- 1Department of Pediatrics, University Children’s Hospital, Bonn, Germany
- 2Global Medical Affairs, Ipsen Pharma GmBH, Munich, Germany
- 3Department of Cardiology, Pediatric Heart Center, University Hospital of Bonn, Bonn, Germany
Liver-related abnormalities are commonly observed in patients with congenital heart disease, and these may lead to secondary manifestations such as pruritus. Odevixibat is an ileal bile acid transporter inhibitor under investigation for the treatment of cholestatic liver diseases. Here, we describe the effects of odevixibat treatment in a pediatric patient with congenital heart disease and severe cholestatic pruritus. A 2-year-old male with Kleefstra syndrome, hypoplastic left heart syndrome, and a history of Giessen procedure and biventricular correction surgery presented to the pediatric cardiology and hepatology outpatient clinics at University Children's Hospital Bonn. Portal hypertension was evident on imaging, and the patient was experiencing severe itching attacks that did not respond to treatment with naltrexone, ursodeoxycholic acid, dimetindene, or rifampicin. Sleep and quality of life were poor. Treatment with odevixibat was initiated off label due to refractory pruritus and elevated serum bile acids. Improvements in pruritus and sleep occurred rapidly with odevixibat and were sustained for the duration of treatment. The patient's serum bile acids decreased from 111 μmol/L before treatment with odevixibat to 24 μmol/L within 1 month of initiating therapy. Relief from pruritus had positive effects on psychomotor development and quality of life. Mild diarrhea lasting 2 days was reported by the patient's mother. In this case report, odevixibat was effective and well tolerated. Together with those of previous studies in patients with progressive familial intrahepatic cholestasis and Alagille syndrome, these results suggest that odevixibat warrants further study as a potential treatment option for patients with cholestatic pruritus of diverse etiologies.
1 Introduction
It is well established that patients with congenital heart disease can develop liver abnormalities and secondary hepatic disease, known as cardiac hepatopathy, as a result of passive venous congestion and/or reduced cardiac output, both in those who have undergone Fontan palliative surgery and in those who have not (1–7). For example, among patients with hypoplastic left heart syndrome [HLHS; a spectrum of cardiac malformations characterized by underdevelopment of the left heart and aorta (8)], a retrospective autopsy study found that 43% (18/42) also had hepatic necrosis (9).
Signs of cardiac hepatopathy can include cholestasis, fibrosis, and hepatocellular carcinoma (1, 10). Cholestasis is a condition of impaired bile flow commonly associated with pruritus, jaundice, and elevated levels of serum bile acids and liver enzymes (11, 12). Although there are some reports of pruritus in patients with heart failure (13, 14), little is known about the prevalence or treatment of cholestatic pruritus in patients with cardiac hepatopathy.
Odevixibat is a potent, selective inhibitor of the ileal bile acid transporter (IBAT). In phase 3 studies in patients with cholestatic liver disease (ie, progressive familial intrahepatic cholestasis (PFIC) and Alagille syndrome) and pruritus, odevixibat treatment significantly reduced symptoms of pruritus and serum bile acid levels (15–17). Based in part on data from these studies, odevixibat was approved by the European Medicines Agency for treatment of PFIC (18) and for treatment of cholestatic pruritus in Alagille syndrome (19) and by the United States Food and Drug Administration for treatment of pruritus in patients with PFIC and Alagille syndrome (20).
Here, we describe the effects of odevixibat treatment in a pediatric patient with HLHS and severe cholestatic pruritus. This patient was also diagnosed with Kleefstra syndrome, a rare genetic disorder associated with intellectual disability, hypotonia, distinct facial features, and occasionally, heart defects, including HLHS (21, 22).
