidence in the partnership and work.”(Provider 10)
Another provider explained how caregiver motivation, or “buy-in,” along with trust in providers, is essential for children who need early intervention services:
“I believe buy-in is essential when it comes to intervention support. One, the parent has to have buy-in that their child actually needs support. And then, two, that the parent has to have a trust factor that the provider is actually going to support their child in that area… I feel a way to alleviate that is for outreach to be paired in tandem with the referral, um, kind of like an appropriate handoff, you know, instead of a call this number.. kind of like, we're going to walk with you in this journey until everyone agrees that this is, this is happening.” (Provider 8)
As this provider noted, caregivers who are highly motivated to secure developmental services for their child may still struggle to navigate enrollment in early intervention or school services. Providers explained that developmental services are more accessible for patients who have a strong connection to a school or hospital system with staff dedicated to helping parents navigate those services. This statement was supported by caregiver anecdotes, as many caregivers who had obtained services for their child credited hospital staff or school educators for helping them schedule appointments.
3.2 Theme 2: caregiver knowledge and beliefs about SCD and development
3.2.1 Breadth of knowledge about SCD varied by professional and personal experience
The most commonly discussed features of SCD were the associated pain and risk of stroke. Most providers could provide more precise answers about the developmental aspects than about the medical aspects of SCD, as most providers had a background in education rather than medicine. Providers identified several developmental delays that children with SCD may be at higher risk for, including issues with memory, attention, adaptive behavior, self-regulation, processing speed, social learning, speech/language, and motor skills. The concerns most often addressed by providers involved attention and speech. Providers also volunteered explanations for why such developmental delays may occur in children with SCD, including pain as a barrier to learning, time in the hospital or low attendance at school, and silent cerebral infarcts or stroke. Four providers were hesitant to attribute developmental delays to SCD itself, noting that the presence of delays in children with SCD is often confounded by other variables, such as low income or limited access to high-quality education. Regarding associated delays, one provider said,
“…a lot of it is, maybe not disease related, but complicated by disease.” (Provider 12)
Most caregivers (80%) had heard of hydroxyurea and more than half (53%) had heard of TCD. Most caregivers who were aware of hydroxyurea or TCD attributed their familiarity to their child's use of hydroxyurea or TCD. Caregivers also offered knowledge about hydroxyurea, with three noting that it can help with avoiding pain crises or hospitalization and three explaining that it is used to prevent blood cells from sickling. Caregivers were less descriptive about their understanding of TCD, with only two caregivers explaining its use to detect stroke.
Four caregivers were unaware that children with SCD are at a higher risk of delays in development than are their peers. Four other caregivers were aware that children with SCD are at risk for developmental delays but did not explain further. Three caregivers noted that speech may be impacted by SCD. Walking delays, difficulties with focus or memory, and problems with everyday activities were each mentioned once.
The mean accuracy score on the KIDI (total correct divided by total attempted) was 61% (SD = 0.07). In comparison, the mean KIDI accuracy score was 83% (SD = 0.16) among participants in a large study of infant/child development (n = 1,358), conducted in the same metropolitan area as the current study (38). Caregivers' accuracy on the KIDI was 1.78 SD below the accuracy observed in the prior study, suggesting knowledge gaps among our sample of SCD caregivers. The mean accuracy score of the current sample was also 1.20 SD below that of mothers whose children attended Head Start (n = 207, M = 70.7, SD = 11.4) (32).
3.2.2 Knowledge comes from direct and professional experiences
Participants learned about SCD, developmental delays, or treatment for developmental delays in one of four ways: direct experience, professional experience, expertise of medical or educational professionals, or formal education. Direct experience was the most frequently discussed source of knowledge, with providers describing workplace interactions with children who have SCD and caregivers describing their own child or family member's experience. Most caregivers who were aware of TCD or hydroxyurea attributed this knowledge to their child's medical experience. Professional experience was largely discussed by providers, who learned about SCD by collaborating with other health professionals. Most caregivers cited expertise from medical or educational experts as their source of knowledge about SCD and development, with many emphasizing their reliance on the hospital's doctors and school program to learn about these topics. One caregiver described how her child's hospital shared information in a way that helped her to retain the new knowledge and build a relationship with hospital staff:
“Each visit that I go to [children’s research hospital], they teach me a little bit more every time. It’s kind of, it’s, it’s so much better than, you know, slamming all that knowledge on a parent when we first, you know, when we first get there.” (Caregiver 13)
Formal education as a source of knowledge about SCD was only mentioned by one provider and one caregiver.
The type and specificity of knowledge about SCD differed between providers and caregivers. Providers had a wider breadth of knowledge about potential risks of SCD, whereas caregivers had a more intimate knowledge of day-to-day life with SCD that was specific to their child's medical and developmental needs.
