- 1Department of Optometry and Pediatric Ophthalmology, Aier Eye Hospital, Jinan University, Guangzhou, Guangdong, China
- 2Department of Pediatric Ophthalmology, Aier Eye Hospital of Wuhan University (Wuhan Aier Eye Hospital), Wuhan, Hubei, China
- 3Department of Optometry and Pediatric Ophthalmology, Hankou Aier Eye Hospital, Wuhan, Hubei, China
- 4Department of Optometry and Pediatric Ophthalmology, Changsha Aier Eye Hospital, Changsha, Hunan, China
Aim: The study aimed to explore the relationship of anthocyanin and its subtypes with myopia in adolescents aged 12–17 years.
Methods: Adolescents data for this cross-sectional study were extracted from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. Anthocyanin and subtypes were obtained using the Nutrient Database for Dietary Studies codes. Myopia was defined as a spherical equivalent of −1.0 diopters or less. The relationships between anthocyanin and subtypes intake and myopia were determined utilizing weighted univariate and multivariate logistic regression models. The relationships were also explored in gender, leisure time, physical activity, sedentary activity, BMI, and serum cotinine subgroups.
Results: A total of 839 adolescents were included for further analysis, among them 245 have myopia. Malvidin (34.98%) was the subtype with the largest anthocyanin intake, followed by cyanidin (22.94%). Compared to adolescents without anthocyanin intake, total anthocyanin intake was related to a lower incidence of myopia (OR = 0.69, 95%CI: 0.51–0.92). Higher intake of cyanidin (OR = 0.69, 95%CI: 0.52–0.92), petunidin (OR = 0.64, 95%CI: 0.42–0.97), and delphinidin (OR = 0.71, 95%CI: 0.51–0.99) were associated with lower odds of myopia in adolescents. Higher total anthocyanin intake was related to lower odds of myopia in those females, leisure time physical activity ≥60 min/day, sedentary time <8 h/day, overweight or obese, and serum cotinine ≥0.05 ng/ml.
Conclusion: Higher total anthocyanin intake, particularly cyanidin, petunidin, and delphinidin, was related to a lower incidence of myopia in adolescents. Increasing dietary anthocyanin intake may be an effective prevention strategy for ocular health.
Introduction
Myopia, or nearsightedness, is a widespread visual condition marked by difficulty in seeing distant objects clearly, affecting millions of adolescents worldwide (1–3). The increasing incidence of myopia in adolescents has heightened concerns about its long-term effects on eye health and quality of life (4, 5). Myopia not only diminishes the quality of life but also creates a reliance on corrective devices like glasses or contact lenses, posing significant public health and economic challenges (6). Recent research has focused on modifiable risk factors, including dietary intake and supplementation, with particular attention to anthocyanin (7).
Anthocyanin is a subclass of flavonoids known for their vivid colors and potential health benefits. They are abundant in foods such as berries, red cabbage, and purple sweet potatoes (8). Anthocyanin plays a role in protecting against oxidative stress and inflammation, which may have potential benefits for vision and eye health (7, 9). A randomized controlled trial reported that oral consumption of 240 mg standardized bilberry extract for 12 weeks relieves the tonic accommodation of the ciliary muscle caused by visual display terminal tasks and near-vision tasks (10). The administration of anthocyanoside oligomer appears to improve visual contrast sensitivity in myopia subjects with asthenopia (11). Based on chick myopia models, compared to the control group, anthocyanin (cyanidin-3-glucoside and cyanidin-3-rutinoside) significantly reduced ocular axial length (12). Delphinidin-3-rutinoside has an inhibitory effect on bovine ciliary smooth muscle contraction (7, 13). Despite these findings, the specific relationship between anthocyanin intake and myopia in adolescents remains underexplored, warranting further investigation.
This study aims to investigate the association between anthocyanin intake, including its various subtypes, and the incidence of myopia in adolescents. Understanding this relationship could inform dietary recommendations and preventive strategies for managing myopia in adolescents, ultimately contributing to better visual health outcomes.