2 Case report
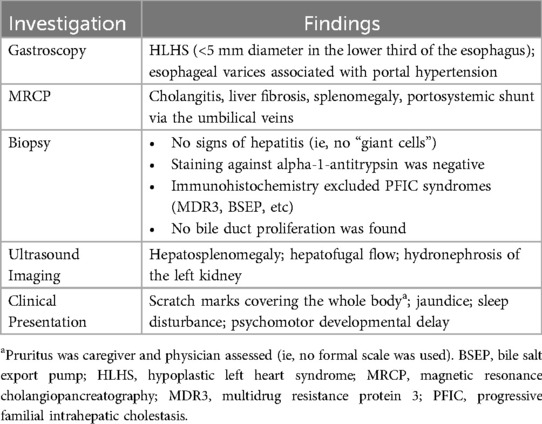
In September 2021, a 2-year-old male with a diagnosis of HLHS presented to the pediatric cardiology outpatient clinic and subsequently to the pediatric hepatology outpatient clinic at University Children's Hospital Bonn for a second opinion on treatment options. The patient had been born in July 2019 at 36 weeks and 6 days of gestation, and serial echocardiograms in the week following birth revealed mitral and aortic stenosis and a hypoplastic aortic arch with ductus-dependent system perfusion. The patient underwent the Giessen hybrid procedure 5 days postnatally, followed by biventricular corrective surgery at 4 months of age. The patient's postoperative course was complicated by development of kidney failure and cholestasis. Magnetic resonance cholangiopancreatography showed cholangitis, liver fibrosis, significant splenomegaly, and portosystemic shunt via the umbilical veins, suggesting portal hypertension. Follow-up gastroscopy and ultrasound revealed 2nd-degree esophageal varices, which confirmed the diagnosis of portal hypertension, and abdominal ultrasound showed hepatofugal flow and hepatosplenomegaly (Table 1). There were no clinical or histological signs indicating typical cholestatic liver diseases such as PFIC or Alagille syndrome. The patient was also diagnosed with Kleefstra syndrome following a genetic test that identified a large deletion on chromosome 9q34.3. In addition to the genetic analysis for Kleefstra syndrome, a next-generation sequencing analysis was performed to exclude genetic nephrologic disease; however, no analysis of genes related to cholestasis was performed.
At the time of presentation to University Children's Hospital Bonn, the patient was in good general condition. He showed no signs of heart failure in everyday life, with oxygen saturation values at 100% and no evidence of tachypnea or increased sweating. The patient's mother reported that he was experiencing severe itching attacks (ie, pruritus), and scratch marks were evident all over his body upon physical examination (Table 1). The patient had to constantly wear gloves to prevent scratching and bleeding, and nighttime sleep was greatly disrupted for both the patient and his parents. Microcephaly and significant psychomotor developmental delay were also observed in the patient.
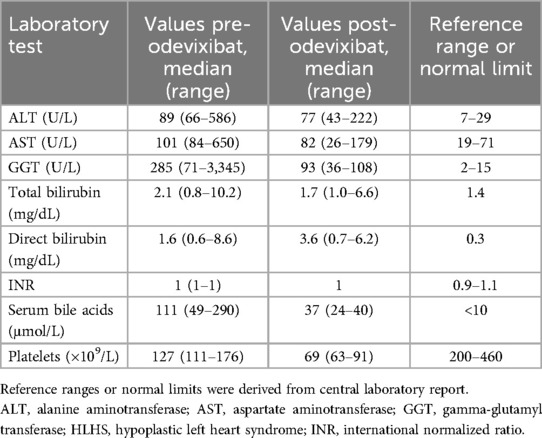
The patient continued to suffer from severe itching and impaired quality of life at 3 years of age. His pruritus was refractory to conventional treatments including naltrexone, ursodeoxycholic acid, or dimetindene oral drops. Rifampicin was also trialed, but because the medication was not well tolerated and resulted in elevations of liver transaminases, it was stopped after 2 weeks. Serum bile acid and liver function test levels were persistently elevated (ie, levels were elevated several fold vs. normal limits across multiple visits) (Table 2). Oral odevixibat 40 µg/kg/day was prescribed off label in November 2022 for ongoing pruritus and elevated serum bile acids; during odevixibat treatment, the patient also received ursodeoxycholic acid (2 × 150 mg).
Within 2 days of initiating odevixibat, marked improvements in pruritus and sleep were observed that were maintained over 6 months. The patient's pruritus was primarily observed by his parents and also by the clinician during physical exam. The patient was no longer scratching or bleeding and did not require gloves; subsequently, the patient's fine motor skills and psychomotor development progressed rapidly. He began to crawl and sleep through the night for the first time since infancy. Overall, the patient's mother reported his quality of life was greatly improved; he was happy, laughing, and better able to focus on learning and playing (Figure 1).
