A Commentary on
By Chen Q, Lin L, Zhang N and Yang Y (2024). Front. Pediatr. 12.1337786. doi:10.3389/fped.2024.1337786
1 Introduction
We have read with great interest the publication by Chen et al. (1) on the co-infection of adenovirus and Mycoplasma pneumoniae among children with severe community-acquired pneumonia (CAP). We especially appreciate the findings about risk factors of severe disease. Several studies have showed whether M. pneumoniae infection contributes to more severe clinical outcomes than other pathogens (2–5). However, the role of M. pneumoniae shoud be considered along with other factors such as age, gender, and co-infection with different pathogens. In this Commentary, we report no association between M. pneumoniae, but an association with age, and severe pneumonia in children.
M. pneumoniae is a common cause of respiratory infections, particularly in children and young adults. Despite its prevalence, the role of M. pneumoniae in the disease severity among children remains controversial (6–9). As a bacterium without a cell wall, M. pneumoniae spreads via respiratory droplets, causing mild, self-limiting infections. It is common in school-aged children and can cause outbreaks in community settings (10, 11).
M. pneumoniae accounts for 10%–40% of pediatric CAP cases with peaks in children aged 5 to 15. Epidemiological studies suggest cyclical outbreaks of M. pneumoniae infections every 4–7 years, with regional and seasonal variation. Severe cases are more common in children with underlying health conditions (12). Research findings on the link between M. pneumoniae and severe pneumonia have been mixed, as summarized in a recent systematic review (9).
2 No association between M. pneumoniae infection and severe pneumonia among children
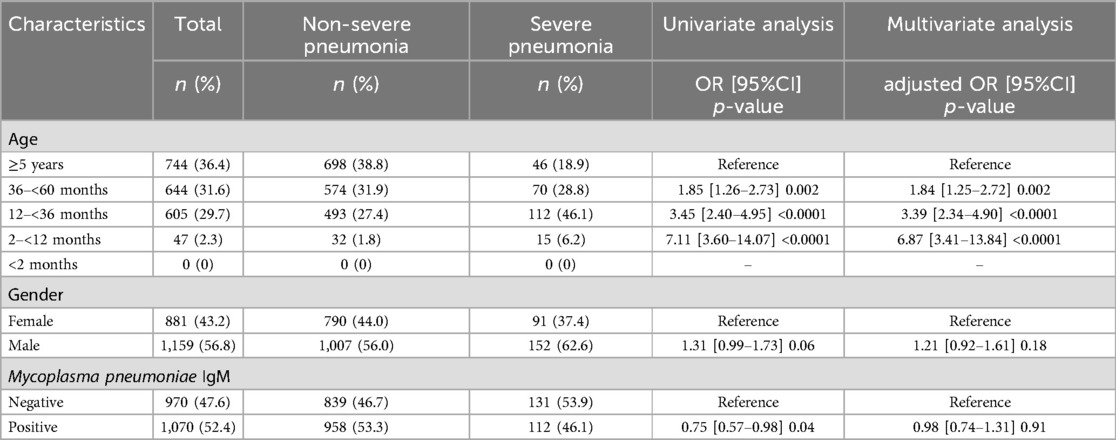
From 01/06/2023 to 31/05/2024, 2040 children with atypical pneumonia were hospitalized at Thai Binh Pediatric Hospital, Thai Binh. The proportion of severe pneumonia was 11.9% (243/2040). Due to limitations in equipment and the fact that health insurance does not cover molecular biological tests for diagnosing respiratory pathogens, the identification of M. pneumoniae infection in children in Thai Binh is only performed using serological methods. The serum samples were quantitatively analyzed for IgM antibodies against Mycoplasma pneumoniae using the Virion/Serion ELISA kit (GmbH Germany, catalog number ESR127M). The antibody levels were expressed in units per milliliter (U/ml). According to the manufacturer's guidelines, the interpretation criteria for M. pneumoniae IgM were as follows: positive (>17 U/ml), negative (<13 U/ml), and borderline (13–17 U/ml). All ELISA assays were carried out strictly following the instructions provided by the manufacturer. A total of 1070 (52.4%) patients were positive with IgM. The distribution of ill children gradually decreased with age.
Univariate and multivariate analysis using logistic regression showed no association between M. pneumoniae infection and male gender with severe pneumonia among children (adjusted OR = 0.98, p = 0.91, and aOR = 1.21, p = 0.18, respectively). However, age was a risk factor closely related to the severity of pneumonia. Compared to children aged ≥5 years old, those aged 2–<12 months were associated with 7 times of risk for severe disease (aOR = 6.87, p < 0.0001), patients aged 12–<36 months and 36–<60 months were associated with 4 times and twice of risk for severe pneumonia (aOR = 3.39, p < 0.0001 and aOR = 1.84, p = 0.002, respectively) (Table 1).
