
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Pediatr. , 21 January 2025
Sec. Pediatric Endocrinology
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1504874
This article is part of the Research Topic Insights in Pediatric Endocrinology: 2024 View all 18 articles
Pubertal onset is characterized by reactivation of the hypothalamic-pituitary-gonadal axis resulting in pulsatile gonadotropin secretion and subsequent sex steroid production. Accurate measurements of the gonadotropins and sex steroids are essential to ensure timely diagnosis of precocious puberty, so as to determine optimal management. This review summarizes the available laboratory testing for the diagnosis of puberty, discussing the different assays used while reviewing the limitations of such testing.
Puberty is the process through which reproductive competence is achieved. Physical characteristics associated with this process include the development of secondary sex characteristics, acceleration in height velocity, and the occurrence of menarche in women and spermatogenesis in men (1). The exact signals that initiate puberty have only recently begun to be understood and several key targets have been identified. Pubertal onset is accompanied by increased kisspeptin and neurokinin B secretion causing the gonadotropin releasing-hormone (GnRH) neurons to secrete GnRH in a pulsatile manner which then stimulates pulsatile pituitary luteinizing hormone (LH) and follicle stimulating hormone (FSH) secretion (2, 3). The LH and FSH further stimulate gonadal sex steroid secretion which promotes development of secondary sex characteristics and influences hypothalamic-pituitary function via negative feedback inhibition (4, 5). In females only, a positive sex steroid feedback develops late in puberty to trigger ovulation (6). Pubertal maturation typically starts between ages 8–13 years in girls and 9–14 years in boys (7). Central precocious puberty (CPP) is associated with early maturation of the hypothalamic-pituitary-gonadal (HPG) axis with premature reactivation of the GnRH pulse generator and sequential maturation of breasts and pubic hair in females and testicular volume, penile enlargement, and pubic hair in males. A significant long-term consequence of untreated CPP is accelerated skeletal maturation, which can result in premature epiphyseal fusion and, consequently a failure to reach genetic target height range (8, 9). Effective CPP treatment could increase final adult height and improve the likelihood of achieving one's genetic target height range (10). Boys with early-onset puberty may have behavioral difficulties and poor psychological adjustment, and girls may experience increased stress from early breast development and onset of menses (11, 12). Therefore, there is a need for accurate diagnosis and identification of children with CPP to optimize treatment plans.
In the evaluation of CPP, in addition to physical examination (for evidence of testicular enlargement in boys and breast development in girls) (13, 14) or ultrasound examination (for evidence of enlarging ovaries/uterus in girls) (15), the most important means of defining the etiology of the precocious sexual development is to determine whether the HPG axis is activated or quiescent by appropriate endocrine tests (16, 17). Those currently available to demonstrate activation of the HPG axis are measurements of one or more of the following: random serum gonadotropin and sex hormone concentrations, urinary gonadotropins, spontaneous nocturnal gonadotropin secretion, and the hormonal (gonadotropin and sex-hormone) response to stimulation with GnRH or a GnRH-agonist (GnRHa). The diagnostic cutoffs of the concentrations of these hormones have varied depending on the assay used and therefore knowledge of the various assays, their sensitivity and specificity are paramount for clinical decision making. These tests are reviewed and their value in diagnosing pubertal onset is appraised below.
Until the 1980's, gonadotropins were quantitated by radioimmunoassay (RIA), a method which allowed reliable measurement of LH and FSH at well detectable (approx. 3–4 IU/L or higher for LH, lower for FSH) serum concentrations, but could not accurately quantitate the low LH levels occurring in the early stages of puberty, due to limited sensitivity and specificity of the assay in the low LH range. This caused an overlap in serum LH concentrations between prepubertal and pubertal children (18, 19). The hormonal diagnosis of puberty required, in most instances, performance of a GnRH stimulation test, in which an LH level of 5 IU/L or greater was usually considered consistent with central puberty. When sensitive and specific immunoradiometric assays (IRMA), based on a “sandwich” assay, employing two antibodies (20, 21) for the subunits of LH, were developed in the mid 80's (22), it became apparent that small nocturnal LH pulses occur in young prepubertal children. There is an increase in frequency and amplitude in the late prepubertal period, so that between late prepuberty and Tanner stage II, LH concentrations become progressively detectable also in random daytime samples, earlier in boys than in girls, as puberty advances (19, 23). Thus, measurements of serum LH concentrations by the new highly sensitive assays allowed, for the first time, the hormonal diagnosis of puberty in random LH samples (19, 24), and appeared to better reflect the LH bioactivity1 (26, 27). The immunometric assays for LH evolved in the following years, with transition from the IRMA (functional sensitivity2 of about 0.15–0.4 IU/L) to ultrasensitive or highly sensitive immunofluorimetric assays (IFMA) (28) and chemiluminescent (ICMA) assays (29). These new assays (functional sensitivity of 0.02–0.05 IU/L) documented the pulsatile LH secretory activity occurring in the late prepubertal and early pubertal stages accurately, and provided reference data for random LH levels in different pediatric ages and across the pubertal stages for both sexes (23, 29–33). With regard to FSH, the highly sensitive assays showed good correlation with the RIA even at lower concentrations (23), but did not improve the diagnostic yield of measuring random FSH values for the hormonal detection of puberty, due to the substantial overlap of both baseline and GnRH-stimulated levels between prepuberty and early-mid puberty. Conversely, very low FSH concentrations, measurable for the first time by immunometric assays, became a useful marker for the diagnosis of hypogonadotropic hypogonadism (34), which can be confirmed by low FSH, LH response to GnRH stimulation as well (33). We refer the reader to a review article of gonadotropin assays (35).
Although highly sensitive assays are essential to detect the early increase in LH levels at the beginning of normal, precocious or delayed puberty in random samples, most small or non-pediatric hospitals in the US, employ LH assays (such as automated ICMAs) on laboratory “platforms” used to test several other analytes. While these assays have sufficient clinical sensitivity (limit of detection 0.1 IU/L, functional sensitivity usually ∼0.2 IU/L) (36, 37) to measure LH in children with mid-advanced puberty, and good specificity, they are often inadequate to detect the low LH concentrations occurring at the onset of puberty. Of note, assays of different sensitivity may be produced by the same manufacturer [for example, HS-DELFIA with an LH functional sensitivity of 0.05 IU/L (38), vs. autoDELFIA (39), with sensitivity of 0.6 IU/L] making it important to focus on the sensitivity of the assay quoted in different papers.
The other critical issue, which is rarely discussed about these assays is that of (excessive) specificity. While the monoclonal antibodies currently employed in most LH immunometric assays have been useful in assuring reproducibility of the assays over time and minimal cross reactivity with beta-hCG, the possibility of “excessive specificity” should be kept in mind as a factor that may lead to “inappropriately” low LH levels. Unlike other hormones secreted in a definite molecular form (such as T3 and T4), the glycoprotein hormones, including LH, circulate in different isoforms, related to post-translational modifications in glycosylation, sialylation and sulfation affecting the mass and content of the carbohydrate moiety, as well as the charge of the molecule. While changes in the carbohydrate component do not interact directly with the antibodies to LH, which are directed against the amino acid/protein epitopes, they may indirectly affect the binding of LH to the Ab in the immunoassay and to its receptor, resulting in variability of the “bioactivity*, the bioactive/immunoreactive ratio and half-life of different LH isoforms (40). A second issue related to interpretation of LH concentrations is the existence of molecular variants of LH with low immunoreactivity and high bio/immune ratio. The most common is a modification in two amino acids of the LH beta subunit (41), which is very common in Finland but may also be relatively common in other countries. Heterozygotes with this variant (about 24% of the Finnish population) show lower than average LH concentrations in most immunometric assays, and homozygotes (approximately 3% in Finland) will have very low or undetectable LH values (42). This variant appears to have normal bioactivity in adult healthy women (41), although it may be associated with a higher risk of PCOS in some women (43) and slower tempo of puberty in adolescent boys (44). This type of “invisible” or “partially visible” LH may cause puzzling clinical conundrums, with conflicting clinical and laboratory findings which may prompt complex and costly investigations (45), as shown in a vignette from our clinic below (see Box 1).
Key Points: Unlike the old LH RIAs, the current immunometric LH assays are sensitive and specific, allowing detection of early pubertal concentrations in unstimulated LH values in a subset of early pubertal children. However, sensitivity may be suboptimal in “platform” assays used in most hospitals. Conversely, excessive specificity, related to the characteristics of the antibodies used in the assay, or the presence of LH molecular variants with low immunoreactivity, may result in underestimation of LH values.
Random LH concentrations are commonly measured in the initial evaluation of children with either premature or delayed pubertal development. Random LH values may be higher in the early morning than at other times during the day (46), although the maximal nocturnal concentrations occur soon after the onset of sleep (33). We and others have shown that random LH concentrations can be used to detect onset of puberty in boys who are “late bloomers”, as LH increases before testicular enlargement is noted (19, 47). The cutoff value considered to be “pubertal” for a random LH value measured with the current, highly sensitive (HS) assays (HS-IFMA, HS ICMA, with limit of quantitation ≤0.05 IU/L), is generally ≥0.3 IU/L (48, 49). The diagnostic sensitivity of a random LH value in the diagnosis of CPP in girls has been evaluated by different groups with variable results, ranging from <50% (50) to 100% (49), with most studies reporting intermediate sensitivity and similarly variable specificity (Table 1). Large studies are more meaningful in this regard. In a large cohort of 449 girls (38), 65% of girls with CPP and 26% of girls with “early normal puberty” (onset between 8 and 9 years) had a random LH > 2 SDS of a control group of girls, and a number of girls with pubertal response to GnRH had prepubertal basal LH values. In another study of over 150 children with CPP, 85% of girls and 97% of boys had a random HS ICMA LH ≥ 0.3 IU/L (48), and the latter subgroup had a generally more advanced pubertal stage than those with lower/undetectable LH. However, some of the girls with stage 4 breast development, and 1 post-menarcheal girl had LH <0.3 IU/L. In another large study (56), the authors noted minimal increase in serum LH concentrations between Tanner B1 and B2, and persistently low LH levels (≤0.2 IU/L) in some children up to stage B4, although this study was marred by using an automated ICMA of suboptimal sensitivity (Immulite 2000 XPi®) which, in our opinion, makes LH values ≤0.3 IU/L difficult to interpret.
