
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr., 25 February 2025
Sec. Neonatology
Volume 13 - 2025 | https://doi.org/10.3389/fped.2025.1416027
This article is part of the Research TopicNeonatal Outcomes - What About Sex, Race(ism) And Social Determinants of Health?View all 11 articles
Introduction: Early newborn mortality, morbidity, and long-term health outcomes are significantly predicted by birth weight. Many babies are born underweight in Ethiopia, but few case–control studies have previously examined the risk variables associated with khat consumption and low birth weight (LBW). Therefore, the aim of this study was to identify maternal khat use and maternal sociodemographic and obstetric risk factors associated with LBW in the Halaba Kulito General Hospital, southern Ethiopia.
Methods: A hospital-based case–control study design was used on 334 neonates (111 cases and 223 controls) at Halaba General Hospital in Halaba Zone, southern Ethiopia, from 01 October 2023 to 27 February 2024. A consecutive sampling method was used to select both the cases and controls. Bi-variable and multivariable logistic regression models were fitted using Stata 14.0 to estimate the effect of maternal khat use and other factors on low birth weight. A p-value of <0.05 was considered a significant difference in low birth weight between the cases and controls.
Results: We found that the mean age of the cases and controls at birth was 25.4 ± 4.57 years and 24.2 ± 3.96 weeks, respectively. Illiteracy [adjusted OR (AOR) = 3.7, 95%CI 1.34, 10.45], rural residence (AOR = 4.1, 95%CI 1.51, 11.35), gestational age <37weeks (AOR = 16.5, 95%CI 7.05, 38.55), maternal mid-upper arm circumference (MUAC) <23 cm (AOR = 4.7, 95%CI 1.89, 11.65), weight gain <12 kg (AOR = 4.8, 95%CI 1.22, 18.59), monthly khat use (AOR = 9.5, 95%CI 2.13, 41.98), weekly khat use (AOR = 11.1, 95%CI 3.69, 33.40), and daily khat use (AOR = 14.1, 95%CI 4.74, 42.03) were the determinant factors for delivering a newborn with low birth weight.
Conclusion: The evidence from this study suggests that illiteracy, rural residence, gestational age <37weeks, maternal MUAC <23 cm, weight gain <12 kg, monthly khat use, weekly khat use, and daily khat use were independent predictors of low birth weight. Suggested strategies involve the early identification and management of identified modifiable variables. We recommend that stakeholders in khat control commit to providing health education and awareness, incorporating khat use among women in the khat control policy, and designing interventions for the cessation of khat use among women.
While for most women, pregnancy and childbirth are happy experiences, there are instances when they result in unfavorable birth outcomes (1). Unfavorable birth outcomes are complex and mostly consist of preterm birth, stillbirth, low birth weight (LBW), macrosomia, congenital anomalies, and infant/neonatal death (2).
Particularly in low- and middle-income nations, LBW is a serious public health concern (3). It increases the rate of newborn and infant deaths and impairment and adds considerably to the burden of childhood diseases (4). A newborn weighing less than 2.5 kg is referred to as LBW, and this is assessed immediately after birth (5). Babies born with LBW may have cerebral palsy, cognitive impairments, motor disabilities, and other psychiatric and behavioral problems (6–9).
While preterm delivery and intrauterine growth retardation are thought to be the causes of LBW, the pathophysiology of LBW is uncertain. The prevalence of LBW in Ethiopia ranges from 8% (10) to 55.9% (11). Some facility-based case–control studies have been done in Ethiopia to identify the determinants of LBW (4 12–16).
Globally, more than 20 million infants are born annually, and 15.5% are born with LBW. Of these, 95.6% are from developing countries. Compared to industrialized countries, the prevalence of LBW in poor countries is more than 20 times higher (17). Approximately 17 million infants are born with LBW in developing countries annually. Regional estimates of LBW include 28% in South Asia, 9% in Latin America, and 13% in sub-Saharan Africa (18). According to an in-depth analysis of the Ethiopian Demographic and Health Survey (EDHS) 2016, the prevalence of LBW in Ethiopia was approximately 29.1% (19). According to a study conducted in Tigray, Ethiopia, the prevalence of LBW is 6.3% in urban areas and 9.9% in rural areas (20).
Many studies have found numerous risk factors for low birth weight. In central Tigray, the sex of the neonate, fewer than four antenatal care (ANC) visits, unplanned pregnancy, and maternal food intake during pregnancy were associated with LBW (21). A study conducted at Gondar University Hospital found that low birth weight was mostly associated with pregnancy-induced hypertension, malaria during pregnancy, female newborns, and a gestational age of less than 37 weeks (22).
A number of cross-sectional and experimental investigations have found that khat consumption during pregnancy can affect pregnancy outcomes. A study of 1,141 Yemeni pregnant women found that those who chewed khat had more LBW neonates than those who did not (23). However, this study showed that there was no increase in congenital abnormalities of neonates and stillbirths born to khat-consuming mothers. Some Ethiopian studies have also shown an association between khat consumption during pregnancy and negative pregnancy outcomes (24–29). A case–control study conducted in the Bale Zone revealed a positive significant association between LBW and khat chewing. The results showed that the odds of having a low-birth-weight baby among mothers who had a history of khat chewing were six times higher than that among non-consumers (24). Similar to this, recent case–control research carried out in Jimma revealed that the likelihood of low birth weight was 12 times higher in neonates from khat-chewing mothers than in neonates from non-consumers (25).
In Ethiopia, only a few analytical epidemiological researches on the association between khat consumption during pregnancy and low birth weight have been conducted. Therefore, we conducted an unmatched case–control study that was designed to measure the association between khat consumption during pregnancy and other determinants and delivering a baby with low birth weight at Halaba Kulito General Hospital, South Ethiopia.
An institution-based unmatched case–control study was conducted in Halaba Kulito General Hospital in southern Ethiopia from 01 October 2023 to 27 February 2024. The hospital was established in 2000 and upgraded to the status of a general hospital in 2022. It is located in Halaba, one of the four districts in the Halaba Zone. The zone has five urban and 79 rural “kebeles” (the lowest administrative division in Ethiopia).
