- 1Department of Clinical Laboratory, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Department of Nursing, Huashan Hospital, Fudan University, Shanghai, China
- 3Department of Laboratory Medicine, Shanghai Children’s Medical Center, Shanghai Jiaotong University School of Medicine, Shanghai, China
Objective: Methicillin-resistant Staphylococcus aureus (MRSA) infection in children has been on the rise, which poses a serious threat to their health and life in China. The purpose of this study was to determine the molecular characteristics, risk factors, and clinical outcomes of MRSA infections among critically ill pediatric patients.
Methods: A retrospective case-control study was performed in the pediatric intensive care unit (PICU) of a tertiary university teaching hospital. All children infected with culture-positive S. aureus in the PICU between January 2016 and December 2021 were included. Univariate and multivariable logistic regression analyses were used to identify potential risk factors for MRSA infection and clinical outcomes of S. aureus infection. All S. aureus isolates were characterized based on antimicrobial resistance, multilocus sequence typing (MLST) and Staphylococcal protein A (spa) typing.
Results: Of 3,974 patients admitted to the PICU, 280 were diagnosed with a S. aureus infection during the 6-year study period. Among them, 43.2% (121/280) were MRSA. All MRSA isolates showed significantly higher rates of resistance to penicillin, erythromycin, clindamycin and tetracycline than MSSA strains. The MRSA strains consisted of 45 spa types and 20 sequence types (STs) (20 clonal complexes), among which the most frequently represented were ST59-t437and ST398-t034. Multivariable logistic regression revealed vaginal delivery, respiratory failure, co-infection with a virus, C-reactive protein (CRP) > 8 mg/L as significant risk factors for MRSA infection. There was no significant difference in all-cause mortality during hospitalization between the MRSA group and the MSSA group. Furthermore, independent predictors for mortality in patients with S. aureus infections were the presence of hypoproteinemia, hematopathy, septic shock, respiratory failure, fever, and white blood cell (WBC) > 15.0 × 109/L.
Conclusions: The study revealed a high proportion of MRSA infections among critically ill pediatric patients, and found significant risk factors for MRSA infection and poor prognosis of S. aureus infection. Methicillin resistance did not contribute to the mortality in the current study. These findings will provide evidence-based practices to make the strategies of prevention and rational use of antibiotics for pediatric patients with S. aureus infection in the ICU.
1 Introduction
S. aureus, one of the most common Gram-positive pathogens, can trigger a variety of infectious diseases in children and adults, including community and hospital-acquired pneumonia, skin and soft tissue infections, infective endocarditis and bloodstream infections (1, 2). With the introduction of semisynthetic penicillins in 1959, methicillin-resistant S. aureus (MRSA) strains rapidly emerged in 1961 (3). Beginning in the 1980s, MRSA spread worldwide to such an extent that hospitals in many countries reported MRSA rates of 50% or more among S. aureus strains (4). Between January 1998 and June 2003, the annual average rate of MRSA in the United States increased to 51.6% in the ICU and 42% in non-ICU wards (5). In China, the prevalence of MRSA in children under the age of 18 still showed an increasing trend from 18.0% in 2005 to 29.8% in 2017 according to the China Antimicrobial Surveillance Network (CHINET) (6). The increase in the incidence of MRSA infection has become a serious problem in clinical fields, which is associated with increased morbidity and mortality, prolonged courses of antibiotics, extra length of hospital stay and excess hospital costs (7–9). In 2017, the World Health Organization (WHO) published a list of bacteria urgently needing new antimicrobial agents, MRSA was listed as a high-priority pathogen (10).
Genetically distinct MRSA lineages have been reported in most countries around the world, some of which were characterized by international epidemics, while others showed restricted geographic ranges (11, 12). For example, ST8 is mainly found in the United States, ST80 is prevalent in Europe, and ST59 is the most dominant ST in the Asia-Pacific region (11, 12). Moreover, the predominant MRSA lineage circulating in a country or region varies with time. Since about 2010, ST59 clone has gradually replaced ST239 as the dominant clone in most hospitals in China (13). In recent decades, there has been a growing body of research literature on MRSA infection in adults (14–16), but very little significant attention has been paid to the molecular characteristics and risk factors of MRSA infection in PICU patients. These patients tend to be critically ill which makes them extremely vulnerable to acquiring MRSA infections and less likely to survive (17).To bridge this gap, we launched a study to determine the molecular characteristics, risk factors, and clinical outcomes of MRSA infections among PICU patients from 2016 to 2021 in a large pediatric teaching hospital in Shanghai, China.
2 Materials and methods
2.1 Study design and population
The retrospective case-control study was performed at the PICU of Shanghai Children's Medical Center (SCMC) from January 2016 to December 2021. As one of the largest pediatric hospitals in Shanghai, SCMC is a teaching hospital affiliated to Shanghai Jiao Tong University, which is in charge of diagnosing, treating, and caring for newborns up to adolescents. The ethics committee of SCMC approved the study (SCMCIRB-K2023151-1) and granted to waive informed consent because of the nature of the retrospective study that minimizes risk to subjects.
During the study period, all children with culture-documented S. aureus infection in the PICU were identified through the Hospital Information System (HIS) and enrolled in this study. S. aureus infection was defined as isolation of S. aureus from at least one culture in addition to symptoms and signs compatible with inflammatory response. If more than one episode of S. aureus infection occurred in the same patient, only the first episode could be included.
To assess risk factors for death in patients with S. aureus infection, patients were divided into a survivor group and a non-survivor group. Survivors were defined as those who remained alive after discharge, and non-survivors as those who were died during hospitalization or discharged as terminally ill and refused treatment. A MRSA case was considered community-acquired if it was isolated from an inpatient within 48 h of hospitalization, and if risk factors for hospital-acquired infections, including recent (within 30 days) hospitalization or medical procedure (such as dialysis, surgery, and catheters), were absent (18).
2.2 Data collection
The following information was retrieved from electronic medical records and included in the database: demographic data (age, gender), information of birth (birth weight, premature, first child, multiple gestation, feeding style and delivery), comorbidities/underlying diseases, concomitant infection with other pathogens, clinical syndromes, medication and intervention therapy within 30 days preceding infection, history of hospitalization, length of hospital stay and PICU stay, clinical outcomes (length of hospital stay and PICU stay after infection, state of patients upon discharge, and hospital cost).