3.2.3 Society lacks SCD awareness
Most providers did not endorse the statement that society lacks caring for those living with SCD. However, all providers who responded this way clarified their response, stating that people with SCD do not get the attention that they need from society. Nine of those providers said that there is a lack of knowledge about people living with SCD, and one provider described SCD as a low priority to those who disseminate knowledge:
"…most of the people with money and resources don't look like the people with sickle cell disease.. I can’t say it's a lack of caring about sickle cell disease, but more about… other priorities.” (Provider 12)
Most providers noted a lack of media coverage about SCD, and multiple providers linked the lack of knowledge and media coverage to the marginalization of Black people in society.
Most caregivers said there was not a lack of caring, but several noted that there is a lack of knowledge about the disease. Some caregivers further explained how the lack of knowledge in society is compounded by the invisible nature of the disease, such that others often trivialized their child's experience with the disease. One parent explained that her answer was informed by experiences of bias against her child with SCD at the hospital and at school. Caregivers almost unanimously said that there is not enough media coverage about SCD or representation of those living with the disease. One caregiver emphasized how the lack of awareness puts the burden on her to justify her child's needs:
“You mostly hear about children with cancer, you don’t too much hear anybody trying to help anybody with sickle cell… When I had my son, nobody, they, they heard of it, but they just don’t know anything about it. So, I’m having to constantly tell people what struggles he go through… It’s like nobody cares.” (Caregiver 13)
Providers and caregivers almost unanimously called for increased awareness of SCD and increased media representation for those who live with it.
3.3 Theme 3: caregiver preferences for developmental services
3.3.1 Caregivers make decisions based on quality, provider trust, and accessibility
Providers and caregivers identified three factors that would influence caregiver acceptance and participation in a developmental education program for preschool-aged children with SCD. All 15 caregivers expressed interest in developmental education programs. Although interested, most caregivers cautioned that they would need to consider additional factors before committing to participate. When asked about how they would make a final decision about participation, their responses fell into the following categories: quality of the program, recommendations, and logistics. Caregiver responses regarding the quality of the program included discussions of the program's content and its perceived relevance to their child's needs. For example, one caregiver explained,
“…me personally, um, I just ask more about the program, what the program consists of… I would just want to see what I’m putting me and my son into it before I just jumped into the – I want to make sure it’s something good for him and not just okay for him.” (Caregiver 1)
Some caregivers placed high value on recommendations, explaining that they would be willing to try a program if it was suggested by a professional they trusted. Program accessibility was the most frequently discussed factor in decision making. Caregivers mentioned logistic considerations including work schedule conflicts, childcare needs, virtual or in-person participation, and time constraints. Providers endorsed the same decision-making considerations, but more frequently pointed out access issues such as lack of transportation or lack of technology.
3.3.2 Logistic preferences vary by family
There was variability in how caregivers described the characteristics of a developmental education program that would be most accessible to their family, suggesting that no single format for intervention programming will meet all caregivers' needs. Although some caregivers were interested in virtual participation, other caregivers stated that they learn better in person. Similarly, some caregivers would prefer to meet regularly on a weeknight, but others stated that their work did not follow a traditional 9 am to 5 pm schedule. To accommodate the diverse needs of families, providers suggested offering virtual/in-person hybrid sessions, offering sessions at multiple times, and providing transportation or daycare to increase in-person attendance.
3.3.3 Caregivers want SCD education and SCD community
Although caregivers had different preferences about the program logistics, they largely agreed on the desired qualitative aspects of the program. Caregivers frequently discussed development and school readiness from an SCD perspective, with a focus on elements to monitor in their child's development and risk factors common in children with SCD. They also discussed what to expect in kindergarten and how to teach their child to communicate their physical needs to teachers, particularly regarding staying hydrated throughout the day and communicating a pain crisis. One caregiver expressed anxiety about her child being in a larger class with less attention from the teacher:
“So, public school, you know, they don’t really baby them.. working with 20 plus kids… I know he’s not going to have as much attention on him, and I was paying attention to him… having a, a crisis since he’s so little he’s… still learning… what to tell me and how… he feels so that’s a concern of mine. But we’re working with him to try to communicate with us better…” (Caregiver 13)
Another recurring desire from caregivers was to learn about strategies to maintain their own mental health while coping with the stress of their child's transition to kindergarten. One caregiver explained this need:
“…I’d want more so, like, a support system to show, you know, manners in which that help as a, as a caregiver to, you know, keep moving forward and, you know, the steps that you can take then as much as you’re taking care of the children, but, like, giving us the education on how to keep ourselves whole in the midst of it.” (Caregiver 14)
Caregivers and providers discussed the idea of using a caregiver educational course as a support group. Multiple providers suggested using a coaching or cohort model rather than lectures. When asked who caregivers wanted to learn from, most suggested that the caregiver educational program be taught by a parent of a child with SCD. Caregivers expressed the need to hear from someone who has successfully gone through the process of preparing their child with SCD for school. Some caregivers also stated their willingness to hear from medical or educational professionals in the sessions. As one caregiver explained, parents should lead the session and then incorporate medical or educational professionals who can “back them up on the facts.” Regarding interventions for children, almost all participants agreed that the main criteria for instructors should be experience with early childhood education, but some caregivers suggested incorporating participation of older children with SCD as role models for the younger children.
