
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 26 February 2025
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1527605
 Hyun Jin Kim1
Hyun Jin Kim1 Ju Young Kim2
Ju Young Kim2 Yoo Min Lee3
Yoo Min Lee3 Yong Hee Hong3
Yong Hee Hong3 Ben Kang4
Ben Kang4 Byung-Ho Choe4
Byung-Ho Choe4 Dae Yong Yi5
Dae Yong Yi5 Eun Hye Lee6
Eun Hye Lee6 Soon Chul Kim7
Soon Chul Kim7 You Jin Choi8
You Jin Choi8 Hyo-Jeong Jang9
Hyo-Jeong Jang9 So Yoon Choi10*
So Yoon Choi10*
Background: Although antinuclear antibody (ANA) is frequently observed in patients with metabolic dysfunction-associated steatotic liver disease (MASLD), its clinical significance in children remains unclear and controversial. In this study, we investigated the prevalence of ANA positivity and the factors associated with it in pediatric MASLD patients without concurrent autoimmune hepatitis.
Methods: We retrospectively reviewed the medical records of patients aged 4–18 years diagnosed with MASLD and tested for ANA from January 2015 to December 2020 at 10 hospitals in Korea. All statistical analyses were carried out using SPSS 26.0 and P-values <0.05 were considered statistically significant.
Results: Out of the 439 patients included, ANAs were present in 89 (20.3%); 51 (57.3%) patients had ANA titer <1:80; 22 (24.7%), <1:160; 10 (11.2%), <1:320; and 6 (6.7%), <1:640. Compared to ANA-negative patients, aspartate aminotransferase (AST, P = 0.003) and alanine aminotransferase (ALT, P = 0.007) levels were significantly higher in ANA-positive patients. The ALT to Platelet Ratio Index (APRI) score was also associated with the ANA-positive patients (P = 0.005). To predict ANA positivity using APRI, the area under receiver operating characteristic (AUROC) curve was 0.597 (p = 0.004), and the APRI cutoff value of >0.893 could predict ANA, with sensitivity and specificity of 42.7% and 72.9%, respectively.
Conclusions: ANA positivity in pediatric MASLD is associated with greater liver enzyme elevation and increased risk of fibrosis, highlighting the need for careful monitoring in ANA-positive patients.
Autoantibodies react with self-antigens and are directed against one or more of the individual's own proteins (1). Non-specific autoantibodies associated with liver disease include antinuclear, anti-smooth muscle, and anti-mitochondrial antibodies (2). Antinuclear and anti-smooth muscle antibodies are frequently positive in patients with autoimmune hepatitis (AIH), and their positivity is one of the diagnostic criteria for AIH, together with hypergammaglobulinemia and typical histological findings (3–5). However, low levels of these autoantibodies are also present in 6%–15% of the healthy population, highlighting their non-specific nature. In patients with chronic liver disease, 7%–52% have been reported to be positive for autoantibodies, as any component of hepatocytes can potentially trigger their production (6, 7).
Metabolic dysfunction-associated steatotic liver disease (MASLD) is diagnosed by exclusion through the presence of hepatic steatosis with no other causes (8, 9). In pediatric obese patients, alanine aminotransferase (ALT) measurement is currently the best screening tool for MASLD, though it has significant limitations. While liver biopsy remains the gold standard for diagnosing MASLD, it is challenging to perform in children due to its invasive nature, need for sedation, and potential complications such as pain, bleeding, and, rarely, mortality (10). Consequently, pediatric MASLD is commonly diagnosed using clinical symptoms, laboratory findings, and imaging rather than biopsy.
Autoantibody testing is often recommended when MASLD is clinically suspected to rule out other potential causes. However, the prevalence and clinical significance of autoantibodies in MASLD, particularly in pediatric patients, are not well established, and studies on ANA in this population are especially limited. In this study, we aimed to investigate the prevalence of antinuclear antibodies and their association with the degree of steatosis and fibrosis in pediatric patients with MASLD.
This was a retrospective multicenter study in the pediatric departments of 10 hospitals in Korea: Chungnam National University Hospital, Chung-Ang University Hospital, Jeonbuk National University Hospital, Kyungpook National University Children's Hospital, Soonchunhyang University Bucheon Hospital, Nowon Eulji Medical Center, Daejeon Eulji Medical Center, Keimyung University Dongsan Medical Center, Inje University Ilsan Paik Hospital, and Kosin University Gospel Hospital.