3 Discussion
The heterogeneity in study designs, populations, and diagnostic methods contributes to the variability in the results assessing the role of M. pneumoniae in severe pediatric pneumonia (6).
One of the primary challenges in establishing a clear association is the presence of confounding factors. Children with severe pneumonia are more likely to undergo extensive diagnostic testing, including polymerase chain reaction (PCR) assays, leading to a detection bias. Additionally, co-infections with other respiratory pathogens, such as viruses or bacteria, can complicate the clinical picture and obscure the role of M. pneumoniae.
The lack of a consistent association between M. pneumoniae infection and severity of disease has important implications for clinical practice (7, 8). Physicians should consider M. pneumoniae a potential cause of pneumonia in children but not assume it is associated with more severe disease without supporting clinical evidence. Treatment decisions should be guided by the overall clinical picture and not solely based on the presence of M. pneumoniae.
Indeed, antibiotic therapy for M. pneumoniae pneumonia typically includes macrolides, such as azithromycin or clarithromycin. However, the emergence of macrolide-resistant M. pneumoniae strains poses a challenge (13, 14). Clinicians must remain vigilant for resistance patterns and consider alternative treatments in older children, such as fluoroquinolones or tetracyclines.
Further research is needed to clarify the relationship between M. pneumoniae and pneumonia severity in children. Prospective studies with standardized diagnostic criteria, robust case definitions, and comprehensive data collection on co-infections and underlying health conditions are essential.
It is also crucial to understand the mechanisms underlying macrolide resistance in M. pneumoniae and develop strategies to minimize its impact. Surveillance programs to monitor resistance patterns and the effectiveness of current treatment regimens can inform clinical guidelines and improve patient outcomes.
The findings of our study should be interpreted with caution due to several limitations. Firstly, although the large sample size, this was a retrospective study conducted at a single center, focusing only on the association of severe pneumonia with age, sex, and M. pneumoniae infection. Other potential factors that might influence the severity of pneumonia in children, such as underlying health conditions, socioeconomic factors, or exposure to environmental pollutants, were not assessed. Including these variables in future research would provide a more comprehensive understanding of the risk factors associated with severe pneumonia in pediatric patients. Furthermore, due to limited laboratory resources, M. pneumoniae infection was identified using a single IgM test, whereas paired sera would provide a more accurate diagnosis. The use of paired sera, with samples collected at least two to four weeks apart, is widely regarded as the preferred approach for confirming M. pneumoniae infection, as it helps to distinguish between acute infection and past exposure. However, the reliability of single IgM serological tests and the requirement for paired testing remain inconsistent (15). However, implementing paired testing poses logistical challenges, especially in children, as it requires additional blood samples over time. Additionally, while paired IgG testing is often used as a reference standard, its relevance to routine clinical practice and its impact on treatment decisions remain limited, especially in pediatric settings.
In conclusion, the current body of evidence does not support a definitive association between M. pneumoniae infection and the severity of pneumonia among children. The variability in study findings highlights the complexity of pneumonia etiology and the influence of multiple factors on disease severity. Clinicians should adopt a holistic approach to diagnosing and managing pediatric pneumonia, considering the potential role of M. pneumoniae without overestimating its impact on disease severity.
