- 1Centre for the Program of AIDS Research in South Africa (CAPRISA), Durban, South Africa
- 2Department of Paediatrics and Child Health, School of Clinical Medicine, University of KwaZulu Natal, Durban, South Africa
- 3Biostatistics Unit, South African Medical Research Council, Tygerberg, South Africa
- 4Division of Epidemiology and Biostatistics, Department of Global Health, University of Stellenbosch, Tygerberg, South Africa
- 5Gilead Sciences Inc, Foster City, CA, United States
- 6South African Medical Research Council, Cape Town, South Africa
- 7Department of Obstetrics and Gynaecology, School of Clinical Medicine, University of KwaZulu Natal, Durban, South Africa
Objective: We evaluated growth metrics in HIV unexposed African breastfed infants in the first 18 months of life in association with in-utero exposure to Tenofovir Diphosphate Fumarate (TDF) containing pre-exposure prophylaxis (PrEP).
Design: We conducted a secondary data analysis of a TDF-PrEP randomized control trial (CAP016 RCT). Pregnant women without HIV were randomized to initiating TDF-PrEP in pregnancy (Immediate-PrEP-IP) or deferred initiation of TDF-PrEP at cessation of breastfeeding (Deferred-PrEP-DP).
Methods: Infant weight (W), length (L), and head circumference (HC) were measured at birth and 6, 26, 50, and 74 weeks of age. Stored dried blood spot samples from pregnant women randomized to the IP arm were used to measure tenofovir-diphosphate (TFV-DP) levels. Age-stratified mean weight-for-age (WAZ), length-for-age (LAZ), weight-for-length (WLZ), and head circumference-for-age (HCAZ) Z-scores were compared between infants exposed to varying TFV-DP concentrations and infants in the DP arm.
Results: A total of 455 mother-infant pairs were included in the secondary analysis, 228 in the IP arm and 227 in the DP arm. WAZ, LAZ, WLZ, and HCAZ scores were comparable between infants in the Deferred-PrEP arm and Immediate-PrEP arm. In a mixed-effects linear regression model adjusting for maternal age, body mass index, socioeconomic and newborn characteristics, in-utero exposure to varying TFV-DP levels was not associated with WAZ (β = −0.52), LAZ (β = −0.46), WLZ (β = −0.43) and HCAZ (β = −0.11) scores over time.
Conclusion: There was no evidence of an association between growth metrics in the first 18 months of life and in-utero exposure to TFV-DP among breastfed HIV unexposed infants.
Introduction
Oral pre-exposure prophylaxis (PrEP), most commonly available as a combination of Tenofovir Disoproxil Fumarate (TDF) and Emtricitabine-triphosphate (FTC), is widely used by pregnant and lactating women since the World Health Organisation (WHO) launched its recommendations in 2017 (1–4). Tenofovir (TFV) is known to readily cross the placenta with cord blood concentrations reaching 70%–100% of maternal plasma concentration (5). While several studies have provided reassuring evidence that in-utero exposure to TDF does not affect pregnancy and newborn outcomes (6–8), a systematic review of adult studies concluded that TDF was associated with lower bone mineral density in adults taking TDF as part of a combination treatment or as PrEP (9). A similar trend was reported for infants who were born to women living with HIV and receiving TDF-containing antiretroviral treatment (10, 11). Consequentially, growth trajectories in HIV-exposed and uninfected infants have been well described, however, studies determining the association between in-utero exposure to TDF-containing antiretroviral treatment and growth have yielded inconsistent findings (12–16).
As more children become exposed to maternal TDF-based PrEP in sub-Saharan Africa, it remains important to understand how this might impact the growth of children already predisposed to nutritional and developmental challenges. Growth outcomes in HIV-unexposed infants and those exposed to in-utero TDF-based PrEP are limited (17–20). Since poor adherence to PrEP use during pregnancy and postpartum period is reported as a common challenge in PrEP implementation studies (21–23), we aimed to determine if there was an association between growth metrics and in-utero exposure to TDF-PrEP as measured by tenofovir diphosphate (TFV-DP) concentration in red blood cells using dried blood spots collected during pregnancy.
Methods
The CAP 016 clinical trial was an open-label randomized control study designed to investigate the safety of TDF/FTC when used as oral PrEP in healthy, HIV-uninfected pregnant women at substantially high risk for HIV infection (7). Pregnant women meeting eligibility criteria and providing informed consent were randomized in a 1:1 ratio to immediate initiation of PrEP in pregnancy (Immediate PrEP Arm) or deferred initiation of PrEP at the cessation of breastfeeding (Deferred PrEP Arm) using a computer-generated permuted block (block size of ten) randomisation list and sealed envelopes with sequential participant study numbers. Gestational age was determined in all participants using ultrasound scanning at the enrolment visit before 28 weeks. Following randomization, women were seen every four weeks until delivery. For women randomized to the Immediate PrEP Arm, the pharmacist dispensed a monthly supply of once-daily oral Truvada (TDF 300 mg and FTC 200 mg). Participants delivered at the regional hospital near the research clinic. A delivery visit was conducted within seven days of delivery at which, labour and delivery data were extracted from the maternity chart and mothers and newborns were examined by the study clinician. All women and infants were given monthly appointments for the first three months after delivery and thereafter three-monthly appointments until 74 weeks. Women remained on their assigned treatment arm until breastfeeding cessation. Data included in this post hoc analysis was collected between September 2017 and December 2020.
