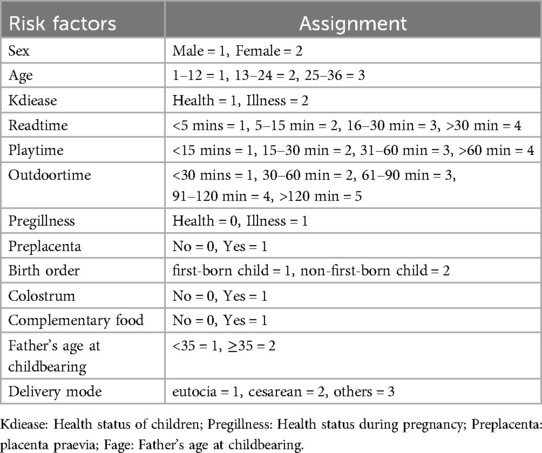
- 1Department of Medical Information, School of Public Health, Jilin University, Jilin, China
- 2Research Development, Shenzhen Health Development Research and Data Management Center, Shenzhen, Guangdong, China
- 3Department of Software Technology, School of Software Engineering, Shenzhen Institute of Information Technology, Shenzhen, Guangdong, China
- 4Department of Management Science, College of Management, Shenzhen University, Shenzhen, Guangdong, China
Background: In light of the global effort to eradicate stunting in childhood, the objective of this research endeavor was to assess the prevalence of stunting and associated factors, simultaneously construct and validate a risk prediction model for stunting among children under the age of three in Shenzhen, China.
Methods: Using the stratified random sampling method, we selected 9,581 children under the age of three for research and analysis. The dataset underwent a random allocation into training and validation sets, adhering to a 8:2 split ratio. Within the training set, a combined approach of LASSO regression analysis and binary logistic regression analysis was implemented to identify and select the predictive variables for the model. Subsequently, model construction was conducted in the training set, encompassing model evaluation, visualization, and internal validation procedures. Finally, to assess the model's generalizability, external validation was performed using the validation set.
Results: A total of 684 (7.14%) had phenotypes of stunt. Utilizing a combined approach of LASSO regression and logistic regression, key predictors of stunting among children under three years of age were identified, including sex, age in months, mother's education, father's age, birth order, feeding patterns, delivery mode, average daily parent-child reading time, average time spent in child-parent interactions, and average daily outdoor time. These variables were subsequently employed to develop a comprehensive prediction model for childhood stunting. A nomogram model was constructed based on these factors, demonstrating excellent consistency and accuracy. Calibration curves validated the agreement between the nomogram predictions and actual observations. Furthermore, ROC and DCA analyses indicated the strong predictive performance of the nomograms.
Conclusions: The developed model for forecasting stunt risk, which integrates a spectrum of variables. This analytical framework presents actionable intelligence to medical professionals, laying down a foundational framework and a pivot for the conception and execution of preemptive strategies and therapeutic interventions.
1 Introduction
Stunting is conceptualized as a protracted insufficiency of adequate nutritional intake in pediatric populations, thereby impeding linear growth trajectories and culminating in cumulative growth deficiencies (1–3). The initial 1,000 days are a pivotal phase in the early growth and development of children, often resulting in enduring adverse outcomes for those who fail to achieve their full developmental capabilities (4). The prompt identification of developmental disorders holds paramount significance for the welfare of children and their respective families.