In addition, the following laboratory indicators were collected from the laboratory information system as well, such as C-reactive protein (CRP), white blood cell (WBC), platelet, hemoglobin, neutrophil/lymphocyte percentage, alanine transaminase (ALT), aspartate aminotransferase (AST), albumin (ALB), serum creatinine and serum urea.
2.3 Antimicrobial susceptibility testing
Bacterial species were firstly identified using an automated Vitek-2 system (bioMerieux, France) and further confirmed by the Vitek MS system (bioMerieux, France). All S. aureus isolates were tested for antibiotic susceptibility using the VITEK2 system with broth microdilution and disk diffusion methods. Sixteen antibiotics employed in the study were as follows: penicillin (P), oxacillin (OXA), cefoxitin (FOX), vancomycin (V), gentamicin (GM), erythromycin (E), tetracycline (TET), tigecycline (TGC), ciprofloxacin (CIP), levofloxacin (LEV), moxifloxacin (MOF), clindamycin (DA), rifampin (RIF), trimethoprim-sulfamethoxazole (SXT), quinupristin/dalfopristin (Q/D), and linezolid (LZD). Results were interpreted by the 2021 Clinical and Laboratory Standards Institute (CLSI) M100-S31 guidelines (19). S. aureus ATCC 29213 was used as the quality control strain.
Additionally, MRSA was defined as S. aureus that was resistant to oxacillin (minimum inhibitory concentration ≥ 4 µg/ml) or cefoxitin (MIC ≥ 8 µg/ml). Multidrug-resistant S. aureus (MDR-SA) was defined as: (i) non-susceptibility to at least one agent in three or more antimicrobial categories, (ii) an MRSA is always considered MDR by virtue of being an MRSA (20).
2.4 Molecular typing
In order to understand the molecular characteristics of S. aureus, all isolates were characterized by multilocus sequence typing (MLST) and staphylococcal protein A (spa) typing. All S. aureus isolates were characterized according to the MLST protocol described by Enright MC et al. (21). Briefly, the chromosomal DNA of 280 S. aureus isolates was extracted by a standard phenol-chloroform extraction procedure. PCR amplicons of seven S. aureus housekeeping genes were obtained from chromosomal DNA. The PCR amplified products were sequenced (Sangon Biotech, Shanghai, China), and the sequences of the PCR products were assigned allele numbers by comparison with the existing sequences available on the MLST website for S. aureus (https://pubmlst.org/), the alleles of the seven genes defined the S. aureus lineage, resulting in an allelic profile designated ST. Clustering was based on STs related at the single-locus-variant level, which were defined as clonal complexes (CCs), was determined using eBURST. Spa typing was determined by using the primers 1514R (CAG CAG TAG TGC CGT TTG CTT) and 1113F (TAA AGA CGA TCC TTC GGT GAG C) to amplify and sequence the polymorphic X-region of the spa, subsequently, spa types were assigned by using the spa database website (http://www.SpaServer.rindom.de).
2.5 Statistical analysis
Baseline and clinical characteristics of patients were summarized using frequencies and proportions for categorical variables, with comparisons performed using Fisher's exact test or Pearson's Chi-square test. For continuous variables exhibiting non-normal distributions, data were reported as medians and interquartile ranges (IQRs), with group differences assessed using the Mann-Whitney U test.
To investigate the associations between variables and the risk of MRSA infection and adverse outcomes of patients with S. aureus infection, we initially conducted regularized lasso regression with L1 regularization using the “glmnet” package in R to address multicollinearity and select relevant independent variables. Following this, stepwise logistic regression analysis was performed on the adjusted independent variables. Variance Inflation Factors (VIF) and tolerance values were calculated to ensure the absence of multicollinearity among the final predictors. Results of the risk factor analysis were reported as odds ratios (ORs) with 95% confidence intervals (CIs) and corresponding p-values. The significance level for all statistical analyses was set at α = 0.05. Correlation analyses were performed using SPSS version 26, while collinearity and logistic regression analyses were conducted using R version 4.4.1.
3 Results
3.1 Secular trend in the prevalence of MRSA infection in the PICU
Of the 3,974 PICU patients, 280 were diagnosed with S. aureus infections after admission to the PICU between January 2016 and December 2021. Among them, 43.2% (121/280) were MRSA, which was similar to the MRSA detection rate (41.4%, 1,004/2,425) in the entire hospital. As shown in Figure 1, the MRSA proportion in PICU showed a stable but slightly upward trend, from 38.7% in 2016 to 43.6% in 2021, whereas that in the entire hospital indicated a declining but fluctuating trend, from 39.4% in 2016 to 36.9% in 2021, which showed a similar tendency with MRSA proportion in group of China (22).
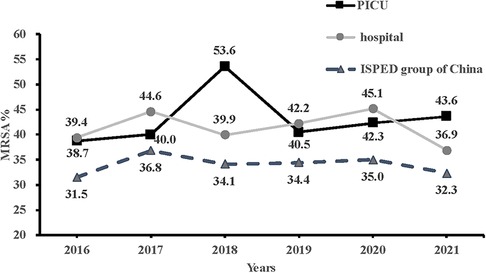
Figure 1. Comparison of trends in methicillin-resistant Staphylococcus aureus in the PICU, the entire hospital and in the ISPED group of China from 2016 to 2021. PICU, pediatric intensive care unit; ISPED, Chinese inspect survey of pediatric consortium.
3.2 Antimicrobial susceptibility profiles among S. aureus isolates
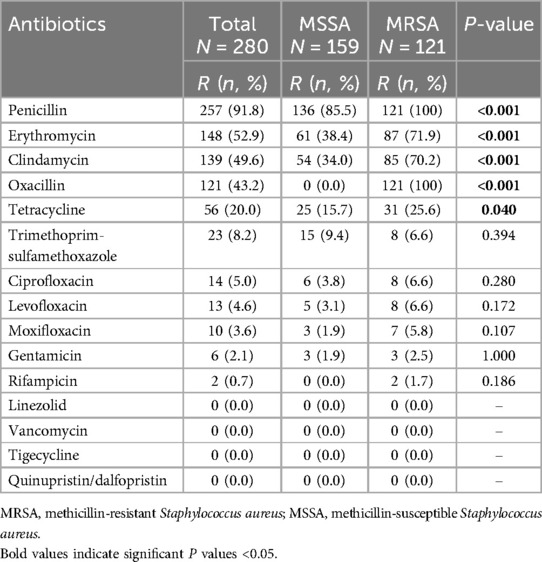
In our study, 144(51.4%) were MDR strains, including all (100%) MRSA strains and 23 (14.5%) MSSA strains. Of 280 S. aureus isolates tested against 16 antibiotics, the rate of resistance to penicillin was the highest (91.8%), followed by erythromycin (52.9%), clindamycin (49.6%), oxacillin (43.2%) and tetracycline (20.0%). However, all isolates were uniformly susceptible to linezolid, vancomycin, tigecycline and quinupristin/dalfopristin. For the remaining antibiotics, the resistance rates were less than 10%.
