- 1Department of Children’s Diseases and Pediatric Surgery, I.Horbachevsky Ternopil National Medical University, Ternopil, Ukraine
- 2Department of Pediatric Infectious Diseases, Ternopil City Hospital N2, Ternopil, Ukraine
Introduction: Long COVID is characterized by diverse symptoms persisting after severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Given the immunomodulatory and neuroprotective properties of vitamin D, understanding its role in long COVID symptoms is of growing interest. This study aimed to determine vitamin D status in children with COVID-19 and assess its impact on the clinical course of disease and long COVID development.
Methods: A prospective cohort study included hospitalized children with confirmed COVID-19, aged 1 month to 18 years, diagnosed between September 2022 and March 2024. Serum 25-hydroxyvitamin D (25(OH)D) concentrations were measured upon hospital admission, and follow-up was done to identify long COVID symptoms.
Results: In total, 162 hospitalized patients with COVID-19 were examined. Vitamin D deficiency was determined in 8.0%, insufficiency in 25.3%, and optimal levels in 66.7% of children with COVID-19. Vitamin D deficiency/insufficiency was observed in 73% of children over 6 years and 21.6% of children under 6 years of age. Comorbid conditions were 1.4 times more frequent in children with vitamin D insufficiency, with undernutrition and obesity playing the most significant roles (p = 0.0023, p = 0.0245, respectively). Serum 25(OH)D concentration depends on COVID-19 severity (p = 0.0405) and children with vitamin D deficiency/insufficiency had a longer hospital stay (4 vs. 3 days, p = 0.0197). The vitamin D status affected the median levels of neutrophils, lymphocytes, their ratio, prothrombin time, fibrinogen levels, and the frequency of increased immunoglobulins M and E levels. Among 134 children who agreed to follow up, 56 (41.8%) experienced long COVID symptoms, while 78 (58.2%) recovered fully. Long COVID was frequently observed in children with vitamin D deficiency/insufficiency (p = 0.0331). The odds of developing long COVID were 2.2 times higher (p = 0.0346) in children with vitamin D deficiency/insufficiency compared to those with optimal levels. Children with vitamin D deficiency/insufficiency more often exhibited neurological (80% vs. 41.9%, p = 0.0040) and musculoskeletal symptoms (16% vs. 0%, p = 0.0208).
Conclusion: The 25(OH)D concentrations in children with COVID-19 depended on their age. Comorbid conditions affect the vitamin D status in children with COVID-19. Vitamin D influenced the COVID-19 severity and duration of hospitalization. There was an increased risk of developing long COVID in children with vitamin D deficiency/insufficiency, and its impact on the development of neurological symptoms associated with long COVID was established.
Introduction
COVID-19, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), primarily affects the respiratory system, leading to conditions like interstitial pneumonia and acute respiratory distress syndrome (1). Although COVID-19 can cause severe complications in adults, especially those with comorbidities, most children experience mild or asymptomatic cases, with very few requiring hospitalization and a low mortality rate globally (2, 3).
As the incidence of SARS-CoV-2 infection increases, concerns are rising regarding persistent symptoms following acute infection, widely known as “long COVID” (4). Long COVID (sometimes referred to as “post-acute sequelae of COVID-19” or “post-COVID-19 syndrome”) is a multisystem condition characterized by a variety of symptoms, including cardiorespiratory, neurological, psychosomatic, sensory, cognitive, and psychological symptoms that arise after infection with coronavirus type 2 (4, 5). As of September 1, 2024, the World Health Organization (WHO) reports that more than 776 million cases of COVID-19 have been documented worldwide, and 10%–30% of non-hospitalized individuals and 50%–70% of hospitalized patients may experience long COVID (6, 7).
Long COVID raises growing health concerns as its persistence can affect multiple organ systems with potentially negative impacts on quality of life (8, 9). Only a few prospective studies collect systematic data in larger cohorts of children with multidisciplinary clinical assessment (4, 5, 10, 11).
Post-COVID syndrome in adults is more often associated with prolonged tissue damage following persistent inflammation caused by the virus, immune dysregulation, autoimmune processes, endothelial damage, and microthrombosis (12, 13). In children and adolescents, similar pathogenic mechanisms are plausible, but the significance of vitamin and micronutrient deficiencies is also being discussed (14). In developing long COVID symptoms in children, age, certain comorbid conditions, and hospitalization in intensive care units were considered important factors (14–16).
Despite the importance of control measures during the COVID-19 pandemic (17–19), prolonged stay at home negatively impacted the health and development of children (20). Children who remain at home for extended periods are more prone to physical inactivity, unhealthy diets, and limited sunlight exposure, which may put them at greater risk of vitamin D deficiency and insufficiency (20–22).
Vitamin D plays a key role in maintaining calcium homeostasis and bone health, as well as having immunoregulatory effects as a potent regulator of innate and adaptive immune responses, influencing the expression of antimicrobial peptides and the inflammatory cascade (23–25). It has been shown to influence gene expression, modulating immune response, inflammation, oxidative stress, and the gut microbiota (26). Optimal serum vitamin D levels are fundamental for promoting health in both pediatric and adult populations (27).
Several studies have shown a connection between symptoms, severity, mortality, and outcomes of COVID-19 and vitamin D concentration in patients, regardless of age (28, 29). However, the majority of studies are related to the adult population. Szerszeń et al. (30) noted a correlation between patient mortality, the need for oxygen therapy, and vitamin D levels in older patients. Some publications have shown the impact of vitamin D status on the development of long COVID, including multisystem inflammatory syndrome (31). However, the studies on the significance of vitamin D in the development and course of COVID-19 in children, and its role in the emergence of long COVID in the pediatric population remain limited and their results are contradictory. The aim of our study was to determine vitamin D status in children with COVID-19 and assess its impact on the clinical course of disease and long COVID development.
Materials and methods
Study design
A prospective cohort study was conducted from September 2022 to March 2024 in the pediatric infectious diseases department of a tertiary-level hospital in Ternopil, Ukraine.
Participants
The study included hospitalized patients aged 1 month to 18 years diagnosed with COVID-19. All cases of SARS-CoV-2 infection were confirmed using polymerase chain reaction (PCR), rapid tests, or serological methods (detection of class M antibodies).
The inclusion criteria for the study were age up to 18 years, confirmed cases of SARS-CoV-2 infection, informed consent from parents or patients, and the ability to determine the concentration of 25(OH)D in serum during hospitalization. Exclusion criteria included parental refusal for examination and unconfirmed cases of COVID-19.
Data collection and laboratory assessments
A thorough collection of baseline and clinical data was conducted upon patient admission. The baseline characteristics included age and sex, while the clinical signs encompassed comorbidities, the severity of COVID-19, and the duration of hospitalization. The severity of COVID-19 was determined according to the WHO definition (32).
A comprehensive laboratory examination at admission included a complete blood count, determination of biochemical blood analysis indicators, coagulogram, and serum immunoglobulins (Ig) A, M, G, and E. Serum immunoglobulins were determined using the Monobind enzyme-linked immunosorbent assay (ELISA) kit, AccuBind ELISA Kits, USA. The evaluation of immunoglobulin levels was conducted according to age-specific norms.
To assess vitamin D status, its quantitative measurement was conducted in serum. For this purpose, the concentration of 25-hydroxyvitamin D (25(OH)D) in serum was determined using the ELISA, FCCu Bind ELISA Microwells, USA.
According to the recommendations of the European Vitamin D Association (EVIDAS), a concentration of 25(OH)D between 30 and 100 ng/ml (75–250 nmol/L) was considered optimal; 20–30 ng/ml (50–75 nmol/L) was classified as vitamin D insufficiency; and concentrations below 20 ng/ml (<50 nmol/L) indicated vitamin D deficiency (33).
