- 1Department of Pediatrics, Division of Neonatology, Memorial University, St. John's, Newfoundland, NF, Canada
- 2Department of OBGYN, Newborn Division, University of Ottawa, The Ottawa Hospital, Ottawa, ON, Canada
- 3Department of Pediatrics, Division of Neonatology, University of Ottawa, Children's Hospital of Eastern Ontario, Ottawa, ON, Canada
- 4Department of Family Medicine, Michigan State University, Jackson, MI, United States
- 5Children's Hospital of Eastern Ontario Research Institute, Ottawa, ON, Canada
- 6Health Sciences Library, University of Ottawa, Ottawa, ON, Canada
Background: Acquired spontaneous intestinal perforation or SIP occurs most commonly in the extremely premature infant population. As the incidence is rising, understanding modifiable factors such as common medication exposures becomes important for individualizing care.
Methods: The primary outcome was SIP in premature infants with exposure to indomethacin, ibuprofen, or acetaminophen. The systematic review and meta-analysis were conducted following the Cochrane methodology and PRISMA guidelines.
Results: The point estimates of three RCTs showed an increase in the risk of SIP with indomethacin exposure compared to no medication, the pooled estimate was not statistically significant. There is no statistically significant association between the risk of SIP for indomethacin with treatment use over prophylactic use and when holding feeds. Ibuprofen conferred less risk than indomethacin, and its route of administration did not alter the risk profile. There was not enough evidence to draw conclusions about the risk of SIP and acetaminophen exposure.
Conclusion: In studies of infants exposed to either indomethacin or ibuprofen in the last 40 years, the incidence of SIP is still commonly within 2–8%. Moving forward modifiable factors such as medication exposure will help guide care to minimize risk where possible.
Systematic Review Registration: https://www.crd.york.ac.uk/, PROSPERO (CRD42017058603).
Introduction
Spontaneous intestinal perforation (SIP) is becoming the most prevalent acquired neonatal intestinal disease in extreme prematurity, outpacing necrotizing enterocolitis (NEC), the former front runner (1, 2). Understanding the architecture of SIP permits analysis and characterization of its relationship to neonatal medications such as non-steroidal anti-inflammatory drugs (NSAIDs) and acetaminophen.
SIP is the localized perforation of the intestine without clinical or histopathological signs of NEC (3). It can be congenital or acquired. Congenital SIP is the rare absence of the intestinal muscularis interna in later gestation infants (4). In acquired SIP, the muscularis layer is present but becomes contracted in the extremely premature and extremely low birth weight populations (4). The clinical hallmark of SIP is the overall stability of the infant (3). SIP presents between 0 and 10 days of life with a shiny distended abdomen, often bluish in color, without the presence of loops (1). On radiography, the abdomen is gasless, and pneumoperitoneum is present without pneumatosis (1). The process is self-limiting with rare cases of recurrence or strictures (5). SIP predominately occurs on the antimesenteric border in the distal ileum (3, 5). On histopathological examination, there is focal hemorrhagic necrosis with a defined border surrounded by healthy appearing bowel (3). The intestinal muscularis propria is thin, with thin-walled vessels in the adjacent submucosa.
There are numerous postulated risk factors for SIP. One of them involves an ischemic hit to the watershed blood supply to the distal ileum (e.g., low APGAR scores, stress, hypoxia, shock, or microemboli), but this does not account for SIP occurring in other locations (3, 6). The patent ductus arteriosus (PDA) is felt to contribute to the risk, due to the decrease in mesenteric blood supply secondary to diastolic steal (7). Other risk factors include prematurity, low birth weight, infection, feeding regime, and antenatal/postnatal medications (2, 3). To understand the potential associations, it is paramount to review the underlying physiology.
Antenatal intestinal growth is driven by insulin-like growth factor -I (IGF-I) (5). Levels are decreased in lower gestation and low birth weight infants, resulting in thinner bowel walls (1, 5). Thicker meconium, associated with premature bowel hypomotility, can increase pressure on the thin intestinal walls (3). Postnatal medications further derange this physiology. NSAIDs including indomethacin and ibuprofen are cyclooxygenase inhibitors that competitively bind to block prostaglandin synthesis (8). In human fetal tissue, indomethacin has pernicious effects occurring at the genomic level disrupting critical metabolic pathways known to elicit a protective response to oxidative stress (9). Additionally, indomethacin reduces the amount of nitric oxide synthase and its precursor arginine during mid-gestation in the human gut (1, 10). Reduced nitric oxide synthase exacerbates intestinal dysmotility. Decreased arginine has deleterious effects on bowel wall tight junctions, disrupting the intestinal barrier, and resulting in increased bacterial translocation (9, 10). The detrimental effects of indomethacin on the gut are compounded when taken concurrently with steroids resulting in depletion of all nitric oxide synthase isoforms, intestinal mucosal hyperplasia, and submucosal thinning (1, 5). When medications are stopped and nitric oxide levels normalize, bowel motility returns, placing pressure on the weakened structure culminating in the potential for SIP. Ibuprofen is another NSAID, with milder effects on cerebral, renal, and mesenteric blood flow compared to indomethacin (11). Acetaminophen also inhibits prostaglandin synthesis through a selective mechanism that does not have the same peripheral vasoconstriction as NSAIDs (8).
The incidence of SIP is increasing, and determining risk factors is cardinal when balancing minimizing peril with maximizing care for this fragile population. The goal of this systematic review was to provide a current analysis of the association of SIP with exposure to indomethacin, ibuprofen, and acetaminophen.
Methods
Protocol registration
The protocol was registered on PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42017058603). The strategy followed methods outlined in the Cochrane Handbook for Systematic Reviews, as well as the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (12, 13). Any differences from the published protocol were decided as a group and outlined in Supplementary Table 1 (14).
Eligibility of studies
The study population was neonates born less than 37 weeks or birth weight less than 2.5 kg. The intervention was exposure to one of the following medications: indomethacin, ibuprofen, or acetaminophen.
The following study designs in any language worldwide were included: randomized controlled trials, clinical trials (for unpublished clinical trials, the corresponding authors were contacted), cohort studies with a control group, and case–control studies.
Eligible studies were required to meet the above criteria as well as the definition of SIP listed below.
Outcomes
Definition of SIP
SIP became a separate recognized entity in 2002 by the National Institute for Child Health and Human Development (NICHD) (4). Historically, SIP and NEC were grouped together with intestinal perforations occurring in both within the same high-risk population, until the 2002 NICHD definition. The timeframe for this systematic review predates the established 2002 definition. To ensure clarity, the definition of SIP in our study was based on the following timeline:
1. Before the 2002 NICHD definition, SIP was defined by either radiological (pneumoperitoneum, intramural echogenicity, and echogenic extramural material on abdominal ultrasonography as pathognomonic signs with no evidence of pneumatosis intestinalis), histopathologic evidence (focal hemorrhagic necrosis, possible hypoplasia of the muscularis layer, and thinning of the submucosa) or surgical evidence of isolated intestinal perforation in the absence of histopathological features of necrotizing enterocolitis (3, 15, 16).
2. After the 2002 NICHD definition, if SIP was listed as an outcome, it was accepted.
Primary outcome
The primary outcome was SIP with comparisons between those exposed to one of the listed medications to either no medication, to an alternate medication from that list, or a prophylactic vs. treatment regimen comparison. In this systematic review, articles designed to compare prophylactic to treatment regimens were pooled regardless of the dose or underlying condition being prevented/treated. Prophylactic medication was given within the first 24 h of life, and treatment was given later than 24 h of life for diagnosed PDA management.
Secondary outcomes
The secondary outcomes (with definitions) were NEC (Bell stage 2 or greater), oliguria (<1 ml/kg/h of urine output), and neonatal death prior to discharge (17). If a study did not meet the definition, the outcome was excluded.
Additional clinical questions and characteristics
The relationship of SIP with medication exposure through the lens of feeding regimes and the ibuprofen route of administration was also explored. Additional characteristics were captured (with definitions) including intraventricular hemorrhage (IVH) (any grade using Papile criteria of IVH), retinopathy of prematurity (ROP) (any stage using the International Classification of ROP), and bronchopulmonary dysplasia (BPD) (requirement of oxygen and/or positive pressure at 36 weeks postmenstrual age or at time of discharge if prior to 36 weeks) (18–20). Antenatal medication information including steroids, magnesium sulfate, and indomethacin, as well as postnatal steroids (not for blood pressure support), was collected.
Search strategy and study selection
A search strategy was developed by a medical librarian in Medline and then translated into the other databases (Embase Classic and OVID, PubMed, LILAC, ScIELO, and Cochrane Central) to retrieve articles. All databases were searched from their dates of inception to 13 November 2016, updated on 19 February 2021, 4 May 2022, and lastly on 30 September 2022 (Supplementary Data Sheet 1). All references were entered into Endnote for processing (21). The initial duplicate screening was performed using Covidence (22). On 9 September 2023, two additional search strategies were performed: systematic review snowballing and systematic review reference check (details in Supplementary Data Sheet 1) (23). Two reviewers independently screened abstracts and full texts of all retrieved articles, and any conflicts were resolved by the biostatisticians.
