
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 12 February 2025
Sec. Pediatric Gastroenterology, Hepatology and Nutrition
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1448507
 Aydan Kansu1*
Aydan Kansu1* Gunsel Kutluk2,†
Gunsel Kutluk2,† Gonul Caltepe3
Gonul Caltepe3 Cigdem Arikan4
Cigdem Arikan4 Nafiye Urganci5
Nafiye Urganci5 Gokhan Tumgor6
Gokhan Tumgor6 Aysel Yuce7
Aysel Yuce7 Ceyda Tuna Kirsaclioglu1
Ceyda Tuna Kirsaclioglu1 Arzu Meltem Demir8,†
Arzu Meltem Demir8,† Fatma Demirbas3
Fatma Demirbas3 Merve Usta5
Merve Usta5 Sibel Yavuz6
Sibel Yavuz6 Duygu Demirtas Guner7
Duygu Demirtas Guner7 Ersin Gumus7
Ersin Gumus7 Buket Dalgic9
Buket Dalgic9 Yasar Dogan10
Yasar Dogan10 Nelgin Gerenli11
Nelgin Gerenli11 Halil Kocamaz12,†
Halil Kocamaz12,† Fulya Gulerman13
Fulya Gulerman13 Elif Sag14
Elif Sag14 Aysugul Alptekin Sarioglu15
Aysugul Alptekin Sarioglu15 Neslihan Eksi Bozbulut9
Neslihan Eksi Bozbulut9 Demet Teker Duztas9
Demet Teker Duztas9 Hatice Altug Demirol10
Hatice Altug Demirol10 Coskun Celtik11
Coskun Celtik11 Olcay Gungor12
Olcay Gungor12 Kaan Demiroren16
Kaan Demiroren16 Aysen Uncuoglu Aydogan17
Aysen Uncuoglu Aydogan17 Ozlem Bekem18
Ozlem Bekem18 Zeynep Arslan13,†
Zeynep Arslan13,† Murat Cakir14
Murat Cakir14 Arzu Ekici19
Arzu Ekici19 Nihal Uyar Aksu17
Nihal Uyar Aksu17 Cigdem Ecevit18
Cigdem Ecevit18 Simge Erdogan15
Simge Erdogan15
Objective: Use of peptide-based formulas supplemented with medium chain triglycerides (MCTs) is considered a beneficial strategy to decrease the tube-feeding associated gastrointestinal tolerance. In children with cerebral palsy (CP), overall effects of enteral tube feeding as well as the utility of peptide-based specialized enteral formulas in those with gastrointestinal intolerance have not been extensively studied. This study aimed to evaluate the utility of enteral tube feeding via specialized peptide-based formula containing MCTs in children with CP in terms of gastrointestinal intolerance, anthropometrics, defecation characteristics and parental satisfaction with enteral formula.
Methods: Children with CP who received enteral tube feeding via specialized peptide-based formula containing MCTs were included in this prospective observational study. Anthropometrics (z scores for weight for age [WFA], weight for height [WFH], triceps skinfold thickness [TSFT] and mid-upper arm circumference [MUAC]), gastrointestinal intolerance symptoms, defecation frequency and stool patterns and formula satisfaction were recorded at baseline and during 6-month follow up.
Results: A total of 96 children with CP (mean ± SD age: 5.6 ± 3.2 years, 56.3% were boys) were included. Significant improvements were noted in MUAC, TSFT and WFH z scores at the 6th month visit. The rate of “severe symptoms” and the likelihood of Type-1/Type-2 (constipation) stool pattern were significantly decreased. Majority of parents were satisfied with the study formula.
Conclusion: Our findings revealed favorable efficacy and safety of using a specialized peptide-based formula containing MCT in provision of enteral tube feeding among children with CP in terms of improved anthropometrics, amelioration of gastrointestinal intolerance symptoms and normalization of bowel movements along with a high parental satisfaction.
Functional feeding disorders and gastrointestinal dysfunction are highly prevalent conditions in children with cerebral palsy (CP), which compromise the adequate nutrient intake in these children by causing vomiting, dysphagia, impaired swallowing, gastroesophageal reflux, aspiration and constipation, in addition to limited self-feeding ability (1–5). Accordingly, many children with CP are considered at high risk of poor nutritional status, particularly those with severe gross motor impairment and oropharyngeal dysfunction (2, 4, 6, 7).
In children with CP, malnutrition leads to growth failure, decreased cerebral function and reduced potential for development, impaired immune, respiratory and gastro-intestinal functions, delayed wound healing, increased morbidity and reduced quality of life (8–10).
Owing to increased life-expectancy in the presence of disability, the prevalence and consequences of feeding difficulties are on the rise in children with CP, making nutritional status an indispensable part of the medical care (9–11). Hence, comprehensive evaluation and treatment of feeding disorders via adequate nutritional support based on better tolerated enteral formulas is considered essential in improving the nutritional status, anthropometrics, energy store and quality of life, as well as in decreasing irritability, spasticity and hospitalization rates in neurologically impaired children including those with CP (12–14).
Enteral tube feeding, via nasogastric or gastrostomy tube feeding depending on the anticipated duration of enteral nutrition, is frequently indicated in children with CP with significant oropharyngeal incoordination who are unable to meet their nutritional requirements orally (11, 12). However, feeding intolerance and related complications comprise a major drawback to enteral tube feeding in children with severe impairment of the central nervous system (15). The formula composition, proteins in particular, is considered critical in provision of best nutritional support in children with CP, given the persisting feeding difficulties in gastrostomy-fed children in addition to adverse effects of highly prevalent gastrointestinal symptoms (possibly related to delayed gastric emptying and gastric dysmotility) on the already impaired nutritional status (1, 16–18).
The higher efficacy and better tolerability of peptide-based formulas, as compared with formulas composed entirely of free amino acids or intact protein, have been reported in malnourished patients, particularly in those with gastrointestinal dysfunction and thus inability to tolerate nutritional supplements containing whole protein or long chain triglycerides (19–21). Patients with impaired gastrointestinal function are considered to require a formula containing hydrolyzed protein and medium chain triglycerides (MCTs) to minimize the need for hydrolysis of protein by the intestinal brush border peptidases for an easier absorption (19, 20, 22). Hence, although being addressed by only a few studies in critically ill patients, use of formulas that are peptide-based and supplemented with MCT and fiber is considered amongst the potential nutritional interventions to decrease the tube-feeding associated gastrointestinal tolerance (21). The overall effects of enteral tube feeding as well as the utility of peptide-based specialized enteral formulas in those with gastrointestinal intolerance have not been extensively studied among children with CP (6, 23–25).
This prospective observational study was designed to evaluate the utility of a specialized peptide-based formula containing MCT in children with CP who had previous tube feeding intolerance on standard enteral formula, in terms of effects on gastrointestinal intolerance, anthropometrics, defecation frequency and stool patterns and parental satisfaction with enteral formula.
Children with CP who received enteral tube feeding with a specialized peptide-based enteral formula containing partially hydrolyzed protein plus MCTs, due to previous tube feeding intolerance on standard enteral formula, were included in this prospective observational multi-center 6-month follow up study conducted at 17 centers across Turkey. Hospitalized or outpatient children with CP (1–12 years of age) who developed gastrointestinal intolerance on enteral tube feeding with standard enteral formula and therefore were planned to receive a specialized peptide-based enteral formula that contain partially hydrolyzed protein plus MCTs were included in the study. Presence of another chronic disease (diabetes, liver disease, kidney disease, endocrine disease, cancer), intestinal obstruction, need for dialysis treatment, artificial respiratory device, parenteral nutrition or normal nutrition in addition to enteral tube feeding were the exclusion criteria of the study. Although 126 children with CP were initially enrolled in the study, 96 children comprised the final study population, since 30 children were excluded due to protocol violation (n = 13), adverse events (n = 7), death (n = 6), dissatisfaction with the nutritional product (n = 2), withdrawal of informed consent form (n = 1) and other reasons (n = 1).
Written informed consent was obtained from parent/legal guardian of each subject. The study was conducted in accordance with the ethical principles stated in the “Declaration of Helsinki” and approved by the Ankara University Faculty of Medicine Clinical Research Ethics Committee (Date of Approval: 24/07/2017; Protocol No: 12-736-17) and the Republic of Turkey Ministry of Health Turkish Medicines and Medical Devices Agency (Date of approval: 16/08/2017; Protocol No: 93189304-514.05.01-E.168888).
Data on demographic characteristics (age, gender), clinical and functional subtypes of CP and gastrointestinal intolerance symptoms were recorded at baseline. Data on clinical nutrition characteristics (type, route, mode of delivery, daily volume), total energy expenditure (TEE), anthropometrics (body mass index [BMI], weight for age [WFA], weight for height [WFH], triceps skinfold thickness [TSFT] and mid-upper arm circumference [MUAC]) expressed as z scores, gastrointestinal intolerance symptoms (severity and frequency), and defecation frequency and stool patterns were recorded at baseline and at three consecutive visits (1st, 3rd and 6th months) during a 6-month follow up. Parent's assessment on feeding and stool patterns and formula satisfaction were also evaluated via responses to the Feeding and Stool Patterns Questionnaire (at baseline and 1st, 3rd and 6th months) and Formula Satisfaction Questionnaire (at 1st, 3rd and 6th months), respectively, which were applied through face-to-face interview method.
Body weight was measured using a digital baby weight scale (10 g precision) in children aged ≤2 years, while with adult electronic scale (100 g precision) in children aged >2 years. Length measurement was performed using a 1 m length measuring tape (0.1 cm precision) in children aged ≤2 years, with a wall mounted stature meter (0.2 cm precision) in children aged >2 years, and with the use of tibial length (measured via a flexible tape measure from the superior border of the medial tibia condyle to the inferior border of the medial malleolus, with both the knee and the ankle at 90 degrees) for the stature estimations when necessary (26). MUAC was measured from the left upper arm flexed slightly at the elbow, at half distance between the acromion and the olecranon using a plastic measuring tape. TSFT was measured from the left arm, and at half distance between the acromion and the olecranon, using a skin fold caliper. Anthropometric data were expressed as z scores for BMI, TSFT and MUAC along with estimation of mean z-scores and percentiles for WFA and WFH (27). Growth was assessed based on the maintenance of anthropometric z-scores (using WHO reference data) during the study (28).