Two hospitals, four clinics, 14 health centers, and 72 health posts provide healthcare services to the population of 301,658 (150,113 men and 151,545 women). Halaba Kulito Hospital provides 24-h uninterrupted healthcare services, including specialized services for both insured and uninsured patients from diverse ethnic and socioeconomic backgrounds. It serves as one of the referral centers for the primary-level healthcare facilities in the rural and urban communities of the municipality.
The source population is all mothers who gave birth and neonates in pairs in Halaba Kulito General Hospital in Halaba Zone. The study population is all mothers who gave birth and neonates in pairs in the Halaba Kulito General Hospital during the study period.
• A newborn weighing less than 2,500 g is referred to as LBW (5). Mothers who gave live birth to infants who weighed less than 2,500 g were considered cases, and mothers who gave live birth to infants who weighed 2,500 g and above were considered controls.
• Mothers who gave birth to single-birth infants were included.
• Mothers who had infants with congenital anomalies were excluded from the study. In addition, mothers who were seriously ill during the data collection period were excluded. Seriously ill refers to a medical condition in which women's health is significantly compromised, often posing a risk to life or requiring intensive medical intervention.
• Those who were unable to communicate were excluded from the study.
The proportion of khat use during pregnancy, khat abuse, and khat use before pregnancy were used to determine the sample size since they were the main exposure variables. Khat use during pregnancy was chosen as an independent variable since it resulted in a higher sample size for the other computed explanatory variables. The sample size was calculated using Epi Info version 7.2.5.0 with the following assumptions: 9.8% proportion of exposure (history of khat chewing during pregnancy) among control groups with an adjusted odds ratio of 2.83 (26), 95% confidence interval (Zα/2 = 1.96), 80% power (Zβ = 0.80), and a 2:1 control-to-case ratio. After adding a non-response rate of 20% (the assumption that women might not give consent after giving birth because of negative birth outcomes, pain, or exhaustion), the total sample size was 334 (111 cases and 223 controls). Eligible cases and two consecutive controls were interviewed by data collectors stationed in each health facility delivery ward until the desired sample size was reached.
Data on maternal sociodemographic and obstetric characteristics, khat use habit, and smoking and alcohol consumption were collected using a pretested questionnaire adopted from various studies (4 12–16). For maternal khat use, case and control women were asked: “How often did you chew khat during pregnancy?” The women chose one of the following responses: every day, weekly, monthly, or never. The women who chewed khat were then asked: “On the days that you chew, how many bundles of khat would you usually have? Women who chewed khat were also asked about the duration of their khat chewing sessions and their simultaneous use of khat and a water pipe.” Obstetric characteristics [gravidity, parity, ANC follow-up, iron-folic acid supplementation, pregnancy-induced hypertension, mode of delivery, and gestational age] were collected from the women's medical record card and delivery summary (Supplementary File S1).
Based on the patients’ medical record numbers, the required medical record cards were drawn from the card room. In addition to the interview and medical record review, the weight of the newborns was measured after birth. The weights of the newborns were measured within 1 h after delivery using a balanced digital Seca scale (Germany), and basic newborn care was provided. The scales were calibrated, and the reading on each scale was reset to zero prior to measuring each newborn. One trained BSc midwife working outside the respective health facilities conducted the interviews, document review, and anthropometric measurements. The data collection procedure was supervised by one MSc-certified midwife. The interviewers were blinded to the case/control status of the participants. The interview was held in a separate room after the woman was stabilized and ready to be discharged. The two respective controls and the cases were interviewed by the same interviewer.
The questionnaire was first prepared in English and translated into the local language, Amharic, and then back to English by independent language experts to check its consistency. A pre-test was conducted on 17 participants out of the study setting, and necessary corrections were made accordingly. The case-to-control ratio used in the pre-test was 1:2 (6 cases and 11 controls). Four days of training were provided to the data collectors and supervisors on the objective of the study, confidentiality of information, respondents` rights, and data collection procedure prior to data collection. Weighing scales were checked and adjusted to zero to check the validity of the measurements. Continuous follow-up and supervision of data collection were conducted by the supervisors. The collected data were checked for completeness, accuracy, and clarity by the investigator and supervisors on a daily basis. Appropriate measures were taken to ensure completeness before data entry.
The data were checked for completeness and inconsistencies and then cleaned and analyzed in Stata (version 14.0). A descriptive analysis was carried out by calculating the mean and standard deviation for normally distributed continuous variables and proportions for categorical variables. The normality of the continuous variables was checked using the Kolmogorov–Smirnov test. Primarily, we conducted bi-variable logistic regression to select variables. The variables that were significant at a p-value of 0.25 in the bi-variable logistic regression analysis were candidate variables for the multivariable analysis. A multivariable binary logistic regression analysis was conducted to evaluate the association between khat chewing and adverse birth outcomes after adjusting for confounding variables. The results are presented as adjusted odds ratios (AORs) with 95% CI, which express the magnitude of the effect of each khat-related factor on the outcome relative to the reference category. Variables that were significant at a p-value of 0.05 and 95% CI in the multivariable logistic regression analysis were considered to be the determinant factors of delivering a baby with low birth weight. Model fitness was tested with the Hosmer–Lemeshow goodness of fit test and omnibus tests of model coefficients. Variance inflation factor and tolerance test were also used to check multicollinearity.
A total of 334 study participants (111 were cases and 223 were controls) were enrolled, resulting in a response rate of 100%. The mean age of the cases and controls was 25.4 ± 4.57 years and 24.2 ± 3.96 years, respectively. Furthermore, 58% of the cases and 30.5% of the controls had no formal education. Nearly similar proportions of the cases (68.5%) and controls (61.4%) were Muslim. Regarding place of residence, more than half (56.8%) of the cases and 13.4% of the controls were resident in rural areas. With respect to occupation status, more than two-thirds of the cases (63.1%) and controls (66.4%) were housewives (Table 1).