Risk of bias appraisal
Two authors conducted risk of bias assessment independently for all included studies. The Cochrane Risk of Bias tool-II (RoB 2) was used for the risk of bias assessment for randomized trials, and the ROBINS-I tool was used for the assessment of non-randomized trials (24, 25). The Cochrane methodology was followed for missing data (26). Any inconsistencies were resolved with the assistance of the biostatisticians.
Data synthesis strategy
Data extraction
All data were extracted and incorporated into Excel (Supplementary Data Sheet 2). This process was conducted independently by two reviewers. Any inconsistencies or queries were resolved by the biostatisticians.
Data analysis
All statistical analyses were conducted using R statistical software (version 4.2.1) (27) with the “meta” package (28). Randomized control trial (RCT) studies and non-RCT studies were grouped and analyzed separately. Odds ratios were pooled for comparative studies using the DerSimonian and Laird random-effects model for meta-analysis (29). In supplementary analyses, incidence proportions were pooled for one group of studies using a random-effects logistic regression model (30). Heterogeneity in the study estimates were calculated using the I2 statistic (31). When I2 was greater than 75%, the heterogeneity was considered high, and pooled estimates were not reported. Qualitative synthesis with either narrative description or tabular representation was presented when studies could not be quantitatively combined due to unacceptable heterogeneity or missing data precluding meta-analysis.
Funnel plots to assess publication bias were not performed as they did not meet the threshold of having at least 10 comparative studies of odds ratios of SIP pooled in a meta-analysis (32).
For both analyses, the risk of SIP with indomethacin exposure was stratified by feeding regimes, and for the risk of SIP with ibuprofen exposure stratified by route of administration, a meta-analysis was conducted pooling odds ratio estimates from comparative studies. Additionally, these questions were examined using data from studies that presented data for one group, and a test for subgroup analysis comparing the pooled incidence ratios estimate for each group was conducted.
A subgroup analysis in addition to Fisher's exact test was performed to compare the SIP proportion for patients taking ibuprofen (not enough studies for Indomethacin) in studies in the last decade compared to prior.
Results
Study selection
The database search identified 1,577 articles. From this, 153 articles were assessed for eligibility, resulting in the inclusion of 45 articles. Through reference searching and systematic review searching, an additional 2, 397 articles were identified giving an additional 20 articles for inclusion bringing the included article total to 65 (Figure 1). Supplementary Figure 1 contains the PRISMA 2020 flow charts for each search. Supplementary Table 2 contains the reasons for article exclusion after full-text screening. A study by Ghanem et al. was not included in the meta-analysis as it did not fit the criteria of RCT or cohort/case–control study (34).
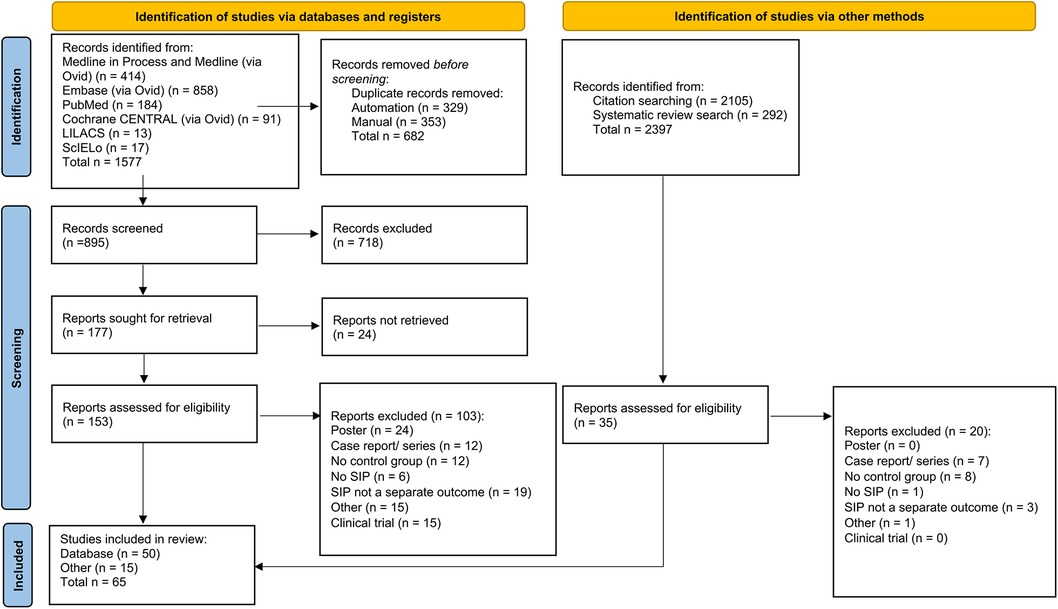
Figure 1. PRISMA 2020 flow diagram for new systematic reviews which included searches of databases, registers, and other sources of study selection process (33).
Study characteristics
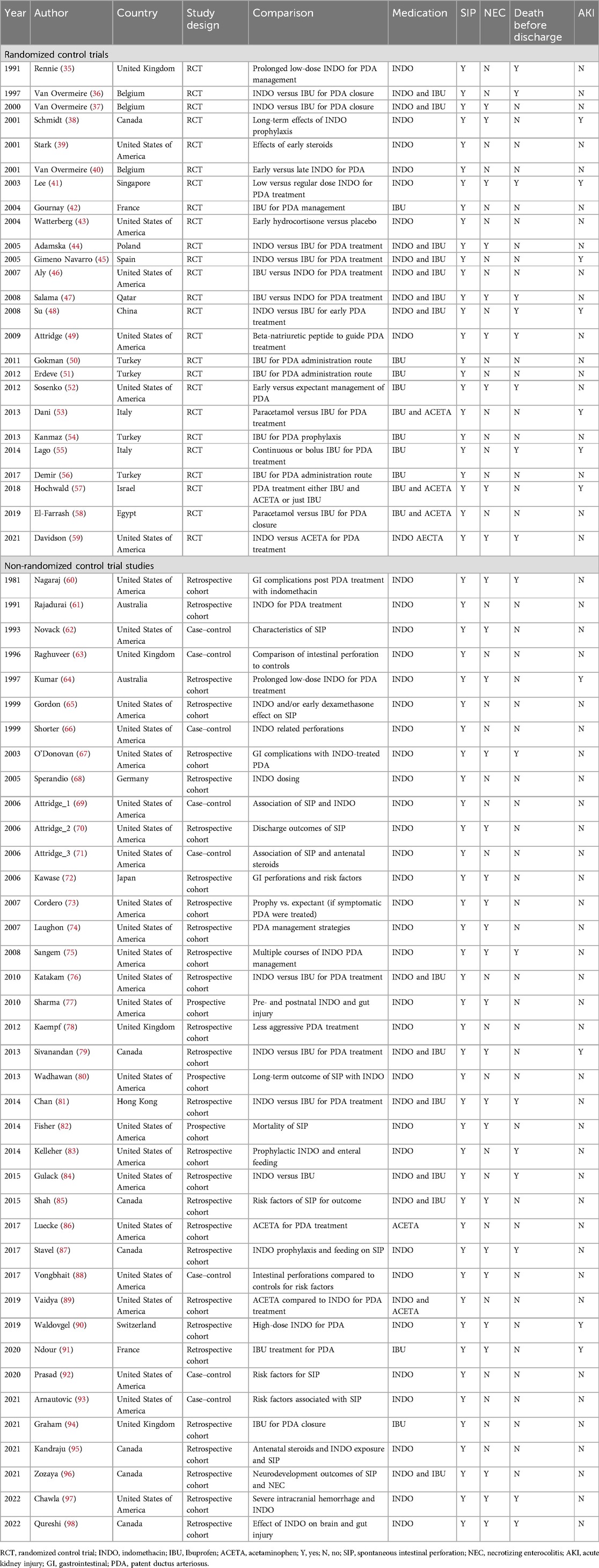
Table 1 displays the characteristics of the RCT and non-RCT studies that were included in the analyses. There were 25 RCT studies, 1 non-randomized trial, 31 cohort studies, and 8 case–control studies. Thirty-six, 10, and 1 study looked at indomethacin, ibuprofen, and acetaminophen, respectively, as the sole medication. There were 18 studies that used more than one medication of interest. As indomethacin and ibuprofen were the most studied medications, the incidence proportion of SIP over time per medication was graphed (Figure 2). It demonstrated a decreasing reported percentage over time until the last few years when it subjectively began to rise.