As a post hoc investigation, TFV-DP was measured in red blood cells using stored dried blood spots (DBS) that were collected at two visits during pregnancy. Extractions from a 50-μl DBS were tested for TFV-DP (fmol/3-mm punch) using high-performance liquid chromatography–tandem mass spectrometry at the University of Cape Town, South Africa. The lower limit of quantification for tenofovir diphosphate was 16.6 fmol/3-mm punch. Extrapolating benchmark adherence metrics from the study by Stranix-Chibanda et al, which estimated the TNF-DP concentration of 93 fmol/punch to correspond to 1 dose/week (24). We used a threshold of 500 fmol/punch which equates to five doses/week representing 70% adherence to the daily oral TDF-PrEP regimen.
At birth, the infant was assessed and the mother's intention to breastfeed was documented. Women were encouraged to breastfeed their infants for as long as possible. Infants were assessed if brought in by the mother at 6, 26, 50, and 74 weeks of age. To ensure optimum retention, these visits coincided with routine infant immunization visits. At each visit, a trained research assistant measured the infant weight, length, and head circumference using a calibrated infant weighing scale, a stadiometer, and a non-stretchable measuring tape respectively. The World Health Organisation (WHO) growth standards were used to calculate age and sex-appropriate Z-scores for weight, length, and head circumference (25). Infant feeding modality was recorded at each visit. Cessation of breastfeeding was defined as 28 days after the last exposure to breast milk.
Maternal sociodemographic characteristics included age, level of education, and monthly household income. Infant birth characteristics included infant sex, gestational age at birth, weight, length, head circumference, infant feeding practice, duration of breastfeeding, and weight-for-age, length-for-age, weight-for-age and head circumference-for-age Z-scores.
Mother-infant pairs were included in this analysis if infants were assessed at more than one scheduled visit at 6, 26, 50, and 74 weeks. The maternal TFV-DP levels were categorized as <500 and >500 fmol/punch. Maternal sociodemographic characteristics and infant birth characteristics were compared between TDF-unexposed infants and infants exposed to maternal TFV-DP level <500 fmol/punch and >500 fmol/punch using t-tests for numeric data and Chi-square tests or Fisher's exact test (small frequencies) for categorical data. Student t-tests assuming unequal variances were used to compare WAZ, LAZ, WLZ, and HCAZ scores at 6, 26, 50, and 74 weeks between TDF-unexposed, maternal TFV-DP level <500 fmol/punch and >500 fmol/punch groups. For the adjusted analysis a mixed linear regression model was used to compare the growth trajectories between groups over time while adjusting for maternal socioeconomic status, age, low birth weight, and infant sex. A correlation coefficient (β) with 95% confidence intervals was reported for WAZ, LAZ, WLZ and HCAZ scores in association with no exposure to TDF-PrEP and in-utero exposure to TFV-DP <500 and >500 fmol/punch. A two-sided P-value of less than 0.05 indicated significance for the primary analyses. SAS 9.4 (NC, USA) was used for all analyses.
Ethical approval of the CAP 016 study was granted by the Institutional Review Board of the University of KwaZulu-Natal (UKZN IRB), BFC 243/16, for which all participants signed a written informed consent. Written informed consent for the original study was obtained before any study procedures were performed and included consent for infant participation following delivery as well as consent for data collected to be used in future research studies.