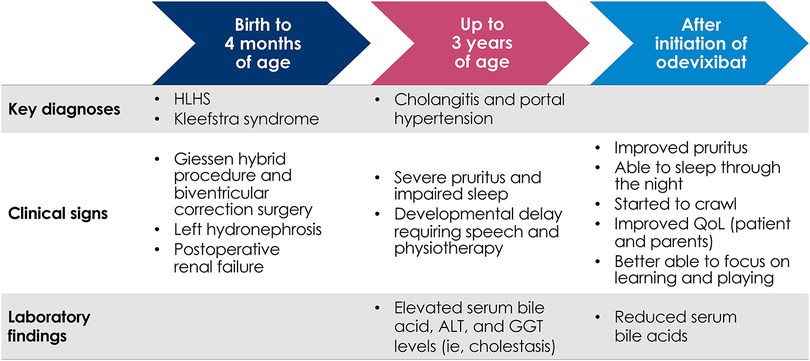
Figure 1. Flow diagram of key clinical milestones for patient with HLHS and severe cholestatic pruritus treated with odevixibat. ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase; HLHS, hypoplastic left heart syndrome; QoL, quality of life.
Serum bile acids decreased from a pretreatment level of 111 μmol/L to 24 μmol/L within 1 month of initiating odevixibat; this reduction in serum bile acids was maintained at the last available assessment in April 2023 (36 μmol/L) (Figure 2). Total bilirubin and alanine aminotransferase (ALT) levels generally remained stable for the first 3 months after odevixibat initiation and then increased. The increase in ALT was transient; at the last available assessment in May 2023, the patient's ALT was lower than pre-odevixibat levels. The last available total bilirubin value in May 2023 was approximately 1.6-fold higher than the upper limit of normal; however, a specific clinical etiology for this perturbation was not apparent, and longer follow-up is not available as the patient passed away during a subsequent heart surgery.
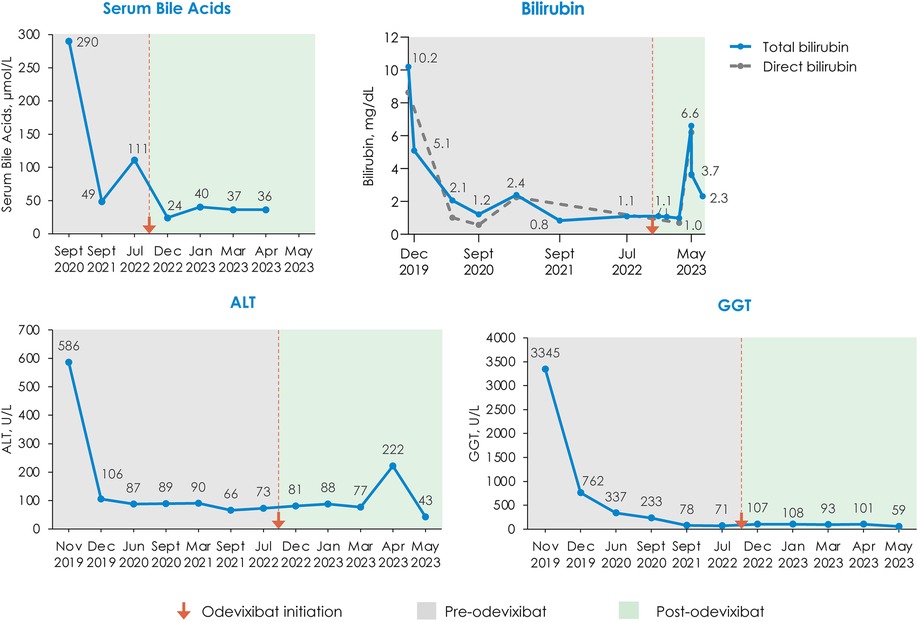
Figure 2. Changes in serum bile acids, bilirubin, ALT, and GGT with odevixibat treatment. All available laboratory values on a per-month basis are plotted. In May 2023, three laboratory assessments were taken within 4 days of each other; the last available value is plotted for ALT and GGT; for total bilirubin, all 3 May 2023 laboratory assessments are plotted. The bilirubin panel shows total bilirubin values with the solid line and direct bilirubin values with the dashed line; values for total bilirubin are labeled. ALT, alanine aminotransferase; GGT, gamma-glutamyl transferase.
Other than mild diarrhea lasting 2 days (reported by the patient's mother) post-odevixibat initiation, odevixibat was well tolerated in the patient.
A lay summary of the case details presented here can be found in the Supplementary Appendix.