Gastrointestinal intolerance symptoms were evaluated in terms of frequency and severity. Symptom frequency scores ranged from 1 to 6 (1: none, 2: once a month, 3: once a week, 4: 2–6 times in a week, 5: 1–2 times per day and 6: ≥3 times per day). Symptom severity scores were grouped as score 1–2 (none-mild), score 3 (moderate) and score 4–5 (severe).
Defecation frequency was evaluated in “at least 2–3 times per day”, “once a day/once in 2–3 days”, “once a week or less” groups. The stool patterns were evaluated using Bristol Scale as categorized into type 1–2 (indicate constipation), type 3–4 (ideal stools as they are easier to pass), and type 5–7 (indicate diarrhea and urgency) (29).
TEE (kcal/day) was estimated using the height-based method for energy requirements of children with CP, including 15 kcal/cm in children without motor dysfunction, 14 kcal/cm for ambulatory children having motor dysfunction and 11 kcal/cm for non-ambulatory children (30).
Statistical analysis was performed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY). The numerical data were analyzed using Friedman test (with pairwise comparisons via Wilcoxon test) where the repeated measurement statistics did not comply with the parametric test assumptions, while repeated measurement statistics were used otherwise. Change over time was evaluated by Wilcoxon test for non-normally distributed variables. Data were expressed as mean ± standard deviation (SD), median (minimum–maximum) and percent (%) where appropriate. p < 0.05 was considered statistically significant.
A total of 96 children with CP were included in the study. The mean age of children with CP was 5.6 years (SD 3.2, range 1.0–12.0 years), and 56.3% of children were boys. Children with spastic CP (78.0%) and severe (30.4%) or total (20.7%) functional limitation comprised the majority of study population. The most common gastrointestinal intolerance symptoms recorded at baseline were gastrointestinal discomfort (65.6%), constipation (62.5%), retching (58.3%), nausea (52.8%), gas (49.5%), stomachache (42.4%) reflux/regurgitation (40.0%) and abdominal distention (37.2%) (Table 1).
Majority of children received Pediasure peptide® 1.0 Cal [Abbott Nutrition; hydrolyzed whey-dominant protein; 7 g (14% E) per 100 ml] at baseline (90.6%, via PEG in 88.5% and via NGT in 11.5%) as well as in the 3rd month (89.6%) and 6th month (92.7%) visits. Peptisorb® [100% whey protein; 2.8 g (11% E) per 100 ml] users comprised the 9.4% (baseline and 3rd month) and 7.3% (6th month) of study population (Table 2). Mode of delivery included bolus injection in 59.6% of children, while pump-assisted administration and gravity-controlled administration were applied in 34.0% and 6.5% of children, respectively. Median daily total volume was 800 ml (61.5 ml/kg) at baseline and 1,000 ml (63.5 ml/kg) at the 6th month visit (Table 2).
Immobile children with 11 kcal/cm height/day energy need comprised the majority of study population in each follow up visit (72.3%, 77.7% and 82.1%, respectively). In these children, the median daily total volume was 800 ml (60.3 ml/kg) at baseline and 1,000 ml (61.1 ml/kg) at 6th month visit (Table 2).
When compared to baseline scores, significant improvement was noted in median (min/max) MUAC z scores [−1.14 (−2.68/−0.19) vs. −1.07 (−2.07/0.32), p = 0.012], TSFT z scores [−0.52 (−1.29/0.40) vs. −0.37 (−1.05/0.92), p = 0.005] and WFH z scores [−2.0 (−3.40/−0.36) vs. −1.52 (−3.11/−0.35), p = 0.003] at 6th month visit. The change from baseline MUAC z score was also significantly higher at 3rd month visit compared to 6th month visit [0.99 (−10.5/3.68) vs. 0.27 (−8.41/4.38), p = 0.012] (Table 3, Figure 1).
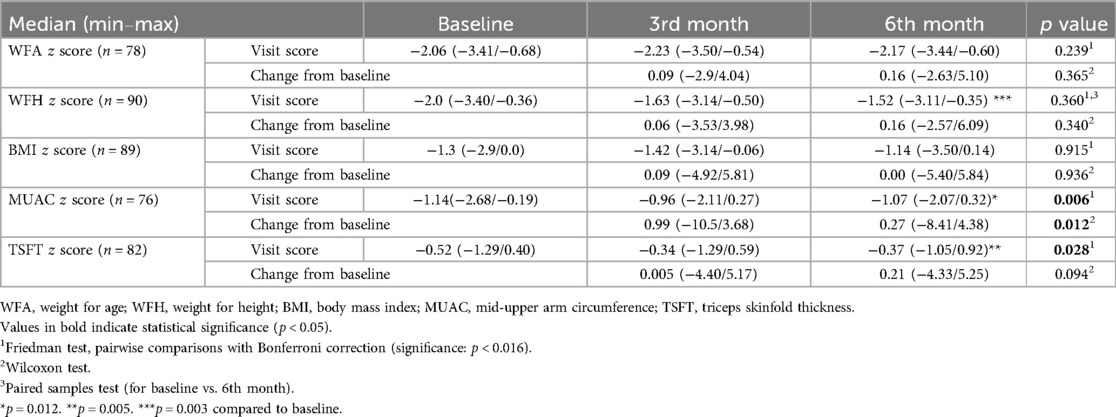
Table 3. Change in anthropometric z scores during study visits in children with CP who received a specialized peptide-based enteral formula.
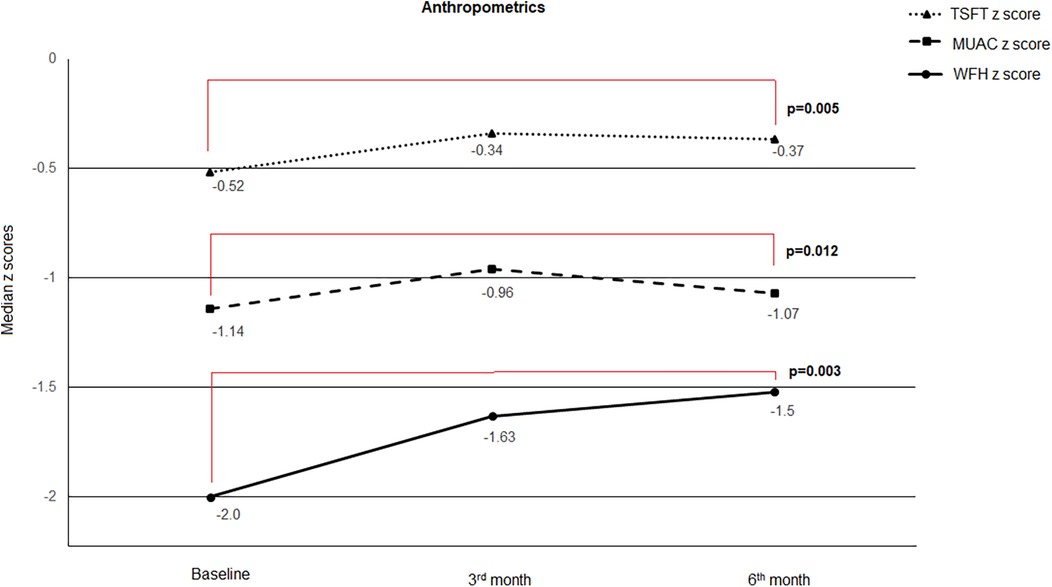
Figure 1. Change in anthropometric z scores during study visits. When compared to baseline scores, significant improvement was noted in median (min/max) MUAC z scores [−1.14(−2.68/−0.19) vs. −1.07(−2.07/0.32), p = 0.012], TSFT z scores [−0.52 (−1.29/0.40) vs. −0.37(−1.05/0.92), p = 0.005] and WFH z scores [−2.0 (−3.40/−0.36) vs. −1.52 (−3.11/−0.35), p = 0.003] at 6th month visit.
When analyzed with respect to clinical classification, WFA z scores in children with mixed CP [−1.14 (−6.85/1.16) vs. −1.04 (−5.77/0.94), p = 0.037] and WFH z scores in those with spastic CP [−2.14 (−7.03/2.9) vs. −1.64 (−7/3.37), p = 0.002] were significantly improved at 6th month compared to baseline (Table 4).