Methods
Study design and participants
Data for this cross-sectional study were extracted from the National Health and Nutrition Examination Survey (NHANES) 2007–2008. The NHANES is a program to assess the health and nutritional status of adults in the United States of America. Participants provided demographic, socioeconomic, and medical information during an in-home interview. A mobile examination center was used to conduct physical and laboratory examinations. The detailed design and data of the NHANES study are available at https://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
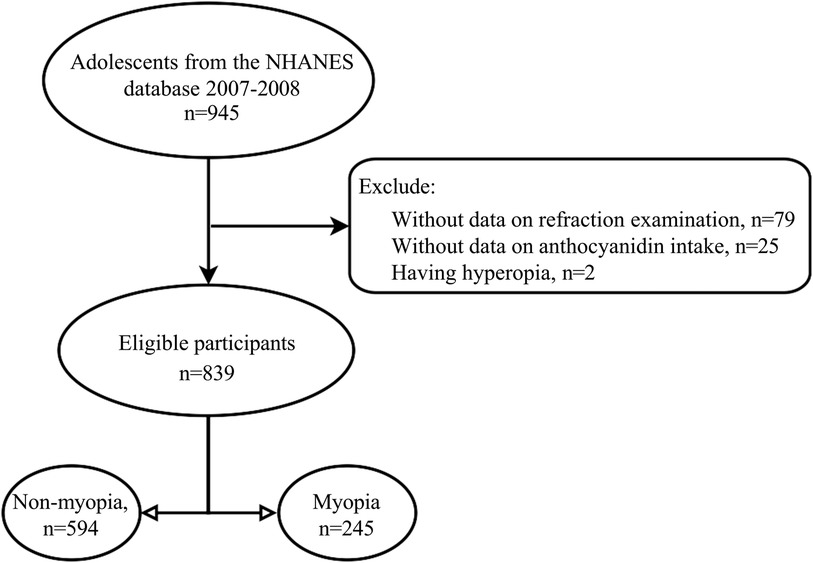
Participants would be included in those meeting the following criteria: (1) age 12–17 years old, (2) receiving a refraction examination, (3) having information on total anthocyanin intake and its sub-classes. Participants would be excluded from those having eye surgery and hyperopia. The selection process was illustrated in Figure 1. The NHANES survey protocol was approved by the National Center for Health Statistics Ethics Review Board. Thus, the Institutional Review Board's approval was exempted from the Ethics Review Board of Hospital.
Anthocyanin intake
This study mainly evaluates anthocyanin intake from foods. Food types were refined using the Nutrient Database for Dietary Studies (FNDDS) codes, which delineate anthocyanin content. The relevant FNDDS version 4.1 was utilized for the survey cycle 2007–2008 (14). The analysis included six anthocyanin types (cyanidin, petunidin, delphinidin, malvidin, pelargonidin, and peonidin) and the total anthocyanin intake from all foods. If the total anthocyanin intake is 0 mg, it is considered as no intake, and if the intake is more than 0 mg, it is considered as intake.
Myopia assessment
Vision examinations were performed for participants aged 12 and above. Refractive status was objectively assessed using an autorefractor (Nidek ARK-760 A, Nidek Co. Ltd., Gamagori, Japan). Each eye was measured three times consecutively, with median values for the sphere, cylinder, and axis recorded. The spherical equivalent refractive error was calculated as the sphere plus half of the column cylinder value. Myopia was defined as a spherical equivalent of −1.0 diopters or less, following previous NHANES refractive error studies (15–17).
Covariates
Vision in adolescents may be influenced by diverse factors, so all characteristics collected in our study were adjusted (18–20). The covariates were as follows: age, gender, ethnicity (divided into Non-Hispanic White, Non-Hispanic Black, Mexican American, and Other Race), poverty income ratio (PIR), household ref person education level (including less than high school, high school or equivalent, and college or above), leisure time physical activity, sedentary activity, body mass index (BMI), serum cotinine, C-reactive protein, and total energy intake. In a typical laboratory, the level of serum cotinine and C-reactive protein were measured.
Statistical analysis
To account for the complex sampling design of the NHANES, statistical analyses were conducted using proper weights (SDMVSTRA, SDMVPSU, WTMEC2YR) for the NHANES sampling. Continuous variables were presented as means and standard errors (S.E), while categorical variables were illustrated as numbers and percentages (%). The differences between anthocyanin intake and non-intake groups were detected utilizing t-tests for continuous variables, and chi-square tests for categorical variables. Weighted univariate and multivariate logistic regression models explored the association between total anthocyanin intake, its subtypes, and myopia in adolescents. The resulted were expressed as odds ratio (OR) and 95 confidence intervals (CIs). Model 1 did not adjust any covariates. Model 2 adjusted socio-demographic covariates. Model 3 adjusted all covariates. In addition, we added sensitive analysis of anthocyanin intake as continuous and categorical variables. Since adolescents with no anthocyanin intake accounted for a certain proportion, when anthocyanin was used as a categorical variable, it was classified as no intake, and the rest was divided according to the median. The results are presented in Supplementary Table S1, Figure S1. The relationships were also explored in gender, leisure time physical activity, sedentary activity, BMI, and serum cotinine subgroups. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA), with two-sided P < 0.05 was considered statistically significant.