As shown in Table 1, apart from oxacillin, resistance to rifampin was only observed in MRSA strains (1.7%). MRSA isolates showed significantly higher rates of resistance to penicillin (100% vs. 85.8%, P < 0.001), erythromycin (71.9% vs. 38.4%, P < 0.001), clindamycin (70.2% vs. 34.0%, P < 0.001), and tetracycline (25.6% vs. 15.7%, P = 0.040) than MSSA isolates.
3.3 Clinical and demographic characteristics of enrolled patients with S. aureus infection
During the six-year study period, all 121 children with MRSA infection in the PICU were classified as the case group, while all 159 children who suffered from MSSA infection were served as the control group. Ultimately, 280 pediatric inpatients who met the inclusion criteria were enrolled in the current study. From the clinical medical records, respiratory infection was the most frequently determined infection type. Among the specimens from which the 280 isolates were recovered, respiratory specimens accounted for 86.7% (243/280), including sputum (239, 85.4%), brochoalveolar lavage fluid (4, 1.4%), followed by blood (28, 10.0%), urine (3,1.1%), ocular and wound secretion (3, 1.1%), pleural fluid (2, 0.7%), and peritoneal fluid (1, 0.4%).
Demographic and clinical characteristics of children associated with S. aureus infection in the PICU are shown in Table 2. Of 280 study patients, 163 (58.2%) patients were male (Male/Female:1.4) and the median age was 0.5 years [interquartile range (IQR), 0.2–2.3 years]. One hundred and thirty-one (46.8%) were firstborn children, and the median birth weight reached 3.2 kg (IQR, 2.8–3.5 kg). 137 (48.9%) delivered vaginally and 162 (57.9%) began breastfeeding after birth.
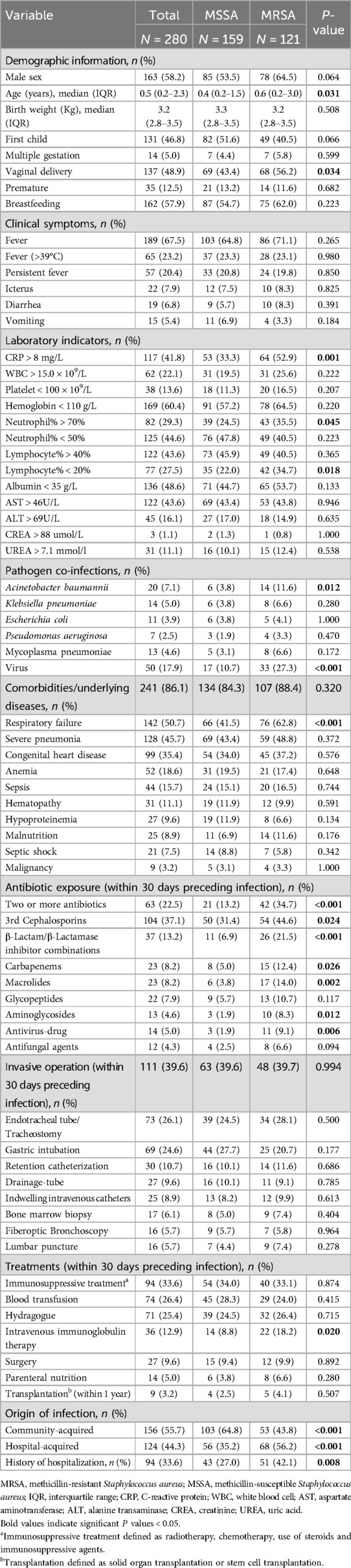
Table 2. Comparison of clinical and epidemiological characteristics between MRSA and MSSA group based on univariate analysis.
The most common clinical symptom was fever, which accounted for more than two-thirds of the study patients. Ninety-four (33.6%) cases had been previously hospitalized and up to 241 (86.1%) patients had at least one underlying disease, among which respiratory failure (142, 50.7%) was the most common, followed by severe pneumonia (128, 45.7%) and congenital heart disease (99, 35.4%). Approximately one-third of patients had received immunosuppressive treatment and 74 (26.4%) had undergone a blood transfusion in a month prior to infection. Furthermore, 22.5% received two or more antibiotics and the most widely used antibiotics were the third-generation cephalosporins. Additionally, 111 (39.6%) children were exposed to some sort of invasive procedure. Among them, tracheal intubation (73, 26.1%) and gastric intubation (69, 24.6%) were the most common.
3.4 Risk factors associated with MRSA infection among critically ill pediatric patients
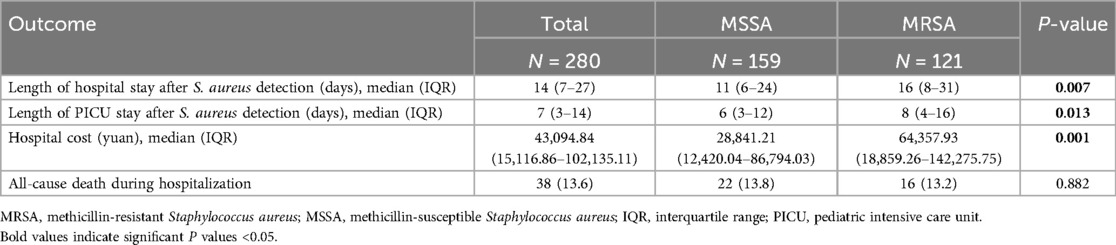
The demographic and clinical characteristics of patients with MRSA and MSSA infection are summarized in Table 2. There was no difference in sex between the two groups. Compared to patients with MSSA infection, those suffered from MRSA infection tend to be older, have a higher proportion of vaginal delivery, respiratory failure, co-infection with Acinetobacter baumannii and virus. Three laboratory indicators, including CRP > 8 mg/L, neutrophil% > 70%, and lymphocyte% < 20%, also more frequently observed in patients with MRSA infection. Furthermore, more patients with MRSA infection received more than two antibiotics, the third-generation cephalosporins, β-lactam/β-lactamase inhibitor combinations, carbapenems, macrolides, aminoglycosides, and antivirus-drug (Table 2). Additionally, the MRSA group had longer hospital stays (p = 0.007), longer PICU stays (P = 0.013) and higher hospitalization expenses (P = 0.001) than the MSSA group (Table 3).