The variable diagnostic sensitivity of random LH concentrations at the time of the initial assessment even when measured by highly sensitive assays, is likely related to multiple factors in different studies, in addition to the characteristics of the assay. These include the different intervals between the onset of detectable breast tissue and the hormonal measurements (Tanner B2 may last for 6 or more months), which may be affected by the patterns of referral in different clinics/countries/health care systems, leading to a selection bias; the different time of the day when LH was measured; preselection criteria such as a “pubertal response” to GnRHa (49); and lack of clinical follow-up to document pubertal progression in some studies. Overall, all the quoted and other papers (51, 58) suggest that, while a baseline LH ≥ 0.3 IU/L by highly sensitive IFMA or ICMA is consistent with CPP, a lower random LH value does not exclude CPP. This was clearly stated in a previous document on the use of GnRHa in children (59), and is keeping with a classical observation (based on the LH response to GnRH in the LH-RIA era), that CPP in girls develops along a continuous spectrum of clinical and hormonal changes (60). This concept is reinforced in the above mentioned recent study by Madsen et al. (56) who, by using a sensitive breast ultrasound (US) technique, noted initial breast changes of puberty approximately 2 years before the onset of palpable breast tissue (Tanner B2) and described in detail the continuum of hormonal changes through the pubertal stages. Data are scantier in boys, but it appears that LH values are higher and more frequently in the detectable range in G2 boys than B2 girls (23, 48). A recent large study in boys correlated random gonadotropin and testosterone levels to pubertal stages, assessed both clinically and by testicular sonogram (57).
Key Points: random serum LH levels ≥0.3 IU/L by highly sensitive assays suggest the hormonal onset of puberty in both sexes. With less sensitive LH assays, a higher cutoff value may need to be employed (we suggest 0.5 IU/L). It is appropriate to confirm the LH elevation with a repeat, early morning sample, if the purpose is to forgo a GnRH or GnRHa stimulation test. However, undetectable LH concentrations (with any assay) do not exclude the hormonal onset of puberty, and may occur in ∼20%–35% of girls and ∼5% of boys with early hormonal changes that can be detected by a stimulation test.
The measurement of spontaneous LH secretion by highly sensitive assays in blood samples, collected every 10–30 min could arguably be considered the most physiological method for detection of the early hormonal changes of puberty (31–33, 35, 61, 62). Unfortunately, the procedure is invasive, costly, requires hospitalization, and is generally unavailable outside of a research setting. We will briefly outline below the data regarding correlation of nocturnal LH levels with GnRH and GnRHa-stimulated LH levels.
Measurement of urinary gonadotropins in timed urine collection by traditional RIA has been available for decades (63, 64), notwithstanding the limited sensitivity of the assay (requiring urine concentration) and reported periodic fluctuations of gonadotropin levels (65). With the advent of the immunometric assays, LH measurement in random or early morning urine samples appeared to provide sufficient sensitivity (66). Urinary gonadotropins increased with advancing age and pubertal development and were detectable in first-morning urinary void, even before physical signs of puberty (67). They were also noted to identify sex-specific gonadotropin changes during early infancy (68). Despite its minimal invasiveness, urinary gonadotropin testing has not become popular in the U.S. However, renewed interest in this procedure has been recently fostered by investigators predominantly from Asian centers (69–71).
Key Points: Urinary gonadotropins (LH particularly) provide “integrated” hormonal concentrations and are a useful adjunct for the diagnosis and treatment of CPP. They have been employed mostly in Europe and Asia, and they have not been as extensively studied as the corresponding serum measurements.
In girls, serum estradiol (E2) concentrations are often undetectable or very low (<15 pg/ml or <55 pmol/L) in random daytime blood samples obtained in early to mid-puberty (Breast stage 2–3). In these early pubertal girls, nocturnal pulses of estradiol occur and are sufficient to induce development of secondary sexual characteristics, so that E2 levels can be quantitated, when measured, by a sensitive assay, during the night or in early morning samples. In this regard, it is important to briefly discuss the unextracted, competitive “platform” estradiol immunoassays commonly used in most hospitals. Even though these assays tend to correlate reasonably well (albeit with variable bias) with the reference liquid chromatography-tandem mass spectrometry (LC-TMS) assay at E2 values of ∼25 pg/ml or higher, they correlate poorly with the reference method at lower E2 concentrations (72), thus providing inadequate sensitivity and specificity and misleading results at the low E2 concentrations encountered in early-mid female puberty (73). As mentioned above, the current “gold standard” for serum E2 measurement in the low range seen in (early) pubertal girls is the LC-TMS method (74, 75), with a functional sensitivity of 1–3 pg/ml. Nonetheless, competitive immunoassays which include extraction and column chromatography (76), or even solvent extraction alone (73) provide good sensitivity (∼2–5 pg/ml) and adequate specificity in early female puberty in laboratories that have no access to the more expensive LC/MS/MS method.
In boys, serum testosterone (T) is secreted by the Leydig cells or derives from conversion of adrenal androgens, so that the small T increase (up to ∼30 ng/dl) in early pubertal, adrenarcheal boys cannot be attributed with certainty to gonadal activation. For this reason, measurement of early increases in serum testosterone in boys has lower diagnostic sensitivity than early detection of E2 increase in girls for the diagnosis of CPP, with the understanding that sex hormone measurements should always be paired with serum LH measurements. Thus, serum T measurement by direct, unextracted assays has been used to define diurnal testosterone rhythms in boys and have shown average testosterone concentrations of ∼30 ng/dl in an early morning sample in early puberty, heralded by testicular volume of 3–6 ml (77). Other clinicians have proposed a serum T level of >40 ng/dl to indicate the onset of puberty in boys (78). Nonetheless, the “gold standard” for T measurement in children is the LC-TMS method (57, 79), or an immunoassay involving manual purification steps (80), as direct testosterone assays correlate poorly with the reference method at low T concentrations (81). As an aside consideration, direct testosterone assays are truly inadequate for assessment of hyperandrogenic conditions in pubertal girls or adult women (82–84).
Lastly, high dose (>1–3 mg/day) biotin (vitamin B7) which is available as an over-the counter supplement used to strengthen nails and hair, interferes with the technical aspects of immunoassays and can lead to either falsely elevated or falsely low results when streptavidin binding is utilized in the assay detection system. Biotin does not interfere with LC-MS/MS assays (85).
Key Points: E2 levels should be measured by highly sensitive assays (LC-TMS or at least extraction methods) to detect early pubertal changes in girls. Even so, E2 may be truly undetectable or very low at stages B2 and early B3. T values >30–40 ng/dl (measured by a sensitive method, LC-TMS or at least extraction assay) are generally consistent with the onset of hormonal puberty in boys. However, the same levels and especially lower levels above prepubertal (>5–10 ng/dl) may be due to adrenarche, gonadarche or a combination thereof and not be truly diagnostic of gonadarche alone. If immunoassays (i.e., methods not based on mass spectrometry) are used and the results do not make sense clinically, use of high-dose biotin supplements should be excluded.
Inhibin B (INHB) is produced in the Sertoli cells of the testis in males, and in the granulosa cells of the ovary in females. It belongs to the transforming growth factor-β super family and regulates the synthesis and secretion of FSH in a negative feedback loop (86). In males, INHB level reflects Sertoli cell number and function and peaks shortly after birth, decreases during childhood, and then increases at puberty due to FSH stimulation. In females, INHB level is related to the number of antral follicles and reflects the ovarian response to gonadotrophins (87). Undetectable or low INHB levels are observed in boys with either congenital or acquired absence of testicular tissue whereas normal or near-normal levels are seen in cryptorchidism and disorders with preserved Sertoli cell function in spite of absence of germ cells or impaired androgen biosynthesis or action (88). While Individual studies have shown basal inhibin B to have good accuracy to predict the onset of puberty, diagnostic thresholds given by different studies are variable and overlapping. Specifically, there is considerable overlap in INHB concentrations between boys with testicular volume 1–3 ml and those with volume >4 ml (89), thus a single diagnostic cutoff for routine clinical practice is still unavailable (90). Recently, FSH stimulated INHB levels has been explored as a promising investigation for prediction of onset of puberty (91).
Key Points: INHB increases at puberty in both sexes, but has been mainly used in males (who have higher values of INHB throughout life), as a marker of puberty. It can be used as an additional hormonal parameter, in the context of a multi-hormonal evaluation, but is inadequate as an isolated maker of puberty in boys.
