- 1Department of Neonatology, Institute of Medical Sciences and SUM Hospital, Bhubaneswar, Orissa, India
- 2Department of Neonatology, Ankura Hospital for Women and Children, Hyderabad, Telangana, India
- 3Department of Neonatology, Kovai Medical Center and Hospital (KMCH), Coimbatore, Tamil Nadu, India
- 4KMCH Research Foundation, Coimbatore, Tamil Nadu, India
- 5Department of Neonatology, ABVIMS and Dr. RML Hospital, New Delhi, India
- 6Health Sciences Librarian, Queen’s University, Kingston, ON, Canada
- 7Neonatal Unit, James Cook University Hospital, Middlesbrough, United Kingdom
- 8Clinical Academic Office, Faculty of Medical Sciences, Newcastle University, Newcastle, United Kingdom
- 9Department of Physics, University of Durham, Durham, United Kingdom
Importance: The direct antiglobulin test (DAT) is commonly used as a screening test for predicting significant neonatal hyperbilirubinemia requiring intervention. However, evidence for this approach is limited.
Objective: The aim of this study was to evaluate the diagnostic utility of DAT in predicting the need for phototherapy and double volume exchange transfusion (DVET) in neonates with ABO and Rhesus (Rh) incompatibility conditions.
Methods: MEDLINE, Embase, CENTRAL, CINAHL, and Web of Science were searched from inception until 1 February 2024. Randomized controlled trials (RCTs) and non-RCTs were eligible for inclusion. Two reviewers screened the titles and abstracts blinded to each other. A Bayesian bivariate random-effects model was employed for the diagnostic test accuracy meta-analyses. Risk of bias was assessed using Quality Assessment for Studies of Diagnostic Accuracy 2 and certainty of evidence (CoE) was adjudged according to the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) guidelines.
Results: In total, 53 studies were included in the systematic review and 28 were synthesized in the meta-analysis. For the need for phototherapy outcome, the pooled sensitivity [95% credible interval (CrI)] and specificity (95% CrI) of DAT in ABO incompatibility (18 studies, n = 10,110) were 56.1% (44.5%–67.8%) and 83.6% (71.6%–90.8%). For Rh incompatibility (three studies, n = 491), the sensitivity and specificity were 40.4% (12.2%–81.7%) and 89.9% (72.7%–94.6%). The CoE was predominantly low. For the need for DVET outcome, the pooled sensitivity and specificity of DAT in ABO incompatibility (three studies, n = 2,652) were 83.6% (35.8%–99.6%) and 74.5% (40.3%–92.7%). For Rh incompatibility (two studies, n = 240), the sensitivity and specificity were 80.3% (34.2%–97.3%) and 68.0% (25.3%–92.1%). The CoE was predominantly very low.
Conclusion: In ABO and Rh incompatibility, DAT probably has moderate specificity and low sensitivity for predicting the need for phototherapy. For DVET, though DAT is possibly a better predictor due to its acceptable sensitivity, the predictive interval was wide. Thus, we do not suggest the routine use of DAT screening to predict the need for phototherapy and DVET. However, it may be used as a second-tier investigation for risk stratification of high-risk neonates.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022297785, PROSPERO (CRD42022297785).
1 Introduction
Neonatal hyperbilirubinemia (NNH) is a common diagnosis in neonates, requiring treatment in the initial postnatal days (1). While the majority of these neonates needing treatment are managed with phototherapy, a small proportion may require additional therapies such as intravenous immunoglobulin (IVIG) and double volume exchange transfusion (DVET). Despite the availability of effective interventions, the risk of serious adverse outcomes including kernicterus, cerebral palsy, hearing loss, and even mortality remains a significant concern (2). These adverse outcomes could be prevented by the timely identification and treatment of neonates at risk of developing severe NNH.
Evidence-based guidelines have recommended the use of various screening tools for early identification and treatment of NNH (1, 3). Despite the incorporation of these tools into clinical practice, instances of serious adverse outcomes secondary to NNH have been reported. This is often attributed to factors such as challenges in identifying high-risk neonates, limitations of the screening tools, and adaptation of policies such as early discharge from healthcare facilities. As a result, regional guidelines suggest risk stratification based on gestational age, bilirubin levels, and the presence of various other morbidities such as sepsis, asphyxia, and ABO, Rhesus (Rh), and other blood group incompatibilities (1, 4, 5). The inclusion of additional tests such as the direct antiglobulin test (DAT) has also been recommended (1, 5).
DAT is commonly performed in suspected cases of hemolytic disease of the newborn due to ABO or Rh incompatibility (6). However, the current clinical guideline does not recommend routine testing of umbilical cord blood for DAT in ABO and Rh incompatibility (5). As the evidence for the same is contentious, many centers continue to use routine DAT testing as a screening test to identify at-risk neonates (6–10).
Thus, we conducted a systematic review and diagnostic test accuracy (DTA) meta-analyses with an aim to evaluate the diagnostic utility of DAT in predicting NNH requiring treatment in neonates with ABO and Rh incompatibility conditions.
2 Methods
The protocol was registered with PROSPERO (CRD42022297785) (11), and the reporting is in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses of Diagnostic Test Accuracy Studies (PRISMA-DTA) (12).
2.1 Study eligibility
Randomized controlled trials (RCTs), non-RCTs, and conference abstracts were eligible for inclusion. Studies that evaluated the diagnostic performance of DAT in predicting the need for treatment of NNH in various mother-neonate blood group pairs, including but not exclusively ABO and Rh incompatibility, were also eligible for inclusion. Case reports, traditional reviews, and systematic reviews were excluded. There were no language restrictions.
2.2 Patient population
We included studies conducted in different healthcare settings in late preterm and term neonates. Studies reporting on preterm neonates of <34 weeks' gestation, NNH secondary to enzyme deficiency, sepsis, liver disorders, and those who had received fetal therapy were excluded.
2.3 Index test
A DAT was performed on umbilical cord blood or during the first 14 days of postnatal life. We included studies that had used the two commonly used methods of DAT measurement (gel or tube agglutination methods). For the generation of the 2 × 2 table, we considered a weakly positive DAT as a positive test.
2.4 Reference standard
A serum bilirubin test was performed in the first 14 days of postnatal life.
2.5 Target condition
We considered the following outcomes: the need for phototherapy, DVET, and IVIG therapy in the first 14 days, irrespective of the treatment thresholds recommended by different guidelines. We did not include outcome parameters such as clinical jaundice and significant hyperbilirubinemia not requiring intervention.
2.6 Information sources
We conducted a comprehensive search of MEDLINE, Embase, CENTRAL, CINAHL, and Web of Science from inception till 1 February 2024. (AS, VK) (Supplementary Table 1). In addition, we searched the bibliographies of included studies and review articles to identify potentially eligible studies for inclusion.
2.7 Study selection and data extraction
All the study titles and abstracts were screened independently in duplicates by two authors (TA, TB) using an online software platform (Rayyan QCRI, Doha) (13). Data extraction was independently performed by the two investigators (VR, VK) using a preformed data extraction form, and disagreements were resolved by consensus. We recorded data items, including blood group, timing of DAT measurement, outcomes, and test accuracy measures [true positives (TPs), true negatives (TNs), false positives (FPs), and false negatives (FNs)].
2.8 Study quality assessment
The risk of bias and applicability concerns of the included studies were assessed using the Quality Assessment for Studies of Diagnostic Accuracy 2 tool (QUADAS-2) (14). Two authors (VK, PK) independently assessed the quality of the studies.