Results
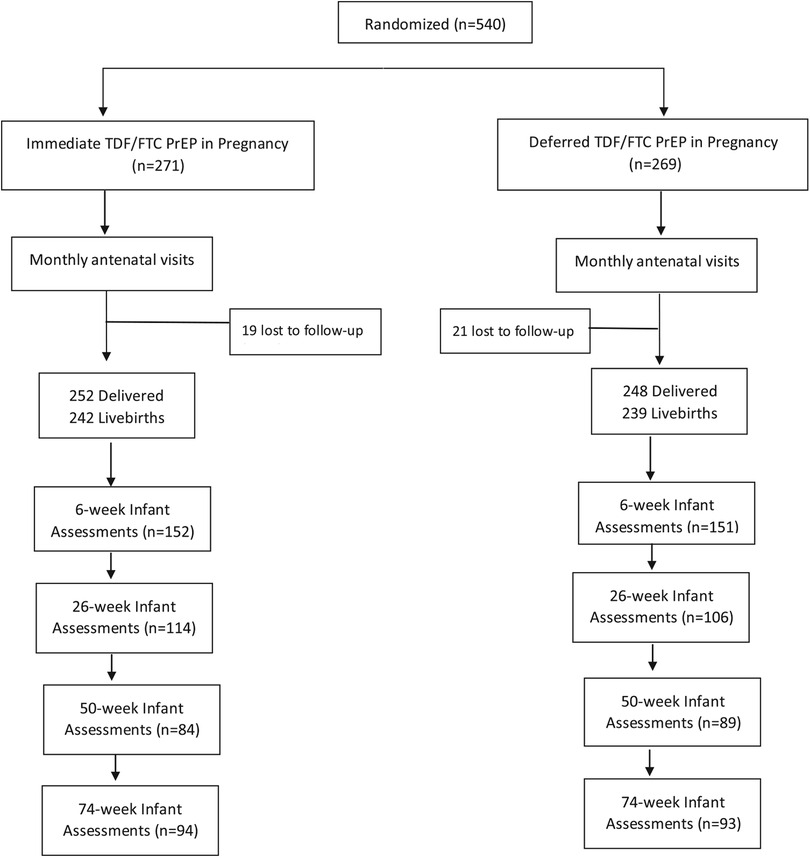
A total of 455 mother-infant pairs were included in the secondary analysis, 228 in the Immediate PrEP arm and 227 in the Deferred PrEP arm. Overall, 303 infants were assessed at 6 weeks, 220 infants at 26 weeks, 173 infants at 50 weeks, and 187 infants at 74 weeks (Figure 1). Among 228 women randomized to the Immediate PrEP arm, the mean TFV-DP level during pregnancy was 310.3 fmol/punch ranging from undetectable to 946.7 fmol/punch. Among the 228 women, 99 (43.4%) women had TFV-DP levels ranging from undetectable to 200 fmol/punch indicating one or less dose/week (<15% adherence); 61 (26.8%) women had TFV-DP levels between 200 and 500 fmol/punch (two to 4 doses/week) and 68 with >500 fmol/punch (five or more doses/week). Overall, we compared the growth metrics of 68 infants exposed to TFV-DP levels >500 fmol/punch with 160 infants exposed to less than 500 fmol/punch and 227 infants in the Deferred PrEP arm.
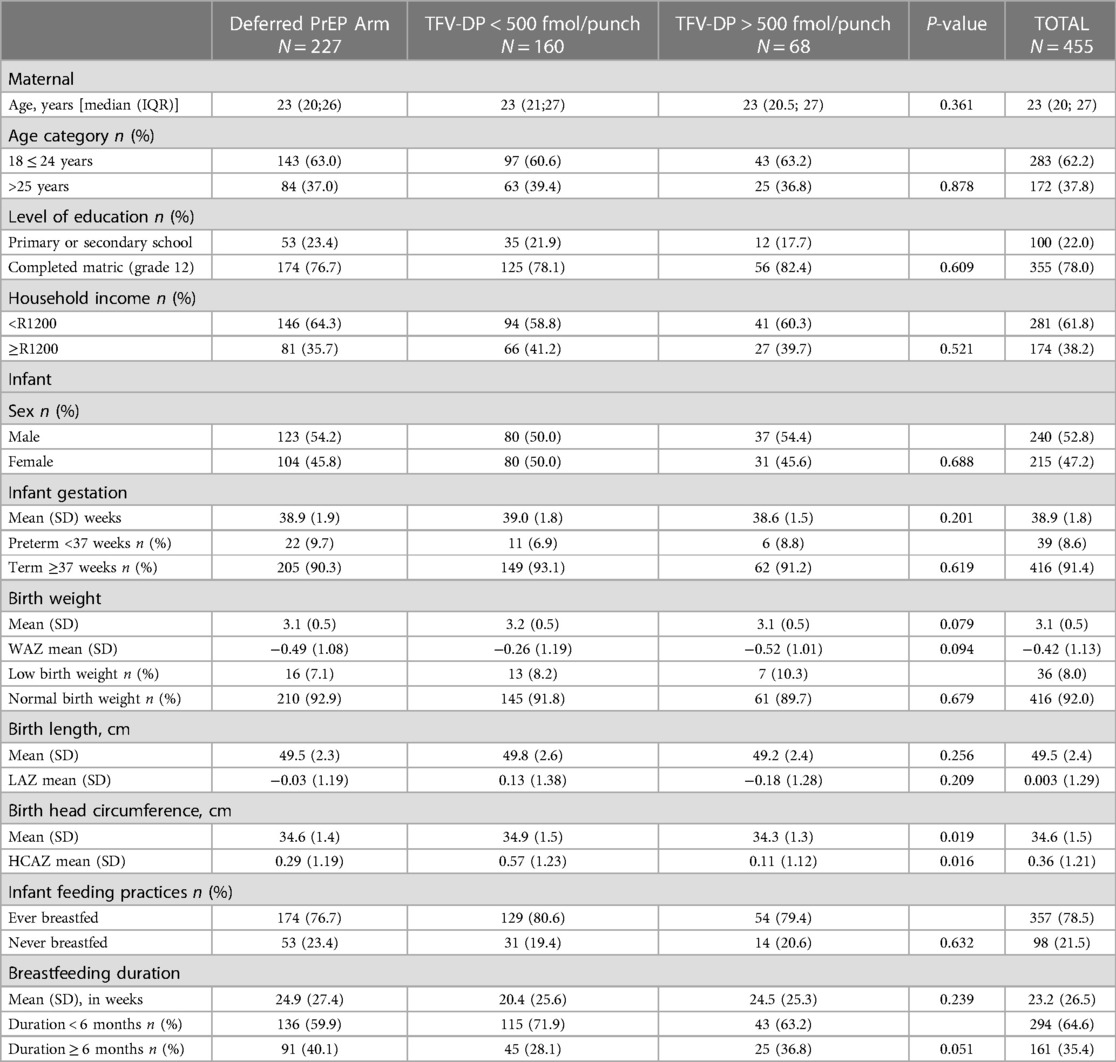
The median age (IQR) of women included in this secondary analysis was 23 (20; 27) with 62% between 18 and 25 years of age. More than half of the women (61.8%) lived in low-income households reporting a monthly income of less than R1200. Baseline sociodemographic characteristics were comparable between women randomized to the deferred PrEP arm, and women randomized to the Immediate PrEP arm with TFV-DP <500 fmol/punch and TFV-DP >500 fmol/punch (Table 1). 