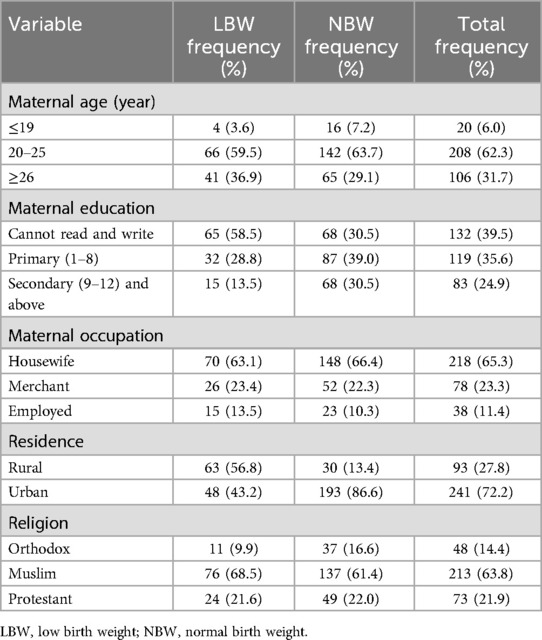
Table 1. Sociodemographic characteristics of mothers who gave birth at Halaba Kulito General Hospital, South Ethiopia.
All the mothers were non-smokers and non-water pipe smokers. The proportion of assisted emergency cesarean section (CS) was 21.6% in the cases, which was higher than the proportion in the controls (9.0%). The proportion of mothers who had four or more ANC visits among the controls was 67.3%, which was higher than that among the cases (30.6%) during the current pregnancy. A higher proportion of cases (26.1%) had a short inter-pregnancy interval than the controls (18.8%). Among the cases, 26.1% of mothers had less than a 2-year gap between the current and previous pregnancies.
Nearly similar proportions of cases (46.6%) and controls (45.0%) were multigravida, while 23.4% of the case group and 28.3% of the control group were primigravida. The majority of the women, 96.4% of the cases and 99.1% of the controls, had no history of pregnancy-induced hypertension. More than half of the cases (53.2%) and 15.2% of the controls had a mid-upper arm circumference (MUAC) of <23 cm. The majority of the participants, 90.1% of the cases and 97.3% of the controls, had no history of abortion before the preceding birth. Finally, 6% of the cases and 2.2% of the controls consumed alcohol during pregnancy (Tables 2 and 3).
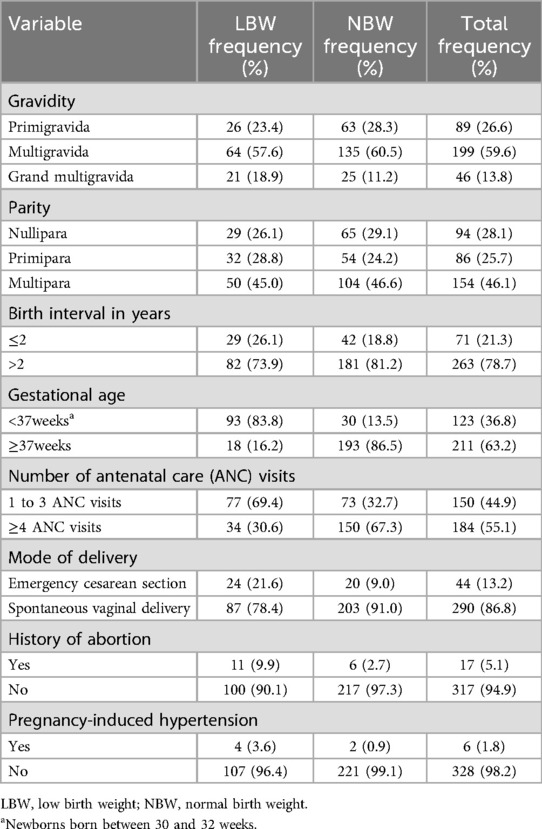
Table 2. Obstetric and health-related characteristics of mothers who gave birth at Halaba Kulito General Hospital, South Ethiopia.
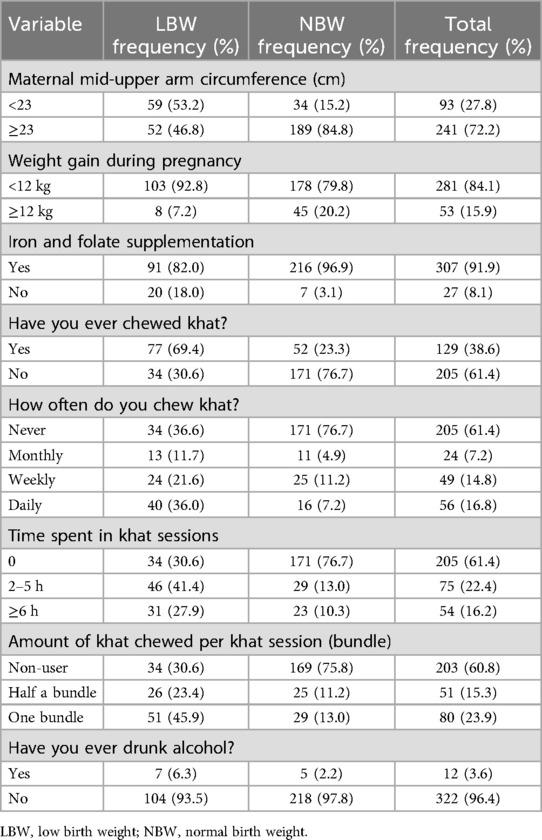
Table 3. Nutritional and substance use characteristics of mothers who gave birth at Halaba Kulito General Hospital, South Ethiopia.
Concerning khat use during pregnancy, a higher proportion of case mothers used khat during pregnancy compared to control mothers (69.4% vs. 23.3%). Furthermore, 11% of the cases and 4.9% of the controls were monthly khat users, while weekly and daily khat use was recorded for 21.6% of the cases and 11.2% of the controls, and 36.0% of the cases and 7.2% of the controls, respectively. Approximately 23% of the cases and 11.0% of the controls used half a bundle of khat per session. Likewise, a higher proportion of cases (45.9%) used one bundle of khat per session than the controls (13.0%). Moreover, 41% of the cases and 13% of the controls chewed khat for 2–5 h per session. Finally, the proportion of women who chewed khat for more than 6 hours per session in the case group was higher than that in the control group (27.9% vs. 10.3%) (Table 3).