In the US, the traditional GnRH stimulation test has been replaced by the GnRHa stimulation test since the mid 1980's, when GnRH became commercially unavailable. Different GnRHa have been used in this country, including parenteral Nafarelin (now unavailable), and, more commonly, subcutaneous (SC) aqueous Leuprolide, which has been used for decades. Triptorelin (which had limited research availability as an aqueous solution in the US) has been used mainly in other countries, and depot preparations of GnRHa have also been used for stimulation, as they release a substantial proportion of the analog rapidly, immediately after injection, resulting in FSH and LH stimulation (92, 93). After an initial study with aqueous SC nafarelin (94), our experience has been limited to aqueous SC leuprolide, which we have been using exclusively for diagnosis of pubertal disorders (95). For monitoring children treated with GnRHa for CPP, we have monitored FSH, LH 1–3 h after stimulation test by aqueous leuprolide and, more recently, by the same Leuprolide depot preparation being used for treatment. We (50, 95) and others (96) have used a Leuprolide dose of 20 mcg/kg. Lower doses of 10 mcg/kg (97) or a single dose of 500 mcg (98) appear to achieve effective stimulation of FSH and LH secretion, but no dose-response studies are available in the 5–20 mcg/kg dose range. Triptorelin has similarly been shown to be effective (55, 99). Although there may be subtle differences in the effectiveness of different GnRHa, probably all of them at the dose commonly employed achieve (sub)maximal FSH and LH stimulation. Few studies have compared the LH response to leuprolide to the response to GnRH stimulation in the same subjects. In a comparison study with testing a few weeks apart, Ibanez (98) showed Leuprolide, 500 mcg SC to induce a LH peak which was almost twice as high as the peak induced by GnRH IV in children with advanced precocious puberty, while response to the 2 agents was similar in prepubertal children. In an analogous, small (N = 8), unpublished study of early-mid pubertal girls (Breast T2–T3) with CPP tested with GnRH IV (2.5 mcg/Kg) 3–5 months after undergoing a Leuprolide stimulation test, we found that SC leuprolide (y) achieved a ∼1,4 times higher LH peak average than GnRH despite these patients being a few months older at the GnRH test. These limited data suggest that Leuprolide achieves at least a similar, and possibly greater stimulation of LH (measured by immunometric method) than native GnRH in children evaluated for CPP. Information regarding correlation between the peak LH to Leuprolide and the spontaneous nocturnal LH secretion is likewise limited. Following a study showing good correlation (r = 0.83) between nocturnal HS- IFMA LH concentrations and (very) low-dose GnRH-stimulated LH peak values (62), we performed an analogous analysis in 28 early to mid-pubertal girls with premature or early pubertal development. The study group included 25 girls with CPP (progressive on follow-up), and 3 with non-progressive exaggerated thelarche (100) (unpublished data). Of the girls with CPP, 7 [age 6.9 ± 0.5 years (mean ± SD), Δ(BA-CA) 1.6 years] were at Tanner stage B2, with LH < 0.25 IU/L in 5, 0.3–0.4 IU/L in 2 girls; baseline (extracted) E2 < 5 pg/ml in 5, 6–7 pg/ml in 2 girls]. Fifteen girls [age 6.8 ± 0.6 years, Δ(BA-CA) 2.2 years], were at stage B3, with baseline LH < 0.25 in 4, 0.25–0.5 in 5, 0.5–1.4 IU/L in 6, and E2 < 5 pg/ml in 7, 5–16 pg/ml in 8 girls). Three girls were at stage B4 [8.7 ± 0.3 years, Δ(BA-CA) 3.8 years], with baseline LH 1.1–2.7 IU/L, E2 10–25 pg/ml. Three girls with exaggerated thelarche [age 1.7 ± 0.3 years, Δ(BA-CA) 1 year] had LH < 0.25 and E2 < 5 pg/ml. In this cohort, we evaluated the relation between 12 h (8 PM to 8 AM) LH-IRMA concentrations (sampled every 30 min) and peak response to Leuprolide (20 mcg/Kg SC). The peak LH (IRMA) after leuprolide showed good correlation with the peak nocturnal LH (r = 0.83, p < 0.001, Figure 1) and the mean nocturnal LH (r = 0.89, p < 0.001). A subsequent study from the U. of Chicago, showed that the peak sleep LH concentration, measured during an approximately 3 h period after the onset of sleep, also correlated well (r > 0.8) with both the peak LH after Leuprolide, as well as the peak E2 after leuprolide, in normal pubertal girls (101).
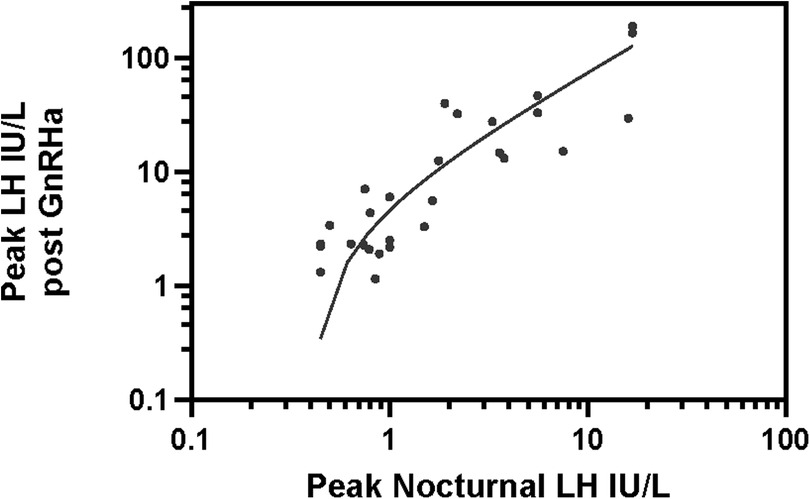
Figure 1. Correlation between nocturnal peak LH vs. Leuprolide stimulated peak LH. y (GnRHa stimulated peak LH) = 7.7 x −3.1 IU/L, where x = peak nocturnal LH. r = 0.83; r2 = 0.69 p < 0.001. LH, luteinizing hormone.
While the above information is useful to validate the GnRHa stimulation test and to interpret clinical studies regarding diagnostic sensitivity and specificity of various tests for the diagnosis of CPP, most pediatric endocrinologists have been seeking a specific cutoff value for stimulated LH to diagnose pubertal activation, when baseline LH levels are non-diagnostic. Clinicians have continued to use a cutoff GnRH-stimulated LH value >5 IU/L for the diagnosis of precocious puberty (37), the same value traditionally used LH-RIA, without taking into consideration the differences between RIA and immunometric assays, between different immunometric assays, and the likely potency difference between GnRH and GnRHa (98). Other investigators have proposed various diagnostic threshold values for LH concentrations in the diagnosis of CPP, based on their clinical data. These cutoffs include 3.3 IU/L for IFMA vs. 4.1 for ICMA (102); 6.9 (girls), 9.6 (boys) IU/L for IFMA (39); 8.0 IU/L for ICMA (98); 6.0 IU/L for automated platform chemiluminescent assay (ECLIA) (55); 7 IU/L (IFMA); 8 IU/L (platform ECLIA) (99). Moreover, we have demonstrated that Leuprolide-stimulated LH values by IRMA (95) or HS-ICMA (50) well below the traditional value of 5 IU/L can be associated with CPP. Other investigators have noted that the diagnostic sensitivity of the leuprolide stimulation test, for a stimulated LH cutoff level of 5 IU/L, is highest when the test is prolonged to 180 min, thus allowing the LH to rise maximally (58). In our opinion, defining a universally applicable GnRHa-stimulated LH cutoff value for the diagnosis of CPP is an elusive goal, given the variability and limited standardization of the different LH assays used, the variable data arising from different populations on which proposed threshold values are based, the duration of the GnRHa tests employed, and the fact that, in the continuum of pubertal progression (103), a “low” LH response at one point in time is not necessarily predictive of the speed of future progression. Other caveats regarding interpretation of LH cutoffs to GnRHa include lower values noted in girls with obesity (104). For all these reasons, we think that, for the diagnosis of CPP in girls, the LH response to stimulation, rather than in absolute values, needs to be interpreted in conjunction with the other clinical, radiological and hormonal parameters, including the sex-hormone response to GnRHa stimulation (discussed below), and, as importantly, an observation period of 3–6 months to evaluate progression in cases that are not clear-cut. Because the LH secretion tends to occur earlier in boys than in girls, for the same degree of sexual development in normal and precocious puberty (23, 48), an LH response to GnRHa > 5 IU/L may be theoretically more universally applicable, however data on cutoff LH response to stimulation are limited for boys with CPP, due to the lower prevalence of this condition in males (105–107).
Different cut-points need to be used to interpret random LH concentrations in girls under two years of age because LH concentrations may normally be higher (following the “minipuberty” of infancy); CPP may frequently be misdiagnosed during this phase of development (108). The interpretation of LH response to GnRH or GnRHa is also difficult in these very young girls with premature sexual development as a subgroup of them with idiopathic premature thelarche may show an LH response >5 IU/L to stimulation (108). Additionally, some girls with atypical or “exaggerated” thelarche may show a robust 20–24 h Estradiol response to leuprolide stimulation (100) and still have a self-limited condition.
Key Points: The LH response to Leuprolide stimulation correlates with the response to GnRH stimulation and with nocturnal LH secretion (with the limitation that correlation studies are few). Although an LH peak >5 IU/L to stimulation has been considered the hallmark of hormonal puberty, pubertal development in girls can be seen at lower LH values, which may be due to different characteristics among LH assays, the continuum of puberty, or other factors. Thus, a universal “pubertal cutoff” for the LH response to GnRHa may be an elusive goal, at least in girls.
