- 1Department of Science, Kazakhstan Medical University “KSPH”, Almaty, Kazakhstan
- 2Department of Assisted Reproductive Technologies, International Clinical Centre of Reproduction “PERSONA”, Almaty, Kazakhstan
- 3Science and Education Department, International Academy of Reproductology, Almaty, Kazakhstan
- 4Science and Education Department, Scientific Center of Pediatrics and Pediatric Surgery, Almaty, Kazakhstan
- 5Department of Outpatient Pediatrics, School of Pediatrics, Asfendiyarov Kazakh National Medical University, Almaty, Kazakhstan
- 6Department of Gynecology, Asfendiyarov Kazakh National Medical University, Almaty, Kazakhstan
- 7Department of Assisted Reproductive Technologies, Institute of Reproductive Medicine, Almaty, Kazakhstan
Objective: The increasing use of assisted reproductive technologies (ART) has led to a growing interest in the health outcomes of offspring. However, the impact of ART on the immune system of children remains poorly understood. While only two publications were found, their findings contradict each other and did not consider other risk factors in their analysis except for ART use. Therefore, this study aimed to examine the potential impact of ART on the immune system of offspring.
Methods: A case-control study was conducted in Kazakhstan to investigate the immune system of ART-conceived children compared to those conceived naturally (NC). The study included participants who met certain criteria, such as having undergone a successful ART program resulting in the birth of either a single or multiple pregnancies. Patients who used donor oocytes/sperm, intrauterine insemination, or surrogacy were excluded. Anamnesis data were collected from children in both groups, and laboratory measurements were performed and analyzed using IBM SPSS Statistic 26.
Results: A total of 120 children conceived by ART and 132 NC children under the age of five were included in our study. We observed that compared with NC group, ART children had lower IgA and IgG levels (p < 0.001), absolute lymphocytosis, high levels of active T-lymphocytes (p = 0.001), and pathological T-helper levels (p = 0.004). Therefore, the clinical presentation of respiratory diseases was lower in ART group. Children born after frozen embryo transfers showed significantly higher levels of T-cytotoxic and active T-lymphocytes compared to children born after fresh embryo transfers (p = 0.007 and p = 0.020, respectively). We utilized ordinal logistic regression to control for confounding variables such as multiple pregnancy, cesarean section, premature birth, and breastfeeding. Despite this, the significant impact of ART on immunogram parameters persisted, indicating the independent and influential nature of ART or other unaccounted factors.
1 Introduction
Assisted reproductive technology (ART) has evolved from an ambitious and experimental procedure into mainstream medicine and has become promising for couples suffering from infertility in the last 40 years (1). Globally, more than 12 million children are born (2), and more than 35,000 are born in Kazakhstan after infertility treatment (3). While birth outcomes and most health aspects are well documented, understanding the influence of ART on the immune system of children is still an area of ongoing research.
Current data indicate that ART-conceived children have a greater incidence of dermatological (4), respiratory (5, 6), gastrointestinal (7), and infectious (8) conditions. Some authors associate this technique with frozen embryo transfer (FET) (9). Children conceived after FET had an increased risk of developing alimentary allergy in early childhood, but no significant association was found with asthma, atopic dermatitis, and allergic rhinitis compared to naturally conceived (NC) children (10). Several systematic reviews have shown a slight increase in the risk of asthma among ART-conceived children compared to NC, though the findings regarding allergies remain limited and inconclusive (11, 12). The GUHS study reported no difference in asthma prevalence but noted greater lung volumes and less bronchial hyperreactivity in ART group (13). Additionally, cases of allergic rhinoconjunctivitis, alimentary allergy, and positive skin prick tests were more common in the ART group, while the rates of atopic dermatitis were similar to those of NC children (14). A cohort study by Wei et al. 2022 (15) demonstrated that the risk of hospitalization in ART-conceived children was 1.23 times greater than that in children in the non-ART group and was associated with infections and allergies compared to that in NC siblings.
Mice conceived via ART displayed less effective immune responses to vaccines and showed altered T-helper (Th) immune responses (16). A study by Xu et al. 2021 (17) showed no significant differences in total T cells and B cells, natural killer (NK), CD4 + T, CD8 + T, T-helper (Th)1 cells, Th17 cells, and regulatory T (Treg) cells among 69 ART-conceived children aged 3–6 years compared to NC (p > 0.05). A 2022 trial conducted by Mikheeva E.M. et al. (18) involved 82 ART infants who showed higher rates of illnesses during the first year. Changes in cellular immunity were established by T-lymphocytopenia in ART infants, and dysfunction of the humoral immune response manifested as dysimmunoglobulinemia (18).
Evidence shows that ART-conceived children tend to have immune disorders (11, 19–21). Understanding the effect of ART on the immune status of children will improve the timely diagnosis and treatment of immune disorders in the future. This study aimed to broaden the sample size compared to that of past studies and assess the impact of ART on the immune system with the inclusion of defined risk factors.
2 Methods
2.1 Study design and patients
This case-control study recruited women who had live births after fresh or frozen embryo transfer between January 2018 and September 2022 at three leading reproductive clinics in Almaty and who were receiving both paid and reimbursement programs. Donor oocytes/sperm or embryo recipients, intrauterine insemination, and surrogacy were excluded. The study population for controls included NC children.
2.2 Data collection
Anamnestic data were collected. Maternal data (obstetric, gynecological, and somatic history) were retrospectively extracted from medical records. The children's medical history, anthropometric data, and blood samples were collected for immunologic analysis at the pediatrician's appointment.