Results
Characteristics of adolescents and anthocyanin intake
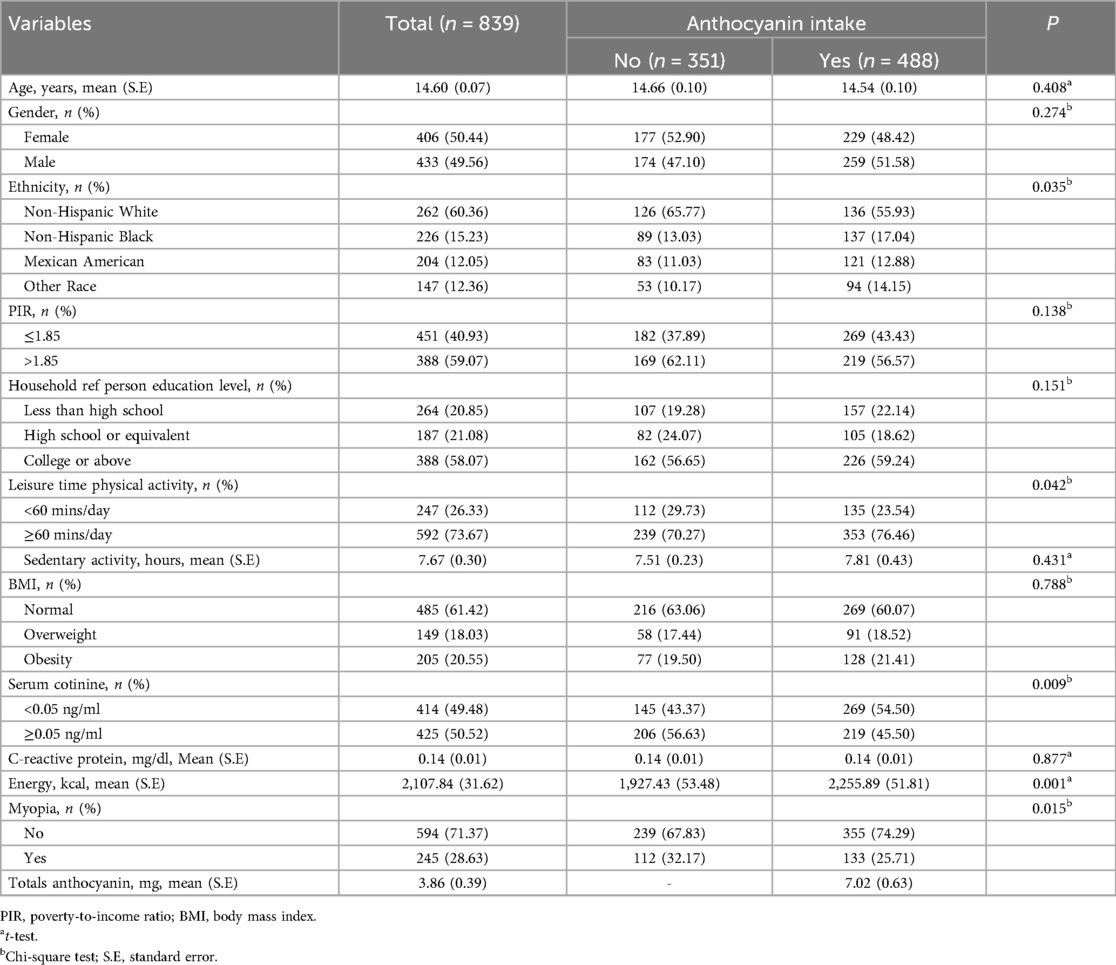
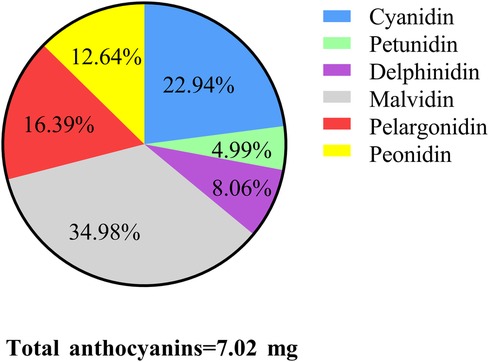
In the study, 106 adolescents were excluded from those without information on refraction examinations (79), without data on anthocyanin intake (n = 25), and hyperopia (n = 2). A total of 839 adolescents were included for further analysis. Among them, the mean age was 14.60 (0.07) years, and 406 (50.44%) were females. 488 adolescents have anthocyanin intake. Statistical differences were observed between the two groups in ethnicity, leisure time physical activity, serum cotinine, total energy intake, and myopia (all P < 0.05). More detailed characteristics were presented in Table 1. Figures 2, 3 depict the intake of anthocyanin intake and its subtypes in adolescents. Malvidin (34.98%) was the subtype with the largest anthocyanin intake, followed by cyanidin (22.94%). Petunidin (4.99%) is the subtype with the lowest proportion of anthocyanin intake in adolescents.