Among patients aged 4–18 years who were diagnosed with MASLD between January 2015 and December 2020, we included only those who had undergone ANA testing at the time of diagnosis. Patients who were diagnosed with autoimmune hepatitis or had other chronic liver diseases were excluded.
Baseline clinical patients' data, such as sex, age, height, weight, and body mass index (BMI), were collected using electronic medical records. Laboratory tests included tests for levels of ALT, aspartate aminotransferase (AST), gamma-glutamyl transferase, total cholesterol, triglyceride, low-density lipoprotein and high-density lipoprotein (HDL) cholesterol, and fasting glucose. ANA tests were performed via indirect immunofluorescence on Hep-2 cells. The ALT to Platelet Ratio Index (APRI) score for noninvasive markers of liver fibrosis was calculated as follows: APRI score = AST level (IU/L)/AST upper limit of normal (IU/L)/platelet count (109/L) (11, 12).
MASLD was diagnosed based on bright or hyperechoic lesions on liver imaging and ALT levels ≥30 IU/L (8). ANA-positivity was defined as ANA titer of ≥1:80 since the detection of low ANA titer is evident even in the healthy population (13). Ultrasonographic evaluation for the diagnosis of fatty liver was conducted by experienced pediatric radiologists who were blinded to the patients' clinical and laboratory data. The diagnosis of hepatic steatosis was based on specific sonographic features, including increased liver parenchymal echogenicity (bright liver) relative to the adjacent kidney and spleen, absence of focal hepatic lesions, enhanced posterior beam attenuation, and reduced clarity of the portal and hepatic vein structures. The severity of hepatic steatosis was graded semiquantitatively as mild (grade 1), moderate (grade 2), or severe (grade 3), following the criteria described by Saadeh et al. (14–16) This assessment inherently carries operator dependency, and neither the hepatorenal index nor artificial intelligence-based image processing techniques were employed in this study. Diabetes mellitus was declared when the fasting plasma glucose level was ≥126 mg/dl or a 2-h oral glucose tolerance test result was ≥200 mg/dl (17, 18). Hypertension was defined as repeated blood pressure values greater than the 95th percentile for the age, sex, and height of that patient at three separate visits (19, 20).
For detecting cirrhosis, using an APRI cutoff score of 2.0 was more specific (91%) but less sensitive (46%). APRI scores of ≤0.3 and ≤0.5 ruled out significant fibrosis and cirrhosis, respectively, and a value of ≥1.5 ruled out significant fibrosis (12, 21).
This study was approved by the Institutional Review Board (IRB) of Chungnam National University Hospital and all other participating centers (IRB number 2019-11-029). This study was conducted according to the guidelines laid down in the Declaration of Helsinki. The need for Informed Consent was waived by the IRB of Chungnam National University Hospital due to the retrospective nature of the study.
Variables were summarized by frequency and percentage for categorical data and mean ± standard deviation for numeric data. Group differences were tested using the chi-squared test or Fisher's exact for categorical data and independent t-test or Mann–Whitney U-test and analysis of variance or Kruskal–Wallis test for numeric data as appropriate. To check if its distribution is normal, we used Shapiro–Wilk's test. Univariate and multivariate logistic regression analysis were performed to identify prognostic factors which are independently related to ANA. The receiver operating characteristic (ROC) curve analysis was used to calculate the area under the curve (AUC) and performed to assess the sensitivity and specificity of APRI for predicting ANA. The cutoff value was determined by Youden's index. All statistical analyses were carried out using SPSS 26.0 statistical software (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp.) and MedCalc Statistical Software version 19.2.6 (MedCalc Software Ltd., Ostend, Belgium). Statistical consultation for the analyses was performed by ACE Statistical Consulting in the Republic of Korea. P-values less than 0.05 was considered statistically significant.
A total of 439 patients were included in the study; 89 (20.3%) were ANA-positive, and 350 (79.7%) were ANA-negative. A comparison of the baseline characteristics of the ANA-positive and ANA-negative groups is presented in Table 1. AST (94.60 ± 91.03 IU/L vs. 72.36 ± 50.22 IU/L, P = 0.009) and ALT (155.37 IU/L ± 96.16 vs. 125.70 ± 82.60 IU/L, P = 0.007) levels were significantly higher in the ANA-positive patient group than in the ANA-negative patient group (Table 2). There was no difference in the degree of steatosis between the two groups, as confirmed by ultrasonography. However, APRI, an indirect indicator of fibrosis, was significantly higher in the ANA-positive group (0.91 ± 0.63 vs. 0.73 ± 0.56, P = 0.005). In addition, the higher the APRI value, the higher the proportion of ANA-positive patients (Figure 1).
