- 1Division of Pediatric Critical Care, Department of Pediatrics, Heersink School of Medicine, The University of Alabama at Birmingham, Birmingham, AL, United States
- 2Division of Nephrology, Department of Pediatrics, Heersink School of Medicine, The University of Alabama at Birmingham, Birmingham, AL, United States
- 3Division of Nephrology, Department of Medicine, Heersink School of Medicine, The University of Alabama at Birmingham, Birmingham, AL, United States
- 4Department of Biostatistics, University of Alabama at Birmingham, Birmingham, AL, United States
Introduction: Fluid accumulation, presently defined as a pathologic state of overhydration/volume overload associated with clinical impact, is common and associated with worse outcomes. At times, deresuscitation, the active removal of fluid via diuretics or ultrafiltration, is necessary. There is no consensus regarding deresuscitation in children admitted to the pediatric intensive care unit. Little is known regarding perceptions and practices among pediatric intensivists and nephrologists regarding fluid provision and deresuscitation.
Methods: Cross-sectional electronic survey of pediatric nephrologists and intensivists from academic societies in the United States designed to better understand fluid management between disciplines. A clinical vignette was used to characterize the perceptions of optimal timing and method of deresuscitation initiation at four timepoints that correspond to different stages of shock.
Results: In total, 179 respondents (140 intensivists, 39 nephrologists) completed the survey. Most 75.4% (135/179) providers believe discussing fluid balance and initiating fluid deresuscitation in pediatric intensive care unit (PICU) patients is “very important”. The first clinical vignette time point (corresponding to resuscitation phase of early shock) had the most dissimilarity between intensivists and nephrologists (p = 0.01) with regards to initiation of deresuscitation. However, providers demonstrated increasing agreement in their responses to initiate deresuscitation as the clinical vignette progressed. Compared to intensivists, nephrologists were more likely to choose “dialysis or ultrafiltration” as a deresuscitation method during the optimization [10.3 vs. 2.9% (p = 0.07)], stabilization [18.0% vs. 3.6% (p < 0.01)], and evacuation [48.7% vs. 23.6% (p < 0.01)] phases of shock. Conversely, intensivists were more likely to utilize scheduled diuretics than nephrologists [47.1% vs. 28.2% (p = 0.04)] later on in the patient course.
Discussion: Most physicians believe that discussing fluid balance and deresuscitation is important. Nevertheless, when to initiate deresuscitation and how to accomplish it differed between nephrologist and intensivists. Widely understood and operationalizable definitions, further research, and eventually evidence-based guidelines are needed to help guide care.
1 Introduction
Intravenous fluids (IVF) are ubiquitously administered in the pediatric intensive care unit (PICU) with the physiologically derived goals of improving cardiac output, meeting daily nutrition needs, and delivering medications/blood products. Unfortunately, this common practice can result in fluid accumulation—a pathologic state of overhydration/volume overload, associated with clinical impact which may vary by age, comorbidity, and phase of illness (1). Some authors utilize the term fluid overload interchangeably with fluid accumulation to describe a pathologic state of positive fluid balance associated with adverse events (2, 3). Excessive fluid results in interstitial edema, which by a variety of mechanisms (impaired oxygen and metabolite diffusion, impaired capillary blood flow, disturbed tissue architecture etc.) can contribute to organ failure (1, 4). It should be noted that patients can have fluid accumulation while still being intravascularly hypovolemic due to increase in total body water. Fluid accumulation is associated with poor outcomes, including increased risk for mortality, increased length of mechanical ventilation, increased hospital and PICU lengths of stay, and increased use of continuous renal replacement therapy (CRRT) (5–12).
One of the most common scenarios among PICU patients that leads to fluid accumulation is shock. While the incidence of shock among critically ill pediatric patients varies in the literature, it is one of the leading causes of morbidity and mortality (13). Characterized by inadequate oxygen and glucose delivery to meet metabolic demand, patients with shock commonly receive administration of fluids in the form of boluses, or continuous administration with the goal to improve oxygen delivery to vital tissues. The “ROSE” conceptual model— resuscitation, optimization, stabilization, evacuation—is a framework that describes four distinct phases of fluid management in shock (14) (Figure 1). Resuscitation (R) is the initial phase of shock, where a patient is at high risk for mortality if oxygen delivery is not improved. R prioritizes organ rescue and often includes aggressive fluid administration (up to 60 ml/kg) (15). As a result of damaged vascular endothelium and the endothelial glycocalyx (most common in septic shock), fluid tends to extravasate from the intravascular space, thus fluid initially deemed helpful can become problematic (16). The second stage, optimization (O) relies on careful titration of fluid administration, optimizing cardiac output, and minimizing additional organ injury by congestion and edema (17). Third, stabilization (S) focuses on organ support as the patient slowly returns to homeostasis. In the stabilization phase, intensivists focus on late conservative fluid management and commonly target an even or net negative fluid balance, which has been associated with decreased mortality (18). Finally, the evacuation (E) phase emphasizes organ recovery and includes strategies to return to a neutral fluid balance. The evacuation phase classically has included deresuscitation—presently defined as the active removal of fluid via ultrafiltration or diuretics (19). Other authors have used the following definition: active fluid removal to treat fluid accumulation causing organ dysfunction (1). Notably, deresuscitation involves more than fluid restriction.