The original observation that GnRHa administration achieves sequential gonadotropin and estradiol secretion in girls with CPP (94, 95) was subsequently expanded to include an equivalent response in boys, adult men and women (78, 97, 109). Given the above noted difficulties in establishing a diagnostic GnRHa-stimulated LH cutoff value to diagnose precocious puberty in girls, the estradiol response to Leuprolide or other GnRHa (and the analogous testosterone response in boys) can be utilized as evidence of the activation of the entire pituitary-gonadal axis at puberty. We reported that ∼20% of girls with progressive CPP undergoing Leuprolide stimulation did not achieve a predetermined “diagnostic” LH peak value of 5 IU/L by ICMA, yet they could be diagnosed by measuring a peak E2 response >50 pg/ml at 24 h (50). The usefulness of the stimulated (20–24 h post injection) E2 level for the diagnosis of CPP has been supported by other investigators (99, 110) and confirmed in our routine clinical experience (unpublished data), but not all (54). In this regard, the proposed cutoff for peak stimulated E2 value to diagnose CPP ranges from 40 pg/ml (98) to 80 pg/ml (54, 99) and a study suggested that a percentage increase of E2 (>28%) between 3 h and 24 h had the best diagnostic accuracy (110). It is likely that these differences are related both to characteristics of the E2 assay employed, and the variably advanced pubertal development in the different populations studied. For the last few decades, we have found that the ranges we employed in our original report (20–24 h E2 responses to leuprolide > 50 pg ml, by an extracted E2 assay, consistent with progressive/advanced CPP, and responses of 25–50 pg/ml consistent with early/slowly progressive CPP) (95) have correlated well with outcome, follow-up and the need for GnRHa treatment. Nonetheless, we realize that any cutoff value is somewhat arbitrary, also in consideration of the continuum of puberty discussed above for stimulated LH cutoff values.
In boys, there seem to be no consensus about cutoff GnRHa-stimulated T levels for the diagnosis of CPP, as reported series showed baseline T levels in a clear pubertal range in Tanner stage 2 (T2) boys (111), and even in T1 boys (109), thus making the stimulated T values clearly elevated and uninterpretable for diagnostic purposes. Studies of boys with delayed puberty may be more informative in this regard. Three adolescent boys with constitutional delay of puberty (Tanner 1) had an average peak T response of 65 ng/dl, significantly higher than the peak of 20 ng/dl achieved by 8 subjects with hypogonadotropic hypogonadism (112). In a cohort of prepubertal (T1) adolescents and young adults, Lanes et al. showed a peak T response of 29 ± 12 ng/dl in 8 subjects with Gonadotropin deficiency compared to 110 ± 20 ng/dl in 14 subjects with delayed puberty (all subjects were T1), however noted overlap of T responses (as well as LH responses) between the 2 groups (96). In the absence of more definite studies, we have empirically interpreted a cutoff (20–24 h) T response ≥100 ng/dl, or an increment (delta) of >60 ng/dl above baseline, as indicative of CPP in boys, with good clinical correlation. We feel, however, that studies of the testosterone response to GnRHa in normal boys (a larger population than boys with sexual precocity) on the brink of puberty (late T1 stage) would be helpful for interpretation and validation of the cutoff T response to GnRHa for the diagnosis of CPP. While we have found that the LH response to GnRHa is generally sufficient to confirm the diagnosis of CPP in the great majority of boys for their consistent increase in LH secretion at stage T2 (23, 48), the T response can be confirmatory and useful in atypical cases of sexual precocity, as described in the vignette from our clinic (see Box 1).
Key Points: As a LH response <5 IU/L (or whatever threshold value is chosen) to GnRHa does not uncommonly occur in girls with CPP, the delayed (20–24 h) E2 response provides an “in vivo” bioassay of the activation of the pituitary-ovarian axis. Serum E2 values of 50 pg/ml (range 40–80) or higher (increasing from a low baseline level) are consistent with CPP. Similarly, T values increasing to 100 ng/dl or higher upon GnRHa stimulation in boys are consistent with puberty, although data are limited due to the low incidence of CPP in males.
The efficacy of treatment with GnRHa should be monitored by clinical, radiological and laboratory parameters, as discussed in various review articles (59, 113–115).
Laboratory evaluation should include ultrasensitive LH, FSH and sensitive and specific sex hormone levels (estradiol in girls, testosterone in boys). While clinicians may minimize or forgo blood tests if clinical indices of response to treatment are reassuring, in our center we use at least an initial laboratory assessment of treatment effectiveness approximately 3–5 months after initiation of GnRHa therapy, typically before the 3rd monthly depot-leuprolide injection, before the 2nd injection of a 12 week or 24-week depot preparation, or 2–3 months after placement of the histrelin implant. We suggest subsequent lab monitoring at least yearly, even if clinical evaluation is reassuring. Although the great majority of children respond to the recommended doses of GnRHa, occasional children show inadequate pubertal suppression and may benefit from an increase in the dosage of the GnRHa (for the depot Leuprolide preparations), more frequent administration (if allowed by insurance/healthcare regulations) or switching from an injectable form to a histrelin implant. Clinical trials for the available GnRHas report a range of response in each of the variables (116–122). The reader is referred to several reviews that are available on the different formulations of GnRHa available for treatment of CPP (59, 123–126).
There is disagreement regarding whether a baseline LH level is sufficient, or a GnRHa- stimulated level is preferable to monitor pubertal hormone suppression (117, 127). We have favored the GnRHa-stimulated values, traditionally “the gold standard” (128) which provide a more sensitive measure of LH suppression, although baseline values may be adequate, and “perfect” suppression of the pubertal hormones may not be necessary for a favorable outcome (129). A GnRHa-stimulated LH level <4 IU/L has been used to define biochemical suppression in most studies (59, 117, 126) although we and others (92) have observed lower values (typically <2.5 IU/L) in the great majority of children adequately responding to GnRHa therapy in our Center. As we have noted above, cutoff value variation in different studies can be related in part to the different immunometric LH assays employed. While we have used an LH value at 60 min after aqueous leuprolide stimulation for assessment of pubertal suppression for all patients in the past, we now use an LH measurement 60–90 min after Leuprolide-depot or triptorelin depot, except, of course, in children with the histrelin implant. The release of a large amount of rapidly absorbable GnRHa from the depot preparation has been shown to provide an intense stimulation of gonadotropins (92, 125).
With regard to random LH values, ultrasensitive LH < 0.6 IU/L has been proposed to define biochemical suppression but has not been rigorously studied (127). Others suggest an LH value <1 (130). Again, the cutoff value may be somewhat assay-dependent, which makes it difficult to compare different studies. It is important to note that random highly sensitive LH levels often fail to revert to a prepubertal range even when the pituitary-gonadal axis is fully suppressed (117, 131).
Estradiol and testosterone levels should be low in children adequately responding to GnRHa therapy, provided they are measured by appropriate sensitive and specific assays, as discussed above. In girls, estradiol levels should be <10 pg/ml and are, in fact, often undetectable even when measured by ultrasensitive assays (129). Testosterone values should be <10 ng/dl in pre-adrenarcheal boys, and <20–30 ng/dl in boys with less or more advanced adrenarche. That being said, occasional children (mostly boys in our experience) may have higher sex hormone levels and still show adequate pubertal suppression clinically and by LH monitoring. For this reason, and the fact that sex hormones are often measured by less sensitive and specific unextracted assays, measurement of sex steroids may have lower diagnostic sensitivity than measurement of LH levels.
Key Points: For laboratory monitoring of GnRHa therapy, measurement of GnRHa-stimulated LH (±FSH) levels is the “gold standard”, with stimulated LH levels <2.5–4 IU/L indicating adequate suppression of the pituitary-gonadal axis. However, baseline LH < 0.6–1 IU/L may suffice to indicate acceptable suppression. Of note, LH values often remain above prepubertal values (0.3 IU/L) in children adequately treated with GnRHa. With good response to GnRHa therapy, serum E2 levels should be suppressed <10 pg/ml in girls, while in boys T levels <10–20 ng/dl are not always achieved and can still be compatible with adequate suppression. Laboratory monitoring should always be used in conjunction with clinical and radiological evidence of response to therapy. This being said, machine learning algorithms to integrate multiple variables for the diagnosis of CPP are being developed (132).
Highly sensitive assays are essential to detect the early increase in LH levels at the beginning of puberty. The GnRHa stimulation test activates the entire pituitary-gonadal axis, thus representing a true in vivo “bioassay” that quantitates the ability of the child to synthesize sex hormones, the effective markers of pubertal effects on the body. This is particularly relevant for the diagnosis of CPP in girls, as a substantial percentage of them may not achieve a” pubertal” LH (usually considered >5 IU/L, but set at different cutoff values in different studies, as discussed above). Those girls with bona fide CPP who do not achieve a “pubertal” LH value on GnRHa stimulation, will most often achieve an E2 peak >50 pg/ml at 20–24 h, indicative of pubertal activation of the pituitary gonadal axis. In boys, measurement of the 20–24 h T response is not as crucial, as most boys with clinical signs of CPP will have stimulated LH responses >5 IU/L, however it may be helpful in corroborating the diagnosis of CPP in atypical cases. Treatment decisions need to be individualized and no one variable alone predicts adequate treatment response.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
KB: Writing – review & editing, Writing – original draft. LG: Writing – review & editing, Writing – original draft.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declare that no Generative AI was used in the creation of this manuscript.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. ^The bioactivity of LH has traditionally been measured by the rat interstitial cell testosterone (RICT)assay described by Dufau et al. (25). In this assay serum from a patient is added to suspension of Leydig cells obtained from rats (or mice in modification of the procedure), and the testosterone released in the medium is quantitated as an index of the LH biopotency.