The association between anthocyanin intake and myopia in adolescents
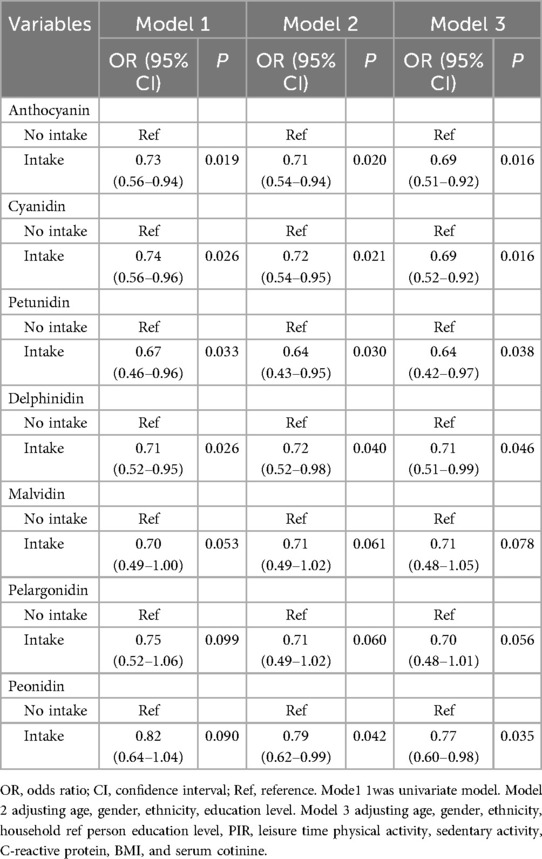
The association between the intake of anthocyanin and its subtypes and myopia in adolescents was illustrated in Table 2. Compared to adolescents without anthocyanin intake, total anthocyanin intake was associated with lower incidence of myopia in model 2 (OR = 0.71, 95%CI: 0.54–0.94) and model 3 (OR = 0.69, 95%CI: 0.51–0.92). After adjusting all covariates, higher intake about subtypes of cyanidin (OR = 0.69, 95%CI: 0.52–0.92), petunidin (OR = 0.64, 95%CI: 0.42–0.97), and delphinidin (OR = 0.71, 95%CI: 0.51–0.99) were related to lower odds of myopia in adolescents. Supplementary Table S1 suggests that there is no statistically significant difference between anthocyanin intake as a continuous variable and the odds of myopia in adolescents. Grouped by median intake of anthocyanin or its subclasses, moderate intake of anthocyanin was associated with myopia in adolescents compared with no anthocyanin intake (OR = 0.60, 95%CI: 0.40–0.92), similar associations were observed in cyanidin, petunidin, and peonidin subclasses. A non-linear association between anthocyanin intake and the incidence of myopia may exist in adolescents. Supplementary Figure S1 shows the distribution of myopia prevalence in different doses of anthocyanin intake.
The association of anthocyanin intake with myopia in gender, leisure time physical activity, sedentary activity, BMI, and serum cotinine subgroups
The associations of anthocyanin intake with myopia were further explored in different subgroups (Table 3). Petunidin (OR = 0.60, 95%CI: 0.39–0.94) and peonidin (OR = 0.63, 95%CI: 0.43–0.94) intake were related to lower odds of myopia in males, while increased total anthocyanin (OR = 0.66, 95%CI: 0.48–0.91) and cyanidin intake (OR = 0.69, 95%CI: 0.50–0.95) were linked to lower incidence of myopia in females. In adolescents with leisure time physical activity ≥60 min/day, total anthocyanin (OR = 0.56, 95%CI: 0.39–0.81), cyanidin (OR = 0.60, 95%CI: 0.42–0.86), petunidin (OR = 0.62, 95%CI: 0.42–0.91), and peonidin (OR = 0.73, 95%CI: 0.54–0.97) intake were related to decreased odds of myopia. In adolescents with sedentary time <8 h/day, total anthocyanin (OR = 0.61, 95%CI: 0.37–0.99) and petunidin (OR = 0.55, 95%CI: 0.31–0.97) intake were related to lower occurrence of myopia. In overweight or obese adolescents, total anthocyanin (OR = 0.59, 95%CI: 0.43–0.82), cyanidin (OR = 0.61, 95%CI: 0.47–0.77), petunidin (OR = 0.60, 95%CI: 0.40–0.91), and peonidin (OR = 0.65, 95%CI: 0.45–0.95) intake were related to lower incidence of myopia. In adolescents with serum cotinine ≥0.05 ng/ml, total anthocyanin (OR = 0.46, 95%CI: 0.24–0.87) and cyanidin (OR = 0.44, 95%CI: 0.26–0.74) intake were linked to lower odds of myopia.