As shown from the results of the bi-variable analysis in Table 4, 16 variables showed a significant association with low birth weight at a 25% level of significance. During the bi-variable analysis of the sociodemographic factors, maternal age ≥26 years [COR = 2.5; (95%CI: 0.79, 8.07)], illiteracy [COR = 4.3; (95%CI: 2.22, 8.21)], and rural residence [COR = 8.4; (95%CI: 4.93, 14.45)] were found to be associated with low birth weight at a 25% level of significance. However, marital occupation and religion were not significantly associated with low birth weight.
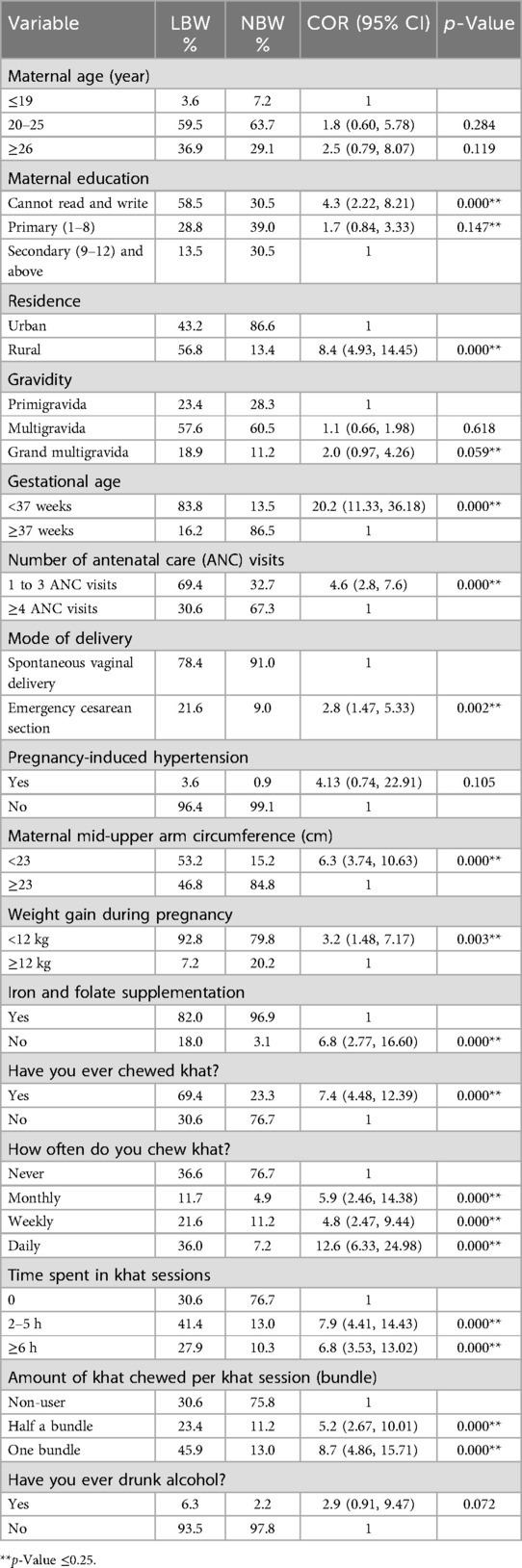
Table 4. Candidate variables for multivariable binary logistic regression to identify determinants of LBW for newborns delivered in Halaba Kulito General Hospital, Halaba Zone, South Ethiopia.
With regard to maternal obstetric and health-related factors, gravidity of five and more [COR = 2.0; (95%CI: 0.97, 4.26)], gestational age of <37 weeks [COR = 20.2; (95%CI: 11.33, 36.18)], between 1 and 3 ANC visits [COR = 4.6; (95%CI: 2.8, 7.6)], emergency CS ([COR = 2.8; (95%CI: 1.47, 5.33)], pregnancy hypertension [COR = 4.13; (95%CI: 0.74, 22.91)], maternal MUAC <23 [COR = 6.3; (95%CI: 3.74, 10.63)], weight gain during pregnancy <12 kg [COR = 3.2; (95%CI: 1.48, 7.17)], without iron supplementation [COR = 6.8; (95%CI: 2.77, 16.60)], maternal khat use [COR = 7.4; (95%CI: 4.48, 12.39)], monthly khat use [COR = 5.9; (95%CI: 2.46, 14.38)], weekly khat use [COR = 4.8; (95%CI: 2.47, 9.44)], daily khat use [COR = 12.6; (95%CI: 6.33, 24.98)], time spent in khat sessions 2–5 h. [COR = 7.9; (95%CI: 4.4, 14.43)], time spent in khat sessions ≥6 h [COR = 6.8; (95%CI: 3.53, 13.02)], half a khat bundle chewed per session [COR = 5.2; (95%CI: 2.67, 10.01)], one khat bundle chewed per session [COR = 8.7; (95%CI: 4.86, 15.71)], and alcohol use [COR = 2.9; (95%CI: 0.91, 9.47)] were significantly associated with low birth weight in the bi-variable analysis. Whereas parity, birth interval with previous birth, and previous history of abortion were not significantly associated with low birth weight (Table 4).
After multivariable logistic regression analysis and controlling for possible confounders, the factors found to be significantly associated with the delivery of LBW babies were maternal education, residence, gestational age, maternal MUAC, weight gain during pregnancy, and frequency of khat use. However, maternal age, iron supplement use, mode of delivery, number of ANC visits, gravidity, and alcohol use were not significantly associated with the delivery of LBW babies. The likelihood of delivery of an LBW baby among women who had no formal education was approximately four times higher compared to those who had at least a secondary education (AOR = 3.7, 95%CI: 1.34, 10.45). Accordingly, the odds of having a low birth weight baby were four times higher among women who lived in rural areas as compared with women who lived in urban areas (AOR = 4.1, 95%CI 1.51, 11.35).
Concerning gestational age, the neonates delivered before 37 weeks of gestational age were 16 times more likely to have low birth weight than neonates delivered at ≥37 weeks of gestational age (AOR = 16.5, 95% CI 7.05, 38.55). Moreover, the odds of having a low birth weight baby among women whose MUAC was less than 23 cm were approximately five times (AOR = 4.7, 95%CI 1.89, 11.65)] higher compared to women whose MUAC was ≥23 cm. Mothers who gained <12 kg during pregnancy were at higher risk of giving birth to a low birth weight baby as compared to mothers with those who gained ≥12 kg (AOR = 4.8, 95%CI 1.22, 18.59). Regarding khat use during pregnancy, the odds of having a low birth weight baby were increased among women who used khat monthly (AOR = 9.5, 95%CI 2.13, 41.98), weekly (AOR = 11.1, 95%CI 3.69, 33.40), and daily (AOR = 14.1, 95%CI 4.74, 42.03) as compared to women who did not use it during pregnancy (Table 5).