91.4% of infants were born at term (>37 weeks) and 92% of normal birth weight (>2,500 g). Birth anthropometric measures were comparable between groups except for the lowest mean HCAZ score (0.11) reported for infants born to women in the TFV-DP >500 fmol/punch group. The vast majority (78.5%) of infants had initiated breastfeeding, however, the mean duration of breastfeeding was relatively short (23 weeks) with the majority (64.6%) breastfed for less than 6 months.
Age-stratified mean WAZ, LAZ, WLZ, and HCAZ scores were comparable between infants born to women randomized to the Deferred PrEP arm and infants exposed to maternal TFV-DP <500 and >500 fmol/punch (Table 2). Mean LAZ scores were consistently low across all groups from 6 weeks, PrEP Unexposed (−1.06), TFV-DP <500 (−0.89) and TFV-DP > 500 (−1.12) through to 74 weeks, PrEP Unexposed (−0.82), TFV-DP <500 (−0.76) and TFV-DP > 500 (−1.03) (Table 2).
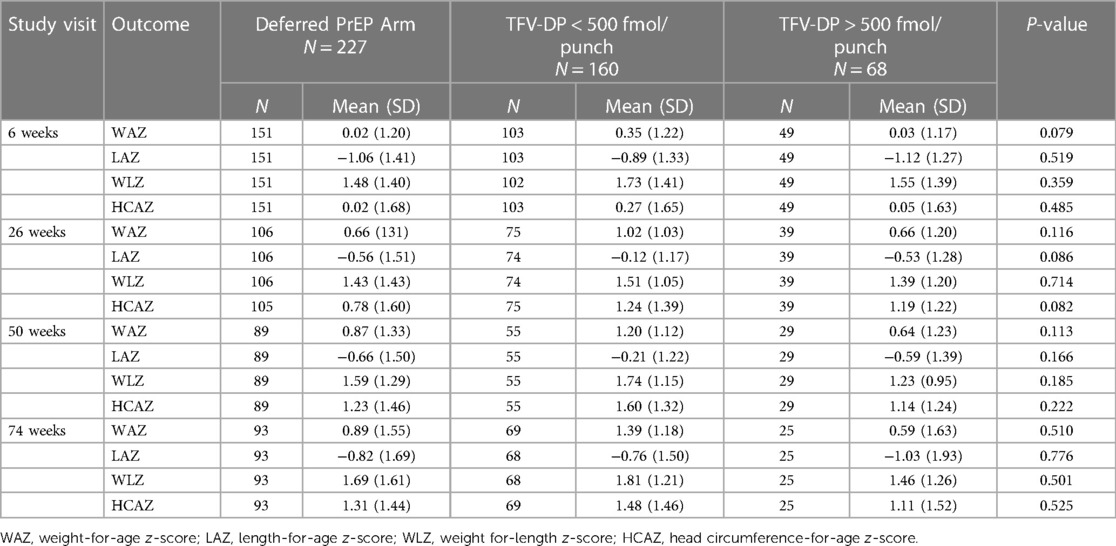
Table 2. Age stratified growth metrics in PrEP unexposed infants vs. Exposed to in-utero TFV-DP Concentration <500 fmol/punch and >500 fmol/punch.
In a mixed-effects linear regression model adjusting for maternal age, BMI, socioeconomic and newborn characteristics, in-utero exposure to high TFV-DP levels (>500 fmol/punch) was not associated with WAZ [β=−0.52 (95%CI −1.10 to 0.06)], LAZ [β=−0.46 (95%CI −1.10 to 0.18)], WLZ [β = −0.43 (95%CI −1.05 to 0.18)] and HCAZ [β = −0.11 (−0.76 to 0.55) scores over time (Table 3).