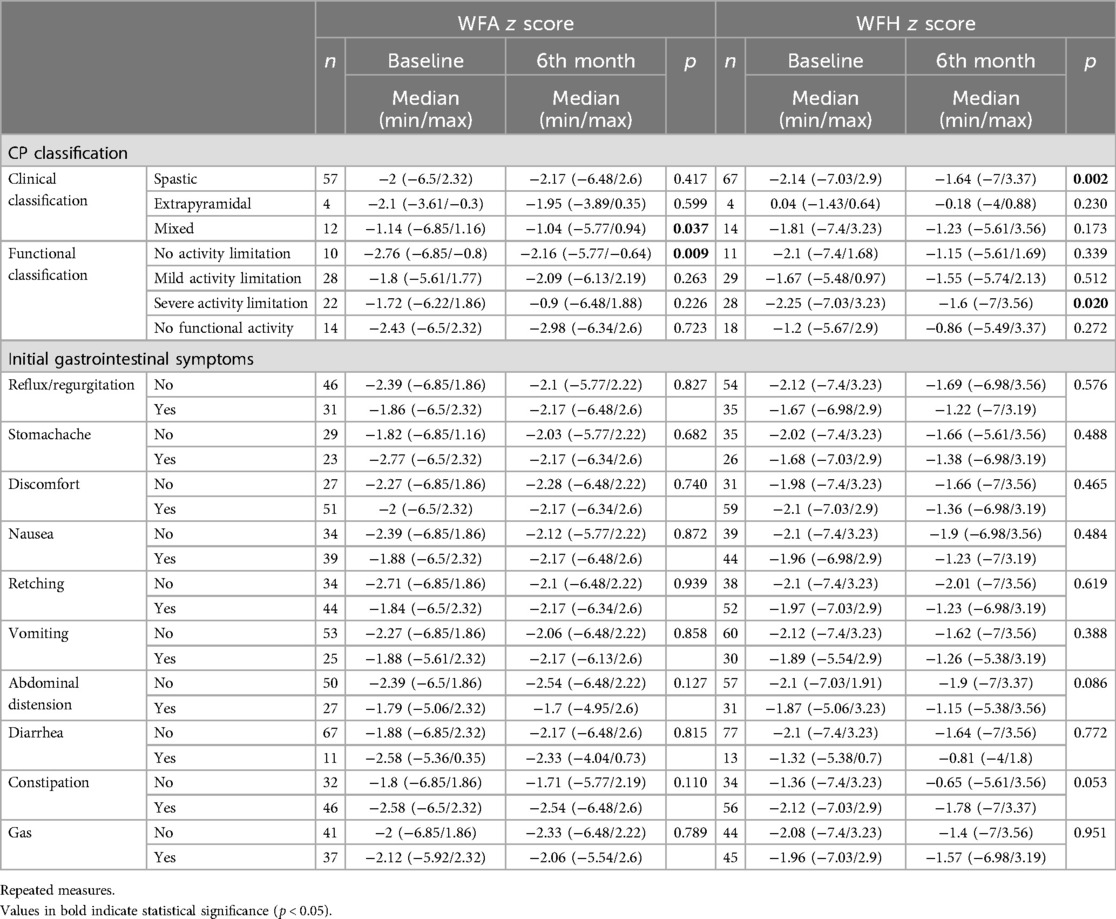
Table 4. WFA and WFH z scores according to CP classification and baseline gastrointestinal symptoms in children with CP who received a specialized peptide-based enteral formula.
When analyzed with respect to functional classification, WFA z scores in children with no activity limitation [−2.76 (−6.85/−0.8) vs. −2.16 (−5.77/−0.64), p = 0.009] and WFH z scores in those with severe activity limitation [−2.25 (−7.03/3.23) vs. −1.6 (−7/3.56), p = 0.020] were significantly improved at 6th month compared to baseline (Table 4).
No significant change was noted in WFA and WFH z scores with respect to type of initial individual gastrointestinal symptoms (Table 4).
Overall, the rate of “severe (score 4–5) symptoms” were significantly decreased, while the rate of “no or mild symptoms” were significantly increased from baseline to 1st month (p < 0.001 for each), 3rd month (p values ranged 0.002 to <0.001) and 6th month (p < 0.001 for each). The improvement (increase in those with no or mild degree symptoms) from baseline was particularly remarkable for discomfort (43.8–86.5%), retching (56.3–87.5%) and abdominal distension (71.9–96.9%) symptoms (Table 5, Figure 2).
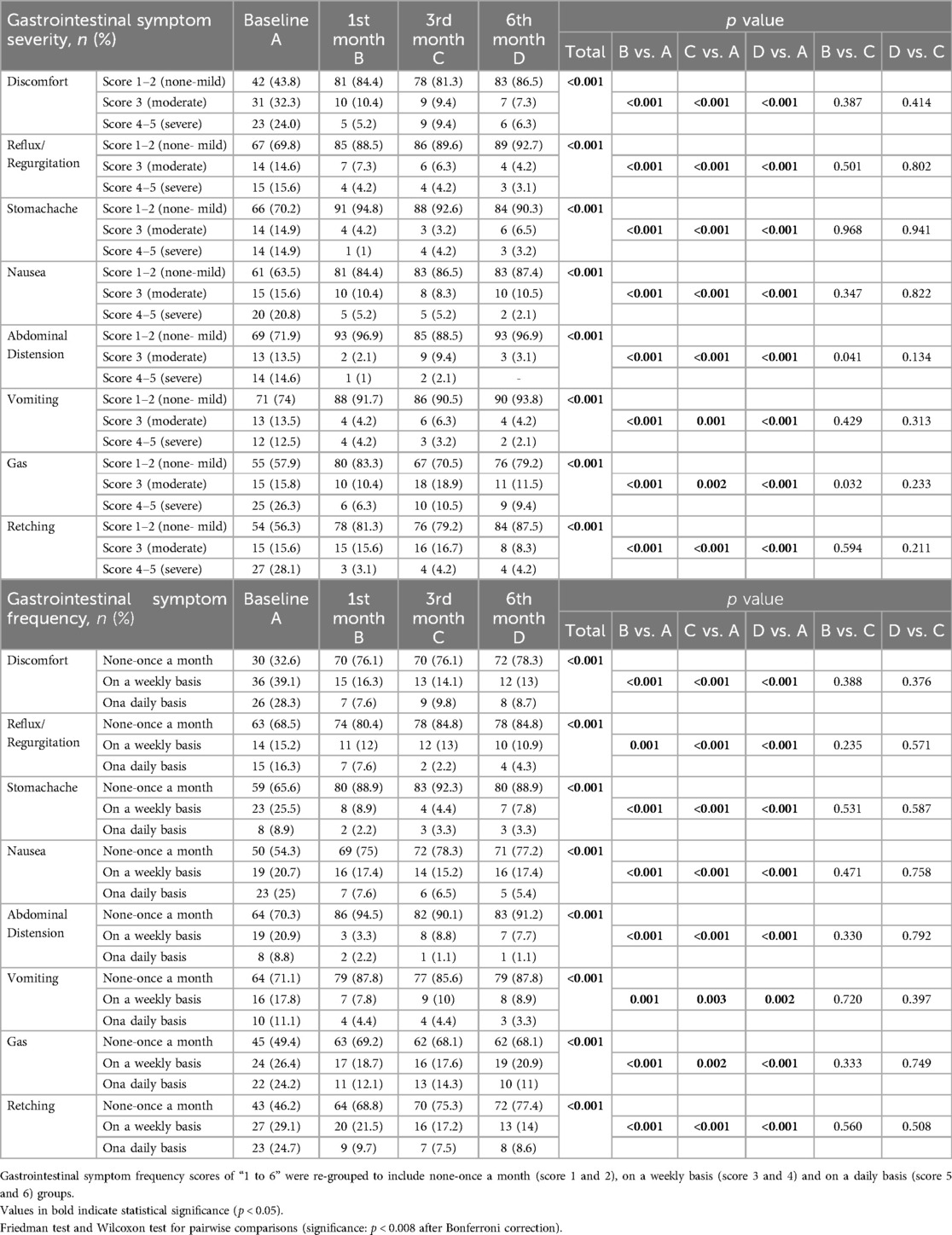
Table 5. Gastrointestinal intolerance symptom severity and frequency in children with CP who received a specialized peptide-based enteral formula.
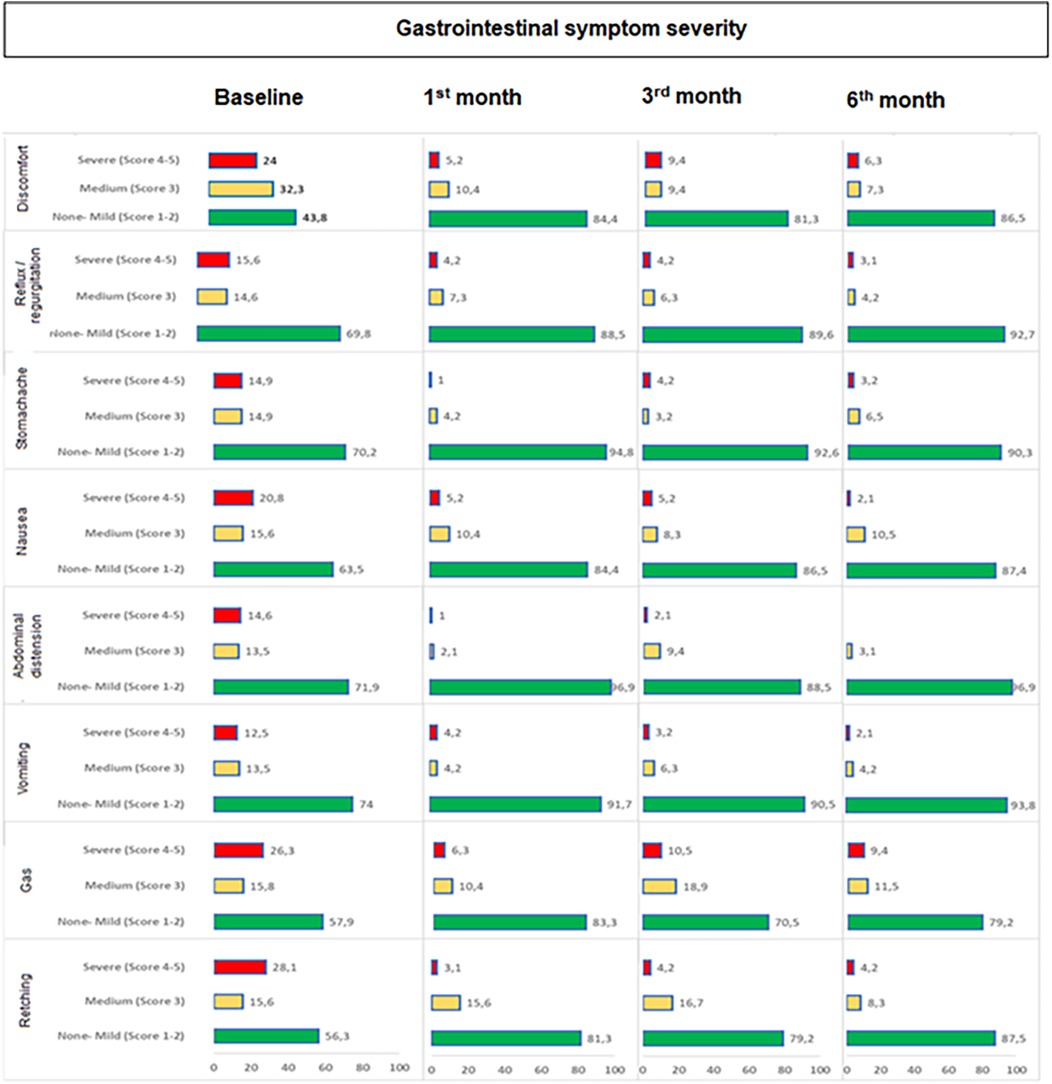
Figure 2. Change in gastrointestinal symptom severity during study visits. Overall, the rate of “severe (score 4−5) symptoms” were significantly decreased, while the rate of “no or mild symptoms” were significantly increased from baseline to 1st month (p < 0.001 for each), 3rd month (p values ranged 0.002 to <0.001) and 6th month (p < 0.001 for each).