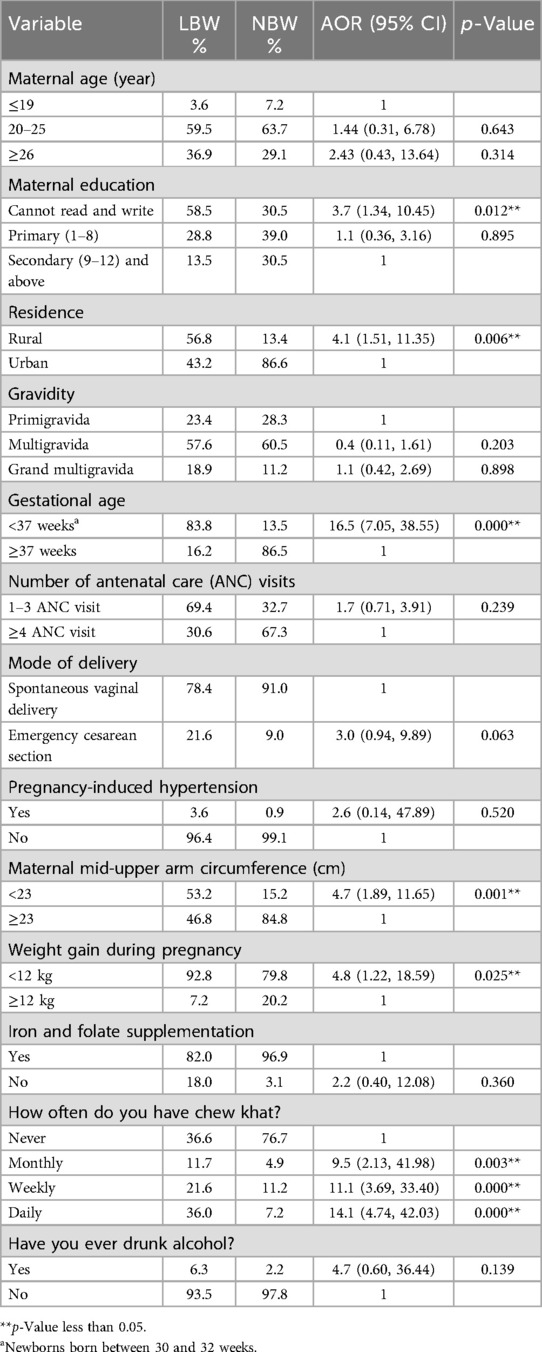
Table 5. Multivariable binary logistic regression to identify determinants of LBW, Halaba Zone, Ethiopia.
An unmatched case–control study was conducted among women who delivered in Kulito General Hospital in southern Ethiopia to determine whether maternal khat use and other factors were associated with the delivery of an LBW baby. This study has identified some sociodemographic, obstetric, khat use-related, and lifestyle-related factors that are associated with the delivery of LBW babies in the study area.
This study found that some sociodemographic factors have a negative impact on the weight of newborns. Maternal age is regarded as an important determinant of healthy outcomes in pregnancy. This study demonstrated no statistical association between maternal age and low birth weight, which conflicts with a study conducted in Nepal that revealed a higher risk of delivering a low birth weight baby by mothers aged less than 20 and more than 30 years (30).
Furthermore, mothers who resided in rural areas were more likely to deliver LBW babies. This finding is in agreement with studies conducted in Tanzania (31) and Ghana (32). The disparity could be attributed to poor rest and constant hard labor during pregnancy among mothers in rural areas. However, this result contrasts with research conducted in the Jimma Zone, Ethiopia (33), and Bangladesh (34) where the risk of delivering low birth weight babies was found to be significantly higher in mothers who resided in urban areas than those living in rural areas. It appears that these results may be attributed to the urban women's attitude towards antenatal care, their interpregnancy gap, and iron and vitamin supplements throughout pregnancy (34).
Other research carried out in similar circumstances found that LBW was more likely among women who gained inadequate gestational weight than among women who obtained enough weight (35). Women who gained less than 12 kg during pregnancy had a three-fold higher risk of delivering an LBW baby compared to women whose weight gain was 12 kg or above. The results reported are identical to the studies conducted in Bangladesh (36) and Mozambique (37). Weight gain during pregnancy is impeded by ill health, poor sanitation, and an inadequately balanced diet, which ultimately hampers the normal growth and development of the baby.
Another factor identified in this study was the MUAC of the mother. Mothers whose MUAC measurement was below 23 cm had a higher risk of giving birth to LBW newborns than mothers whose MUAC measurement was ≥23 cm. This finding was consistent with studies conducted in Addis Ababa (38); Sidama, southern Ethiopia (39); Kersa, Oromia, Ethiopia (40); and Sawula, southern Ethiopia (41). A systematic review of 12 longitudinal studies indicated that 50% of the studies investigated the association between low maternal MUAC and LBW babies, and all of these reported a considerably elevated risk of LBW among mothers with low MUAC during pregnancy (42).
Antenatal consumption of various substances has been recognized to be significant (43). The WHO Expert Committee on Drug Dependence (ECDD) critical review results showed that substance use during pregnancy may have different obstetric effects, including low birth weight (44). Our study showed that the odds of having an LBW baby were increased among women who used khat monthly, weekly, and daily compared to women who did not use it during pregnancy. Consistent with this conclusion, studies from Africa and the Middle East show a connection between khat consumption and low birth weight (45–47). In a recent systematic review and meta-analysis, the pooled odds of giving birth to an LBW baby among mothers who used khat during pregnancy were three times higher than the non-users (48). This relationship between antenatal khat usage and LBW may be attributed to the sympathomimetic activity of cathinone, the primary element of khat responsible for its vasoconstrictive effects, which complicate pregnancy and birth outcomes. The vasoconstrictive effects include maternal tachycardia, preeclampsia, decreased placental blood flow, and fetal hypoperfusion, leading to intrauterine fetal hypoxia and limited fetal growth (49).