The growth and development of children has been investigated and studied in many countries. As per the latest estimates reported in the 2017 Lancet Series, approximately 249 million children (43%) under the age of 5 in low- and middle-income countries (LMICs) faced a heightened risk of suboptimal development in 2010, attributed primarily to stunting or extreme poverty exposure, with the highest concentration observed in South Asia and sub-Saharan Africa (5). A search assessed the nutritional condition of 293 infants and toddlers aged between 0 and 24 months in the Ecuadorian highlands, discovering that stunting was present in 56.2% of the children (6). Furthermore, a comprehensive study revealed that 19.5% of Argentinean children exhibited a predisposition to neurodevelopmental disorders (7). Another study encompassed 330,613 children across 63 nations. Collectively, for all the countries studied, approximately 25% of the children were suspected of having stunt. Regionally, the prevalence spanned from 10% in Europe and Central Asia to a noteworthy 42% in West and Central Africa (8). A prospective birth cohort analysis in Shanghai, China revealed that the overall prevalence of suspected developmental delay at 2, 6, and 12 months of age was 15.6%, 15.8%, and 12.6% respectively (9). An additional investigation disclosed that 14.6% of children exhibited suboptimal scores on the Early Childhood Development Index (ECDI) in the cognitive domain. Furthermore, a positive correlation was observed between these low development scores and the presence of stunting (10). In impoverished regions of China, an alarming 39.7% of children under three years of age are at a heightened risk of developmental delays (11).
To devise effective preventative strategies, it is paramount to gain a comprehensive understanding of the risk factors associated with stunting. Iwayama's research revealed that both advancing maternal and paternal age significantly related to child development and growth in the general populace (12). A comparative analysis of matched groups indicates that being underweight and experiencing a shortened gestation period may contribute to suboptimal weight gain and impaired head growth during infancy (13). Pursuant to the World Health Organization's national assessment of suboptimal infant and young child feeding (IYCF) practices, the duration of breastfeeding is classified as highly satisfactory, whereas the early initiation of breastfeeding and exclusive breastfeeding (EBF) are deemed as satisfactory (14). Animal-sourced foods, which are abundant in essential amino acids, play a crucial role in promoting linear growth and developmental outcomes among young children residing in low- and middle-income countries (15). A research initiative undertaken between 2009 and 2012, encompassing 1,324 children within the age bracket of 0–24 months and domiciled in rural Pakistan, demonstrated that child diet and mother-child interactions improved children's cognitive, language and motor development (16). White matter hyperintensity (WMH) in patients with cerebral small vessel disease (CSVD) is strongly associated with cognitive impairment (17).
The Bayley Scales of Infant Development (BSID) has been a long-standing and extensively utilized instrument for the identification and diagnostic assessment of neurodevelopmental delays among infants. The Bayley-III assessment has attained widespread recognition as the “gold standard” in diagnostic evaluations for early childhood development (ECD) (18). However, BSID is impractical for routine screenings in low-resource settings and must be performed by a trained professional (19–21). The necessity to track the development of large cohorts of children necessitates a reliable yet cost-effective method of assessment. Individual examinations by professionals for each child would be prohibitively expensive. A viable alternative is to directly inquire parents regarding their infant's behavioral patterns. The Parents’ Evaluation of Developmental Status (PEDS), the Child Development Inventories (CDI), and the Ages and Stages Questionnaires (ASQ) have been highly regarded by the American Academy of Pediatrics as outstanding instruments, exhibiting excellent psychometric properties (22). The ASQ is an inexpensive instrument that necessitates an approximate 15-minute time investment for its screening and assessment (23). Furthermore, ASQ has been translated into 16 languages in low- and middle- income countries (LMICs), with at least 23 LMICs having used the questionnaire (24). Many studies have formally addressed the reliability and application of the Ages and Stages Questionnaires 3rd edition (ASQ-3) (25–28). What's more, the United Nations International Children's Emergency Fund (UNICEF) has endorsed the utilization of the ASQ-3, as a means to verify whether children are experiencing normal neurological development (7).
Although there are a number of studies exploring the associated factors of stunting, they all have some limitations. For example, the sample size is mostly concentrated below 2,000, and the data collection area is also relatively concentrated, which may limit their representativeness of the sampling (29, 30). Additionally, few studies have deeply explored the relationship between gestational diseases and developmental delay.
Against this backdrop, the present study comprehensively identified risk factors for stunting, thereby establishing a predictive model with high accuracy. Additionally, it aimed to assess the prevalence of stunting and its associated factors among children under three years of age in Shenzhen, China (31). Nomograms, serving as efficient and precise assessment tools, have the potential to assist clinical medical personnel in objectively identifying children under three years of age who are vulnerable to growth stunting.