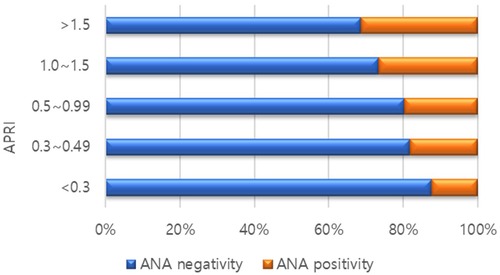
Figure 1. Proportion of antinuclear antibody (ANA) positivity according to ALT to platelet ratio Index (APRI) scores.
Risk factor analysis for ANA is shown in Table 3. In the univariate analysis, ALT [odds ratio (OR) 1.00, 95% confidence interval (CI): 1.00–1.01, P = .004], AST [OR 1.01, 95% CI: 1.00–1.01, P = .019], and APRI score [OR 1.62, 95% CI: 1.12–2.34, P = .011] were related to ANA although only ALT [OR 1.00, 95% CI: 1.00–1.01, P = .004] was related to ANA in the multivariate analysis.
A receiver operating characteristic (ROC) curves of APRI to predict ANA is shown in Table 4 and Figure 2. The area under receiver operating characteristic (AUROC) curve was 0.597 (p = 0.004), and the APRI cutoff value of >0.893 could predict positive-ANA, with sensitivity and specificity of 42.7% and 72.9%, respectively.

Table 4. Receiver operating characteristic curve analysis for predicting positive anti-nuclear antibody.
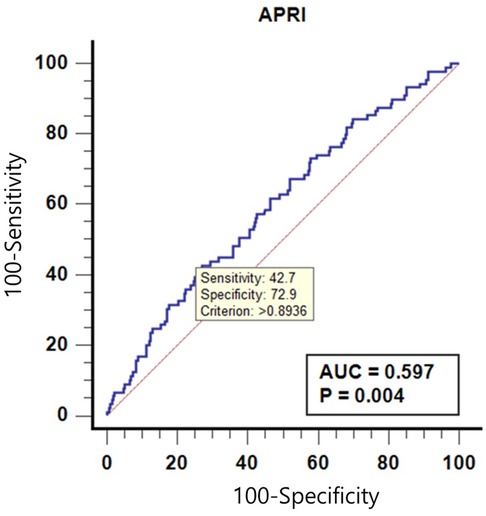
Figure 2. Receiver operating characteristic curves of alanine aminotransferase to platelet ratio Index (APRI) to predict anti-nuclear antibody (ANA).
In this study, we found that 20.3% of pediatric patients with MASLD tested positive for ANA, with varying titers observed across the cohort. Notably, ANA-positive patients exhibited significantly higher levels of AST and ALT compared to ANA-negative patients, suggesting that ANA positivity is associated with greater liver inflammation. However, there was no significant difference in the degree of hepatic steatosis between the two groups as assessed by ultrasonography. Additionally, the APRI score, a non-invasive index commonly used to assess liver fibrosis, was significantly elevated in ANA-positive patients, supporting a potential link between ANA positivity and increased fibrosis risk in pediatric MASLD.
Interestingly, in our multivariate analysis, only ALT remained significantly associated with ANA positivity, while other factors, including AST and APRI, did not retain statistical significance after adjustment. This may be explained by the fact that ALT is a more specific marker of hepatocellular injury compared to AST, which is also found in other tissues, including muscle and the heart (22). The stronger association of ALT with ANA positivity may reflect its closer link to ongoing liver inflammation specifically related to MASLD, whereas AST elevations may be influenced by extrahepatic factors. Additionally, ALT elevation may indicate subclinical immune-mediated hepatocellular injury that could be linked to autoimmune responses reflected by ANA positivity (23). The absence of significant associations with other variables suggests that ALT may serve as a more sensitive marker of immune-related liver injury in this population.
The association between ANA positivity and MASLD may be partially explained by insulin resistance. Previous studies have reported a close link between high-titer ANA positivity and elevated indices of insulin resistance, a well-known factor in MASLD pathogenesis (24, 25). Hepatic NKT cell accumulation, which promotes fibrosis in liver disease, can also produce autoantibodies in MASLD (26). However, the clinical significance of ANA in patients with MASLD is conflicting and controversial. Yodoshi et al. found a strong association between positive ANA and higher steatosis scores, while Adams et al. demonstrated that ANA-positive NASH patients had more severe liver necroinflammation and fibrosis than ANA-negative patients (27, 28). In contrast, Kohut et al. observed no association between autoantibodies and the degree of liver inflammation, steatosis, or fibrosis, though they noted that combined ALT and ANA positivity could improve identification of patients at higher risk for NASH (29). In our study, while there was no difference in the degree of steatosis between ANA-positive and ANA-negative groups, ALT and APRI levels were significantly higher in ANA-positive patients.