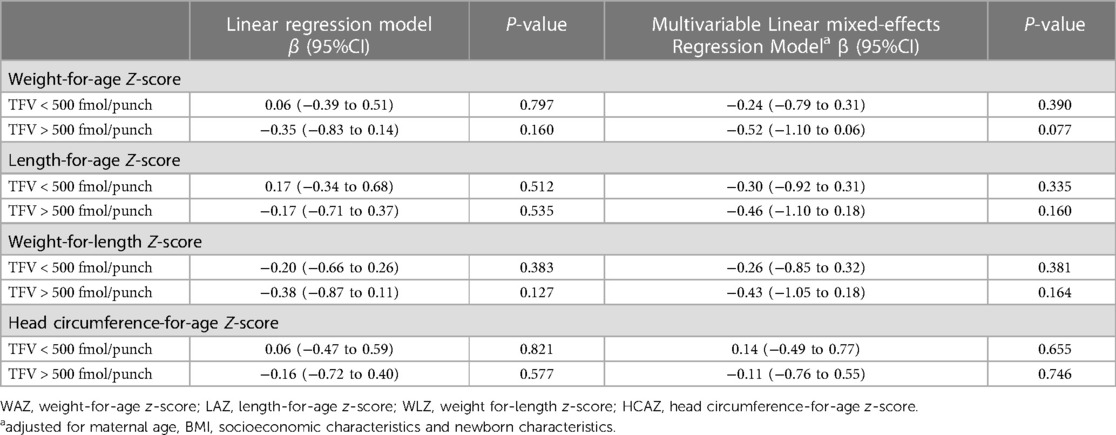
Table 3. In-utero exposure to TFV-DP concentration as predictors for WAZ, LAZ, WLZ and HCAZ scores in HIV unexposed infants.
Between 6 and 50 weeks, mean LAZ, WAZ, WLZ and HCAZ scores were lowest with in-utero exposure of TFV-DP >500 fmol/punch while there was much overlap in the LAZ, WAZ and WLZ profiles for the PrEP unexposed and TNF-DP <500 fmol/punch exposed infants in the first 26 weeks of life (Figure 2). Beyond 50 weeks, while other growth metrics showed an upward trend, only the mean LAZ score decreased for the PrEP unexposed group to levels below that of the TFV exposed groups.
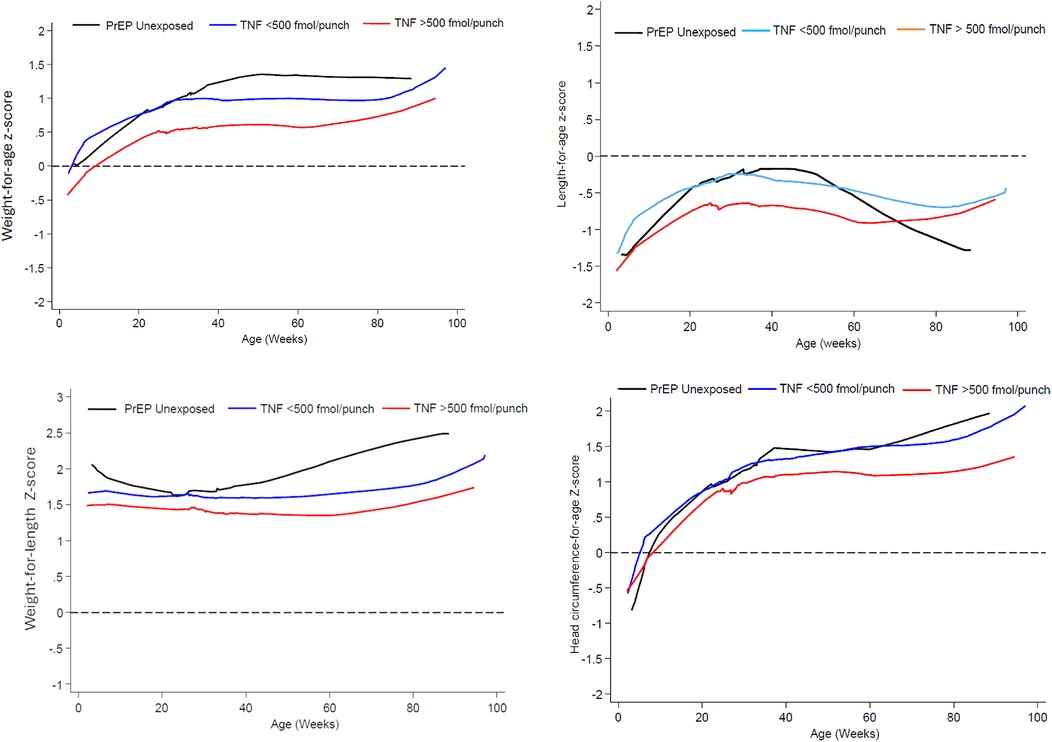
Figure 2. Mean weight-for-age, length-for-age, weight-for-length and head circumference-for-age Z-score from 6 t0 74 weeks by prEP exposure and in-utero TFV-DP concentration exposure.