2.9 Data synthesis
Data synthesis was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy (15). We estimated individual sensitivities and specificities, along with their 95% confidence intervals using DTA measures. These findings are presented using forest plots and the summary receiver operating characteristic (SROC) space. In addition, we used a Bayesian bivariate random-effects approach to estimate median pooled sensitivity and specificity, along with 95% credible intervals (CrI). Bayesian analysis was performed using METABayesDTA version 1.4 and R software version 4.3.1 (R Foundation for Statistical Computing, Vienna, Austria) using the “meta4diag' and “meta” packages (16, 17). Vague priors were used. A Stan sampler with two chains, 500 warm-up iterations, and 1,500 total iterations was utilized. Model fit was assessed by visualizing the correlation residual plot and the frequency table probability residual plot. Model convergence was confirmed by examining the trace and density plots.
A meta-analysis was performed for each outcome: the need for phototherapy, the need for DVET, and the requirement of IVIG therapy. Within each outcome, the DTA measures were estimated for specific maternal and neonatal blood group combinations. For the DTA meta-analysis, we included only studies with ABO or Rh incompatibility, as these are the two commonly encountered clinical scenarios. Studies that evaluated DAT in blood group scenarios other than ABO or Rh incompatibility were included in the narrative review. ABO or Rh blood group incompatibilities were categorized into ABO incompatibility [Mother blood group (MBG): O; Rh positive and baby blood group (BBG): A or B irrespective of Rh status], Rh incompatibility (MBG: A, or B or AB or O with Rh negative and BBG: A or B or AB or O with Rh positive), or ABO/Rh incompatibility (MBG: O with Rh positive and BBG: A or B with Rh positive or negative OR MBG: A, B, AB, O with Rh negative and BBG: A or B or AB or O with Rh positive).
2.9.1 Subgroup and sensitivity analysis
Heterogeneity was explored using subgroup analysis of the covariates, DAT testing methods and treatment thresholds, as per the guidelines. We performed a post-hoc sensitivity analysis by excluding studies with applicability concerns in the patient selection domain. For publication bias, we examined the regression coefficient line for plot asymmetry. A funnel plot of the log diagnostic odds ratio against 1/(effective sample size)1/2 was generated. For assessing the certainty of the evidence, we used GRADEPro, following GRADE guidelines for DTA meta-analysis for each target condition and blood group combination (18).
3 Results
3.1 Study selection
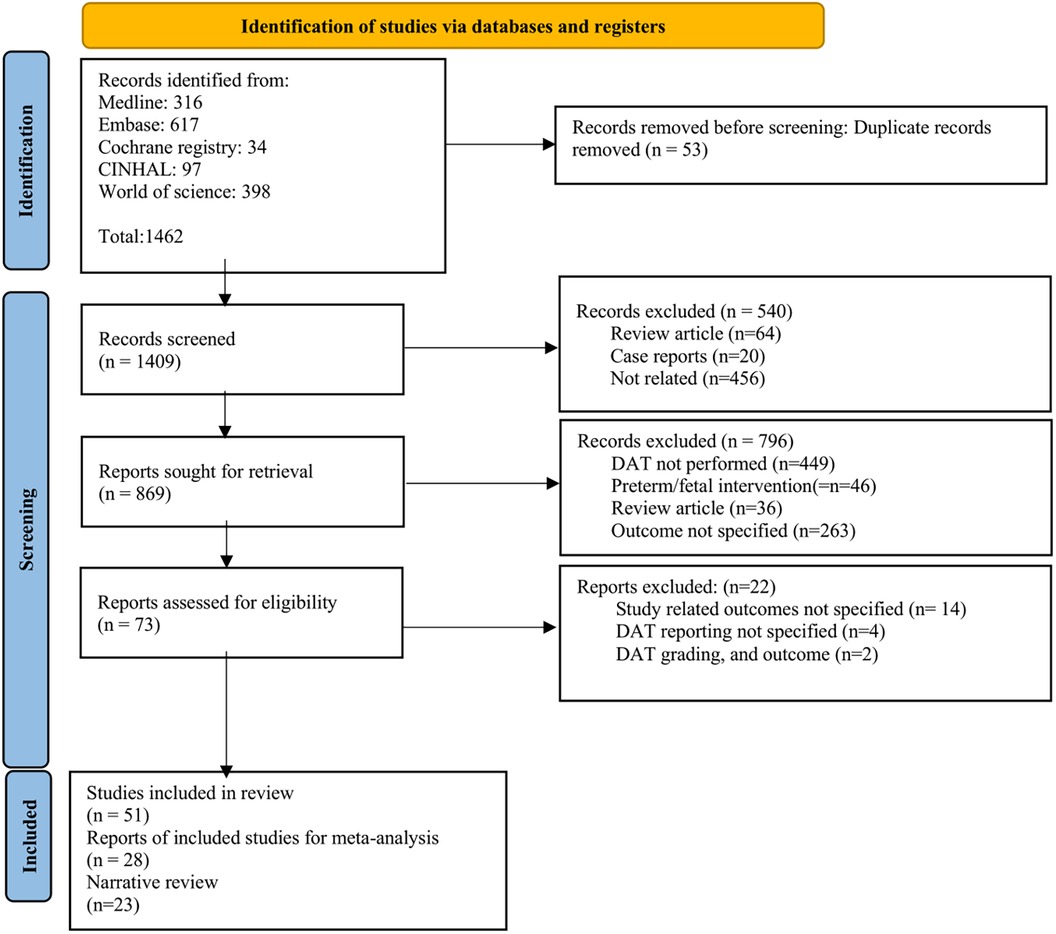
After the removal of duplicates, 1,409 titles and abstracts were screened. Of these, 73 were selected for full-text review. Among them, 51 were included in the systemic review. Of the included studies, 28 were synthesized in a DTA meta-analysis, and 23 studies were included in the narrative review (Figure 1). The excluded studies with valid reasons for their exclusion are listed in Supplementary Table 2.