2.3 Laboratory methods
Fasting blood samples were collected during the morning (08:00–10:30) after an overnight fast of at least 8 h. The absolute lymphocyte counts and indicators of cellular (CD3, CD4, CD8, CD16, CD19, CD25, CD56, CD95, and CD3 HLA DR+) and humoral (IgM, IgA, and IgG) immunity were determined at the Scientific Center of Pediatrics and Pediatric Surgery. Immune status was investigated on a BD FACSLyric flow cytofluorimeter (USA) in the BD FACSuite Clinical v1.5 program using monoclonal antibodies against CD3, CD19, CD4, CD8, CD45, and CD56 presented in the BD Multitest 6-Color TBNK kit and single monoclonal antibodies (anti-HLA-DR). The samples were stained with monoclonal antibodies (mAb) using reagents from the manufacturer Becton Dickinson (BD) according to generally accepted recommendations. To do this, 5 µl of mAb was added to a tube with 50 µl of the sample under study, in our case, peripheral blood, mixed on a Vortex and incubated for 15 min at room temperature in the dark. After adding 1 ml of 1 × 10 BD FACS™ lysing reagent 10× Concentrate (1 ml of primary solution and 9 ml of distilled water), the mixture was vortexed and incubated for 10 min at room temperature in the dark. The mixture was centrifuged for 5 min at 300 g/min, after which the supernatant was removed. Then, 0.5 ml of BD CellWash solution was added.
2.4 Statistical analysis
The sample size calculation used the Lehr formula for average values, assuming a study power of 90% and a significance level of 0.01 (22). The minimum clinically significant difference in the lymphocyte content was determined based on a pilot study involving 20 children (5 with an 11.5 standard deviation). Upon inputting these values into the Lehr formula, we deduced the minimum required size for each of the compared groups. Consequently, a minimum of 112 patients was required for each group to meet the parameters set by the study.
All the statistical analyses were performed using SPSS statistical software (version 26; SPSS, Inc., USA). Quantitative data are presented as medians (Me) and interquartile ranges (IQRs) because of the nonnormal distribution of variables. Comparisons between groups were carried out by the Mann–Whitney U-test for nonnormally distributed continuous data, and Fisher's exact test and χ2 test for categorical data. Odds ratios (ORs) with 95% confidence intervals (CIs) were computed for all maternal somatic, obstetric, and postnatal care history variables for ART relative to spontaneous conception. If the frequency of occurrence of a trait in one of the groups was 0, the Haldane–Enscombe correction was used to calculate the odds ratio (OR). The calculation was performed using an online calculator: https://www.medcalc.org/calc/odds_ratio.php. Spearman's correlation test was applied to compare variables with non-normal distribution. The limit of statistical significance was P < 0.05. The ordinal logistic regression was used to assess the independent influence of ART with the elimination of confounders on meaningful indicators of the immunogram. Missing values were less than 1%.
2.5 Trial registration
The protocol was registered on ClinicalTrials.gov (NCT01369355) on October 17, 2023.
2.6 Ethical approval
This study complied with the Declaration of Helsinki and was approved by the local Ethics Committee of the “Scientific Center of Pediatrics and Pediatric Surgery” on April 13, 2022 (reference number: 2). Informed consent was obtained from all the legally authorized representatives of the research participants before enrollment in the trial.
3 Results
3.1 Cohort description
A total of 252 children aged 1 to 55 months who met the inclusion criteria were enrolled. The study group included 120 ART-conceived children, while the control group included 132 NC children. The characteristics of the study population are presented in Table 1.
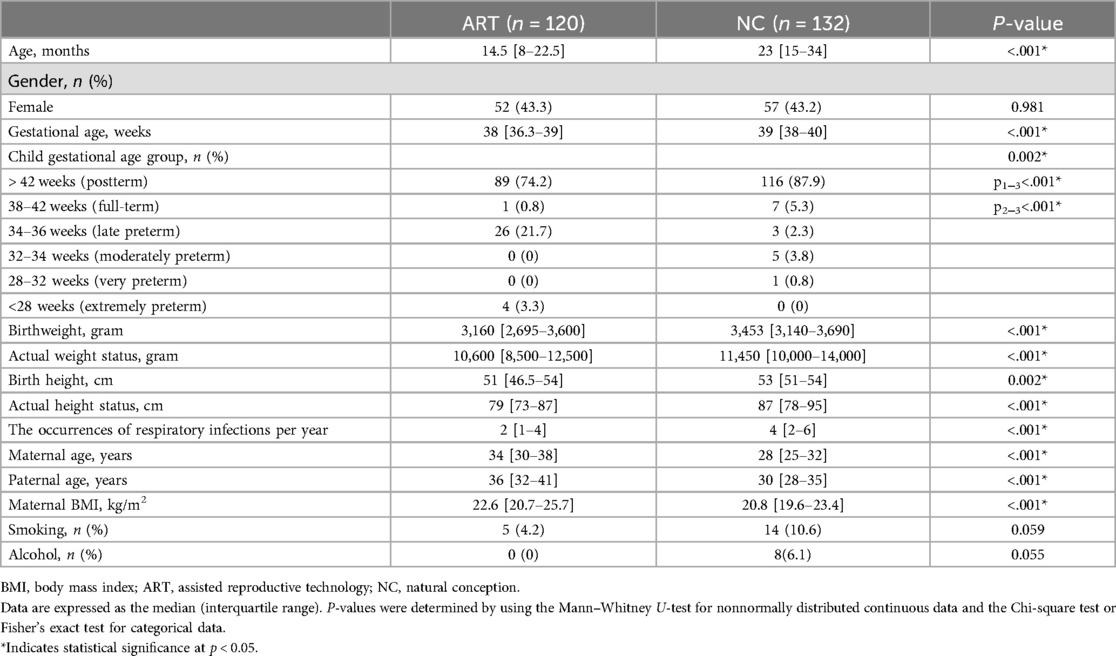
Table 1. Characteristics of the study population conceived by assisted reproductive technology and naturally conceived.
Late premature infants significantly prevailed in ART group (p < 0.001). Based on the medical history data of the mothers (Table 2), the odds of multiple pregnancy in ART group were 9.65 times greater than in NC group (95% CI: 2.81–33.18).