Discussion
In a post hoc analysis of a randomized non-inferiority PrEP in pregnancy clinical trial, in-utero exposure to high concentrations of TFV-DP, as opposed to lower TFV-DP concentrations or no-exposure to TDF-PrEP, was not associated with adverse growth trajectories in HIV-unexposed breastfed infants in the first 18 months of life. WAZ, LAZ, WLZ and HCAZ scores were lowest among infants exposed to high concentration of TFV-DP but not significantly lower than the other study groups.
Suboptimal adherence to daily oral PrEP in pregnancy and postpartum as demonstrated through TFV-DP concentrations in maternal blood has been previously reported (22, 23) and our study confirms the suboptimal adherence using an objective assessment. Our findings of the lack of an association between growth measures and in-utero TDF-based PrEP exposure are consistent with other TDF-based PrEP safety studies, although few studies explored the effect of high in-utero exposure to TFVDP on growth anthropometrics. Although studies such as the PrEP Implementation for Mothers in Antenatal Care (PrIMA) intend to report TFV-DP levels as an objective method of assessing adherence, recently released growth monitoring beyond 12 months did not include findings that were dependent on an objective assessment of adherence (17). At 24 months, there was no difference in mean weight, mean height, frequency of underweight, and stunting between children with and without prenatal PrEP exposure (18). Growth measures remained comparable at 30 months. In the Partners Demonstration Project, in sensitivity analysis restricted to infants whose mothers had tenofovir detected there was no evidence that 17 infants born to women with detectable TFV-DP levels had poorer growth profiles by 12 months of age when compared to the 49 PrEP unexposed infants (19). In the PrEP Implementation in Young Women and Adolescents (PrIYA) program that delivered PrEP to pregnant and postpartum women integrated within routine maternal and child health clinics in Kenya, early growth monitoring revealed that WAZ, WLZ and LAZ scores did not differ between PrEP exposed and unexposed infants at 6 weeks of age (20). This is probably one of the larger cohorts (206 PrEP exposed and 1,324 PrEP unexposed), but the short time frame for growth monitoring and the lack of an objective assessment of true in-utero exposure to TFV-DP renders these findings inconclusive.
Mean LAZ scores in our study were consistently lower than zero at all visits between 6 weeks and 18 months but not associated with TDF exposure. While the LAZ scores were lowest for the high-TFV-DP exposed group, it appeared to normalize after 12 months of age. On the other hand, infants not exposed to PrEP had higher LAZ scores in the first year of life and subsequently with decreased scores between 12 and 18 months of life. Lower LAZ scores and a higher prevalence of stunting among HIV exposed uninfected (HEU) infants in comparison to HIV unexposed uninfected (HUU) infants have previously been reported (13). These studies have implicated the shorter breastfeeding duration among HEU infants in poor linear growth. In our study, HUU infants in the non-PrEP arm were breastfed for longer however none had breastfed for longer than 12 months and cessation of breastfeeding before 12 months may be related to decreased linear growth after 12 months.
WAZ scores were lowest for infants exposed to higher TFV-DP concentrations and unlike other growth metrics in our study that improved over time, the WAZ score remained lower throughout the study follow-up period until 18 months. Further follow-up of growth parameters in infants beyond 18 months would be essential and the PrIMA-x study could provide valuable data after considering true in-utero TFV-DP exposure (18). Our study was not without limitations. The retention rate was low with many missed follow-up visits and early study terminations often seen as a result of internal migration common amongst women in the postpartum period. Furthermore, the study follow-up period overlapped with the COVID-19 pandemic affecting all clinical trial-related activities and operations. As such there were far fewer infants than anticipated that contributed to the data analysis.
Conclusion
Although our findings are consistent with other PrEP studies, we provide additional evidence of a lack of association between growth restriction and in-utero TDF exposure using an objective assessment of PrEP exposure. While the small sample size makes interpretation of these results difficult, limiting its generalisability to other settings, it nevertheless provides important reassuring safety data on growth outcomes of infants exposed to maternal TDF-based PrEP.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of KwaZulu Natal Biomedical Research Ethics Committee. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MN: Conceptualization, Writing – original draft, Data curation, Investigation, Methodology, Writing – review & editing. KN: Conceptualization, Writing – original draft, Writing – review & editing, Supervision. CL: Data curation, Formal Analysis, Writing – review & editing. AD: Data curation, Investigation, Methodology, Writing – review & editing. RC: Resources, Writing – review & editing. JR: Investigation, Resources, Writing – review & editing. GG: Investigation, Resources, Writing – review & editing. DM: Conceptualization, Funding acquisition, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study was funded by Gilead Sciences Inc (Grant Number CO-EU-276-4399) and the South African Medical Research Council (Grant Number 2753/CO324/AP/2020). Study drugs were supplied by Gilead Sciences Inc. The funder (Gilead Sciences Inc.) was not involved in the study design, collection, analysis and interpretation of data, writing of this article or the decision to submit it for publication.