3.2 Characteristics of included studies
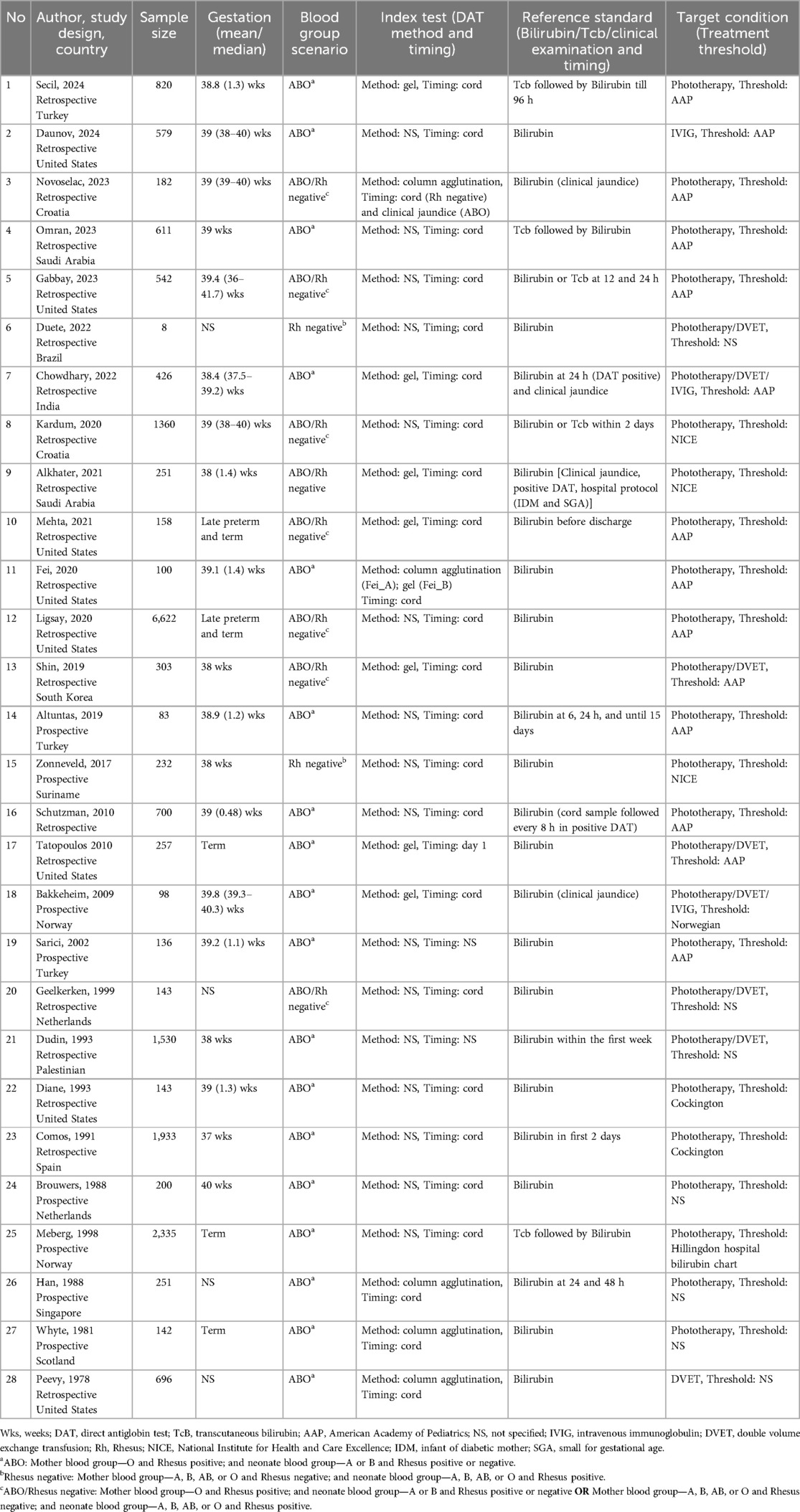
A total of 28 studies (n = 20,935) (10, 19–44) were included in the meta-analysis, with 20 studies (10, 19, 26, 28, 30, 32–44) (n = 11,385), 3 studies (10, 22, 29) (n = 491), and 9 studies (10, 20, 21, 23–25, 27, 31) (n = 9,561) assessed DAT in the ABO, Rh and ABO/Rh incompatibility scenarios, respectively. Only two studies (23, 25) included late preterm along with term neonates, while the others only included term neonates. Furthermore, 16 studies (20, 22, 24, 25, 28–33, 36, 39–42, 45) did not specify the method of DAT assessment, 7 studies (10, 19, 23, 27, 35, 37, 38) utilized the gel method, and 5 studies (21, 26, 34, 43, 44) used the column agglutination method. Most DAT assessments were performed with cord blood samples (10, 19, 20, 22–31, 33–37, 40–44), while two studies (21, 38) performed the assessments during the initial postnatal days. Of the included studies, 28 studies (10, 19–44) (n = 20,162) reported the outcome measure of the need for phototherapy, 7 studies (19, 22, 27, 29, 31, 32, 44) (n = 3,338) were on DVET, and 2 studies (19, 45) (n = 1,005) were on IVIG therapy. The characteristics of the included studies synthesized in the meta-analysis are provided in Table 1, and those in the narrative review are summarized in Appendix I in the Supplementary Material.
3.3 Risk of bias and applicability
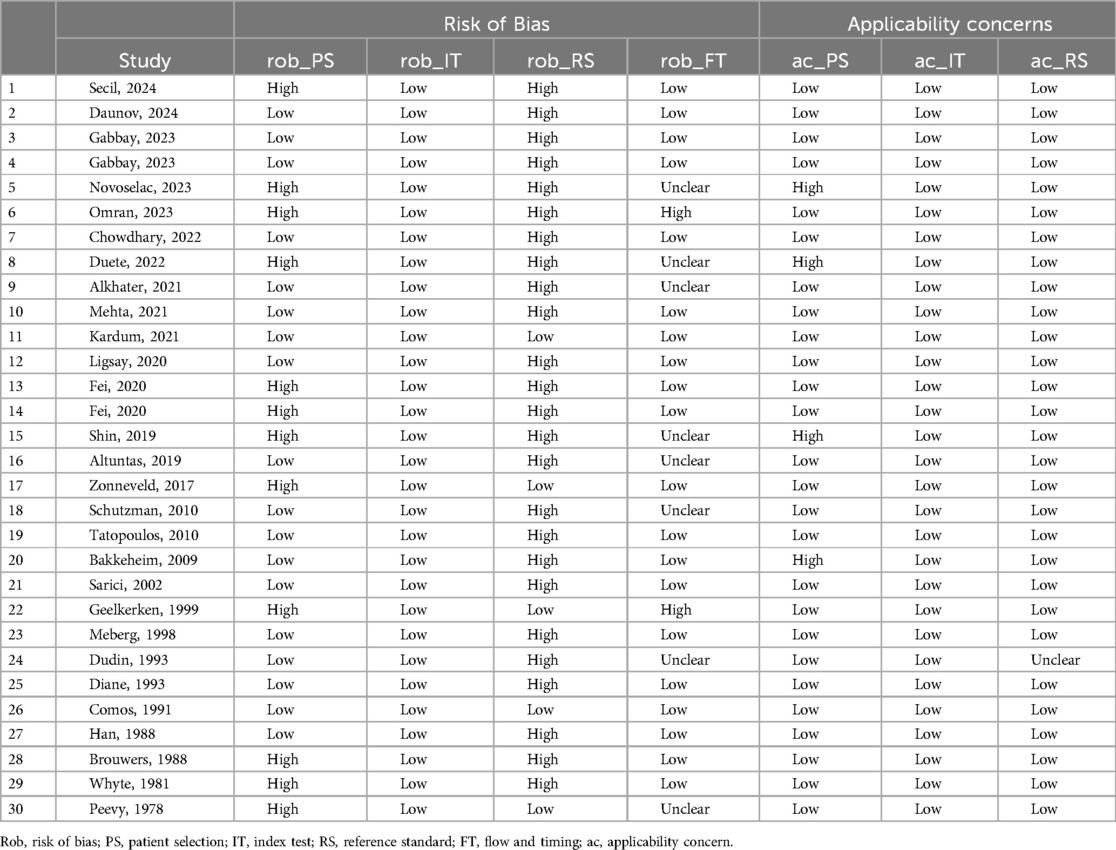
Overall, the studies were adjudged to have a moderate risk of bias (Table 2). The common reason to adjudge studies as having a high risk of bias was in the domain of reference standard, as the DAT result was not blinded to the clinicians. For patient selection, high risk was attributed to the non-consecutive inclusion of neonates. Applicability concerns in the patient selection domain were related to the selective inclusion of neonates by prior testing. The proportion of studies assessed to have an overall risk of bias of “low,” “high,” or “unclear” is presented in Figure 2.
