- 1School of Medicine and Surgery, University of Milano-Bicocca, Monza, Italy
- 2Department of Medicine and Surgery, Bicocca Bioinformatics Biostatistics and Bioimaging Research Centre-B4, University of Milano-Bicocca, Monza, Italy
- 3Biostatistics and Clinical Epidemiology, Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy
- 4Clinical Chemistry Laboratory Medicine, Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy
- 5Pediatrics, Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy
- 6Tettamanti Center, Fondazione IRCCS San Gerardo dei Tintori, Monza, Italy
Introduction: Down syndrome (DS) is linked to unique hematopoietic characteristics that affect complete blood count (CBC) parameters. Accurate reference ranges are essential for proper CBC interpretation in this population.
Methods: This retrospective study analyzed 2,627 CBCs from 481 DS patients, aged 31 days to 18 years, at a tertiary care center in Italy. Patients with significant comorbidities were excluded to ensure a homogeneous group.
Results: CBC parameters were assessed to establish age- and sex-specific reference ranges. Centile charts were developed for each parameter, and an online tool was created to allow clinicians to compare individual CBC results with the new ranges. Comparisons with the general pediatric population revealed significant differences, particularly in hemoglobin, hematocrit, and mean corpuscular volume, which were higher in DS (p < 0.001). In contrast, a significant percentage of CBCs showed white blood cell counts below the 2.5th centile of healthy controls (p < 0.001), except for the 31 days–1 year age group. A similar trend was observed for lymphocytes (p < 0.001) in the 1-18 years group.
Discussion: These newly established DS-specific reference ranges provide clinicians with a crucial tool for evaluating CBC results, potentially reducing unnecessary tests and emphasizing the need for tailored clinical assessment in managing this unique population.
Introduction
Down syndrome (DS) is the most common chromosomal aneuploidy among live births and the most frequent cause of intellectual disability related to a demonstrable microscopic chromosomal aberration (1), with an incidence of 1:700–1,000 live births and a prevalence of 1:400–3,000 in the general population (2). From a hematological perspective, patients with trisomy 21 may exhibit alterations in all hematopoietic lineages, as a consequence of the overexpression of several genes involved in the regulation of the hematopoietic system (3). In fetuses and neonates with DS, studies on hematopoiesis have shown a marked expansion of megakaryocytic and erythroid progenitors, alterations in myeloid progenitors and severe impairment in lymphocyte development (4–6). These alterations appear to persist throughout infancy, but systematic studies have not yet been conducted to confirm this assumption (6). Accordingly, newborns with DS may present with a wide spectrum of hematological disorders, including polycythemia, thrombocytopenia, neutrophilia, and lymphocytopenia (7–10). Conversely, macrocytosis and leukopenia are findings more commonly recorded among children, adolescents and adults (11–15). Macrocytosis is often regarded as the result of an increased expression of the cystathionine beta-synthase gene (CBS), leading to an increase in folate re-methylation pathways and, subsequently, to a prolonged cell cycle and increased red blood cell volume (12). Complete blood count (CBC) abnormalities, in most cases, are not associated to any other underlying pathological condition and have no clinical repercussions. Conversely, DNA mutations occurring in the setting of increased cellular proliferation in hematopoietic sites have been extensively described in children with DS, leading to pathological phenomena ranging from transient abnormal myelopoiesis in newborns (prevalence: 4%–10%) (16) to acute leukemia in infants, children and adolescents (prevalence: 3%) (17).
Given the above-mentioned specificities of children with DS, the development of syndrome-specific CBC reference ranges would prevent clinicians from labelling normal findings as pathological and, accordingly, from prescribing unnecessary biochemical monitoring. Conversely, reference values would promote timely detection of the few abnormalities deserving prompt additional assessment.
To the best of our knowledge, only two published studies have reported the distribution of CBC parameters in pediatric cohorts of individuals with DS (18, 19). As peripheral blood counts show remarkable changes along the maturation process that leads from fetal to adult erythropoiesis, the interpretation of CBC parameters is based on the comparison with reference ranges of normality drawn from the otherwise healthy pediatric population (20). The aim of this study is to outline the normal distribution of CBC values in pediatric subjects with DS, in order to identify the age- and gender-specific reference ranges for each hematological parameter and to compare them with those published for the general pediatric population (21, 22).
Methods
Data sources and patients
We designed a retrospective, observational, monocentric analysis. Eligible patients were retrieved from the pediatric outpatient Clinic of Fondazione IRCCS San Gerardo dei Tintori Hospital, Monza (Italy).
We included children and adolescents diagnosed with DS, aged between 31 days and 18 years, of both sexes and with different ethnical background. Only CBCs withdrawn in otherwise healthy children, on an outpatient basis and as a part of routine follow-up were gathered. We excluded CBCs of patients diagnosed with underlying clinical conditions, i.e., hematological or oncological diseases, cyanogenic congenital heart diseases, severe/chronic lung diseases, and severe obstructive sleep apnea syndrome. Also, CBCs assessed in the setting of infectious events, acute illnesses, emergency or following transfusional events or recent surgery were excluded. Finally, we ruled out patients treated with ongoing myelosuppressive medications. The blood counts of DS subjects with celiac disease were included in the analysis only if performed after 12 months of a gluten-free diet.
The study was conducted in accordance with the Declaration of Helsinki. Informed consent was obtained from patients and their families, and the investigation was approved by the local ethical board. All data were anonymized.