The percentage of children who experience symptoms on a daily basis significantly decreased along with significant increase in the percentage of children with no or once monthly symptoms baseline to 1st month (p values ranged 0.001 to <0.001 for each), 3rd month (p values ranged 0.003 to <0.001) and 6th month (p values ranged 0.002 to <0.001). In general, improvement in frequency occurred within one month and was sustained through 6 months with increase in the percentage of children with lesser symptom frequency to ∼70% or 75% depending on symptom which stayed at that level throughout. The improvement (decrease in those who experience symptoms on a daily basis) from baseline was particularly remarkable for discomfort (28.3 to 8.7%), nausea (25.0 to 5.4%), retching (24.7 to 8.6%) and gas (24.2 to 11.0%) symptoms (Table 5, Figure 3).
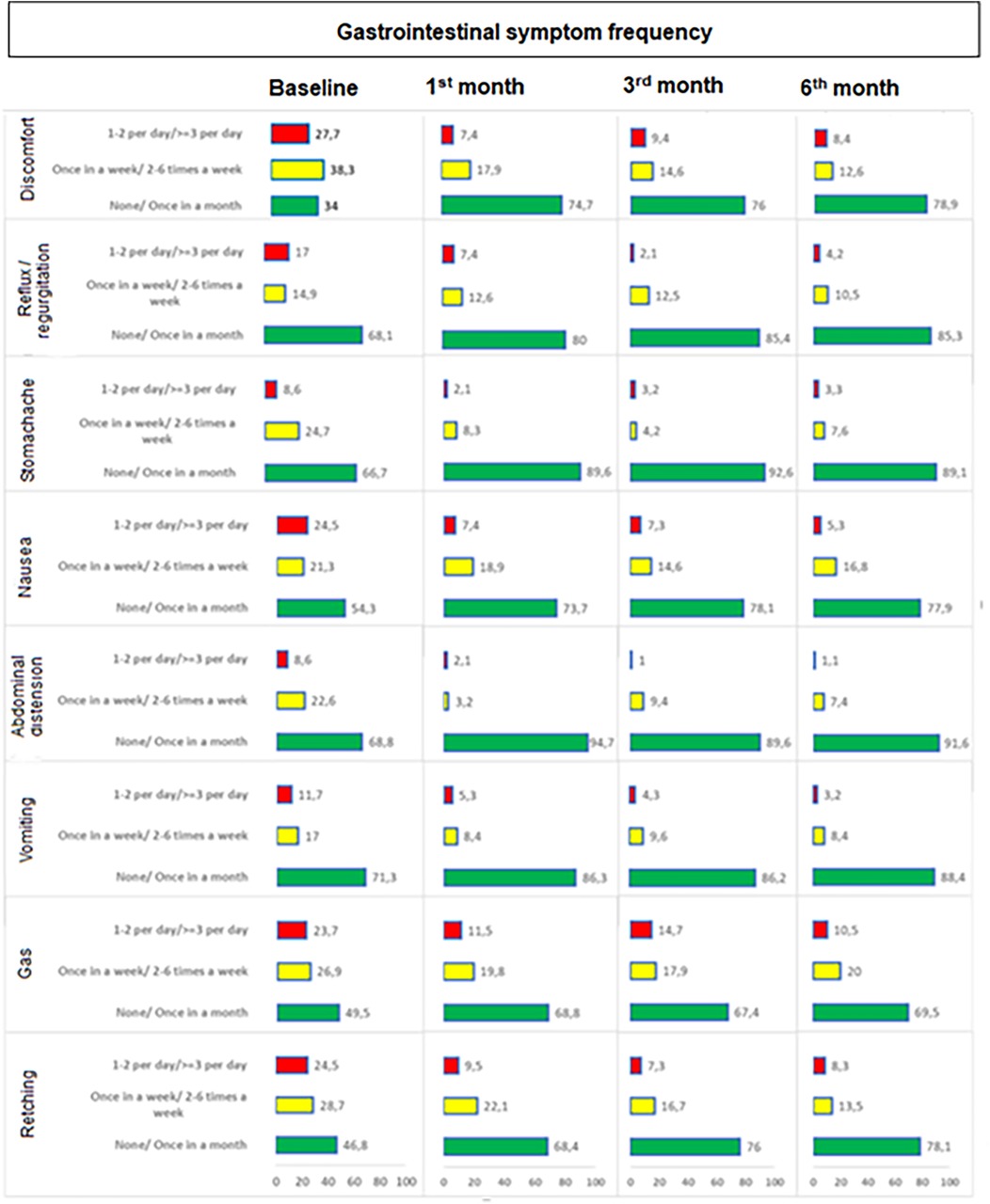
Figure 3. Change in gastrointestinal symptom frequency during study visits. The percentage of children who experience symptoms on a daily basis significantly decreased along with significant increase in the percentage of children with no or once monthly symptoms baseline to 1st month (p values ranged 0.001 to <0.001 for each), 3rd month (p values ranged 0.003 to <0.001) and 6th month (p values ranged 0.002 to <0.001).
When compared to baseline, 1st month, 3rd month and 6th month assessments revealed significant decrease in the likelihood of Type-1/Type-2 (constipation) stool pattern (from 31.6% at baseline to 7.4%, 2.1% and 3.2%, respectively, p < 0.001 for each) (Table 6).
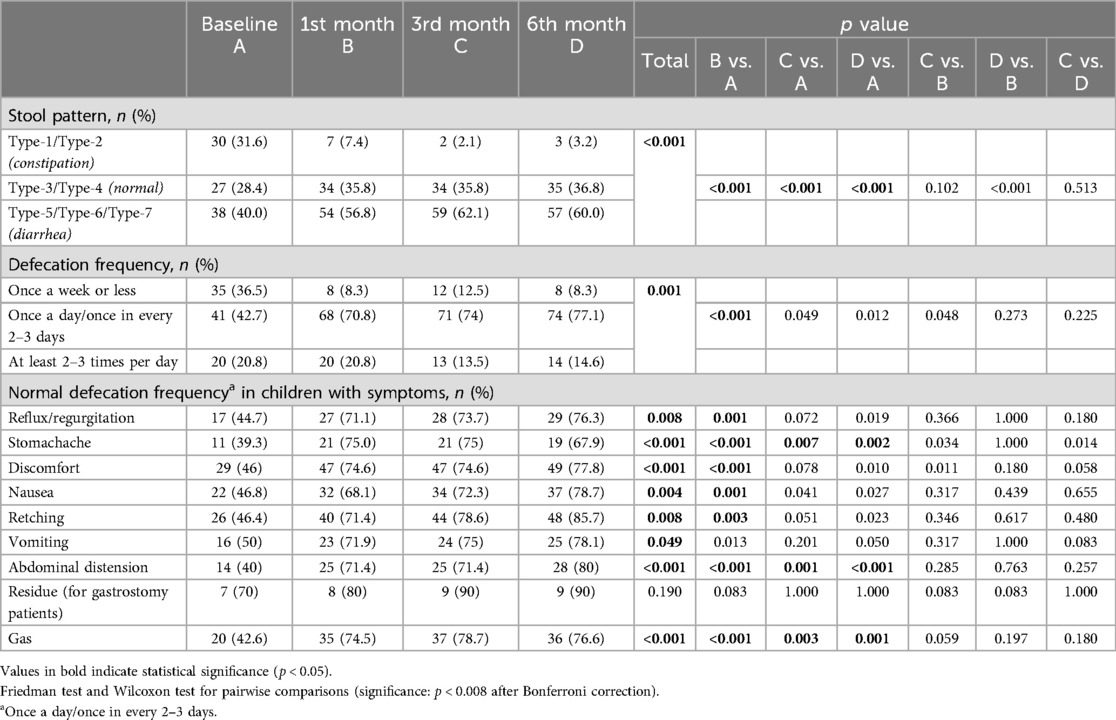
Table 6. Defecation frequency and stool patterns in children with CP who received a specialized peptide-based enteral formula.
The rate of normal (once a day or in every 2–3 days) defecation frequency was also increased from 42.7% at baseline to 70.8% at 1st month (p < 0.001) and this increase was maintained at 3rd and 6th month visits, particularly in the presence of stomachache (from 39.3 to 75% at 3rd month, p = 0.007 and to 67.9% at 6th month, p = 0.002), abdominal distension (from 40.0 to 71.4% and 80.0%, p = 0.001 and p < 0.001, respectively) and gas (from 42.6 to 78.7% and 76.6%, p = 0.003 and p = 0.001, respectively) (Table 6).