Author contributions
DLP: Data curation, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. MMT: Data curation, Methodology, Validation, Writing – original draft. KDL: Data curation, Validation, Writing – review & editing. TDP: Data curation, Validation, Writing – review & editing. CTV: Data curation, Validation, Writing – review & editing. KLD: Data curation, Validation, Writing – review & editing. TLD: Data curation, Formal Analysis, Methodology, Validation, Writing – original draft, Writing – review & editing. VTH: Conceptualization, Data curation, Formal Analysis, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Chen Q, Lin L, Zhang N, Yang Y. Adenovirus and Mycoplasma pneumoniae co-infection as a risk factor for severe community-acquired pneumonia in children. Front Pediatr. (2024) 12:1337786. doi: 10.3389/fped.2024.1337786
2. Yun KW, Wallihan R, Desai A, Alter S, Ambroggio L, Cohen DM, et al. Clinical characteristics and etiology of community-acquired pneumonia in US children, 2015–2018. Pediatr Infect Dis J. (2022) 41:381. doi: 10.1097/INF.0000000000003475
3. Lee K-L, Lee C-M, Yang T-L, Yen T-Y, Chang L-Y, Chen J-M, et al. Severe Mycoplasma pneumoniae pneumonia requiring intensive care in children, 2010–2019. J Formos Med Assoc. (2021) 120:281–91. doi: 10.1016/j.jfma.2020.08.018
4. Zheng B, Zhao J, Cao L. The clinical characteristics and risk factors for necrotizing pneumonia caused by Mycoplasma pneumoniae in children. BMC Infect Dis. (2020) 20:391. doi: 10.1186/s12879-020-05110-7
5. Shim JY. Current perspectives on atypical pneumonia in children. Clin Exp Pediatr. (2020) 63:469–76. doi: 10.3345/cep.2019.00360
6. Wang S, Tang J, Tan Y, Song Z, Qin L. Prevalence of atypical pathogens in patients with severe pneumonia: a systematic review and meta-analysis. BMJ Open. (2023) 13:e066721. doi: 10.1136/bmjopen-2022-066721
7. Gong C, Zhang T, Luo M, Li A, Dong M, Li M, et al. Distribution of the atypical pathogens of community-acquired pneumonia to disease severity. J Thorac Dis. (2018) 10(11):5991–6001. doi: 10.21037/jtd.2018.10.50
8. Gordon O, Oster Y, Michael-Gayego A, Marans RS, Averbuch D, Engelhard D, et al. The clinical presentation of pediatric Mycoplasma pneumoniae infections—a single center cohort. Pediatr Infect Dis J. (2019) 38:698. doi: 10.1097/INF.0000000000002291
9. Kevat PM, Morpeth M, Graham H, Gray AZ. A systematic review of the clinical features of pneumonia in children aged 5–9 years: implications for guidelines and research. J Glob Health. (2002) 12:10002. doi: 10.7189/jogh.12.10002
10. Lee E, Kim C-H, Lee YJ, Kim H-B, Kim B-S, Kim HY, et al. Annual and seasonal patterns in etiologies of pediatric community-acquired pneumonia due to respiratory viruses and Mycoplasma pneumoniae requiring hospitalization in South Korea. BMC Infect Dis. (2020) 20:132. doi: 10.1186/s12879-020-4810-9
11. Biagi C, Cavallo A, Rocca A, Pierantoni L, Antonazzo D, Dondi A, et al. Pulmonary and extrapulmonary manifestations in hospitalized children with Mycoplasma pneumoniae infection. Microorganisms. (2021) 9:2553. doi: 10.3390/microorganisms9122553
12. Brown RJ, Nguipdop-Djomo P, Zhao H, Stanford E, Spiller OB, Chalker VJ. Mycoplasma pneumoniae epidemiology in England and Wales: a national perspective. Front Microbiol. (2016) 7:157. doi: 10.3389/fmicb.2016.00157
13. Leng M, Yang J, Zhou J. The molecular characteristics, diagnosis, and treatment of macrolide-resistant Mycoplasma pneumoniae in children. Front Pediatr. (2023) 11:1115009. doi: 10.3389/fped.2023.1115009
14. Ding G, Zhang X, Vinturache A, van Rossum AMC, Yin Y, Zhang Y. Challenges in the treatment of pediatric Mycoplasma pneumoniae pneumonia. Eur J Pediatr. (2024) 183:3001–11. doi: 10.1007/s00431-024-05519-1
15. Canadian Agency for Drugs and Technologies in Health. Serum IgM and Molecular Tests for Mycoplasma pneumoniae Detection: A Review of Diagnostic Test Accuracy, Clinical Effectiveness, Cost-Effectiveness, and Guidelines [Internet]. Canadian Agency for Drugs and Technologies in Health. (2015):1115009. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK333304/ (accessed September 08, 2024).
Keywords: Mycoplasma pneumoniae, pneumonia, children, risk factors, adenovirus
Citation: Phi DL, To MM, Le KD, Pham TD, Vu CT, Duong KL, Dao TL and Hoang VT (2024) Commentary: Adenovirus and Mycoplasma pneumoniae co-infection as a risk factor for severe community-acquired pneumonia in children. Front. Pediatr. 12:1464813. doi: 10.3389/fped.2024.1464813
Received: 15 July 2024; Accepted: 20 November 2024;
Published: 2 December 2024.
Edited by:
Tongqiang Zhang, Tianjin Children's Hospital, ChinaReviewed by:
Malik Aydin, Witten/Herdecke University, GermanyCopyright: © 2024 Phi, To, Le, Pham, Vu, Duong, Dao and Hoang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Van Thuan Hoang, dGh1YW55dGIzNmNAZ21haWwuY29t; dGh1YW5odkB0YnVtcC5lZHUudm4=
 Duc Long Phi
Duc Long Phi Thi Loi Dao
Thi Loi Dao Van Thuan Hoang
Van Thuan Hoang