- Department of Pediatrics Department, Dongyang People’s Hospital, Dongyang, Zhejiang, China
The mortality of pulmonary embolism in children is high, and there is no standardized treatment protocol. We present a case of successful treatment of Mycoplasma pneumoniae (M pneumoniae) with pulmonary embolism in a child using rivaroxaban and doxycycline, thereby exploring a more appropriate treatment option. A 10-year-old male presented with fever, cough, and chest pain as the main symptoms. M pneumoniae polymerase chain reaction of bronchoalveolar lavage fluid was positive, and computed tomography angiography indicated pulmonary embolism. Azithromycin, doxycycline, and piperacillin-tazobactam were administered sequentially for infection control, while methylprednisolone was given to control inflammation and heparin and rivaroxaban for sequential treatment, resulting in a satisfactory recovery.
Introduction
The incidence rate of pulmonary embolism in children has been increasing in recent years. The incidence rate of pulmonary embolism in children in the community ranges from approximately 1.4 to 9 cases per one million (1), and in American children, it increased by 200% between 2001 and 2014 (2). The mortality of pulmonary embolism is high in children, with reports indicating up to 26% (3). Currently, there is no standardized treatment for pulmonary embolism caused by Mycoplasma pneumoniae (M pneumoniae) in children. This study presents a case of M pneumoniae accompanied by pulmonary embolism in a pediatric patient who was successfully treated with rivaroxaban and doxycycline. Promptly initiating treatment in children with this dual diagnosis resulted in favorable outcomes, such as shortened hospitalization, improved safety, and good patient adherence.
Case description
A previously healthy 10-year-old male child was admitted to our hospital with a history of fever and cough for 8 days and chest pain for 1 day. Initial examination revealed a C-reactive protein (CRP) level of 112 mg/L (reference range: 0–10 mg/L), and pulmonary computed tomography (CT) revealed multiple inflammatory areas in both lungs (Figure 1A).
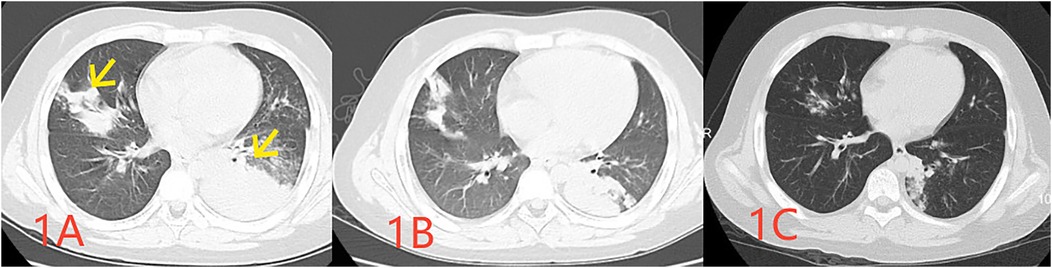
Figure 1. Pulmonary CT images obtained at 1 week (A indicating the infected lesion with a yellow arrow), 2 weeks (B indicating absorption at the site of infection), and 1 month (C demonstrating the essentially absorbed focus of infection) following the disease onset.
Before admission, the patient received ceftriaxone for 1 day, azithromycin for 5 days, and concurrently, methylprednisolone for 4 days without any improvement.
One Day before admission, the patient developed chest pain, and reexamination revealed a CRP level of 133 mg/L. A pharyngeal swab test indicated a positive M pneumoniae polymerase chain reaction (PCR). A repeat pulmonary CT demonstrated multiple inflammatory areas in both lungs, accompanied by focal consolidation (Figure 1B). During the course of the disease, the child exhibited no other symptoms. At the time of examination, the child weighed 50.5 kg, measured 142 cm in height, had a respiratory rate of 40 breaths per minute, coarse breath sounds, and audible wet rales in both lungs.
No other pathogenic bacteria were detected following admission. Two days post-admission, a bronchoscopy at our hospital found thick sputum in the right bronchus. Bronchoalveolar lavage fluid tested positive for pneumonia type M pneumoniae via PCR, with no other pathogens like tuberculosis detected.