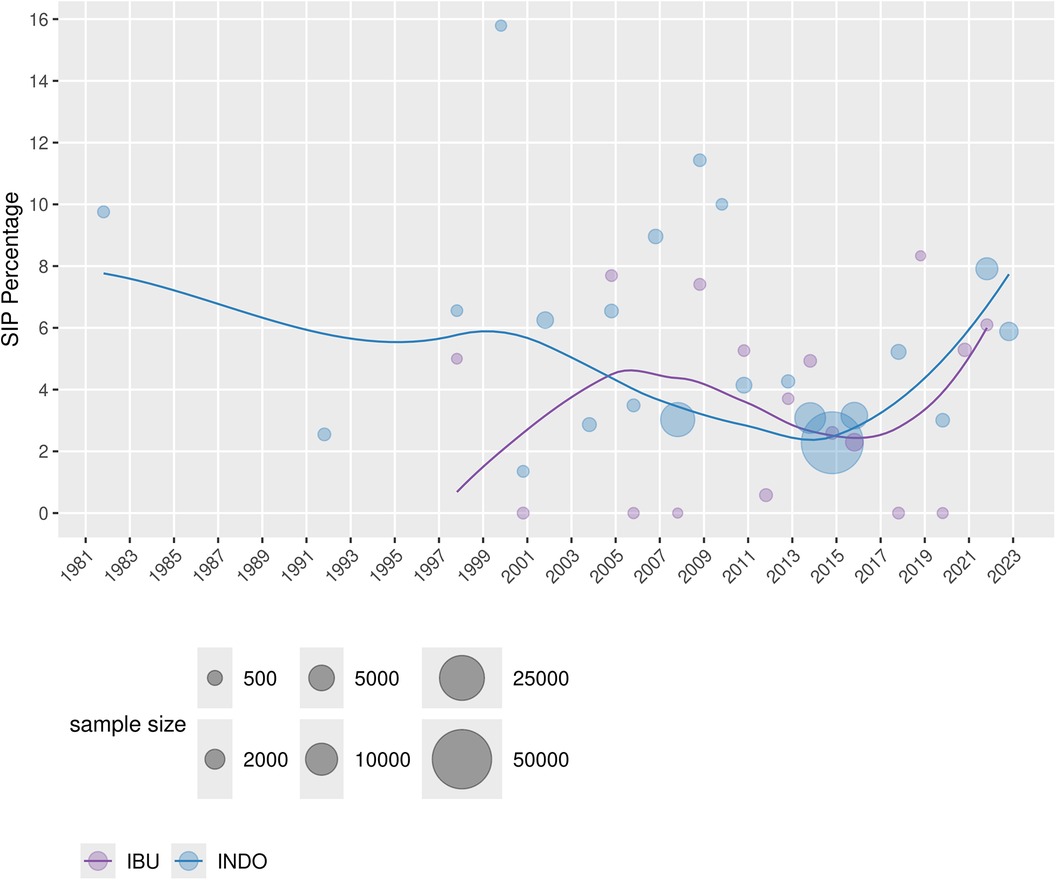
Figure 2. Percentage of SIP in the included studies across the years for both INDO and IBU, with a smoothing line (weighted by sample size).
Meta-analysis
Descriptive statistics and tables of evidence summary are in Supplementary Data Sheet 3.
Primary outcome—SIP
Table 2 depicts the results for the primary outcome.

Table 2. Risk of SIP in premature infants who received NSAID medication combinations for comparison for primary outcome analysis.
Indomethacin
Several indomethacin comparisons were explored including indomethacin vs. no medication and prophylactic vs. treatment use. The RCT pooled estimate showed no evidence for the risk of SIP in premature infants with indomethacin compared to the no medication group. Three RCTs looked at indomethacin prophylactic vs. treatment use. Of the three, two had larger sample sizes and suggested that indomethacin treatment use may have less risk of SIP compared to prophylactic use; however, the pooled estimate was borderline (Figure 3).
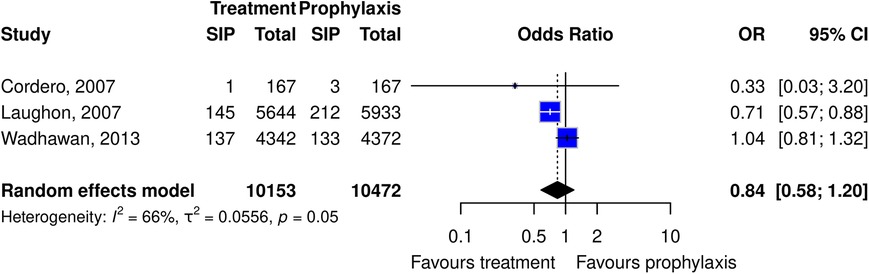
Figure 3. Forest plot of the risk or SIP in premature infants taking indomethacin as a treatment vs. prophylaxis. (All studies are cohort studies).
Ibuprofen
The association of SIP in premature infants exposed to ibuprofen compared to no medication was examined. Based on RCT data, there was no evidence to support increased odds of SIP in infants taking ibuprofen compared to the no medication group. Given the rise in ibuprofen use over the last decade, a time-based comparison of prevalence was made, comparing the last decade (2012 to present) to the previous (prior to 2012). From this, there was no difference in the prevalence of SIP in premature infants exposed to ibuprofen in the last decade.
Indomethacin vs. ibuprofen
Studies designed to compare indomethacin to ibuprofen included six RCT studies and five cohort studies. For both study designs, the pooled estimates did not reach significance; however, the results trended toward less risk of SIP in those taking ibuprofen vs. indomethacin.
Acetaminophen
There were significantly fewer articles exploring acetaminophen and SIP with no articles looking at SIP and acetaminophen exposure compared to no medication. Due to the limited number of articles, meta-analysis of the acetaminophen studies was not possible.
Secondary outcomes
The secondary outcomes explored included the risk of NEC, oliguria, and death before discharge with the same medication exposure combinations as the primary outcome. See Figures S10–S29 in Supplementary Data Sheet 3.
Additional clinical questions and characteristics
Risk of various outcomes with indomethacin exposure stratified by feeding regime
When layering on the complexity of feeding regimens with medication exposure, there were only studies exploring indomethacin. In analyzing the risk of SIP, NEC, and death before discharge in premature infants with indomethacin exposure—stratified by feeding vs. not feeding during treatment—there were two studies that explored this relationship. It was evident that premature infants on assisted feeding, and taking indomethacin, had a higher risk of SIP, NEC, and death before discharge compared to those who did not take indomethacin (Figure 4).
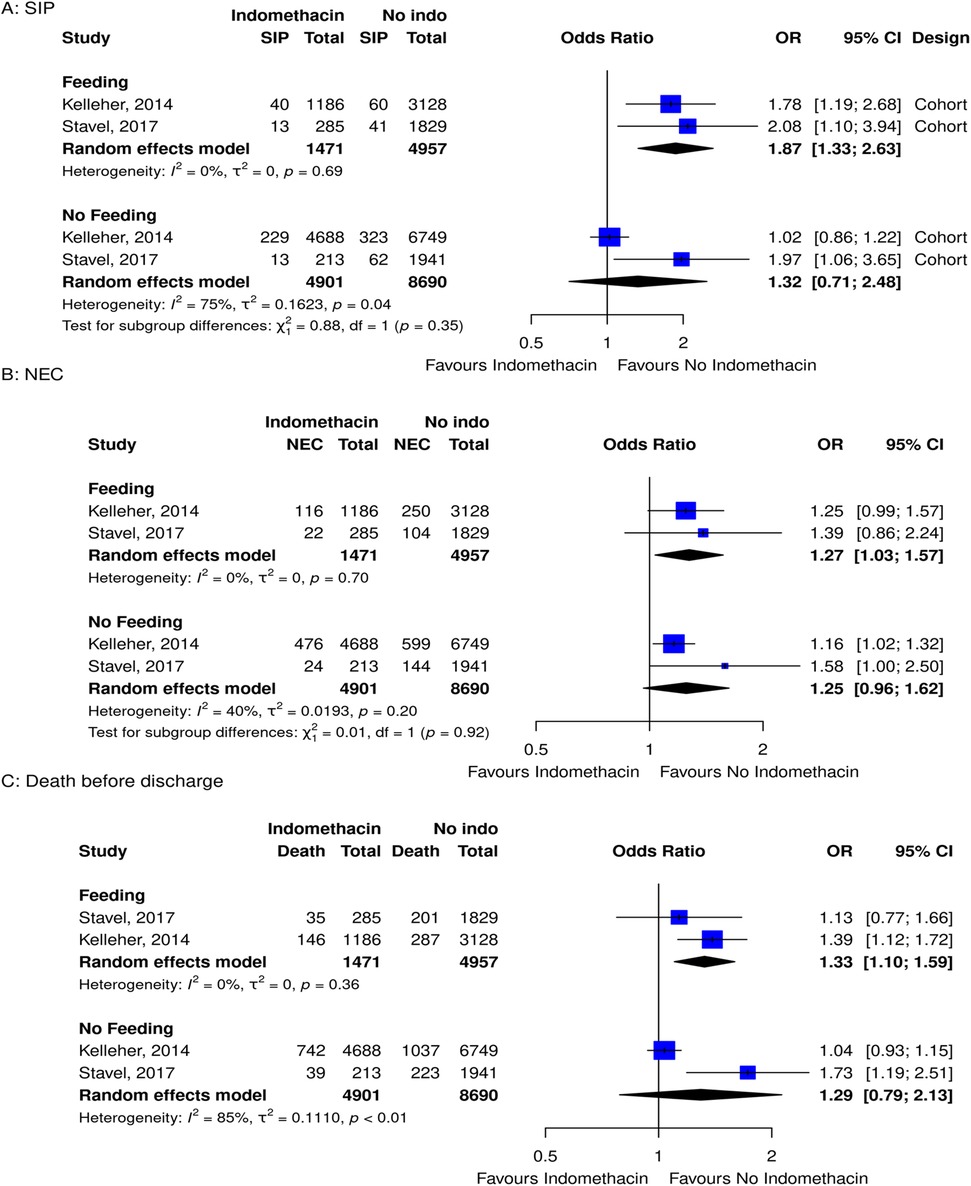
Figure 4. Forest plots for subgroup meta-analyses for the risk of (A) SIP, (B) NEC, and (C) death before discharge grouped by feeding and no feeding groups in infants that took indomethacin vs. no medication.