Hematological parameters
In our institution, CBC is performed annually in pediatric patients diagnosed with DS, in accordance with the guidelines of American Academy of Pediatrics (AAP). CBCs were performed between 2004 and 2023 by two different hematological analyzers: Sysmex XN-10 and Beckman Coulter DxH800. The following parameters were retrieved: red blood cells (RBC, ×1012/L), hemoglobin (HB, g/L), mean corpuscular volume (MCV, fl), hematocrit (HCT, %), white blood cells (WBC, ×109/L), absolute neutrophil count (NEU, ×109/L), absolute lymphocyte count (LYM, ×109/L), and platelets (PLT, ×109/L).
Statistical analyses
CBC parameters assessed by two different lab analyzers were compared by non parametric regression model and reported as time trendlines by cubic splines. Mean (SD), or median (interquartile ranges, IQR) were used to describe continuous variables and frequencies (percentages) for qualitative variables. Age and genders-specific CBC parameters reference ranges were estimated via regression quantiles. The lower and the upper limits of each Gaussian distribution was referred with reference to the 2.5th and 97.5th percentiles, respectively. Inequality constraints were used to ensure both monotonicity and non-crossing of the estimated quantile curves, and penalized splines were employed to model the nonlinear patterns concerning age. The distribution of each blood count parameter was graphically delineated with sex-specific nomograms, displaying the 2.5th, 10th, 25th, 50th, 75th, 90th, and 97.5th percentiles of the recorded age-specific data. Age groups were defined for each parameter, in order to establish reference ranges and compare them with those of the general pediatric population (21, 22) and with two recent studies conducted on children with DS (18, 19). A one-sample test on proportion was used to compare reference ranges of our patients with DS and healthy controls (21, 22) with respect to specific reference thresholds. The tests were two-sided, and the significance level was set at.05. Wilcoxon-test was used to compare CBCs distribution, by age groups and sexes, of our study with those of the healthy control population and DS patients from Harvey et al. (19) and those of DS patients from Garcia de la Puente et al. (18); the results were also graphically represented. The significance level used to reject the null hypothesis is 0.05. All the statistical analyses were performed with the opensource R software v.4.4.2.
Results
Study population
Four-hundred-eighty-one patients out of the 559 eligible from our Institution met the criteria for inclusion in the study. Overall, 2,627 CBCs were collected. For this cohort, the average number of CBCs for each patient was found to be 5.67 ± 3.7 SD. Of the 481 patients enrolled, 249 were males (51.8%). From an ethnical perspective, 438 (91.1%) were Caucasian, 20 (4.2%) originating from North Africa, 8 (1.6%) from Central Africa, 8 (1.6%) from Southeast Asia, 6 (1.2%) from Central/South America and 1 patient of Chinese origin. With reference genetic background, karyotype was retrievable for 451 out of 481 individuals, with the following distribution: 431 (96%) free trisomy of chromosome 21, 10 (2%) mosaicism and the remaining 10 (2%) Robertsonian translocation. As for comorbidities, 197 DS individuals were born with a non-cyanotic congenital heart defect (41%), 86 (18%) were found to have a thyroid disorder (including autoimmune conditions), 56 (11%) were diagnosed with celiac disease, 18 (3.7%) had a gastrointestinal defect, 10 (2%) with epilepsy, 4 (0.8%) with alopecia, and 2 (0.4%) with diabetes mellitus type 1.
Hematological parameters: normal distribution of values in the study population
Out of the 2,627 CBCs recorded, 1,062 were assessed by the Sysmex analyzer, and 1,565 with Beckman Coulter. The comparison by a non-parametric regression model using cubic splines demonstrated superimposable results, with satisfactory degree of agreement (Supplementary Figure S1). Figure 1 reports a graphical representation of the normal distribution of CBC parameters in the study population. The sex-specific nomograms display the 2.5th, 10th, 25th, 50th, 75th, 90th, and 97.5th percentiles of the recorded age-specific data. Finally, Table 1 presents percentiles for each parameter of CBCs in DS, categorized by age groups. The last two age groups (10–12 years and 13–18 years), corresponding to the age ranges in which the influence of pubertal development is expected, are further differentiated based on sex.
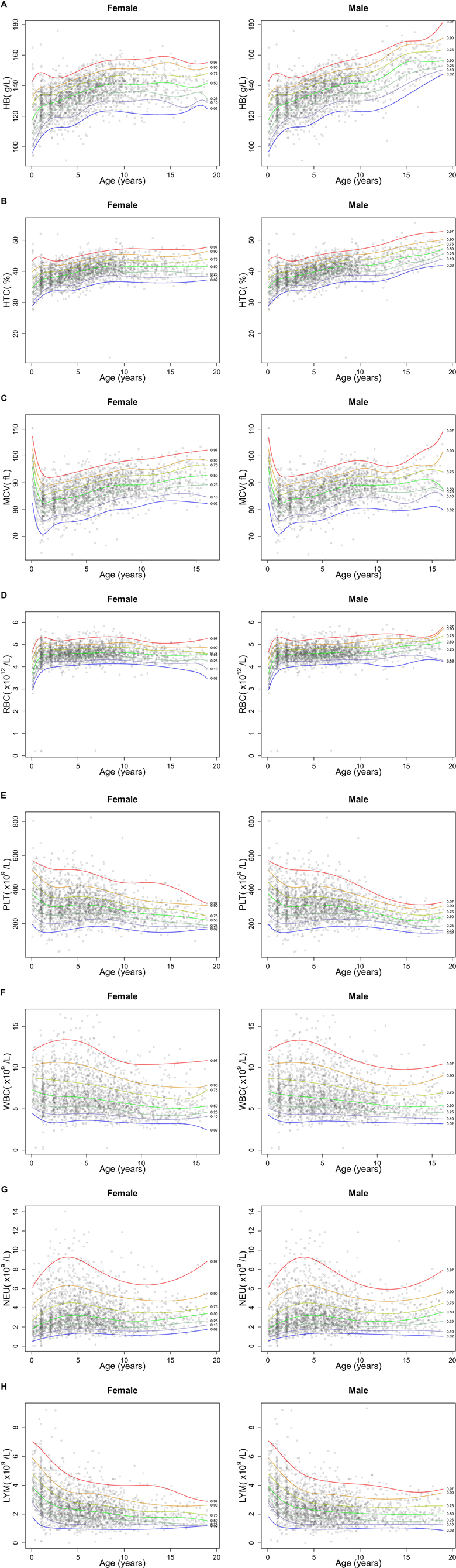
Figure 1. Graphical representation of centiles of CBC parameters in the study cohort of children and adolescents with DS. The figure shows the centile distribution for hemoglobin (HB, panel A), hematocrit (HTC, panel B), mean corpuscular volume (MCV, panel C), red blood cells (RBC, panel D), platelets (PLT, panel E), white blood cells (WBC, panel F), neutrophils (NEU, panel G) and lymphocyte (LYM, panel H).