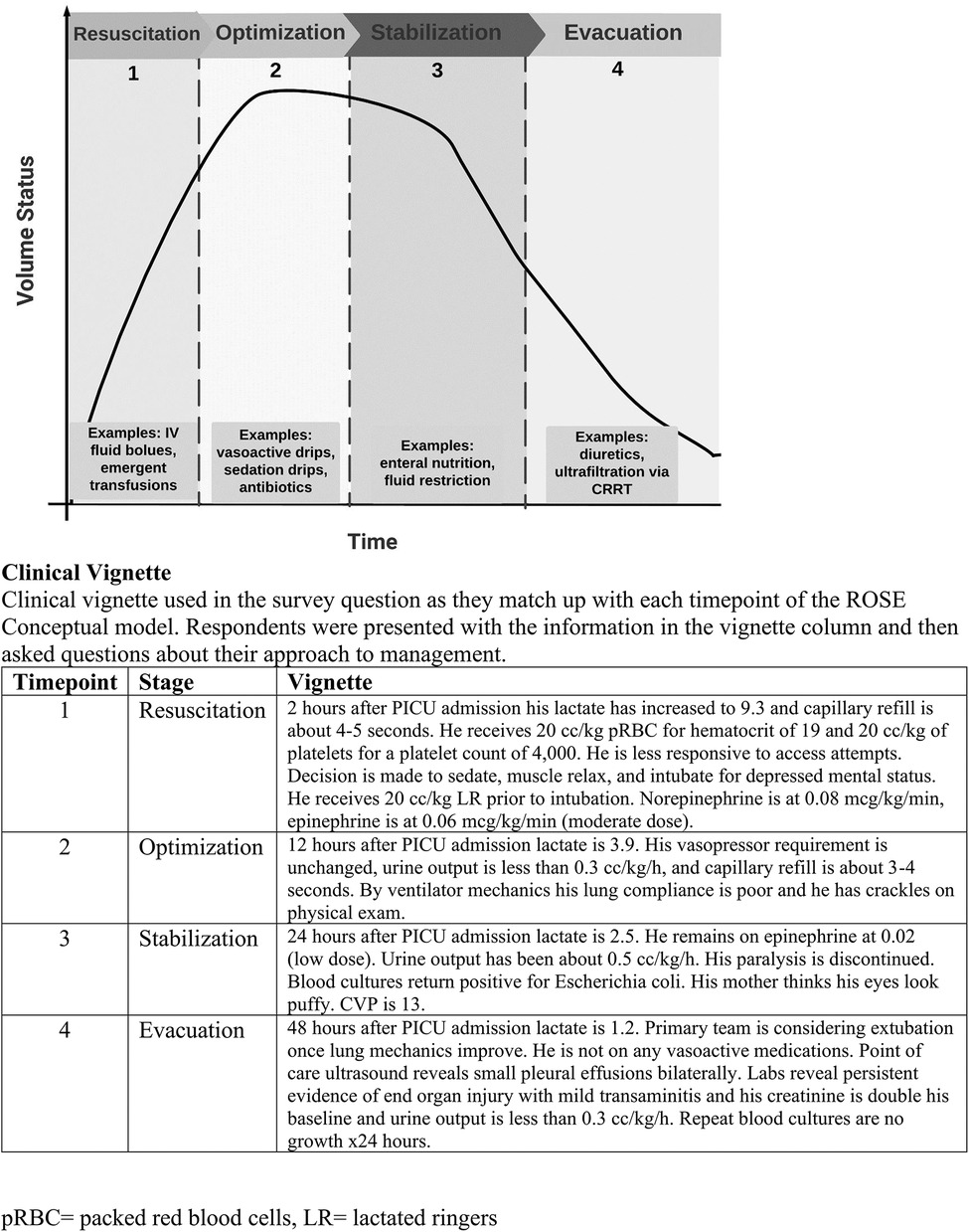
Figure 1. ROSE diagram. ROSE diagram that illustrates a conceptual framework of fluid management in shock. A patient's volume status ’s plotted over the clinical course. (Adapted from Malbrain, 2024).
While consensus-based guidelines offer more concrete recommendations on appropriate resuscitation of the critically ill child in shock, consensus guidelines for timing or method of fluid deresuscitation do not currently exist (15). In critically ill patients, deresuscitation involves close communication between intensivists and nephrologists, especially when CRRT is being considered for patients with acute kidney injury, significant fluid accumulation, or electrolyte derangements (20). Whether differences between pediatric nephrologist and intensivists exist in the perceived best strategies to prevent and treat fluid accumulation has not been fully evaluated.
In order to improve our understanding of providers’ perceptions of fluid accumulation and subsequently deresuscitation, we performed a survey-based study anchored on a clinical vignette. We hypothesized that differences exist between pediatric intensivists and pediatric nephrologists regarding (a) how providers determine the transition point between resuscitation and deresuscitation and (b) what strategies providers use to mitigate and correct fluid accumulation.
2 Methods
2.1 Study population
We performed a cross-sectional survey-based study to assess perceptions and approaches to pediatric patients with fluid accumulation. We surveyed attending physicians in pediatric nephrology and pediatric critical care as they are the two groups most closely involved in evaluating fluid status and managing the consequences of fluid accumulation in the PICU population. The surveyed population represents academic clinicians in majority US-based hospitals and utilized convenience-based sampling by disseminating through the Pediatric Society of Critical Care Medicine website and the following listservs: pediatric Continuous Renal Replacement Therapy (pCRRT), Pediatric Acute Lung Injury and Sepsis Investigators Network (PALISI), and American Society of Pediatric Nephrology (ASPN). A nonresponse rate was not calculated because the total sample size was unknown. This is due in part to inability to quantify number of providers targeted because of duplicate email addresses included in the listservs. According to the American Board of Pediatrics, 729 pediatricians currently maintain Pediatric Nephrology certification, while 3,128 maintain certification in Pediatric Critical Care Medicine (21).