Ibuprofen oral vs. intravenous administration route in premature infants
There are three possible administration routes for ibuprofen: rectal, oral, or intravenous. Rectal administration was excluded as there was only one article, leaving oral and intravenous administration routes for comparison. In a comparative analysis, there were two studies that compared the ibuprofen administration route (IV vs. oral); however, only one study had one case of SIP, the second study had no SIP cases so we could not pool the estimate (Supplementary Data Sheet 3, see Figure S9C). When pooling estimates from non-comparative studies, there were 12 RCTs (8 had IV administration, and 4 had oral administration), we pooled SIP proportions for each route, and a test for subgroup difference showed no difference in the pooled proportion risk of SIP with ibuprofen administration orally vs. intravenous route. (Supplementary Data Sheet 3, see Figure S9B).
Additional characteristics
For the incidence of ROP, BPD, and IVH as well as analysis of antenatal medications (steroids, indomethacin, magnesium sulfate) and postnatal steroids, see Supplementary Data Sheet 3, pages 41–46.
Risk of bias assessment
The RCT risk of bias assessment revealed one out of the twenty-five studies that showed some concern while the remainder were assessed at low risk for bias (Figure 5). A concern within the RCT studies was not declaring if the analysis was done in an intention-to-treat manner. The study that had some concerns did not provide details about the randomization process (49). The intervention group had the total doses of medication determined by lab values. Measures for concealing groups were not discussed.
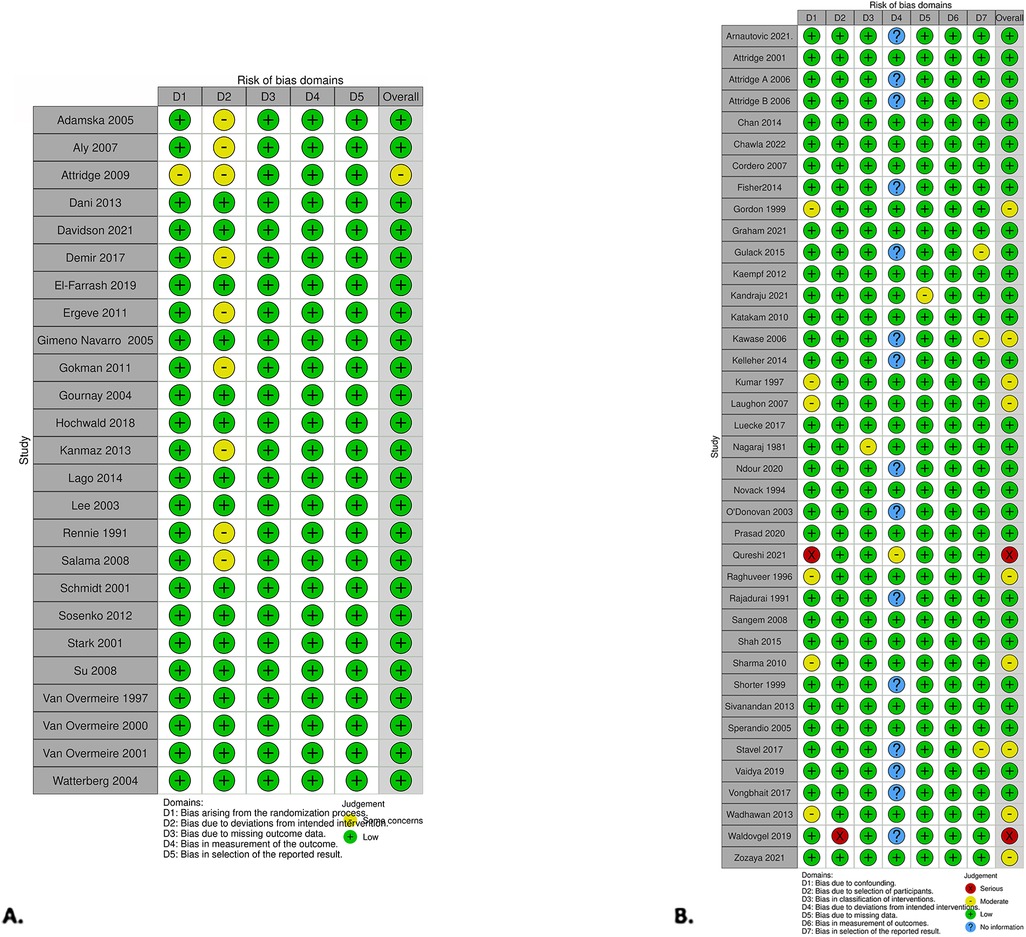
Figure 5. Risk of bias was presented using the risk-of-bias visualization tool. (A) RCT studies using RoB2. (B) Non-RCT studies using the ROBINS-I tool (99).
Within the non-RCT studies, two showed serious concern, nine had moderate concern, and the remaining were assessed as low risk for bias (Figure 5). Qureshi et al. had serious concerns (98). While exploring infants who had prophylactic indomethacin and outcomes, the authors noted which infants had a PDA but did not adjust for treatment of PDA with indomethacin or comment if it was considered. The number of infants with a PDA was not significantly different between the two groups. Waldvogel et al. also had serious concerns (90). The study's design was to compare standard to high-dose indomethacin for PDAs. All infants received a standard dose of indomethacin, and then when the PDA did not close, they went on to have a high dose, making the direct comparison between the groups unequal.
Discussion
One in twenty premature infants born at less than 2.5 kg, exposed to indomethacin, are at risk of developing SIP. As the incidence of SIP is on the rise, medication exposure is a modifiable variable, allowing for individualized care. To aid in these crucial decisions, one must look at the medications both in isolation and in the global context within neonatology.
The landscape of neonatology is ever-changing, with the evolution of indomethacin use as an example, initially used for the prevention of IVH and later for PDA management (98, 100, 101). It was the most prevalent medication captured in this review. Over time numerous clinical questions around the use of indomethacin and the risk of SIP have risen including the following: what is the general risk of SIP when exposed to indomethacin, does using it for prophylaxis vs. treatment modalities alter the risk of SIP, and is there an increased risk of SIP when taken indomethacin while feeding compared to feeds being held? We identified three RCT studies looking at SIP with exposure to indomethacin vs. no medication, and together they did not demonstrate an increased risk of SIP when taking indomethacin.
Three different RCTs explored treatment vs. prophylactic indomethacin use. It suggested a trend for a lower risk of SIP with indomethacin treatment compared to prophylaxis use. Moreover, all three RCTs had different clinical designs/questions, but each compared early/prophylactic use within the first 24 h (either for IVH or PDA prevention) and compared it to PDA treatment use after 24 h of life. Given that PDA is a risk factor for SIP, one could postulate that the treatment use should demonstrate a higher risk of SIP over prophylactic use but that was not the trend. There are several reasons why the inverse could be possible. The first is based on the total numbers exposed to indomethacin. By exposing indomethacin to only those infants who require it for treatment, the total number of SIPs in relation to indomethacin would be reduced. Another reason for prophylactic use to confer a higher risk of SIP could be in relation to timing. SIP occurs most commonly in the first 3 days of life which is the same window when indomethacin prophylaxis is administered, while treatment use typically starts later than the first days of life (1). It is possible that giving indomethacin in this early window might add insurmountable strain in an already high-risk period. To parse out those finer details, knowing the precise timing of medication administration to SIP would be required.