Acknowledgments
We thank the participants who volunteered for this trial; the staff at Umlazi Clinical Research Site and the CAPRISA Data Management Team.
Conflict of interest
JR and RC are employed by Gilead Sciences.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1447173/full#supplementary-material
References
1. Thomson KA, Baeten JM, Mugo NR, Bekker LG, Celum CL, Heffron R. Tenofovir-based oral preexposure prophylaxis prevents HIV infection among women. Curr Opin HIV AIDS. (2016) 11(1):18–26. doi: 10.1097/COH.0000000000000207
2. Kinuthia J, Pintye J, Abuna F, Mugwanya KK, Lagat H, Onyango D, et al. PrEP implementation for young women and adolescents (PrIYA) programme. Pre-exposure prophylaxis uptake and early continuation among pregnant and post-partum women within maternal and child health clinics in Kenya: results from an implementation programme. Lancet HIV. (2020) 7(1):e38–48. doi: 10.1016/S2352-3018(19)30335-2
3. Joseph Davey DL, Bekker LG, Gorbach PM, Coates TJ, Myer L. Delivering preexposure prophylaxis to pregnant and breastfeeding women in Sub-Saharan Africa: the implementation science frontier. AIDS. (2017) 31(16):2193–7. doi: 10.1097/QAD.0000000000001604
4. WHO Technical brief. Preventing HIV During Pregnancy and Breastfeeding in the Context of pre-Exposure Prophylaxis (PrEP). Geneva: World Health Organization (2017).
5. Best BM, Burchett S, Li H, Stek A, Hu C, Wang J, et al. Pharmacokinetics of tenofovir during pregnancy and postpartum. HIV Med. (2015) 16(8):502–11. doi: 10.1111/hiv.12252
6. Heffron R, Mugo N, Hong T, Celum C, Marzinke MA, Ngure K, et al. Pregnancy outcomes and infant growth among babies with in-utero exposure to tenofovir-based preexposure prophylaxis for HIV prevention. AIDS. (2018) 32(12):1707–13. doi: 10.1097/QAD.0000000000001867
7. Moodley D, Lombard C, Govender V, Naidoo M, Desmond AC, Naidoo K, et al. Pregnancy and neonatal safety outcomes of timing of initiation of daily oral tenofovir disoproxil fumarate and emtricitabine pre-exposure prophylaxis for HIV prevention (CAP016): an open-label, randomised, non-inferiority trial. Lancet HIV. (2023) 10(3):e154–63. doi: 10.1016/S2352-3018(22)00369-1
8. Joseph Davey DL, Nyemba DC, Mvududu R, Mashele N, Johnson L, Bekker LG, et al. Pregnancy outcomes following self-reported and objective-measured exposure to oral preexposure prophylaxis in South Africa. AIDS. (2024) 38(1):75–83. doi: 10.1097/QAD.0000000000003729
9. Baranek B, Wang S, Cheung AM, Mishra S, Tan DH. The effect of tenofovir disoproxil fumarate on bone mineral density: a systematic review and meta-analysis. Antivir Ther. (2020) 25(1):21–32. doi: 10.3851/IMP3346
10. Siberry GK, Jacobson DL, Kalkwarf HJ, Wu JW, DiMeglio LA, Yogev R, et al. Lower newborn bone mineral content associated with maternal use of tenofovir disoproxil fumarate during pregnancy. Clin Infect Dis. (2015) 61(6):996–1003. doi: 10.1093/cid/civ437
11. Vhembo T, Baltrusaitis K, Tierney C, Owor M, Dadabhai S, Violari A, et al. IMPAACT P1084s study team. Bone and renal health in infants with or without breastmilk exposure to tenofovir-based maternal antiretroviral treatment in the PROMISE randomized trial. J Acquir Immune Defic Syndr. (2023) 93(5):431–7. doi: 10.1097/QAI.0000000000003218
12. Le Roux MS, Jao J, Brittain K, Phillips TK, Olatunbosun S, Ronan A, et al. Tenofovir exposure in utero and linear growth in HIV-exposed, uninfected infants. AIDS. (2017) 31(1):97–104. doi: 10.1097/QAD.0000000000001302
13. Neary J, Langat A, Singa B, Kinuthia J, Itindi J, Nyaboe E, et al. Higher prevalence of stunting and poor growth outcomes in HIV-exposed uninfected than HIV-unexposed infants in Kenya. AIDS. (2022) 36(4):605–10. doi: 10.1097/QAD.0000000000003124
14. Ramokolo V, Kuhn L, Lombard C, Jackson D, Goga AE. Impact of antenatal antiretroviral drug exposure on the growth of children who are HIV-exposed uninfected: the national South African prevention of mother to child evaluation cohort study. BMC Infect Dis. (2022) 22(1):908. doi: 10.1186/s12879-022-07847-9
15. Szanyi J, Walles JK, Tesfaye F, Gudeta AN, Björkman P. Intrauterine HIV exposure is associated with linear growth restriction among Ethiopian children in the first 18 months of life. Trop Med Int Health. (2022) 27(9):823–30. doi: 10.1111/tmi.13805
16. Powis KM, Smeaton L, Hughes MD, Tumbare EA, Souda S, Jao J, et al. In-utero triple antiretroviral exposure associated with decreased growth among HIV-exposed uninfected infants in Botswana. AIDS. (2016) 30(2):211–20. doi: 10.1097/QAD.0000000000000895
17. Gomez L, Pintye J, Stern J, Ngumbau N, Ochieng B, Marwa M, et al. No association between prenatal PrEP exposure and adverse growth outcomes among Kenyan infants: a prospective study. [Abstract]. In: IAS 2021. Berlin, Germany: International AIDS Society (2021). p. 248–9.