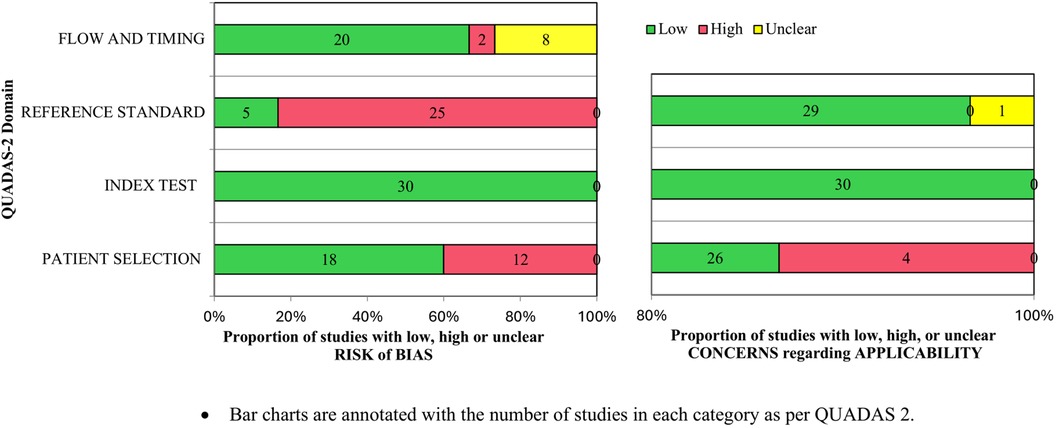
Figure 2. Proportions of studies with low, high, or unclear risk of bias and applicability concerns as per QUADAS-2.
3.4 Main results
We have summarized the study-specific sensitivity and specificity of all the included studies for the target conditions: the need for phototherapy (Supplementary Figures 1, 2), DVET (Supplementary Figures 3, 4), and IVIG therapy (Supplementary Figures 5, 6), employing forest plots and the SROC curves.
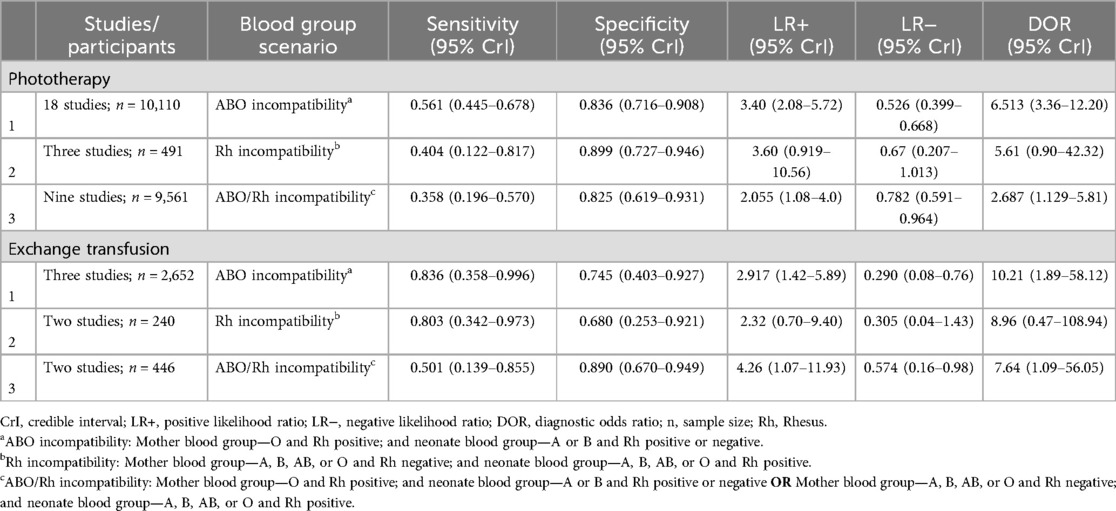
For the primary target condition, the need for phototherapy, a total of 28 studies (n = 20,162) (10, 19–44) were included. In the meta-analysis of studies evaluating DAT in ABO incompatibility (18 studies; n = 10,110) (10, 19, 26, 28, 30, 32–43), the posterior median sensitivity and specificity were 56.1% (95% CrI: 44.5%–67.8%) and 83.6% (95% CrI: 71.6%–90.8%), respectively. For the Rh isoimmunization scenario (three studies; n = 491) (10, 22, 29), the pooled sensitivity and specificity were 40.4% (95% CrI: 12.2%–81.7%) and 89.9% (95% CrI: 72.7%–94.6%), respectively. Studies that had evaluated DAT in ABO/Rh incompatibility (nine studies; n = 9,561) (10, 20, 21, 23–25, 27, 31) yielded a pooled sensitivity and specificity of 35.8% (95% CrI: 19.6%–57.0%) and 82.5% (95% CrI: 61.9%–93.1%), respectively (Figures 3, 4, Table 3).
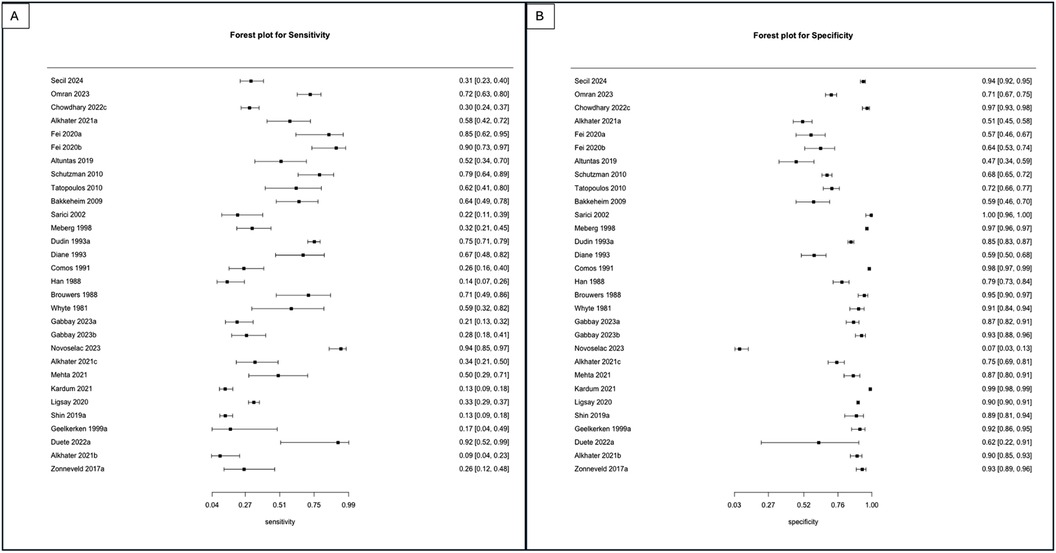
Figure 3. (A) Forest plot depicting the sensitivity of studies utilizing the DAT as an index test to predict the need for phototherapy in ABO, Rh, and ABO/Rh incompatibility scenarios. (B) Forest plot depicting the specificity of studies utilizing the DAT as an index test to predict the need for phototherapy in ABO, Rh, and ABO/Rh incompatibility scenarios.