2.2 Survey design
The survey was 25 questions long and took less than 10 min to complete (Supplementary File S1). It was generated using structured questions intended to discern provider perceptions regarding deresuscitation. The initial survey questions provided respondents an opportunity to share their perspectives on fluid accumulation, deresuscitation, and the clinical culture at their institution. A 5-point Likert scale was utilized when possible. A clinical vignetter was designed with four time points that align with the ROSE framework (Resuscitation, Optimization, Stabilization, and Evacuation) for fluid management in shock (Figure 1). It outlined the clinical course of a 4-year-old patient with leukemia presenting in shock (heart rate 140 beats per minute, blood pressure 68/39 mmHg, respiratory rate 26 breaths per minute, temperature 103°F, lactate 7.0 mmol/L) who received 3 × 20 ml/kg fluid boluses (lactated ringers) and was placed on catecholamine infusions to maintain mean arterial blood pressure > 10th percentile for age. Following this vignette introduction, respondents were given follow-up details as the case progressed and then asked to decide whether they believed the patient was still in shock; whether initiation of deresuscitation was appropriate; and if so, what strategies they would utilize to perform deresuscitation. The survey concluded by asking demographic information about the provider (e.g., total years in practice) and their institution (e.g., number of PICU beds).
The survey was anonymous, voluntary, and responses were confidential. Consent from the provider, mentioned at the beginning of the survey, was assumed upon completion. Since this survey was novel and designed by our study team, no current validity evidence exists in the literature. To address internal consistency, the survey was independently pre-tested and reviewed by 2 expert pediatric intensivists and 2 expert pediatric nephrologists. After reaching agreement through iterative review, the survey was entered into REDCap which served as the end-user survey interface and the data management platform (20, 21). Data was stored within REDCap study database and on password protected institutional devices. Data collection began in November 2023 and concluded in May 2024. We were unable to control for respondents filling out the survey multiple times as we did not collect any personal identifiers. The institutional review board for the University of Alabama at Birmingham approved this study (IRB-300011177).
2.3 Statistical analysis
All responses were mandatory, so we did not have to account for missing data. Descriptive analyses (means (standard deviations), medians (interquartile ranges), and frequency distributions (%)) described study participants and relevant characteristics. Categorical responses were summarized as frequency (%) and compared between groups using Chi-square or Fischer exact test. Proportion difference with 95% confidence interval were reported. Continuous measures were compared between groups using t-test or Wilcoxon rank-sum tests as appropriate. Similar analyses were performed for the subgroups—whether responses differ by years of experience or the size of the institution within each group as well as overall. For years of experience, we chose 7 years as a cut-off as this was the median number of years post-training for intensivists. We chose number of ICU beds as a surrogate for size of institution and opted for a cut-off of 26 beds as this was the median for both intensivists’ and nephrologists’ responses. All statistical tests were two-tailed with p value <0.05 used to indicate statistical significance. Analyses were performed using SAS version 9.4 software (SAS Institute, Inc.; Cary, NC).
3 Results
3.1 Demographics
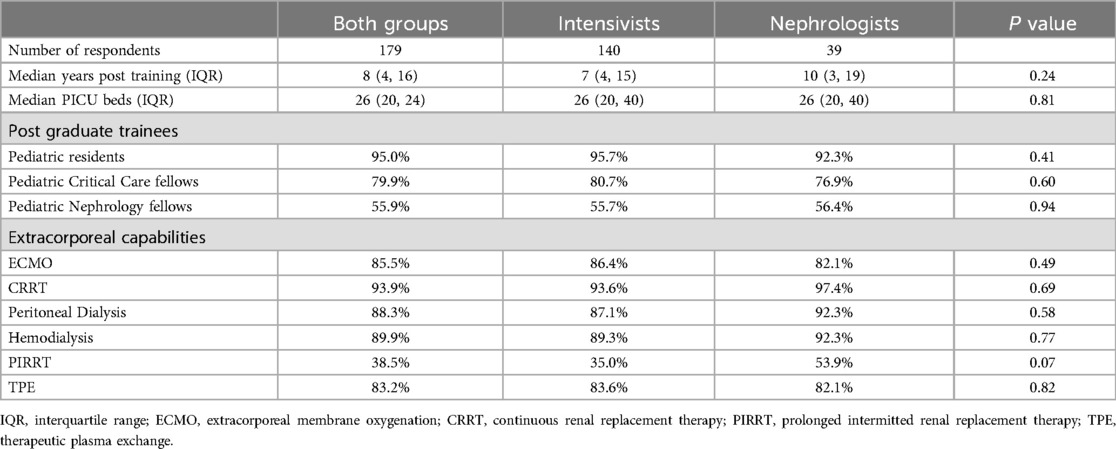
The target study population of this survey was pediatric intensivists (including cardiac intensivists but excluding neonatologists) and pediatric nephrologists who have finished training. The survey was completed by 140 pediatric intensivists and 39 pediatric nephrologists for a total of 179 respondents. Only 4 providers opened but did not complete the survey (2.2%). The median (IQR) number of years post-medical training was 7 (4, 15) for intensivists and 10 (3, 19) for nephrologists (p = 0.24). Providers from both groups self-reported a median (IQR) of 26 (20, 40) PICU beds at their institution. Regarding post-graduate trainees at their institution, 95.0% of providers reported having pediatric residents, 79.9% reported having pediatric critical care fellows, and 55.9% reported pediatric nephrology fellows. CRRT capabilities were reported by 93.3% of survey respondents and extracorporeal membrane oxygenation capabilities were reported by 85.5% of providers (Table 1).
3.2 Importance of fluid balance
The overall perspective regarding fluid balance was not significantly different between intensivists and nephrologists. At large, 135/179 (75.4%) of providers believed discussing fluid balance and initiating fluid deresuscitation in PICU patients is “very important”, but when asked how their institution views this practice only 79/179 (44.1%) responded “very important”. Fluid balance is explicitly discussed “all of the time” when rounding on critically ill patients by 56.0% of intensivists and 43.6% of nephrologists (p = 0.15). When asked if there is consistency among providers within the respondent's specialty in determining clinical stability prior to initiating deresuscitation, less than half of respondents agreed or strongly agreed that practice habits are consistent (strongly disagree 6.1%, disagree 29.6%, neither disagree nor agree 24.0%, agree 30.7%, and strongly agree 9.5%). Institutional deresuscitation protocols were rare with only 2.2% of providers reporting following one.