3 Discussion
This case report is the first to demonstrate the sustained effectiveness of the IBAT inhibitor odevixibat in treating pruritus in a child with cardiac hepatopathy. While cardiac hepatopathy usually occurs many years after a Fontan procedure, the liver in patients with congenital heart disease may experience damage from the time of birth, and relevant liver fibrosis may be present in younger years (6, 7, 23–25). The patient described here with HLHS experienced chronic itching that led to significant impairments in sleep and quality of life. Following failure of other antipruritic agents, treatment with odevixibat rapidly and robustly improved the patient's pruritus, sleep, and quality of life and lowered serum bile acid levels.
This case also highlights the substantial burden of disease associated with chronic pruritus. Consistent with observations in patients with PFIC and Alagille syndrome (26–30), pruritus was among the most debilitating symptoms experienced by this patient and directly contributed to poor quality of life in both the patient and his family. The pruritus reductions with odevixibat were accompanied by improvements in the patient's mood, attention, and motor coordination (eg, ability to crawl and play).
Odevixibat was well tolerated in this patient, with diarrhea being the only side effect reported by the patient's mother. The diarrhea event was mild, transient, and consistent with results observed in previous studies of IBAT inhibitors [ie, odevixibat or maralixibat] (15, 17, 31, 32). Following initiation of odevixibat, there were some fluctuations in the patient's ALT and bilirubin values, which is consistent with previously reported findings from a phase 3 trial of odevixibat in patients with PFIC (17). In that study, while some patients generally had improvements in hepatic laboratory values with odevixibat, some mean values remained elevated (17).
In summary, odevixibat had rapid and sustained effects on itching, sleep, and serum bile acids in a patient with congenital heart disease and severe cholestatic pruritus. Together with those of previous studies in patients with PFIC and Alagille syndrome, these results suggest that odevixibat warrants further study as a potential treatment option for patients with cholestatic pruritus of diverse etiologies.
Data availability statement
The datasets presented in this article are not readily available to preserve the individual's privacy. Reasonable requests to access the datasets should be directed to the corresponding author.
Ethics statement
Written informed consent from the patient's parents was obtained prior to initiating any treatment, and this, as well as the expanded access program protocol, were submitted to and approved by an Independent Ethics Committee. In addition, informed consent was obtained from the parents of the patient for publication of the case details presented here.
Author contributions
RG: Conceptualization, Investigation, Writing – original draft, Writing – review & editing. CM: Conceptualization, Writing – original draft, Writing – review & editing. PR: Conceptualization, Writing – original draft, Writing – review & editing. MS: Conceptualization, Investigation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Editorial and medical writing support was provided by Peloton Advantage, an OPEN Health company, and was funded by Albireo Pharma, Inc., an Ipsen company.
Acknowledgments
We thank the patient's family, who contributed to this case report.
Conflicts of interest
RG is a consultant to Albireo Pharma, an Ipsen company, and Mirum. CM was previously employed by Albireo Pharma, an Ipsen company. PR is a current employee of Ipsen.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction Note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1443338/full#supplementary-material
Abbreviations
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BSEP, bile salt export pump; GGT, gamma-glutamyl transferase; HLHS, hypoplastic left heart syndrome; IBAT, ileal bile acid transporter; INR, international normalized ratio; MRCP, magnetic resonance cholangiopancreatography; MDR3, multidrug resistance protein 3; PFIC, progressive familial intrahepatic cholestasis; QoL, quality of life.