2. ^Functional sensitivity, also referred to as the limit of quantitation, is the minimal concentration at which the intra-assay coefficient of variation (CV) is <20%. The limit of detection, or “sensitivity”, is usually defined as the minimal concentration above 2 SD of the 0 standard.
1. Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. (2016) 4(3):254–64. doi: 10.1016/S2213-8587(15)00418-0
2. Garcia JP, Guerriero KA, Keen KL, Kenealy BP, Seminara SB, Terasawa E. Kisspeptin and neurokinin B signaling network underlies the pubertal increase in GnRH release in female rhesus monkeys. Endocrinology. (2017) 158(10):3269–80. doi: 10.1210/en.2017-00500
4. Wood CL, Lane LC, Cheetham T. Puberty: normal physiology (brief overview). Best Pract Res Clin Endocrinol Metab. (2019) 33(3):101265. doi: 10.1016/j.beem.2019.03.001
5. Grumbach MM. The neuroendocrinology of human puberty revisited. Harm Res. (2002) 57(Suppl 2):2–14. doi: 10.1159/000058094
6. Kauffman AS. Neuroendocrine mechanisms underlying estrogen positive feedback and the LH surge. Front Neurosci. (2022) 16:953252. doi: 10.3389/fnins.2022.953252
7. Cabrera SM, Bright GM, Frane W, Blethen SL, Lee PA. Age of thelarche and menarche in contemporary US females: a cross-sectional analysis. Pediatr Endocrinol Metab. (2014) 27(1-2):47–51. doi: 10.1515/jpem-2013-0286
8. Shangold MM, Kelly M, Berkeley AS, Freedman KS, Groshen S. Relationship between menarcheal age and adult height. Southern Med J. (1989) 82(4):443–5. doi: 10.1097/00007611-198904000-00009
9. Biro FM, McMahon RP, Striegel-Moore R, Crawford PB, Obarzanek E, Morrison JA, et al. Impact of timing of pubertal maturation on growth in black and white female adolescents: the national heart, lung, and blood institute growth and health study. J Pediatr. (2001) 138(5):636–43. doi: 10.1067/mpd.2001.114476
10. Li P, Li Y, Yang CL. Gonadotropin releasing hormone agonist treatment to increase final stature in children with precocious puberty. Medicine (Baltimore). (2014) 93(27):e260. doi: 10.1097/MD.0000000000000260
11. Mendie J, Ryan RM, McKone KMP. Early menarche and internalizing and externalizing in adulthood: explaining the persistence of effects. I Adolescent Health. (2019) 65(5):599–606. doi: 10.1016/j.jadohealth.2019.06.004
12. Mendie J, Ryan RM, McKone KMP. Age at menarche, depression, and antisocial behavior in adulthood. Pediatrics. (2018) 141(1):e2017–1703. doi: 10.1542/peds.2017-1703
13. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. (1970) 45(239):13–23. doi: 10.1136/adc.45.239.13
14. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child. (1969) 44(235):291–303. doi: 10.1136/adc.44.235.291
15. Sathasivam A, Rosenberg HK, Shapiro S, Wang H, Rapaport R. Pelvic ultrasonography in the evaluation of central precocious puberty: comparison with leuprolide stimulation test. J Pediatr. (2011) 159(3):490–5. doi: 10.1016/j.jpeds.2011.02.032
16. Chen M, Eugster EA. Central precocious puberty: update on diagnosis and treatment. Pediatr Drugs. (2015) 17(4):273–81. doi: 10.1007/s40272-015-0130-8
17. Bangalore Krishna K, Silverman LA. Diagnosis of central precocious puberty. Endocrinol Metab Clin North Am. (2024) 53(2):217–27. doi: 10.1016/j.ecl.2024.02.002
18. Apter D, Cacciatore B, Alfthan H, Stenman UH. Serum luteinizing hormone concentrations increase 100-fold in females from 7 years to adulthood, as measured by time-resolved immunofluorometric assay. J Clin Endocrinol Metab. (1989) 68(1):53–7. doi: 10.1210/jcem-68-1-53
19. Garibaldi LR, Picco P, Magier S, Chevli R, Aceto T Jr. Serum luteinizing hormone concentrations, as measured by a sensitive immunoradiometric assay, in children with normal, precocious or delayed pubertal development. J Clin Endocrinol Metab. (1991) 72(4):888–98. doi: 10.1210/jcem-72-4-888
20. Ehrlich PH, Moyle WR, Canfield RE. Methods in enzymology. Methods Enzymol. (1985) 109:638–55. doi: 10.1016/0076-6879(85)09120-0
21. Soos M, Siddle K. Characterisation of monoclonal antibodies for human luteinising hormone, and mapping of antigenic determinants on the hormone. Clin Chim Acta. (1983) 133(3):263–74. doi: 10.1016/0009-8981(83)90270-X
22. Hunter WM, Bennie JG, Kellett HA, Micklem LR, Scott A, James K. A monoclonal antibody-based immunoradiometric assay for h-LH. Ann Clin Biochem. (1984) 21(Pt 4):275–83. doi: 10.1177/000456328402100408
23. Partsch CJ, Hummelink R, Sippell WG. Reference ranges of lutropin and follitropin in the luliberin test in prepubertal and pubertal children using a monoclonal immunoradiometric assay. J Clin Chem Clin Biochem. (1990) 28(1):49–52.2107274
24. De Hertogh R, Wolter R, Van Vliet G, Vankrieken L. Serum gonadotropins levels in childhood. Critical comparison between immunoradiometric assays and radioimmunoassays. Acta Endocrinol (Copenh). (1989) 121(1):141–6. doi: 10.1530/acta.0.1210141
25. Dufau ML, Pock R, Neubauer A, Catt KJ. In vitro bioassay of LH in human serum: the rat interstitial cell testosterone (RICT) assay. J Clin Endocrinol Metab. (1976) 42(5):958–69. doi: 10.1210/jcem-42-5-958
26. Huhtaniemi I, Ding YQ, Tahtela R, Valimaki M. The bio/immuno ratio of plasma luteinizing hormone does not change during the endogenous secretion pulse: reanalysis of the concept using improved immunometric techniques. J Clin Endocrinol Metab. (1992) 75(6):1442–5. doi: 10.1210/jcem.75.6.1464645
27. Jaakkola T, Ding YQ, Kellokumpu-Lehtinen P, Valavaara R, Martikainen H, Tapanainen J, et al. The ratios of serum bioactive/immunoreactive luteinizing hormone and follicle-stimulating hormone in various clinical conditions with increased and decreased gonadotropin secretion: reevaluation by a highly sensitive immunometric assay. J Clin Endocrinol Metab. (1990) 70(6):1496–505. doi: 10.1210/jcem-70-6-1496
28. Khosravi MJ, Morton RC, Diamandis EP. Sensitive, rapid procedure for time resolved immunofluorometry of lutropin. Clin Chem. (1988) 34(8):1640–4. doi: 10.1093/clinchem/34.8.1640
29. Neely EK, Hintz RL, Wilson DM, Lee PA, Gautier T, Argente J, et al. Normal ranges for immunochemiluminometric gonadotropin assays. J Pediatr. (1995) 127(1):40–6. doi: 10.1016/S0022-3476(95)70254-7
30. Wennink JM, Delemarre-van de Waal HA, Schoemaker R, Schoemaker H, Schoemaker J. Luteinizing hormone and follicle stimulating hormone secretion patterns in boys throughout puberty measured using highly sensitive immunoradiometric assays. Clin Endocrinol (Oxf). (1989) 31(5):551–64. doi: 10.1111/j.1365-2265.1989.tb01279.x
31. Wennink JM, Delemarre-van de Waal HA, Schoemaker R, Schoemaker H, Schoemaker J. Luteinizing hormone and follicle stimulating hormone secretion patterns in girls throughout puberty measured using highly sensitive immunoradiometric assays. Clin Endocrinol (Oxf). (1990) 33(3):333–44. doi: 10.1111/j.1365-2265.1990.tb00498.x
32. Wennink JM, Delemarre-van de Waal HA, van Kessel H, Mulder GH, Foster JP, Schoemaker J. Luteinizing hormone secretion patterns in boys at the onset of puberty measured using a highly sensitive immunoradiometric assay. J Clin Endocrinol Metab. (1988) 67(5):924–8. doi: 10.1210/jcem-67-5-924
33. Wu FC, Butler GE, Kelnar CJ, Stirling HF, Huhtaniemi I. Patterns of pulsatile luteinizing hormone and follicle-stimulating hormone secretion in prepubertal (midchildhood) boys and girls and patients with idiopathic hypogonadotropic hypogonadism (Kallmann’s syndrome): a study using an ultrasensitive time-resolved immunofluorometric assay. J Clin Endocrinol Metab. (1991) 72(6):1229–37. doi: 10.1210/jcem-72-6-1229
34. Grinspon RP, Ropelato MG, Gottlieb S, Keselman A, Martinez A, Ballerini MG, et al. Basal follicle-stimulating hormone and peak gonadotropin levels after gonadotropin-releasing hormone infusion show high diagnostic accuracy in boys with suspicion of hypogonadotropic hypogonadism. J Clin Endocrinol Metab. (2010) 95(6):2811–8. doi: 10.1210/jc.2009-2732
35. Apter D. Ultrasensitive new immunoassays for gonadotropins in the evaluation of puberty. Curr Opin Pediatr. (1993) 5(4):481–7. doi: 10.1097/00008480-199308000-00019