3.3 Measuring fluid balance
The majority of intensivists (70.0%) and nephrologists (84.6%) report using weight in addition to intake/output method to assess fluid balance. Ultrasonography to assess fluid balance was infrequent (intensivists 31.4% vs. nephrologists 18.0%, p = 0.11). Greater than 90% of providers from both groups selected each of the following as crucial components to their assessment of fluid balance: physical exam, urine output, intake and output, and daily weights (Supplementary Figure S1). The majority of providers (83.2%) stated they have the tools necessary to accurately assess and recommend fluid goals, but upon free text questioning common responses to factors limiting fluid balance assessment included inaccurate weights, inconsistently charted intake and output, and informal electronic medical record recording and ordering.
3.4 Deresuscitation initiation
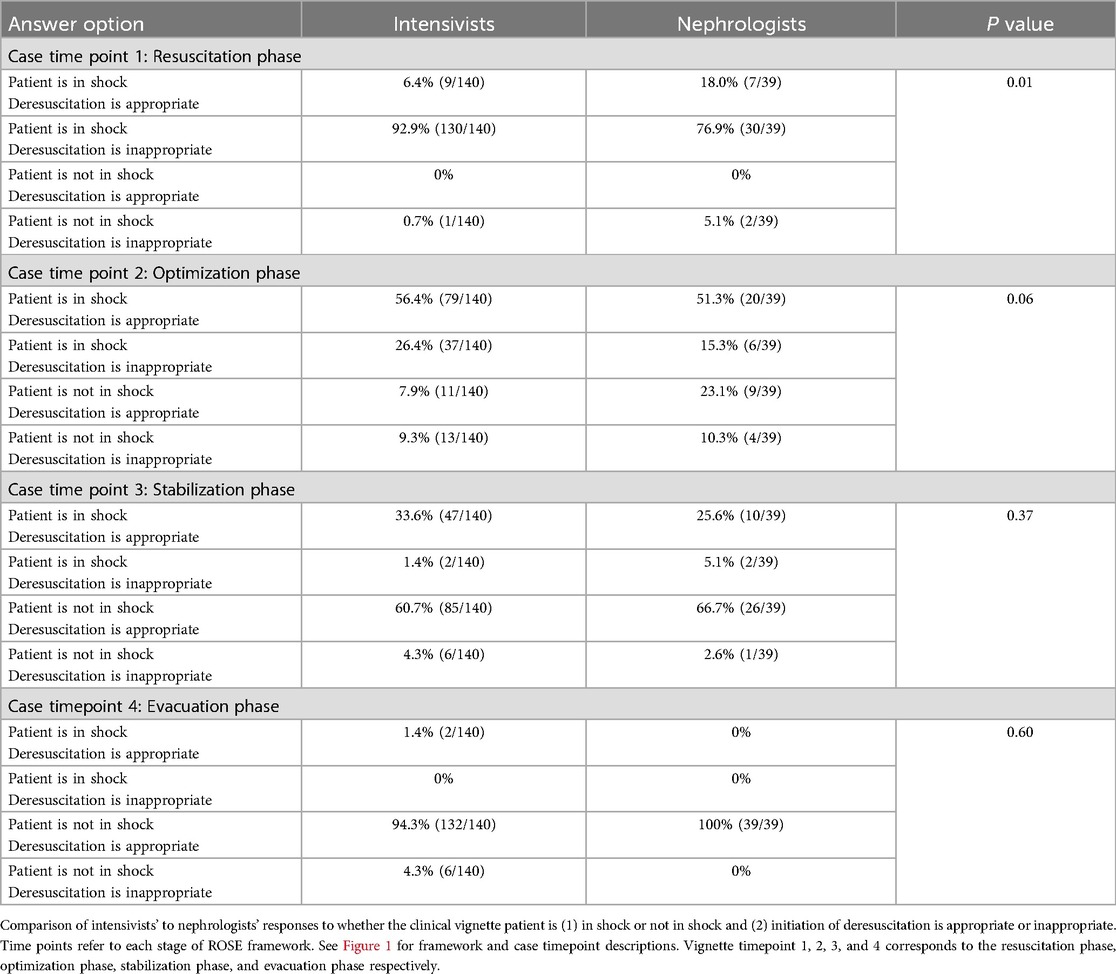
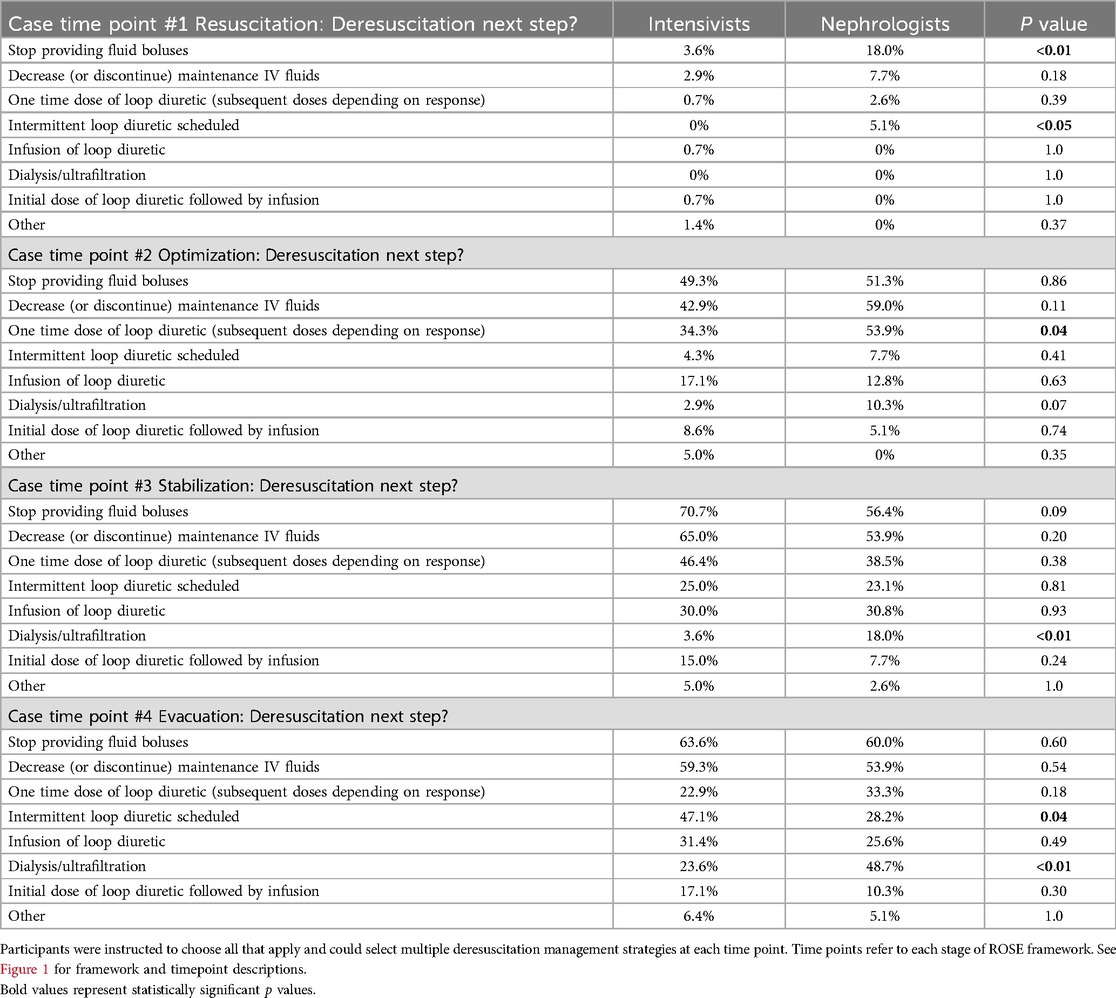
A clinical vignette was used to characterize the perceptions of optimal timing and method of deresuscitation initiation. The first time point (corresponding to resuscitation) had the most dissimilarity between intensivists and nephrologists with nephrologists more likely to initiate deresuscitation [18.0% vs. 6.4% (p = 0.01)] (Table 2). More than half of all providers favored initiating deresuscitation by the second timepoint (optimization) which remained consistent throughout the remainder of the vignette. Nephrologists were more likely than intensivists to choose “Dialysis or ultrafiltration” for deresuscitation at time points 3 and 4 respectively (18.0% vs. 3.6% [p < 0.05], and 48.7% vs. 23.6% [p < 0.01]). This pattern persisted for timepoint 2, though did not reach statistical significance 10.3% vs. 2.9% [p = 0.07]. Selection of specific fluid management (e.g., decreasing maintenance IVF) or diuretic management (e.g., infusion of loop diuretic) was similar between groups with the exception of the fourth time point where intensivists were more likely to select intermittent loop diuretic use [47.1% vs. 28.2% (p = 0.04)] (Table 3). Within specialties there was inconsistency among respondents when it came to method for deresuscitation and very few methods were selected by a majority of providers.
3.5 Subgroup analysis