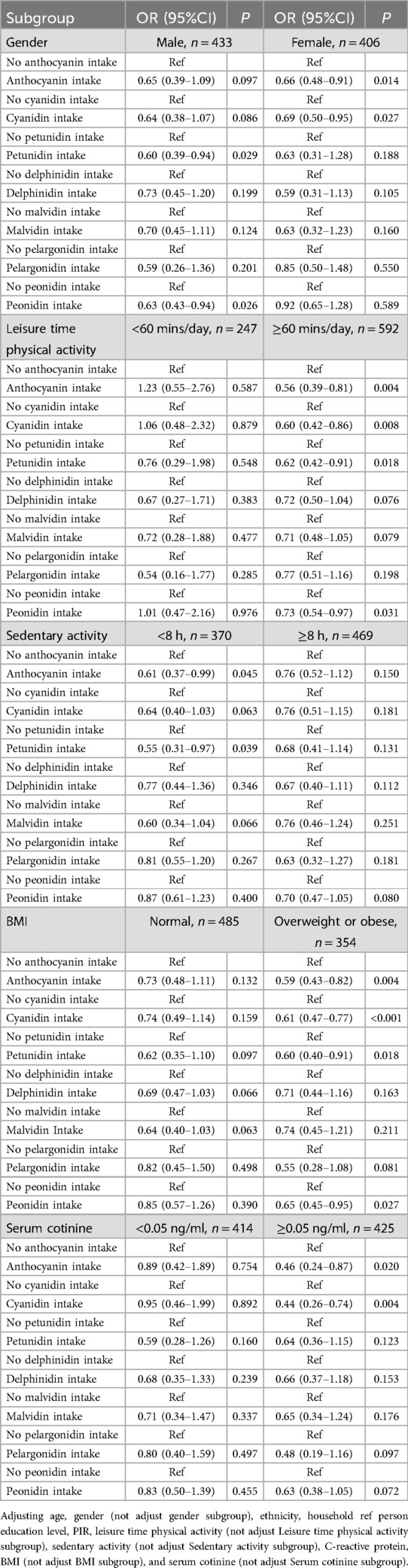
Table 3. The association between anthocyanin intake and myopia in different gender, leisure time physical activity, sedentary activity, BMI, and serum cotinine subgroups.
Discussion
Increased anthocyanin intake is associated with a reduced incidence of myopia in adolescents aged 12–18 years. Specifically, higher overall anthocyanin intake was associated with a lower odds of developing myopia. Furthermore, when examining the effects of anthocyanin subtypes, increased intake of cyanidin, petunidin, and delphinidin was similarly linked to reduced odds of myopia. The findings suggest that dietary anthocyanins, particularly these specific subtypes, may play a role in mitigating the risk of myopia in adolescents.
The findings align with previous research that has indicated the beneficial effects of dietary antioxidants on eye health (7, 21, 22). Anthocyanins may enhance rhodopsin regeneration, thus accelerating rod cell light sensitivity (7). Fan et al. (23) also found that anthocyanin oligomer could improve dry eye disease. Cyanidin improves retinal pigment epithelium cell barrier function by reducing endoplasmic reticulum stress-induced apoptosis, with noted antioxidant activity (24, 25). Delphinidin could protect human retinal pigment epithelial cells from H2O2− induced oxidative damage through anti-apoptotic and antioxidant effects (26). Our study broadens these findings to myopia, an increasingly common condition in adolescents, suggesting that anthocyanin might provide extensive ocular health benefits.
The potential mechanisms between higher anthocyanin intake and lower odds of myopia involve several biological pathways. Anthocyanin is well-recognized for its antioxidant properties, which have a vital role in mitigating oxidative stress in the retina and lens (27). The retina is particularly susceptible to oxidative damage due to its high metabolic activity and exposure to light (28, 29). Cyanidin, petunidin, and delphinidin, as specific anthocyanin subtypes, have been shown to reduce oxidative stress by scavenging free radicals and enhancing antioxidant defenses (8). The reduction in oxidative stress could potentially protect retinal cells from damage that contributes to the development of myopia. Additionally, anthocyanin has anti-inflammatory effects that may further contribute to its protective role. Chronic inflammation is known to affect ocular tissues and may influence myopia progression (30). By reducing inflammation, anthocyanin could help in mitigating the structural integrity and function of the eye. Cyanidin has been shown to inhibit pro-inflammatory cytokines and enzymes, thereby reducing inflammatory responses in various tissues (31). Finally, anthocyanins might influence ocular growth directly. They are thought to impact the regulation of eye growth through effects on signaling pathways involved in the development of myopia. Studies have shown that anthocyanin can modulate pathways such as those involving retinal pigment epithelium cells, which are crucial in maintaining proper ocular growth (32, 33). Delphinidin, in particular, has been shown to enhance blood flow to the retina, potentially supporting better ocular health and growth regulation (34).
The potential impact of anthocyanin on gut health and the microbiome should also be considered. Anthocyanins, plant-derived polyphenols, are metabolized primarily in the large intestine, where they undergo bacterial fermentation. This process not only facilitates the absorption of bioactive compounds but also influences the composition and activity of the gut microbiota (35). Increased intake of anthocyanins may lead to a greater diversity of gut bacteria, as certain strains thrive on these polyphenols and their metabolites. This modulation of gut microbiota can enhance the production of short-chain fatty acids (SCFAs), which have been shown to exhibit anti-inflammatory effects and improve gut barrier function (36). Consequently, a healthier gut microbiome may contribute to improved systemic health, potentially influencing ocular development and reducing the risk of myopia. Conversely, an alteration in gut microbiome composition due to excessive anthocyanin metabolism could lead to imbalances, promoting the growth of pathogenic bacteria and resulting in dysbiosis. Dysbiosis has been linked to various health issues, including inflammation and metabolic disorders, which may indirectly affect ocular health (37).