Following admission, the patient received azithromycin (10 mg/kg/day for 2 days), followed by doxycycline (2 mg/kg/dose every 12 h for 10 days) combined with piperacillin-tazobactam (4.5 g/dose every 8 h for 9 days) for infection management, and methylprednisolone for anti-inflammatory treatment. On the second day of admission, the patient's chest pain disappeared, and body temperature normalized. D-dimer levels were measured at 16.88 μg/ml (reference range: <0.5 μg/ml), and enoxaparin calcium was administered subcutaneously at 4,100 International Units per day. On the third day, pulmonary artery computed tomography angiography (CTA) revealed a right upper pulmonary artery branch embolism (Figure 2A). Heparin was discontinued, and oral rivaroxaban (10 mg/time orally twice daily for one month, followed by 15 mg/time once daily for 2 months) was administered. There were no other symptoms, such as swelling or pain in the limbs, observed throughout the process. The following laboratory parameters were monitored: Hemoglobin (106–136 g/L; reference range: ≥120 g/L), Platelet count (173–352 × 109/L; reference range: 100–453 × 109/L), International Normalized Ratio (INR; 1.1–1.4; reference range: 0.8–1.3), thrombin time (TT) (13.6–16.3 s; reference range: 13.6–16.3 s), plasma prothrombin time (PT) (13.6–6.3 s; reference range: 13.6–6.3 s), activated partial thromboplastin time (APTT) (39–50 s; reference range: 28–44 s), alanine aminotransferase (49 U/L; reference range: 9–50 U/L), aspartate aminotransferase (48 U/L; reference range: 9–50 U/L), urea (3.57 mmol/L; reference range 3.1–8 mmol/L), and creatinine (49 µmol/L; reference range: 57–97 µmol/L).

Figure 2. Pulmonary CTA images obtained at 10 days (A highlighting embolus formation in the lung with a red arrow) and 3 months (B indicating absorption of the pulmonary embolus post-treatment with a yellow arrow) following the disease onset.
However, two weeks after discharge, the patient experienced an episode of increased coughing, with a CRP level of 63.22 mg/L. The D-dimer level was measured at 0.54 μg/L, and a follow-up pulmonary CT indicated resolution of the previous bilateral multiple inflammation, with new cavities in the lesion of the left lower lobe (Figure 1C). After 10 days of additional treatment with oral cefixime, the cough resolved with no recurrence. Three months after discharge, the patient's D-dimer level was 0.22 μg/ml, and no cough symptoms were present. The original embolic lesions were absorbed, and pulmonary CTA revealed no pulmonary embolism (Figure 2B). The child was monitored for 6 months after discontinuing rivaroxaban without any discomfort (Table 1).
Discussion
Children have a significantly increased incidence of pulmonary embolism and a high mortality rate (1–3). M pneumoniae is one of the most common pathogens in children (4) and can directly or indirectly cause pulmonary embolism (5). Reports of pulmonary embolism caused by M pneumoniae in children have significantly increased in recent years (6, 7).
The exact pathogenesis of M pneumoniae is unclear. It may involve cytokine induction causing vascular damage and obstruction, or lipoprotein sugars from M pneumoniae inducing procoagulant activity, thereby resulting in a hypercoagulable state (5, 8). When coagulation occurs, thrombin activates the fibrinolytic system, resulting in the formation of D-dimer (8). Previous studies have identified D-dimer as an independent risk factor for M pneumoniae associated with pulmonary embolism (9, 10). In our case, we examined a child with pulmonary embolism and found no symptoms of embolism. We monitored coagulation markers (INR, APTT, PT, TT, and D-dimer) and observed a significant increase in D-dimer levels during the embolism, which normalized once the embolism resolved. However, additional examinations were not conducted to further investigate the underlying cause of pulmonary embolism formation, and no specific coagulation defects have been identified.
Anticoagulation is crucial for managing children with M pneumoniae and embolism; however, no standardized treatment protocol exists for complicated cases of pulmonary embolism. Currently, the primary drugs used for treating pulmonary embolism in children include heparin, vitamin K antagonists such as warfarin, and direct oral anticoagulants (DOACs), including rivaroxaban, apixaban, and dabigatran (6, 7, 11, 12).
Although the conventional anticoagulant warfarin is widely utilized for the prevention and treatment of deep vein thrombosis, it requires regular monitoring of the INR and is affected by dietary factors, such as the consumption of spinach and wolfberry, which can elevate the risk of bleeding (13). Furthermore, the efficacy of warfarin in treating pediatric patients with M pneumoniae complicated by pulmonary embolism remains inconsistent. One child in the United States (14), four in Shanghai (6), and thirty-eight in Beijing (15) exhibited a good prognosis with M pneumoniae and pulmonary embolism; however, a fatal case was reported in Zhengzhou (16). However, among seven cases of M pneumoniae complicated by pulmonary embolism in Jilin, China, five children revealed no response to heparin and warfarin treatment and underwent surgical treatment, whereas two patients died of acute respiratory distress syndrome after surgery (17).