36. Soldin OP, Hoffman EG, Waring MA, Soldin SJ. Pediatric reference intervals for FSH, LH, estradiol, T3, free T3, cortisol, and growth hormone on the DPC IMMULITE 1000. Clin Chim Acta. (2005) 355(1-2):205–10. doi: 10.1016/j.cccn.2005.01.006
37. Suh J, Choi MH, Kwon AR, Kim YJ, Jeong JW, Ahn JM, et al. Factors that predict a positive response on gonadotropin-releasing hormone stimulation test for diagnosing central precocious puberty in girls. Ann Pediatr Endocrinol Metab. (2013) 18(4):202–7. doi: 10.6065/apem.2013.18.4.202
38. Mogensen SS, Aksglaede L, Mouritsen A, Sorensen K, Main KM, Gideon P, et al. Diagnostic work-up of 449 consecutive girls who were referred to be evaluated for precocious puberty. J Clin Endocrinol Metab. (2011) 96(5):1393–401. doi: 10.1210/jc.2010-2745
39. Brito VN, Batista MC, Borges MF, Latronica AC, Kohek MB, Thirone AC, et al. Diagnostic value of fluorometric assays in the evaluation of precocious puberty. J Clin Endocrinol Metab. (1999) 84(10):3539–44. doi: 10.1210/jcem.84.10.6024
40. Bergendah M, Veldhuis JD. Is there a physiological role for gonadotrophin oligosaccharide heterogeneity in humans? III. Luteinizing hormone heterogeneity: a medical physiologist’s perspective. Hum Reprod. (2001) 16(6):1058–64. doi: 10.1093/humrep/16.6.1058
41. Haavisto AM, Pettersson K, Bergendahl M, Virkamaki A, Huhtaniemi I. Occurrence and biological properties of a common genetic variant of luteinizing hormone. J Clin Endocrinol Metab. (1995) 80(4):1257–63. doi: 10.1210/jcem.80.4.7714098
42. Pettersson K, Ding YQ, Huhtaniemi I. An immunologically anomalous luteinizing hormone variant in a healthy woman. J Clin Endocrinol Metab. (1992) 74(1):164–71. doi: 10.1210/jcem.74.1.1727817
43. Tapanainen JS, Koivunen R, Fauser BC, Taylor AE, Clayton RN, Rajkowa M, et al. A new contributing factor to polycystic ovary syndrome: the genetic variant of luteinizing hormone. J Clin Endocrinol Metab. (1999) 84(5):1711–5. doi: 10.1210/jcem.84.5.5702
44. Raivio T, Huhtaniemi I, Anttila R, Siimes MA, Hagenas L, Nilsson C, et al. The role of luteinizing hormone-beta gene polymorphism in the onset and progression of puberty in healthy boys. J Clin Endocrinol Metab. (1996) 81(9):3278–82. doi: 10.1210/jcem.81.9.8784083
45. Martin-Du-Pan RC, Horak M, Bischof P. Clinical significance of invisible or partially visible luteinizing hormone. Hum Reprod. (1994) 9(11):1987–90. doi: 10.1093/oxfordjournals.humrep.a138379
46. Kang YS, Yoo DY, Chung IH, Yoo EG. Diurnal variation of gonadotropin levels in girls with early stages of puberty. Ann Pediatr Endocrinol Metab. (2017) 22(3):183–8. doi: 10.6065/apem.2017.22.3.183
47. Binder G, Schweizer R, Blumenstock G, Braun R. Inhibin B plus LH vs GnRH agonist test for distinguishing constitutional delay of growth and puberty from isolated hypogonadotropic hypogonadism in boys. Clin Endocrinol (Oxf). (2015) 82(1):100–5. doi: 10.1111/cen.12613
48. Logan LA, Eugster EA. A comparison of patients with central precocious puberty who have a pubertal versus prepubertal ultrasensitive LH at presentation. Horm Res Paediatr. (2021) 93(11-12):651–5. doi: 10.1159/000513934
49. Neely EK, Wilson DM, Lee PA, Stene M, Hintz RL. Spontaneous serum gonadotropin concentrations in the evaluation of precocious puberty. J Pediatr. (1995) 127(1):47–52. doi: 10.1016/S0022-3476(95)70255-5
50. Sathasivam A, Garibaldi L, Shapiro S, Godbold J, Rapaport R. Leuprolide stimulation testing for the evaluation of early female sexual maturation. Clin Endocrinol (Oxf). (2010) 73(3):375–81. doi: 10.1111/j.1365-2265.2010.03796.x
51. Houk CP, Kunselman AR, Lee PA. Adequacy of a single unstimulated luteinizing hormone level to diagnose central precocious puberty in girls. Pediatrics. (2009) 123(6):e1059–63. doi: 10.1542/peds.2008-1180
52. Pasternak Y, Friger M, Loewenthal N, Haim A, Hershkovitz E. The utility of basal serum LH in prediction of central precocious puberty in girls. Eur J Endocrinol. (2012) 166(2):295–9. doi: 10.1530/EJE-11-0720
53. Harrington J, Palmeri MR. Clinical review: distinguishing constitutional delay of growth and puberty from isolated hypogonadotropic hypogonadism: critical appraisal of available diagnostic tests. J Clin Endocrinol Metab. (2012) 97(9):3056–67. doi: 10.1210/jc.2012-1598
54. Carretto F, Salinas-Vert I, Granada-Yvern ML, Murillo-Valles M, GomezGomez C, Puig-Domingo M, et al. The usefulness of the leuprolide stimulation test as a diagnostic method of idiopathic central precocious puberty in girls. Horm Metab Res. (2014) 46(13):959–63. doi: 10.1055/s-0034-1387790
55. Poomthavorn P, Khlairit P, Mahachoklertwattana P. Subcutaneous gonadotropin-releasing hormone agonist (triptorelin) test for diagnosing precocious puberty. Horm Res. (2009) 72(2):114–9. doi: 10.1159/000232164
56. Madsen A, Bruserud IS, Bertelsen BE, Roelants M, Oehme NHB, Viste K, et al. Hormone references for ultrasound breast staging and endocrine profiling to detect female onset of puberty. J Clin Endocrinol Metab. (2020) 105(12):e4886–95. doi: 10.1210/clinem/dgaa679
57. Madsen A, Oehme NB, Roelants M, Bruserud JS, Eide GE, Viste K, et al. Testicular ultrasound to stratify hormone references in a cross-sectional Norwegian study of male puberty. J Clin Endocrinol Metab. (2020) 105(6):1888–98. doi: 10.1210/clinem/dgz094
58. Nijjar JK, Weiss JJ, Misra M, Stanley TN. Utility and duration of leuprolide stimulation test in children. J Pediatr Endocrinol Metab. (2020) 33(8):1073–81. doi: 10.1515/jpem-2019-0414
59. Bangalore Krishna K, Fuqua JS, Rogol AD, Klein KO, Popovic J, Houk CP, et al. Use of gonadotropin-releasing hormone analogs in children: update by an international consortium. Horm Res Paediatr. (2019) 91(6):357–72. doi: 10.1159/000501336
60. Pescovitz OH, Hench KD, Barnes KM, Loriaux DL, Cutler GB Jr. Premature thelarche and central precocious puberty: the relationship between clinical presentation and the gonadotropin response to luteinizing hormone-releasing hormone. J Clin Endocrinol Metab. (1988) 67(3):474–9. doi: 10.1210/jcem-67-3-474
61. Wu FC, Butler GE, Kelnar CJ, Sellar RE. Patterns of pulsatile luteinizing hormone secretion before and during the onset of puberty in boys: a study using an immunoradiometric assay. J Clin Endocrinol Metab. (1990) 70(3):629–37. doi: 10.1210/jcem-70-3-629
62. Goji K, Tanikaze S. Comparison between spontaneous gonadotropin concentration profiles and gonadotropin response to low-dose gonadotropin releasing hormone in prepubertal and early pubertal boys and patients with hypogonadotropic hypogonadism: assessment by using ultrasensitive, time-resolved immunofluorometric assay. Pediatr Res. (1992) 31(5):535–9. doi: 10.1203/00006450-199205000-00027
63. Kulin HE, Bell PM, Santen RJ, Ferber AJ. Integration of pulsatile gonadotropin secretion by timed urinary measurements: an accurate and sensitive 3-hour test. J Clin Endocrinol Metab. (1975) 40(5):783–89. doi: 10.1210/jcem-40-5-783
64. Kulin HE, Santner SJ. Timed urinary gonadotropin measurements in normal infants, children, and adults, and in patients with disorders of sexual maturation. J Pediatr. (1977) 90(5):760–5. doi: 10.1016/S0022-3476(77)81243-2
65. Maesaka H, Suwa S, Tachibana K, Kikuchi N. Quantitation of urinary gonadotropins in normal children. Pediatr Res. (1990) 28(4):401–4. doi: 10.1203/00006450-199010000-00019
66. Demir A, Voutilainen R, Juul A, Dunkel L, Alfthan H, Skakkebaek NE, et al. Increase in first morning voided urinary luteinizing hormone levels precedes the physical onset of puberty. J Clin Endocrinol Metab. (1996) 81(8):2963–7. doi: 10.1210/jcem.81.8.8768859
67. Kolby N, Busch AS, Aksglaede L, Sorensen K, Petersen H, Andersson AM, et al. Nocturnal urinary excretion of FSH and LH in children and adolescents with normal and early puberty. J Clin Endocrinol Metab. (2017) 102(10):3830–8. doi: 10.1210/jc.2017-01192
68. Demir A, Voutilainen R, Hero M. Quantification of urinary gonadotropins by specific assays may improve the evaluation of sex-specific hormonal changes in early infancy. Clin Endocrinol (Oxf). (2024) 101(2):114–20. doi: 10.1111/cen.15064