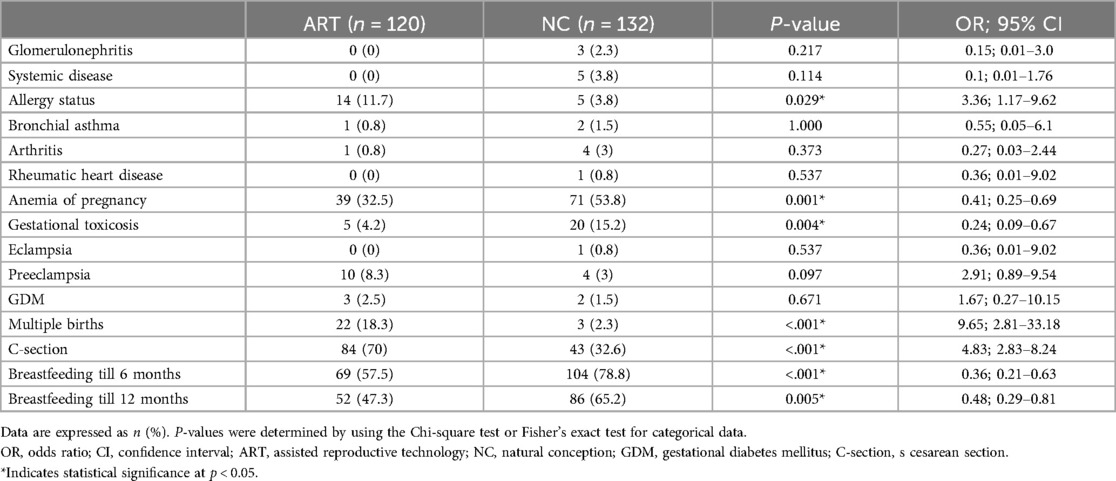
Table 2. Comparison of somatic, obstetric, and postnatal medical histories between women who underwent assisted reproductive technology and those who conceived naturally.
The parents of the NC group were younger than the parents of the ART group (p < 0.001). Additionally, the weight and BMI of the mothers were lower in the NC group (p = 0.002 and p < 0.001, respectively). For extragenital diseases in women in ART group, the odds of having an allergic disease were 3.36 times greater in ART group than in NC group (95% CI: 1.17–9.62). The obstetric history showed that ART-conceived women were 2.43 times less likely to experience anemia during pregnancy (95% CI = 0.25–0.69) and 4.17 times less likely to experience gestational toxicosis (95% CI = 0.09–0.67). For ART group, the odds of having a cesarean section (C-section) were 4.8 times greater than that for NC group (95% CI: 2.83–8.24). No significant differences were found in the odds of other maternal medical history data. The odds of breastfeeding in the first 6 months were 2.8 times lower for ART-conceived children than for NC-conceived children (95% CI: 0.21–0.63). The number of respiratory infections per year was significantly greater in NC children (р < 0.001).
3.2 Comparison of immune status between NC and ART children
Table 3 shows the results of the cellular and humoral immunity assessments in ART and NC children. The analyses were evaluated based on laboratory guidelines and categorized as falling within reference ranges, high, or low levels.
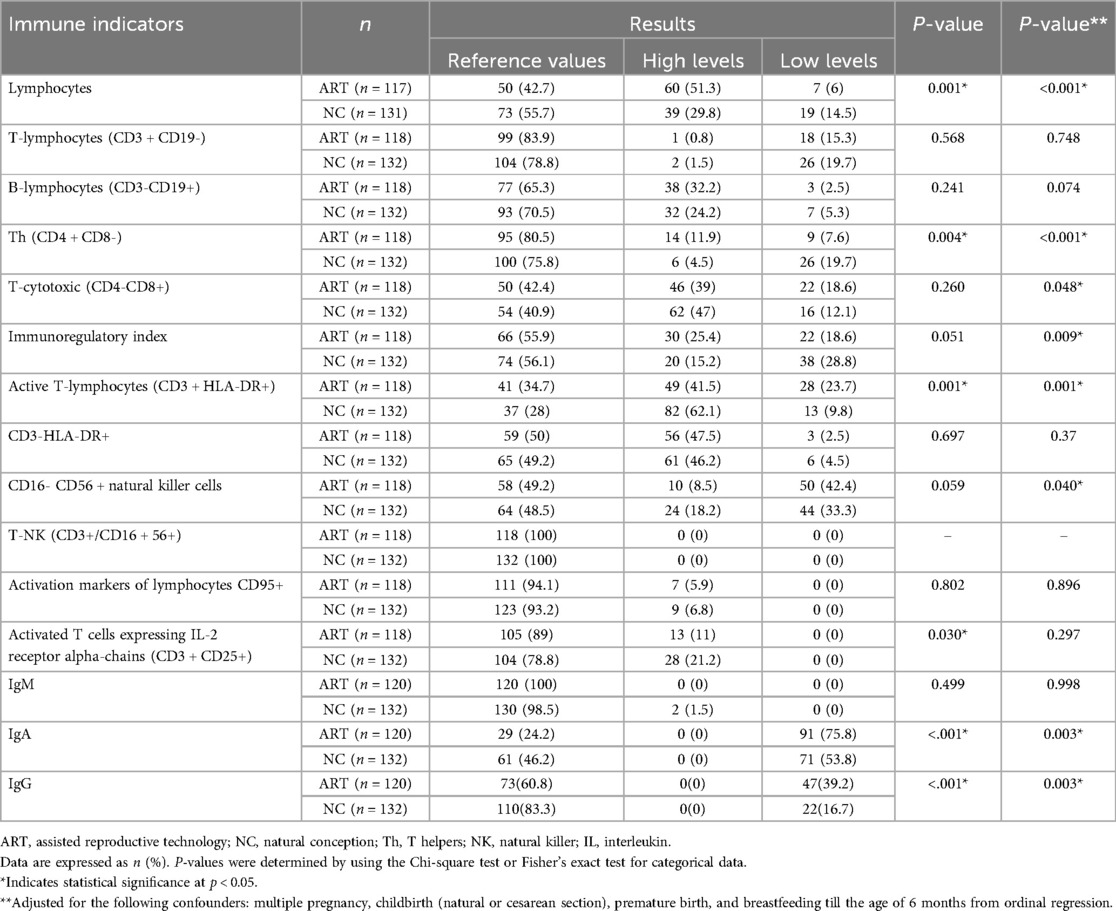
Table 3. Comparative assessment of immune cell profiles in children conceived by assisted reproductive technology versus those conceived naturally.