18. Gomez L, Kinuthia J, Larsen A, Stern J, Dettinger J, Marwa M, et al. Association of prenatal PrEP exposure with neurodevelopmental and growth outcomes beyond 24 months among Kenyan children. [Abstract]. In: AIDS 2022. Montreal, Canada: International AIDS Society (2022). https://programme.aids2022.org/Abstract/Abstract/?abstractid=11814 (Accessed July 19, 2024).
19. Heffron R, Mugo N, Hong T, Celum C, Marzinke MA, Ngure K, et al. Partners demonstration project and the partners PrEP study teams. Pregnancy outcomes and infant growth among babies with in-utero exposure to tenofovir-based preexposure prophylaxis for HIV prevention. AIDS. (2018) 32(12):1707–13. doi: 10.1097/QAD.0000000000001867
20. Dettinger JC, Kinuthia J, Pintye J, Abuna F, Begnel E, Mugwanya K, et al. Perinatal outcomes following maternal pre-exposure prophylaxis (PrEP) use during pregnancy: results from a large PrEP implementation program in Kenya. J Int AIDS Soc. (2019) 22(9):e25378. doi: 10.1002/jia2.25378
21. Joseph Davey D, Nyemba DC, Castillo-Mancilla J, Wiesner L, Norman J, Mvududu R, et al. Adherence challenges with daily oral pre-exposure prophylaxis during pregnancy and the postpartum period in South African women: a cohort study. J Int AIDS Soc. (2022) 25(12):e26044. doi: 10.1002/jia2.26044
22. Young AM, Saidi F, Phanga T, Tseka J, Bula A, Mmodzi P, et al. Male partners’ support and influence on pregnant women’s oral PrEP use and adherence in Malawi. Front Reprod Health. (2023) 5:1206075. doi: 10.3389/frph.2023.1206075
23. Pintye J, Kinuthia J, Abuna F, Mugwanya K, Lagat H, Dettinger JC, et al. Frequency and predictors of tenofovir-diphosphate detection among young Kenyan women in a real-world pre-exposure prophylaxis implementation program. Clin Infect Dis. (2020) 71(9):e509–12. doi: 10.1093/cid/ciaa181
24. Stranix-Chibanda L, Anderson PL, Kacanek D, Hosek S, Huang S, Nematadzira TG, et al. tenofovir diphosphate concentrations in dried blood spots from pregnant and postpartum adolescent and young women receiving daily observed pre-exposure prophylaxis in Sub-Saharan Africa. Clin Infect Dis. (2021) 73(7):e1893–900. doi: 10.1093/cid/ciaa1872
Keywords: in-utero, exposure, preexposure prophylaxis (PrEP), growth, breastfeeding
Citation: Naidoo M, Naidoo KL, Lombard C, Desmond AC, Clark R, Rooney JF, Gray G and Moodley D (2024) In-utero exposure to tenofovir disoproxil fumarate pre-exposure prophylaxis and growth metrics in HIV unexposed breastfed infants in South Africa: a post hoc analysis of the CAP 016 PrEP in pregnancy RCT. Front. Pediatr. 12:1447173. doi: 10.3389/fped.2024.1447173
Received: 11 June 2024; Accepted: 16 July 2024;
Published: 9 August 2024.
Edited by:
Eline Tommelein, Vrije University Brussels, BelgiumReviewed by:
Devasena Gnanashanmugam, Cepheid, United StatesElizabeth Egieyeh, University of the Western Cape, South Africa
© 2024 Naidoo, Naidoo, Lombard, Desmond, Clark, Rooney, Gray and Moodley. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dhayendre Moodley, bW9vZGxleWQxQHVrem4uYWMuemE=
 Megeshinee Naidoo
Megeshinee Naidoo Kimesh L. Naidoo
Kimesh L. Naidoo Carl Lombard
Carl Lombard Alicia C. Desmond1
Alicia C. Desmond1 Dhayendre Moodley
Dhayendre Moodley