Long COVID definition and monitoring
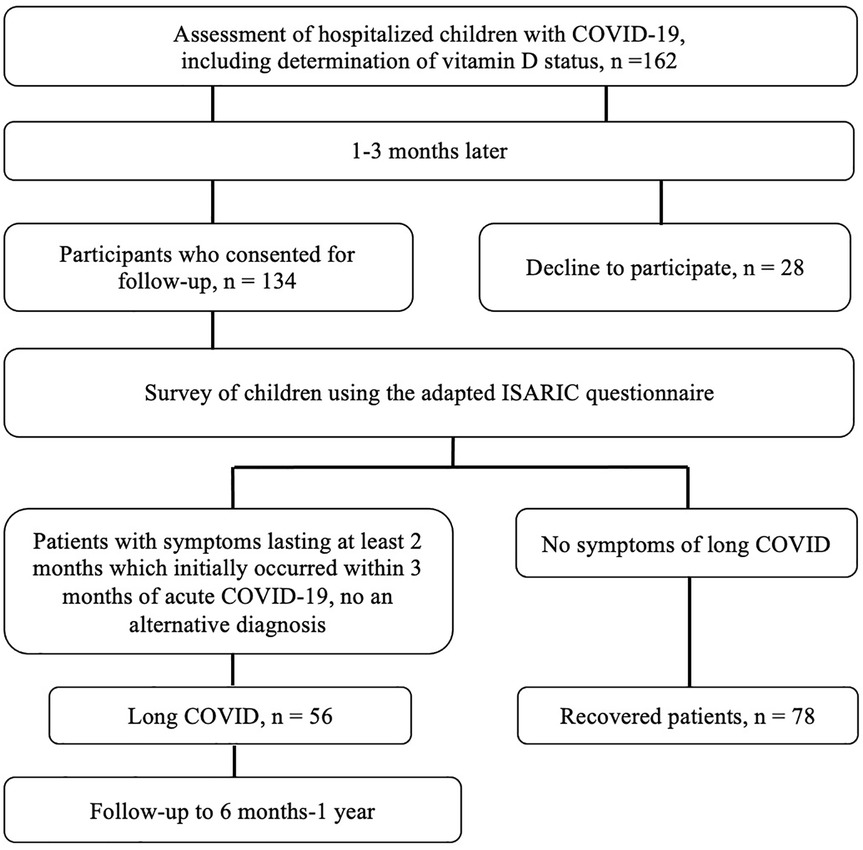
After discharge from the hospital, patients were monitored for the presence of long-term symptoms of COVID-19. For this purpose, we conducted surveys at intervals of 1–3, 3–6, 6–9, and 9–12 months after the acute phase of infection, using the questionnaire developed by the International Severe Acute Respiratory and Emerging Infection Consortium (ISARIC)/IP4C Global Pediatric COVID-19 Follow-Up Form. Patients or their parents, in cases where the children were under 8 years old, answered the questions. The presence of “long COVID” was determined according to WHO criteria, defined as the continuation or development of new symptoms at least 3 months after the initial SARS-CoV-2 infection, with a duration of at least 2 months with no other explanations (6).
Patients who did not have symptoms during the follow-up period after the onset of acute COVID-19 symptoms for at least 8 weeks were defined as fully recovered. The selection of patients for the study is shown in Figure 1.
Ethical considerations
Throughout our study, we adhered to all recommendations of the 1975 Declaration of Helsinki (as revised in 2000). The study was approved by the I. Horbachevsky Ternopil National Medical University Ethics Committee (Minutes № 70 from August 1, 2022). Upon admission, all parents or children who reached the age of 16 signed an informed, voluntary consent for the study, as well as for the use of diagnostic and treatment results in scientific works.
Statistical analysis
Statistical analysis of the results was performed using the STATISTICA 12 software. All data were described as the mean ± standard deviation (SD) for normally distributed data or median and interquartile range (IQR) for skewed distributions and categorical variables expressed as frequency (percentage). Differences in variables with a normal distribution between two independent samples were compared using the Student's t-test, while the results with a non-normal distribution were analyzed using the Mann–Whitney U-test, and categorical variables were compared using the Chi-square test. A p-value of less than 0.05 was defined as statistically significant and highlighted in the tables in bold font.
Odds ratio (OR) and 95% confidence intervals (CI) were determined to explore the influence of vitamin D deficiency on the development of long COVID. For this purpose, we used only statistically significant features.
Results
Characteristics of children with COVID-19
In total, 162 hospitalized patients with COVID-19 were examined. Clinical and laboratory characteristics of children with COVID-19 are presented in Table 1. The average age of hospitalized patients with COVID-19 was 3.62 ± 4.55 years, ranging from 1 month to 18 years. Boys predominated over girls in the overall cohort of patients (56.8%).
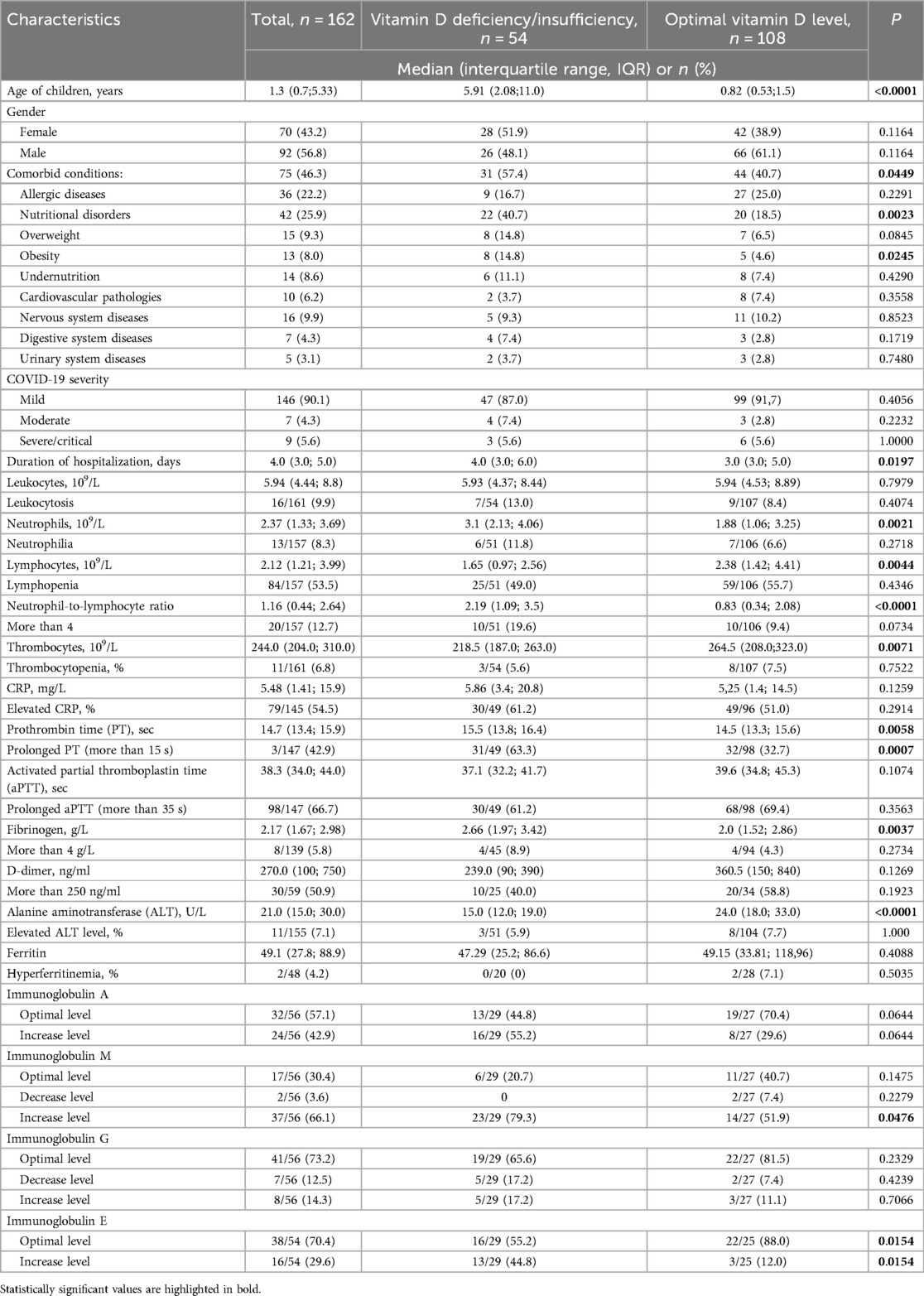
Table 1. Clinical characteristics of the patients with COVID-19 and their dependence on vitamin D status.