4 Discussion
Patients with SCD comprise a historically marginalized population that face substantial health disparities (22, 39, 40). To establish an equitable developmental intervention for families of young children with SCD, we conducted a mixed-methods investigation through a health equity lens (25). Through analysis of both qualitative and quantitative data, we identified three broad themes that frame how caregivers and providers view developmental services (i.e., services to promote children's cognitive, language, and physical development). These themes include the quality of medical and educational experiences, caregiver knowledge and beliefs about SCD and development, and caregiver preferences for developmental services.
There were notable discrepancies in the barriers endorsed by caregivers and providers. Most caregivers did not endorse expecting or experiencing significant barriers to obtain developmental services for their child, whereas providers frequently endorsed numerous barriers for patient families. Providers described negative medical and educational experiences of caregivers. In contrast, caregivers mostly emphasized their own positive experiences. These discrepancies are consistent with prior literature assessing medical adherence in patients with SCD based on caregiver and provider reporting. Caregivers of children with SCD tend to overestimate rates of medical adherence (41, 42) and report fewer barriers to disease management (43) than do providers. Our results suggest that this response pattern extends to developmental services for children with SCD. Caregivers may not endorse these barriers due to stigma and fear of judgement or racism (44, 45). The differing lived experiences of providers and caregivers can lead to feelings of stigma and discrimination among families of patients with SCD (22). These feelings result in strained relationships and limited trust that can impact how barriers are reported (46). Furthermore, caregivers can only provide their own personal experience, whereas providers may have observed a wider range of barriers among caregivers. Conversely, although providers may have observed certain barriers in a few families, they may generalize these barriers to all families of children with SCD. Discrepancies in racial and ethnic backgrounds between providers and caregivers may also partially explain their experiences with these barriers.
Several caregivers highlighted the difficulties of balancing priorities when seeking developmental services for their child. For example, some caregivers noted that they often must prioritize their child's medical care or their own medical care, and others shared that it was difficult to maintain employment because their jobs do not provide the flexibility needed to attend appointments. Some caregivers (n = 3) and most providers (n = 12) shared that transportation or distance from the provider was a barrier to care. To overcome these barriers, developmental programs for children with SCD may prioritize virtual/telehealth services and offer more services outside working hours. Virtual/telehealth services may also have barriers due to limited internet access, but these issues can be addressed by providing hotspots or other low-cost internet options. Telehealth-based developmental services have been deemed feasible and acceptable for underserved and low-income families (47, 48). Telemedicine approaches for children and adolescents with SCD have received high levels of satisfaction from patients, providers, and caregivers (49–51).
Caregivers of young children with SCD showed limited knowledge of infant and child development, and providers identified these knowledge gaps as a barrier to accessing services. On a standardized questionnaire (KIDI) assessing parent knowledge of infant norms and milestones, principles of development, and parenting strategies, the caregivers in our study showed more limited knowledge than other caregivers living in the same metro area or caregivers with children enrolled in Head Start (32, 38). If caregivers are unfamiliar with normative expectations and principles of development, they may struggle to identify whether their child is delayed or to implement positive parenting strategies. Many caregivers were also unaware that children with SCD are at a higher risk of experiencing developmental delays or how these delays may manifest. Multiple providers reported observing these knowledge gaps and noted that a lack of knowledge about child development may limit follow-through with developmental services. Specifically, providers raised concerns that caregivers may not acknowledge that their child has a developmental delay or may not believe that such delays warrant further action. The discrepancy between caregivers' perceptions and normative expectations highlights a significant barrier to the utilization of developmental services. If caregivers are referred for a developmental evaluation or service, follow-through may be poor if they do not view their child's development as a concern. Caregivers of young children with SCD need education on normative child development and parenting strategies, but knowledge alone is unlikely to lead to significant behavior change (52). Instead, providers can use strategies such as motivational interviewing (53, 54) to promote caregivers' self-efficacy (55, 56) allowing caregivers to feel confident in their ability to follow through with provider recommendations. Culturally tailored parent education programs that utilize motivational interviewing and emphasize cultural pride may be particularly effective (57, 58).