The DOAC rivaroxaban has been approved for use in children in the United States (18). Studies have indicated that DOACs have sufficient safety and efficacy in adolescents (11). In a Phase III trial investigating the safety and efficacy of rivaroxaban for treating venous thrombosis in children, the results were similar to those observed in adults (19). A real-world study demonstrated that rivaroxaban was safe for treating venous thrombosis in children, with a thrombosis recurrence rate of 3.6% (20). Studies have revealed that adult patients taking rivaroxaban for deep vein thrombosis or pulmonary embolism experience shorter hospital stays, lower costs, and a lower rate of post-thrombotic syndrome (0.53% vs. 0.55%) compared to those taking warfarin (21, 22). However, reports on rivaroxaban for treating M pneumoniae with pulmonary embolism in children have focused on Tianjin, China (7, 23, 24). A study demonstrated nine cases of children diagnosed with M pneumoniae and pulmonary embolism at Tianjin Children's Hospital between 2,018 and 2021 (7). These children received a sequential treatment regimen involving low-molecular-weight heparin and rivaroxaban. The follow-up period ranged from 0.5–9 months, during which no cases of thrombosis were observed (7).
The patient was discharged after 9 days of hospitalization. During the three months of treatment, the child was monitored only for coagulation and D-dimer levels several times while receiving rivaroxaban. After 6 months of rivaroxaban treatment, the child was free of cough and other symptoms. A CTA review indicated the disappearance of the embolism, and both the D-dimer levels and coagulation function were normal, enabling the child to engage in appropriate physical activity. The child's hospitalization lasted 9 days, significantly shorter than the typical duration for those on warfarin [Chen et al., 13–23 days (6); Song et al., at least 15–21 day s (7)], and there was no need for regular INR monitoring. Family members expressed high satisfaction with the entire treatment process and reported a good prognosis.
Conclusion
Rivaroxaban is a safe and convenient treatment option for pulmonary embolism resulting from M pneumoniae in children, with a positive prognosis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of Dongyang People's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
FX: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. LH: Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The author would like to thank Wuhuanhuan for her constant advice.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Stein PD, Kayali F, Olson RE. Incidence of venous thromboembolism in infants and children: data from the national hospital discharge survey. J Pediatr. (2004) 145:563–5. doi: 10.1016/j.jpeds.2004.06.021
2. Carpenter SL, Richardson T, Hall M. Increasing rate of pulmonary embolism diagnosed in hospitalized children in the United States from 2001 to 2014. Blood Adv. (2018) 2:1403–8. doi: 10.1182/bloodadvances.2017013292
3. Rajpurkar M, Biss TT, Amankwah EK, Martinez D, Williams S, van Ommen CH, et al. Pulmonary embolism and in situ pulmonary artery thrombosis in paediatrics. Thromb Haemost. (2017) 117:1199–207. doi: 10.1160/TH16-07-0529
4. Kutty PK, Jain S, Taylor TH, Bramley AM, Diaz MH, Ampofo K, et al. Mycoplasma pneumoniae among children hospitalized with community-acquired pneumonia. Clin Infect Dis. (2019) 68:5–12. doi: 10.1093/cid/ciy419
5. Narita M. Classification of extrapulmonary manifestations due to Mycoplasma pneumoniae infection on the basis of possible pathogenesis. Front Microbiol. (2016) 7:23. doi: 10.3389/fmicb.2016.00023
6. Chen S, Ke S, Vinturache A, Dong X, Ding G. Pulmonary embolism associated with Mycoplasma pneumoniae pneumonia in children. Pediatr Pulmonol. (2023) 58:3605–8. doi: 10.1002/ppul.26691
7. Song S, Xu Y. A retrospective study of the clinical characteristics of 9 children with pulmonary embolism associated with Mycoplasma pneumoniae pneumonia. BMC Pediatr. (2023) 23:370. doi: 10.1186/s12887-023-04188-7
8. Ma L, Wang XH, Li N. Progress on Mycoplasma pneumoniae pneumonia coagulation abnormalities and related indexes in children. Int J Pediatr. (2023) 50:678–83. 10.3760/cma.j.issn.1673¯4408.2023.10.007