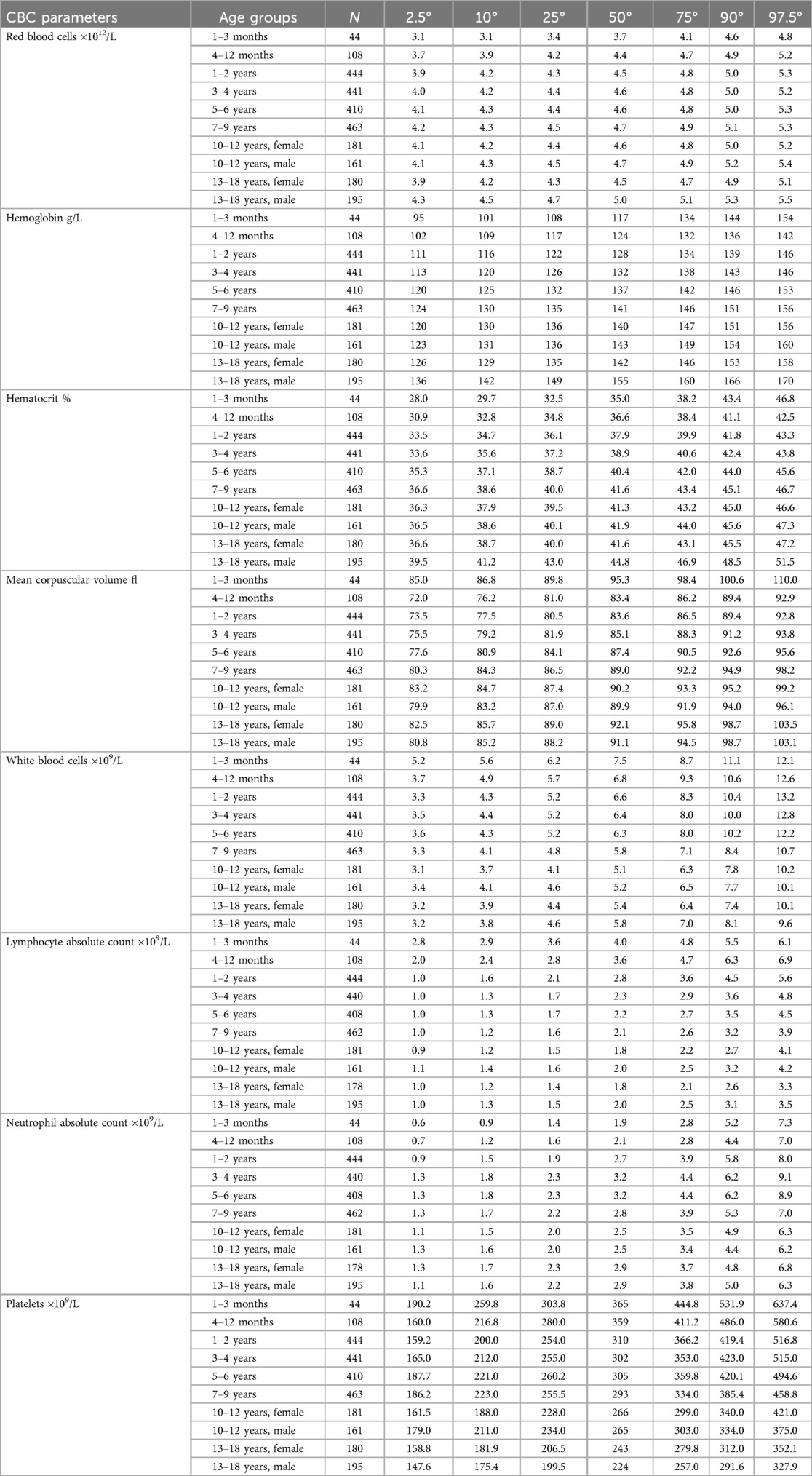
Table 1. Percentiles of the distribution of CBC parameters in the study population of Down syndrome individuals, declined by age class.
Online informatic tool to assess CBC's parameters centile
We developed a computational web tool aimed at verifying whether hemogram parameters fall within newly defined normal ranges for individuals with DS. By filling in the blanks, the tool provides the calculated centile for each determination with reference to our data and graphically plots up to 3 sequentially assessed values. The tool is available online at: https://b4-uni25-5627493duksfy852qr80fewbsn3986g43jkgkzie8.shinyapps.io/HematologyReferenceTrisomy21/.