a priori we determined provider level of experience (<7 years vs. ≥7 years) and the number of PICU beds at the respondents’ institution (<26 beds vs. ≥26 beds, as a surrogate for patient volume) as relevant factors that could impact a provider's response regarding if a patient was in shock and if deresuscitation was appropriate. In this subgroup analysis, a statistically significant difference between specialties was observed at time point 1 and 2 among providers practicing at institutions with ≥26 PICU beds (Supplementary Table S1), whereas no difference was seen at this time point in the full cohort, at institutions with <26 PICU beds, or when analyzed by years of experience (Supplementary Tables S1 and S2). We also looked at provider responses to these same questions within specialty groups to see if institution size or experience elucidated differences to practice approach. Supplementary Table S3 define nephrologists’ responses comparing institution size and years of experience. Supplementary Table S4 represent intensivists’ responses comparing institution size and years of experience. Within subspecialties, the subgroup analysis did not detect any differences in responses when separated by our cut offs.
4 Discussion
This cross-sectional survey of pediatric intensivists and nephrologists provides insight into providers’ perceptions and practices regarding fluid deresuscitation in critically ill children. As previously alluded to, fluid accumulation is associated with poor clinical outcomes in children admitted to the PICU (5–11, 22, 23). This negative association has been shown in a dose dependent manner (12) and remains important even early in a PICU course (24). We hypothesized there would be significant differences between intensivists’ and nephrologists’ responses regarding optimal timing and preferred strategies to initiate deresuscitation. Ultimately, we found that both groups agreed when it came to general fluid management principles. The respondents of this survey seem to acknowledge the importance of identifying and managing fluid accumulation as 75.4% of providers believed discussing fluid balance and initiating fluid deresuscitation in PICU patients is “very important”. Notably however, this response of “very important” falls to 44.1% when respondents were asked about their colleagues’ views on importance of fluid discussions suggesting room for improvement. Regarding specifics of initiating deresuscitation there was less consistency across providers. Nephrologists deemed patients ready for ready for deresuscitation earlier in the course than intensivists, however this difference narrowed at later case time points.