There were two retrospective cohort studies that looked at feeding and indomethacin. From their comparisons, it was evident that feeding premature infants and taking indomethacin had a higher risk of SIP, NEC, and death before discharge compared to those who did not take indomethacin. When infants were not feeding, there was no evidence of an increased risk of SIP, NEC, or death before discharge. These results are in keeping with the non-selective action of indomethacin and subsequent decrease in mesenteric blood flow (11). When blood flow to the gut is reduced, challenging it with feeds may add additional stress. The articles did not explore advancing feeds while taking indomethacin, but caution should be taken.
There has been a slow shift away from indomethacin RCTs with only one article (Davidson, 2021) using indomethacin since 2012 (59). Whether this is due to a lack of equipoise or due to decreased use is uncertain. A 2023 Cochrane review showed that while indomethacin reduces severe IVH, it does not affect the composite outcome of moderate/severe neurodevelopmental disability (101). Additionally, with prophylactic low-dose hydrocortisone protocols now coming into focus for BPD reduction, recommendations are to not use indomethacin and steroids concurrently (102). For PDA closure indomethacin and ibuprofen have similar efficacy, with ibuprofen having a more selective mechanism (47). Cumulatively, the evidence demonstrates that the targeted population for indomethacin is perhaps narrower than first envisioned.
As PDA management evolved, ibuprofen has proven to be a contender. With respect to ibuprofen exposure and the risk of SIP, the major clinical questions include the following: what is the general risk of SIP, is it riskier than indomethacin, and does the administration route alter the risk profile? Our meta-analysis revealed that there was no difference in risk of developing SIP when comparing ibuprofen to no medication. When comparing it head-to-head with indomethacin, both study designs (RCTs and cohort) did not reach significance; however, the results trended toward less risk of SIP in those taking ibuprofen vs. those taking indomethacin. The same trends held true for the secondary outcomes of NEC and oliguria. In general, this is in keeping with the notion that while both indomethacin and ibuprofen are NSAIDs, the side effects profile for ibuprofen is milder. For PDA management oral ibuprofen has been shown to be more efficacious than intravenous (51). Making the next logical question, does route of ibuprofen administration change the risk of SIP. Based on our analysis there was no difference in the pooled proportion of SIP in premature infants taking ibuprofen via oral or IV routes. Overall, the meta-analysis trends showed ibuprofen to confer less risk of SIP than indomethacin, and there was no difference in the proportion of SIP based on administration route.
In PDA treatment, there are less trials looking at acetaminophen which was reinforced in this review. There were three RCT studies that explored acetaminophen compared to other medications. Of the 107 patients exposed to acetaminophen, there were 3 cases of SIP (Supplementary Data Sheet 3). Given the low sample size, this is not enough to draw any conclusions. Acetaminophen is the medication of choice when there are contraindications to NSAIDs; thus, looking only at retrospective cohort data would introduce selection bias. Until it becomes more prominent the comparative evidence between acetaminophen and NSAIDs, characterizing SIP will be insufficient.
Another way to map the clinical practice landscape is to look at the trends over time. This can be done on a large scale exploring the incidence of SIP and medication exposure over time or alternatively by focusing on a shorter period to reduce potential background noise. Figure 2 depicts the incidence (as a percentage) of SIP over time, showing first the indomethacin era followed by ibuprofen. In keeping with the trends of the meta-analysis, ibuprofen has a lower percentage of SIP than indomethacin. Interestingly, both were decreasing until the last few years when it subjectively started and continued to rise. One factor that may have contributed to the rise in SIP is the lower age of viability. The more immature the infant, the more immature the gastrointestinal function and motility, thus increasing the risk of SIP. One way to explore this postulation would be to look at the incidence of SIP by gestational age. Additionally, diagnostic capabilities are improving, allowing more cases of SIP to be detected. The combination of these factors could be adding to the increase in SIP. This review did not limit medications based on their therapeutic indication and used a wide publication time frame. To limit this scope, we completed a subgroup analysis looking at just the last decade of ibuprofen RCTs. The expectation was decreased heterogeneity. However, the pooled proportion of SIP within the last decade compared to the prior was the same, with similar heterogeneity. This suggests that despite a changing landscape the risk of developing SIP with ibuprofen exposure has remained unchanged. This, combined with the increasing incidence of SIP, further supports the idea that earlier age of viability and better detection are contributing to the increase in the incidence of SIP.
This systematic review is the first step in comprehensively gathering what is known to date about common medications and SIP in premature neonates. In laying that foundation, it was important to keep the scope wide to analyze the general trends. From here, one can narrow the scope, via time, definition, which medication, the medication administration route, or geography to flesh out more granular details.
Limitations
The results of our analyses must be viewed with an understanding of the limitations. The diversity of the content resulted in high heterogeneity which had several contributing factors including numerous research questions, geographical care differences, and early SIP nomenclature concerns. Articles were not selected based on SIP being the primary outcome; rather, regardless of the research question, the data were probed to determine medication exposures and the occurrence of SIP. Earlier articles looked to find commonalities among cohorts, while others focused on PDA treatment with SIP as an adverse outcome.
One of the first hurdles in paper accruement was crafting a definition of SIP that predated the now-accepted version. In numerous formative papers, authors would include SIP under a general NEC umbrella. In the included list of RCT articles, 19 of the 25 were from after the 2002 official NICHD definition of SIP (4). It is possible that while the attempt was to provide an in-depth review of SIP solely, many cases have not been captured due to nomenclature issues as the neonatal field was actively expanding and evolving. This was most evident when discussing SIP but was also true for secondary outcomes. Having consistent reporting of definitions and standards would improve what data can be extracted for future meta-analysis comparisons.
Another paper accruement issue was that the many papers identified used NSAIDs for PDA management. Unless the abstract mentioned SIP it would not have been detected in the search strategy. Possible next steps could entail searching for all papers involving PDA management with NSAIDs and then excluding articles during full-text screen that did not report SIP.
An additional contributor to the high heterogeneity could be differences in global practice styles. To increase catchment, searches were worldwide with no language restrictions. A subgroup analysis based on geography was not conducted. Of note, 36 of the 65 articles included arose from North America. With the incidence of SIP increasing, there is a need for focused initiatives to decrease risk where possible, and exploring regionalized themes will be of future clinical relevance.
Understanding the timing of medication exposure to the presentation of SIP was a limiting factor. Studies recorded medication exposure and SIP in a binary fashion. However, to discern the true relationship, knowing the timing of medication exposure to the onset of SIP is crucial. The closest to this was the comparison of prophylaxis to the treatment use of indomethacin. Understating the temporal relationship could help identify a potential high-risk timing window.
Conclusions
This is the first systematic review exploring the impact of common NSAID medications on the risk of neonatal SIP over the last four decades. While cases of SIP with indomethacin or ibuprofen exposure were declining, it is now on the rise. We documented the challenges of nomenclature as SIP evolved into a unique entity. Indomethacin or ibuprofen alone vs. no medication does not increase the risk of SIP. However, treatment use trended toward less risk of SIP than prophylactic use. When exploring the relationship of indomethacin and feeds, infants not fed had less risk of SIP. Indomethacin should be restricted to a narrower subpopulation of premature infants with the implementation of stricter criteria. Broadly, ibuprofen was gentler than indomethacin, and the route of ibuprofen administration does not alter the risk of SIP.