Comorbidities were present in 75 (46.3%) patients, with 31 children (19.1%) having two or more conditions. Nutritional disorders were the most common comorbidities (25.9%), followed by allergic conditions (22.2%). Disorders of the nervous, cardiovascular, gastrointestinal systems, and kidneys were less frequently observed (Table 1).
Children with mild COVID-19 predominated in the cohort (90.1%). Moderate cases were observed in 4.3%, severe cases in 4.3%, and critical cases in 1.2% of patients. Eight patients (4.9%) developed COVID-19-related pneumonia. Seven patients (4.3%) required treatment in the intensive care unit, and four children (2.5%) required oxygen therapy during their treatment. Oxygen therapy was administered using a nasal cannula or a face mask, with the duration of oxygen supply depending on the effectiveness of the therapy and the severity of respiratory disorders. The average duration of oxygen therapy was 3 days. Mechanical ventilation was provided briefly for two children who developed acute respiratory failure. No deaths were reported among the children in this cohort. The average length of hospitalization was 4.6 ± 3.0 days, ranging from 1 to 20 days.
Leukocytosis was observed in 9.9% of children, lymphopenia in 53.5%, and neutrophilia in 8.3% of patients. A neutrophil-to-lymphocyte ratio greater than 4 was noted in 12.7% of patients. In most children with COVID-19 (85.7%), platelet counts were within the normal range. An elevated C-reactive protein (CRP) level was found in 54.5% of patients.
A reduced fibrinogen level was identified in 42.5% of patients, while an elevated fibrinogen level was noted in 5.8%. D-dimer levels were elevated in 50.9% of patients upon admission. Ferritin levels ranged from 4.03 to 440 ng/ml, with elevated levels observed in only two cases (4.2%).
An increase in IgA was observed in 42.9% of children, IgM in 66.1%, IgG in 14.3%, and IgE in 29.6% of children. Additionally, decreased levels of IgM were detected in 3.6% of children and IgG in 12.5% of children.
Vitamin D status in patients with the acute phase of SARS-CoV-2 infection
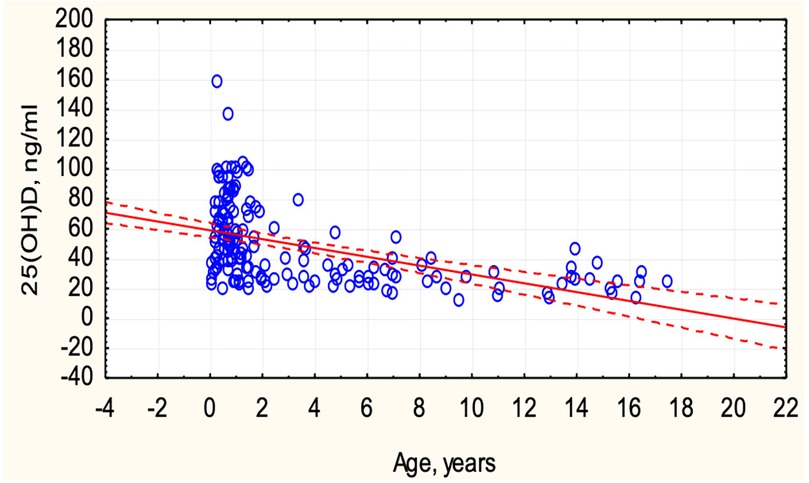
Vitamin D deficiency was identified in 13 (8.0%), insufficiency in 41 (25.3%), and optimal levels in 108 (66.7%) children with COVID-19. Vitamin D status depended on the age of the children (Figure 2). In children under 6 years old, optimal vitamin D levels were more frequently observed (78.4% vs. 21.6%, p < 0.0001), while in children over 6 years, only 27% had optimal levels, and 73% exhibited deficiency and insufficiency. An inverse correlation was observed between the concentration of 25(OH)D and the age of the children (r = −0.4989, p < 0.05) (Figure 3).
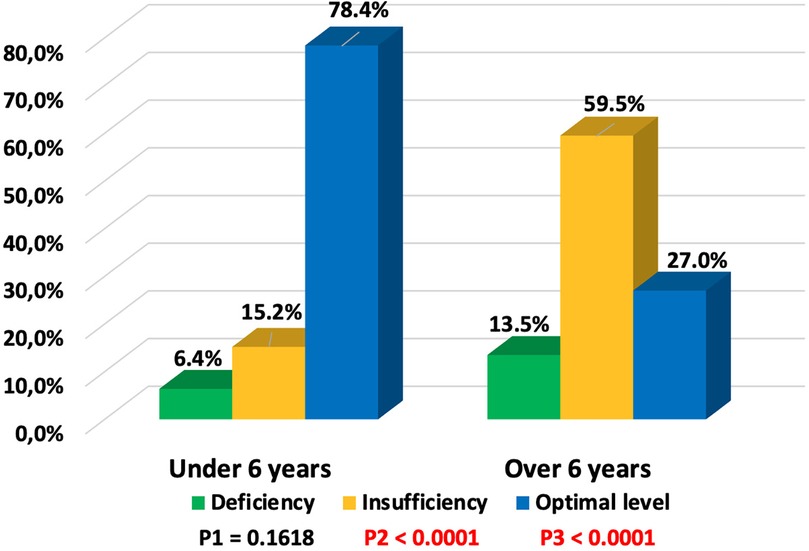
Figure 2. Dependence of vitamin D status on age in children with COVID-19 (P1—value between groups under 6 years and over 6 years with vitamin D deficiency, P2—value between groups under 6 years and over 6 years with vitamin D insufficiency, P3—value between groups under 6 years and over 6 years with optimal vitamin D levels).
Comparison of clinical characteristics of COVID-19 based on vitamin D status
In the first group, with 25(OH)D concentrations below 30.0 ng/ml, there were 54 (33.3%) children, while the remaining 108 (66.7%) were classified in the second group with optimal vitamin D levels (30.0–100.0 ng/ml). The comparison of clinical characteristics of COVID-19 patients based on vitamin D status is presented in Table 1.
Overall, comorbid conditions were 1.4 times more prevalent in children with vitamin D insufficiency, and this difference was statistically significant (p = 0.0449). Specifically, nutritional disorders, particularly obesity, were significantly more common in children with low vitamin D levels (p = 0.0023 and p = 0.0245, respectively).
We did not find a correlation between the severity of COVID-19 and vitamin D status. However, the mean level of serum 25(OH)D concentration in children with mild course was significantly higher than in children with moderate and severe/critical course (49.19 ng/ml vs. 34.40 ng/ml, p = 0.0405) (Figure 4). Children with vitamin D deficiency and insufficiency had longer hospital stays (4 vs. 3 days, p = 0.0197).
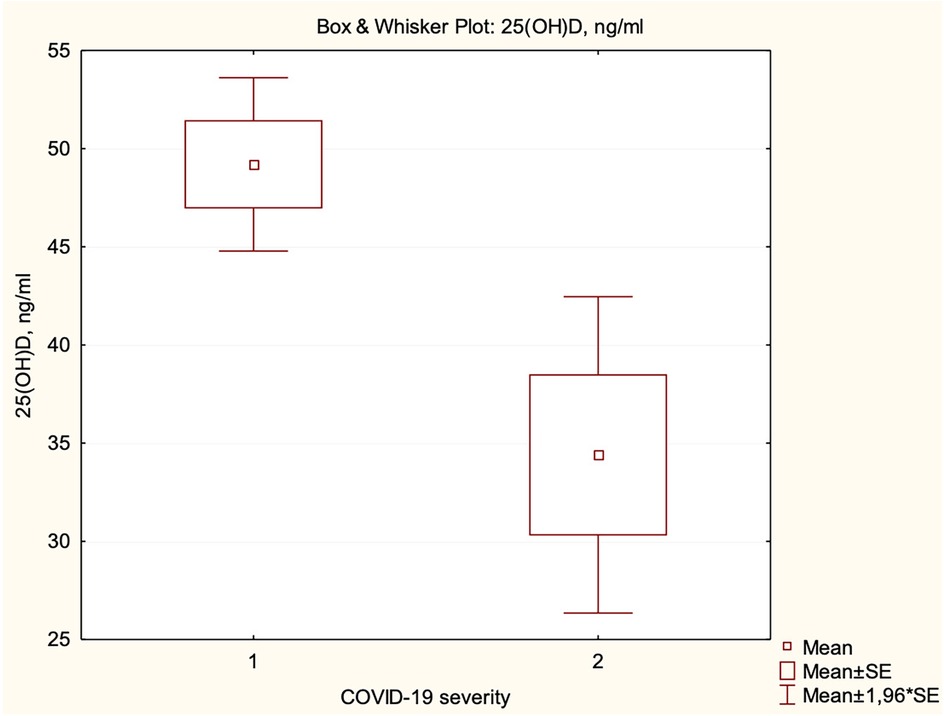
Figure 4. Dependence of serum 25(OH)D concentration on the severity of COVID-19 (1—mild, 2—moderate and severe/critical).