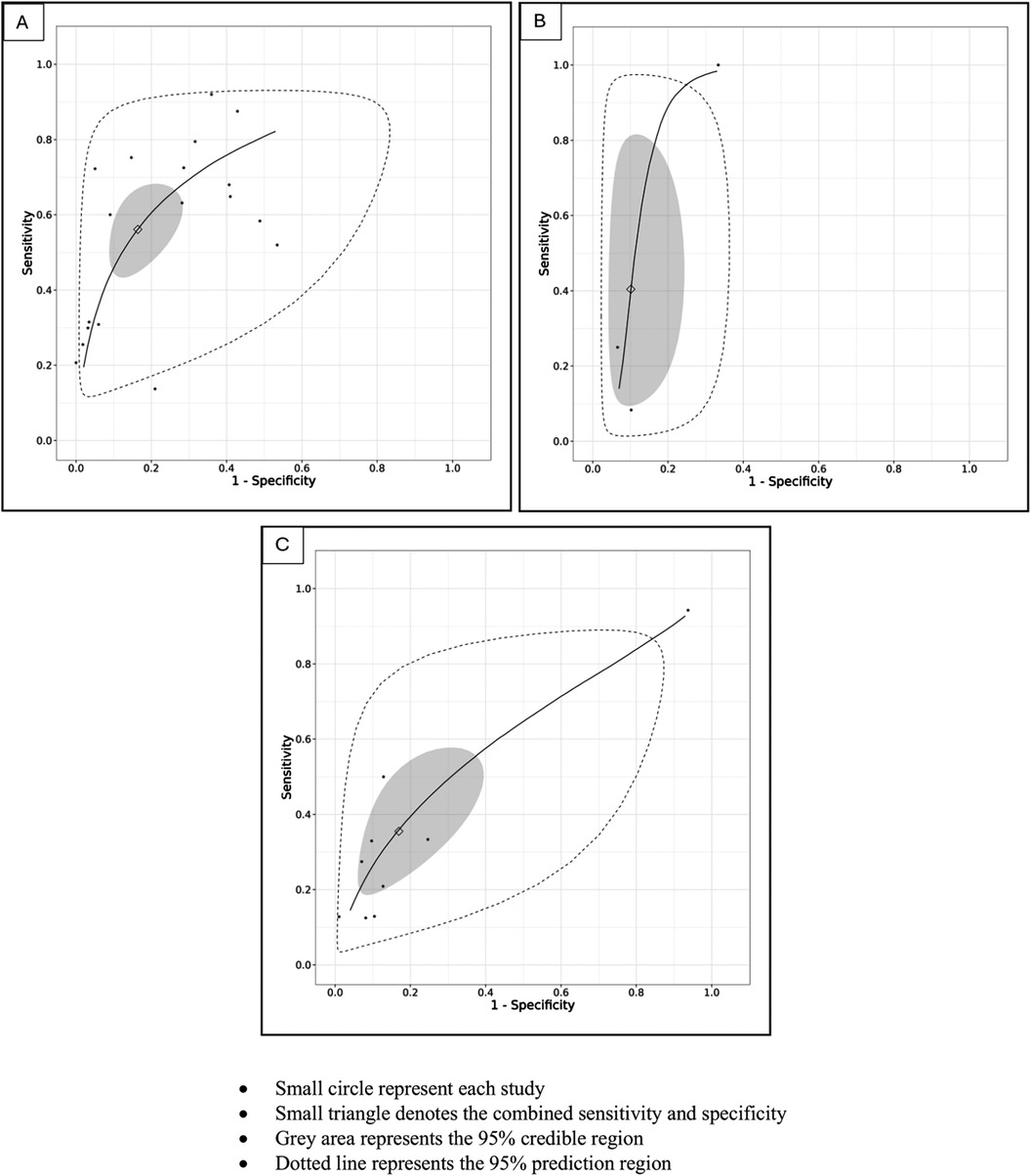
Figure 4. (A) SROC plot of studies utilizing the DAT as an index test to predict the need for phototherapy in ABO incompatibility scenarios. (B) SROC plot of studies utilizing the DAT as an index test to predict the need for phototherapy in Rh incompatibility scenarios. (C) SROC plot of studies utilizing the DAT as an index test to predict the need for phototherapy in ABO/Rh incompatibility scenarios.
For the target condition, the need for DVET, a total of seven studies (n = 3,338) (19, 22, 27, 29, 31, 32, 44) were included. In ABO incompatibility settings (three studies; n = 2,652) (19, 32, 44), the pooled sensitivity and specificity were 83.6% (95% CrI: 35.8%–99.6%) and 74.5% (95% CrI: 40.3%–92.7%). In Rh incompatibility (two studies; 240 infants) (22, 29), the pooled sensitivity and specificity were 80.3% (95% CrI: 34.2%–97.3%) and 68.0% (95% CrI: 25.3%–92.1%). Whereas for ABO/Rhesus incompatibility (two studies; n = 446) (27, 31), the pooled sensitivity and specificity were 50.1% (95% CrI: 13.9%–85.5%) and 89.0% (95% CrI: 67.0%–94.9%) (Table 3 and Supplementary Figure 7).
For the target condition, the need for IVIG (two studies; n = 1,005) (19, 45), we could not perform a meta-analysis as only a limited number of studies on each blood group scenario had reported on this outcome.
3.4.1 Subgroup analysis
3.4.1.1 Treatment thresholds
Subgroup analysis was conducted for the need for phototherapy for the studies which used the American Academy of Pediatrics (AAP) threshold chart in the ABO (nine studies, n = 3,231) (19, 26, 28, 30, 35, 36, 38, 39) and ABO/Rh incompatibility (six studies, n = 7,807) (20, 21, 23, 25, 27) scenarios. Whereas subgroup analyses were performed for studies using National Institute for Health and Care Excellence (NICE) threshold charts in the Rh (two studies, n = 483) (10, 29) and ABO/Rh (two studies; n = 1,611) (10, 24) incompatibility scenarios. There were no significant differences in the subgroup analyses except for an improvement in sensitivity when using AAP charts for the need for phototherapy in ABO incompatibility scenarios. The sensitivity and specificity were 80.3% (95% CrI: 34.2%–97.3%) and 68.0% (95% CrI: 25.3%–92.1%), respectively (Supplementary Table 3).
3.4.1.2 DAT method
The subgroup analysis based on the method used for DAT measurement (gel vs. tube method) showed no significant difference for the outcomes of phototherapy and DVET in any of the blood group scenarios (Supplementary Table 3).
3.4.2 Post-hoc sensitivity analysis
We also performed a post-hoc sensitivity analysis by excluding studies with applicability concerns in patient selection (21, 22, 27, 37). For the need for phototherapy, we found no differences from the primary analysis for ABO incompatibility (17 studies, n = 10,012) (10, 19, 26, 28, 30, 32–36, 38–43), Rh incompatibility (two studies, n = 483) (10, 29), and ABO/Rh incompatibility (seven studies, n = 9,076) (10, 20, 23–25, 31) (Supplementary Table 4).
3.4.3 Certainty of evidence
The certainty of evidence for the outcome measure, the need for phototherapy, was very low for the pooled sensitivity in ABO, Rh, and ABO/Rh incompatibility. This was primarily due to inconsistency, followed by the risk of bias and imprecision. In contrast, the certainty of evidence for specificity varied between low to moderate in ABO, Rh, and ABO/Rh incompatibility, mainly due to the risk of bias and inconsistency. For the outcome measure, the need for DVET, the certainty of evidence for sensitivity was very low. For specificity, it remained very low to low across ABO, Rh, and ABO/Rh incompatibility scenarios. Downrating the certainty of evidence was done mainly due to the risk of bias and inconsistency (Table 4).