Moving forward, the field needs nomenclature unification and timing of medication exposure to the onset of SIP to tease out the details of gastrointestinal pathology in premature neonates. As more data become available, limiting the search field time-wise, making geographic comparisons, and continued use of strict outcome definitions could lend itself to a stronger consensus for classification. This will lead to a transparent interpretation of the data and will delineate further details on the evolving relationship between commonly used medications and neonatal SIP.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JH: Data curation, Investigation, Writing – original draft, Writing – review & editing, Conceptualization, Methodology, Project administration. WS: Data curation, Investigation, Writing – review & editing. LH: Data curation, Formal Analysis, Methodology, Software, Writing – review & editing. MC: Conceptualization, Investigation, Writing – review & editing. NB: Data curation, Formal Analysis, Methodology, Software, Writing – review & editing. LS: Methodology, Writing – review & editing. EF: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Funding
The authors declare financial support was not received for the research, authorship, and/or publication of this article. This study was not funded and was supported through the Research Institute at the Children's Hospital of Eastern Ontario.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1450121/full#supplementary-material
References
1. Swanson JR, Hair A, Clark RH, Gordon PV. Spontaneous intestinal perforation (SIP) will soon become the most common form of surgical bowel disease in the extremely low birth weight (ELBW) infant. J Perinatol. (2022) 42(4):423–9. doi: 10.1038/s41372-022-01347-z
2. Fatemizadeh R, Mandal S, Gollins L, Shah S, Premkumar M, Hair A. Incidence of spontaneous intestinal perforations exceeds necrotizing enterocolitis in extremely low birth weight infants fed an exclusive human milk-based diet: a single center experience. J Pediatr Surg. (2021) 56(5):1051–6. doi: 10.1016/j.jpedsurg.2020.09.015
3. Pumberger W, Mayr M, Kohlhauser C, Weninger M. Spontaneous localized intestinal perforation in very-low-birth-weight infants: a distinct clinical entity different from necrotizing enterocolitis. J Am Coll Surg. (2002) 195(6):796–803. doi: 10.1016/S1072-7515(02)01344-3
4. Gordon PV, Attridge JT. Understanding clinical literature relevant to spontaneous intestinal perforations. Am J Perinatol. (2009) 26(4):309–16. doi: 10.1055/s-0028-1103514
5. Gordon PV. Understanding intestinal vulnerability to perforation in the extremely low birth weight infant. Pediatr Res. (2009) 65(2):138–44. doi: 10.1203/PDR.0b013e31818c7920
6. Hwang H, Murphy JJ, Gow KW, Magee JF, Bekhit E, Jamieson D. Are localized intestinal perforations distinct from necrotizing enterocolitis? J Pediatr Surg. (2003) 38(5):763–7. doi: 10.1016/jpsu.2003.50162
7. Rao R, Bryowsky K, Mao J, Bunton D, McPherson C, Mathur A. Gastrointestinal complications associated with ibuprofen therapy for patent ductus arteriosus. J Perinatol. (2011) 31(7):465–70. doi: 10.1038/jp.2010.199
8. El-Mashad AE, El-Mahdy H, El Amrousy D, Elgendy M. Comparative study of the efficacy and safety of paracetamol, ibuprofen, and indomethacin in closure of patent ductus arteriosus in preterm neonates. Eur J Pediatr. (2017) 176(2):233–40. doi: 10.1007/s00431-016-2830-7
9. Perron N, Tremblay E, Ferretti E, Babakissa C, Seidman EG, Levy E, et al. Deleterious effects of indomethacin in the mid-gestation human intestine. Genomics. (2013) 101(3):171–7. doi: 10.1016/j.ygeno.2012.12.003
10. Ferretti E, Tremblay E, Thibault MP, Grynspan D, Burghardt KM, Bettolli M, et al. The nitric oxide synthase 2 pathway is targeted by both pro- and anti-inflammatory treatments in the immature human intestine. Nitric Oxide. (2017) 66:53–61. doi: 10.1016/j.niox.2017.03.003
11. Rao SC, Basani L, Simmer K, Samnakay N, Deshpande G. Peritoneal drainage versus laparotomy as initial surgical treatment for perforated necrotizing enterocolitis or spontaneous intestinal perforation in preterm low birth weight infants. Cochrane Database Syst Rev. (2011) 6:CD006182. doi: 10.1002/14651858.CD006182.pub2
12. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10(10):ED000142. doi: 10.1002/14651858.ED000142
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
14. Hudson J-A, Shabbir W, Chan MLM, Hayawi L, Barrowman N, Sikora L, et al. What is the association between common medications (indomethacin, ibuprofen and acetaminophen) and spontaneous intestinal perforations in premature infants? A systematic review protocol. F1000Res. (2022) 11:1258. doi: 10.12688/f1000research.125132.1
15. Fischer A, Vachon L, Durand M, Cayabyab RG. Ultrasound to diagnose spontaneous intestinal perforation in infants weighing 1000 g at birth. J Perinatol. (2015) 35(2):104–9. doi: 10.1038/jp.2014.169
16. Ibrahim AH HA, Alsherbiny H, Hussein MRA, Khan SA. Spontaneous intestinal perforation followed by necrotizing enterocolitis in an extremely low birth weight neonate: case report and review of the literature. Annals of Pediatr Surg. (2020) 16:1–4. doi: 10.1186/s43159-020-00027-x
17. Patel RM, Ferguson J, McElroy SJ, Khashu M, Caplan MS. Defining necrotizing enterocolitis: current difficulties and future opportunities. Pediatr Res. (2020) 88(Suppl 1):10–5. doi: 10.1038/s41390-020-1074-4
18. Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, et al. International classification of retinopathy of prematurity, third edition. Ophthalmology. (2021) 128(10):e51–68. doi: 10.1016/j.ophtha.2021.05.031
19. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. (1978) 92(4):529–34. doi: 10.1016/S0022-3476(78)80282-0
20. Thebaud B, Goss KN, Laughon M, Whitsett JA, Abman SH, Steinhorn RH, et al. Bronchopulmonary dysplasia. Nat Rev Dis Primers. (2019) 5(1):78. doi: 10.1038/s41572-019-0127-7
22. Covidence Systematic Review Software, Veritas Health Innovation Melbourne, Australia. Available online at: www.covidence.org.
23. Wohlin C. Guidelines for snowballing in systematic literature studies and a replication in software engineering. Proceedings of the 18th International Conference on Evaluation and Assessment in Software Engineering (2014). p. 1–10
24. Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. Br Med J. (2016) 355:i4919. doi: 10.1136/bmj.i4919
25. Sterne JAC, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
26. Higgins J. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] (2011). Available online at: www.handbook.cochrane.org.
27. Team RC. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Vienna, Austria: Scientific Research Publishing (2021).
28. Balduzzi S, Rucker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health. (2019) 22(4):153–60. doi: 10.1136/ebmental-2019-300117
29. DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. (2007) 28(2):105–14. doi: 10.1016/j.cct.2006.04.004
30. Stijnen T, Hamza TH, Ozdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med. (2010) 29(29):3046–67. doi: 10.1002/sim.4040
31. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. (2002) 21(11):1539–58. doi: 10.1002/sim.1186
32. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. Br Med J. (2011) 343:d4002. doi: 10.1136/bmj.d4002
33. Ahle S, Badru F, Damle R, Osei H, Munoz-Abraham AS, Bajinting A, et al. Multicenter retrospective comparison of spontaneous intestinal perforation outcomes between primary peritoneal drain and primary laparotomy. J Pediatr Surg. (2020) 55(7):1270–5. doi: 10.1016/j.jpedsurg.2019.07.007
34. Ghanem S, Mostafa M, Shafee M. Effect of oral ibuprofen on patent ductus arteriosus in premature newborns. J Saudi Heart Assoc. (2010) 22(1):7–12. doi: 10.1016/j.jsha.2010.03.002
35. Rennie JM, Cooke RW. Prolonged low dose indomethacin for persistent ductus arteriosus of prematurity. Arch Dis Child. (1991) 66(1 Spec No):55–8. doi: 10.1136/adc.66.1_Spec_No.55
36. Van Overmeire B, Follens I, Hartmann S, Creten WL, Van Acker KJ. Treatment of patent ductus arteriosus with ibuprofen. Arch Dis Child Fetal Neonatal Ed. (1997) 76(3):F179–84. doi: 10.1136/fn.76.3.F179
37. Van Overmeire B, Smets K, Lecoutere D, Van de Broek H, Weyler J, Degroote K, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. (2000) 343(10):674–81. doi: 10.1056/NEJM200009073431001
38. Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, et al. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. (2001) 344(26):1966–72. doi: 10.1056/NEJM200106283442602
39. Stark AR, Carlo WA, Tyson JE, Papile LA, Wright LL, Shankaran S, et al. Adverse effects of early dexamethasone treatment in extremely-low-birth-weight infants. National Institute of Child Health and Human Development Neonatal Research Network. N Engl J Med. (2001) 344(2):95–101. doi: 10.1056/NEJM200101113440203
40. Van Overmeire B, Van de Broek H, Van Laer P, Weyler J, Vanhaesebrouck P. Early versus late indomethacin treatment for patent ductus arteriosus in premature infants with respiratory distress syndrome. J Pediatr. (2001) 138(2):205–11. doi: 10.1067/mpd.2001.110528
41. Lee J, Rajadurai VS, Tan KW, Wong KY, Wong EH, Leong JY. Randomized trial of prolonged low-dose versus conventional-dose indomethacin for treating patent ductus arteriosus in very low birth weight infants. Pediatrics. (2003) 112(2):345–50. doi: 10.1542/peds.112.2.345
42. Gournay V, Roze JC, Kuster A, Daoud P, Cambonie G, Hascoet JM, et al. Prophylactic ibuprofen versus placebo in very premature infants: a randomised, double-blind, placebo-controlled trial. Lancet. (2004) 364(9449):1939–44. doi: 10.1016/S0140-6736(04)17476-X
43. Watterberg KL, Gerdes JS, Cole CH, Aucott SW, Thilo EH, Mammel MC, et al. Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics. (2004) 114(6):1649–57. doi: 10.1542/peds.2004-1159
44. Adamska E, Helwich E, Rutkowska M, Zacharska E, Piotrowska A. Comparison of the efficacy of ibuprofen and indomethacin in the treatment of patent ductus arteriosus in prematurely born infants. Med Wieku Rozwoj. (2005) 9(3 Pt 1):335–54.16547381
45. Gimeno Navarro A, Cano Sanchez A, Fernandez Gilino C, Carrasco Moreno JI, Izquierdo Macian I, Gutierrez Laso A, et al. Ibuprofen versus indomethacin in the treatment of patent ductus arteriosus in preterm infants. An Pediatr. (2005) 63(3):212–8. doi: 10.1157/13078483
46. Aly H, Lotfy W, Badrawi N, Ghawas M, Abdel-Meguid IE, Hammad TA. Oral Ibuprofen and ductus arteriosus in premature infants: a randomized pilot study. Am J Perinatol. (2007) 24(5):267–70. doi: 10.1055/s-2007-976550
47. Husam Salama AA, Al-Rifai H, Shaddad A, Samawal L, Habboub L, Masoud A. A randomized controlled trial on the use of oral ibuprofen to close patent ductus arteriosus in premature infants. J Neonatal Perinatal Med. (2008) 1(3):153–8.