Comparison of CBC parameters in Down syndrome vs. controls
The lower and upper limit of the new defined reference ranges (percentiles 2.5 and 97.5) of CBC parameters, categorized by age groups and sex, were compared with those of the general pediatric population (21), as depicted in Figure 2. The proportion of HB, HCT, and MCV values above the 97.5th centile in the study population was statistically greater than in the general pediatric population, as reported in Table 2. A statistically significant proportion of WBC values were below the 2.5th centile reported in DS population (p < 0.001), except for the age group 31 days–1 year. The same result was observed for LYM (p < 0.001), from 1 year to 18 years old. The proportion of NEU falling above the 97.5th centile or below the 2.5th centile was statistically greater and lower, respectively, over the timespan assessed (see Table 2 for additional details). Considering PTL, the proportion of values below the 2.5th centile for healthy children achieved statistical significance only for some of the age classes assessed. Conversely, we found no significant differences concerning the upper PTL limit between our DS groups and healthy controls.
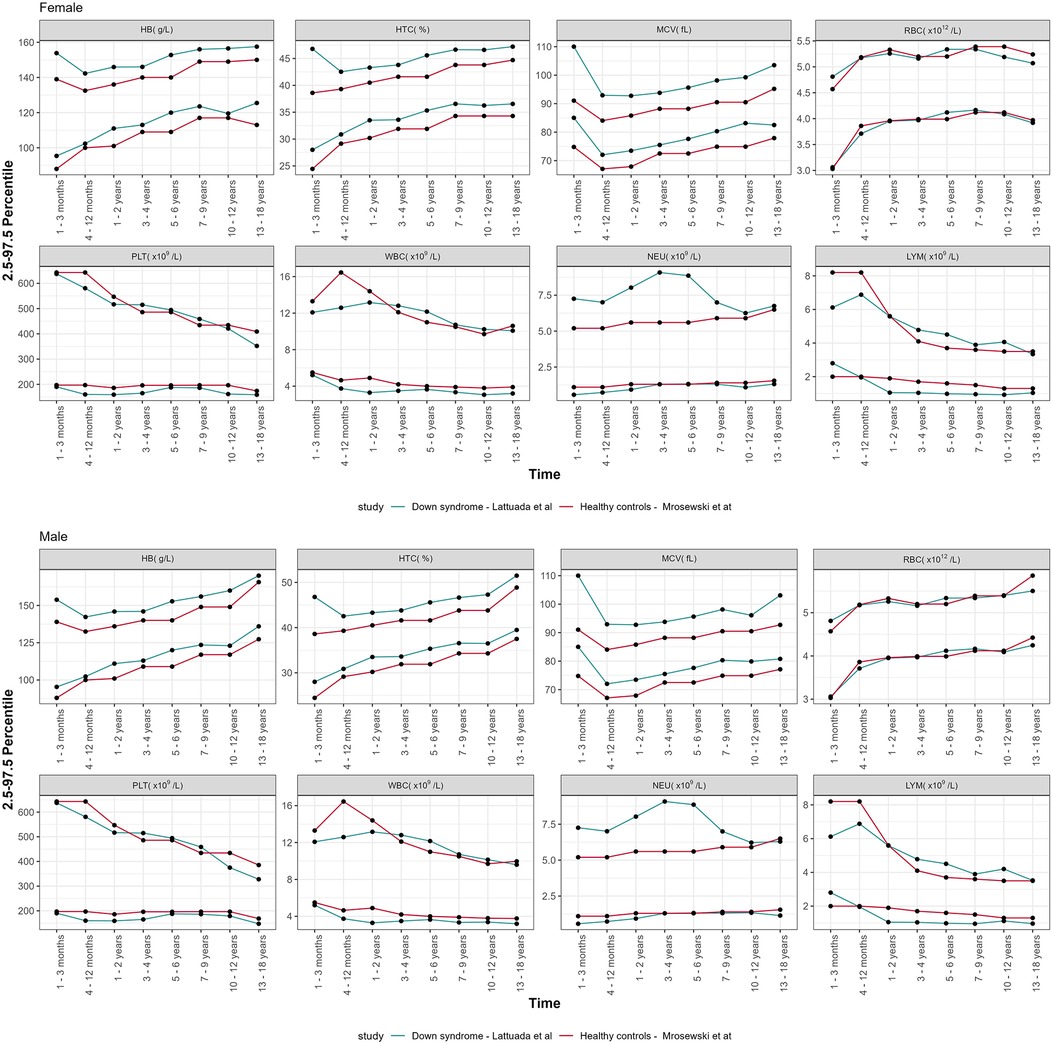
Figure 2. Comparison of the centile distribution of CBC parameters vs. healthy controls from the literature, declined by age groups and sexes.