2 Methods
2.1 Sample selection
Participants for the study comprised 9,581 infants and toddlers (0–3 years of age) who received health checkups at the Child Health Clinic of Shenzhen Social Health Center between August 2021 and June 2022. Screening is performed on children and completed by parents or primary caregivers to identify children at risk for developmental delay. The questionnaire investigators were all child health care doctors who were professionally trained and passed the assessment.
2.2 Inclusion and exclusion criteria
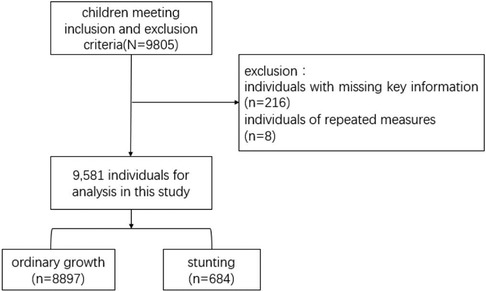
The following are the criteria for inclusion: (1) infants and toddlers must be between 0 and 36 months; (2) voluntary participation and informed consent; (3) the primary caregiver must be present at the assessment site concurrently with the children. The exclusion criteria include: (1) infants and young children with developmental delays or serious congenital hereditary diseases; (2) parents (caregivers) suffering from intellectual disability or emotional problems; (3) children older than 36 months or refuse to sign the informed consent; and (4) non-primary caregivers present at the assessment site. Based on 9,805 infants under the age of three, 212 individuals with missing main information and 8 individuals with repeated measurements were excluded, and 9,581 individuals meeting the inclusion and exclusion criteria were selected to participate in the analysis.
2.3 Outcome variable
The stunt of infants and toddler children were screened and assessed by ASQ-3. ASQ-3 is extensively utilized on a global basis for comprehensive developmental screening of children aged 1–66 months scale (32, 33), and it is the most validated and recommended by the UNICEF to determine whether children have normal neurological development (7). A study has substantiated the high reliability of the ASQ, thereby establishing its efficacy as a screening method for developmental delays (33).
The evaluation results of ASQ-3 are divided into: (1) above the threshold, the total score >2 -1 s, indicating that the child is developing normally; (2) near the threshold, the total score is 2 −2 s∼2 −1 s, indicating that the child may need additional help in one or more areas, but do not show obvious abnormalities and require further monitoring; (3) below the threshold, the total score is <2 −2 s, indicating an abnormal development of the child. The ASQ results in one or more areas below the threshold indicate developmental abnormalities, while those above and near the threshold are normal.
2.4 Variables
Collect information about infants and their parents through the basic information table, mainly include:
(1) Basic information of children: age and gender;
(2) Birth status: gestational week, mode of delivery, birth weight and birth order;
(3) Feeding situation: feeding mode within six months of age (Feeding methods of infants within 6 months of age), these include exclusive breastfeeding (only the mother's milk without any other dairy or animal milk), mixed feeding (breastfeeding along with milk powder), and bottle-feeding (only milk powder). Complementary food (Food other than breast and milk powder in infancy) and colostrum feeding (Breast milk secreted by the mother within 2–3 days after delivery);
(4) Parental information: parental education level, parental childbearing age, maternal employment status, occupation and maternal health status during pregnancy (gestational diabetes mellitus, hypertension during pregnancy, anemia during pregnancy, bacterial vaginitis, placenta previa, prenatal depression);
(5) Family social and economic status: family type, housing area and district;
(6) Others: average parent-child reading time, average daily time of interaction with other children, and average time of outdoor exercise per day.