Multivariable logistic regression analysis revealed that vaginal delivery (P = 0.010), respiratory failure (P = 0.006), co-infection with a virus (P = 0.004) and CRP > 8 mg/L (P = 0.005) remained independent risk factors for acquiring MRSA infection (Table 4).
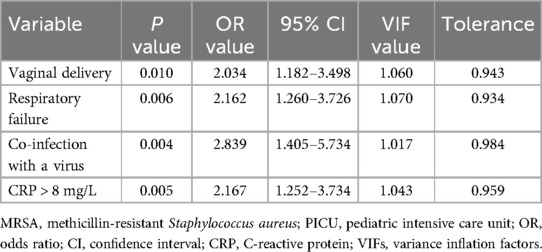
Table 4. Multivariable logistic regression analysis of risk factors for MRSA infection among critically ill pediatric patients.
3.5 Clinical outcomes and risk factors of death of patients with S. aureus infection
During the hospital stay, 38 of 280 patients died, of which 25 patients discharged as terminally ill and refused treatment, the all-cause mortality rate was 13.6% in this study. The analysis of clinical outcomes and risk factors associated with mortality in patients with S. aureus infection is presented in Tables 5, 6, respectively. HA-MRSA infections occurred more frequently in the non-survival group (63.2% vs. 41.3%, P = 0.012), whereas CA-MRSA was more common in the survival group (36.8% vs. 58.7%, P = 0.012) (Table 5).
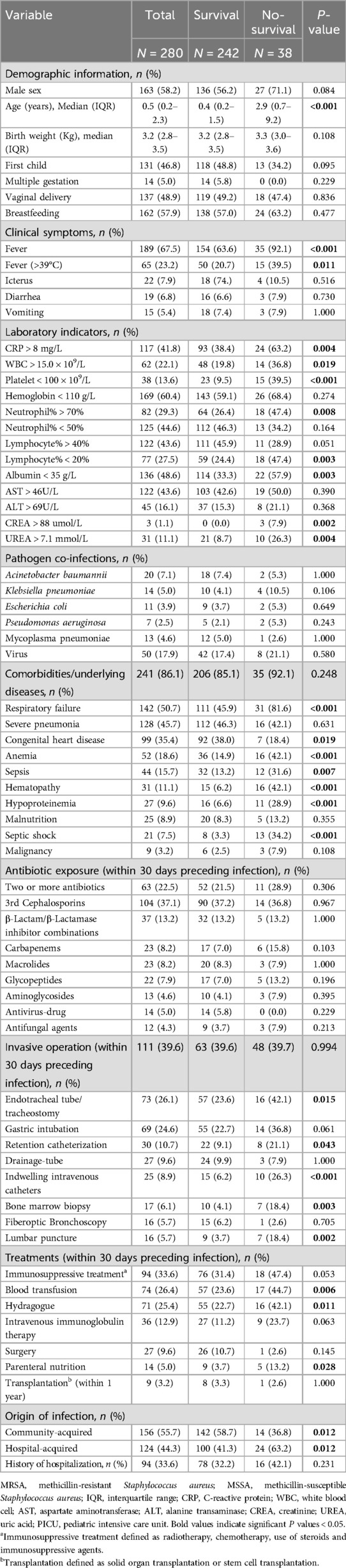
Table 5. Univariate analysis for factors associated with mortality of children with Staphylococcus aureus infections.
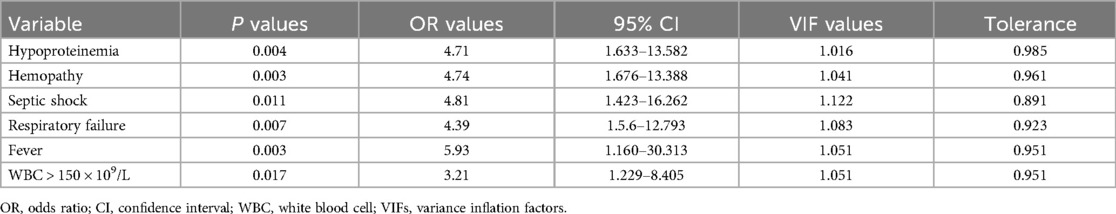
Table 6. Multivariable logistic regression analysis of variables related to mortality of children with Staphylococcus aureus infection.
Compared with survivors, non-survivors had a higher proportion of fever, especially fever >39℃, CRP > 8 mg/L, WBC >15.0 × 109/L, platelet <100 × 109/L, neutrophil% > 70%, lymphocyte% < 20%, albumin < 35 g/L, serum creatinine > 88 umol/L, and serum urea > 7.1 mmol/L. Moreover, some underlying diseases, including respiratory failure, anemia, sepsis, hematopathy, hypoproteinemia, and septic shock were more frequent in non-survivors. Besides, those who received specific treatments before S. aureus isolation, including endotracheal tube/tracheostomy, retention catheterization, indwelling intravenous catheters, bone marrow biopsy, lumbar puncture, blood transfusion, parenteral nutrition, and hydragogue (Table 5) were more likely to be non-survivors.
In the multivariable logistic regression model, independent risk factors associated with mortality in children with S. aureus infection included hypoproteinemia (P = 0.004), hepatopathy (P = 0.003), septic shock (P = 0.011), respiratory failure (P = 0.007), fever (P = 0.003) and WBC >15.0 × 109/L (P = 0.017) (Table 6). There was no significant difference in the mortality between the MRSA group and the MSSA group (13.2% vs. 13.8%, P = 0.882) (Table 3).