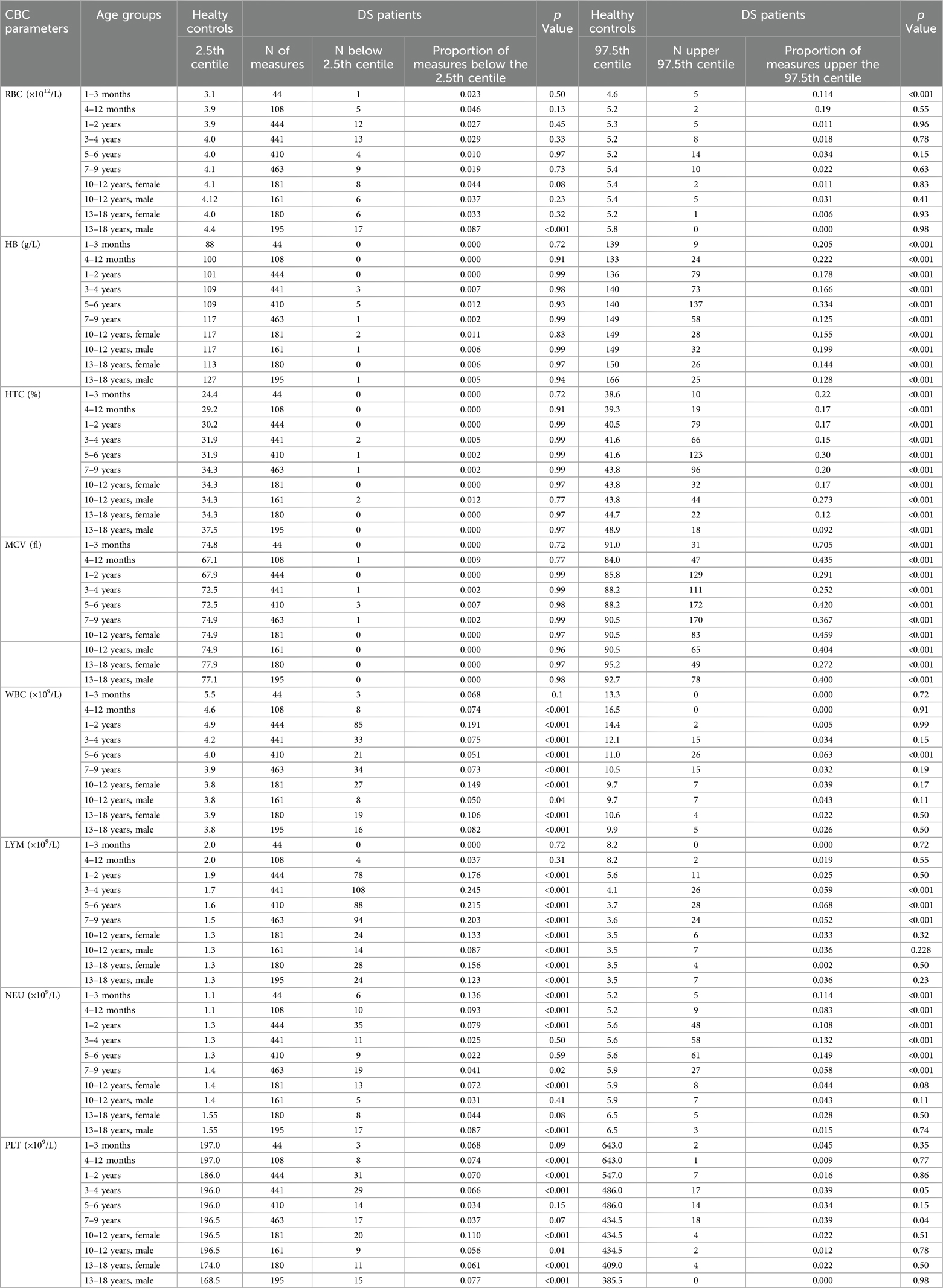
Table 2. Comparison of reference ranges of our patients with DS and healthy controls using one-sample test on proportion.
Figure 3 summarizes the comparison between our data and two recent studies conducted on children with DS (18, 19). The mean and SD for all our parameters, collected in four age groups (90 days–2 years, 2–5 years, 6–1 years, 12–18 years), for both sexes, were compared with Garcia's DS population, Harvey's DS population and Harvey's healthy controls, as reported in Table 3.
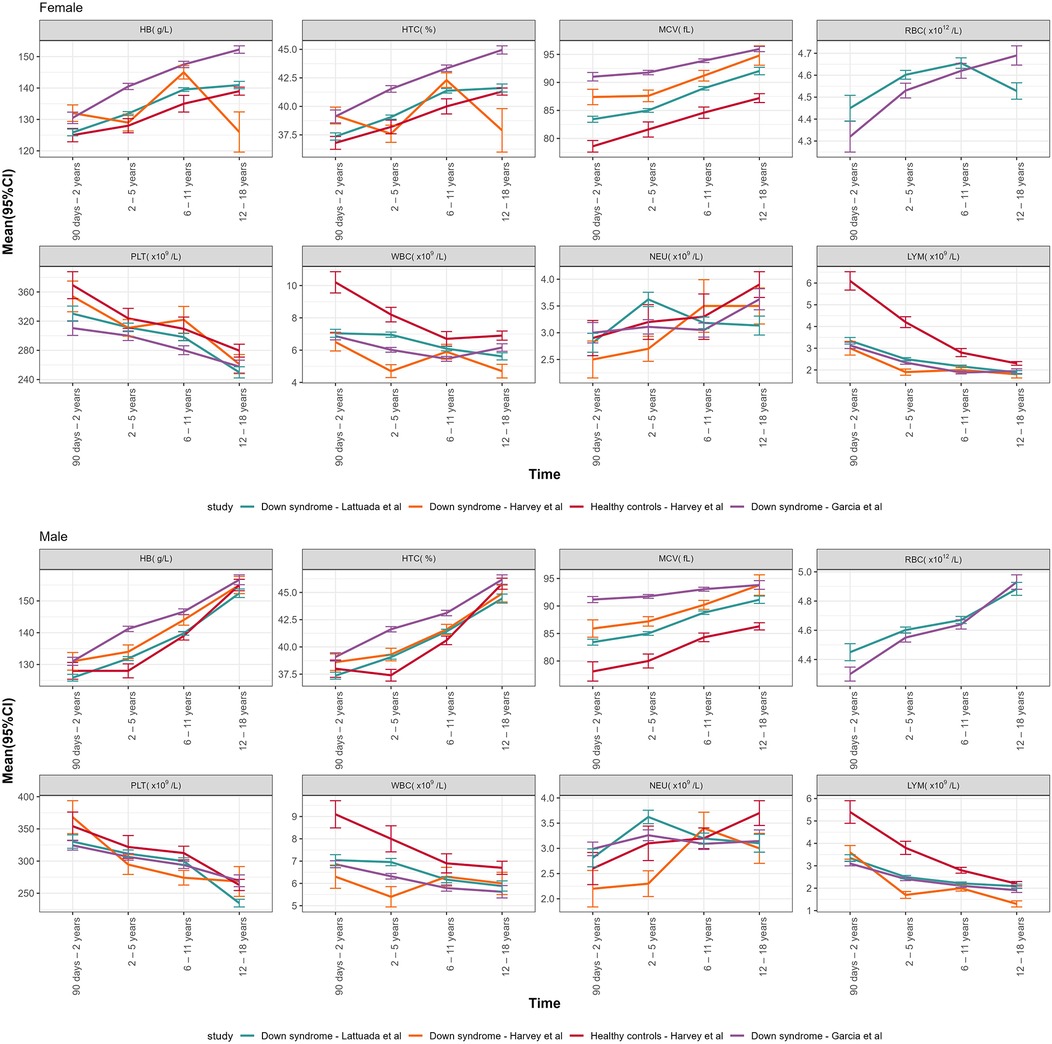
Figure 3. Graphical representation of the mean (95% CI) of CBC DS parameters of our study vs. Garcia's DS population, Harvey's DS population and Harvey's healthy controls, declined by age groups and sexes.