48. Su BH, Lin HC, Chiu HY, Hsieh HY, Chen HH, Tsai YC. Comparison of ibuprofen and indometacin for early-targeted treatment of patent ductus arteriosus in extremely premature infants: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. (2008) 93(2):F94–9. doi: 10.1136/adc.2007.120584
49. Attridge JT, Kaufman DA, Lim DS. B-type natriuretic peptide concentrations to guide treatment of patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed. (2008) 94(3):F178–82. doi: 10.1136/adc.2008.147587
50. Gokmen T, Erdeve O, Altug N, Oguz SS, Uras N, Dilmen U. Efficacy and safety of oral versus intravenous ibuprofen in very low birth weight preterm infants with patent ductus arteriosus. J Pediatr. (2011) 158(4):549–554.e1. doi: 10.1016/j.jpeds.2010.10.008
51. Erdeve O, Yurttutan S, Altug N, Ozdemir R, Gokmen T, Dilmen U, et al. Oral versus intravenous ibuprofen for patent ductus arteriosus closure: a randomised controlled trial in extremely low birthweight infants. Arch Dis Child Fetal Neonatal Ed. (2012) 97(4):F279–83. doi: 10.1136/archdischild-2011-300532
52. Sosenko IR, Fajardo MF, Claure N, Bancalari E. Timing of patent ductus arteriosus treatment and respiratory outcome in premature infants: a double-blind randomized controlled trial. J Pediatr. (2012) 160(6):929–935.e1. doi: 10.1016/j.jpeds.2011.12.031
53. Dani C, Lista G, Bianchi S, Mosca F, Schena F, Ramenghi L, et al. Intravenous paracetamol in comparison with ibuprofen for the treatment of patent ductus arteriosus in preterm infants: a randomized controlled trial. Eur J Pediatr. (2021) 180(3):807–16. doi: 10.1007/s00431-020-03780-8
54. Kanmaz G, Erdeve O, Canpolat FE, Oguz SS, Uras N, Altug N, et al. Serum ibuprofen levels of extremely preterm infants treated prophylactically with oral ibuprofen to prevent patent ductus arteriosus. Eur J Clin Pharmacol. (2013) 69(5):1075–81. doi: 10.1007/s00228-012-1438-8
55. Lago P, Salvadori S, Opocher F, Ricato S, Chiandetti L, Frigo AC. Continuous infusion of ibuprofen for treatment of patent ductus arteriosus in very low birth weight infants. Neonatology. (2013) 105(1):46–54. doi: 10.1159/000355679
56. Demir N, Peker E, Ece I, Balahoroglu R, Tuncer O. Efficacy and safety of rectal ibuprofen for patent ductus arteriosus closure in very low birth weight preterm infants. J Matern Fetal Neonatal Med. (2017) 30(17):2119–25. doi: 10.1080/14767058.2016.1238897
57. Hochwald O, Mainzer G, Borenstein-Levin L, Jubran H, Dinur G, Zucker M, et al. Adding paracetamol to ibuprofen for the treatment of patent ductus arteriosus in preterm infants: a double-blind, randomized, placebo-controlled pilot study. Am J Perinatol. (2018) 35(13):1319–25. doi: 10.1055/s-0038-1653946
58. El-Farrash RA, El Shimy MS, El-Sakka AS, Ahmed MG, Abdel-Moez DG. Efficacy and safety of oral paracetamol versus oral ibuprofen for closure of patent ductus arteriosus in preterm infants: a randomized controlled trial. J Matern Fetal Neonatal Med. (2019) 32(21):3647–54. doi: 10.1080/14767058.2018.1470235
59. Davidson JM, Ferguson J, Ivey E, Philip R, Weems MF, Talati AJ. A randomized trial of intravenous acetaminophen versus indomethacin for treatment of hemodynamically significant PDAs in VLBW infants. J Perinatol. (2021) 41(1):93–9. doi: 10.1038/s41372-020-0694-1
60. Nagaraj HS, Sandhu AS, Cook LN, Buchino JJ, Groff DB. Gastrointestinal perforation following indomethacin therapy in very low birth weight infants. J Pediatr Surg. (1981) 16(6):1003–7. doi: 10.1016/S0022-3468(81)80865-2
61. Rajadurai VS, Yu VY. Intravenous indomethacin therapy in preterm neonates with patent ductus arteriosus. J Paediatr Child Health. (1991) 27(6):370–5. doi: 10.1111/j.1440-1754.1991.tb00422.x
62. Novack CM, Waffarn F, Sills JH, Pousti TJ, Warden MJ, Cunningham MD. Focal intestinal perforation in the extremely-low-birth-weight infant. J Perinatol. (1994) 14(6):450–3.7876936
63. Raghuveer G, Speidel B, Marlow N, Porter H. Focal intestinal perforation in preterm infants is an emerging disease. Acta Paediatr. (1996) 85(2):237–9. doi: 10.1111/j.1651-2227.1996.tb14000.x
64. Kumar RK, Yu VY. Prolonged low-dose indomethacin therapy for patent ductus arteriosus in very low birthweight infants. J Paediatr Child Health. (1997) 33(1):38–41. doi: 10.1111/j.1440-1754.1997.tb00988.x
65. Gordon P, Rutledge J, Sawin R, Thomas S, Woodrum D. Early postnatal dexamethasone increases the risk of focal small bowel perforation in extremely low birth weight infants. J Perinatol. (1999) 19(8 Pt 1):573–7. doi: 10.1038/sj.jp.7200269
66. Shorter NA, Liu JY, Mooney DP, Harmon BJ. Indomethacin-associated bowel perforations: a study of possible risk factors. J Pediatr Surg. (1999) 34(3):442–4. doi: 10.1016/S0022-3468(99)90495-5
67. O'Donovan DJ, Baetiong A, Adams K, Chen A, Smith EO, Adams JM, et al. Necrotizing enterocolitis and gastrointestinal complications after indomethacin therapy and surgical ligation in premature infants with patent ductus arteriosus. J Perinatol. (2003) 23(4):286–90. doi: 10.1038/sj.jp.7210911
68. Sperandio M, Beedgen B, Feneberg R, Huppertz C, Brussau J, Poschl J, et al. Effectiveness and side effects of an escalating, stepwise approach to indomethacin treatment for symptomatic patent ductus arteriosus in premature infants below 33 weeks of gestation. Pediatrics. (2005) 116(6):1361–6. doi: 10.1542/peds.2005-0293
69. Attridge JT, Clark R, Walker MW, Gordon PV. New insights into spontaneous intestinal perforation using a national data set: (1) SIP is associated with early indomethacin exposure. J Perinatol. (2006) 26(2):93–9. doi: 10.1038/sj.jp.7211429
70. Attridge JT, Herman AC, Gurka MJ, Griffin MP, McGahren ED, Gordon PV. Discharge outcomes of extremely low birth weight infants with spontaneous intestinal perforations. J Perinatol. (2006) 26(1):49–54. doi: 10.1038/sj.jp.7211407
71. Attridge JT, Clark R, Gordon PV. New insights into spontaneous intestinal perforation using a national data set (3): antenatal steroids have no adverse association with spontaneous intestinal perforation. J Perinatol. (2006) 26(11):667–70. doi: 10.1038/sj.jp.7211589
72. Kawase Y, Ishii T, Arai H, Uga N. Gastrointestinal perforation in very low-birthweight infants. Pediatr Int. (2006) 48(6):599–603. doi: 10.1111/j.1442-200X.2006.02282.x
73. Cordero L, Nankervis CA, Delooze D, Giannone PJ. Indomethacin prophylaxis or expectant treatment of patent ductus arteriosus in extremely low birth weight infants? J Perinatol. (2007) 27(3):158–63. doi: 10.1038/sj.jp.7211659
74. Laughon M, Bose C, Clark R. Treatment strategies to prevent or close a patent ductus arteriosus in preterm infants and outcomes. J Perinatol. (2007) 27(3):164–70. doi: 10.1038/sj.jp.7211662
75. Sangem M, Asthana S, Amin S. Multiple courses of indomethacin and neonatal outcomes in premature infants. Pediatr Cardiol. (2008) 29(5):878–84. doi: 10.1007/s00246-007-9166-z
76. Katakam LI, Cotten CM, Goldberg RN, Dang CN, Smith PB. Safety and effectiveness of indomethacin versus ibuprofen for treatment of patent ductus arteriosus. Am J Perinatol. (2010) 27(5):425–9. doi: 10.1055/s-0029-1243371