Most of parents reported that peptide-based enteral formula was associated with normalization of bowel movements (>85%) via decreasing the likelihood of increased bowel movements (>68%) as well as the constipation (>76%), with no vomiting after feeding (>82%) and an improved general status of the child (>79%), regardless of the follow up visit (Table 7).
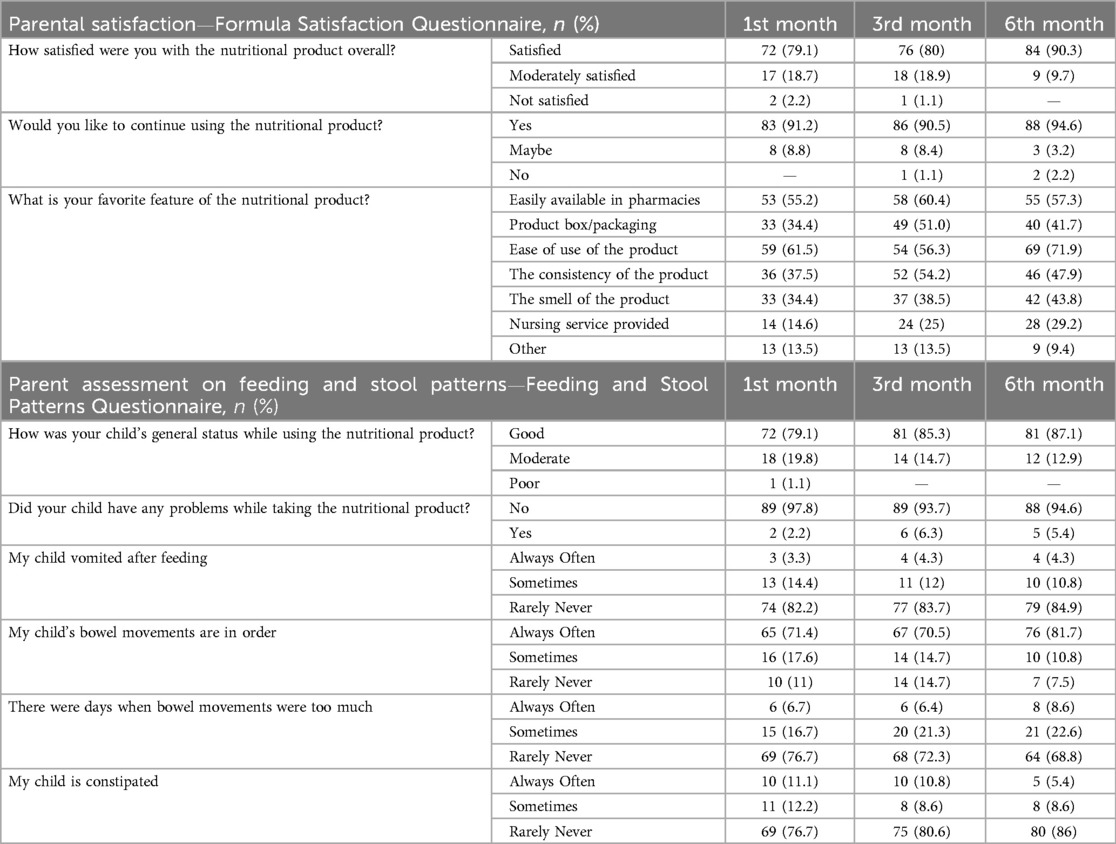
Table 7. Parent assessment on feeding and stool patterns and parental satisfaction with the nutritional product in children with CP.
Majority of parents were satisfied with the study formula (97.8% at 1st month visit, 98.9% at 3rd month visit and 100.0% at 6th month visit), reported no problems (97.8%, 93.7% and 94.6%, respectively) and they wished to continue using the enteral formula (91.2%, 90.5% and 94.6%, respectively) (Table 7).
The favorite feature of the product was considered to be ease of use (by 61.5%, 56.3% and 71.9% of parents at 1st, 3rd and 6th month visits, respectively) and its availability in pharmacies (by 55.2%, 60.4% and 57.3% of parents at 1st, 3rd and 6th month visits, respectively) (Table 7).
Other than significantly increased percentage of patients with high serum albumin (13.6 vs. 47.5%, p < 0.001), no significant change was noted in blood analysis findings during follow up visits. In total, 28 adverse events were reported in 22 children during study period, and the most frequently reported AEs were death (n = 6), pneumonia (n = 6), discomfort with PEG (n = 6) and upper respiratory infection (n = 5) (Table 8).
Our findings revealed that use of a specialized peptide-based formula containing MCT (Pediasure® peptide in majority of cases) in children with CP and feeding intolerance on previous tube feeding with standard enteral formula was associated with improved anthropometrics, amelioration in gastrointestinal intolerance symptoms both in terms of severity and frequency, normalization of defecation frequency and stool patterns and a high parental satisfaction with the formula. In general, improvement in frequency occurred within one month and was sustained through 6 months with increase in the percentage of children with lesser symptom frequency to ∼70% or 75% depending on symptom which stayed at that level throughout.
Spastic CP as accompanied with severe or total functional limitation was the leading diagnosis in our study population, while gastrointestinal discomfort, constipation and retching were the most common gastrointestinal intolerance symptoms recorded at baseline. Similarly, in a study among 1,108 children with CP from Turkey, the quadriplegic spastic CP was reported in majority of children in addition to Gross Motor Function Classification System (GMFCS) level V motor dysfunction, co-morbid gastrointestinal problems (constipation, lack of appetite and difficulty in swallowing) and malnutrition, especially in those with higher levels of gross motor dysfunction (7). Data from the multi-country PURPLE N (Profiling Children and Youth with Cerebral Palsy in Relation to Feeding and Nutrition) study, including Turkey, also revealed the severe form of CP (spastic CP in 84%, quadriplegia in 55%, and GMFCS level VI-V in 50%) along with gastrointestinal problems (i.e., constipation, gastrointestinal reflux, vomiting and retching) in most of children and a higher risk of malnutrition particularly in those with higher GMFCS level (31).
In fact, prevalence of malnutrition reported by different studies in children with CP ranges from 40% to 90%, depending on the study population and anthropometric tool (32–35). Previous multicenter studies in our country also revealed a considerable difference in the prevalence of malnutrition in children with CP, which was reported to be 57.2%, 92.6% to 94.3% and 91.3%, based on physicians' clinical judgment, Gomez classification of WFA percentiles and Waterlow classification of HFA percentiles, respectively (7, 36). Hence, use of growth charts for general pediatric population for anthropometric assessment in children with CP is considered to be associated with a risk of overestimating malnutrition in these children (7, 32, 37). Indeed, while anthropometric references exist for children with CP, they are not currently recommended because they only allow to know the growth from children with different degrees of severity, not the desirable growth, so the nutritional objective is restricted (38, 39).
Nonetheless, supporting the consideration of non-ambulatory status amongst the strongest risk factors for malnutrition in children with CP, several studies indicated association of higher levels of gross motor impairment with an increased risk of malnutrition and anthropometric deficits in curves in children with CP (7, 12, 18, 31, 40, 41). In addition, children with CP are suggested to have significantly lower anthropometric values in case of severe comorbidities and gastrointestinal problems, emphasizing the potential role of nutritional assessment and management as part of their overall care (42). The favorable efficacy and tolerability profile of peptide-based enteral formula in our children with severely disabled CP and intolerance on previous tube feeding with standard enteral formula is important in this regard.
Stunted growth and underdeveloped fat-free mass are considered to be more pronounced in more severe forms of CP, and therefore using screening tools for growth and fat mass is suggested to be more efficient in children with higher the GMFCS levels (37, 43). Notably, the combined use of MUAC, age, and GMFCS level is recommended in accurate prediction of weight in children with CP as well as the use of circumferences, primarily MUAC, in combination with other methods (i.e., TSFT) in the anthropometric assessment (37, 44, 45). Accordingly, our findings related to achievement of significantly improved MUAC z scores and TSFT z scores, as the highly sensitive measures of diagnosing malnutrition in the setting of CP, seem consistent with the multimodal effects of peptide-based enteral formula in terms of bone, muscle, and the adipose panicle (the energetic balance) (45–48).
In addition, WFH z scores were significantly improved at 6th month in our overall study population as well as in the subgroups of children with “spastic CP and severe activity limitation”, while WFA z scores were significantly improved at 6th month in those with “mixed CP and no activity limitation”. These findings seem notable given the increased likelihood of feeding difficulties in children with CP who have more severe motor impairment (i.e., tetraparesis) and more varied motor symptoms (i.e., mixed CP) (4). Other studies also reported the change in nutritional improvement according to the muscle tonicity in children with CP on PEG tube feeding, such as improved BMI for-age z-score and percent ideal body weight in the hypertonic group but not in the hypotonic group (49, 50). This emphasizes the possibility of different growth patterns and thus different nutritional support needs depending on the CP type (49, 50). Also, in a study addressing the factors related to caregiver burden on activities of daily living (ADLs) in 69 children with CP, GMFCS grade and intellectual disability were reported to be associated increased caregiver burden score, whereas, greater difficulty in performing ADLs was noted with lower weight z scores, BMI z scores and fat mass, regardless of the degree of GMFCS and intellectual disability (51). Accordingly, in addition to the degree of clinical impairment, nutritional status is also considered a key factor affecting the caregiver's difficulty in performing the ADLs in children with CP (51).
Immobile children with 11 kcal/cm height/day energy need comprised the majority of our study population in each follow up visit (72.3%, 77.7% and 82.1%, respectively) and the median values for TEE in these children were 1,168 kcal/day at baseline and 1,199 kcal/day at 1st month. Likewise, in a study among 13 children with CP, nutritional support for four weeks was reported to reveal a significant increase in TEE (from 1,121 kcal/day at baseline to 1,189 kcal/day at 30 days) (52). In this regard, our findings support the likelihood of normalization of TEE via an adequate energy intake in children with CP, which is lower than in healthy children due to adaptation of being fed with low energy diets over a prolonged period of time (52, 53).