3.6 Molecular characterization of S. aureus strains isolated from critically ill children
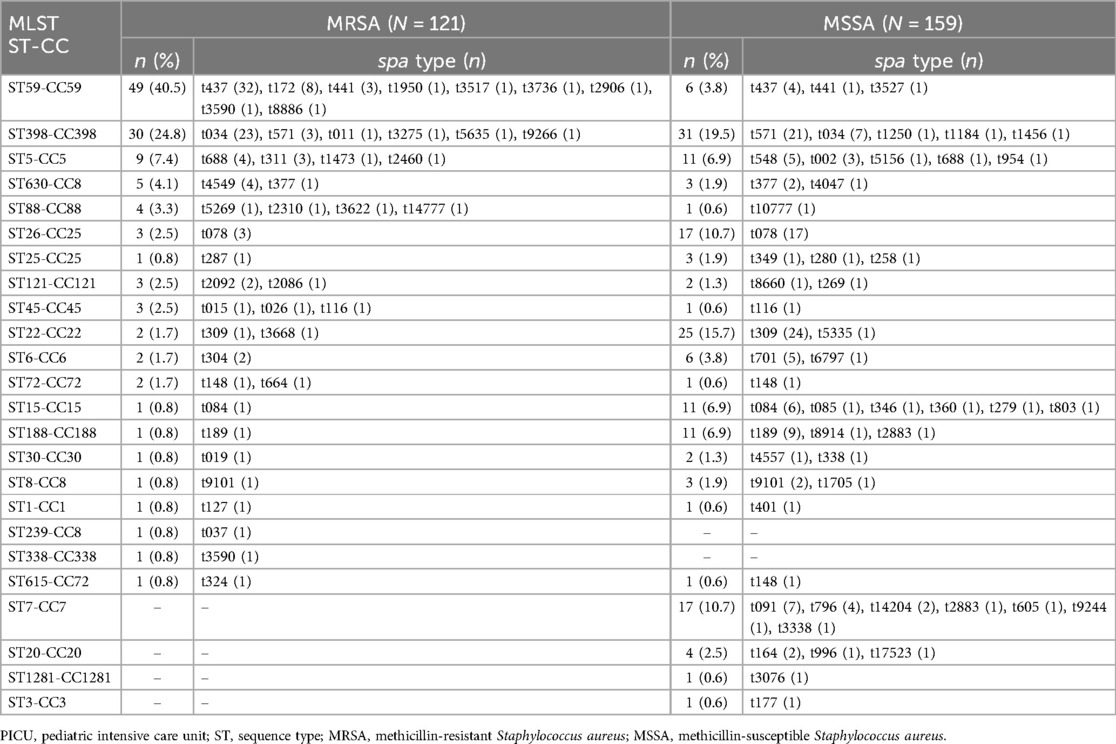
The genetic diversity of all S. aureus isolates from children admitted to the PICU was analyzed by MLST and spa typing. There were 24 distinct sequence types (STs) identified within the 280 isolates, which belonged to 23 CCs (Table 7). CC398, CC59, CC22, CC25, and CC5 were the most prevalent CCs, including 21.8% (61/280), 19.6% (55/280), 9.6% (27/280), 8.5% (24/280), and 7.1% (20/280) of the isolated, respectively. While, the most frequently represented STs genotype was ST398 (21.8%, 61/280), followed by ST59 (19.6%, 55/280) and ST22 (9.6%, 27/280). The top three STs accounted for half of all S. aureus isolates. The spa typing discriminated 280 strains into 86 types, with t437 (12.9%, 36/280) as the most frequently represented type, followed by t034 (10.7%, 30/280), t309 (8.9%, 25/280), t571 (8.6%, 24/280) and t078 (7.1%, 20/280). Additionally, the percentage of ST59 genotype among the MRSA population, including the ST59-t437 genotype, showed an upward trend from 2016 to 2020 (except for 2021), whilst the proportion of ST398 genotype, including the ST398-t034 genotype, exhibited a wave-like uplift from 2016 to 2021 (Figure 2).
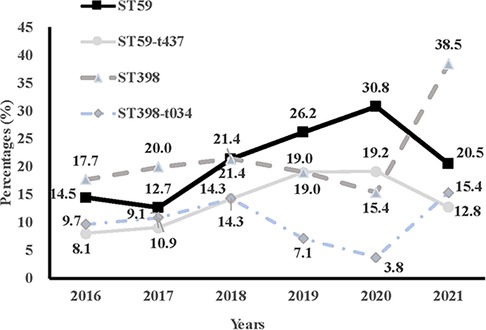
Figure 2. Comparison of trends of percentages of ST59, ST59-t437, ST398, and ST398-t034 in methicillin-resistant Staphylococcus aureus from 2016 to 2021.
Twenty distinct STs and 45 spa types were identified among MRSA strains, whereas 22 STs and 53 spa types were found with MSSA isolates. Among them, ST22-t309, ST398-t571, and ST26-t078 were the most common types of MSSA isolates, whereas ST59-t437 and ST398-t034 were overrepresented among MRSA isolates (OR = 13.933, P < 0.001, OR = 5.096, P < 0.001). Of note, ST398 was a unique genotype prevalent in both MRSA and MSSA. ST239 and ST338 genotypes were uniquely identified in MRSA strains, while ST7, ST20, ST1281 and ST3 were only found in MSSA strains. The remaining STs including ST398, ST59, ST22, ST26, ST5, ST15, ST188, ST6, ST630, ST121, ST88, ST25, ST45, ST8, ST30, ST72, ST1 and ST615 were found in both MRSA and MSSA.
Of the major prevalent strains, 89.1% of ST59 strains were MRSA, 49.2% of ST398 strains were MRSA and 7.4% of ST22 strains were MRSA. ST59 strains showed significantly higher rates of resistance to penicillin, erythromycin, clindamycin, and tetracycline than ST398 or ST22 strains (Supplementary Table S1). Except for methicillin resistance, ST398 and ST22 strains had no significant difference in resistance to other antibiotics.
4 Discussion
MRSA infections are a growing problem in pediatric patients, associated with high morbidity and mortality. In this retrospective study, we described the molecular characteristics, risk factors, and clinical outcomes of MRSA infections among critically ill pediatric patients between January 2016 and December 2021.