2.5 Data analysis
To balance the risk of overfitting and underfitting, while ensuring that the model can fully learn the data features during the training stage, the partition ratio of the dataset was selected by 8:2. The dataset was randomly partitioned into training and validation sets, with a 8:2 ratio, to ensure appropriate model generalization. The training set facilitated the selection of salient features and the subsequent development of the predictive model. Conversely, the validation set served to evaluate the performance of the trained model. Categorical variables were presented in terms of frequency (percentage), and the chi-square test was employed to compare variations between distinct groups. To address potential collinearity among candidate variables, LASSO regression models were implemented, enabling the identification of optimal predictor variables. Subsequently, a logistic regression analysis was executed to ascertain the odds ratios and their respective 95% confidence intervals in both univariate and multivariate contexts.
In the current investigation, the discriminative capacity of the model was assessed using the area under the receiver operating characteristic curve (AUROC). Additionally, a calibration curve was employed to quantify the concordance between predicted probabilities and actual observations. To evaluate the clinical validity of the model, decision curve analysis (DCA) was performed. All data were processed utilizing the R software package (version 4.3.1). All statistical tests were conducted with two-tailed significance, and a P-value threshold of <0.05 was considered statistically significant.
3 Results
3.1 Descriptive analysis
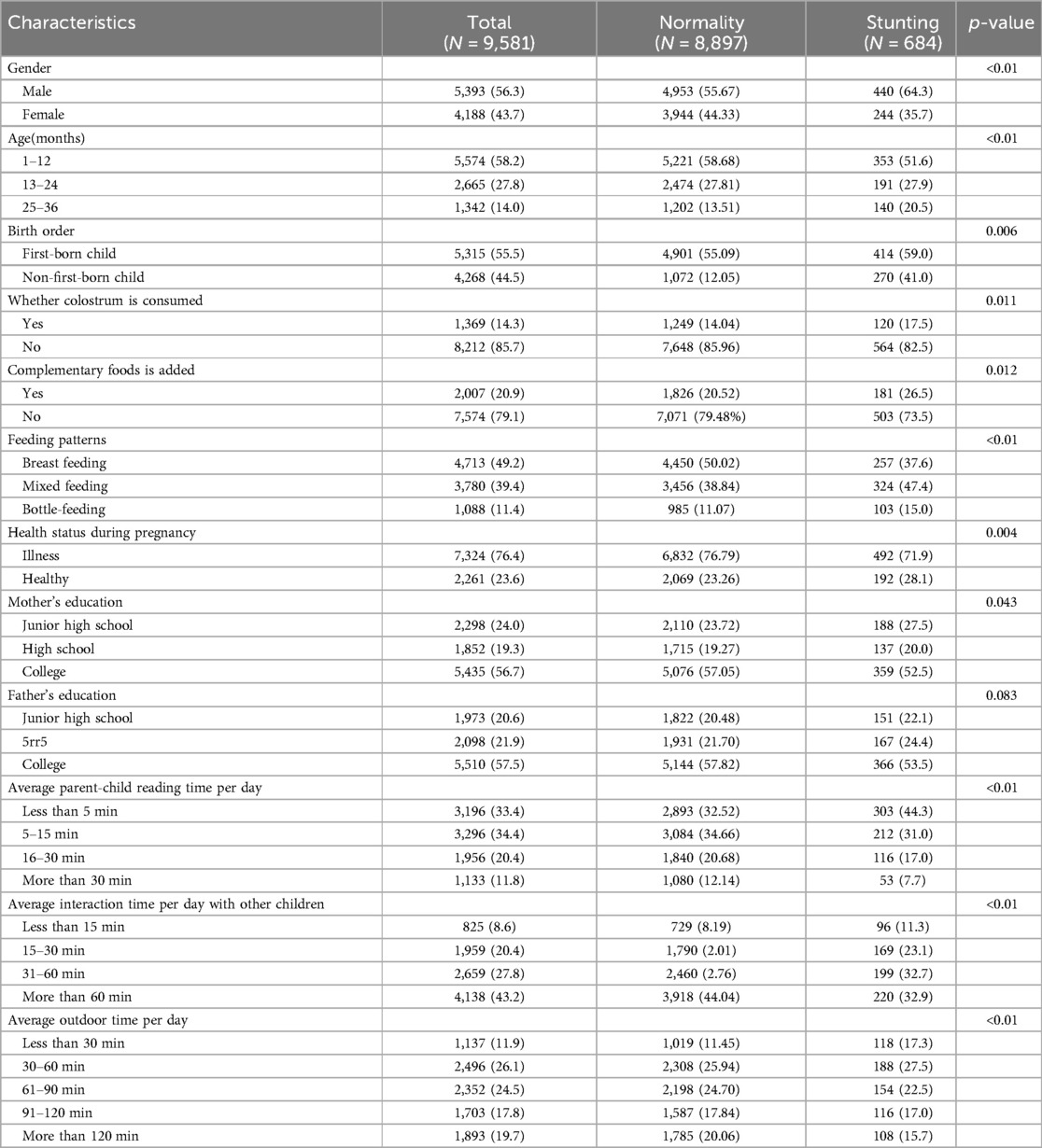