Although, use of PEG (standard polymeric enteral formula) in children with CP has been reported to ameliorate malnutrition with improved anthropometrics, achievement of most of the catch-up growth and nutritional correction in approximately 6 months after PEG tube insertion (24, 49, 54, 55), a substantial population of children with CP and enteral tube feeding are affected by persistent feeding intolerance (56, 57). In this regard, our findings highlight the evaluation of gastrointestinal problems together with nutritional status and neurological and neuromuscular impairment in children with CP, since the concomitant presence of constipation, diarrhea, gastroesophageal reflux and feeding intolerance may necessitate alteration in formula and feeding regimens (18, 58). Moreover, selecting the appropriate type of enteral formula is of critical importance in maintaining or recovering the nutritional status in children with CP, as a promising strategy to overcome feeding intolerance and to reduce the related adverse health outcomes (i.e., increased risk of severe illness, mortality and nosocomial infections) (15, 49, 59).
In a systematic review of 15 randomized clinical trials on nutritional and dietary interventions in children with CP, the authors suggested that certain dietary and nutritional interventions offer potential benefits in clinical improvement, such as use of whey-based or pectin-enriched enteral formulas for gastroesophageal reflux, supplementation with lipid mixture or diet with high-density energy for improvements in anthropometric measures, supplementation with probiotics, prebiotics, symbiotics or magnesium for constipation and use of a nutritional support system for gross motor function (60). However, while authors indicated that some promising dietary and nutritional interventions may promote important clinical improvements for patients with CP, they also emphasized that the evidence is weak, due to availability of few published clinical trials along with many methodological errors, leading to a high risk of bias (60).
In our children with severely disabled CP and gastrointestinal intolerance, peptide-based enteral formula ameliorated gastrointestinal symptom severity and frequency starting from the 1st month of nutritional support. The improvement in symptom severity was particularly remarkable for discomfort, retching and abdominal distension, while the improvement in symptom frequency was more pronounced for discomfort, nausea, retching and gas. Hence, our findings support the likelihood of protein composition in enteral formulas to ameliorate the gastrointestinal symptoms among gastrostomy-fed children with CP, possibly in relation to effects on gastric emptying and dysmotility (17, 61). It has also been suggested that children with CP, frequently having concomitant gastrointestinal problems including foregut dysmotility, may be more sensitive to type of protein in the meal than healthy children (17, 62).
The unique characteristics of MCTs such as not requiring a complex process of digestion and their facile absorption without the need for bile or pancreatic enzymes confer significant advantage over most other lipid molecules, particularly in the setting of gastrointestinal disorders (63, 64). Besides the amelioration of gastrointestinal intolerance symptoms, peptide-based enteral formula containing MCTs also revealed additional benefits in terms of normalization of bowel movements with a significant decrease in type 1 and a significant increase in type 4 stool patterns in our children. These effects appeared starting from the 1st month of clinical nutrition and maintained throughout the follow up, even in the presence of stomachache, abdominal distension and gas. This seems notable given that chronic constipation in CP has a significant impact on child's well-being, as can be associated with gastrointestinal manifestations (recurrent vomiting, abdominal discomfort and early satiety, compromising dietary intake), urinary symptoms (poorly voiding bladder, recurrent urinary tract infection and deterioration of vesicoureteric reflux) and impaired quality of life (11, 58, 59, 65).
Majority of parents in the current study were satisfied with the study formula, reported significantly improved bowel movements and stool patterns and general status of the child with no adverse reactions and they wished to continue using it. Similarly, evidence of caregiver satisfaction with gastrostomy tube feeding was reported in the majority of studies, including PEG-fed children with CP, regarding the ease of feeding, improvement in child's disposition and nutrition, enhanced child's comfort and abilities, less stress and low risk of long-term adverse events (6, 11, 25, 65, 66).
In addition, a high degree of agreement was noted in child feeding and stool patterns as determined by parents and clinicians in the current study, which is important given that family-assessed measurements or family reports, as recently become more popular and important, are considered to be advantageous in terms of providing data not limited to a clinical setting or a visit-time (67, 68).
Given that the nutritional status of children with CP is affected by several factors inherent to their own condition and those beyond dietary intake, such as GMFCS level, oral-motor dysfunction, feeding skills, gastrointestinal disorders, physical activity levels and altered energy requirements, multidisciplinary monitoring and evaluation of nutrition support for children with CP has an important role in timely identification and management of nutritional status and potential complications (28, 58, 59).
Major strength of the present study seems to be availability of a comprehensive assessment of nutritional and gastrointestinal data in a large-scale cohort of children with CP as well as use of a specialized peptide-based enteral tube feeding in the presence of gastrointestinal intolerance symptoms. Certain limitations to this study should be considered. First, due to observational nature, non-randomized allocation and thereby the likelihood of main selection bias and confounding is possible. Second lack of data on co-morbid gastrointestinal dysfunction such as dysphagia, gastroesophageal reflux and oral motor dysfunction is another limitation which otherwise would extend the knowledge achieved in the current study.
In conclusion, our findings revealed that choice of a specialized peptide-based formula containing MCT in provision of enteral tube feeding among children with CP and feeding intolerance on previous standard enteral formula is an effective strategy that leads to improved anthropometrics, amelioration of gastrointestinal intolerance, and normalization of bowel movements along with a high parental satisfaction. Our findings suggest that nutritional and gastrointestinal problems should concomitantly be assessed in children with CP and those with concomitant gastrointestinal problems may benefit from provision of enteral tube feeding with use of a specialized peptide-based enteral formula. Hence, appropriate identification of nutritional support needs depending on the clinical and functional CP types is important in children with CP as well as the timely recognition of feeding intolerance that may necessitate alteration in formula and feeding regimens for maintaining or recovering the nutritional status. The utility of specialized peptide-based enteral formulas in enteral tube feeding among children with CP should be further addressed by longer term and larger scale studies in terms of nutritional, gastrointestinal and survival outcomes.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by Ankara University Faculty of Medicine Clinical Research Ethics Committee (Date of Approval: 24/07/2017; Protocol No: 12-736-17) and the Republic of Turkey Ministry of Health Turkish Medicines and Medical Devices Agency (Date of approval: 16/08/2017; Protocol No: 93189304-514.05.01-E.168888). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
AK: Conceptualization, Methodology, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing. GK: Formal Analysis, Methodology, Resources, Writing – review & editing. GC: Formal Analysis, Methodology, Resources, Writing – review & editing. CA: Data curation, Investigation, Resources, Writing – review & editing. NU: Data curation, Investigation, Resources, Writing – review & editing. GT: Data curation, Visualization, Writing – review & editing. AY: Resources, Supervision, Writing – review & editing. CT: Investigation, Writing – review & editing. AD: Investigation, Writing – review & editing. FD: Investigation, Writing – review & editing. MU: Investigation, Writing – review & editing. SY: Investigation, Writing – review & editing. DD: Investigation, Writing – review & editing. EG: Investigation, Writing – review & editing. BD: Investigation, Writing – review & editing. YD: Investigation, Writing – review & editing. NG: Investigation, Writing – review & editing. HK: Investigation, Writing – review & editing. FG: Investigation, Writing – review & editing. ES: Investigation, Writing – review & editing. AA: Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Visualization, Writing – review & editing. NE: Investigation, Writing – review & editing. DT: Investigation, Writing – review & editing. HA: Investigation, Writing – review & editing. CC: Investigation, Writing – review & editing. OG: Investigation, Writing – review & editing. KD: Investigation, Writing – review & editing. AU: Investigation, Writing – review & editing. OB: Investigation, Writing – review & editing. ZA: Investigation, Writing – review & editing. MC: Investigation, Writing – review & editing. AE: Investigation, Writing – review & editing. NU: Investigation, Writing – review & editing. CE: Investigation, Writing – review & editing. SE: Funding acquisition, Project administration, Resources, Visualization, Writing – review & editing.
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by Abbott Nutrition Turkey.
The authors would like to thank Cagla Ayhan, MD and Prof. Sule Oktay, MD, PhD. from Kappa Training Consultancy Research LL (Izmir, Turkey) who provided editorial support funded by Abbott Nutrition Turkey, Sinan Ozgur Aydin and Evrim Koseoglu from CRM Contract Research Organization (Ankara, Turkey) for statistical analysis funded by Abbott Nutrition Turkey, and Klinar Contract Research Organization (Ankara, Turkey).