Although providers and caregivers gave contradictory responses about barriers to developmental services, the two groups agreed on facilitators to participation. Both groups highlighted the importance of a positive caregiver–provider relationship to facilitate access to developmental services. Previous studies have documented distrust and poor communication between families of patients with SCD and their providers (59–61). Poor caregiver–provider communication is associated with more hospitalizations and worse health literacy (62). Thus, to facilitate access and engagement in developmental services, the medical team and developmental providers must use communication strategies that build trust. This may be accomplished by implementing family-centered communication, which involves obtaining and understanding the families' perspectives, considering psychosocial and cultural context, and reaching a shared understanding of health concerns and treatment options (59, 63). Interventions emphasizing family centered communication engage families as partners in designing and implementing interventions and seek routine feedback.
Few caregivers reported symptoms of depression on the PROMIS and most denied stress or depression as a barrier to accessing services. In contrast, most providers endorsed caregiver stress and/or depression as a barrier to accessing services. Compared to normative expectations, both caregivers of (64) and child/adolescent (65) patients with SCD do not display elevated rates of depression. Yet, caregiver stress and depression is associated with their child's pain intensity and functional impairment (64), which may become more apparent as their child grows older. Assessing caregiver mental health and promoting coping strategies should be incorporated into family-centered communication (59, 63) with providers.
Caregivers consistently endorsed a strong preference to learn from other families and to build a community of caregivers with shared experiences. The desire to learn from other caregivers or patients with SCD is consistent with findings from other studies (66, 67). These findings may reflect cultural values, as African Americans families tend to be more communalistic and value interdependence more than do European American families (68). Caregivers noted that other families with SCD can comment on the challenges of the lived experience and provide solutions that are practical and approachable. Caregivers expressed a desire to interact with other families for social connectedness and community building, as they experience feelings of isolation related to their child's SCD diagnosis and have limited interactions with other families with shared experiences. These feelings of isolation were exacerbated by the perception that there is a lack of caring or media coverage of SCD within the hospital system and broader society, due in part to racism. Caregivers expressed the burden of having to frequently educate others about SCD. Families living with a chronic disease may develop a strong social identity related to the disease (69–71). Peer-to-peer or group interactions that build upon this identity provide a sense of shared meaning, support, and efficacy that can reduce depression and loneliness (72). Thus, developmental services that foster these connections among caregivers may have better engagement and may more successfully promote caregiver self-efficacy and mental health.
By collecting information from both providers and caregivers, we obtained unique perspectives on developmental services for young children with SCD. The convergent mixed-methods approach incorporating standardized quantitative assessments and qualitative data comprehensively captured potential barriers and facilitators and caregiver knowledge of developmental norms. However, several study limitations exist. Not all caregivers had experience with developmental services and therefore could only relay expectations based on medical care or preschool experiences for their child. We intentionally included caregivers with or without these exposures to gain perspectives from a diverse sample of caregivers rather than from only those who had concerns about their child's development. All data were collected from caregivers served at a single institution and from providers in the surrounding area, potentially limiting generalizability of the results. Caregiver and provider experiences may differ based on geographical location, hospital resources, and broader community resources. Therefore, further investigation is needed through multi-center studies using diverse geographic sampling to evaluate if results are consistent across centers.
5 Conclusions
Patients with SCD and their families experience health disparities that negatively impact quality of life. Using the Health Equity Implementation Framework (25), we identified determinants of access to and quality of developmental services according to caregivers and providers. There were notable discrepancies in barriers to care based on caregiver and provider responses. Caregivers of young children with SCD showed limited knowledge of infant and child development, and providers identified these knowledge gaps as a barrier to accessing and utilizing services. To increase engagement and follow-through of developmental services, interventions should use family-centered communication to facilitate shared understanding and incorporate peer support to foster self-efficacy among caregivers. These findings will support the creation of accessible developmental interventions for children with SCD.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the St. Jude Children's Research Hospital institutional review board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MT: Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. EM: Data curation, Formal analysis, Investigation, Project administration, Writing – original draft, Writing – review & editing. NC: Data curation, Writing – original draft, Writing – review & editing. EK: Conceptualization, Writing – original draft, Writing – review & editing, Methodology. MF: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. JSP: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. HMC: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. AAK: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. JSH: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. AMH: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the American Lebanese Syrian Associated Charities (ALSAC). AMH was supported by K23HL166697 (National Heart, Lung, and Blood Institute) during the time of this study. AAK was supported by K12HL137942 and K24HL148305 (National Heart, Lung, and Blood Institute) during the time of the study.
Acknowledgments
The authors thank the caregivers and providers for their study participation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1530457/full#supplementary-material
Supplementary File 1 | Caregiver interview guide. This semi structured interview guide was used with each of the caregivers interviewed for the present study.