A comparison of the blood absolute lymphocyte count, Th (CD4 + CD8-), active T-lymphocyte (CD3 + HLA-DR+), activated T cell expressing IL-2 receptor alpha-chain (CD3 + CD25+), IgA, and IgG in the blood between the study groups revealed significant differences (p = 0.001, p = 0.004, p = 0.001, p = 0.030, p < 0.001, p < 0.001, respectively). The differences were explained by absolute lymphocytosis, increased levels of Th (CD4 + CD8-), and pathological levels of active T lymphocytes (CD3 + HLA-DR+) in ART children, a greater prevalence of absolute lymphocytopenia, increased levels of active T-lymphocytes (CD3 + HLA-DR+) and activated T cells expressing IL-2 receptor alpha chains (CD3 + CD25+) in NC children. Both groups exhibited low IgA and IgG levels; however, ART group displayed lower levels more frequently.
ART group exhibited a higher prevalence of multiple pregnancies, premature births via cesarean section, and a lack of breastfeeding—factors known to influence the development of children's immunity significantly (23–29). In our analysis, we employed ordinal logistic regression to control these confounding variables. The results indicated that the statistically significant effects of ART on the levels of lymphocytes, T-helper cells (CD4 + CD8-), active T-lymphocytes (CD3 + HLA-DR+), IgA, and IgG remained prominent (p < 0.001, p < 0.001, p = 0.001, p = 0.003, and p = 0.003, respectively). However, the statistically significant impact of ART on the levels of activated T-lymphocytes expressing the alpha chain of the IL-2 receptor, CD3 + CD25+, became non-significant (p = 0.299). Additionally, we observed that the statistically significant impact of ART on the levels of T-cytotoxic cells (CD4-CD8+), immunoregulatory index, and CD16- CD56 + natural killer cells has emerged (p = 0.048, p = 0.009, and p = 0.040, respectively).
Figure 1 describes the correlation between immune markers such as lymphocytes (%), T-lymphocytes (CD3 + CD19-) (%), B-lymphocytes (CD3-CD19+) (%), and Th (CD4 + CD8-) (%) in ART and NC groups.

Figure 1. Correlation analysis of immune markers in children conceived by assisted reproductive technology and naturally conceived. (A) Correlation heatmap for ART-conceived children. (B) Correlation heatmap for NC children.
Both in ART and NC groups, T-lymphocytes (CD3 + CD19-) showed a significant moderate positive correlation with Th (p < 0.001) and a significant moderate negative correlation with B lymphocytes (p < 0.001). In ART group Th showed a significant weak positive correlation with lymphocytes (p = 0.001) and a significant weak negative correlation with B lymphocytes (p = 0.015).
3.3 Comparison of immune status between FET group and fresh-ET group
We assessed the immune status of children born after FET or fresh embryo transfer (Fresh-ET) (Table 4). Overall, 24.2% (n = 29) of the children conceived using Fresh-ET, and 75.8% conceived using FET (n = 91).
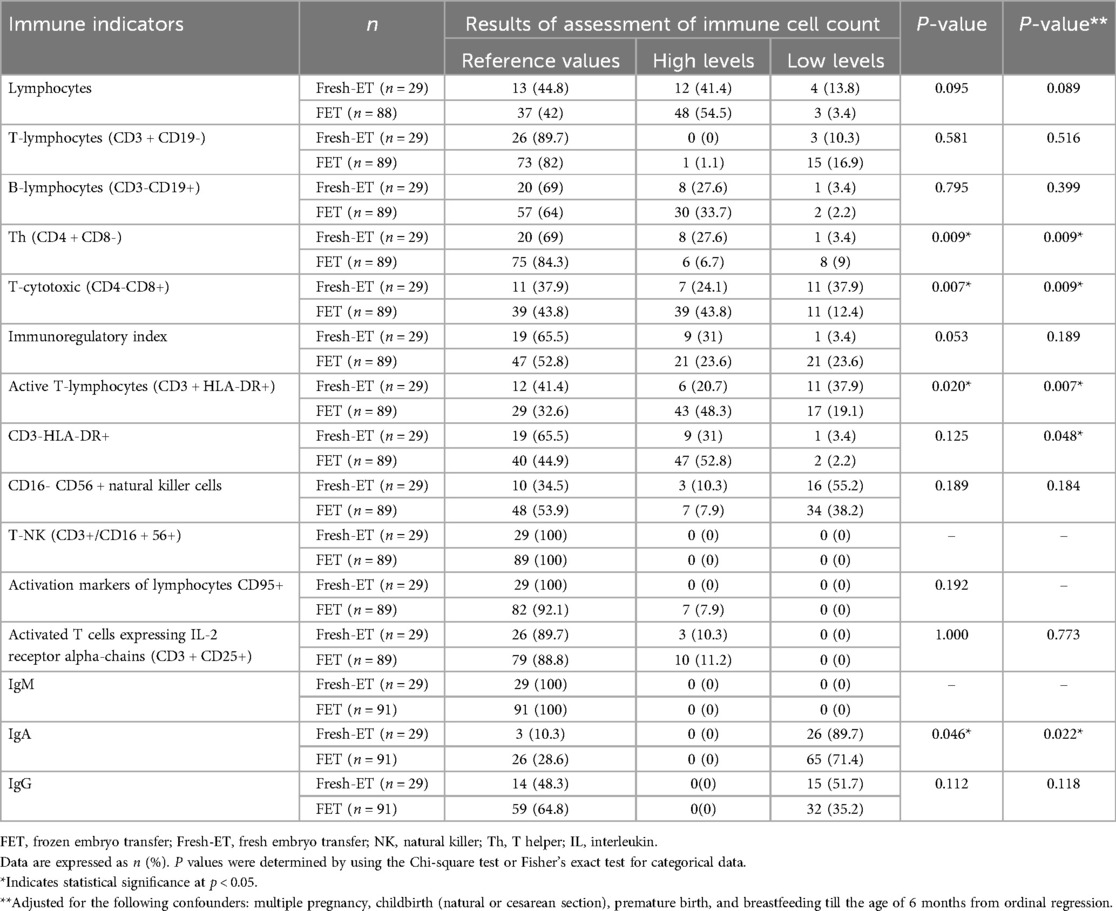
Table 4. Comparative assessment of immune cell profiles in children conceived by frozen vs. fresh embryo transfer.