Table 3. Comparison of the mean and standard deviation of CBC DS parameters of our study vs. Garcia's DS population, Harvey's DS population and Harvey's healthy controls.
Discussion
In DS, hematopoiesis is affected by the over-expression of several genes involved in the regulation and maturation of the hematopoietic system (3), located on chromosome 21. In addition, the occurrence rates of abnormal hematological findings can be regarded in some patients as the direct effect of clinical comorbidities, such as cyanogenic cardiac defects or infectious diseases. One of the most deeply characterized genes is RUNX1, a transcription factor that regulates hematopoiesis and megakaryopoiesis (23). Other genes thought to be responsible for abnormal hematopoiesis include GATA1, ERG, ETS2, BACH1, TIAM1, IFNAR1, GART, SON, SOD1, HMGN1, and USP16 (3, 24, 25). In addition, several genes encoding interferon receptors are located on chromosome 21. Accordingly, trisomy 21 can lead to an increased expression of interferon-stimulated genes, resulting in a mild interferonopathy in the microenvironment of bone marrow, with inhibitory effects on hematopoietic precursors (26).
Along with clinical and anamnestic data, the availability of reference ranges is a pivotal element in the decision-making process that eventually leads to labelling biochemical findings as pathological (27). The reliability of reference intervals mostly depends on the homogeneity of the population from which they are drawn and avoiding selection biases is pivotal to outline reproducible results (28). Given the demonstrated specificities of hematopoiesis in individuals with DS, it is advisable to compare the CBC findings with syndrome-specific reference intervals.
As in pediatrics the integrated result of growth, immune system maturation, and pubertal attainment results in physiological changes of hematopoiesis over time, percentile plotted against age can be regarded as the most reliable representation of the continuous and dynamic modifications in CBC parameters (29). Therefore, we outlined the normal distribution of CBC parameters from 31 days to 18 years of age and reported the data drawn in our population with sex-specific nomograms.
As expected, males showed a progressive increase in red blood cell parameters from the onset of puberty onwards, as a result of the promoting action of testosterone on erythropoiesis (30).
The comparison between the distribution of CBC parameters in DS and healthy controls from the literature (21, 22) outlines several differences. With reference to red blood cells parameters, individuals with DS showed a statistically significant upward shift of the distribution of hemoglobin, mean cell volume and hematocrit compared to controls, while the distribution of the concentration of RBC did not statistically differ in the two cohorts for most of the age span included in the analysis. Finally, the qualitative evaluation of the trendlines of red blood cells parameters over time showed a similar pattern in DS compared to controls.
Considering white blood cells parameters, subjects with DS had a statistically significant downward shift of the lower percentile of white blood cells and lymphocytes. Neutrophils, instead, showed a significant upward shift of the 97.5° centile compared to controls.
The distribution of PLT appeared overall superimposable between our cohort and controls.
The demonstrated statistically significant specificities in the CBC findings among individuals with DS compared to controls was consistent with the data reported in pediatric cohorts of individuals with DS from Colorado (19) and Mexico (18). The comparison between averages in our study vs. Harvey vs. Garcia de la Puente vs. controls further highlights higher mean values of HB, HTC, MCV and lower mean values of PLT, WBC, LYM in the Down population.
Neutrophils, in our DS population, were significantly higher in the age group between 2 and 5 years compared to other studies on DS. It should be noted this is the hematological parameter that is mostly affected by common intercurrent events such as infectious events, typical of pre-school childhood; although we excluded infected patients, pre-clinical or asymptomatic pictures might have justified such discrepancy.
The differences observed among the various population groups with DS could be linked to the geographic origins of the compared DS group, characterized by different genetic, epigenetic and environmental factors (i.e., altitudes, diets). Furthermore, in African and Arab populations it's observed a higher prevalence of the genetic variant of the Duffy antigen receptor for chemokines (DARC), which results in benign ethnic neutropenia (31).
The strict eligibility criteria, the uniformity of data determined by the monocentric nature of the study, the wide sample size that included more than 2,000 CBCs and the creation of an informatic tool to determine centiles, represent the strengths of our analysis.