The leukocyte level, as well as the percentage of children with leukocytosis, did not depend on vitamin D status in COVID-19 patients. The median neutrophil level was higher in the group of patients with low vitamin D levels (p = 0.0021), while the median lymphocyte level was higher in patients with optimal vitamin D levels (p = 0.0044). When examining the ratio of neutrophils to lymphocytes, it was found that the ratio was lower in the group with normal vitamin D levels, p < 0.0001. However, the percentage of children with neutrophilia, lymphopenia, and an elevated neutrophil-to-lymphocyte ratio did not differ between the two groups, which may indicate a greater age dependence, as these indicators change with age, and considering that most children under 6 years had optimal vitamin D levels, while those over 6 predominantly had vitamin D insufficiency. However, the number of children with a neutrophil-to-lymphocyte ratio greater than 4 was twice as high among those with vitamin D deficiency/insufficiency, p = 0.0734. In patients with optimal vitamin D levels, the median platelets count was significantly higher (p = 0.0071), but there was no significant difference in the frequency of thrombocytopenia (p = 0.6587).
The CRP level, although somewhat higher in the group of patients with low vitamin D levels, did not differ significantly (Table 1).
Only 11 out of 146 (7.5%) hospitalized children with COVID-19 had all coagulation parameters assessed in this study within the normal range. The medians of PT and fibrinogen were higher in the cohort of patients with low vitamin D levels (p = 0.0058 and p = 0.0037, respectively). The proportion of children with prolonged PT and elevated fibrinogen levels was twice as high in patients with vitamin D deficiency/insufficiency and COVID-19, although the difference was statistically significant only for PT. The median values of aPTT and D-dimer were not dependent on vitamin D status.
The median ALT level was higher in children with optimal vitamin D levels; however, the proportion of children with elevated ALT and ferritin levels did not differ between the two groups of children with COVID-19 (Table 1).
When comparing immunoglobulin levels in children with deficient or insufficient vitamin D levels to those with optimal vitamin D levels, it was found that children with vitamin D deficiency/insufficiency more frequently exhibited elevated levels of IgA (55.2% vs. 29.6%), IgM (79.3% vs. 51.9%), IgG (17.2% vs. 11.1%), and IgE (44.8% vs. 12.0%). The differences were statistically significant for IgM (p = 0.0476) and IgE (p = 0.0154), with a trend toward increased IgA levels (p = 0.0644).
Clinical characteristics of children with long COVID
Of the 162 patients included in the study, 134 consented to further observation. Among them, symptoms of long COVID were identified in 56 (41.8%) children, while 78 (58.2%) recovered fully. The observation period ranged from 6 months to 1 year, with an average of 10.4 months. Among the symptoms in children with long COVID, general manifestations predominated, observed in 37 (66.1%) children. These included fatigue, general weakness, decreased appetite, reduced physical activity, and difficulties starting tasks. Neurological symptoms included insomnia or excessive sleepiness, headache, increased irritability, emotional lability, decreased memory and attention, and inability to concentrate on tasks. This group of symptoms was present in 33 (58.9%) children. Gastroenterological symptoms, such as hepatopathy, abdominal pain, nausea, and constipation, were observed in 9 (16.1%) patients. Cardiological manifestations, including tachycardia or conduction disturbances, were less common, occurring in 2 (3.6%) children, while musculoskeletal symptoms (myalgias and arthralgias) were noted in 4 patients (7.1%). Additionally, 20 (35.7%) children experienced frequent acute respiratory infections, which occurred significantly more often than before the SARS-CoV-2 infection.
Long COVID symptoms depend on vitamin D status
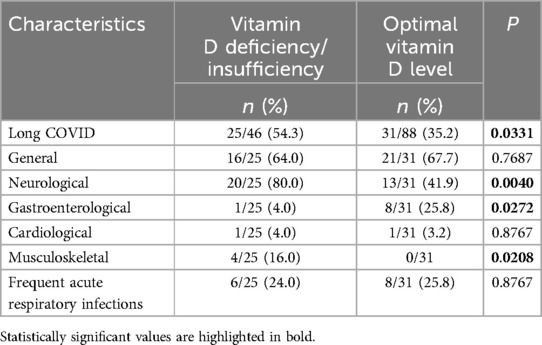
The vitamin D status in patients with long COVID and the symptoms of long COVID based on vitamin D status are shown in Table 2. Vitamin D deficiency was observed in 7 (12.5%) children, insufficiency in 18 (32.1%), and optimal levels in 31 (55.4%) children who subsequently developed long COVID symptoms, while among recovered children, optimal vitamin D levels were found in 57 (73.1%). Long COVID was more frequently observed in children with vitamin D deficiency/insufficiency) (Figure 5), and this difference was statistically significant (p = 0.0331). The odds of developing long COVID were 2.2 times higher (OR = 2.1889, 95% CI: 1.0585–4.5267; p = 0.0346) in children with vitamin D deficiency/insufficiency compared to those with optimal vitamin D levels. It should be noted that vitamin D status in children with symptoms of both long COVID and those who recovered depended on the age of the children. Deficiency/insufficiency of vitamin D was observed in 15/18 (83.3%) children over 6 years old with long COVID symptoms and in 11/14 (78.5%) children who recovered (p = 0.7321). The median concentration of 25(OH)D in patients with long COVID symptoms was lower (34.52 ng/ml; IQR: 23.69; 67.31 ng/ml) compared to those who recovered (44.52 ng/ml; IQR: 28.65; 70.03 ng/ml), but the difference was not statistically significant, p = 0.1451.
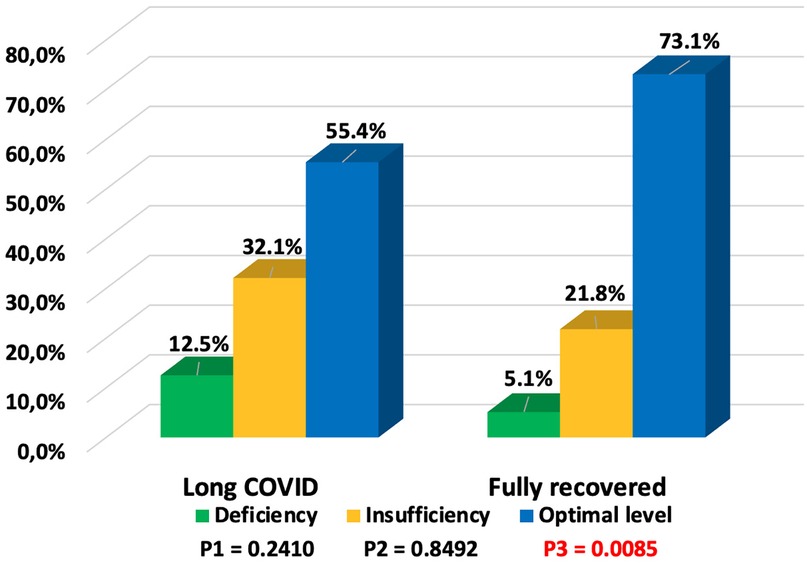
Figure 5. Vitamin D status in children with long COVID and fully recovered (P1—value between groups of children with long COVID and fully recovered with vitamin D deficiency, P2—value between groups of children with long COVID and fully recovered with vitamin D insufficiency, P3—value between groups of children with long COVID and fully recovered with optimal vitamin D levels).