The APRI's predictive value for ANA positivity was modest, with an AUROC curve of 0.597. An APRI cutoff of >0.893 showed a sensitivity of 42.7% and specificity of 72.9% for predicting ANA positivity. While these values indicate limited utility for APRI in reliably identifying ANA-positive patients, they suggest that higher APRI scores might warrant closer monitoring of pediatric MASLD patients, especially those with elevated liver enzymes.
Additionally, previous studies have suggested that MASLD may worsen clinical outcomes in patients with AIH. Johnson et al. reported that patients with combined AIH and NASH experienced poorer survival and more adverse outcomes than those with AIH alone, underscoring the need for caution when ANA is positive in patients with MASLD or concurrent AIH (30).
Long-term studies of ANA-positive MASLD patients, especially in children, remain limited. One recent study noted that ANA-positive MASLD patients had a higher prevalence of NASH at diagnosis; however, long-term outcomes, including hepatocellular carcinoma occurrence, extrahepatic malignancy, and overall survival, were similar to those of ANA-negative patients (31). In our study, a high ANA titer (1:320) was not associated with significant fibrosis in MASLD patients, nor was there a significant elevation in AST, ALT, or APRI levels in patients with higher ANA titers. This aligns with previous findings that significant ANA positivity (ANA ≥1:160) is not necessarily linked to advanced histological features in MASLD (32). Interestingly, the APRI cutoff value for predicting ANA positivity (0.893) in our study was higher than the optimal APRI score of 0.64, typically used to predict advanced fibrosis (F3/F4) in chronic hepatitis C patients (33).
This study had several limitations. First, its retrospective design may have affected the consistency of some variables. Second, the operator-dependent nature of ultrasonography in diagnosing hepatic steatosis is a notable limitation. Since this was a multicenter study, sonographic assessments were conducted by multiple radiologists, which may have introduced inter-operator variability and influenced the evaluation of hepatic steatosis severity. Recent studies have shown artificial intelligence-based ultrasonographic algorithms can automatically calculate the hepatorenal index, significantly improving the diagnostic performance for mild hepatic steatosis and reducing operator dependency (34). Such AI-driven tools are expected to contribute to more accurate and standardized assessments of hepatic steatosis in the future. While ALT levels were analyzed in relation to ANA, they were not adjusted for BMI, which represents a limitation of our study. Despite these limitations, our study is valuable in that it evaluates the significance of ANA positivity in a relatively large cohort of pediatric MASLD patients.
In conclusion, ANA positivity in pediatric MASLD is found to be associated with elevated liver enzymes and increased fibrosis risk, as indicated by higher APRI scores in ANA-positive patients. These results underscore the need for careful monitoring and potentially more aggressive management strategies in ANA-positive children with MASLD. Future research should aim to further elucidate the role of ANA in pediatric MASLD pathogenesis and assess its utility as a biomarker for disease severity and progression.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving humans were approved by Chungnam National University Hospital (IRB number 2019-11-029). The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because the retrospective nature of the study.