AA, SE are Abbott employees.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1448507/full#supplementary-material
1. Sullivan PB, Lambert B, Rose M, Ford-Adams M, Johnson A, Griffiths P. Prevalence and severity of feeding and nutritional problems in children with neurological impairment: Oxford Feeding Study. Dev Med Child Neurol. (2000) 42(10):674–80. doi: 10.1017/s0012162200001249
2. Dahl M, Thommessen M, Rasmussen M, Selberg T. Feeding and nutritional characteristics in children with moderate or severe cerebral palsy. Acta Pediatr. (1996) 85(6):697–701. doi: 10.1111/j.1651-2227.1996.tb14129.x
3. Calis EA, Veugelers R, Sheppard JJ, Tibboel D, Evenhuis HM, Penning C. Dysphagia in children with severe generalized cerebral palsy and intellectual disability. Dev Med Child Neurol. (2008) 50(8):625–30. doi: 10.1111/j.1469-8749.2008.03047.x
4. García Ron A, González Toboso RM, Bote Gascón M, de Santos MT, Vecino R, Bodas Pinedo A. Nutritional status and prevalence of dysphagia in cerebral palsy: usefulness of the eating and drinking ability classification system scale and correlation with the degree of motor impairment according to the gross motor function classification system. Neurologia (Engl Ed). (2023) 38(1):35–40. doi: 10.1016/j.nrleng.2019.12.006
5. Dahlseng MO, Finbråten AK, Júlíusson PB, Skranes J, Andersen G, Vik T. Feeding problems, growth and nutritional status in children with cerebral palsy. Acta Paediatr. (2012) 101(1):92–8. doi: 10.1111/j.1651-2227.2011.02412.x
6. Sleigh G, Brocklehurst P. Gastrostomy feeding in cerebral palsy: a systematic review. Arch Dis Child. (2004) 89(6):534–9.15155398
7. Aydin K, Turkish Cerebral Palsy Study Group. A multicenter cross-sectional study to evaluate the clinical characteristics and nutritional status of children with cerebral palsy. Clin Nutr ESPEN. (2018) 26:27–34. doi: 10.1016/j.clnesp.2018.05.002
8. Penagini F, Borsani B, Bosetti A, Mameli C, Dilillo D, Ramponi G, et al. Resting energy expenditure in children with cerebral palsy: accuracy of available prediction formulae and development of a population-specific formula. Clin Nutr ESPEN. (2018) 25:44–9. doi: 10.1016/j.clnesp.2018.04.006
9. Sullivan PB. Nutrition and growth in children with cerebral palsy: setting the scene. Eur J Clin Nutr. (2013) 67(Suppl 2):S3–4. doi: 10.1038/ejcn.2013.222
10. Trivić I, Hojsak I. Evaluation and treatment of malnutrition and associated gastrointestinal complications in children with cerebral palsy. Pediatr Gastroenterol Hepatol Nutr. (2019) 22(2):122–31. doi: 10.5223/pghn.2019.22.2.122
11. Sullivan PB. Pros and cons of gastrostomy feeding in children with cerebral palsy. Paediatr Child Health. (2013) 24(8):351–4. doi: 10.1016/j.paed.2013.11.004
12. Quitadamo P, Thapar N, Staiano A, Borrelli O. Gastrointestinal and nutritional problems in neurologically impaired children. Eur J Paediatr Neurol. (2016) 20(6):810–5. doi: 10.1016/j.ejpn.2016.05.019
13. Rempel GR, Colwell S, Nelson RP. Growth in children with cerebral palsy fed via gastrostomy. Pediatrics. (1988) 82(6):857–62. doi: 10.1542/peds.82.6.857
14. Sanders KD, Cox K, Cannon R, Blanchard D, Pitcher J, Papathakis P, et al. Growth response to enteral feeding by children with cerebral palsy. JPEN J Parenter Enteral Nutr. (1990) 14(1):23–6. doi: 10.1177/014860719001400123
15. Hauer J. Feeding intolerance in children with severe impairment of the central nervous system: strategies for treatment and prevention. Children (Basel). (2017) 5(1):1. doi: 10.3390/children5010001
16. Stevenson RD, Conaway M, Chumlea WC, Rosenbaum P, Fung EB, Henderson RC, et al. Growth and health in children with moderate-to-severe cerebral palsy. Pediatrics. (2006) 118(3):1010–8. doi: 10.1542/peds.2006-0298
17. Brun AC, Størdal K, Johannesdottir GB, Bentsen BS, Medhus AW. The effect of protein composition in liquid meals on gastric emptying rate in children with cerebral palsy. Clin Nutr. (2012) 31(1):108112. doi: 10.1016/j.clnu.2011.07.009
18. Campanozzi A, Capano G, Miele E, Romano A, Scuccimarra G, Del Giudice E, et al. Impact of malnutrition on gastrointestinal disorders and gross motor abilities in children with cerebral palsy. Brain Dev. (2007) 29(1):25–9. doi: 10.1016/j.braindev.2006.05.008
19. Nelson JL. A pilot intervention study to evaluate compliance to a peptide-based oral nutritional supplement in an adult population with impaired gastrointestinal function. Clin Nutr Exp. (2019) 28:123–30. doi: 10.1016/j.yclnex.2019.08.001
20. Silk DBA, Fairclough P, Clark ML, Hegarty JE, Addison JM, Burston D, et al. Use of a peptide rather than free amino acid nitrogen source in chemically defined ‘elemental’ diets. JPEN J Parenter Enteral Nutr. (1980) 4(6):548–53. doi: 10.1177/0148607180004006548
21. Seres DS, Ippolito PR. Pilot study evaluating the efficacy, tolerance and safety of a peptide-based enteral formula versus a high protein enteral formula in multiple ICU settings (medical, surgical, cardiothoracic). Clin Nutr. (2017) 36(3):706–9. doi: 10.1016/j.clnu.2016.04.016
22. Keller J, Layer P. The pathophysiology of malabsorption. Viszeralmedizin. (2014) 30(3):150–4. doi: 10.1159/000364794
23. Gantasala S, Sullivan PB, Thomas AG. Gastrostomy feeding versus oral feeding alone for children with cerebral palsy. Cochrane Database Syst Rev. (2013) 2013(7):CD003943. doi: 10.1002/14651858.CD003943.pub3
24. Sullivan PB, Juszczak E, Bachlet AM, Lambert B, Vernon-Roberts A, Grant HW, et al. Gastrostomy tube feeding in children with cerebral palsy: a prospective, longitudinal study. Dev Med Child Neurol. (2005) 47(2):77–85. doi: 10.1017/s0012162205000162
25. Sullivan PB, Alder N, Bachlet AM, Grant H, Juszczak E, Henry J, et al. Gastrostomy feeding in cerebral palsy: too much of a good thing? Dev Med Child Neurol. (2006) 48(11):877–82. doi: 10.1017/S0012162206001927
26. Stevenson RD. Use of segmental measures to estimate stature in children with cerebral palsy. Arch Pediatr Adolesc Med. (1995) 149:658–62. doi: 10.1001/archpedi.1995.02170190068012
27. McDowell MA, Fryar CD, Ogden CL, Flegal KM. Anthropometric reference data for children and adults: United States, 2003–2006. Natl Health Stat Report. (2008) (10):1–48.25585443
28. WHO Multicentre Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Acta Paediatr Suppl. (2006) 450:76–85. doi: 10.1111/j.1651-2227.2006.tb02378.x
29. O’Donnell LJD, Virjee J, Heaton KW. Detection of pseudodiarrhoea by simple clinical assessment of intestinal transit rate. Br Med J. (1990) 300:439–40. doi: 10.1136/bmj.300.6722.439
30. Andrew MJ, Sullivan PB. Growth in cerebral palsy. Nutr Clin Pract. (2010) 25(4):357–61. doi: 10.1177/0884533610374061
31. Fogarasi A, Fazzi E, Smorenburg ARP, Mazurkiewicz-Beldzinska M, Dinopoulos A, Pobiecka A, et al. The PURPLE N study: objective and perceived nutritional status in children and adolescents with cerebral palsy. Disabil Rehabil. (2022) 44(22):6668–75. doi: 10.1080/09638288.2021.1970255
32. Tekin H, Tekgül H, Yılmaz S, Arslangiray D, Reyhan H, Serdaroğlu G, et al. Prevalence and severity of malnutrition in pediatric neurology outpatients with respect to underlying diagnosis and comorbid nutrition and feeding related problems. Turk J Pediatr. (2018) 60(6):709–17. doi: 10.24953/turkjped.2018.06.012
33. Soylu OB, Unalp A, Uran N, Dizdarer G, Ozgonul FO, Conku A, et al. Effect of nutritional support in children with spastic quadriplegia. Pediatr Neurol. (2008) 39(5):330–4. doi: 10.1016/j.pediatrneurol.2008.07.020
34. Wang F, Cai Q, Shi W, Jiang H, Li N, Ma D, et al. A cross-sectional survey of growth and nutritional status in children with cerebral palsy in West China. Pediatr Neurol. (2016) 58:90–7. doi: 10.1016/j.pediatrneurol.2016.01.002
35. Caram AL, Morcillo AM, Costa-Pinto EA. Nutritional status of children with cerebral palsy in a Brazilian tertiary care teaching hospital. Dev Med Child Neurol. (2008) 50(12):956. doi: 10.1111/j.1469-8749.2008.03128.x
36. Carman KB, Aydın K, Kilic Aydin B, Cansu A, Direk MC, Durmus S, et al. Evaluation of micronutrient levels in children with cerebral palsy. Pediatr Int. (2022) 64(1):e15005. doi: 10.1111/ped.15005
37. Sørensen SJ, Brekke G, Kok K, Sørensen JL, Born AP, Mølgaard C, et al. Nutritional screening of children and adolescents with cerebral palsy: a scoping review. Dev Med Child Neurol. (2021) 63(12):1374–81. doi: 10.1111/dmcn.14981
38. Romano C, van Wynckel M, Hulst J, Broekaert I, Bronsky J, Dall'Oglio L, et al. European society for paediatric gastroenterology, hepatology and nutrition guidelines for the evaluation and treatment of gastrointestinal and nutritional complications in children with neurological impairment. J Pediatr Gastroenterol Nutr. (2017) 65(2):242–64. doi: 10.1097/MPG.0000000000001646
39. Samson-Fang L, Bell KL. Assessment of growth and nutrition in children with cerebral palsy. Eur J Clin Nutr. (2013) 67(Suppl 2):S5–8. doi: 10.1038/ejcn.2013.223