The levels of T-cytotoxic (CD4-CD8+) and active T-lymphocytes (CD3 + HLA-DR+) were significantly greater in FET group than in the Fresh-ET group (p = 0.007 and p = 0.020, respectively). The level of Th (CD4 + CD8-) was higher in Fresh-ET group (p = 0.009), while the level of IgA was significantly lower in both groups. In our analysis using ordinal logistic regression to mitigate confounding factors such as multiple pregnancy, cesarean section, premature birth, and breastfeeding, observed a statistically significant influence of Fresh-ET on the levels of T-helper cells (CD4 + CD8-), T-cytotoxic (CD4-CD8+), active T-lymphocytes (CD3 + HLA-DR+), CD3-HLA-DR+, and IgA (p = 0.009, p = 0.009, p = 0.007, p = 0.048, and p = 0.022, respectively).
4 Discussion
The key findings of this case-control study are as follows: (i) a greater incidence of allergic diseases was observed in ART mothers; (ii) a statistically significant difference in immune parameters was found in ART children; and (iii) a statistically significant difference in immune parameters was found in FET group.
The data consistently showed that women who underwent ART were diagnosed more often with allergic diseases (95% CI: 1.17–9.62), confirming the results of several studies on the relationship between allergic diseases in women and subfertility, thereby increasing the risk of immune pathologies in offspring (21, 30, 31). Maternal allergies can trigger the development of allergies in offspring, either through inherited allergic predispositions or by creating an environment that promotes allergic disorders (32). Having a parental history of allergic disease increases a child's risk of developing allergies (33), with the combined influence of both parents posing a greater risk than either parent alone (34). Certain genes that regulate the T-cell response have been linked to the predisposition for developing allergic disorders (35). Furthermore, it has been found that maternal drug allergies are associated with a higher risk of long-term infectious hospitalizations in offspring, suggesting a potential inheritance of immune dysfunctions from mother to child (36).
Low levels of IgA and IgG were observed in both groups (p < 0.001 and p < 0.001, respectively). However, lower IgA and IgG levels were more common in ART group which confirmed the data of Mikheeva E.M. et al. 2022 (18). Notably, IgG deficiency, which is involved in the secondary immune response, reduces the effectiveness of vaccination, which confirms the results of a study of mice conceived using ART (16). Absolute lymphocytosis and increased Th levels were significantly more common in ART group (p = 0.001 and p = 0.004, respectively). Furthermore, a statistically significant correlation between these variables was established (p = 0.001). Additionally, a notable negative correlation between B-lymphocytes and Th levels was identified (p < 0.001), indicating that B cell dysfunction contributed to a diminished immunoglobulin response. Th cells are a diverse group of cells essential for adaptive immunity (37). They have a direct and indirect impact on nearly every aspect of the immune response. This population includes effector cells, which are designed to defend against infections, and Tregs, which help prevent excessive reactions to both self-antigens and harmful external antigens. Th cells play a crucial role in assisting B cells in generating antibodies during the initial immune response (humoral immunity). Changes in Th status can significantly contribute to the development of autoimmune, inflammatory, and allergic disorders in ART offspring in the future (37). Reduced levels of IgG and Th may significantly contribute to the diminished efficacy of vaccination in ART-conceived children, which warrants further investigation to elucidate the underlying mechanisms involved. Both groups showed high levels of active T-lymphocytes and activated T cells expressing IL-2 receptor alpha chains (CD3 + CD25+), but 23.7% of children in ART group had decreased levels of active T-lymphocytes (CD3 + HLA-DR+). When comparing the immune status of a group of children conceived using FET and Fresh-ET, the levels of T-cytotoxic (CD4-CD8+) and active T-lymphocytes (CD3 + HLA-DR+) were significantly greater in FET group and lower in Fresh-ET group (p = 0.007 and p = 0.020, respectively). The Th level was greater in Fresh-ET group (p = 0.009), while the IgA level was significantly lower in both groups (p = 0.046). The activation of cellular immunity was revealed, which indicated more frequent infectious diseases and respiratory diseases in FET group than in Fresh-ET group, confirming the findings of previous studies (9). Our data on the occurrence of respiratory infections per year in ART-conceived children differ from those in the study by Mikheeva E.M. et al. (18), where ART-conceived children were classified as frequently ill. According to our study, the occurrence of respiratory infections per year was lower in ART group than in the control group, which indicates a reduced immune response in this cohort.
Previous studies on the immune status of ART-conceived children failed to consider risk factors other than ART use in their analysis. This study factored in medical history data and revealed that ART-conceived children were mostly born by C-section (95% CI: 2.83–8.24) and were significantly less likely to be breastfed for at least the first 6 months (95% CI: 0.21–0.63). In addition, ART-conceived children were more likely to be born prematurely compared to NC group (p < 0.001).
The development of the gut microbiota at an early age is crucial for the balanced preparation of the immune system, which occurs in the early stages of life in the so-called “window of opportunity” (23). C-section birth is associated with adverse effects on immune development (24), particularly in the inflammatory response to infection and in cells involved in the allergic response, during infancy and early childhood (25, 26). Prematurity is associated with a high risk of dysbiosis and delayed colonization with decreased diversity (27). Furthermore, a decrease in breastfeeding success exacerbates changes in the early development of the gut microbiota (28, 29).
In our analysis, we utilized ordinal logistic regression to eliminate confounding variables and accurately assess the impact of multiple pregnancy, cesarean section, premature birth, and breastfeeding. Despite our rigorous approach, the statistically significant impact of ART on the immunogram parameters remained, underscoring the independent and influential nature of ART or other unaccounted factors.