Supplementary File 2 | Provider interview guide. This semi structured interview guide was used with each of the providers interviewed for the present study.
References
1. Piel FB, Steinberg MH, Rees DC. Sickle cell disease. N Engl J Med. (2017) 376(16):1561–73. doi: 10.1056/NEJMra1510865
2. Hassell KL. Population estimates of sickle cell disease in the U.S. Am J Prev Med. (2010) 38(4 Suppl):S512–21. doi: 10.1016/j.amepre.2009.12.022
3. Debaun MR, Derdeyn CP, McKinstry RC. Etiology of strokes in children with sickle cell anemia. Ment Retard Dev Disabil Res Rev. (2006) 12(3):192–9. doi: 10.1002/mrdd.20118
4. Guilliams KP, Fields ME, Dowling MM. Advances in understanding ischemic stroke physiology and the impact of vasculopathy in children with sickle cell disease. Stroke. (2019) 50(2):266–73. doi: 10.1161/STROKEAHA.118.020482
5. DeBaun MR, Armstrong FD, McKinstry RC, Ware RE, Vichinsky E, Kirkham FJ. Silent cerebral infarcts: a review on a prevalent and progressive cause of neurologic injury in sickle cell anemia. Blood. (2012) 119(20):4587–96. doi: 10.1182/blood-2011-02-272682
6. Baldeweg T, Hogan AM, Saunders DE, Telfer P, Gadian DG, Vargha-Khadem F, et al. Detecting white matter injury in sickle cell disease using voxel-based morphometry. Ann Neurol. (2006) 59(4):662–72. doi: 10.1002/ana.20790
7. Heitzer AM, Cohen DL, Okhomina VI, Trpchevska A, Potter B, Longoria J, et al. Neurocognitive functioning in preschool children with sickle cell disease. Pediatr Blood Cancer. (2022) 69(3):e29531. doi: 10.1002/pbc.29531
8. Wang W, Freeman M, Hamilton L, Carroll Y, Kang G, Moen J, et al. Developmental screening of three-year-old children with sickle cell disease compared to controls. Br J Haematol. (2021) 195(4):621–8. doi: 10.1111/bjh.17780
9. Kanter J, Jordan LB. Improving the healthcare model for management of adults with sickle cell disease in the PPACA era. J Hematol Transfus. (2015) 3(1):1037.
10. Britto MT, Garrett JM, Dugliss MAJ, Johnson CA, Majure JM, Leigh MW. Preventive services received by adolescents with cystic fibrosis and sickle cell disease. Arch Pediatr Adolesc Med. (1999) 153(1):27–32. doi: 10.1001/archpedi.153.1.27
11. Reeves SL, Madden B, Freed GL, Dombkowski KJ. Transcranial Doppler screening among children and adolescents with sickle cell anemia. JAMA Pediatr. (2016) 170(6):550. doi: 10.1001/jamapediatrics.2015.4859
12. Heitzer AM, Hamilton L, Stafford C, Gossett J, Ouellette L, Trpchevska A, et al. Academic performance of children with sickle cell disease in the United States: a meta-analysis. Front Neurol. (2021) 12:786065. doi: 10.3389/fneur.2021.786065
13. Wang W, Enos L, Gallagher D, Thompson R, Guarini L, Vichinsky E, et al. Neuropsychologic performance in school-aged children with sickle cell disease: a report from the cooperative study of sickle cell disease. J Pediatr. (2001) 139(3):391–7. doi: 10.1067/mpd.2001.116935
14. Heitzer AM, Longoria J, Okhomina V, Wang WC, Raches D, Potter B, et al. Hydroxyurea treatment and neurocognitive functioning in sickle cell disease from school age to young adulthood. Br J Haematol. (2021) 195(2):256–66. doi: 10.1111/bjh.17687
15. Nordhov SM, Rønning JA, Dahl LB, Ulvund SE, Tunby J, Kaaresen PI. Early intervention improves cognitive outcomes for preterm infants: randomized controlled trial. Pediatrics. (2010) 126(5):e1088. doi: 10.1542/peds.2010-0778
16. Ramey CT, Ramey SL. Early intervention and early experience. Am Psychol. (1998) 53(2):109. doi: 10.1037/0003-066X.53.2.109
17. Burchinal MR, Campbell FA, Bryant DM, Wasik BH, Ramey CT. Early intervention and mediating processes in cognitive performance of children of low-income African American families. Child Dev. (1997) 68(5):935. doi: 10.2307/1132043
18. Ramey CT, Ramey SL. Prevention of intellectual disabilities: early interventions to improve cognitive development. Prev Med. (1998) 27(2):224–32. doi: 10.1006/pmed.1998.0279