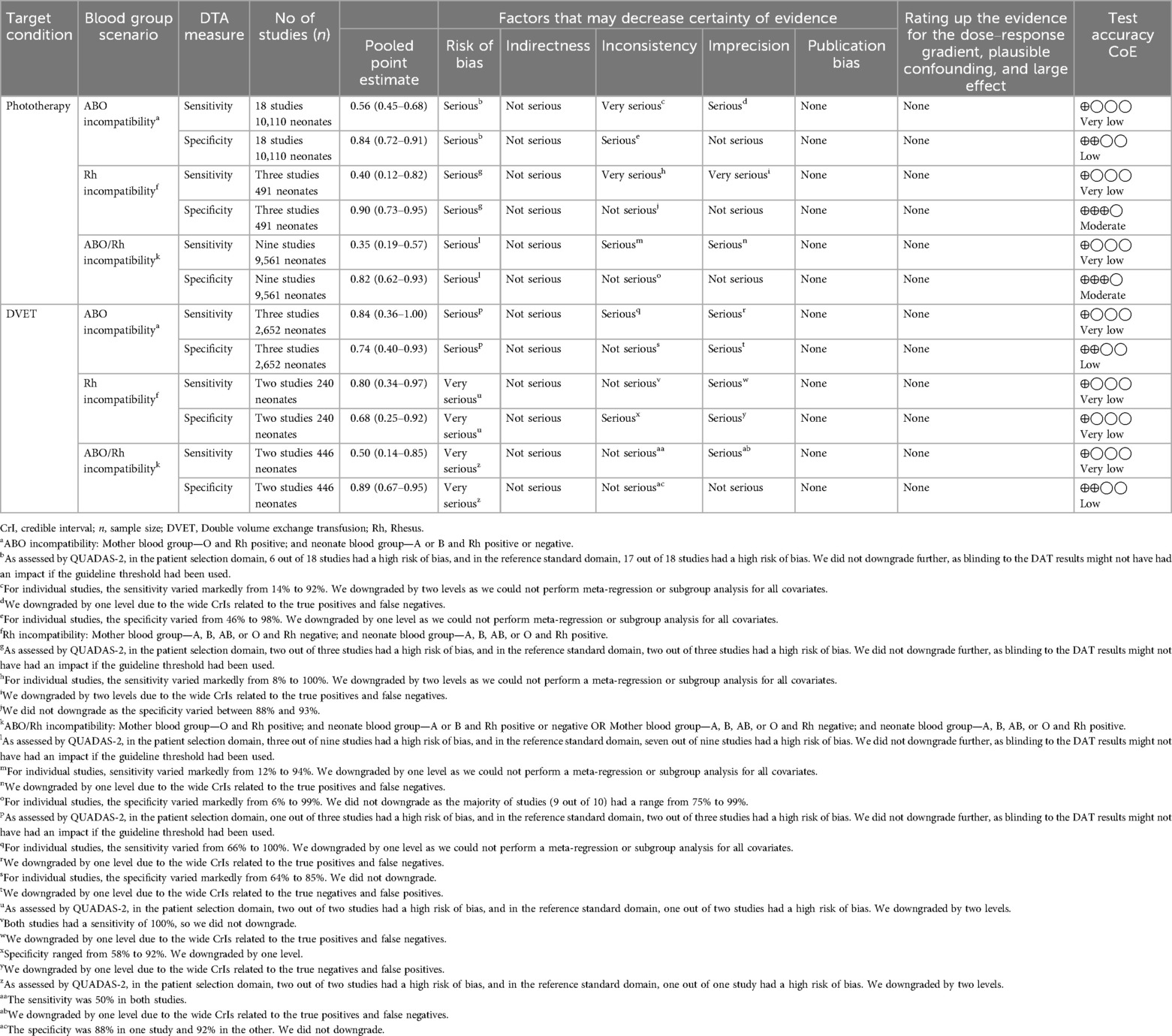
Table 4. GRADE assessment for the certainty of evidence for the effect estimates of the sensitivity and specificity of DAT in predicting the need for phototherapy and DVET.
3.4.4 Publication bias
For the outcome measures, the need for phototherapy and DVET, we did not detect publication bias as Deek's plot for asymmetry was not statistically significant (Supplementary Figures 8, 9).
4 Discussion
In our systematic review and DTA meta-analysis, we found that the DAT had low sensitivity but moderate specificity in predicting the need for phototherapy in ABO and Rh incompatibility scenarios. The evidence certainty for sensitivity ranged from very low to low, while for specificity, it was low to moderate. For the outcome measure, the need for DVET, the DAT showed varied sensitivity and specificity across ABO and Rh incompatibility scenarios, with the evidence certainty ranging from very low to low for both sensitivity and specificity.
The sensitivity of DAT for predicting the need for phototherapy was 56.1% in ABO incompatibility, 40.4% in Rh incompatibility, and 35.8% in studies that had evaluated either ABO or Rh incompatibility pairs, with only modest changes in specificity in the aforementioned groups, which was moderate. In addition, the predictive interval for Rh and ABO/Rh incompatibility was as low as 14%, indicating that the DAT is a poor predictor of the need for phototherapy in these scenarios. The poor predictive ability of DAT in Rh and ABO/Rh incompatibility could likely be attributed to a false positive rate of approximately 15% due to the routine use of maternal anti-D immunoglobulin in Rh-negative mothers and the passive transfer of these immunoglobulins to infants (46). The specificity of DAT for predicting the need for phototherapy was 83.6%, 89.9%, and 82.5% in ABO, Rh, and ABO/Rh incompatibility, respectively. However, not accounting for other causes of NNH, such as G6PD deficiency and extravasation, likely could have impacted the specificity of DAT. In contrast, the sensitivity and specificity of the DAT for predicting the need for DVET varied markedly across different blood group incompatibility scenarios. For ABO and Rh incompatibility, the sensitivity was above 80%, while the specificity was relatively lower.
To illustrate the practical implications of our study findings, we could consider 1,000 neonates with ABO incompatibility with a 20% probability [based on Gabbay et al. 2023 (20)] of them needing phototherapy for NNH. In this scenario, there would be 243 positive test results. However, 131 (41%) neonates would be falsely diagnosed as having ongoing hemolysis (false positive), whereas, among the 757 negative DAT results, 88 (12%) would be incorrect diagnoses (false negative). This high rate of false positives could lead to delayed discharge, additional investigations, an increased burden on the healthcare system, and heightened parental anxiety. On the other side, false negatives pose a significant risk, as high-risk neonates might be discharged earlier and followed up less frequently, placing the infants at risk of significant hyperbilirubinemia. In addition, the cost implications are considerable. Nevertheless, the moderate specificity of DAT for ABO incompatibility would allow 669 (88%) out of the 757 cases with negative results to have the correct diagnosis (true negatives). This improved specificity may facilitate early discharge and reduce costs.
The studies we included had a moderate risk of bias, especially in the reference standard domain. This was due to the non-blinding of the DAT test results to clinicians, which could significantly influence the need for an intervention. In addition, there was uncertainty in the flow and timing domain due to the poor reporting of the included studies.
Our review has crucial clinical implications, as it is the first comprehensive review addressing the utility of DAT in the management of neonates with blood group incompatibility. Previously, conflicting evidence on the utility of DAT led to varied recommendations by academic bodies. The AAP recommends a routine umbilical cord blood DAT for all mothers with a history of antenatal antibody screen being positive or unknown or with an Rh-negative blood group (1). The NICE advises against using routine umbilical cord blood DATs to predict significant NNH (4). As a DAT has a low sensitivity in most situations, a DAT may not be used as a screening tool for predicting the need for NNH treatment. Our findings imply that a negative DAT result in an incompatibility scenario can reasonably identify neonates who are less likely to require treatment for NNH and may be restricted to at-risk neonates to assess their risk stratification. Moreover, the sensitivity and specificity of a DAT in the prediction of DVET varied markedly with a wide predictive interval and very low to low evidence certainty. Therefore, we do not suggest the use of the DAT as a screening test for predicting severe hyperbilirubinemia requiring DVET.