The differences in anthocyanin intake and myopia between males and females may reflect underlying physiological and hormonal variations. In males, the association of higher petunidin and peonidin intake with reduced myopia odds might be attributed to these compounds’ specific effects on retinal health and visual function, potentially mediated by gender-specific metabolic pathways or hormonal influences. In females, the relationship between higher total anthocyanin and cyanidin intake and lower myopia odds suggests that these compounds may more broadly influence ocular health. The association between higher anthocyanin intake and reduced incidence of myopia in adolescents with leisure time physical activity ≥60 min/day, indicates the potential synergistic effects of diet and physical activity on ocular health. The additional intake of anthocyanin could amplify these beneficial effects. Obesity is often linked to increased systemic inflammation and oxidative stress, which are known risk factors for myopia (3). Anthocyanin, such as cyanidin and petunidin, has shown potential in reducing oxidative stress and inflammation, thus potentially counteracting the negative impact of excess weight on ocular health (7, 38). Similarly, anthocyanin could attenuate smoking-induced acute endothelial dysfunction and improve peripheral temperature in young smokers (39).
Dietary interventions may as a preventive measure against myopia in adolescents. Given the rising prevalence of myopia in younger populations, incorporating anthocyanin-rich foods into the diet could offer a cost-effective and non-invasive strategy to mitigate the risk of developing myopia. Nutritional recommendations emphasizing foods rich in anthocyanin, such as berries and certain vegetables, could be beneficial as part of broader public health initiatives aimed at reducing myopia rates among adolescents. It is important to consider the anthocyanin content in these foods alongside realistic intake levels and absorption rates to ensure effective protective benefits. For instance, typical servings of these fruits and vegetables may yield varying amounts of anthocyanins, which may influence their overall efficacy. Future studies should focus on establishing specific intake targets that reflect not only the anthocyanin composition of these foods but also the bioavailability and potential protective mechanism of these compounds in relation to myopia development. By understanding these factors, we can provide more precise and actionable dietary guidelines that can be realistically adopted in everyday diets, thus maximizing their potential health benefits for adolescents.
Nevertheless, the study has several limitations. First, the research utilized a public dataset with a cross-sectional design, preventing the establishment of a causal relationship between anthocyanin intake and myopia in adolescents. Second, although many covariates were adjusted for, unmeasured covariates in the database may influence our findings. Third, since anthocyanin intake reflects only the current status, and lack of validation studies specifically related to anthocyanin intake assessment, prospective cohort studies are needed to explore its association with myopia in adolescents. Finally, the current analysis is not based on direct measurements of anthocyanin levels in blood or urine, but rather on estimates derived from dietary data. More objective measurements of anthocyanin intake should be investigated in the future.
Conclusion
Higher anthocyanin intake, particularly cyanidin, petunidin, and delphinidin, was associated with a lower incidence of myopia in adolescents. The findings support the potential for dietary anthocyanin to contribute to myopia prevention strategies. Future research should focus on longitudinal studies and clinical trials to validate these findings and further explore the underlying mechanisms of anthocyanins’ effects on ocular health.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: NHANES, https://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
YC: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing. QX: Conceptualization, Project administration, Supervision, Writing – original draft, Writing – review & editing. LL: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing, Funding acquisition. YL: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. ZZ: Data curation, Formal Analysis, Investigation, Methodology, Writing – review & editing. ZY: Conceptualization, Project administration, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was supported by the Science and Technology Foundation of Health and Family Planning Commission of WuHan (No. WG20B06).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statements