Understanding what contributes to fluid accumulation may assist in modifying it. Fluid boluses are an important component of the resuscitation phase; however, volume of maintenance and replacement IVF have been shown to significantly exceed total volume of resuscitation fluids. Fluids for resuscitation, maintenance, and replacement account for a smaller proportion of cumulative fluid balance than fluid creep—unintentional volume administered as a vehicle for other medications or electrolytes (25). A retrospective study in pediatric cardiac surgical patients concluded that patients with greater fluid creep had greater odds of cumulative fluid balance exceeding 10% and mortality (26). From our survey, the most commonly identified steps during the resuscitation phase that could limit positive fluid balance were a reduction in boluses or decrease maintenance fluids. For the remaining three case points, these choices continued to be the top 2 most selected management options. While limiting boluses or decreasing maintenance fluids are potentially modifiable contributors to fluid accumulation, adult data reveals fluid restriction alone has limited efficacy in preventing mortality (27–29).
In addition to limiting total fluid in, diuretic therapy can be utilized when initiating deresuscitation. Our survey identified that nephrologists were more likely to introduce diuretics early in the patient course, while intensivists were more likely to utilize diuretics later in the course. We also identified inconsistencies regarding approaches to diuretics (ex. infusion vs. intermittent dosing) and few options reached a majority. Responses related to diuretic options exceeded 100%, which indicates some providers selected multiple diuretic based options. This insinuates the approach to diuretic prescription is nuanced and highlights the lack of guidance in the literature. In one study, PICU patients who receive furosemide were less likely to develop a cumulative fluid balance >5%, but there is a paucity of pediatric literature about optimal timing of diuretic initiation and safety of doing so while still in shock (30).
One reason nephrologists may have been less likely to select diuretic use during the evacuation phase could be that they were more likely to select ultrafiltration (48.7% vs. 23.6%). Notably, nephrologists’ preference for ultrafiltration exceeded intensivists’ in the optimization and stabilization phase as well. This may be explained by differences in specialized training—nephrologists being more familiarized with prescription of ultrafiltration vs. intensivists being more familiar with technical skills/sedation required for dialysis catheter placement. Future work should focus on factors that intensivists and nephrologists weigh in deciding between diuretics and ultrafiltration.
Like diuretic administration, the pediatric literature guiding CRRT initiation for fluid accumulation is lacking and limited to retrospective or observational data (20). Prior randomized control trials in adults that use thresholds of serum creatinine suggest neither accelerated nor postponed CRRT initiation is best (31, 32). Historically in pediatrics, CRRT initiation has been based off severity of fluid accumulation (33) though an evidence-based threshold does not exist (20). There is observational data that reveal significant increase in mortality if CRRT is initiated at 20% positive cumulative fluid balance compared to 10% (34). TAKING FOCUS 2 recently described a clinical decision support algorithm to help guide CRRT initiation which found that CRRT days and rates of mortality decreased after algorithm implementation (33).
Still, it stands there are no consensus-based guidelines for how and when to perform deresuscitation which is reinforced in our survey as only 2.2% of providers report utilizing an institutional protocol. Complicating this matter is that identifying fluid imbalance has innate inaccuracies that our survey briefly addressed by some free text answers. Malbrain et al. suggests deresuscitation is appropriate once “salvage resuscitation” is finished—but the specifics of what defines salvage resuscitation is imprecise (17). The Pediatric Acute Disease Quality Initiative collaborators identified that rigorous science dedicated to managing fluid balance and fluid accumulation are lacking and recommended a working group dedicated to better understanding fluid balance (3). Notably, deresuscitation protocols have been shown to be feasible with potential clinical benefits in adults, yet a significant gap remains in implementing standardized deresuscitation practices in pediatrics (35, 36).
Our study is unique in including both intensivists and nephrologists, who frequently collaborate in caring for critically ill patients with acute kidney injury and fluid accumulation. However, this study has a few notable limitations. Innate to survey-based studies, the results represent providers’ perceptions of their practice, not their actual practice patterns. It is vulnerable to nonresponse bias, measurement bias, and coverage bias. We were also unable to control for respondents filling out multiple times. We think our survey was brief with high yield questions, thus limiting incomplete survey responses. There were only 4 providers who started but did not finish the survey. We are unfortunately unable to report a nonresponse rate due to methodology of dissemination. There was a discrepancy in number of intensivists compared to number of nephrologists that completed the survey (i.e., 140 to 39). While this ratio reflects the ratio of providers who maintain board certification in the respective specialties as discussed in the methods, the incongruity could skew the results. We also did not perform reliability testing to measure validity of our tool. Some components of the survey included medical information more specific to daily practice of intensivists (ex. vasoactive infusions or lung compliance) which may have skewed responses from nephrologists less familiar with these topics. Finally, our survey was disseminated via avenues that target physicians involved in American academic societies, and thus, their opinions and practices may differ from those in private practice or those in low resource or international settings. Survey respondents reported greater perceived personal emphasis on fluid balance than their institution which highlights the possibility that survey respondents are more interested in the topic at hand.
5 Conclusion
In conclusion, pediatric nephrologists and intensivists typically agree when it is appropriate to initiate fluid deresuscitation, except from early in shock. The way deresuscitation is accomplished had greater variability, especially regarding ultrafiltration and/or dialysis. This study highlights the necessity of widely accepted and operationalizable definitions, additional research, and eventual development of evidence-based consensus guidelines on deresuscitation strategies with the goal of improving patient outcomes.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study involving human participants were approved by University of Alabama, Institutional Review Board 300011177. This study was conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent upon completion of the survey.
Author contributions
CB: Writing – review & editing, Writing – original draft, Visualization, Methodology, Investigation, Conceptualization. DA: Writing – review & editing, Supervision, Conceptualization. JN: Writing – review & editing, Supervision. PP: Writing – review & editing, Supervision, Conceptualization. FR: Writing – review & editing, Formal Analysis, Conceptualization. TW: Writing – review & editing, Supervision, Conceptualization. JO: Writing – review & editing, Visualization, Supervision, Methodology, Investigation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors would like to acknowledge Emily Mixon for her expertise in REDCap survey dissemination.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
For full disclosure, we provide here an additional list of other author’s commitments and funding sources that are not directly related to this study: In the last 24 months, DA has consulted with Nuwellis, Seastar, and Abbott. His institution has received funding for education and research from Nuwellis, Bioporto, Leadiant and Seastar. He has financial interests in patent/innovations pending in the area of kidney support therapies and urine collection devices. He is the Founder and Chief Scientific Officer for Zorro-Flow Inc.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1484893/full#supplementary-material
Abbreviations
CRRT, Continuous renal replacement therapy; IVF, intravenous fluids; PICU, pediatric intensive care unit; ROSE, resuscitation optimization stabilization evacuation.