77. Sharma R, Hudak ML, Tepas JJ 3rd, Wludyka PS, Teng RJ, Hastings LK, et al. Prenatal or postnatal indomethacin exposure and neonatal gut injury associated with isolated intestinal perforation and necrotizing enterocolitis. J Perinatol. (2010) 30(12):786–93. doi: 10.1038/jp.2010.59
78. Kaempf JW, Wu YX, Kaempf AJ, Kaempf AM, Wang L, Grunkemeier G. What happens when the patent ductus arteriosus is treated less aggressively in very low birth weight infants? J Perinatol. (2012) 32(5):344–8. doi: 10.1038/jp.2011.102
79. Sivanandan S, Bali V, Soraisham AS, Harabor A, Kamaluddeen M. Effectiveness and safety of indomethacin versus ibuprofen for the treatment of patent ductus arteriosus in preterm infants. Am J Perinatol. (2013) 30(9):745–50. doi: 10.1055/s-0032-1332800
80. Wadhawan R, Oh W, Vohr BR, Saha S, Das A, Bell EF, et al. Spontaneous intestinal perforation in extremely low birth weight infants: association with indometacin therapy and effects on neurodevelopmental outcomes at 18-22 months corrected age. Arch Dis Child Fetal Neonatal Ed. (2013) 98(2):F127–32. doi: 10.1136/archdischild-2011-300659
81. Chan NM, Law CW, Kwan KF. Ibuprofen versus indomethacin treatment of patent ductus arteriosus: comparative effectiveness and complications. Hong Kong Med J. (2014) 20(3):205–12. doi: 10.12809/hkmj134080
82. Fisher JG, Jones BA, Gutierrez IM, Hull MA, Kang KH, Kenny M, et al. Mortality associated with laparotomy-confirmed neonatal spontaneous intestinal perforation: a prospective 5-year multicenter analysis. J Pediatr Surg. (2014) 49(8):1215–9. doi: 10.1016/j.jpedsurg.2013.11.051
83. Kelleher J, Salas AA, Bhat R, Ambalavanan N, Saha S, Stoll BJ, et al. Prophylactic indomethacin and intestinal perforation in extremely low birth weight infants. Pediatrics. (2014) 134(5):e1369–77. doi: 10.1542/peds.2014-0183
84. Gulack BC, Laughon MM, Clark RH, Sankar MN, Hornik CP, Smith PB. Comparative effectiveness and safety of indomethacin versus ibuprofen for the treatment of patent ductus arteriosus. Early Hum Dev. (2015) 91(12):725–9. doi: 10.1016/j.earlhumdev.2015.08.003
85. Shah J, Singhal N, da Silva O, Rouvinez-Bouali N, Seshia M, Lee SK, et al. Intestinal perforation in very preterm neonates: risk factors and outcomes. J Perinatol. (2015) 35(8):595–600. doi: 10.1038/jp.2015.41
86. Luecke CM, Liviskie CJ, Zeller BN, Vesoulis ZA, McPherson C. Acetaminophen for patent ductus arteriosus in extremely low-birth-weight neonates. J Pediatr Pharmacol Ther. (2017) 22(6):461–6. doi: 10.5863/1551-6776-22.6.461
87. Stavel M, Wong J, Cieslak Z, Sherlock R, Claveau M, Shah PS. Effect of prophylactic indomethacin administration and early feeding on spontaneous intestinal perforation in extremely low-birth-weight infants. J Perinatol. (2017) 37(2):188–93. doi: 10.1038/jp.2016.196
88. Vongbhavit K, Underwood MA. Intestinal perforation in the premature infant. J Neonatal Perinatal Med. (2017) 10(3):281–9. doi: 10.3233/NPM-16148
89. Vaidya R, Wilson D, Paris Y, Madore L, Singh R. Use of acetaminophen for patent ductus arteriosus treatment: a single center experience. J Matern Fetal Neonatal Med. (2020) 33(16):2723–9. doi: 10.1080/14767058.2018.1559810
90. Waldvogel S, Atkinson A, Wilbeaux M, Nelle M, Berger MR, Gerull R. High dose indomethacin for patent ductus arteriosus closure increases neonatal morbidity. Am J Perinatol. (2021) 38(7):707–13. doi: 10.1055/s-0039-3400996
91. Ndour D, Bouamari H, Berthiller J, Claris O, Plaisant F, Nguyen KA. Adverse events related to ibuprofen treatment for patent ductus arteriosus in premature neonates. Arch Pediatr. (2020) 27(8):452–5. doi: 10.1016/j.arcped.2020.08.007
92. Prasad U, Mohnani A, Hussain N. Spontaneous intestinal perforation associated with premature twin infants. J Neonatal Perinatal Med. (2021) 14(3):403–9. doi: 10.3233/NPM-200541
93. Arnautovic TI, Longo JL, Trail-Burns EJ, Tucker R, Keszler M, Laptook AR. Antenatal risk factors associated with spontaneous intestinal perforation in preterm infants receiving postnatal indomethacin. J Pediatr. (2021) 232:59–64.e1. doi: 10.1016/j.jpeds.2021.01.011
94. Catherine Graham YS. Efficacy and adverse effects of ibuprofen in the treatment of haemodynamically significant patent ductus arteriosus (PDA) in preterm infants. Arch Dis Child. (2021) 106:A53–4.
95. Kandraju H, Kanungo J, Lee KS, Daspal S, Adie MA, Dorling J, et al. Association of co-exposure of antenatal steroid and prophylactic indomethacin with spontaneous intestinal perforation. J Pediatr. (2021) 235:34–41.e1. doi: 10.1016/j.jpeds.2021.03.012
96. Zozaya C, Shah J, Pierro A, Zani A, Synnes A, Lee S, et al. Neurodevelopmental and growth outcomes of extremely preterm infants with necrotizing enterocolitis or spontaneous intestinal perforation. J Pediatr Surg. (2021) 56(2):309–16. doi: 10.1016/j.jpedsurg.2020.05.013
97. Chawla S, Natarajan G, Laptook AR, Chowdhury D, Bell EF, Ambalavanan N, et al. Model for severe intracranial hemorrhage and role of early indomethacin in extreme preterm infants. Pediatr Res. (2022) 92(6):1648–56. doi: 10.1038/s41390-022-02012-z
98. Qureshi M, Shah PS, Abdelgadir D, Ye XY, Afifi J, Yuen R, et al. Gestational age-dependent variations in effects of prophylactic indomethacin on brain injury and intestinal injury. J Pediatr. (2021) 235:26–33.e2. doi: 10.1016/j.jpeds.2021.02.073
99. McGuinness LA, Higgins WT. Risk-of-bias visualization (robvis): an R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. (2021) 12(1):55–61. doi: 10.1002/jrsm.1411
100. Ment LR, Duncan CC, Ehrenkranz RA, Kleinman CS, Pitt BR, Taylor KJ, et al. Randomized indomethacin trial for prevention of intraventricular hemorrhage in very low birth weight infants. J Pediatr. (1985) 107(6):937–43. doi: 10.1016/S0022-3476(85)80197-9
101. Mitra S, de Boode WP, Weisz DE, Shah PS. Interventions for patent ductus arteriosus (PDA) in preterm infants: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. (2023) 4(4):CD013588.37039501
Keywords: spontaneous intestinal perforation (SIP), premature (babies), non-steroidal anti-inflammatory drug (NSAID), systematic review, acetaminophen
Citation: Hudson Jo-Anna BJ, Shabbir W, Hayawi LM, Chan MLM, Barrowman N, Sikora L and Ferretti E (2024) Use of NSAIDs and acetaminophen and risk of spontaneous intestinal perforations in premature infants: a systematic review and meta-analysis. Front. Pediatr. 12:1450121. doi: 10.3389/fped.2024.1450121
Received: 17 June 2024; Accepted: 9 October 2024;
Published: 22 November 2024.
Edited by:
Thangaraj Abiramalatha, Kovai Medical Center and Hospitals (KMCH), IndiaReviewed by:
Sam Ebenezer Athikarisamy, University of Western Australia, AustraliaJoanna Seliga-Siwecka, Medical University of Warsaw, Poland
Copyright: © 2024 Hudson, Shabbir, Hayawi, Chan, Barrowman, Sikora and Ferretti. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Emanuela Ferretti, ZWZlcnJldHRpQHRvaC5jYQ==
 Jo-Anna B. J. Hudson
Jo-Anna B. J. Hudson Wardha Shabbir4
Wardha Shabbir4 Emanuela Ferretti
Emanuela Ferretti