69. Lee SY, Kim JM, Kim YM, Lim HH. Single random measurement of urinary gonadotropin concentration for screening and monitoring girls with central precocious puberty. Ann Pediatr Endocrinol Metab. (2021) 26(3):178–84. doi: 10.6065/apem.2040208.104
70. Shim YS, An SH, Lee HJ, Kang MJ, Yang S, Hwang IT. Random urinary gonadotropins as a useful initial test for girls with central precocious puberty. Endocr J. (2019) 66(10):891–903. doi: 10.1507/endocrj.EJ19-0071
71. Xu D, Zhou X, Wang J, Cao X, Liu T. The value of urinary gonadotropins in the diagnosis of central precocious puberty: a meta-analysis. BMC Pediatr. (2022) 22(1):453. doi: 10.1186/s12887-022-03481-1
72. Won EJ, Yi A, Ko YJ. Analytical performance evaluation for estradiol using liquid chromatography-tandem mass spectrometry. Clin Biochem. (2023) 113:59–63. doi: 10.1016/j.clinbiochem.2023.01.003
73. Ankarberg-Lindgren C, Norjavaara E. A purification step prior to commercial sensitive immunoassay is necessary to achieve clinical usefulness when quantifying serum 17beta-estradiol in prepubertal children. Eur J Endocrinol. (2008) 158(1):117–24. doi: 10.1530/EJE-07-0403
74. Denver N, Khan S, Homer NZM, Maclean MR, Andrew R. Current strategies for quantification of estrogens in clinical research. J Steroid Biochem Mo Biol. (2019) 192:105373. doi: 10.1016/j.jsbmb.2019.04.022
75. Won E, Yi A, Ko YJ, Kim S, Kang SH, Park G, et al. Establishment of Korean pediatric reference intervals for estradiol using ultra-high-performance liquid chromatography-tandem mass spectrometry. Clin Biochem. (2023) 113:52–8. doi: 10.1016/j.clinbiochem.2023.01.004
76. Geisler J, Ekse D, Helle H, Duong NK, Lonning PE. An optimised, highly sensitive radioimmunoassay for the simultaneous measurement of estrone, estradiol and estrone sulfate in the ultra-low range in human plasma samples. J Steroid Biochem Mo Biol. (2008) 109(1-2):90–5. doi: 10.1016/j.jsbmb.2007.12.011
77. Ankarberg-Lindgren C, Norjavaara E. Changes of diurnal rhythm and levels of total and free testosterone secretion from pre to late puberty in boys: testis size of 3ml is a transition stage to puberty. Eur J Endocrinol. (2004) 151(6):747–57. doi: 10.1530/eje.0.1510747
78. Ghai K, Rosenfield RL. Maturation of the normal pituitary-testicular axis, as assessed by gonadotropin-releasing hormone agonist challenge. J Clin Endocrinol Metab. (1994) 78(6):1336–40. doi: 10.1210/jcem.78.6.8200935
79. Kushnir MM, Blamires T, Rockwood AL, Roberts WL, Yue B, Erdogan E, et al. Liquid chromatography-tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. (2010) 56(7):1138–47. doi: 10.1373/clinchem.2010.143222
80. Stanczyk FZ. Measurement of androgens in women. Semin Reprod Med. (2006) 24(02):078–85. doi: 10.1055/s-2006-939566
81. Ankarberg-Lindgren C, Dahlgren J, Andersson MX. High-sensitivity quantification of serum androstenedione, testosterone, dihydrotestosterone, estrone and estradiol by gas chromatography-tandem mass spectrometry with sex- and puberty-specific reference intervals. J Steroid Biochem Mol Biol. (2018) 183:116–24. doi: 10.1016/j.jsbmb.2018.06.005
82. Herold DA, Fitzgerald RL. Immunoassays for testosterone in women: better than a guess? Clin Chem. (2003) 49(8):1250–1. doi: 10.1373/49.8.1250
83. Taieb J, Mathian B, Millot F, Patricot MC, Mathieu E, Queyrel N, et al. Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem. (2003) 49(8):1381–95. doi: 10.1373/49.8.1381
84. Stanczyk FZ. Diagnosis of hyperandrogenism: biochemical criteria. Best Pract Res Clin Endocrinol Metab. (2006) 20(2):177–91. doi: 10.1016/j.beem.2006.03.007
85. French D. Clinical utility of laboratory developed mass spectrometry assays for steroid hormone testing. J Mass Spectrom Adv Clin Lab. (2023) 28:13–9. doi: 10.1016/j.jmsacl.2023.01.006
86. Anderson RA, Sharpe RM. Regulation of inhibin production in the human male and its clinical applications. Int J Androl. (2000) 23(3):136–44. doi: 10.1046/j.1365-2605.2000.00229.x
87. Gao Y, Du Q, Liu L, Liao Z. Serum inhibin B for differentiating between congenital hypogonadotropic hypogonadism and constitutional delay of growth and puberty: a systematic review and meta-analysis. Endocrine. (2021) 72(3):633–43. doi: 10.1007/s12020-020-02582-0
88. Andersson AM, Skakkebaek NE. Serum inhibin B levels during male childhood and puberty. Mol Cell Endocrinol. (2001) 180(1-2):103–7. doi: 10.1016/S0303-7207(01)00520-2
89. Borelli-Kjaer A, Aksglaede L, Jensen RB, Hagen CP, Ljubicic ML, Busch AS, et al. Serum concentrations of inhibin B in healthy females and males throughout life. J Clin Endocrinol Metab. (2024) 110(1):70–7. doi: 10.1210/clinem/dgae439
90. Rohayem J, Nieschlag E, Kliesch S, Zitzmann M. Inhibin B, AMH, but not INSL3, IGFl or DHEAS support differentiation between constitutional delay of growth and puberty and hypogonadotropic hypogonadism. Andrology. (2015) 3(5):882–7. doi: 10.1111/andr.12088
91. Chaudhary S, Walia R, Bhansali A, Dayal D, Sachdeva N, Singh T, et al. FSH stimulated lnhibin B (FSH-iB): a novel marker for the accurate prediction of pubertal outcome in delayed puberty. J Clin Endocrinol Metab. (2021) 106(9):e3495–e505. doi: 10.1210/clinem/dgab357
92. Bhatia S, Neely EK, Wilson DM. Serum luteinizing hormone rises within minutes after depot leuprolide injection: implications for monitoring therapy. Pediatrics. (2002) 109(2):E30. doi: 10.1542/peds.109.2.e30
93. Brito VN, Latronico AC, Arnhold I, Mendonca BB. A single luteinizing hormone determination 2h after depot leuprolide is useful for therapy monitoring of gonadotropin-dependent precocious puberty in girls. J Clin Endocrinol Metab. (2004) 89(9):4338–42. doi: 10.1210/jc.2003-031537
94. Rosenfield RL, Garibaldi LR, Moll GW Jr, Watson AC, Burstein S. The rapid ovarian secretory response to pituitary stimulation by the gonadotropin-releasing hormone agonist nafarelin in sexual precocity. J Clin Endocrinol Metab. (1986) 63(6):1386–9. doi: 10.1210/jcem-63-6-1386
95. Garibaldi LR, Aceto T Jr, Weber C, Pang S. The relationship between luteinizing hormone and estradiol secretion in female precocious puberty: evaluation by sensitive gonadotropin assays and the leuprolide stimulation test. J Clin Endocrinol Metab. (1993) 76(4):851–6. doi: 10.1210/jcem.76.4.8473395
96. Lanes R, Gunczler P, Osuna JA, Palacios A, Carrillo E, Ramirez X, et al. Effectiveness and limitations of the use of the gonadotropin-releasing hormone agonist leuprolide acetate in the diagnosis of delayed puberty in males. Harm Res. (1997) 48(1):1–4. doi: 10.1159/000185421
97. Rosenfield RL, Perovic N, Ehrmann DA, Barnes RB. Acute hormonal responses to the gonadotropin releasing hormone agonist leuprolide: dose-response studies and comparison to nafarelin-a clinical research center study. J Clin Endocrinol Metab. (1996) 81(9):3408–11. doi: 10.1210/jcem.81.9.8784105
98. Ibanez L, Potau N, Zampolli M, Virdis R, Gussinye M, Carrascosa A, et al. Use of leuprolide acetate response patterns in the early diagnosis of pubertal disorders: comparison with the gonadotropin-releasing hormone test. J Clin Endocrinol Metab. (1994) 78(1):30–5. doi: 10.1210/jcem.78.1.7507123
99. Freire AV, Escobar ME, Gryngarten MG, Arcari AJ, Ballerini MG, Bergada I, et al. High diagnostic accuracy of subcutaneous triptorelin test compared with GnRH test for diagnosing central precocious puberty in girls. Clin Endocrinol (Oxf). (2013) 78(3):398–404. doi: 10.1111/j.1365-2265.2012.04517.x
100. Garibaldi LR, Aceto T Jr, Weber C. The pattern of gonadotropin and estradiol secretion in exaggerated thelarche. Acta Endocrinol (Copenh). (1993) 128(4):345–50. doi: 10.1530/acta.0.1280345
101. Rosenfield RL, Bordini B, Yu C. Comparison of detection of normal puberty in girls by a hormonal sleep test and a gonadotropin-releasing hormone agonist test. J Clin Endocrinol Metab. (2013) 98(4):1591–601. doi: 10.1210/jc.2012-4136
102. Resende EA, Lara BH, Reis JD, Ferreira BP, Pereira GA, Borges MF. Assessment of basal and gonadotropin-releasing hormone-stimulated gonadotropins by immunochemiluminometric and immunofluorometric assays in normal children. J Clin Endocrinol Metab. (2007) 92(4):1424–9. doi: 10.1210/jc.2006-1569