References
1. Malbrain MLNG, Wong A, Nasa P, Ghosh S. Rational Use of Intravenous Fluids in Critically Ill Patients. 1 ed. Cham: Springer Nature (2024). p. 598.
2. Gorga SM, Selewski DT, Goldstein SL, Menon S. An update on the role of fluid overload in the prediction of outcome in acute kidney injury. Pediatr Nephrol. (2024) 39(7):2033–48. doi: 10.1007/s00467-023-06161-z
3. Selewski DT, Barhight MF, Bjornstad EC, Ricci Z, de Sousa Tavares M, Akcan-Arikan A, et al. Fluid assessment, fluid balance, and fluid overload in sick children: a report from the Pediatric Acute Disease Quality Initiative (ADQI) conference. Pediatr Nephrol. (2024) 39(3):955–79. doi: 10.1007/s00467-023-06156-w
4. Lintz VC, Vieira RA, Carioca FL, Ferraz IS, Silva HM, Ventura AMC, et al. Fluid accumulation in critically ill children: a systematic review and meta-analysis. EClinicalMedicine. (2024) 74:102714. doi: 10.1016/j.eclinm.2024.102714
5. Virk M, Akcan-Arikan A. Fluid overload in critically ill children is associated with increased mortality. J Pediatr. (2018) 198:322–5. doi: 10.1016/j.jpeds.2018.04.059
6. Barbaro R, Fan E. In critically ill children, fluid overload is consistently associated with worse outcomes. BMJ Evid Based Med. (2019) 24(1):41–2. doi: 10.1136/bmjebm-2018-111009
7. Flori HR, Church G, Liu KD, Gildengorin G, Matthay MA. Positive fluid balance is associated with higher mortality and prolonged mechanical ventilation in pediatric patients with acute lung injury. Crit Care Res Pract. (2011) 2011:854142. doi: 10.1155/2011/854142
8. Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL. Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med. (2012) 13(3):253–8. doi: 10.1097/PCC.0b013e31822882a3
9. Lex DJ, Tóth R, Czobor NR, Alexander SI, Breuer T, Sápi E, et al. Fluid overload is associated with higher mortality and morbidity in pediatric patients undergoing cardiac surgery. Pediatr Crit Care Med. (2016) 17(4):307–14. doi: 10.1097/PCC.0000000000000659
10. Selewski DT, Askenazi DJ, Bridges BC, Cooper DS, Fleming GM, Paden ML, et al. The impact of fluid overload on outcomes in children treated with extracorporeal membrane oxygenation: a multicenter retrospective cohort study. Pediatr Crit Care Med. (2017) 18(12):1126–35. doi: 10.1097/PCC.0000000000001349
11. Hassinger AB, Wald EL, Goodman DM. Early postoperative fluid overload precedes acute kidney injury and is associated with higher morbidity in pediatric cardiac surgery patients. Pediatr Crit Care Med. (2014) 15(2):131–8. doi: 10.1097/PCC.0000000000000043
12. Sutherland SM, Zappitelli M, Alexander SR, Chua AN, Brophy PD, Bunchman TE, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. (2010) 55(2):316–25. doi: 10.1053/j.ajkd.2009.10.048
13. Bjorklund A, Resch J, Slusher T. Pediatric shock review. Pediatr Rev. (2023) 44(10):551–65. doi: 10.1542/pir.2022-005630
14. Malbrain ML, Marik PE, Witters I, Cordemans C, Kirkpatrick AW, Roberts DJ, et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. (2014) 46(5):361–80. doi: 10.5603/AIT.2014.0060
15. Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Pediatr Crit Care Med. (2020) 21(2):e52–e106. doi: 10.1097/PCC.0000000000002198
16. Zampieri FG, Bagshaw SM, Semler MW. Fluid therapy for critically ill adults with sepsis: a review. JAMA. (2023) 329(22):1967–80. doi: 10.1001/jama.2023.7560
17. Malbrain MLNG, Martin G, Ostermann M. Everything you need to know about deresuscitation. Intensive Care Med. (2022) 48(12):1781–6. doi: 10.1007/s00134-022-06761-7
18. Silversides JA, Fitzgerald E, Manickavasagam US, Lapinsky SE, Nisenbaum R, Hemmings N, et al. Deresuscitation of patients with iatrogenic fluid overload is associated with reduced mortality in critical illness. Crit Care Med. (2018) 46(10):1600–7. doi: 10.1097/CCM.0000000000003276
19. Malbrain MLNG, Van Regenmortel N, Saugel B, De Tavernier B, Van Gaal PJ, Joannes-Boyau O, et al. Principles of fluid management and stewardship in septic shock: it is time to consider the four D’s and the four phases of fluid therapy. Ann Intensive Care. (2018) 8(1):66. doi: 10.1186/s13613-018-0402-x
20. Neumayr TM, Bayrakci B, Chanchlani R, Deep A, Morgan J, Arikan AA, et al. Programs and processes for advancing pediatric acute kidney support therapy in hospitalized and critically ill children: a report from the 26th Acute Disease Quality Initiative (ADQI) consensus conference. Pediatr Nephrol. (2024) 39(3):993–1004. doi: 10.1007/s00467-023-06186-4
21. The American Board of Pediatrics RD. Pediatric subspecialists ever certified. Chapel Hill, NC: The American Board of Pediatrics (2022). Available online at: https://www.abp.org/dashboards/pediatric-subspecialists-ever-certified (accessed June 05, 2024).