Clarifying resistance trends of MRSA is beneficial to guide clinical anti-infective treatment. In the United States, the incidence of MRSA bloodstream infections in hospitals and communities decreased by 74% and 40%, respectively, from 2006 to 2015 (23). In recent years, the prevalence of MRSA in the hospital has declined in some European countries, e.g., Austria, France, Ireland, the UK and Greece (24). In China, the proportions of MRSA among S. aureus infections in adults decreased from 69.94% in 2005 to 31.00% in 2020 (25, 26). In contrast, the prevalence of MRSA infections among critically ill pediatric patients found in our study remained in the range of 38.70%–43.5% during the six-year study period. These discrepancies may be attributed in part to the study population or the rise of community-associated MRSA (CA-MRSA) in children (27). Furthermore, all MRSA isolates showed a significantly higher rates of resistance to penicillin, erythromycin, clindamycin, and tetracycline than MSSA isolates. Consequently, MRSA infection should draw more attention in consideration of high drug resistance rate.
In recent years, there have been increasing studies on the molecular characteristics of MRSA infections in adults. However, the molecular profile of MRSA among Chinese children is limited. A study of S. aureus strains isolated from pediatric patients in Suzhou found that ST22 and ST59 were the most typical MRSA strains (28). Data from Guangzhou showed ST45 and ST59 were found to be major MRSA lineages among school-aged children across five schools (29). In contrast to these results, our results indicated that ST59 and ST398 were the dominant types of pediatric MRSA isolates. Among them, ST59-t437 and ST398-t034 genotypes are of particular concern, as they are 14 and 5 times more likely to cause MRSA infections in children, respectively. We observed the proportion of ST59 strain in all S. aureus strains increased year by year, mainly due to the increase in the proportion of MRSA-ST59-t437 infections. In comparison to an ST239-t030 strain, ST59-t437 MRSA lineage was reported to be characteristic of fast growth ability, high survival rate resistance, high toxin secretion levels, and cytotoxicity (30), which might facilitate its spread among pediatric patients, and maintain the most predominant MRSA clone in China (31). ST398 is a typical livestock-associated MRSA globally, which was first observed among pig and pig farmers in the Netherlands in 2003, and then found in Austria, Germany, and other countries (32). ST398-MRSA has been found more and more in humans and is associated with serious diseases. The virulence and biofilm formation of the ST398-MRSA subtype have been found to favor their adaptability in community and medical settings (33). Notably, we observed a further significant increase in the proportion of ST398 strain, from 17.7% in 2016 to 38.5% in 2021, especially the ST398-t034 lineage. Therefore, active surveillance is necessary to control and prevent the clinical impact of ST398-MRSA infections.
This study shed light on risk factors for MRSA infection in critically ill children. Patients with vaginal delivery, respiratory failure, co-infection with a virus, and CRP > 8 mg/L were significantly more likely to develop MRSA infection than MSSA infection. Pregnant women usually showed mild immunosuppression, elevated estrogen levels and blood glucose levels (34), which makes the MRSA colonization rate in pregnant women more than twice higher than in the general community (35). Furthermore, vaginal carriage represented a major risk factor for vertical transmission of this pathogen from mother to newborn (36), this may explain why pediatric patients with vaginal delivery are two times more likely to develop MRSA infections.
Our data revealed that respiratory failure is not only an independent risk factor for MRSA infection, but also for death due to S. aureus infection. Patients suffering from respiratory failure are often severely ill and tend to undergo invasive procedures such as mechanical ventilation support, which leads to an increased chance of bacterial infections, including MRSA (37). Moreover, severity of illness (such as Charlson complications score, septic shock and hematology tumor) has been significantly associated with poor prognosis in patients with S. aureus infection in many literatures (38, 39). It has even been reported that serious underlying diseases can lead to higher mortality rates regardless of the aetiologic agent (40). In addition, co-infection with a virus and high CRP value also increased the odds ratio for MRSA infection. Co-infection with a virus seems to be at an increased risk of severe diseases such as the development of respiratory failure, and patients would have a weaker immune system and are more vulnerable to MRSA infection. The high CRP values may be due in part to the fact that patients with MRSA infection have a higher proportion of co-viral infections and severe underlying diseases than those with MSSA infection.
In the current study, we found patients with MRSA infection had longer hospital stays and increased hospitalization costs than those with MSSA infection. However, no significant difference in all-cause mortality was observed between the two groups. In addition to microbial factors, host factors also play an important role in the progress of the disease (41). Serious underlying disease has ever been reported to be independently associated with mortality in patients with S. aureus infection (38, 39). Consistent with these studies, we found the presence of hypoproteinemia, hematopathy, septic shock and respiratory failure were independent predictors of worse outcomes for patients with S. aureus infection. Moreover, fever and WBC > 15.0 × 109/L were another two predictors for mortality as well. WBC count was an inflammatory biomarker that reflected the underlying biological processes (42). Although it is not disease-specific, it is widely used to measure the severity of disease, tissue inflammation and infection.
There are several limitations in our study. Firstly, it was performed at a single center in China, and may not be generalizable to other institutions or settings. Secondly, there is no data exploring the virulence genes of S. aureus isolates, which would shed new light on the prognosis of patients with S. aureus infection. Thirdly, in our study, the S. aureus isolates were mainly recovered from sputum samples, accounting for 85.4%, which makes it more difficult to identify whether the pathogen is a colonized or an infected strain and easily leads to overestimate the detection rate of MRSA. Despite the above-mentioned limitations, this study spans a long time and recruited study patients through very strict inclusion and exclusion criteria, in order to minimize the selection bias, and strengthen the accuracy of data and validation of our results.
5 Conclusions
Through the 6-year study, a high proportion of MRSA infections were found in critically ill pediatric patients. These MRSA isolates consisted of 20 spa types and 45 sequence types (STs), among which the most frequently represented were ST59-t437 and ST398-t034. Multivariable logistic regression revealed vaginal delivery, respiratory failure, co-infection with a virus, C-reactive protein (CRP) > 8 mg/L as significant risk factors for MRSA infection. Methicillin resistance didn't contribute to the mortality associated with S. aureus infection in the current study. Furthermore, the presence of hypoproteinemia, hematopathy, septic shock, respiratory failure, fever, and WBC > 15.0 × 109/L were independent predictors of mortality due to S. aureus infection. These findings will provide evidence-based practices to make the strategies of prevention and rational use of antibiotics for pediatric patients with S. aureus infection stay in the ICU.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Shanghai Children's Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this was a retrospective observational study, and it did not cause any disruption to patients.