An experimental animal investigation found a reduction in placental blood flow due to vasoconstriction in the uteroplacental vessels among khat-fed animals compared to controls (50), which may have led to fetal growth restriction. The normal growth of the unborn fetus throughout intrauterine life is highly dependent on the healthy growth and appropriate attachment of the umbilical cord to the placenta (51). Abnormal cord insertion (marginal), abnormal umbilical cord coiling, and true umbilical cord knots were found to be significantly more prevalent among births in khat user cohorts compared to the births of their non-khat user counterparts (52).
Khat also has an anorectic effect (53–55), which could lead to decreased food intake by the pregnant woman. Chewing khat may diminish the appetite of pregnant mothers; therefore, pregnant mothers who chew khat may consume less, which may significantly decrease the nutrients for the unborn fetus and hence influence its growth. In a similar manner, an experimental study indicated that there were significant reductions in fetal weight and crown-rump length at different amounts of khat consumption (56). Furthermore, pregnant mothers who chew khat, and even those who are poor, may prioritize purchasing khat, and not have enough nutritional foods at home and thus consume less food, which may not meet the needs of the unborn fetus, affecting its growth.
The selection of cases and controls was based on the records of the maternal and neonatal register, therefore, it is less likely that this study has misclassification biases in both the exposure and case–control categories. The most important limitation of the current study lies in the fact that the data used for the analyses were primarily collected for routine healthcare services and not for research purposes or for a specific intervention. Errors may have occurred during the documentation of the records. Another limitation of our study is that we were unable to identify whether LBW was largely caused by gestational duration or khat consumption. Future research should investigate this association by including more specific data on gestational age and other relevant variables.
Furthermore, the study analyzed data from one hospital, and the findings may not be generalizable to mothers who attended other hospitals or those who delivered at home. Another limitation of this study was that most of the information was self-reported; therefore, it was prone to reporting bias. However, we provided extensive training to our data collectors to retrieve participant's information as accurately as possible. Ideally, serum cathinone levels would have been a better measure; however, it was not possible to obtain blood samples in our study.
This study showed that sociodemographic, nutritional, obstetric, and maternal khat use factors were significantly associated with delivering an LBW baby. Rural residency, women who had no formal education, neonates delivered before 37 weeks of gestational age, women whose MUAC was less than 23 cm, and mothers who gained <12 kg were determinants of LBW. Our study underscores the importance of antenatal care and health education about the effects of khat use and other factors during pregnancy that may lead to LBW.
We recommend engagement with stakeholders in khat control to provide health education and awareness, incorporate khat use among women in khat control policy, and design interventions for khat use cessation among women. Specifically, the Ministry of Health should develop guidelines that incorporate the effect of khat on maternal health and newborn health outcomes. Additionally, health workers and local community and religious leaders should emphasize the provision of health education regarding the damage resulting from pregnant mothers chewing khat, with a special focus on the effect on their fetuses. Further studies, preferably prospective cohort studies that include evaluation of khat use at each trimester and psychological factors, are needed to identify and characterize the underlying causes of LBW in Halaba and elsewhere.
The original contributions presented in the study are included in the article/Supplementary Material, and further inquiries can be directed to the corresponding author.
The studies involving humans were approved by the Wolaita Sodo University's Institutional Research Ethics Review Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. All participants provided written informed consent. In the case of children under the age of 18, verbal consent was sought from their families, followed by assent from the participant/minor. To maintain confidentiality, the information received from the participant was saved in a file that did not reveal the individual's identity. Women who were unaware of the hazards associated with khat usage were provided health information about the effects of khat on health and social interaction. Women who frequently used khat were instructed to develop health-seeking behaviors, and therefore, an effective link to healthcare services was established.
BW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. TD: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. EW: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Arbaminch College of Sciences provided funding for this study. However, the sponsoring institution had no part in the study's design, data collection, analysis, interpretation, or article writing.
We would like to acknowledge the support of the administrator of Kulito General Hospital in facilitating the data collection process.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1416027/full#supplementary-material
AOR, adjusted odds ratio; COR, crude odds ratio; CI, confidence interval; EDHS, Ethiopian Demographic and Health Survey; LBW, low birth weight; MUAC, mid-upper arm circumference; SD, standard deviation; VIF, variance inflation factor; IUGR, intrauterine growth retardation; ECCD, Expert Committee on Drug Dependence; NBW, normal birth weight; ANC, antenatal care; WHO, World Health Organization.
1. Lawn JE, Lee AC, Kinney M, Sibley L, Carlo WA, Paul VK, et al. Two million intrapartum-related stillbirths and neonatal deaths: where, why, and what can be done? Int J Gynaecol Obstet. (2009) 107:S5–S19. doi: 10.1016/j.ijgo.2009.07.016
2. Hornstra G, Uauy R, Yang X. The Impact of Maternal Nutrition on the Offspring. Basel: Karger Medical and Scientific Publishers (2005).