22. McCammond AN, Axelrod DM, Bailly DK, Ramsey EZ, Costello JM. Pediatric cardiac intensive care society 2014 consensus statement: pharmacotherapies in cardiac critical care fluid management. Pediatr Crit Care Med. (2016) 17(3 Suppl 1):S35–48. doi: 10.1097/PCC.0000000000000633
23. Gelbart B, Serpa Neto A, Stephens D, Thompson J, Bellomo R, Butt W, et al. Fluid accumulation in mechanically ventilated, critically ill children: retrospective cohort study of prevalence and outcome. Pediatr Crit Care Med. (2022) 23(12):990–8. doi: 10.1097/PCC.0000000000003047
24. Selewski DT, Gist KM, Basu RK, Goldstein SL, Zappitelli M, Soranno DE, et al. Impact of the magnitude and timing of fluid overload on outcomes in critically ill children: a report from the multicenter international assessment of worldwide acute kidney injury, renal angina, and epidemiology (AWARE) study. Crit Care Med. (2023) 51(5):606–18. doi: 10.1097/CCM.0000000000005791
25. Van Regenmortel N, Verbrugghe W, Roelant E, Van den Wyngaert T, Jorens PG. Maintenance fluid therapy and fluid creep impose more significant fluid, sodium, and chloride burdens than resuscitation fluids in critically ill patients: a retrospective study in a tertiary mixed ICU population. Intensive Care Med. (2018) 44(4):409–17. doi: 10.1007/s00134-018-5147-3
26. Hudkins MR, Miller-Smith L, Evers PD, Muralidaran A, Orwoll BE. Nonresuscitation fluid accumulation and outcomes after pediatric cardiac surgery: single-center retrospective cohort study. Pediatr Crit Care Med. (2023) 24(12):1043–52. doi: 10.1097/PCC.0000000000003373
27. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. (2006) 354(24):2564–75. doi: 10.1056/NEJMoa062200
28. Meyhoff TS, Hjortrup PB, Wetterslev J, Sivapalan P, Laake JH, Cronhjort M, et al. Restriction of intravenous fluid in ICU patients with septic shock. N Engl J Med. (2022) 386(26):2459–70. doi: 10.1056/NEJMoa2202707
29. Shapiro NI, Douglas IS, Brower RG, Brown SM, Exline MC, Ginde AA, et al. Early restrictive or liberal fluid management for sepsis-induced hypotension. N Engl J Med. (2023) 388(6):499–510. doi: 10.1056/NEJMoa2212663
30. Dai X, Chen J, Li W, Bai Z, Li X, Wang J, et al. Association between furosemide exposure and clinical outcomes in a retrospective cohort of critically ill children. Front Pediatr. (2020) 8:589124. doi: 10.3389/fped.2020.589124
31. Bagshaw SM, Wald R, Adhikari NKJ, Bellomo R, da Costa BR, Dreyfuss D, et al. Timing of initiation of renal-replacement therapy in acute kidney injury. N Engl J Med. (2020) 383(3):240–51. doi: 10.1056/NEJMoa2000741
32. Gaudry S, Hajage D, Martin-Lefevre L, Lebbah S, Louis G, Moschietto S, et al. Comparison of two delayed strategies for renal replacement therapy initiation for severe acute kidney injury (AKIKI 2): a multicentre, open-label, randomised, controlled trial. Lancet. (2021) 397(10281):1293–300. doi: 10.1016/S0140-6736(21)00350-0
33. Goldstein SL, Krallman KA, Roy JP, Collins M, Chima RS, Basu RK, et al. Real-time acute kidney injury risk stratification-biomarker directed fluid management improves outcomes in critically ill children and young adults. Kidney Int Rep. (2023) 8(12):2690–700. doi: 10.1016/j.ekir.2023.09.019
34. Alobaidi R, Morgan C, Basu RK, Stenson E, Featherstone R, Majumdar SR, et al. Association between fluid balance and outcomes in critically ill children: a systematic review and meta-analysis. JAMA Pediatr. (2018) 172(3):257–68. doi: 10.1001/jamapediatrics.2017.4540
35. Silversides JA, McMullan R, Emerson LM, Bradbury I, Bannard-Smith J, Szakmany T, et al. Feasibility of conservative fluid administration and deresuscitation compared with usual care in critical illness: the Role of Active Deresuscitation After Resuscitation-2 (RADAR-2) randomised clinical trial. Intensive Care Med. (2022) 48(2):190–200. doi: 10.1007/s00134-021-06596-8
Keywords: fluid accumulation, deresuscitation, critical care, nephrology, CRRT—continuous renal replacement therapy
Citation: Braun CG, Askenazi DJ, Neyra JA, Prabhakaran P, Rahman AKMF, Webb TN and Odum JD (2024) Fluid deresuscitation in critically ill children: comparing perspectives of intensivists and nephrologists. Front. Pediatr. 12:1484893. doi: 10.3389/fped.2024.1484893
Received: 22 August 2024; Accepted: 9 October 2024;
Published: 28 October 2024.
Edited by:
Julie Fitzgerald, Children’s Hospital of Philadelphia, United StatesReviewed by:
Colin Sallee, University of California, Los Angeles, United StatesMatthew Barhight, Ann & Robert H. Lurie Children’s Hospital of Chicago, United States
Celeste Dixon, Children’s Hospital of Philadelphia, United States
Copyright: © 2024 Braun, Askenazi, Neyra, Prabhakaran, Rahman, Webb and Odum. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chloe G. Braun, Y2dtZXllckB1YWJtYy5lZHU=
 Chloe G. Braun
Chloe G. Braun David J. Askenazi2
David J. Askenazi2 Priya Prabhakaran
Priya Prabhakaran