Author contributions
CD: Data curation, Software, Formal Analysis, Investigation, Writing – review & editing. WJ: Investigation, Methodology, Conceptualization, Formal Analysis, Writing – review & editing. YZ: Investigation, Methodology, Conceptualization, Writing – review & editing, Formal Analysis. WH: Writing – review & editing, Investigation, Data curation. HW: Funding acquisition, Supervision, Writing – review & editing, Methodology, Project administration, Writing – original draft. XW: Supervision, Writing – review & editing, Methodology, Project administration.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by the Foundation of Chinese medicine scientific research project of Shanghai Municipal Health Commission (grant number: 2020JZ001).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1457645/full#supplementary-material
References
1. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. (2015) 28(3):603–61. doi: 10.1128/CMR.00134-14
2. Lakhundi S, Zhang K. Methicillin-resistant Staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. (2018) 31(4):e00020–18. doi: 10.1128/CMR.00020-18
3. Barber M. Methicillin-resistant staphylococci. J Clin Pathol. (1961) 14(4):385–93. doi: 10.1136/jcp.14.4.385
4. Diekema DJ, Pfaller MA, Schmitz FJ, Smayevsky J, Bell J, Jones RN, et al. Survey of infections due to Staphylococcus species: frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the western pacific region for the SENTRY antimicrobial surveillance program, 1997–1999. Clin Infect Dis. (2001) 32(Suppl 2):S114–32. doi: 10.1086/320184
5. NNIS System. National nosocomial infections surveillance (NNIS) system report, data summary from January 1992 through June 2003, issued August 2003. Am J Infect Control. (2003) 31(8):481–98. doi: 10.1016/j.ajic.2003.09.002
6. Hu F, Zhu D, Wang F, Wang M. Current Status and trends of antibacterial resistance in China. Clin Infect Dis. (2018) 67(suppl_2):S128–34. doi: 10.1093/cid/ciy657
7. Rodrigues R, Passadouro R, Gomes O, Castro R. Risk factors, length of stay and in-hospital mortality of methicillin-resistant Staphylococcus aureus infections: a case-control study. Acta Med Port. (2020) 33(3):174–82. doi: 10.20344/amp.10952
8. Otto M. Methicillin-resistant Staphylococcus aureus infection is associated with increased mortality. Future Microbiol. (2012) 7(2):189–91. doi: 10.2217/fmb.11.156
9. David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. (2010) 23(3):616–87. doi: 10.1128/CMR.00081-09
10. Organization, W.H. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics (2017). Available online at: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1 (accessed April 23, 2021).
11. Mediavilla JR, Chen L, Mathema B, Kreiswirth BN. Global epidemiology of community-associated methicillin resistant Staphylococcus aureus (CA-MRSA). Curr Opin Microbiol. (2012) 15(5):588–95. doi: 10.1016/j.mib.2012.08.003
12. Witte W. Community-acquired methicillin-resistant Staphylococcus aureus: what do we need to know? Clin Microbiol Infect. (2009) 15(Suppl 7):17–25. doi: 10.1111/j.1469-0691.2009.03097.x
13. Li S, Sun S, Yang C, Chen H, Yin Y, Li H, et al. The changing pattern of population structure of Staphylococcus aureus from bacteremia in China from 2013 to 2016: sT239-030-MRSA replaced by ST59-t437. Front Microbiol. (2018) 9:332. doi: 10.3389/fmicb.2018.00332
14. Koga S, Takazono T, Kido T, Muramatsu K, Tokutsu K, Tokito T, et al. Evaluation of the effectiveness and use of anti-methicillin-resistant Staphylococcus aureus agents for aspiration pneumonia in older patients using a nationwide Japanese administrative database. Microorganisms. (2023) 11(8):1905. doi: 10.3390/microorganisms11081905
15. Shirata M, Ito I, Tanabe N, Konishi S, Oi I, Hamao N, et al. Risk factors associated with methicillin-resistant Staphylococcus aureus isolation from serially collected sputum samples of patients hospitalized with pneumonia. J Infect Chemother. (2021) 27(9):1323–8. doi: 10.1016/j.jiac.2021.04.022
16. Hu X, Hu K, Liu Y, Zeng L, Hu N, Chen X, et al. Risk factors for methicillin-resistant Staphylococcus aureus colonization and infection in patients with human immunodeficiency virus infection: a systematic review and meta-analysis. J Int Med Res. (2022) 50(1):3000605211063019. doi: 10.1177/03000605211063019
17. Yilmaz M, Elaldi N, Balkan İİ, Arslan F, Batırel AA, Bakıcı MZ, et al. Mortality predictors of Staphylococcus aureus bacteremia: a prospective multicenter study. Ann Clin Microbiol Antimicrob. (2016) 15:7. doi: 10.1186/s12941-016-0122-8
18. Geng W, Yang Y, Wu D, Huang G, Wang C, Deng L, et al. Molecular characteristics of community-acquired, methicillin-resistant Staphylococcus aureus isolated from Chinese children. FEMS Immunol Med Microbiol. (2010) 58(3):356–62. doi: 10.1111/j.1574-695X.2009.00648.x
19. Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing. Wayne, PA: CLSI Document (2021). M100–S31.
20. Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. (2012) 18(3):268–81. doi: 10.1111/j.1469-0691.2011.03570.x
21. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J Clin Microbiol. (2000) 38(3):1008–15. doi: 10.1128/JCM.38.3.1008-1015.2000
22. Wu X, Wang C, He L, Xu H, Jiang C, Chen Y, et al. Antimicrobial resistance profile of methicillin-resistant Staphylococcus aureus isolates in children reported from the ISPED surveillance of bacterial resistance, 2016–2021. Front Cell Infect Microbiol. (2023) 13:1102779. doi: 10.3389/fcimb.2023.1102779
23. Kavanagh KT, Abusalem S, Calderon LE. The incidence of MRSA infections in the United States: is a more comprehensive tracking system needed? Antimicrob Resist Infect Control. (2017) 6:34. doi: 10.1186/s13756-017-0193-0
24. Stefani S, Chung DR, Lindsay JA, Friedrich AW, Kearns AM, Westh H, et al. Meticillin-resistant Staphylococcus aureus (MRSA): global epidemiology and harmonisation of typing methods. Int J Antimicrob Agents. (2012) 39(4):273–82. doi: 10.1016/j.ijantimicag.2011.09.030
25. Hu FP, Guo Y, Zhu DM, Wang F, Jiang XF, Xu YC, et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin Microbiol Infect. (2016) 22(Suppl 1):S9–14. doi: 10.1016/j.cmi.2016.01.001
26. Hu F, Guo Y, Zhu D, Wang F, Jiang XF, Xu YC, et al. CHINET surveillance of bacterial resistance: results of 2020. Chin. J Infect Chemother. (2021) 21(4):377–87. doi: 10.16718/j.1009-7708.2021.04.001
27. Wang X, Li X, Liu W, Huang W, Fu Q, Li M. Molecular characteristic and virulence gene profiles of community-associated methicillin-resistant Staphylococcus aureus isolates from pediatric patients in Shanghai, China. Front Microbiol. (2016) 7:1818. doi: 10.3389/fmicb.2016.01818
28. Zhou Y, Yu S, Su C, Gao S, Jiang G, Zhou Z, et al. Molecular characteristics of methicillin-resistant and susceptible Staphylococcus aureus from pediatric patients in eastern China. Pathogens. (2023) 12(4):549. doi: 10.3390/pathogens12040549
29. Liang B, Liang X, Gao F, Long Y, Mai J, Ai X, et al. Active surveillance, drug resistance, and genotypic profiling of Staphylococcus aureus among school-age children in China. Front Med (Lausanne). (2021) 8:701494. doi: 10.3389/fmed.2021.701494
30. Liao F, Gu W, Fu X, Yuan B, Zhang Y. Comparison of virulence-related determinants between the ST59-t437 and ST239-t030 genotypes of methicillin-resistant Staphylococcus aureus. BMC Microbiol. (2021) 21(1):264. doi: 10.1186/s12866-021-02329-5
31. Wang B, Xu Y, Zhao H, Wang X, Rao L, Guo Y, et al. Methicillin-resistant Staphylococcus aureus in China: a multicentre longitudinal study and whole-genome sequencing. Emerg Microbes Infect. (2022) 11(1):532–42. doi: 10.1080/22221751.2022.2032373
32. Fluit AC. Livestock-associated Staphylococcus aureus. Clin Microbiol Infect. (2012) 18(8):735–44. doi: 10.1111/j.1469-0691.2012.03846.x
33. Lu H, Zhao L, Si Y, Jian Y, Wang Y, Li T, et al. The surge of hypervirulent ST398 MRSA lineage with higher biofilm-forming ability is a critical threat to clinics. Front Microbiol. (2021) 12:636788. doi: 10.3389/fmicb.2021.636788
34. Robinson DP, Klein SL. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav. (2012) 62(3):263–71. doi: 10.1016/j.yhbeh.2012.02.023
35. Shiadeh MN, Sepidarkish M, Mollalo A, As’adi N, Khani S, Shahhosseini Z, et al. Worldwide prevalence of maternal methicillin-resistant Staphylococcus aureus colonization: a systematic review and meta-analysis. Microb Pathog. (2022) 171:105743. doi: 10.1016/j.micpath.2022.105743
36. Lin J, Yao Z. Maternal-infant correlation of multidrug-resistant Staphylococcus aureus carriage: a prospective cohort study. Front Pediatr. (2018) 6:384. doi: 10.3389/fped.2018.00384
37. Sacar S, Sayin Kutlu S, Turgut H, Cevahir N, Hircin Cenger D, Tekin K. Epidemiology and associated factors for nosocomial methicillin-resistant Staphylococcus aureus infection in a tertiary-care hospital. Epidemiol Infect. (2010) 138(5):697–701. doi: 10.1017/S0950268809991063
38. Wang JL, Chen SY, Wang JT, Wu GH, Chiang WC, Hsueh PR, et al. Comparison of both clinical features and mortality risk associated with bacteremia due to community-acquired methicillin-resistant Staphylococcus aureus and methicillin-susceptible S. aureus. Clin Infect Dis. (2008) 46(6):799–806. doi: 10.1086/527389
39. Kim J, Park SY, Sohn KM, Kim B, Joo EJ. Methicillin resistance increased the risk of treatment failure in native joint septic arthritis caused by Staphylococcus aureus. Antibiotics (Basel). (2023) 12(11):1628. doi: 10.3390/antibiotics12111628
40. Topeli A, Unal S, Akalin HE. Risk factors influencing clinical outcome in Staphylococcus aureus bacteraemia in a turkish university hospital. Int J Antimicrob Agents. (2000) 14(1):57–63. doi: 10.1016/S0924-8579(99)00147-8
41. Simor AE, Pelude L, Golding G, Fernandes R, Bryce E, Frenette C, et al. Canadian nosocomial infection surveillance program. Determinants of outcome in hospitalized patients with methicillin-resistant Staphylococcus aureus bloodstream infection: results from national surveillance in Canada, 2008–2012. Infect Control Hosp Epidemiol. (2016) 37(4):390–7. doi: 10.1017/ice.2015.323
Keywords: methicillin-resistant staphylococcus aureus, children, genotype, risk factors, mortality
Citation: Dai C, Ji W, Zhang Y, Huang W, Wang H and Wang X (2024) Molecular characteristics, risk factors, and clinical outcomes of methicillin-resistant Staphylococcus aureus infections among critically ill pediatric patients in Shanghai, 2016–2021. Front. Pediatr. 12:1457645. doi: 10.3389/fped.2024.1457645
Received: 3 July 2024; Accepted: 20 September 2024;
Published: 17 October 2024.
Edited by:
Ana Friães, University of Lisbon, PortugalReviewed by:
Marcos Daniel Pinho, University of Lisbon, PortugalRutan Zhang, University of Washington, United States
Copyright: © 2024 Dai, Ji, Zhang, Huang, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiying Wang, bGlhbmcxOTk2MTExMUBzaHV0Y20uZWR1LmNu; Xing Wang, d3hfNTE2NkAxNjMuY29t
†These authors have contributed equally to this work
 Congyi Dai
Congyi Dai Wenting Ji2,†
Wenting Ji2,† Yufei Zhang
Yufei Zhang Weichun Huang
Weichun Huang Haiying Wang
Haiying Wang Xing Wang
Xing Wang