3. Tshotetsi L, Dzikiti L, Hajison P, Feresu S. Maternal factors contributing to low birth weight deliveries in Tshwane district, South Africa. PLoS One. (2019) 14(3):e0213058. doi: 10.1371/journal.pone.0213058
4. Hailu LD, Kebede DL. Determinants of low birth weight among deliveries at a referral hospital in northern Ethiopia. BioMed Res Int. (2018) 2018. doi: 10.1155/2018/8169615
5. Muchemi OM, Echoka E, Makokha A. Factors associated with low birth weight among neonates born at Olkalou district hospital, central region, Kenya. Pan Afr Med J. (2015) 20(1):5. doi: 10.11604/pamj.2015.20.108.4831
6. Chang H-Y, Sung Y-H, Wang S-M, Lung H-L, Chang J-H, Hsu C-H, et al. Short-and long-term outcomes in very low birth weight infants with admission hypothermia. PLoS One. (2015) 10(7):e0131976. doi: 10.1371/journal.pone.0131976
7. Fan RG, Portuguez MW, Nunes ML. Cognition, behavior and social competence of preterm low birth weight children at school age. Clinics. (2013) 68:915–21. doi: 10.6061/clinics/2013(07)05
8. Fitzgibbons SC, Ching Y, Yu D, Carpenter J, Kenny M, Weldon C, et al. Mortality of necrotizing enterocolitis expressed by birth weight categories. J Pediatr Surg. (2009) 44(6):1072–6. doi: 10.1016/j.jpedsurg.2009.02.013
9. Mathewson KJ, Chow CH, Dobson KG, Pope EI, Schmidt LA, Van Lieshout RJ. Mental health of extremely low birth weight survivors: a systematic review and meta-analysis. Psychol Bull. (2017) 143(4):347. doi: 10.1037/bul0000091
10. Madebo T. A two year retrospective study of birth weight in Sidamo regional hospital. Ethiop Med J. (1994) 32(4):255–60.7835354
11. Korsak V. Incidence and some perinatal problems of multiple pregnancies in a central referral hospital, Addis Ababa. Ethiop Med J. (1989) 27(4):217–21.2598909
12. Baye Mulu G, Gebremichael B, Wondwossen Desta K, Adimasu Kebede M, Asmare Aynalem Y, Getahun M B. Determinants of low birth weight among newborns delivered in public hospitals in Addis Ababa, Ethiopia: case-control study. Pediatric Health Med Ther. (2020) 11:119–26. doi: 10.2147/PHMT.S246008
13. Demelash H, Motbainor A, Nigatu D, Gashaw K, Melese A. Risk factors for low birth weight in Bale zone hospitals, south-east Ethiopia: a case–control study. BMC Pregnancy Childbirth. (2015) 15:1–10. doi: 10.1186/s12884-015-0677-y
14. Desta SA, Damte A, Hailu T. Maternal factors associated with low birth weight in public hospitals of Mekelle city, Ethiopia: a case-control study. Ital J Pediatr. (2020) 46(1):124. doi: 10.1186/s13052-020-00890-9
15. Ejeta Chibsa S, Adem Hussen M, Bayisa K, Tefera Kefeni B. Determinants of low birth weight among newborns delivered at Mettu Karl comprehensive specialized hospital, southwest Ethiopia: a case–control study. Sci Rep. (2024) 14(1):4399. doi: 10.1038/s41598-024-54248-w
16. Girma S, Fikadu T, Agdew E, Haftu D, Gedamu G, Dewana Z, et al. Factors associated with low birthweight among newborns delivered at public health facilities of Nekemte town, west Ethiopia: a case control study. BMC Pregnancy Childbirth. (2019) 19(1):220. doi: 10.1186/s12884-019-2372-x
19. Alemu T, Umeta M. Prevalence and predictors of” small size” babies in Ethiopia: in-depth analysis of the Ethiopian demographic and health survey, 2011. Ethiop J Health Sci. (2016) 26(3):243–50. doi: 10.4314/ejhs.v26i3.7
20. Teklehaimanot N, Hailu T, Assefa H. Prevalence and factors associated with low birth weight in Axum and Laelay Maichew districts, north Ethiopia: a comparative cross sectional study. Int J Nutr Food Sci. (2014) 3(6):560–66. doi: 10.11648/j.ijnfs.20140306.21
21. Mumbare SS, Maindarkar G, Darade R, Yenge S, Tolani MK, Patole K. Maternal risk factors associated with term low birth weight neonates: a matched-pair case control study. Indian Pediatr. (2012) 49(1):25–8. doi: 10.1007/s13312-012-0010-z
22. Adane AA, Ayele TA, Ararsa LG, Bitew BD, Zeleke BM. Adverse birth outcomes among deliveries at Gondar university hospital, northwest Ethiopia. BMC Pregnancy Childbirth. (2014) 14:90. doi: 10.1186/1471-2393-14-90
23. Eriksson M, Ghani NA, Kristiansson B. Khat-chewing during pregnancy-effect upon the off-spring and some characteristics of the chewers. East Afr Med J. (1991) 68(2):106–11.2040229
24. Demelash H, Motbainor A, Nigatu D, Gashaw K, Melese A. Risk factors for low birth weight in Bale zone hospitals, south-east Ethiopia: a case-control study. BMC Pregnancy Childbirth. (2015) 15(264):015–0677.
25. Tesfay K, Abera M, Wondafrash M, Tesfaye M. Effect of khat use during pregnancy on the birth weight of newborn in Jimma, Ethiopia. Int J Ment Health Addict. (2019) 17(6):1432–41. doi: 10.1007/s11469-018-9888-6
26. Dendir E, Deyessa N. Substance use and birth weight among mothers attending public hospitals: a case control study. Ethiop J Health Dev. (2017) 31(1):27–35.
27. Nahla S E, Abdul-Mughni A, Dessouki A, Hassan S. Effect of the prenatal exposure of khat on the skeleton of developing rabbit embryo: morphometric and gross anatomical study. Fetal Pediatr Pathol. (2022) 41(3):381–95. doi: 10.1080/15513815.2020.1827319
28. Abd El-Aziz GS. Effect of khat administration on the intrauterine fetal growth in the rat. Ethiop J Health Sci. (1996) 6(2):66–83.