Despite including several studies, the certainty of evidence remained very low to low for sensitivity and low to moderate for specificity for the need for phototherapy outcome measure, implying the need for future studies with improved study designs.
4.1 Strengths and limitations
To the best of our knowledge, this is the first systematic review to assess the predictive ability of DAT for determining the need for an intervention in NNH. We conducted a thorough literature search and performed clinically relevant analyses based on various blood group combinations, including various subgroup and post-hoc sensitivity analyses. We adhered to the standards recommended by the Cochrane Screening and Diagnostic Tests Methods group and followed the PRISMA reporting guidelines. We assessed the certainty of evidence as guided by the GRADE working group. However, our study had some limitations. Initially, we planned to include all blood group combinations in the meta-analyses, but we restricted the analysis to clinically relevant incompatibility scenarios. We were unable to do many of the pre-planned subgroup analyses such as based on gestational age and DAT strength due to the non-availability of data. We could not evaluate the effect of the updated AAP guidelines on phototherapy and DVET on the sensitivity and specificity of DAT, as none of the included studies evaluated these thresholds. Finally, we did not account for ethnic differences or consider other causes of significant hyperbilirubinemia.
5 Conclusions
In ABO and Rh incompatibility, DAT probably has moderate specificity but low sensitivity for predicting the need for phototherapy. For the need for DVET, DAT is possibly a poor predictor as the sensitivity and specificity varied markedly across blood groups with wide predictive intervals. Thus, we do not suggest the use of DAT as a screening test for predicting hyperbilirubinemia requiring either phototherapy or DVET.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
VK: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. VR: Conceptualization, Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. TA: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. TB: Conceptualization, Writing – original draft, Writing – review & editing. AS: Writing – original draft, Writing – review & editing. PK: Conceptualization, Formal Analysis, Methodology, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1475623/full#supplementary-material
References
1. Kemper AR, Newman TB, Slaughter JL, Maisels MJ, Watchko JF, Downs SM, et al. Clinical practice guideline revision: management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. (2022) 150(3):e2022058859. doi: 10.1542/peds.2022-058859
2. American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics. (2004) 114(1):297–316. doi: 10.1542/peds.114.1.297
3. Ten Kate L, van Oorschot T, Woolderink J, Teklenburg-Roord S, Bekhof J. Transcutaneous bilirubin accuracy before, during, and after phototherapy: a meta-analysis. Pediatrics. (2023) 152(6):e2023062335. doi: 10.1542/peds.2023-062335
4. (NICE) NIfHaCE. Jaundice in newborn babies under 28 days. (2010). Available online at: https://www.nice.org.uk/guidance/cg98 (Accessed June 06, 2024).
5. 2023 exceptional surveillance of jaundice in newborns under 28 days (NICE guideline CG98). National Institute for Health and Care Excellelnce. (2023). Available online at: https://www.nice.org.uk/guidance/cg98/resources/2023-exceptional-surveillance-of-jaundice-in-newborns-under-28-days-nice-guideline-cg98-13197079837/chapter/Surveillance-decision?tab=evidence (Accessed June 06, 2024).
6. Keir A, Agpalo M, Lieberman L, Callum J. How to use: the direct antiglobulin test in newborns. Arch Dis Child Educ Pract Ed. (2015) 100(4):198–203. doi: 10.1136/archdischild-2013-305553
7. Shahid R, Graba S. Outcome and cost analysis of implementing selective Coombs testing in the newborn nursery. J Perinatol. (2012) 32(12):966–9. doi: 10.1038/jp.2012.26
8. Kaplan M, Hammerman C, Vreman HJ, Wong RJ, Stevenson DK. Direct antiglobulin titer strength and hyperbilirubinemia. Pediatrics. (2014) 134(5):e1340–4. doi: 10.1542/peds.2014-1290
9. Oztekin O, Kalay S, Tezel G, Barsal E, Bozkurt S, Akcakus M, et al. Is the strength of direct antiglobulin test important for the duration of phototherapy? J Matern Fetal Neonatal Med. (2014) 27(5):534–6. doi: 10.3109/14767058.2013.819335
10. AlKhater SA, Albalwi RA, Alomar SA, Alsultan AA, Almuhaidib HR, Almousa RA, et al. Value of the direct antiglobulin test in predicting the need for phototherapy in newborns. J Blood Med. (2021) 12:53–61. doi: 10.2147/JBM.S291606
11. NationalInstituteforHealthResearch. PROSPERO home page. Available online at: https://www.crd.york.ac.uk/prospero/ (Accessed June 06, 2024).
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
13. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev. (2016) 5(1):210. doi: 10.1186/s13643-016-0384-4
14. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
15. Macaskill P, Gatsonis C, Deeks J, Harbord R, Takwoingi Y. Cochrane handbook for systematic reviews of diagnostic test accuracy. Available online at: https://methods.cochrane.org/sites/methods.cochrane.org.sdt/files/public/uploads/ Chapter%2010%20-%20Version%201.0.pdf (Accessed June 06, 2024).
16. R Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing (2023). https://www.R-project.org/
17. Guo J, Riebler A. meta4diag: Bayesian Bivariate Meta-Analysis of Diagnostic Test Studies for Routine Practice. arXiv 2018. arXiv preprint arXiv:151206220
18. Yang B, Mustafa RA, Bossuyt PM, Brozek J, Hultcrantz M, Leeflang MMG, et al. GRADE Guidance: 31. Assessing the certainty across a body of evidence for comparative test accuracy. J Clin Epidemiol. (2021) 136:146–56. doi: 10.1016/j.jclinepi.2021.04.001
19. Chowdhary S, Devi U, Giridhar S. Predicting significant hyperbilirubinemia in ABO incompatibility: is cord direct antiglobulin test useful? Indian J Hematol Blood Transfus. (2022) 38(3):591–5. doi: 10.1007/s12288-021-01513-x
20. Gabbay JM, Agneta EM, Turkington S, Bajaj BM, Sinha B, Geha T. Rates of phototherapy among ABO-incompatible newborns with a negative direct antiglobulin test. J Perinatol. (2023) 43(11):1357–62. doi: 10.1038/s41372-023-01650-3
21. Novoselac J, Buzina Marić K, Rimac V, Selak I, Raos M, Golubić Cepulić B. Significance of immunohematologic testing in mother and newborn ABO incompatibility. Immunohematology. (2023) 39(2):55–60. doi: 10.21307/immunohematology-2023-009
22. Duete ÚR, Brunetta DM, Araujo Júnior E, Tonni G, Carvalho FHC. Maternal-fetal alloimmunization: perinatal outcomes in a reference hospital in Northeastern Brazil. Rev Assoc Med Bras (1992). (2022) 68(5):670–4. doi: 10.1590/1806-9282.20220047
23. Mehta R, Petrova A. Direct antiglobulin test in the prediction of hyperbilirubinemia and predischarge bilirubin levels in infants with mother-infant blood type incompatibility. Pediatr Neonatol. (2021) 62(4):406–11. doi: 10.1016/j.pedneo.2021.04.002
24. Kardum D, Serdarusic I, Biljan B, Santic K, Zivkovic V, Kos M. Cord blood bilirubin and prediction of neonatal hyperbilirubinemia and perinatal infection in newborns at risk of hemolysis. J Pediatr (Rio J). (2021) 97(4):440–4. doi: 10.1016/j.jped.2020.08.009
25. Ligsay A, Hubbard EM, Koola J, Longhurst C. Neonatal hemolysis risk assessment in the era of universal bilirubin screening. J Invest Med. (2020) 68(1):A117–8.