HK: Data curation, Formal Analysis, Investigation, Writing – original draft. JK: Data curation, Methodology, Writing – review & editing. YL: Data curation, Project administration, Resources, Writing – review & editing. YH: Data curation, Resources, Software, Writing – review & editing. BK: Data curation, Project administration, Resources, Writing – review & editing. B-HC: Software, Supervision, Validation, Writing – review & editing. DY: Data curation, Investigation, Software, Writing – review & editing. EL: Methodology, Project administration, Resources, Writing – review & editing. SK: Software, Supervision, Validation, Writing – review & editing. YC: Methodology, Project administration, Software, Writing – review & editing. H-JJ: Investigation, Resources, Software, Writing – review & editing. SC: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Supervision, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by Kosin University College of Medicine (2022) and the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2022R1G1A1010896).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Elkon K, Casali P. Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. (2008) 4(9):491–8. doi: 10.1038/ncprheum0895
2. Czaja AJ, Homburger HA. Autoantibodies in liver disease. Gastroenterology. (2001) 120(1):239–49. doi: 10.1053/gast.2001.20223
3. Invernizzi P, Lleo A, Podda M. Interpreting serological tests in diagnosing autoimmune liver diseases. Semin Liver Dis. (2007) 27(2):161–72. doi: 10.1055/s-2007-979469
4. Zeman MV, Hirschfield GM. Autoantibodies and liver disease: uses and abuses. Can J Gastroenterol. (2010) 24(4):225–31. doi: 10.1155/2010/431913
5. Zachou K, Rigopoulou E, Dalekos GN. Autoantibodies and autoantigens in autoimmune hepatitis: important tools in clinical practice and to study pathogenesis of the disease. J Autoimmune Dis. (2004) 1(1):2. doi: 10.1186/1740-2557-1-2
6. Homburger HA, Cahen YD, Griffiths J, Jacob GL. Detection of antinuclear antibodies: comparative evaluation of enzyme immunoassay and indirect immunofluorescence methods. Arch Pathol Lab Med. (1998) 122(11):993–9.9822128
7. Lenzi M, Bellentani S, Saccoccio G, Muratori P, Masutti F, Muratori L, et al. Prevalence of non-organ-specific autoantibodies and chronic liver disease in the general population: a nested case-control study of the Dionysos cohort. Gut. (1999) 45(3):435–41. doi: 10.1136/gut.45.3.435
8. Vos MB, Abrams SH, Barlow SE, Caprio S, Daniels SR, Kohli R, et al. NASPGHAN clinical practice guideline for the diagnosis and treatment of nonalcoholic fatty liver disease in children: recommendations from the expert committee on NAFLD (ECON) and the North American society of pediatric gastroenterology, hepatology and nutrition (NASPGHAN). J Pediatr Gastroenterol Nutr. (2017) 64(2):319–34. doi: 10.1097/MPG.0000000000001482
9. Badraoui R, Ben-Nasr H, Bardakçi F, Rebai T. Pathophysiological impacts of exposure to an endocrine disruptor (tetradifon) on α-amylase and lipase activities associated metabolic disorders. Pestic Biochem Physiol. (2020) 167:104606. doi: 10.1016/j.pestbp.2020.104606
10. Joy D, Thava VR, Scott BB. Diagnosis of fatty liver disease: is biopsy necessary? Eur J Gastroenterol Hepatol. (2003) 15(5):539–43.12702913
11. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. (2003) 38(2):518–26. doi: 10.1053/jhep.2003.50346
12. Loaeza-del-Castillo A, Paz-Pineda F, Oviedo-Cárdenas E, Sánchez-Avila F, Vargas-Vorácková F. AST to platelet ratio index (APRI) for the noninvasive evaluation of liver fibrosis. Ann Hepatol. (2008) 7(4):350–7. doi: 10.1016/S1665-2681(19)31836-8
13. Andersen P. Correlation of smooth-muscle and nuclear antibodies in normal subjects. Clin Exp Immunol. (1977) 27(1):74–7.300307
14. Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. (1999) 94(9):2467–74. doi: 10.1111/j.1572-0241.1999.01377.x
15. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. (2002) 123(3):745–50. doi: 10.1053/gast.2002.35354
16. Ferraioli G, Monteiro LBS. Ultrasound-based techniques for the diagnosis of liver steatosis. World J Gastroenterol. (2019) 25(40):6053. doi: 10.3748/wjg.v25.i40.6053
17. American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. (2008) 31 Suppl 1:S55–60. doi: 10.2337/dc08-S055
18. American Diabetes Association. Screening for type 2 diabetes. Diabetes Care. (2004) 27 Suppl 1:S11–4. doi: 10.2337/diacare.27.2007.s11