General and cardiological symptoms were observed equally frequently in both groups, regardless of vitamin D status. Neurological manifestations were almost twice as common in children with vitamin D deficiency/insufficiency compared to those with optimal levels (p = 0.0040). Low vitamin D levels also affected musculoskeletal manifestations (16% vs. 0%, p = 0.0208). The odds of developing neurological symptoms of long COVID were 5.5 times higher (OR = 5.5385, 95% CI: 1.6480–18.6133; p = 0.0056) in children with vitamin D deficiency/insufficiency compared to those with optimal vitamin D levels. For musculoskeletal symptoms, OR = 13.1860, 95% CI: 0.6746–257.7413; p = 0.0890. In contrast, gastroenterological symptoms were more frequently observed in patients with normal vitamin D levels (p = 0.0272). The increased incidence of respiratory diseases was nearly the same in both groups of children.
Discussion
Our study revealed a decrease in vitamin D levels in one-third of children with COVID-19. However, findings from other studies regarding the prevalence of hypovitaminosis D among children with acute SARS-CoV-2 infection remain contradictory. Bayramoğlu et al. (29) reported hypovitaminosis D in 35.4% of hospitalized children with mild and moderate disease. This aligns with our findings, as most children in our cohort had mild COVID-19. In contrast, Bayrak et al. (34) found a higher prevalence of vitamin D deficiency or insufficiency (67.1%) among hospitalized children with COVID-19. Furthermore, the average 25(OH)D concentration in their study was significantly lower compared to that of our cohort.
Vitamin D status is influenced not only by the disease itself but also by various external factors. In our cohort, vitamin D status was notably age-dependent, with vitamin D deficiency or insufficiency observed in 73% of children over six years of age. Other studies have also highlighted an age-related dependence of vitamin D levels in hospitalized COVID-19 patients (35), with adolescents being more susceptible to developing hypovitaminosis D. This increased susceptibility among adolescents may stem from their tendency toward sedentary lifestyles, particularly during extended periods of staying at home, with greater access to computers, televisions, and smartphones (36, 37).
The COVID-19 pandemic has contributed to prolonged home confinement for both children and adults, significantly reducing sun exposure time (38, 39). Epidemiological studies have indicated that the pandemic has altered vitamin D levels, particularly among preschool- and school-aged children (40).
Our study revealed differences in mean 25(OH)D concentrations based on COVID-19 severity and the length of hospital stay among children with different vitamin D status. These findings align with other studies that report a direct impact of vitamin D levels on symptoms, severity, and outcomes of COVID-19 (28–30, 41).
Notably, among our patients, pneumonia was diagnosed in only 8 children (4.9%), and 6 (75.0%) of whom had hypovitaminosis D. Nicolae et al. (42) confirmed that hypovitaminosis D could contribute to the development of pneumonia due to its significant influence on immunological processes. Regression analysis in another study also identified low vitamin D levels as a risk factor for developing respiratory distress (41). Furthermore, Kosmeri et al. (43) demonstrated an association between vitamin D insufficiency and the severity of COVID-19 in adults. Their study highlighted 25(OH)D concentration as an independent risk factor for COVID-19 infection and the likelihood of requiring hospitalization.
Other studies have demonstrated the impact of vitamin D status on COVID-19 mortality, especially in adult patients (30, 44, 45). A population-based study revealed a negative correlation between 25(OH) vitamin D average levels and COVID-19 mortality in 19 European countries (44). Seal et al. demonstrated that 25(OH)D concentrations were associated with COVID-19-related hospitalization and mortality in a cohort of veterans (45). A meta-analysis also declared an association between COVID-19 severity and mortality and low serum vitamin D levels (46).
The association between vitamin D deficiency and disease severity or increased mortality is likely related to impaired immune responses. Vitamin D plays a critical role in supporting the immune system. It modulates innate and adaptive immune responses, enhancing the body's ability to fight infections and reducing excessive inflammatory responses (24, 27, 36). Through its effects on immune cells such as macrophages, dendritic cells, and T cells, vitamin D promotes the production of antimicrobial peptides like cathelicidins and defensins, which are vital for neutralizing pathogens (25). Furthermore, vitamin D stabilizes endothelial function and regulates vascular permeability, reducing the risk of cytokine storm and associated complications often observed in severe infections like COVID-19 (29).
An analysis of laboratory characteristics revealed that the median levels of neutrophils and the neutrophil-to-lymphocyte ratio were significantly higher in children with low vitamin D levels (p = 0.0021; p < 0.0001, respectively), whereas median levels of lymphocytes and platelets were significantly lower in this group of patients (p = 0.0044; p = 0.0071, respectively). Alpcan et al. (41) reported a positive correlation between vitamin D levels and leukocyte, lymphocyte, and platelet counts. Similarly, another study found that lower vitamin D levels were associated with increased clinical severity and more pronounced inflammatory markers (29). In our study, CRP was elevated in 55.6% of children with COVID-19 and vitamin D deficiency/insufficiency; however, no statistically significant difference was observed compared to patients with optimal vitamin D levels. Ferritin levels were elevated in only two patients, and no dependence on vitamin D status was identified. By contrast, another study demonstrated significantly higher levels of CRP and ferritin in adult patients with low vitamin D levels (47). Moreover, elevations in CRP, fibrinogen, and lymphopenia were more frequently observed in cases of vitamin D deficiency rather than insufficiency (29). In our cohort, the proportion of children with vitamin D deficiency was relatively small, comprising only 8.0% of the population, while the majority exhibited vitamin D insufficiency. This distribution may explain the lack of statistically significant differences in certain inflammatory markers.
We also observed changes in coagulation markers, specifically increased PT and fibrinogen levels (p = 0.0058; p = 0.0037, respectively). Our previous research demonstrated age-related characteristics of coagulation markers in children with COVID-19 (48). Other studies have also reported a relationship between vitamin D levels and thrombotic complications in COVID-19 patients (47, 49–51). Cooper et al. (50) noted that vitamin D activation reduces the risk of respiratory infections and decreases coagulation and thrombosis.
Increased IgA levels were nearly twice as common, IgM levels were 1.5 times more frequent, IgE levels were 3.7 times more frequent, and changes in IgG levels were 1.8 times more frequent in children with vitamin D deficiency/insufficiency compared to those with optimal levels. Our findings align with results from other researchers, who demonstrated that the immunomodulatory effects of vitamin D are associated with the inhibition of B-cell proliferation, blockage of their differentiation, and a significant decrease in immunoglobulin secretion (52).
On one hand, the more frequent elevation of immunoglobulin levels in cases of vitamin D deficiency/insufficiency may be linked to a more severe course of illness, as was also shown in our study. Specifically, Peraire et al. (53) investigated the relationship between circulating immunoglobulins (IgA, IgG, IgM) and COVID-19 pneumonia. They established that IgM, IgA, and IgG concentrations were significantly higher in patients with COVID-19 pneumonia (mild, severe, and critical forms) compared to those in the ambulatory group (P ≤ 0.001).
On the other hand, increased immunoglobulin production in children with reduced vitamin D levels may potentially contribute to the development of autoimmunity and/or symptoms of long COVID (8, 13, 26). Notably, several studies have demonstrated a rise in autoimmune diseases following the COVID-19 pandemic (54).
In our study, symptoms of long COVID were observed in 41.8% of hospitalized children. The prevalence of patients with long COVID symptoms is highly variable, ranging from 3.4% in symptomatic patients (55) to 81.4% in hospitalized patients (56). Numerous factors can influence the prevalence of long COVID, including age, study cohort, follow-up duration, comorbidities, COVID-19 vaccination status, etc. Rao et al. reported that the frequency of at least one systemic, syndromic, or drug-induced sign of post-acute complications of SARS-CoV-2 infection was 41.9% among children with a positive test for the virus (57), which aligns with the results of our study. The authors highlighted a higher prevalence of long COVID symptoms among children under 5 years of age and those with comorbidities. The high prevalence of comorbid conditions in our cohort (46.3%) and the predominance of children under 6 years of age (77.2%) could explain the high frequency of long COVID symptoms. It should also be noted that we studied the presence of symptoms specifically in hospitalized patients, which may have contributed to the high frequency of long COVID in our findings.