40. Herrera-Anaya E, Angarita-Fonseca A, Herrera-Galindo VM, Martínez-Marín RD, Rodríguez-Bayona CN. Association between gross motor function and nutritional status in children with cerebral palsy: a cross-sectional study from Colombia. Dev Med Child Neurol. (2016) 58(9):936–41. doi: 10.1111/dmcn.13108
41. Brunner MLM R, Cieri ME, Rodriguez Marco MP, Schroeder AS, Cuestas E. Nutritional status of children with cerebral palsy attending rehabilitation centers. Dev Med Child Neurol. (2020) 62(12):1383–8. doi: 10.1111/dmcn.14667
42. Boudokhane S, Ben Jeddou K, Migaou H, Salah S, Jellad A, Ben Salah Frih Z. Anthropometric and nutritional assessment of children with severe cerebral palsy: about a Tunisian population. Ann Phys Rehab Med. (2015) 58(Suppl 1):e141. doi: 10.1016/j.rehab.2015.07.335
43. Whitney DG, Gross-Richmond P, Hurvitz EA, Peterson MD. Total and regional body fat status among children and young people with cerebral palsy: a scoping review. Clin Obes. (2019) 9(5):e12327. doi: 10.1111/cob.12327
44. Brunner MLM R, Cieri ME, Butler C, Cuestas E. Development of equations and software for estimating weight in children with cerebral palsy. Dev Med Child Neurol. (2021) 63(7):860–5. doi: 10.1111/dmcn.14857
45. Aydın K, Dalgıç B, Kansu A, Ozen H, Selimoğlu MA, Tekgül H, et al. The significance of MUAC z-scores in diagnosing pediatric malnutrition: a scoping review with special emphasis on neurologically disabled children. Front Pediatr. (2023) 11:1081139. doi: 10.3389/fped.2023.1081139
46. Mramba L, Ngari M, Mwangome M, Muchai L, Bauni E, Walker AS, et al. A growth reference for mid upper arm circumference for age among school age children and adolescents, and validation for mortality: growth curve construction and longitudinal cohort study. Br Med J. (2017) 358:j3423. doi: 10.1136/bmj.j3423
47. Gurka M, Kuperminc M, Busby M, Bennis JA, Grossberg RI, Houlihan CM, et al. Assessment and correction of skinfold thickness equations in estimating body fat in children with cerebral palsy. Dev Med Child Neurol. (2010) 52(2):35–41. doi: 10.1111/j.1469-8749.2009.03474.x
48. Leonard M, Dain E, Pelc K, Dan B, De Laet C. Nutritional status of neurologically impaired children: impact on comorbidity. Arch Pediatr. (2020) 27(2):95–103. doi: 10.1016/j.arcped.2019.11.003
49. Suh CR, Kim W, Eun BL, Shim JO. Percutaneous endoscopic gastrostomy and nutritional interventions by the pediatric nutritional support team improve the nutritional status of neurologically impaired children. J Clin Med. (2020) 9(10):3295. doi: 10.3390/jcm9103295
50. Więch P, Ćwirlej-Sozańska A, Wiśniowska-Szurlej A, Kilian J, Lenart-Domka E, Bejer A, et al. The relationship between body composition and muscle tone in children with cerebral palsy: a case-control study. Nutrients. (2020) 12(3):864. doi: 10.3390/nu12030864
51. Martínez de Zabarte Fernández JM, Ros Arnal I, Peña Segura JL, García Romero R, Rodríguez Martínez G. Carga del cuidador del paciente con parálisis cerebral moderada-grave: ¿influyeelestadonutricional? [Caregiver burden in patients with moderate-severe cerebral palsy. The influence of nutritional status]. An Pediatr (Engl Ed). (2021) 94(5):311–7. doi: 10.1016/j.anpedi.2020.06.020
52. García-Contreras AA, Vasquez-Garibay EM, Romero-Velarde E, Ibarra-Gutierrez AI, Troyo-Sanroman R. Energy ex-penditure in children with cerebral palsy and moderate/severe malnutrition during nutritional recovery. Nutr Hosp. (2015) 31(5):2062–9. doi: 10.3305/nh.2015.31.5.8588
53. Arrowsmith FE, Allen JR, Gaskin KJ, Somerville H, Birdsall J, Barzi F, et al. Nutritional rehabilitation increases the resting energy expenditure of malnourished children with severe cerebral palsy. Dev Med Child Neurol. (2012) 54(2):170–5. doi: 10.1111/j.1469-8749.2011.04166.x
54. Civan HA, Bektas G, Dogan AE, Ozdener F. Percutaneous endoscopic gastrostomy feeding in children with cerebral palsy. Neuropediatrics. (2021) 52(4):326–32. doi: 10.1055/s-0041-1731007
55. Dipasquale V, Catena MA, Cardile S, Romano C. Standard polymeric formula tube feeding in neurologically impaired children: a five-year retrospective study. Nutrients. (2018) 10(6):684. doi: 10.3390/nu10060684
56. Jersak T, Kim SS, Noritz G, Testa M, Humphrey L. Defining persistent total parenteral nutrition use in patients with neurologic impairment. J Palliat Med. (2022) 25(4):577–83. doi: 10.1089/jpm.2021.0086
57. McCallum Z, Delany C, Gillam L. Crossing the line? Ethics of parenteral nutrition in paediatricneurodisability complicated by intestinal failure. Arch Dis Child. (2023) 108(1):11–4. doi: 10.1136/archdischild-2021-323500
58. Andrew MJ. Nutrition in children with neurodisability. Paediatr Child Health. (2019) 29(10):437–40. doi: 10.1016/j.paed.2019.07.004
59. Sousa KT, Ferreira GB, Santos AT, Nomelini QSS, Minussi LOA, Rezende ÉRMA, et al. Assessment of nutritional status and frequency of complications associated to feeding in patients with spastic quadriplegic cerebral palsy. Rev Paul Pediatr. (2020) 38:e2018410. doi: 10.1590/1984-0462/2020/38/2018410
60. Rebelo F, Mansur IR, Miglioli TC, Meio MDB, Junior SCG. Dietary and nutritional interventions in children with cerebral palsy: a systematic literature review. PLoS One. (2022) 17(7):e0271993. doi: 10.1371/journal.pone.0271993
61. Fried MD, Khoshoo V, Secker DJ, Gilday DL, Ash JM, Pencharz PB. Decrease in gastric emptying time and episodes of regurgitation in children with spastic quadriplegia fed a whey-based formula. J Pediatr. (1992) 120(4 Pt 1):569–72. doi: 10.1016/s0022-3476(10)80003-4
62. Sullivan PB. Gastrointestinal disorders in children with neurodevelopmental disabilities. Dev Disabil Res Rev. (2008) 14(2):128–36. doi: 10.1002/ddrr.18
63. Shah ND, Limketkai BN. The use of medium-chain triglycerides in gastrointestinal disorders. Pract Gastroenterol. (2017) 160:26–8.
64. Watanabe S, Tsujino S. Applications of medium-chain triglycerides in foods. Front Nutr. (2022) 9:802805. doi: 10.3389/fnut.2022.802805
65. Sullivan PB, McIntyre E. Gastrointestinal problems in disabled children. Curr Paediatr. (2005) 15(4):347–53. doi: 10.1016/j.cupe.2005.04.014
66. Sullivan PB, Juszczak E, Bachlet AM, Thomas AG, Lambert B, Vernon-Roberts A, et al. Impact of gastrostomy tube feeding on the quality of life of carers of children with cerebral palsy. Dev Med Child Neurol. (2004) 46(12):796–800. doi: 10.1017/s0012162204001392
67. Mutlu A, Kara ÖK, Livanelioğlu A, Karahan S, Alkan H, Yardımcı BN, et al. Agreement between parents and clinicians on the communication function levels and relationship of classification systems of children with cerebral palsy. Disabil Health J. (2018) 11(2):281–6. doi: 10.1016/j.dhjo.2017.11.001
Keywords: cerebral palsy, peptide-based formula, gastrointestinal intolerance, anthropometrics, parental satisfaction
Citation: Kansu A, Kutluk G, Caltepe G, Arikan C, Urganci N, Tumgor G, Yuce A, Tuna Kirsaclioglu C, Demir AM, Demirbas F, Usta M, Yavuz S, Demirtas Guner D, Gumus E, Dalgic B, Dogan Y, Gerenli N, Kocamaz H, Gulerman F, Sag E, Alptekin Sarioglu A, Eksi Bozbulut N, Teker Duztas D, Altug Demirol H, Celtik C, Gungor O, Demiroren K, Uncuoglu Aydogan A, Bekem O, Arslan Z, Cakir M, Ekici A, Uyar Aksu N, Ecevit C and Erdogan S (2025) Use of a specialized peptide-based enteral formula containing medium-chain triglycerides for enteral tube feeding in children with cerebral palsy and previous tube feeding intolerance on standard enteral formula: a prospective observational TolerUP study. Front. Pediatr. 13:1448507. doi: 10.3389/fped.2025.1448507
Received: 13 June 2024; Accepted: 15 January 2025;
Published: 12 February 2025.
Edited by:
Andrew S. Day, University of Otago, New ZealandReviewed by:
Stephanie Brown, University of Otago, New ZealandCopyright: © 2025 Kansu, Kutluk, Caltepe, Arikan, Urganci, Tumgor, Yuce, Tuna Kirsaclioglu, Demir, Demirbas, Usta, Yavuz, Demirtas Guner, Gumus, Dalgic, Dogan, Gerenli, Kocamaz, Gulerman, Sag, Alptekin Sarioglu, Eksi Bozbulut, Teker Duztas, Altug Demirol, Celtik, Gungor, Demiroren, Uncuoglu Aydogan, Bekem, Arslan, Cakir, Ekici, Uyar Aksu, Ecevit and Erdogan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aydan Kansu, YXlkYW5rYW5zdUBnbWFpbC5jb20=
†Present Addresses: Gunsel Kutluk,Department of Pediatric Gastroenterology, University of Health Sciences Basaksehir Cam and Sakura City Hospital, Istanbul, Türkiye
Arzu Meltem Demir,Department of Pediatrics, Division of Pediatric Gastroenterology, Hepatology and Nutrition, Ankara University Faculty of Medicine, Ankara, Türkiye
Halil Kocamaz,Department of Pediatrics, Division of Pediatric Gastroenterology, Dicle University Faculty of Medicine, Diyarbakir, Türkiye
Zeynep Arslan,Department of Developmental-Behavioral Pediatrics, Bilkent City Hospital, Ankara, Türkiye
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.