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1503926/full#supplementary-material
References
1. Holden BA, Fricke TR, Wilson DA, Jong M, Naidoo KS, Sankaridurg P, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology. (2016) 123(5):1036–42. doi: 10.1016/j.ophtha.2016.01.006
2. Rudnicka AR, Kapetanakis VV, Wathern AK, Logan NS, Gilmartin B, Whincup PH, et al. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: implications for aetiology and early prevention. Br J Ophthalmol. (2016) 100(7):882–90. doi: 10.1136/bjophthalmol-2015-307724
3. Grzybowski A, Kanclerz P, Tsubota K, Lanca C, Saw SM. A review on the epidemiology of myopia in school children worldwide. BMC Ophthalmol. (2020) 20(1):27. doi: 10.1186/s12886-019-1220-0
4. Modjtahedi BS, Abbott RL, Fong DS, Lum F, Tan D. Reducing the global burden of myopia by delaying the onset of myopia and reducing myopic progression in children: the academy’s task force on myopia. Ophthalmology. (2021) 128(6):816–26. doi: 10.1016/j.ophtha.2020.10.040
5. Naidoo KS, Fricke TR, Frick KD, Jong M, Naduvilath TJ, Resnikoff S, et al. Potential lost productivity resulting from the global burden of myopia: systematic review, meta-analysis, and modeling. Ophthalmology. (2019) 126(3):338–46. doi: 10.1016/j.ophtha.2018.10.029
6. Sankaridurg P, Tahhan N, Kandel H, Naduvilath T, Zou H, Frick KD, et al. Imi impact of myopia. Invest Ophthalmol Vis Sci. (2021) 62(5):2. doi: 10.1167/iovs.62.5.2
7. Nomi Y, Iwasaki-Kurashige K, Matsumoto H. Therapeutic effects of anthocyanins for vision and eye health. Molecules. (2019) 24(18):3311. doi: 10.3390/molecules24183311
8. Mattioli R, Francioso A, Mosca L, Silva P. Anthocyanins: a comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules. (2020) 25(17):3809. doi: 10.3390/molecules25173809
9. Speer H, D'Cunha NM, Alexopoulos NI, McKune AJ, Naumovski N. Anthocyanins and human health-a focus on oxidative stress, inflammation and disease. Antioxidants (Basel). (2020) 9(5):366. doi: 10.3390/antiox9050366
10. Kosehira M, Machida N, Kitaichi N. A 12-week-long intake of bilberry extract (Vaccinium Myrtillus L.) improved objective findings of ciliary muscle contraction of the eye: a randomized, double-blind, placebo-controlled, parallel-group comparison trial. Nutrients. (2020) 12(3):600. doi: 10.3390/nu12030600
11. Lee J, Lee HK, Kim CY, Hong YJ, Choe CM, You TW, et al. Purified high-dose anthocyanoside oligomer administration improves nocturnal vision and clinical symptoms in myopia subjects. Br J Nutr. (2005) 93(6):895–9. doi: 10.1079/BJN20051438
12. Iida H, Nakamura Y, Matsumoto H, Kawahata K, Koga J, Katsumi O. Differential effects of black currant anthocyanins on diffuser- or negative Lens-induced ocular elongation in chicks. J Ocul Pharmacol Ther. (2013) 29(6):604–9. doi: 10.1089/jop.2012.0224
13. Matsumoto H, Kamm KE, Stull JT, Azuma H. Delphinidin-3-Rutinoside relaxes the bovine ciliary smooth muscle through activation of etb receptor and No/cgmp pathway. Exp Eye Res. (2005) 80(3):313–22. doi: 10.1016/j.exer.2004.10.002
14. Sebastian RS, Wilkinson Enns C, Goldman JD, Moshfegh AJ. Dietary flavonoid intake is inversely associated with cardiovascular disease risk as assessed by body mass index and waist circumference among adults in the United States. Nutrients. (2017) 9(8):827. doi: 10.3390/nu9080827
15. Lyu IJ, Oh SY. Association between age at menarche and risk of myopia in the United States: NHANES 1999–2008. PLoS One. (2023) 18(5):e0285359. doi: 10.1371/journal.pone.0285359
16. Qiu M, Wang SY, Singh K, Lin SC. Association between myopia and glaucoma in the United States population. Invest Ophthalmol Vis Sci. (2013) 54(1):830–5. doi: 10.1167/iovs.12-11158
17. Zhou Z, Li S, Yang Q, Yang X, Liu Y, Hao K, et al. Association of N-3 polyunsaturated fatty acid intakes with juvenile myopia: a cross-sectional study based on the NHANES database. Front Pediatr. (2023) 11:1122773. doi: 10.3389/fped.2023.1122773
18. He X, Sankaridurg P, Xiong S, Li W, Naduvilath T, Lin S, et al. Prevalence of myopia and high myopia, and the association with education: Shanghai child and adolescent large-scale eye study (scale): a cross-sectional study. BMJ Open. (2021) 11(12):e048450. doi: 10.1136/bmjopen-2020-048450
19. Chiang SY, Weng TH, Lin CM, Lin SM. Ethnic disparity in prevalence and associated risk factors of myopia in adolescents. J Formos Med Assoc. (2020) 119(1 Pt 1):134–43. doi: 10.1016/j.jfma.2019.03.004
20. Zheng T, Jiang S, Fu W, Liu H, Ding S, Xv D, et al. Prevalence of and risk factors for myopia among urban and rural children in northeast China: protocol for a school-based cross-sectional study. BMJ Open. (2024) 14(2):e077735. doi: 10.1136/bmjopen-2023-077735
21. Khoo HE, Ng HS, Yap WS, Goh HJH, Yim HS. Nutrients for prevention of macular degeneration and eye-related diseases. Antioxidants (Basel). (2019) 8(4):85. doi: 10.3390/antiox8040085