Our study showed that children with vitamin D deficiency or insufficiency had more than twice the risk of developing long COVID compared to those with optimal vitamin D levels (OR = 2.1889, p = 0.0346). These findings align with results from other studies. For instance, lower 25(OH) vitamin D concentrations were reported six months after the acute phase of the disease in adults with long COVID compared to those who fully recovered from coronavirus infection (20.1 vs. 23.2 ng/ml, p = 0.0300) (58). Similarly, Chen et al. demonstrated that vitamin D deficiency was associated with delayed recovery in adult patients with long COVID (59). A study by Guerrero-Romero et al. highlighted a threefold increase in the risk of developing long COVID in adult patients with insufficient vitamin D and magnesium levels (60). However, several investigations involving adult populations did not find a significant association between low serum vitamin D levels and long COVID (59, 61, 62). Pizzini et al. conducted a prospective, multicenter study on the long-term sequelae of COVID-19 and their association with 25(OH)D concentrations (63). While vitamin D deficiency was commonly observed among COVID-19 patients, it was not linked to long-term disease outcomes. Similarly, other researchers found reduced concentrations of vitamins D, A, and E in adult patients with COVID-19 but reported no significant impact on the development of long COVID symptoms (64).
In children, research has primarily focused on vitamin D status concerning multisystem inflammatory syndrome (MIS-C) associated with COVID-19. Studies have shown that children with MIS-C had significantly lower vitamin D levels than those in the non-MIS-C group (65), and vitamin D deficiency was found in 72% of children with MIS-C (31).
The impact of vitamin D levels on long COVID symptoms in children has also been analyzed. We observed an almost twofold increase in the frequency of neurological symptoms in patients with vitamin D deficiency (p = 0.0040) and a higher incidence of musculoskeletal symptoms (p = 0.0208). However, the odds ratio indicated only an increased risk of neurological symptoms. Another study found lower 25(OH)D levels in patients with neurocognitive symptoms six months after recovering from COVID-19 (60). A multivariable regression analysis conducted by Townsend et al. showed no relationship between persistent fatigue and reduced exercise tolerance following COVID-19 (66).
Given vitamin D's ability to modulate both immune and nerve cells, its role in neuroimmune modulation, anti-inflammatory action, neuroprotection, and endothelial function suggests that it could play a positive role in preventing and managing neuropsychiatric, neuroinflammatory, and other processes in long COVID (67, 68). However, further research is needed to determine optimal dosages and the duration of treatment.
Strengths and limitations of the study
This study provides valuable insight into the role of vitamin D in pediatric COVID-19 patients, an area where data remains limited. Most studies have focused on adults, making this work a significant contribution to understanding long COVID in children. The study includes a prospective cohort design with thorough follow-up, allowing for detailed observations of long COVID symptoms in relation to vitamin D status.
However, the study has several limitations. The sample size, particularly for children with long COVID, is relatively small, which may affect the generalizability of the results. The study population was drawn from a tertiary-level pediatric hospital, meaning the patients may differ from those in other pediatric hospitals. Additionally, no control group of healthy children did not contract COVID-19, which could have provided additional comparative data on vitamin D status in non-infected populations. The study was conducted during a specific time frame, and seasonal variations in sunlight exposure, which could affect vitamin D levels, were not fully accounted for. Another limitation is the method used to measure vitamin D levels—ELISA—which is less sensitive and specific compared to chemiluminescence and mass spectrometry, the internationally recommended methods.
Conclusion
The 25(OH)D concentrations in children with COVID-19 depended on their age. Vitamin D deficiency/insufficiency was observed in 73% of children over 6 years of age and 21.6% of children under 6 years of age with COVID-19. The presence of comorbid conditions, particularly undernutrition and obesity, affected the vitamin D status in children with COVID-19. Vitamin D influenced the COVID-19 severity and duration of hospitalization. There was an increased risk of developing long COVID in children with vitamin D deficiency/insufficiency, and its impact on the development of neurological symptoms associated with long COVID was established. Further research is needed to determine independent predictors of long COVID development.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by I. Horbachevsky Ternopil National Medical University Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
VP: Conceptualization, Data curation, Formal Analysis, Methodology, Resources, Software, Visualization, Writing – original draft, Writing – review & editing. TK: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – review & editing, Writing – original draft. OD: Data curation, Formal Analysis, Writing – review & editing, Writing – original draft. LV: Conceptualization, Data curation, Resources, Writing – review & editing, Writing – original draft. OB: Conceptualization, Data curation, Formal Analysis, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was financed by the Ministry of Health of Ukraine with funds from the state budget, project title “Assessment of the quality of life and psychological state of children with long COVID-19 in time of war”, state registration number 0123U100301, implementation period - 2023–2025.
Acknowledgments
We would like to express our sincere thanks to all the medical workers of the Ternopil City Children's Hospital for their great help in attracting patients to our work. We are very grateful to all patients and their parents who agreed to participate in the study and diligently followed all requirements and requests. We are also grateful to ISARIC and the International Post-Covid Condition in Children Collaboration for providing permission to use the standardized pediatric COVID-19 Follow-up Case Report Form.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Gemelli Against COVID-19 Post-Acute Care Study Group. Post-COVID-19 global health strategies: the need for an interdisciplinary approach. Aging Clin Exp Res. (2020) 32(8):1613–20. doi: 10.1007/s40520-020-01616-x
2. Swann OV, Holden KA, Turtle L, Pollock L, Fairfield CJ, Drake TM, et al. Clinical characteristics of children and young people admitted to hospital with COVID-19 in United Kingdom: prospective multicentre observational cohort study. Br Med J. (2020) 370:m3249. doi: 10.1136/bmj.m3249
3. Boyarchuk O, Predyk L, Yuryk I. COVID-19 in patients with juvenile idiopathic arthritis: frequency and severity. Reumatologia. (2021) 59(3):197–9. doi: 10.5114/reum.2021.107590
4. Borch L, Holm M, Knudsen M, Ellermann-Eriksen S, Hagstroem S. Long COVID symptoms and duration in SARS-CoV-2 positive children - a nationwide cohort study. Eur J Pediatr. (2022) 181(4):1597–607. doi: 10.1007/s00431-021-04345-z
5. Zimmermann P, Pittet LF, Curtis N. How common is long COVID in children and adolescents? Pediatr Infect Dis J. (2021) 40(12):e482–7. doi: 10.1097/INF.0000000000003328
6. World Health Organization. COVID-19 Dashboard. World Health Organization. (2024). Available online at: https://data.who.int/dashboards/covid19/cases (Accessed July 22, 2023).
7. World Health Organization. Post COVID-19 Condition (Long COVID). World Health Organization (2023). Available online at: https://www.who.int/europe/news-room/fact-sheets/item/postcovid-19-condition#:∼:text=It%20is%20defined%20as%20the,months%20with%20no%20other%20explanation (accessed July 11, 2023).
8. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
9. Venkatesan P. NICE guideline on long COVID. Lancet Respir Med. (2021) 9(2):129. doi: 10.1016/S2213-2600(21)00031-X
10. Ashkenazi-Hoffnung L, Shmueli E, Ehrlich S, Ziv A, Bar-On O, Birk E, et al. Long COVID in children: observations from a designated pediatric clinic. Pediatr Infect Dis J. (2021) 40(12):e509–11. doi: 10.1097/INF.0000000000003285
11. Mizrahi B, Sudry T, Flaks-Manov N, Yehezkelli Y, Kalkstein N, Akiva P, et al. Long COVID outcomes at one year after mild SARS-CoV-2 infection: nationwide cohort study. Br Med J. (2023) 380:e072529. doi: 10.1136/bmj-2022-072529
12. Volianska LA, Burbela EI, Kosovska TM, Perestiuk VO, Boyarchuk OR. Long COVID in children: frequency and diagnostic challenges. Ukr J Perinatol Pediatr. (2023) 3(95):101–6. doi: 10.15574/PP.2023.95.101
13. Boyarchuk O, Volianska L. Autoimmunity and long COVID in children. Reumatologia. (2023) 61(6):492–501. doi: 10.5114/reum/176464
14. Piazza M, Di Cicco M, Pecoraro L, Ghezzi M, Peroni D, Comberiati P. Long COVID-19 in children: from the pathogenesis to the biologically plausible roots of the syndrome. Biomolecules. (2022) 12(4):556. doi: 10.3390/biom12040556
15. Fainardi V, Meoli A, Chiopris G, Motta M, Skenderaj K, Grandinetti R, et al. Long COVID in children and adolescents. World J Pediatr. (2021) 17(2):495. doi: 10.1007/s12519-021-00457-6
16. Osmanov IM, Spiridonova E, Bobkova P, Gamirova A, Shikhaleva A, Andreeva M, et al. Risk factors for post-COVID-19 condition in previously hospitalised children using the ISARIC global follow-up protocol: a prospective cohort study. Eur Respir J. (2022) 59(2):2101341. doi: 10.1183/13993003.01341-2021
17. Fong MW, Gao H, Wong JY, Xiao J, Shiu EYC, Ryu S, et al. Nonpharmaceutical measures for pandemic influenza in nonhealthcare settings—social distancing measures. Emerg Infect Dis. (2020) 26(5):976–84. doi: 10.3201/eid2605.190995
18. Wong SCY, Kwong RT, Wu TC, Chan JWM, Chu MY, Lee SY, et al. Risk of nosocomial transmission of coronavirus disease 2019: an experience in a general ward setting in Hong Kong. J Hosp Infect. (2020) 105(2):119–27. doi: 10.1016/j.jhin.2020.03.036
19. Cowling BJ, Ali ST, Ng TWY, Tsang TK, Li JCM, Fong MW, et al. Impact assessment of non-pharmaceutical interventions against coronavirus disease 2019 and influenza in Hong Kong: an observational study. Lancet Public Health. (2020) 5(5):e279–88. doi: 10.1016/S2468-2667(20)30090-6
20. Wang G, Zhang Y, Zhao J, Zhang J, Jiang F. Mitigate the effects of home confinement on children during the COVID-19 outbreak. Lancet. (2020) 395(10228):945–7. doi: 10.1016/S0140-6736(20)30547-X
21. Nowson CA, McGrath JJ, Ebeling PR, Haikerwal A, Daly RM, Sanders KM, et al. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust. (2012) 196(11):686–7. doi: 10.5694/mja11.10301
22. Yu L, Ke HJ, Che D, Luo SL, Guo Y, Wu JL. Effect of pandemic-related confinement on vitamin D status among children aged 0–6 years in Guangzhou, China: a cross-sectional study. Risk Manag Healthc Policy. (2020) 13:2669–75. doi: 10.2147/RMHP.S282495
23. Norman AW. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr. (2008) 88(2):491S–9. doi: 10.1093/ajcn/88.2.491S
24. Lips P. Vitamin D physiology. Prog Biophys Mol Biol. (2006) 92(1):4–8. doi: 10.1016/j.pbiomolbio.2006.02.016
25. Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. (2009) 4:1151–65. doi: 10.2217/fmb.09.87
26. Aribi M, Mennechet FJD, Touil-Boukoffa C. Editorial: the role of vitamin D as an immunomodulator. Front Immunol. (2023) 14:1186635. doi: 10.3389/fimmu.2023.1186635
27. Marino R, Misra M. Extra-skeletal effects of vitamin D. Nutrients. (2019) 11(7):1460. doi: 10.3390/nu11071460
28. Iacopetta D, Ceramella J, Catalano A, Saturnino C, Pellegrino M, Mariconda A, et al. COVID-19 at a glance: an up-to-date overview on variants, drug design and therapies. Viruses. (2022) 14(3):573. doi: 10.3390/v14030573
29. Bayramoğlu E, Akkoç G, Ağbaş A, Akgün Ö, Yurdakul K, Selçuk Duru HN, et al. The association between vitamin D levels and the clinical severity and inflammation markers in pediatric COVID-19 patients: single-center experience from a pandemic hospital. Eur J Pediatr. (2021) 180(8):2699–705. doi: 10.1007/s00431-021-04030-1
30. Szerszeń MD, Kucharczyk A, Bojarska-Senderowicz K, Pohorecka M, Śliwczyński A, Engel J, et al. Effect of vitamin D concentration on course of COVID-19. Med Sci Monit. (2022) 28:e937741. doi: 10.12659/MSM.937741
31. Darren A, Osman M, Masilamani K, Habib Ali S, Kanthimathinathan HK, Chikermane A, et al. Vitamin D status of children with paediatric inflammatory multisystem syndrome temporally associated with severe acute respiratory syndrome coronavirus 2 (PIMS-TS). Br J Nutr. (2022) 127(6):896–903. doi: 10.1017/S0007114521001562
32. World Health Organization. Clinical Management of COVID-19: Interim Guidance, 27 May 2020. World Health Organization (2020). Available online at: https://iris.who.int/handle/10665/332196 (Accessed May 27, 2020).
33. Pludowski P. COVID-19 and other pleiotropic actions of vitamin D: proceedings from the fifth international conference “vitamin D—minimum, maximum, optimum” under the auspices of the European vitamin D association (EVIDAS). Nutrients. (2023) 15(11):2530. doi: 10.3390/nu15112530
34. Bayrak H, Öztürk D, Bolat A, Ünay B. Association between vitamin D levels and COVID-19 infection in children: a case-control study. Turk Arch Pediatr. (2023) 58(3):250–5. doi: 10.5152/TurkArchPediatr.2023.22217
35. Karimian P, Tahami MS, Sayyahfar S, Aghajani Delavar M. Association of vitamin D and severity of COVID-19 in children. Eur J Transl Myol. (2022) 32(2):10453. doi: 10.4081/ejtm.2022.10453
36. Kinash MI, Boyarchuk OR. Fat-soluble vitamins and immunodeficiency: mechanisms of influence and opportunities for use. Vopr Pitan. (2020) 89(3):22–32. doi: 10.24411/0042-8833-2020-10026
37. Vierucci F, Del Pistoia M, Fanos M, Gori M, Carlone G, Erba P, et al. Vitamin D status and predictors of hypovitaminosis D in Italian children and adolescents: a cross-sectional study. Eur J Pediatr. (2013) 172(12):1607–17. doi: 10.1007/s00431-013-2119-z
38. Volianska L, Burbela E, Kosovska T, Perestiuk V, Boyarchuk O. The role of vitamin D in the course of SARS-CoV-2 infection and long COVID in children (literature review). Child's Health. (2023) 18(3):225–30. doi: 10.22141/2224-0551.18.3.2023.1590
39. Boyarchuk OR, Monastyrska OI, Suvalko SI, Perestiuk VO, Hariyan TV. Impact of COVID-19, pandemic, and full-scale war on the health of schoolchildren: survey results. Ukr J Perinatol Pediatr. (2024) 2(98):77–85. doi: 10.15574/PP.2024.98.77
40. Mosca C, Colucci A, Savoia F, Calì C, Del Bene M, Ranucci G, et al. Vitamin D levels in the pre- and post-COVID-19 pandemic periods and related confinement at pediatric age. Nutrients. (2023) 15(9):2089. doi: 10.3390/nu15092089
41. Alpcan A, Tursun S, Kandur Y. Vitamin D levels in children with COVID-19: a report from Turkey. Epidemiol Infect. (2021) 149:e180. doi: 10.1017/S0950268821001825
42. Nicolae M, Mihai CM, Chisnoiu T, Balasa AL, Frecus CE, Mihai L, et al. Immunomodulatory effects of vitamin D in respiratory tract infections and COVID-19 in children. Nutrients. (2023) 15(15):3430. doi: 10.3390/nu15153430