Conversely, potential limitations include the retrospective nature of the study and the potential role of common comorbidities in Down syndrome (i.e., autoimmune diseases) in affecting blood count parameters.
Individuals with DS frequently present with alterations in complete blood count parameters, mostly not related to underlying pathological conditions and asymptomatic. The identification of syndrome-specific reference intervals, that define the physiological distribution of CBC parameters in children with DS, can play a pivotal role in clinical practice. Reference data may provide clinicians with a practical guide, also supported by the development of a dedicated informatic tool designed to estimate the centile of each recorded value with reference to the above-mentioned syndrome-specific ranges. By providing an immediate comparison of real-life findings with syndrome-specific references, the tool may prevent clinicians from prescribing unnecessary investigations. On the other hand, it may prompt timely identification of altered values that deserve urgent hematological evaluation.
Overall, it is important to emphasize that, as with any other laboratory parameter, CBC values should always be integrated with physical examination, medical history, and eventual assessment of additional biochemical parameters.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Comitato etico territoriale Lombardia 3. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
ML: Data curation, Methodology, Writing – original draft. GC: Data curation, Formal Analysis, Investigation, Methodology, Software, Writing – original draft. MC: Conceptualization, Data curation, Supervision, Writing – review & editing. AL: Conceptualization, Data curation, Writing – review & editing. OM: Conceptualization, Data curation, Writing – review & editing. GF: Conceptualization, Supervision, Writing – review & editing. CF: Conceptualization, Supervision, Writing – review & editing. AB: Supervision, Writing – review & editing. AC: Conceptualization, Methodology, Supervision, Writing – review & editing. PC: Conceptualization, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The project is supported (not financially) by the European Reference Network on Rare Hematological Diseases (ERN-EuroBloodNet)—Project ID No. 10108571. ERN-EuroBloodNet is partly co-funded by the European Union within the framework of the Fourth EU Health Programme.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1510733/full#supplementary-material
References
1. Cuckle H, Benn P. Review of epidemiological factors (other than maternal age) that determine the prevalence of common autosomal trisomies. Prenat Diagn. (2021) 41(5):536–44. doi: 10.1002/pd.5822
2. Chen L, Wang L, Wang Y, Hu H, Zhan Y, Zeng Z, et al. Global, regional, and national burden and trends of down syndrome from 1990 to 2019. Front Genet. (2022) 13:908482. doi: 10.3389/fgene.2022.908482
3. Osato M, Ito Y. Increased dosage of the RUNX1/AML1 gene: a third mode of RUNX leukemia? Crit Rev Eukaryot Gene Expr. (2005) 15(3):217–28. doi: 10.1615/critreveukargeneexpr.v15.i3.40
4. Tunstall-Pedoe O, Roy A, Karadimitris A, de la Fuente J, Fisk NM, Bennett P, et al. Abnormalities in the myeloid progenitor compartment in down syndrome fetal liver precede acquisition of GATA1 mutations. Blood. (2008) 112(12):4507–11. doi: 10.1182/blood-2008-04-152967
5. Bruwier A, Chantrain CF. Hematological disorders and leukemia in children with down syndrome. Eur J Pediatr. (2012) 171(9):1301–7. doi: 10.1007/s00431-011-1624-1
6. Roy A, Cowan G, Vyas P, Roberts I. The impact of trisomy 21 on early human hematopoiesis. Cell Cycle. (2013) 12(4):533–4. doi: 10.4161/cc.23667
7. Henry E, Walker D, Wiedmeier SE, Christensen RD. Hematological abnormalities during the first week of life among neonates with down syndrome: data from a multihospital healthcare system. Am J Med Genet A. (2007) 143A(1):42–50. doi: 10.1002/ajmg.a.31442
8. Martínez-Macías FJ, Bobadilla-Morales L, Gonzalez-Cruz J, Quiles-Corona M, Corona-Rivera A, Peña-Padilla C, et al. Descriptive study of the complete blood count in newborn infants with down syndrome. Am J Med Genet A. (2017) 173(4):897–904. doi: 10.1002/ajmg.a.38097
9. Karakurt N, Uslu İ, Aygün C, Albayrak C. Hematological disturbances in down syndrome: single centre experience of thirteen years and review of the literature. Turk J Pediatr. (2019) 61(5):664–70. doi: 10.24953/turkjped.2019.05.004
10. Kim DW, Kim HR, Shin MG, Baek HJ, Kook H, Hwang TJ, et al. Distinctive hematological abnormalities in East Asian neonates and children with down syndrome. Int J Lab Hematol. (2011) 33(4):369–77. doi: 10.1111/j.1751-553X.2011.01299.x
11. Kivivuori SM, Rajantie J, Siimes MA. Peripheral blood cell counts in infants with down’s syndrome. Clin Genet. (1996) 49(1):15–9. doi: 10.1111/j.1399-0004.1996.tb04318.x
12. David O, Fiorucci GC, Tosi MT, Altare F, Valori A, Saracco P, et al. Hematological studies in children with down syndrome. Pediatr Hematol Oncol. (1996) 13(3):271–5. doi: 10.3109/08880019609030827