19. Hoyt CR, King AA, Heitzer A, MacArthur E, Wilson A, Leaver H. Utilization of early intervention among children with sickle cell disease. Blood. (2023) 142(Supplement 1):3737. doi: 10.1182/blood-2023-180852
20. Ciribassi RM, Patil CL. We don't wear it on our sleeve": sickle cell disease and the (in)visible body in parts. Soc Sci Med. (2016) 148:131–8. doi: 10.1016/j.socscimed.2015.11.033
21. Jacob SA, Daas R, Feliciano A, Lamotte JE, Carroll AE. Caregiver experiences with accessing sickle cell care and the use of telemedicine. BMC Health Serv Res. (2022) 22(1):239. doi: 10.1186/s12913-022-07627-w
22. Blakey AO, Lavarin C, Brochier A, Amaro CM, Eilenberg JS, Kavanagh PL, et al. Effects of experienced discrimination in pediatric sickle cell disease: caregiver and provider perspectives. J Racial Ethn Health Disparities. (2023) 10(6):3095–106. doi: 10.1007/s40615-022-01483-4
23. Hardy SJ, Bills SE, Meier ER, Schatz JC, Keridan KJ, Wise S, et al. A randomized controlled trial of working memory training in pediatric sickle cell disease. J Pediatr Psychol. (2021) 46(8):1001–14. doi: 10.1093/jpepsy/jsab030
24. Hoyt CR, Heitzer AM, Hardy SJ. Commentary on the ASH 2020 guidelines on cognitive screening and intervention in sickle cell disease. Blood Adv. (2023) 7(14):3680–2. doi: 10.1182/bloodadvances.2023009851
25. Woodward EN, Matthieu MM, Uchendu US, Rogal S, Kirchner JE. The health equity implementation framework: proposal and preliminary study of hepatitis C virus treatment. Implement Sci. (2019) 14:1–18. doi: 10.1186/s13012-019-0861-y
26. Creswell JW, Clark VLP. Designing and Conducting Mixed Methods Research. Thousand Oaks, CA: Sage publications (2017).
27. Hankins JS, Estepp JH, Hodges JR, Villavicencio MA, Robison LL, Weiss MJ, et al. Sickle cell clinical research and intervention program (SCCRIP): a lifespan cohort study for sickle cell disease progression from the pediatric stage into adulthood. Pediatr Blood Cancer. (2018) 65(9):e27228. doi: 10.1002/pbc.27228
28. Smedley BD, Stith AY, Nelson AR. Assessing Potential Sources of Racial and Ethnic Disparities in Care: Patient-and System-level factors. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. US: National Academies Press (2003).
29. Cella D, Riley W, Stone A, Rothrock N, Reeve B, Yount S, et al. The patient-reported outcomes measurement information system (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. (2010) 63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011
30. Health NIo. PROMIS Score Cut Points: Health Measures. Available online at: https://www.healthmeasures.net/score-and-interpret/interpret-scores/promis/promis-score-cut-points (updated 11/3/2023).
31. Liu H, Cella D, Gershon R, Shen J, Morales LS, Riley W, et al. Representativeness of the patient-reported outcomes measurement information system internet panel. J Clin Epidemiol. (2010) 63(11):1169–78. doi: 10.1016/j.jclinepi.2009.11.021
33. Hamilton MA, Orme JG. Examining the construct validity of three parenting knowledge measures using LISREL. Soc Serv Rev. (1990) 64(1):121–43. doi: 10.1086/603745
35. Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. (2008) 62(1):107–15. doi: 10.1111/j.1365-2648.2007.04569.x
36. Lindgren B-M, Lundman B, Graneheim UH. Abstraction and interpretation during the qualitative content analysis process. Int J Nurs Stud. (2020) 108:103632. doi: 10.1016/j.ijnurstu.2020.103632
37. Creamer EG. Building theoretical insight through joint displays in mixed methods research. Sch Psychol. (2023) 38(6):399–409. doi: 10.1037/spq0000574
38. Sontag-Padilla LM, Burns RM, Shih RA, Griffin BA, Martin LT, Chandra A, et al. The Urban Child Institute CANDLE Study. Methodological Overview and Baseline Sample Description. Santa Monica, CA: RAND Corporation (2015). p. 63–82.