22. Zhu F. Anthocyanins in cereals: composition and health effects. Food Res Int. (2018) 109:232–49. doi: 10.1016/j.foodres.2018.04.015
23. Fan M, Kim SA, Choi YJ, Tang Y, Yang HP, Kim EK. Anthocyanin oligomer (grape skin extract) administration improves dry eye disease: a randomised, double-blind, placebo-controlled study. Clin Exp Ophthalmol. (2023) 51(2):122–30. doi: 10.1111/ceo.14207
24. Peng W, Wu Y, Peng Z, Qi W, Liu T, Yang B, et al. Cyanidin-3-glucoside improves the barrier function of retinal pigment epithelium cells by attenuating endoplasmic reticulum stress-induced apoptosis. Food Res Int. (2022) 157:111313. doi: 10.1016/j.foodres.2022.111313
25. Anfuso CD, Giurdanella G, Longo A, Cosentino A, Agafonova A, Rusciano D, et al. Antioxidant activity of cyanidin-3-O-glucoside and verbascoside in an in vitro model of diabetic retinopathy. Front Biosci (Landmark Ed). (2022) 27(11):308. doi: 10.31083/j.fbl2711308
26. Ni T, Yang W, Xing Y. Protective effects of delphinidin against H2O2-induced oxidative injuries in human retinal pigment epithelial cells. Biosci Rep. (2019) 39(8):BSR20190689. doi: 10.1042/bsr20190689
27. Tao Z, Zhang R, Zuo W, Ji Z, Fan Z, Chen X, et al. Association between dietary intake of anthocyanidins and heart failure among American adults: NHANES (2007–2010 and 2017–2018). Front Nutr. (2023) 10:1107637. doi: 10.3389/fnut.2023.1107637
28. Ozawa Y. Oxidative stress in the light-exposed retina and its implication in age-related macular degeneration. Redox Biol. (2020) 37:101779. doi: 10.1016/j.redox.2020.101779
29. Böhm EW, Buonfiglio F, Voigt AM, Bachmann P, Safi T, Pfeiffer N, et al. Oxidative stress in the eye and its role in the pathophysiology of ocular diseases. Redox Biol. (2023) 68:102967. doi: 10.1016/j.redox.2023.102967
30. Xu R, Zheng J, Liu L, Zhang W. Effects of inflammation on myopia: evidence and potential mechanisms. Front Immunol. (2023) 14:1260592. doi: 10.3389/fimmu.2023.1260592
31. Jin X, Wang C, Wu W, Liu T, Ji B, Zhou F. Cyanidin-3-Glucoside alleviates 4-hydroxyhexenal-induced Nlrp3 inflammasome activation via jnk-C-jun/ap-1 pathway in human retinal pigment epithelial cells. J Immunol Res. (2018) 2018:5604610. doi: 10.1155/2018/5604610
32. Liu Y, Liu M, Chen Q, Liu GM, Cao MJ, Sun L, et al. Blueberry polyphenols ameliorate visible light and lipid-induced injury of retinal pigment epithelial cells. J Agric Food Chem. (2018) 66(48):12730–40. doi: 10.1021/acs.jafc.8b05272
33. Wang C, Wang K, Li P. Blueberry anthocyanins extract attenuated diabetic retinopathy by inhibiting endoplasmic Reticulum stress via the mir-182/Ogg1 axis. J Pharmacol Sci. (2022) 150(1):31–40. doi: 10.1016/j.jphs.2022.06.004
34. Yamazaki K, Ishida K, Otsu W, Muramatsu A, Nakamura S, Yamada W, et al. Delphinidins from maqui berry (Aristotelia Chilensis) ameliorate the subcellular organelle damage induced by blue light exposure in murine photoreceptor-derived cells. BMC Complement Med Ther. (2024) 24(1):3. doi: 10.1186/s12906-023-04322-z
35. Ayvaz H, Cabaroglu T, Akyildiz A, Pala CU, Temizkan R, Ağçam E, et al. Anthocyanins: metabolic digestion, bioavailability, therapeutic effects, current pharmaceutical/industrial use, and innovation potential. Antioxidants (Basel). (2023) 12(1):48. doi: 10.3390/antiox12010048
36. Verediano TA, Stampini Duarte Martino H, Dias Paes MC, Tako E. Effects of anthocyanin on intestinal health: a systematic review. Nutrients. (2021) 13(4):1331. doi: 10.3390/nu13041331
37. Li B, Wang L, Bai W, Chen W, Chen F, Shu C. Unveiling the metabolic modulatory effect of anthocyanin and gut Microbiota involvement. In Anthocyanins: Chemistry, Processing & Bioactivity. Singpore: Springer Nature Singapore (2021). doi: 10.1007/978-981-16-7055-8_16
38. Lee YM, Yoon Y, Yoon H, Park HM, Song S, Yeum KJ. Dietary anthocyanins against obesity and inflammation. Nutrients. (2017) 9(10):1089. doi: 10.3390/nu9101089
Keywords: adolescent, myopia, adolescents, cyanidin, petunidin, delphinidin
Citation: Chen Y, Xu Q, Lv L, Liu Y, Zhang Z and Yang Z (2024) The association between anthocyanin intake and myopia in adolescents: a cross-sectional study of NHANES. Front. Pediatr. 12:1503926. doi: 10.3389/fped.2024.1503926
Received: 30 September 2024; Accepted: 31 October 2024;
Published: 15 November 2024.
Edited by:
Tim S. Nawrot, University of Hasselt, BelgiumReviewed by:
Patrick De Boever, University of Antwerp, BelgiumKenneth Vanbrabant, University of Hasselt, Belgium
Copyright: © 2024 Chen, Xu, Lv, Liu, Zhang and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhikuan Yang, emt5YW5nX2V5ZUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Ying Chen1,2,3,†
Ying Chen1,2,3,† Zhikuan Yang
Zhikuan Yang