The cross-sectional analysis included 9,581 respondents, with average age of 13.42 ± 9.16 months (Mean ± SD) and a 43.7% female proportion. The majority of sample’ birth weights were within the normal range (93.2%). Additionally, 49.2% of the infants were exclusively breastfed until 6 months of age. More than half of infants were first-born (55.5%). When considering family factors, it is noteworthy that over half of mothers (56.7%) possess a college education. Concerning social and environmental factors, 11.8% of respondents read more than half an hour per day, while 19.8% spent more than 2 h outdoors per day (Table 1). The selection process of subjects is shown in Figure 1.
3.2 Prevalence and associated variables of stunting
The prevalence of delayed growth and development was found to be 7.14% in this study. The 18 variables, including the gender, age in months, maternal education, health status during pregnancy, father age, birth order, delivery mode, feeding mode, average parent-child reading time and average outdoor time per day, average interaction time per day with other children, average outdoor time per day, health status during pregnancy, and placenta praevia were significantly different from the normal group (p < 0.05) (Table 1).
3.3 LASSO logistic regression
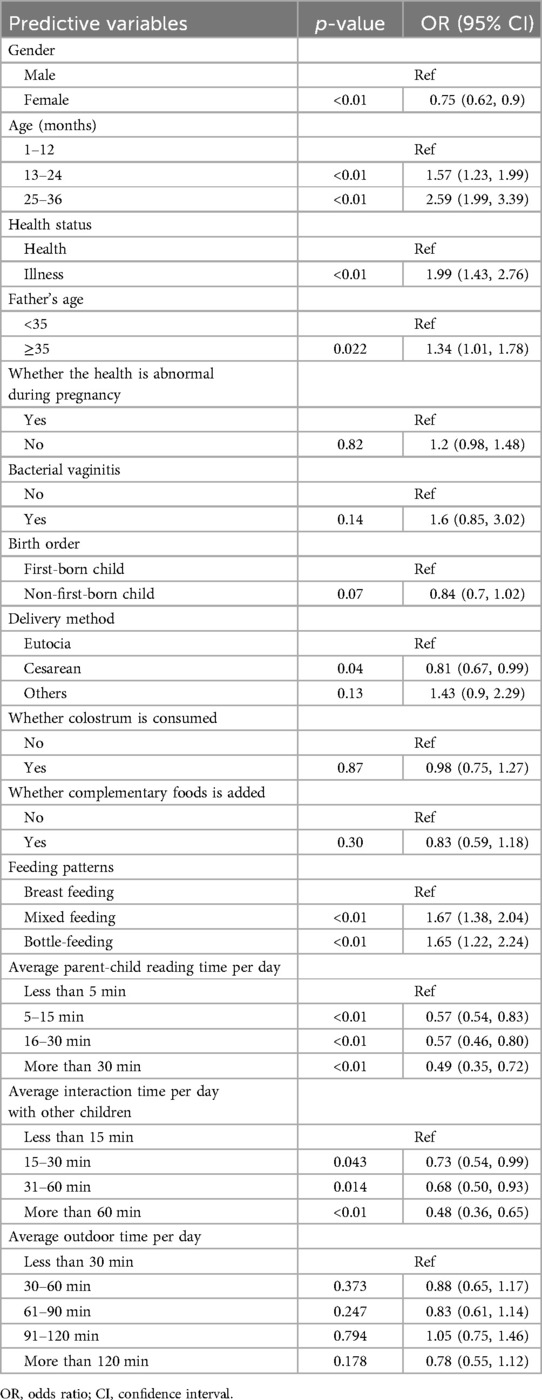
To reduce noise, variables that proved to be insignificant during the initial univariate analysis were excluded from the LASSO regression. This research utilized a LASSO regression model to pinpoint potential predictors of stunt (Figures 2A,B). Following this, the identified factors linked to stunt were integrated into a logistic regression model. In the end, it was determined that elements like gender, age in months, health status of children, birth order, feeding patterns, average parent-child reading time per day, average interaction time per day with other children and average outdoor time per day were correlated with growth retardation (Table 2).