It is biologically conceivable that ART could contribute to health issues in children. One theory posits that the mechanical and hormonal intervention of gametes and embryos might trigger epigenetic modifications, which could impact the immune system and lower disease resistance. ART procedures take place during a critical stage of development that is vital for epigenetic reprogramming. As a result, technical interventions during this delicate period may lead to a modified epigenetic profile of the conceived child (38). Several studies have observed altered epigenetic characteristics in gametes from infertile couples, raising concerns about a higher risk of imprinting disorders and somatic epigenetic alterations in children conceived via ART (39–41). Changes in gene expression related to immune response have also been observed in the placentas of individuals undergoing IVF, suggesting a possible effect on the immune response of their offspring (42). Additionally, pregnancies conceived using ART have been associated with a greater risk of tumor development, which may indicate an increased immune tolerance to tumor antigens (43). The application of ART involves administering sex hormones. Even normal hormonal changes during pregnancy can trigger an inflammatory response, along with metabolic and endocrine alterations (44). Current evidence suggests that women who conceive through IVF are more likely to develop gestational diabetes mellitus compared to those who conceive spontaneously. This correlation remains significant even after accounting for factors such as maternal age, gestational age, and parity (45, 46). Moreover, gestational diabetes mellitus can lead to immune dysregulation, inflammation, changes in the microbiota, and allergic diseases in the offspring due to epigenetic changes brought about by a hyperglycemic environment in the uterus (47, 48). These research findings and clinical observations suggest that ART could affect the immune profile of the offspring.
To enhance the immune system health of ART-conceived children and improve the efficacy of vaccinations, medical leaders in Kazakhstan are advised to incorporate consultations with immunologists to assess the immune status within the first year of life. Continuous monitoring of the immune system of ART children is also recommended to promptly address any changes that may occur.
4.1 Limitations
First, the size of the study group does not reflect the wider population. Lehr's formula proposed a minimum number of patients sufficient to compare immune parameters between ART-conceived and NC children. Second, our study is limited by a short follow-up period due to the complexity and cost of longitudinal studies, which limits the ability to assess the long-term effects of ART procedures on the immune system of offspring. To enhance this understanding, future research must prioritize this consideration. Third, we assessed a few risk factors, excluding others, such as infertility itself, drugs used for ovulation induction, placental pathology, differences in lifestyle choices, other diseases, and high levels of anxiety in couples. Fourth, in addition to considered confounders (multiple pregnancy, cesarean section, premature birth, and breastfeeding), there are significant differences in other general characteristics (e.g., gestational age, birth weight, and others), that could affect the reliability of the study's findings, which would be good to take into account in future research. Fifth, significant confounding factors, including the type of ART procedures, as well as the protocols for ovarian stimulation, were not sufficiently controlled in the current study. These factors may considerably influence the immune outcomes of the offspring and should be addressed in future research to enhance the accuracy and reliability of the findings. Sixth, this study included only a sample from Kazakhstan. It is necessary to expand the geography of studies to increase the sample size and increase the applicability of findings to other ethnic groups. Thus, longitudinal studies should be conducted considering various risk factors for immune diseases to obtain a clear overview of the long-term effects of ART on the immune status of offspring.
5 Conclusion
Despite the limited number of studies on the immune system of ART-conceived offspring, our study confirmed the altered immune profiles in ART-conceived children. Specifically, they tend to have lower levels of IgA and IgG, absolute lymphocytosis, and increased Th cell counts which can result in a poor immune response and vaccine effectiveness. However, considering the analysis of risk factors, further research is needed to comprehensively distinguish between the role of ART treatment and parental factors in determining the immune outcomes of offspring.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by local Ethics Committee of the "Scientific Center of Pediatrics and Pediatric Surgery" on April 13, 2022 (reference number: 2). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
SI: Data curation, Formal Analysis, Investigation, Writing – original draft, Writing – review & editing. VL: Conceptualization, Data curation, Methodology, Resources, Supervision, Writing – review & editing. AP: Writing – review & editing. LM: Funding acquisition, Methodology, Project administration, Resources, Writing – review & editing. ZN: Investigation, Writing – review & editing. FK: Investigation, Writing – review & editing. АB: Writing – review & editing. VN: Investigation, Writing – review & editing. AA: Investigation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This research was supported by a “Somatic and psychosocial status of children after ART determination with the development of prediction model and principles of child management” grant from the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan (No. ИРН АР14872103).
Acknowledgments
We would like to thank all clinical and scientific staff of the International Clinical Center for Reproductology “PERSONA”, Institute of Reproductive Medicine for their help with the recruitment of patients and the Scientific Center of Pediatrics and Pediatric Surgery for conducting clinical trial.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1447956/full#supplementary-material
References
1. Isenova SSH, Aripkhanova AS, Sultanmuratova DD, Kazybaeva AS, Tileukul NA, Boran AM. Management strategies for thrombophilic patients undergoing assisted reproductive technologies. Akusherstvo i Ginekologiya (Russian Federation). (2023) 2023:5–10. doi: 10.18565/aig.2023.177
2. Factsheets and infographics. Available online at: https://www.eshre.eu/Europe/Factsheets-and-infographics (accessed March 28, 2024).