9. Li Y-T, Zhang J, Wang M-Z, Ma Y-M, Zhi K, Dai F-L, et al. Changes in coagulation markers in children with Mycoplasma pneumoniae pneumonia and their predictive value for Mycoplasma severity. Ital J Pediatr. (2023) 49:143. doi: 10.1186/s13052-023-01545-1
10. Qiu J, Ge J, Cao L. D-dimer: the risk factor of children’s severe Mycoplasma Pneumoniae pneumonia. Front Pediatr. (2022) 10. doi: 10.3389/fped.2022.828437
11. Corrales-Medina FF, Raffini L, Recht M, Santos J, Thornburg CD, Davila J. Direct oral anticoagulants in pediatric venous thromboembolism: experience in specialized pediatric hemostasis centers in the United States. Res Pract Thromb Haemost. (2023) 7:100001. doi: 10.1016/j.rpth.2022.100001
12. Brandão LR, Albisetti M, Halton J, Bomgaars L, Chalmers E, Mitchell LG, et al. Safety of dabigatran etexilate for the secondary prevention of venous thromboembolism in children. Blood. (2020) 135:491. doi: 10.1182/blood.2019000998
13. Tan CSS, Lee SWH. Warfarin and food, herbal or dietary supplement interactions: asystematic review. Br J Clin Pharmacol. (2021) 87:352–74. doi: 10.1111/bcp.14404
14. Graw-Panzer KD, Verma S, Rao S, Miller ST, Lee H. Venous thrombosis and pulmonary embolism in a child with pneumonia due to Mycoplasma pneumoniae. J Natl Med Assoc. (2009) 101:956–8. doi: 10.1016/S0027-9684(15)31045-2
15. Chen L, Yin J, Liu X, Liu J, Xu B, Shen K. Thromboembolic complications of Mycoplasma pneumoniae pneumonia in children. Clin Respir J. (2023) 17:187–96. doi: 10.1111/crj.13584
16. Zhuo Z, Li F, Chen X, Jin P, Guo Q, Wang H. Mycoplasma pneumonia combined with pulmonary infarction in a child. Int J Clin Exp Med. (2015) 8:1482–6. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4358614/ (accessed November 28, 2023).25785159
17. Sheng C-Q, Yang C-F, Ao Y, Zhao Z-Y, Li Y-M. Mycoplasma pneumoniae pneumonia with pulmonary embolism: a study on pediatric cases in Jilin province of China. Exp Ther Med. (2021) 21:201. doi: 10.3892/etm.2021.9634
18. Research C for DE and. FDA approves drug to treat, help prevent types of blood clots in certain pediatric populations. FDA (2021) Available online at: https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-treat-help-prevent-types-blood-clots-certain-pediatric-populations (accessed February 4, 2024).
19. Spiezia L, Campello E, Tormene D, Simioni P. Venous thromboembolism in children: the rivaroxaban experience. Semin Thromb Hemost. (2024) 50(6):866–72. doi: 10.1055/s-0043-1778106
20. Hassan E, Motwani J. Real world experience of efficacy and safety of rivaroxaban in paediatric venous thromboembolism. Thromb Res. (2023) 221:92–6. doi: 10.1016/j.thromres.2022.11.027
21. Margolis JM, Deitelzweig S, Kline J, Tran O, Smith DM, Bookhart B, et al. Shorter hospital stays and lower costs for rivaroxaban compared with warfarin for venous thrombosis admissions. J Am Heart Assoc. (2016) 5:e003788. doi: 10.1161/JAHA.116.003788
22. Søgaard M, Nielsen PB, Skjøth F, Kjældgaard JN, Coleman CI, Larsen TB. Rivaroxaban versus warfarin and risk of post-thrombotic syndrome among patients with venous thromboembolism. Am J Med. (2018) 131:787–794.e4. doi: 10.1016/j.amjmed.2018.01.041
23. Han C, Zhang T, Zheng J, Jin P, Zhang Q, Guo W, et al. Analysis of the risk factors and clinical features of Mycoplasma pneumoniae pneumonia with embolism in children: a retrospective study. Ital J Pediatr. (2022) 48:153. doi: 10.1186/s13052-022-01344-0
Keywords: mycoplasma, pulmonary embolism, rivaroxaban, doxycycline, case report
Citation: Xiaoqian F and Hemin L (2025) Successful rivaroxaban and doxycycline therapy for Mycoplasma pneumonia with pulmonary embolism in children: a case report. Front. Pediatr. 13:1449493. doi: 10.3389/fped.2025.1449493
Received: 15 June 2024; Accepted: 24 January 2025;
Published: 12 February 2025.
Edited by:
Ya Gao, Lanzhou University, ChinaReviewed by:
Camilla Calvieri, Sapienza University of Rome, ItalyLuca Spiezia, University of Padua, Italy
Copyright: © 2025 Xiaoqian and Hemin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu Hemin, SGVtaW5rZXlAMTYzLmNvbQ==
 Fang Xiaoqian
Fang Xiaoqian Lu Hemin
Lu Hemin