13. Roizen NJ, Amarose AP. Hematologic abnormalities in children with down syndrome. Am J Med Genet. (1993) 46(5):510–2. doi: 10.1002/ajmg.1320460509
14. McLean S, McHale C, Enright H. Hematological abnormalities in adult patients with down’s syndrome. Ir J Med Sci. (2009) 178(1):35–8. doi: 10.1007/s11845-008-0223-2
15. Hamaguchi Y, Kondoh T, Fukuda M, Yamasaki K, Yoshiura KI, Moriuchi H, et al. Leukopenia, macrocytosis, and thrombocytopenia occur in young adults with down syndrome. Gene. (2022) 835:146663. doi: 10.1016/j.gene.2022.146663
16. Zipursky A. Transient leukaemia—a benign form of leukaemia in newborn infants with trisomy 21. Br J Kaematol. (2003) 120(6):930–8. doi: 10.1046/j.1365-2141.2003.04229.x
17. Triarico S, Trombatore G, Capozza MA, Romano A, Mastrangelo S, Attinà G, et al. Hematological disorders in children with down syndrome. Expert Rev Hematol. (2022) 15(2):127–35. doi: 10.1080/17474086.2022.2044780
18. García de la Puente S, Flores-Arizmendi KA, Guerrero-Tapia YY, Vargas-Robledo TT, López-Santiago NC. Blood cytology in children with down syndrome. BMC Pediatr. (2022) 22(1):387. doi: 10.1186/s12887-022-03450-8
19. Harvey S, Wolter-Warmerdam K, Hickey F, Daniels D, DomBourian M, Ambruso DR. Blood counts in children with down syndrome. Pediatr Blood Cancer. (2022) 69(12):e30002. doi: 10.1002/pbc.30002
20. Bohn MK, Higgins V, Tahmasebi H, Hall A, Liu E, Adeli K, et al. Complex biological patterns of hematology parameters in childhood necessitating age- and sex-specific reference intervals for evidence-based clinical interpretation. Int J Lab Hematol. (2020) 42(6):750–60. doi: 10.1111/ijlh.13306
21. Mrosewski I, Dähn T, Hehde J, Kalinowski E, Lindner I, Meyer TM, et al. Indirectly determined hematology reference intervals for pediatric patients in Berlin and Brandenburg. Clin Chem Lab Med. (2021) 60(3):408–32. doi: 10.1515/cclm-2021-0853
22. Mrosewski I, Dähn T, Hehde J, Kalinowski E, Lindner I, Meyer TM, et al. Indirectly determined reference intervals for automated white blood cell differentials of pediatric patients in Berlin and Brandenburg. Clin Chem Lab Med. (2023) 61(6):1116–22. doi: 10.1515/cclm-2022-1265
23. Ng CEL, Yokomizo T, Yamashita N, Cirovic B, Jin H, Wen Z, et al. A Runx1 intronic enhancer marks hemogenic endothelial cells and hematopoietic stem cells. Stem Cells. (2010) 28:1869–81. doi: 10.1002/stem.507
24. Stankiewicz MJ, Crispino JD. ETS2 and ERG promote megakaryopoiesis and synergize with alterations in GATA-1 to immortalize hematopoietic progenitor cells. Blood J Am Soc Hematol. (2009) 113:3337–47. doi: 10.1182/blood-2008-08-174813
25. Adorno M, Sikandar S, Mitra SS, Kuo A, Nicolis Di Robilant B, Haro-Acosta V, et al. Usp16 contributes to somatic stem-cell defects in down’s syndrome. Nature. (2013) 501(7467):380–4. doi: 10.1038/nature12530
26. Chung H, Green PHR, Wang TC, Kong XF. Interferon-Driven immune dysregulation in down syndrome: a review of the evidence. J Inflamm Res. (2021) 14:5187–200. doi: 10.2147/JIR.S280953
27. Cattoni A, Molinari S, Capitoli G, Masera N, Nicolosi ML, Barzaghi S, et al. Thyroid function tests in children and adolescents with trisomy 21: definition of syndrome-specific reference ranges. J Clin Endocrinol Metab. (2023) 108(11):2779–88. doi: 10.1210/clinem/dgad333
28. Ritchie RF, Palomaki G. Selecting clinically relevant populations for reference intervals. Clin Chem Lab Med. (2004) 42(7):702–9. doi: 10.1515/CCLM.2004.120
29. Zierk J, Hirschmann J, Toddenroth D, Arzideh F, Haeckel R, Bertram A, et al. Next-generation reference intervals for pediatric hematology. Clin Chem Lab Med. (2019) 57(10):1595–607. doi: 10.1515/cclm-2018-1236
30. Warren AM, Grossmann M. Haematological actions of androgens. Best Pract Res Clin Endocrinol Metab. (2022) 36(5):101653. doi: 10.1016/j.beem.2022.101653
Keywords: Down syndrome, hematopoiesis, complete blood count, reference ranges, pediatric
Citation: Lattuada M, Capitoli G, Casati M, Lazzerotti A, Maglia O, Ferrari GM, Fossati C, Biondi A, Cattoni A and Corti P (2024) Reference ranges for complete blood count in children and adolescents with Down syndrome. Front. Pediatr. 12:1510733. doi: 10.3389/fped.2024.1510733
Received: 13 October 2024; Accepted: 20 November 2024;
Published: 11 December 2024.
Edited by:
Tomasz Szczepanski, Medical University of Silesia, PolandReviewed by:
Tomasz Urasinski, Pomeranian Medical University, PolandCsongor Kiss, University of Debrecen, Hungary
Copyright: © 2024 Lattuada, Capitoli, Casati, Lazzerotti, Maglia, Ferrari, Fossati, Biondi, Cattoni and Corti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martina Lattuada, bS5sYXR0dWFkYTlAY2FtcHVzLnVuaW1pYi5pdA==
†These authors have contributed equally to this work and share first authorship
‡These authors have contributed equally to this work and share senior authorship
 Martina Lattuada
Martina Lattuada Giulia Capitoli
Giulia Capitoli Marco Casati4
Marco Casati4 Andrea Biondi
Andrea Biondi Alessandro Cattoni
Alessandro Cattoni