43. Kosmeri C, Balomenou F, Rallis D, Baltogianni M, Giapros V. The role of serum vitamin 25(OH)D concentration in the COVID-19 pandemic in children. Br J Nutr. (2023) 130(3):417–22. doi: 10.1017/S0007114522003476
44. Ahmad AS, Juber NF, Al-Naseri H, Heumann C, Ali R, Oliver T. Association between average vitamin D levels and COVID-19 mortality in 19 European countries-A population-based study. Nutrients. (2023) 15(22):4818. doi: 10.3390/nu15224818
45. Seal KH, Bertenthal D, Carey E, Grunfeld C, Bikle DD, Lu CM. Association of vitamin D Status and COVID-19-related hospitalization and mortality. J Gen Intern Med. (2022) 37(4):853–61. doi: 10.1007/s11606-021-07170-0
46. Akbar MR, Wibowo A, Pranata R, Setiabudiawan B. Low serum 25-hydroxyvitamin D (vitamin D) level is associated with susceptibility to COVID-19, severity, and mortality: a systematic review and meta-analysis. Front Nutr. (2021) 8:660420. doi: 10.3389/fnut.2021.660420
47. Susianti H, Wahono CS, Rahman PA, Pratama MZ, Wulanda IA, Hartanti KD, et al. Low levels of vitamin D were associated with coagulopathy among hospitalized coronavirus disease-19 (COVID-19) patients: a single-centered study in Indonesia. J Med Biochem. (2021) 40(4):341–50. doi: 10.5937/jomb0-30228
48. Boyarchuk O, Perestiuk V, Kosovska T, Volianska L. Coagulation profile in hospitalized children with COVID-19: pediatric age dependency and its impact on long COVID development. Front Immunol. (2024) 15:1363410. doi: 10.3389/fimmu.2024.1363410
49. Salamanna F, Maglio M, Sartori M, Landini MP, Fini M. Vitamin D and platelets: a menacing duo in COVID-19 and potential relation to bone remodeling. Int J Mol Sci. (2021) 22(18):10010. doi: 10.3390/ijms221810010
50. Cooper ID, Crofts CAP, DiNicolantonio JJ, Malhotra A, Elliott B, Kyriakidou Y, et al. Relationships between hyperinsulinaemia, magnesium, vitamin D, thrombosis and COVID-19: rationale for clinical management. Open Heart. (2020) 7(2):e001356. doi: 10.1136/openhrt-2020-001356
51. Getachew B, Landis HE, Manaye KF, Tizabi Y. COVID-19-associated coagulopathy: role of vitamins D and K. Curr Pharm Biotechnol. (2023) 24(3):401–10. doi: 10.2174/1389201023666220527110455
52. Chen S, Sims GP, Chen XX, Gu YY, Chen S, Lipsky PE. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol. (2007) 179(3):1634–47. doi: 10.4049/jimmunol.179.3.1634
53. Peraire J, García-Pardo G, Chafino S, Sánchez A, Botero-Gallego M, Olona M, et al. Immunoglobulins in COVID-19 pneumonia: from the acute phase to the recovery phase. Eur J Med Res. (2024) 29(1):223. doi: 10.1186/s40001-024-01824-5
54. Votto M, Castagnoli R, Marseglia GL, Licari A, Brambilla I. COVID-19 and autoimmune diseases: is there a connection? Curr Opin Allergy Clin Immunol. (2023) 23(2):185–92. doi: 10.1097/ACI.0000000000000888
55. Perlis RH, Green J, Santillana M, Lazer D, Ognyanova K, Simonson M, et al. Persistence of symptoms up to 10 months following acute COVID-19 illness. medRxiv [preprint]. (2021) 03:2021.03.07.21253072. doi: 10.1101/2021.03.07.21253072
56. Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez-Mayordomo V, Rodríuez-Jiménez J, Palacios-Ceña M, Velasco-Arribas M, et al. Long-term post-COVID symptoms and associated risk factors in previously hospitalized patients: a multicenter study. J Infect. (2021) 83(2):237–79. doi: 10.1016/j.jinf.2021.04.036
57. Rao S, Lee GM, Razzaghi H, Lorman V, Mejias A, Pajor NM, et al. Clinical features and burden of postacute sequelae of SARS-CoV-2 infection in children and adolescents. JAMA Pediatr. (2022) 176(10):1000–9. doi: 10.1001/jamapediatrics.2022.2800
58. di Filippo L, Frara S, Nannipieri F, Cotellessa A, Locatelli M, Rovere Querini P, et al. Low vitamin D levels are associated with long COVID syndrome in COVID-19 survivors. J Clin Endocrinol Metab. (2023) 108(10):e1106–16. doi: 10.1210/clinem/dgad207
59. Mohamed Hussein AAR, Galal I, Amin MT, Moshnib AA, Makhlouf NA, Makhlouf HA, et al. Prevalence of vitamin D deficiency among patients attending post COVID-19 follow-up clinic: a cross-sectional study. Eur Rev Med Pharmacol Sci. (2022) 26(8):3038–45. doi: 10.26355/eurrev_202204_28635
60. Chen KY, Lin CK, Chen NH. Effects of vitamin D and zinc deficiency in acute and long COVID syndrome. J Trace Elem Med Biol. (2023) 80:127278. doi: 10.1016/j.jtemb.2023.127278
61. Guerrero-Romero F, Gamboa-Gómez CI, Rodríguez-Morán M, Orrante M, Rosales-Galindo E, Cisneros-Ramírez I, et al. Hypomagnesemia and 25-hydroxyvitamin D deficiency in patients with long COVID. Magnes Res. (2023) 36(3):30–6. doi: 10.1684/mrh.2023.0519
62. Hikmet RG, Wejse C, Agergaard J. Effect of vitamin D in long COVID patients. Int J Environ Res Public Health. (2023) 20(22):7058. doi: 10.3390/ijerph20227058
63. Wu JY, Liu MY, Hsu WH, Tsai YW, Liu TH, Huang PY, et al. Association between vitamin D deficiency and post-acute outcomes of SARS-CoV-2 infection. Eur J Nutr. (2024) 63(2):613–22. doi: 10.1007/s00394-023-03298-3
64. Pizzini A, Aichner M, Sahanic S, Böhm A, Egger A, Hoermann G, et al. Impact of vitamin D deficiency on COVID-19—a prospective analysis from the CovILD registry. Nutrients. (2020) 12:2775. doi: 10.3390/nu12092775
65. Zengin N, Bal A, Goren TA, Bayturan SS, Alkan F, Akcali S. Serum vitamin D levels in relation to development of multisystem inflammatory syndrome in pediatric COVID-19. J Pediatr Infect Dis. (2022) 17(06):308–16. doi: 10.1055/s-0042-1756713
66. Townsend L, Dyer AH, McCluskey P, O'Brien K, Dowds J, Laird E, et al. Investigating the relationship between vitamin D and persistent symptoms following SARS-CoV-2 infection. Nutrients. (2021) 13(7):2430. doi: 10.3390/nu13072430
67. Chen TB, Chang CM, Yang CC, Tsai IJ, Wei CY, Yang HW, et al. Neuroimmunological effect of vitamin D on neuropsychiatric long COVID syndrome: a review. Nutrients. (2023) 15(17):3802. doi: 10.3390/nu15173802
68. Mundula T, Russo E, Curini L, Giudici F, Piccioni A, Franceschi F, et al. Chronic systemic low-grade inflammation and modern lifestyle: the dark role of gut microbiota on related diseases with a focus on COVID-19 pandemic. Curr Med Chem. (2022) 29(33):5370–96. doi: 10.2174/0929867329666220430131018
Keywords: COVID-19, SARS-CoV-2 infection, long COVID, vitamin D, 25(OH)D, children vitamin D, long COVID
Citation: Perestiuk V, Kosovska T, Dyvoniak O, Volianska L and Boyarchuk O (2025) Vitamin D status in children with COVID-19: does it affect the development of long COVID and its symptoms? Front. Pediatr. 13:1507169. doi: 10.3389/fped.2025.1507169
Received: 7 October 2024; Accepted: 27 January 2025;
Published: 14 February 2025.
Edited by:
Mourad Aribi, University of Abou Bekr Belkaïd, AlgeriaReviewed by:
Israel Parra-Ortega, Federico Gómez Children’s Hospital, MexicoJessie Zurita-Cruz, Hospital Infantil de México Federico Gómez, Mexico
Copyright: © 2025 Perestiuk, Kosovska, Dyvoniak, Volianska and Boyarchuk. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vita Perestiuk, cGVyZXN0aXVrX3ZvQHRkbXUuZWR1LnVh
 Vita Perestiuk
Vita Perestiuk Tetyana Kosovska
Tetyana Kosovska Olha Dyvoniak2
Olha Dyvoniak2 Liubov Volianska
Liubov Volianska Oksana Boyarchuk
Oksana Boyarchuk