References
1. Komatsu H, Inui A, Kishiki K, Kawai H, Yoshio S, Osawa Y, et al. Liver disease secondary to congenital heart disease in children. Expert Rev Gastroenterol Hepatol. (2019) 13(7):651–66. doi: 10.1080/17474124.2019.1621746
2. Cromme-Dijkhuis AH, Hess J, Hählen K, Henkens CM, Bink-Boelkens MT, Eygelaar AA, et al. Specific sequelae after fontan operation at mid- and long-term follow-up. Arrhythmia, liver dysfunction, and coagulation disorders. J Thorac Cardiovasc Surg. (1993) 106(6):1126–32. doi: 10.1016/S0022-5223(19)33989-3
3. Kaulitz R, Luhmer I, Bergmann F, Rodeck B, Hausdorf G. Sequelae after modified Fontan operation: postoperative haemodynamic data and organ function. Heart. (1997) 78(2):154–9. doi: 10.1136/hrt.78.2.154
4. Narkewicz MR, Sondheimer HM, Ziegler JW, Otanni Y, Lorts A, Shaffer EM, et al. Hepatic dysfunction following the Fontan procedure. J Pediatr Gastroenterol Nutr. (2003) 36(3):352–7. doi: 10.1097/00005176-200303000-00009
5. van Nieuwenhuizen RC, Peters M, Lubbers LJ, Trip MD, Tijssen JG, Mulder BJ. Abnormalities in liver function and coagulation profile following the Fontan procedure. Heart. (1999) 82(1):40–6. doi: 10.1136/hrt.82.1.40
6. Schwartz MC, Sullivan L, Cohen MS, Russo P, John AS, Guo R, et al. Hepatic pathology may develop before the Fontan operation in children with functional single ventricle: an autopsy study. J Thorac Cardiovasc Surg. (2012) 143(4):904–9. doi: 10.1016/j.jtcvs.2011.08.038
7. Schwartz MC, Glatz AC, Daniels K, Goldberg DJ, Rand E, Epelman MS, et al. Hepatic abnormalities are present before and early after the Fontan operation. Ann Thorac Surg. (2015) 100(6):2298–304. doi: 10.1016/j.athoracsur.2015.06.071
8. Tchervenkov CI, Jacobs JP, Weinberg PM, Aiello VD, Béland MJ, Colan SD, et al. The nomenclature, definition and classification of hypoplastic left heart syndrome. Cardiol Young. (2006) 16(4):339–68. doi: 10.1017/S1047951106000291
9. Weinberg AG, Bolande RP. The liver in congenital heart disease. Effects of infantile coarctation of the aorta and the hypoplastic left heart syndrome in infancy. Am J Dis Child. (1970) 119(5):390–4. doi: 10.1001/archpedi.1970.02100050392002
10. Myers RP, Cerini R, Sayegh R, Moreau R, Degott C, Lebrec D, et al. Cardiac hepatopathy: clinical, hemodynamic, and histologic characteristics and correlations. Hepatology. (2003) 37(2):393–400. doi: 10.1053/jhep.2003.50062
11. Feldman AG, Sokol RJ. Neonatal cholestasis: emerging molecular diagnostics and potential novel therapeutics. Nat Rev Gastroenterol Hepatol. (2019) 16(6):346–60. doi: 10.1038/s41575-019-0132-z
12. Kamath BM, Stein P, Houwen RHJ, Verkade HJ. Potential of ileal bile acid transporter inhibition as a therapeutic target in Alagille syndrome and progressive familial intrahepatic cholestasis. Liver Int. (2020) 40(8):1812–22. doi: 10.1111/liv.14553
13. Niklasson O, Boman K, Stenberg B. The prevalence and characteristics of pruritus in patients with heart failure. Br J Dermatol. (2015) 172(6):1541–6. doi: 10.1111/bjd.13682
14. Zambroski CH, Moser DK, Bhat G, Ziegler C. Impact of symptom prevalence and symptom burden on quality of life in patients with heart failure. Eur J Cardiovasc Nurs. (2005) 4(3):198–206. doi: 10.1016/j.ejcnurse.2005.03.010
15. Thompson RJ, Arnell H, Artan R, Baumann U, Calvo PL, Czubkowski P, et al. Odevixibat treatment in progressive familial intrahepatic cholestasis: a randomised, placebo-controlled, phase 3 trial. Lancet Gastroenterol Hepatol. (2022) 7(9):830–42. doi: 10.1016/S2468-1253(22)00093-0
16. Ovchinsky N, Aumar M, Baker A, Baumann U, Bufler P, Cananzi M, et al. Efficacy and safety of odevixibat in patients with Alagille syndrome (ASSERT): a phase 3, double-blind, randomised, placebo-controlled trial. Lancet Gastroenterol Hepatol. (2024) 9(7):632–45. doi: 10.1016/S2468-1253(24)00074-8
17. Thompson RJ, Artan R, Baumann U, Calvo PL, Czubkowski P, Dalgic B, et al. Interim results from an ongoing, open-label, single-arm trial of odevixibat in progressive familial intrahepatic cholestasis. JHEP Rep. (2023) 5(8):100782. doi: 10.1016/j.jhepr.2023.100782