103. Oerter KE, Uriarte MM, Rose SR, Barnes KM, Cutler GB Jr. Gonadotropin secretory dynamics during puberty in normal girls and boys. J Clin Endocrinol Metab. (1990) 71(5):1251–8. doi: 10.1210/jcem-71-5-1251
104. Sakornyutthadej N, Mahachoklertwattana P, Wankanit S, Poomthavorn P. Peak serum luteinising hormone cut-off during gonadotropin-releasing hormone analogue test for diagnosing central precocious puberty was lower in girls with obesity as compared with girls with normal weight. Clin Endocrinol (Oxf). (2024) 100(4):368–78. doi: 10.1111/cen.15026
105. Kang S, Park MJ, Kim JM, Yuk JS, Kim SH. Ongoing increasing trends in central precocious puberty incidence among Korean boys and girls from 2008 to 2020. PLoS One. (2023) 18(3):e0283510. doi: 10.1371/journal.pone.0283510
106. Maione L, Bouvattier C, Kaiser UB. Central precocious puberty: recent advances in understanding the aetiology and in the clinical approach. Clin Endocrinol. (2021) 95(4):542–55. doi: 10.1111/cen.14475
107. Soriano-GuiJJen L, Corripio R, Labarta JI, Canete R, Castro-Feijoo L, Espino R, et al. Central precocious puberty in children living in Spain: incidence, prevalence, and influence of adoption and immigration. J Clin Endocrinol Metab. (2010) 95(9):4305–13. doi: 10.1210/jc.2010-1025
108. Bizzarri C, Spadoni GL, Bottaro G, Montanari G, Giannone G, Cappa M, et al. The response to gonadotropin releasing hormone (GnRH) stimulation test does not predict the progression to true precocious puberty in girls with onset of premature thelarche in the first three years of life. J Clin Endocrinol Metab. (2014) 99(2):433–9. doi: 10.1210/jc.2013-3292
109. Potau N, Ibanez L, Sentis M, Carrascosa A. Sexual dimorphism in the maturation of the pituitary-gonadal axis, assessed by GnRH agonist challenge. Eur J Endocrinol. (1999) 141(1):27–34. doi: 10.1530/eje.0.1410027
110. Chin VL, Cai Z, Lam L, Shah B, Zhou P. Evaluation of puberty by verifying spontaneous and stimulated gonadotropin values in girls. J Pediatr Endocrinol Metab. (2014) 28(3-4):387–92. doi: 10.1515/jpem-2014-0135
111. Cuttler L, Rosenfield RL, Ehrmann DA, Kreiter M, Burstein S, Cara JF, et al. Maturation of gonadotropin and sex steroid responses to gonadotropin-releasing hormone agonist in males. J Clin Endocrinol Metab. (1993) 76(2):362–6. doi: 10.1210/jcem.76.2.8432780
112. Ehrmann DA, Rosenfield RL, Cuttler L, Burstein S, Cara JF, Levitsky LL. A new test of combined pituitary-testicular function using the gonadotropin-releasing hormone agonist nafarelin in the differentiation of gonadotropin deficiency from delayed puberty: pilot studies. J Clin Endocrinol Metab. (1989) 69(5):963–7. doi: 10.1210/jcem-69-5-963
113. Brito VN, Spinola-Castro AM, Kochi C, Kopacek C, Silva PC, Guerra-Junior G. Central precocious puberty: revisiting the diagnosis and therapeutic management. Arch Endocrinol Metab. (2016) 60(2):163–72. doi: 10.1590/2359-3997000000144
114. Latronico AC, Brito VN, Carel JC. Causes, diagnosis, and treatment of central precocious puberty. Lancet Diabetes Endocrinol. (2016) 4(3):265–74. doi: 10.1016/S2213-8587(15)00380-0
115. Kilberg MJ, Vogiatzi MG. Approach to the patient: central precocious puberty. J Clin Endocrinol Metab. (2023) 108(8):2115–23. doi: 10.1210/clinem/dgad081
116. Neely EK, Lee PA, Bloch CA, Larsen L, Yang D, Mattia-Goldberg C, et al. Leuprolide acetate I-month depot for central precocious puberty: hormonal suppression and recovery. Int J Pediatr Endocrinol. (2010) 2010:398639. doi: 10.1186/1687-9856-2010-398639
117. Neely EK, Silverman LA, Geffner ME, Danoff TM, Gould E, Thornton PS. Random unstimulated pediatric luteinizing hormone levels are not reliable in the assessment of pubertal suppression during histrelin implant therapy. Int J Pediatr Endocrinol. (2013) 2013(1):20. doi: 10.1186/1687-9856-2013-20
118. Lee PA, Klein K, Mauras N, Neely EK, Bloch CA, Larsen L, et al. Efficacy and safety of leuprolide acetate 3-month depot 11.25 milligrams or 30 milligrams for the treatment of central precocious puberty. J Clin Endocrinol Metab. (2012) 97(5):1572–80. doi: 10.1210/jc.2011-2704
119. Lee PA, Klein K, Mauras N, Lev-Vaisler T, Bacher P. 36-month treatment experience of two doses of leuprolide acetate 3-month depot for children with central precocious puberty. J Clin Endocrino LMetab. (2014) 99(9):3153–9. doi: 10.1210/jc.2013-4471
120. Eugster EA, Clarke W, Kletter GB, Lee PA, Neely EK, Reiter EO, et al. Efficacy and safety of histrelin subdermal implant in children with central precocious puberty: a multicenter trial. J Clin Endocrinol Metab. (2007) 92(5):1697–704. doi: 10.1210/jc.2006-2479
121. Klein K, Yang J, Aisenberg J, Wright N, Kaplowitz P, Lahlou N, et al. Efficacy and safety of triptorelin 6-month formulation in patients with central precocious puberty. J Pediatr Endocrinol Metab. (2016) 29(11):1241–8. doi: 10.1515/jpem-2015-0376
122. Klein KO, Freire A, Gryngarten MG, Kletter GB, Benson M, Miller BS, et al. Phase 3 trial of a small-volume subcutaneous 6-month duration leuprolide acetate treatment for central precocious puberty. J Clin Endocr Metab. (2020) 105(10):e3660–3671. doi: 10.1210/clinem/dgaa479
123. Popovic J, Geffner ME, Rogol AD, Silverman LA, Kaplowitz PB, Mauras N, et al. Gonadotropin-releasing hormone analog therapies for children with central precocious puberty in the United States. Front Pediatr. (2022) 10:1–12. doi: 10.3389/fped.2022.968485
124. Gohil A, Eugster EA. Gonadotropin-releasing hormone analogs for treatment of central precocious puberty in children younger than 2 years of age. J Pediatr. (2022) 244:215–8. doi: 10.1016/j.jpeds.2021.12.030
125. Silverman LA, Geffner ME, Benson M. Long-acting gonadotropin-releasing hormone analogues for central precocious puberty, including 45-mg 6-month subcutaneous leuprolide acetate: use for treatment and treatment monitoring. Horm Res Paediatr. (2024):1–8. doi: 10.1159/000539020
126. Carel JC, Eugster EA, Rogol A, Ghizzoni L, Palmeri MR, Antoniazzi F, et al. Consensus statement on the use of gonadotropin-releasing hormone analogs in children. Pediatrics. (2009) 123(4):e752–62. doi: 10.1542/peds.2008-1783
127. Lee PA, Luce M, Bacher P. Monitoring treatment of central precocious puberty using basal luteinizing hormone levels and practical considerations for dosing with a 3-month leuprolide acetate formulation. J Pediatr Endocrinol Metab. (2016) 29(11):1249–57. doi: 10.1515/jpem-2016-0026
128. Carel JC, Leger J. Clinical practice. Precocious puberty. N Engl J Med. (2008) 358(22):2366–77. doi: 10.1056/NEJMcp0800459
129. Kunz GJ, Sherman T, Klein KO. Luteinizing hormone (LH) and estradiol suppression and growth in girls with central precocious puberty: is more suppression better? Are pre-injection LH levels useful in monitoring treatment? J Pediatr Endocrinol Metab. (2007) 20(11):1189–98. doi: 10.1515/jpem.2007.20.11.1189
130. Klein KO, Miller BS, Mauras N. Unstimulated luteinizing hormone for assessment of suppression during treatment of central precocious puberty with 6- month subcutaneous leuprolide acetate: correlations with clinical response. Horm Res Paediatr. (2024):1–10. doi: 10.1159/000539110
131. Lewis KA, Eugster EA. Random luteinizing hormone often remains pubertal in children treated with the histrelin implant for central precocious puberty. J Pediatr. (2013) 162(3):562–5. doi: 10.1016/j.jpeds.2012.08.038
Keywords: puberty, precocious puberty, GnRHa, LH, FSH
Citation: Bangalore Krishna K and Garibaldi L (2025) Critical appraisal of diagnostic laboratory tests in the evaluation of central precocious puberty. Front. Pediatr. 12:1504874. doi: 10.3389/fped.2024.1504874
Received: 1 October 2024; Accepted: 26 November 2024;
Published: 21 January 2025.
Edited by:
Sally Radovick, The State University of New Jersey, United StatesReviewed by:
Rodolfo A. Rey, Hospital de Niños Ricardo Gutiérrez, ArgentinaCopyright: © 2025 Bangalore Krishna and Garibaldi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kanthi Bangalore Krishna, YmFuZ2Fsb3Jla3Jpc2huYWsyQHVwbWMuZWR1
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.