3. Ilmuratova S, Manzhuova L, Lokshin V. Health status of children born after assisted reproductive technologies. Reproductive Medicine. (2022) 1(50):15–22. doi: 10.37800/RM.1.2022.15-22
4. Krieger Y, Wainstock T, Sheiner E, Harlev A, Landau D, Horev A, et al. Long-term pediatric skin eruption-related hospitalizations in offspring conceived via fertility treatment. Int J Dermatol. (2018) 57:317–23. doi: 10.1111/ijd.13903
5. Imterat M, Wainstock T, Sheiner E, Landau D, Walfisch A, Harlev A. Fertility treatments and the risk of pediatric obstructive sleep apnea in the offspring—results from a population-based cohort study. Pediatr Pulmonol. (2019) 54:1534–40. doi: 10.1002/ppul.24428
6. Mitter VR, Håberg SE, Magnus MC. Early childhood respiratory tract infections according to parental subfertility and conception by assisted reproductive technologies. Hum Reprod. (2022) 37:2113–25. doi: 10.1093/humrep/deac162
7. Shachor N, Wainstock T, Sheiner E, Harlev A. Fertility treatments and gastrointestinal morbidity of the offspring. Early Hum Dev. (2020) 144:105021. doi: 10.1016/j.earlhumdev.2020.105021
8. Wainstock T, Sheiner E, Yoles I, Sergienko R, Landau D, Harlev A. Fertility treatments and offspring pediatric infectious morbidities: results of a population-based cohort with a median follow-up of 10 years. Fertil Steril. (2019) 112:1129–35. doi: 10.1016/j.fertnstert.2019.07.1325
9. Hwang SS, Dukhovny D, Gopal D, Cabral H, Diop H, Coddington CC, et al. Health outcomes for Massachusetts infants after fresh versus frozen embryo transfer. Fertil Steril. (2019) 112:900–7. doi: 10.1016/j.fertnstert.2019.07.010
10. Luo Q-Y, Su K, Dong Z-H, Feng T-N, Zhang C, Hao Y-H, et al. Association between frozen embryo transfer and childhood allergy: a retrospective cohort study. Reprod Biomed Online. (2024) 49(5):104320. doi: 10.1016/j.rbmo.2024.104320
11. Wijs LA, Fusco MR, Doherty DA, Keelan JA, Hart RJ. Asthma and allergies in offspring conceived by ART: a systematic review and meta-analysis. Hum Reprod Update. (2021) 28:132–48. doi: 10.1093/humupd/dmab031
12. Tsabouri S, Lavasidis G, Efstathiadou A, Papasavva M, Bellou V, Bergantini H, et al. Association between childhood asthma and history of assisted reproduction techniques: a systematic review and meta-analysis. Eur J Pediatr. (2021) 180:2007–17. doi: 10.1007/s00431-021-03975-7
13. Hart RJ, Penova-Veselinovic B, Wijs LA, Yovich JL, Burton P. Cohort profile: the growing up healthy study (GUHS)-A prospective and observational cohort study investigating the long-term health outcomes of offspring conceived after assisted reproductive technologies. PLoS One. (2022) 17(7):e0272064. doi: 10.1371/JOURNAL.PONE.0272064
14. Hart RJ, Wijs LA. The longer-term effects of IVF on offspring from childhood to adolescence. Frontiers in Reproductive Health. (2022) 4:1045762. doi: 10.3389/frph.2022.1045762
15. Wei SQ, Luu TM, Bilodeau-Bertrand M, Auger N. Assisted reproductive technology and childhood morbidity: a longitudinal cohort study. Fertil Steril. (2022) 118:360–8. doi: 10.1016/j.fertnstert.2022.04.025
16. Ahmadi H, Fathi F, Karimi H, Amidi F, Mehdinejadiani S, Moeini A, et al. Altered TH1, TH2, TH17 balance in assisted reproductive technology conceived mice. J Reprod Immunol. (2020) 139:103117. doi: 10.1016/j.jri.2020.103117
17. Xu X, Wu H, Bian Y, Cui L, Man Y, Wang Z, et al. The altered immunological status of children conceived by assisted reproductive technology. Reprod Biol Endocrinol. (2021) 19(1):171. doi: 10.1186/S12958-021-00858-2
18. Mikheeva EM, Penkina NI. Immunological status in children of the first year of life born as a result of assisted reproductive technologies. Practical Med. (2022) 20(7):41–5. doi: 10.32000/2072-1757-2022-7-41-45
19. Cui L, Zhou W, Xi B, Ma J, Hu J, Fang M, et al. Increased risk of metabolic dysfunction in children conceived by assisted reproductive technology. Diabetologia. (2020) 63:2150–7. doi: 10.1007/s00125-020-05241-1
20. Nwaru BI, Mccleary N, Erkkola M, Kaila M, Virtanen SM, Sheikh A. Assisted reproductive technology and risk of asthma and allergy in the offspring: protocol for a systematic review and meta-analysis. BMJ Open. (2016) 6(4):e010697. doi: 10.1136/bmjopen-2015-010697
21. Polinski KJ, Stevens DR, Mendola P, Lin TC, Sundaram R, Bell E, et al. Infertility treatment associated with childhood asthma and atopy. Hum Reprod. (2022) 37:1609–18. doi: 10.1093/humrep/deac070
22. Lehr R. Sixteen S-squared over D-squared: a relation for crude sample size estimates. Stat Med. (1992) 11:1099–102. doi: 10.1002/sim.4780110811
23. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science. (2016) 352:539–44. doi: 10.1126/science.aad9378
24. Kristensen K, Henriksen L. Cesarean section and disease associated with immune function. J Allergy Clin Immunol. (2016) 137:587–90. doi: 10.1016/j.jaci.2015.07.040
25. Thompson AL. Caesarean delivery, immune function and inflammation in early life among Ecuadorian infants and young children. J Dev Orig Health Dis. (2019) 10:555–62. doi: 10.1017/S2040174419000047
26. Reyman M, van Houten MA, van Baarle D, Bosch AATM, Man WH, Chu MLJN, et al. Impact of delivery mode-associated gut microbiota dynamics on health in the first year of life. Nat Commun. (2019) 10(1):1–12. doi: 10.1038/s41467-019-13014-7
27. Xiang Q, Yan X, Shi W, Li H, Zhou K. Early gut microbiota intervention in premature infants: application perspectives. J Adv Res. (2023) 51:59–72. doi: 10.1016/j.jare.2022.11.004
28. Camacho-Morales A, Caba M, García-Juárez M, Caba-Flores MD, Viveros-Contreras R, Martínez-Valenzuela C. Breastfeeding contributes to physiological immune programming in the newborn. Front Pediatr. (2021) 9:744104. doi: 10.3389/fped.2021.744104