19. Kayfanda [summary of product characteristics]. Boulogne-Billancourt, France: Ipsen Pharma (2024).
21. Willemsen MH, Vulto-van Silfhout AT, Nillesen WM, Wissink-Lindhout WM, van Bokhoven H, Philip N, et al. Update on Kleefstra syndrome. Mol Syndromol. (2012) 2(3–5):202–12. doi: 10.1159/000335648
22. Campbell CL, Collins RT 2nd, Zarate YA. Severe neonatal presentation of kleefstra syndrome in a patient with hypoplastic left heart syndrome and 9q34.3 microdeletion. Birth Defects Res A Clin Mol Teratol. (2014) 100(12):985–90. doi: 10.1002/bdra.23324
23. Gordon-Walker TT, Bove K, Veldtman G. Fontan-associated liver disease: a review. J Cardiol. (2019) 74(3):223–32. doi: 10.1016/j.jjcc.2019.02.016
24. de Lange C, Möller T, Hebelka H. Fontan-associated liver disease: diagnosis, surveillance, and management. Front Pediatr. (2023) 11:1100514. doi: 10.3389/fped.2023.1100514
25. Ghaferi AA, Hutchins GM. Progression of liver pathology in patients undergoing the Fontan procedure: chronic passive congestion, cardiac cirrhosis, hepatic adenoma, and hepatocellular carcinoma. J Thorac Cardiovasc Surg. (2005) 129(6):1348–52. doi: 10.1016/j.jtcvs.2004.10.005
26. Kamath BM, Baker A, Houwen R, Todorova L, Kerkar N. Systematic review: the epidemiology, natural history, and burden of Alagille syndrome. J Pediatr Gastroenterol Nutr. (2018) 67(2):148–56. doi: 10.1097/MPG.0000000000001958
27. Elisofon SA, Emerick KM, Sinacore JM, Alonso EM. Health status of patients with Alagille syndrome. J Pediatr Gastroenterol Nutr. (2010) 51(6):759–65. doi: 10.1097/MPG.0b013e3181ef3771
28. Kamath BM, Spino C, McLain R, Magee JC, Fredericks EM, Setchell KD, et al. Unraveling the relationship between itching, scratch scales, and biomarkers in children with Alagille syndrome. Hepatol Commun. (2020) 4(7):1012–8. doi: 10.1002/hep4.1522
29. Baker A, Kerkar N, Todorova L, Kamath BM, Houwen RHJ. Systematic review of progressive familial intrahepatic cholestasis. Clin Res Hepatol Gastroenterol. (2019) 43(1):20–36. doi: 10.1016/j.clinre.2018.07.010
30. Srivastava A. Progressive familial intrahepatic cholestasis. J Clin Exp Hepatol. (2014) 4(1):25–36. doi: 10.1016/j.jceh.2013.10.005
31. Shneider BL, Spino C, Kamath BM, Magee JC, Bass LM, Setchell KD, et al. Placebo-controlled randomized trial of an intestinal bile salt transport inhibitor for pruritus in Alagille syndrome. Hepatol Commun. (2018) 2(10):1184–98. doi: 10.1002/hep4.1244
Keywords: odevixibat, cholestasis, hypoplastic left heart syndrome, pruritus, case report
Citation: Ganschow R, Maucksch C, Rauschkolb P and Schneider MBE (2025) Odevixibat treatment in a child with hypoplastic left heart syndrome and severe cholestatic pruritus: a case report. Front. Pediatr. 12:1443338. doi: 10.3389/fped.2024.1443338
Received: 3 June 2024; Accepted: 3 December 2024;
Published: 23 January 2025;
Corrected: 22 August 2025.
Edited by:
Claudia Mandato, University of Salerno, ItalyReviewed by:
Tudor Lucian Pop, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaPatryk Lipiński, Maria Sklodowska-Curie Medical Academy, Poland
Jian-She Wang, Fudan University, China
Angela Pepe, Ospedale San Carlo, Italy
Copyright: © 2025 Ganschow, Maucksch, Rauschkolb and Schneider. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rainer Ganschow, cmFpbmVyLmdhbnNjaG93QHVrYm9ubi5kZQ==
†ORCID:
Rainer Ganschow
orcid.org/0000-0003-0588-774X
 Rainer Ganschow
Rainer Ganschow Christof Maucksch2
Christof Maucksch2