26. Fei F, Marques MB, Staley EM, Williams LA. An automated method for direct antiglobulin testing and the resulting amount of phototherapy used at a large academic medical center. Lab Medicine. (2020) 51(1):50–5. doi: 10.1093/labmed/lmz029
27. Shin KH, Lee HJ, Song D, Lee SM, Kim IS, Kim H, et al. Characteristics of bilirubin according to the results of the direct antiglobulin test and its impact in hemolytic disease of the newborn. Lab Med. (2019) 50(2):138–44. doi: 10.1093/labmed/lmy050
28. Altuntas N, Akpinar Tekgunduz S, Ozkan Kirgin B, Dogan OC, Kislal FM. Is the phototherapy requirement in neonatal hyperbilirubinemia due to AB0 incompatibility predictable? Turk J Pediatr Dis. (2019) 13(5):330–4.
29. Zonneveld R, Lamers M, Schonewille H, Brand A, Kanhai HHH, Zijlmans WCWR. Prevalence of positive direct antiglobulin test and clinical outcomes in Surinamese newborns from D-negative women. Transfusion. (2017) 57(10):2496–501. doi: 10.1111/trf.14229
30. Schutzman DL, Sekhon R, Hundalani S. Hour-specific bilirubin nomogram in infants with ABO incompatibility and direct Coombs-positive results. Arch Pediatr Adolesc Med. (2010) 164(12):1158–64. doi: 10.1001/archpediatrics.2010.242
31. Van Geelkerken LAFE, Mantel M, Oudesluys-Murphy AM. Predictive value of the direct antiglobulin test in cordblood. Nederlands Tijdschrift Voor de Klinische Chemie. (1999) 24(6):324–6.
32. Dudin AA, Rambaud-Cousson A, Badawi S, Da’na NA, Thalji A, Hannoun A. ABO and Rh(D) blood group distribution and their implication for feto-maternal incompatibility among the Palestinian population. Ann Trop Paediatr. (1993) 13(3):249–52. doi: 10.1080/02724936.1993.11747654
33. Bel Comos J, Ribera Crusafont A, Natal Pujol A, Coroleu Lletget W, Pujol Posch N, Prats Vinas J. [Value of the Coombs test in ABO incompatibility]. Valor del test de Coombs en la incompatibilidad ABO. (1991) 35(4):248–50.
34. Han P, Kiruba R, Ong R, Joseph R, Tan KL, Wong HB. Haematolytic disease due to ABO incompatibility: incidence and value of screening in an Asian population. Aust Paediatr J. (1988) 24(1):35–8. doi: 10.1111/j.1440-1754.1988.tb01329.x
35. Ercin S, Coskun Y, Kayas K, Kavas N, Gursoy T. Positive direct antiglobulin test: is it a risk factor for significant hyperbilirubinemia in neonates with ABO incompatibility? Am J Perinatol. (2024) 41(4):505–10. doi: 10.1055/a-1709-5036
36. Al-Omran AM, Shapan HA, Al-Abdi SY. A retrospective comparison of phototherapy need in O-B versus O-A incompatibility in a single Saudi institution. J Neonatal Perinatal Med. (2023) 16(2):311–7. doi: 10.3233/NPM-221136
37. Bakkeheim E, Bergerud U, Schmidt-Melbye AC, Akkök CA, Liestøl K, Fugelseth D, et al. Maternal IgG anti-A and anti-B titres predict outcome in ABO-incompatibility in the neonate. Acta Paediatr. (2009) 98(12):1896–901. doi: 10.1111/j.1651-2227.2009.01478.x
38. Tatopoulos A, Hubert C, Vieux R, Hascoët JM. [What blood tests to predict severe hyperbilirubinemia in early maternity discharge?]. J Gynecol Obstet Biol Reprod (Paris). (2010) 39(3):218–23. doi: 10.1016/j.jgyn.2010.02.006
39. Sarici SU, Yurdakök M, Serdar MA, Oran O, Erdem G, Tekinalp G, et al. An early (sixth-hour) serum bilirubin measurement is useful in predicting the development of significant hyperbilirubinemia and severe ABO hemolytic disease in a selective high-risk population of newborns with ABO incompatibility. Pediatrics. (2002) 109(4):e53. doi: 10.1542/peds.109.4.e53
40. Madlon-Kay DJ. The clinical significance of ABO blood group incompatibility. Arch Fam Med. (1993) 2(3):285–7. doi: 10.1001/archfami.2.3.285
41. Brouwers HA, Overbeeke MA, van Ertbruggen I, Schaasberg W, Alsbach GP, van der Heiden C, et al. What is the best predictor of the severity of ABO-haemolytic disease of the newborn? Lancet. (1988) 2(8612):641–4. doi: 10.1016/S0140-6736(88)90466-7
42. Meberg A, Johansen KB. Screening for neonatal hyperbilirubinaemia and ABO alloimmunization at the time of testing for phenylketonuria and congenital hypothyreosis. Acta Paediatr. (1998) 87(12):1269–74. doi: 10.1111/j.1651-2227.1998.tb00950.x
43. Whyte J, Graham H. Prediction of the severity of ABO haemolytic disease of the newborn by cord blood tests. Acta Paediatr Scand. (1981) 70(2):217–22. doi: 10.1111/j.1651-2227.1981.tb05545.x
44. Peevy KJ, Wiseman HJ. ABO Hemolytic disease of the newborn: evaluation of management and identification of racial and antigenic factors. Pediatrics. (1978) 61(3):475–8. doi: 10.1542/peds.61.3.475
45. Daunov M, Schlosser A, Malay S, Adams J, Clark R, Ferrerosa L, et al. A description of IVIG use in term neonates with ABO incompatibility. Am J Perinatol. (2024) 41(13):1761–1766. doi: 10.1055/a-2255-8772
Keywords: neonatal jaundice, Coombs test, meta-analysis, newborn, exchange transfusion
Citation: Kumar Krishnegowda V, Ramaswamy VV, Abiramalatha T, Bandyopadhyay T, S AKP and Kannan Loganathan P (2025) Direct antiglobulin test for the prediction of neonatal hyperbilirubinemia needing an intervention: a systematic review and diagnostic test accuracy meta-analysis. Front. Pediatr. 12:1475623. doi: 10.3389/fped.2024.1475623
Received: 4 August 2024; Accepted: 27 December 2024;
Published: 28 January 2025.
Edited by:
Francesco Pegoraro, University of Florence, ItalyReviewed by:
Tudor Lucian Pop, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaRamesh Vidavalur, Cayuga Medical Center, United States
Copyright: © 2025 Kumar Krishnegowda, Ramaswamy, Abiramalatha, Bandyopadhyay, S and Kannan Loganathan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Prakash Kannan Loganathan, cGthbm5hbmxvZ2FuYXRoYW5AbmhzLm5ldA==
†ORCID:
Prakash Kannan Loganathan
orcid.org/0000-0003-3717-8569
 Vijay Kumar Krishnegowda
Vijay Kumar Krishnegowda Viraraghavan Vadakkencherry Ramaswamy
Viraraghavan Vadakkencherry Ramaswamy Thangaraj Abiramalatha3,4
Thangaraj Abiramalatha3,4 Tapas Bandyopadhyay
Tapas Bandyopadhyay Abdul Kareem Pullattayil S
Abdul Kareem Pullattayil S Prakash Kannan Loganathan
Prakash Kannan Loganathan