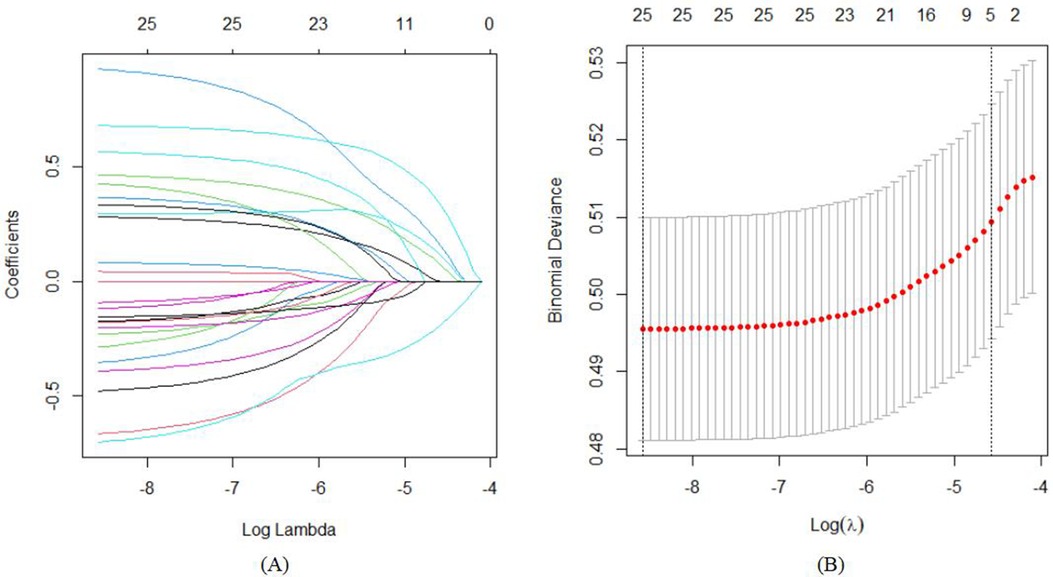
Figure 2. The variable filtering process of the Lasso regression. (A) Lasso coefficient profiles of the candidate features; (B) the selection of optimal parameters (lambda) by tenfold cross-validation, in which dotted vertical lines were drawn at the optimal values by using the minimum criteria and limits defined by 1 standard deviation.
3.4 Developing predictive models
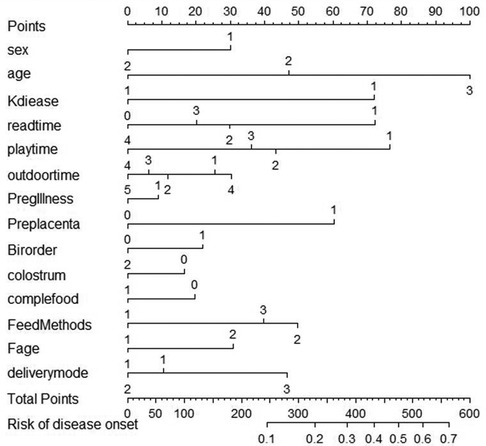
By implementing a tenfold cross-validation strategy, the Least Absolute Shrinkage and Selection Operator (LASSO) regression technique was leveraged to discern the most influential predictors that constitute the model's architecture. Subsequently, a comprehensive multiple logistic regression analysis was executed to construct the predictive model. An evaluation of the variance inflation factor (VIF) indicated that all variables possessed VIF values beneath the critical threshold of 4, indicating the absence of multicollinearity and a favorable model fit. The model was developed utilizing RStudio, facilitated by the “rms” package, and a nomogram was subsequently created leveraging the “nomogram” function from the same package (Figure 3; Table 3).