19. Falkner B, Gidding SS, Portman R, Rosner B. Blood pressure variability and classification of prehypertension and hypertension in adolescence. Pediatrics. (2008) 122(2):238–42. doi: 10.1542/peds.2007-2776
20. Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical practice guideline for screening and management of high blood pressure in children and adolescents. Pediatrics. (2017) 140(3):e20171904. doi: 10.1542/peds.2017-1904
21. Chou R, Wasson N. Blood tests to diagnose fibrosis or cirrhosis in patients with chronic hepatitis C virus infection: a systematic review. Ann Intern Med. (2013) 158(11):807–20. doi: 10.7326/0003-4819-158-11-201306040-00005
22. Vuppalanchi R, Chalasani N. 5—laboratory tests in liver disease. In: Saxena R, editor. Practical Hepatic Pathology: A Diagnostic Approach. Saint Louis: W.B. Saunders (2011). p. 55–62. doi: 10.1016/B978-0-443-06803-4.00005-8
23. Chentoufi AA, Serov YA, Alazmi M, Baba K. Immune components of liver damage associated with connective tissue diseases. J Clin Transl Hepatol. (2014) 2(1):37–44. doi: 10.14218/JCTH.2014.00001
24. Loria P, Lonardo A, Leonardi F, Fontana C, Carulli L, Verrone AM, et al. Non-organ-specific autoantibodies in nonalcoholic fatty liver disease: prevalence and correlates. Dig Dis Sci. (2003) 48(11):2173–81. doi: 10.1023/B:DDAS.0000004522.36120.08
25. Kitade H, Chen G, Ni Y, Ota T. Nonalcoholic fatty liver disease and insulin resistance: new insights and potential new treatments. Nutrients. (2017) 9(4):387. doi: 10.3390/nu9040387
26. Syn WK, Htun Oo Y, Pereira TA, Karaca GF, Jung Y, Omenetti A, et al. Accumulation of natural killer T cells in progressive nonalcoholic fatty liver disease. Hepatology. (2010) 51(6):1998–2007. doi: 10.1002/hep.23599
27. Yodoshi T, Orkin S, Arce-Clachar AC, Bramlage K, Xanthakos SA, Mouzaki M, et al. Significance of autoantibody seropositivity in children with obesity and non-alcoholic fatty liver disease. Pediatr Obes. (2021) 16(1):e12696. doi: 10.1111/ijpo.12696
28. Adams LA, Lindor KD, Angulo P. The prevalence of autoantibodies and autoimmune hepatitis in patients with nonalcoholic Fatty liver disease. LWW. (2004) 99(7):1316–20. doi: 10.1111/j.1572-0241.2004.30444.x
29. Kohut T, Shah A, Russo P, Panganiban J. Autoimmune antibodies in children and adolescents with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. (2022) 75(3):264–8. doi: 10.1097/MPG.0000000000003534
30. Luca-Johnson D, Wangensteen KJ, Hanson J, Krawitt E, Wilcox R. Natural history of patients presenting with autoimmune hepatitis and coincident nonalcoholic fatty liver disease. Dig Dis Sci. (2016) 61(9):2710–20. doi: 10.1007/s10620-016-4213-3
31. Younes R, Govaere O, Petta S, Miele L, Tiniakos D, Burt A, et al. Presence of serum antinuclear antibodies does not impact long-term outcomes in nonalcoholic fatty liver disease. Am J Gastroenterol. (2020) 115(8):1289–92. doi: 10.14309/ajg.0000000000000676
32. Vuppalanchi R, Gould RJ, Wilson LA, Unalp-Arida A, Cummings OW, Chalasani N, et al. Clinical significance of serum autoantibodies in patients with NAFLD: results from the nonalcoholic steatohepatitis clinical research network. Hepatol Int. (2012) 6(1):379–85. doi: 10.1007/s12072-011-9277-8
33. Papadopoulos N, Vasileiadi S, Papavdi M, Sveroni E, Antonakaki P, Dellaporta E, et al. Liver fibrosis staging with combination of APRI and FIB-4 scoring systems in chronic hepatitis C as an alternative to transient elastography. Ann Gastroenterol. (2019) 32(5):498. doi: 10.20524/aog.2019.0406
Keywords: metabolic dysfunction-associated steatotic liver disease, antinuclear antibody, aspartate aminotransferase, alanine aminotransferase, pediatrics - children
Citation: Kim HJ, Kim JY, Lee YM, Hong YH, Kang B, Choe BH, Yi DY, Lee EH, Kim SC, Choi YJ, Jang H-J and Choi SY (2025) Association of antinuclear antibody positivity with liver disease severity in pediatric metabolic dysfunction-associated steatotic liver disease. Front. Pediatr. 13:1527605. doi: 10.3389/fped.2025.1527605
Received: 13 November 2024; Accepted: 5 February 2025;
Published: 26 February 2025.
Edited by:
Thomai Karagiozoglou- Lampoudi, International Hellenic University, GreeceReviewed by:
Ilaria Farella, University of Bari Aldo Moro, ItalyCopyright: © 2025 Kim, Kim, Lee, Hong, Kang, Choe, Yi, Lee, Kim, Choi, Jang, Choi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: So Yoon Choi, a3MyMDA1NDZAa29zaW5tZWQub3Iua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.