29. Yadeta TA, Egata G, Seyoum B, Marami D. Khat chewing in pregnant women associated with prelabor rupture of membranes, evidence from eastern Ethiopia. Pan Afr Med J. (2020) 36(1):3. doi: 10.11604/pamj.2020.36.1.22528
30. KC A, Basel PL, Singh S. Low birth weight and its associated risk factors: health facility-based case-control study. PLoS One. (2020) 15(6):e0234907. doi: 10.1371/journal.pone.0234907
31. Siza J. Risk factors associated with low birth weight of neonates among pregnant women attending a referral hospital in northern Tanzania. Tanzan J Health Res. (2008) 10(1):1–8. doi: 10.4314/thrb.v10i1.14334
32. Mohammed S, Bonsing I, Yakubu I, Wondong WP. Maternal obstetric and socio-demographic determinants of low birth weight: a retrospective cross-sectional study in Ghana. Reprod Health. (2019) 16(1):70. doi: 10.1186/s12978-019-0742-5
33. Tema T. Prevalence and determinants of low birth weight in Jimma zone, southwest Ethiopia. East Afr Med J. (2006) 83(7):3. doi: 10.4314/eamj.v83i7.9448
34. Azimul S, Matin A, Shabnam J, Shamianaz S, Bannerje M. Maternal factors affecting low birth weight in urban area of Bangladesh. J Dhaka Med Coll. (2009) 18(1):64–9. doi: 10.3329/jdmc.v18i1.6309
35. Gondwe A, Ashorn P, Ashorn U, Dewey KG, Maleta K, Nkhoma M, et al. Pre-pregnancy body mass index (BMI) and maternal gestational weight gain are positively associated with birth outcomes in rural Malawi. PLoS One. (2018) 13(10):e0206035. doi: 10.1371/journal.pone.0206035
36. Hassoune S, Bassel S, Nani S, Elbouri H, Zine K, Maaroufi A. Maternal factors associated with low birth weight: case-control study in a Moroccan public hospital. Pan Afr Med J. (2015) 20:303. doi: 10.11604/pamj.2015.20.303.2659
37. Osman NB, Challis K, Cotiro M, Nordahl G, Berström S. Perinatal outcome in an obstetric cohort of Mozambican women. J Trop Pediatr. (2001) 47(1):30–8. doi: 10.1093/tropej/47.1.30
38. Toru T, Anmut W. Assessment of low birth weight and associated factors among neonates in Butajira general hospital, south Ethiopia, cross sectional study, 2019. Int J Pediatr. (2020) 2020:5841963. doi: 10.1155/2020/5841963
39. Gebremedhin S, Enquselassie F, Umeta M. Independent and joint effects of prenatal zinc and vitamin A deficiencies on birthweight in rural Sidama, southern Ethiopia: prospective cohort study. PLoS One. (2012) 7(12):e50213. doi: 10.1371/journal.pone.0050213
40. Assefa N, Berhane Y, Worku A. Wealth status, mid upper arm circumference (MUAC) and antenatal care (ANC) are determinants for low birth weight in Kersa, Ethiopia. PLoS One. (2012) 7(6):e39957. doi: 10.1371/journal.pone.0039957
41. Abera Z, Ejara D, Gebremedhin S. Nutritional and non-nutritional factors associated with low birth weight in Sawula town, Gamo Gofa zone, southern Ethiopia. BMC Res Notes. (2019) 12(1):540. doi: 10.1186/s13104-019-4529-0
42. Tang AM, Dong K, Deitchler M, Chung M, Maalouf-Manasseh Z, Tumilowicz A, et al. Use of Cutoffs for mid-upper arm Circumference (MUAC) as an Indicator or Predictor of Nutritional and Health-related outcomes in Adolescents and Adults: A Systematic Review. Washington, DC: FHI (2013). p. 360.
44. World Health Organization. WHO Expert Committee on Drug Dependence: Thirty-fourth report. Geneva: World Health Organization (2006).
45. Hassan N, Gunaid A, Murray Lyon IM. Khat [Catha edulis]: health aspects of khat chewing. East Mediterr Health J. (2007) 13(3):706–18.17687845
46. Khawaja M, Al-Nsour M, Saad G. Khat (Catha edulis) chewing during pregnancy in Yemen: findings from a national population survey. Matern Child Health J. (2008) 12:308–12. doi: 10.1007/s10995-007-0231-2
47. World Health Organization. Global Nutrition Targets 2025: Stunting Policy Brief. Geneva: World Health Organization (2014).
48. Bayih WA, Belay DM, Ayalew MY, Tassew MA, Chanie ES, Feleke DG, et al. The effect of substance use during pregnancy on neonatal outcomes in Ethiopia: a systematic review and meta-analysis. Heliyon. (2021) 7(4):e06740. doi: 10.1016/j.heliyon.2021.e06740
49. Mwenda J, Arimi M, Kyama M, Langat D. Effects of khat (Catha edulis) consumption on reproductive functions: a review. East Afr Med J. (2003) 80(6):318–23. doi: 10.4314/eamj.v80i6.8709
50. Jansson T, Kristiansson B, Qirbi A. Effect of khat on uteroplacental blood flow in awake, chronically catheterized, late-pregnant Guinea pigs. J Ethnopharmacol. (1988) 23(1):19–26. doi: 10.1016/0378-8741(88)90111-0
51. Tantbirojn P, Saleemuddin A, Sirois K, Crum CP, Boyd TK, Tworoger S, et al. Gross abnormalities of the umbilical cord: related placental histology and clinical significance. Placenta. (2009) 30(12):1083–8. doi: 10.1016/j.placenta.2009.09.005
52. Wondemagegn AT, Bekana M, Bekuretsion Y, Afework M. The effect of possible mediators on the association between chewing khat during pregnancy and fetal growth and newborn size at birth in eastern Ethiopia. BMC Pregnancy Childbirth. (2024) 24(1):63. doi: 10.1186/s12884-024-06243-2
53. Al-Habori M. The potential adverse effects of habitual use of Catha edulis (khat). Expert Opin Drug Saf. (2005) 4(6):1145–54. doi: 10.1517/14740338.4.6.1145
54. Heymann T, Bhupulan A, Zureikat N, Bomanji J, Drinkwater C, Giles P, et al. Khat chewing delays gastric emptying of a semi-solid meal. Aliment Pharmacol Ther. (1995) 9(1):81–3. doi: 10.1111/j.1365-2036.1995.tb00356.x
55. Tucci SA. Phytochemicals in the control of human appetite and body weight. Pharmaceuticals. (2010) 3(3):748–63. doi: 10.3390/ph3030748
Keywords: low birth weight, khat, pregnant, determinants, Ethiopia
Citation: Wogayehu B, Demissie T, Wolka E and Alemayehu M (2025) Association between maternal khat use and other determinants and low birth weight in Halaba Zone, South Ethiopia: an unmatched case–control study. Front. Pediatr. 13:1416027. doi: 10.3389/fped.2025.1416027
Received: 11 April 2024; Accepted: 28 January 2025;
Published: 25 February 2025.
Edited by:
Rachana Singh, Tufts University, United StatesReviewed by:
Enrique Gomez-Pomar, University of Kentucky, United StatesCopyright: © 2025 Wogayehu, Demissie, Wolka and Alemayehu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Biruk Wogayehu, YmlydWs5MDB6ZWxhbGVtQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.