29. Kalbermatter C, Fernandez Trigo N, Christensen S, Ganal-Vonarburg SC. Maternal Microbiota, early life colonization and breast milk drive immune development in the newborn. Front Immunol. (2021) 12:683022. doi: 10.3389/fimmu.2021.683022
30. Adamczak R, Ukleja-Sokołowska N, Bartuzi Z. Fertility and allergy: is there a correlation? Postepy Hig Med Dosw. (2019) 73:440–6. doi: 10.5604/01.3001.0013.4536
31. Esfandiari N, Nesbit C, Litzky J, Dela Cruz D, Gibson S, DeMars L, et al. High prevalence of allergy in patients undergoing in vitro fertilization and embryo transfer. J Assist Reprod Genet. (2020) 37:311–20. doi: 10.1007/s10815-020-01691-z
32. Grijincu M, Buzan MR, Zbîrcea LE, Păunescu V, Panaitescu C. Prenatal factors in the development of allergic diseases. Int J Mol Sci. (2024) 25:6359. doi: 10.3390/ijms25126359
33. Saito-Abe M, Yamamoto-Hanada K, Pak K, Iwamoto S, Sato M, Miyaji Y, et al. How a family history of allergic diseases influences food allergy in children: the Japan environment and children’s study. Nutrients. (2022) 14:4323. doi: 10.3390/nu14204323
34. Hossenbaccus L, Linton S, Ramchandani R, Gallant MJ, Ellis AK. Insights into allergic risk factors from birth cohort studies. Annals of allergy. Asthma Immunol. (2021) 127:312–7. doi: 10.1016/j.anai.2021.04.025
35. Gao X, Yan Y, Zeng G, Sha T, Liu S, He Q, et al. Influence of prenatal and early-life exposures on food allergy and eczema in infancy: a birth cohort study. BMC Pediatr. (2019) 19:1–9. doi: 10.1186/s12887-019-1623-3
36. Mahamid A, Wainstock T, Sheiner E, Rosenberg E, Kluwgant D, Pariente G. Perinatal outcome and long-term infectious hospitalizations of offspring born to women with known drug allergy. Am J Reprod Immunol. (2022) 88:e13608. doi: 10.1111/aji.13608
37. Santarlasci V, Cosmi L, Maggi L, Liotta F, Annunziato F. IL-1 and T helper immune responses. Front Immunol. (2013) 4:52955. doi: 10.3389/fimmu.2013.00182
38. Monk D, Mackay DJG, Eggermann T, Maher ER, Riccio A. Genomic imprinting disorders: lessons on how genome, epigenome and environment interact. Nat Rev Genet. (2019) 20:235–48. doi: 10.1038/s41576-018-0092-0
39. Choux C, Binquet C, Carmignac V, Bruno C, Chapusot C, Barberet J, et al. The epigenetic control of transposable elements and imprinted genes in newborns is affected by the mode of conception: aRT versus spontaneous conception without underlying infertility. Hum Reprod. (2018) 33:331–40. doi: 10.1093/humrep/dex366
40. Cortessis VK, Azadian M, Buxbaum J, Sanogo F, Song AY, Sriprasert I, et al. Comprehensive meta-analysis reveals association between multiple imprinting disorders and conception by assisted reproductive technology. J Assist Reprod Genet. (2018) 35:943–52. doi: 10.1007/s10815-018-1173-x
41. Henningsen AA, Gissler M, Rasmussen S, Opdahl S, Wennerholm UB, Spangsmose AL, et al. Imprinting disorders in children born after ART: a nordic study from the CoNARTaS group. Hum Reprod. (2020) 35:1178–84. doi: 10.1093/humrep/deaa039
42. Paolino M, Koglgruber R, Cronin SJF, Uribesalgo I, Rauscher E, Harreiter J, et al. RANK Links thymic regulatory T cells to fetal loss and gestational diabetes in pregnancy. Nature. (2020) 589:442–7. doi: 10.1038/s41586-020-03071-0
43. Hargreave M, Jensen A, Hansen MK, Dehlendorff C, Winther JF, Schmiegelow K, et al. Association between fertility treatment and cancer risk in children. JAMA. (2019) 322:2203–10. doi: 10.1001/jama.2019.18037
44. Coussa A, Hasan HA, Barber TM. Impact of contraception and IVF hormones on metabolic, endocrine, and inflammatory status. J Assist Reprod Genet. (2020) 37(6):1267–72. doi: 10.1007/s10815-020-01756-z
45. Bosdou JK, Anagnostis P, Goulis DG, Lainas GT, Tarlatzis BC, Grimbizis GF, et al. Risk of gestational diabetes mellitus in women achieving singleton pregnancy spontaneously or after ART: a systematic review and meta-analysis. Hum Reprod Update. (2020) 26:514–44. doi: 10.1093/humupd/dmaa011
46. Mohammadi M, Khedmati Morasae E, Maroufizadeh S, Almasi-Hashiani A, Navid B, Amini P, et al. Assisted reproductive technology and the risk of gestational diabetes mellitus: a systematic review and meta-analysis. Middle East Fertil Soc J. (2020) 25:1–12. doi: 10.1186/s43043-020-0018-6
47. Chu AHY, Godfrey KM. Gestational diabetes Mellitus and developmental programming. Ann Nutr Metab. (2021) 76:4–15. doi: 10.1159/000509902
Keywords: assisted reproductive technology (ART), children, immune system, immune dysregulation, C-section=caesarean section, breastfeeding
Citation: Ilmuratova S, Lokshin V, Prodeus A, Manzhuova L, Nurgaliyeva Z, Kussainova F, Bazarbaeva A, Nekhorosheva V and Abshekenova A (2024) Immune profiling of ART-conceived children in Kazakhstan: a case-control study. Front. Pediatr. 12:1447956. doi: 10.3389/fped.2024.1447956
Received: 12 June 2024; Accepted: 30 October 2024;
Published: 22 November 2024.
Edited by:
Andrew S. Day, University of Otago, New ZealandReviewed by:
Zongzhi Liu, Chinese Academy of Sciences (CAS), ChinaHamid Ahmadi, University of Pécs, Hungary
Xiuye Xing, Haidian Maternal and Child Health Hospital of Beijing, China
Copyright: © 2024 Ilmuratova, Lokshin, Prodeus, Manzhuova, Nurgaliyeva, Kussainova, Bazarbaeva, Nekhorosheva and Abshekenova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sevara Ilmuratova, aWxtdXJhdG92YS5zQGdtYWlsLmNvbQ==
 Sevara Ilmuratova
Sevara Ilmuratova Vyacheslav Lokshin
Vyacheslav Lokshin Andrey Prodeus
Andrey Prodeus Lyazzat Manzhuova4
Lyazzat Manzhuova4 Zhanar Nurgaliyeva
Zhanar Nurgaliyeva Farida Kussainova
Farida Kussainova Aygul Bazarbaeva
Aygul Bazarbaeva Valeriya Nekhorosheva
Valeriya Nekhorosheva Aygerim Abshekenova
Aygerim Abshekenova