39. Lee L, Smith-Whitley K, Banks S, Puckrein G. Reducing health care disparities in sickle cell disease: a review. Public Health Rep. (2019) 134(6):599–607. doi: 10.1177/0033354919881438
40. Power-Hays A, McGann PT. When actions speak louder than words—racism and sickle cell disease. N Engl J Med. (2020) 383(20):1902–3. doi: 10.1056/NEJMp2022125
41. Drotar D. Treatment adherence in patients with sickle cell Anemia. J Pediatr. (2010) 156(3):350–1. doi: 10.1016/j.jpeds.2009.10.035
42. Creary SE, Modi AC, Stanek JR, Chisolm DJ, O’Brien SH, Nwankwo C, et al. Allocation of treatment responsibility and adherence to hydroxyurea among adolescents with sickle cell disease. J Pediatr Psychol. (2019) 44(10):1196–204. doi: 10.1093/jpepsy/jsz061
43. Modi AC, Crosby LE, Guilfoyle SM, Lemanek KL, Witherspoon D, Mitchell MJ. Barriers to treatment adherence for pediatric patients with sickle cell disease and their families. Childrens Health Care. (2009) 38(2):107–22. doi: 10.1080/02739610902813245
44. Bulgin D, Tanabe P, Jenerette C. Stigma of sickle cell disease: a systematic review. Issues Ment Health Nurs. (2018) 39(8):675–86. doi: 10.1080/01612840.2018.1443530
45. Wesley KM, Zhao M, Carroll Y, Porter JS. Caregiver perspectives of stigma associated with sickle cell disease in adolescents. J Pediatr Nurs. (2016) 31(1):55–63. doi: 10.1016/j.pedn.2015.09.011
46. Smedley BD. The lived experience of race and its health consequences. Am J Public Health. (2012) 102(5):933–5. doi: 10.2105/AJPH.2011.300643
47. McIntyre LL, Neece CL, Sanner CM, Rodriguez G, Safer-Lichtenstein J. Telehealth delivery of a behavioral parent training program to Spanish-speaking Latinx parents of young children with developmental delay: applying an implementation framework approach. School Psych Rev. (2022) 51(2):206–20. doi: 10.1080/2372966X.2021.1902749
48. Rooks-Ellis D, Howorth SK, Kunze M, Boulette S, Sulinski E. Effects of a parent training using telehealth: equity and access to early intervention for rural families. J Childh Educ Soc. (2020) 1(2):141–66. doi: 10.37291/2717638X.20201242
49. Speller-Brown B, Carhuas C, Stone A, Connolly M, Martin B, Zhang A, et al. Telemedicine in sickle cell disease: patient, parent, and provider perspectives. Pediatr Blood Cancer. (2023) 70(3):e30125. doi: 10.1002/pbc.30125
50. Zhang S, Xue W, Boorman EP, Ruvalcaba E, David M, Lanzkron SM, et al. Accessible care with high patient satisfaction: telemedicine use in sickle cell disease. Telemed e-Health. (2023) 29(7):1068–77. doi: 10.1089/tmj.2022.0387
51. Jacob SA, Carroll AE, Bennett WE Jr. A feasibility study of telemedicine for paediatric sickle cell patients living in a rural medically underserved area. J Telemed Telecare. (2021) 27(7):431–5. doi: 10.1177/1357633X19883558
52. Jensen SA, Elkin TD, Hilker K, Jordan S, Iyer R, Smith MG. Caregiver knowledge and adherence in children with sickle cell disease: knowing is not doing. J Clin Psychol Med Settings. (2005) 12:333–7. doi: 10.1007/s10880-005-7819-8
53. Erickson SJ, Gerstle M, Feldstein SW. Brief interventions and motivational interviewing with children, adolescents, and their parents in pediatric health care settings: a review. Arch Pediatr Adolesc Med. (2005) 159(12):1173–80. doi: 10.1001/archpedi.159.12.1173
54. Resnicow K, DiIorio C, Soet JE, Borrelli B, Hecht J, Ernst D. Motivational interviewing in health promotion: it sounds like something is changing. Health Psychol. (2002) 21(5):444. doi: 10.1037/0278-6133.21.5.444
55. Coleman PK, Trent A, Bryan S, King B, Rogers N, Nazir M. Parenting behavior, mothers’ self-efficacy beliefs, and toddler performance on the Bayley scales of infant development. Early Child Dev Care. (2002) 172(2):123–40. doi: 10.1080/03004430210888
56. Pelletier J, Brent JM. Parent participation in children’school readiness: the effects of parental self-efficacy, cultural diversity and teacher strategies. Int J Early Childh. (2002) 34(1):45–60. doi: 10.1007/BF03177322
57. Self KJ, Borsari B, Ladd BO, Nicolas G, Gibson CJ, Jackson K, et al. Cultural adaptations of motivational interviewing: a systematic review. Psychol Serv. (2023) 20(S1):7. doi: 10.1037/ser0000619
58. McCabe KM, Yeh M, Zerr AA. Personalizing behavioral parent training interventions to improve treatment engagement and outcomes for culturally diverse families. Psychol Res Behav Manag. (2020) 13:41–53. doi: 10.2147/PRBM.S230005
59. Carlson MM, Bear B, Alderfer MA, Schultz CL, Monroe DP, Crosby LE, et al. Family-centered communication in pediatric sickle cell disease. Pediatr Blood Cancer. (2022) 69(12):e30016. doi: 10.1002/pbc.30016
60. Heitzer AM, Macarthur E, Tamboli M, Wilson A, Hankins JS, Hoyt CR. Awareness, access, and communication: provider perspectives on early intervention services for children with sickle cell disease. Front Pediatr. (2024) 12:1366522. doi: 10.3389/fped.2024.1366522
61. Cox ED, Swedlund MP, Young HN, Moreno MA, Schopp JM, Rajamanickam V, et al. Family engagement in pediatric sickle cell disease visits. Health Commun. (2017) 32(1):51–9. doi: 10.1080/10410236.2015.1099503
62. Cronin RM, Yang M, Hankins JS, Byrd J, Pernell BM, Kassim A, et al. Association between hospital admissions and healthcare provider communication for individuals with sickle cell disease. Hematology. (2020) 25(1):229–40. doi: 10.1080/16078454.2020.1780737
63. Hashim MJ. Patient-centered communication: basic skills. Am Fam Physician. (2017) 95(1):29–34.28075109
64. Sil S, Woodward KE, Johnson YL, Dampier C, Cohen LL. Parental psychosocial distress in pediatric sickle cell disease and chronic pain. J Pediatr Psychol. (2021) 46(5):557–69. doi: 10.1093/jpepsy/jsaa130
65. Heitzer AM, Longoria J, Porter JS, MacArthur E, Potter B, Ding J, et al. Internalizing symptoms in adolescents with sickle cell disease. J Pediatr Psychol. (2023) 48(1):91–103. doi: 10.1093/jpepsy/jsac068
66. Martin BM, Thaniel LN, Speller-Brown BJ, Darbari DS. Comprehensive infant clinic for sickle cell disease: outcomes and parental perspective. J Pediatr Health Care. (2018) 32(5):485–9. doi: 10.1016/j.pedhc.2018.04.018
67. Nikolajski C, O'Brien J, Nardo E, Szigethy E, Jonassaint C. Tailoring a digital mental health program for patients with sickle cell disease: qualitative study. JMIR Ment Health. (2023) 10:e44216. doi: 10.2196/44216
68. Coll CG, Crnic K, Lamberty G, Wasik BH, Jenkins R, Garcia HV, et al. An integrative model for the study of developmental competencies in minority children. Child Dev. (1996) 67(5):1891–914. doi: 10.2307/1131600
69. Su J, Dugas M, Guo X, Gao G. Building social identity-based groups to enhance online peer support for patients with chronic disease: a pilot study using mixed-methods evaluation. Transl Behav Med. (2022) 12(5):702–12. doi: 10.1093/tbm/ibac008
70. Cameron JE, Voth J, Jaglal SB, Guilcher SJT, Hawker G, Salbach NM. “In this together”: social identification predicts health outcomes (via self-efficacy) in a chronic disease self-management program. Soc Sci Med. (2018) 208:172–9. doi: 10.1016/j.socscimed.2018.03.007
71. Carroll L, Chippior J, Karmali S, Sriram D, Ysseldyk R. We are caregivers: social identity is associated with lower perceived stress among rural informal caregivers. Can J Aging. (2019) 38(1):59–75. doi: 10.1017/S0714980818000430
Keywords: sickle cell, mixed-methods, intervention, developmental, caregivers
Citation: Tamboli M, MacArthur E, Collins N, Kang E, Fernandez M, Porter JS, Conklin HM, King AA, Hankins JS and Heitzer AM (2025) Caregiver and provider perspectives on developmental services for children with sickle cell disease: a mixed methods analysis. Front. Pediatr. 13:1530457. doi: 10.3389/fped.2025.1530457
Received: 18 November 2024; Accepted: 10 March 2025;
Published: 21 March 2025.
Edited by:
Raffaella Colombatti, University Hospital of Padua, ItalyReviewed by:
Najibah Galadanci, University of Alabama at Birmingham, United StatesVincenzo Voi, San Luigi Gonzaga University Hospital, Italy
Copyright: © 2025 Tamboli, MacArthur, Collins, Kang, Fernandez, Porter, Conklin, King, Hankins and Heitzer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrew M. Heitzer, YW5kcmV3LmhlaXR6ZXJAc3RqdWRlLm9yZw==
 Mollie Tamboli
Mollie Tamboli Erin MacArthur1
Erin MacArthur1 Eunyoung Kang
Eunyoung Kang Maria Fernandez
Maria Fernandez Heather M. Conklin
Heather M. Conklin Allison A. King
Allison A. King Jane S. Hankins
Jane S. Hankins Andrew M. Heitzer
Andrew M. Heitzer