3.5 Validating predictive models
3.5.1 Assessment of the model-based discriminative ability
The Area Under the Curve (AUC), or area under the ROC curve, serves as a statistical measure to gauge the efficacy of a classification algorithm, highlighting the likelihood that a randomly positive sample will rank higher than a randomly chosen negative sample. This metric is frequently applied to evaluate the performance of machine learning classifiers. In this context, the predictive model's discriminative capacity was appraised by analyzing the occurrence of developmental delays among infants aged 0–3 years in both the training set and the validation set. As illustrated in Figures 4A,B, the AUC for the predictive model, when applied to the training cohort, was calculated to be 0.678 with a 95% confidence interval ranging from 0.6555 to 0.7002. In contrast, the AUC for the validation cohort yielded a slightly higher value of 0.734, accompanied by a 95% confidence interval extending from 0.6892 to 0.7782. The nomogram demonstrates robust discriminative capabilities and predictive accuracy, effectively distinguishing between typical developmental trajectories and growth impediments.
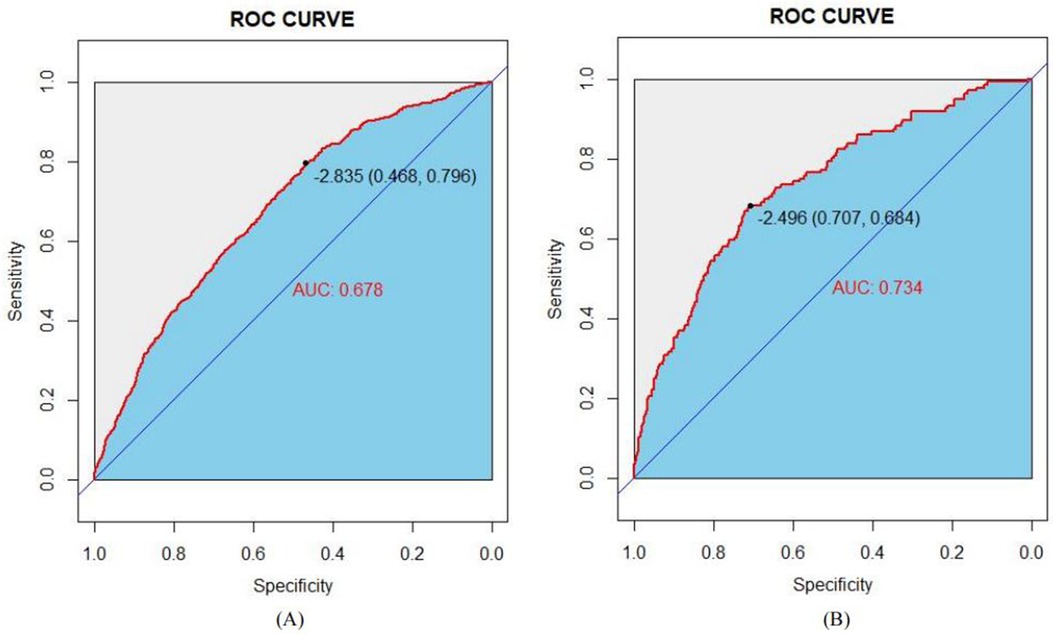
Figure 4. ROC curves of the study's generated nomogram in the study: (A) for the training set; and (B) for the internal validation set.
3.5.2 Correcting the predictive model
The model's congruence was evaluated utilizing calibration plots and the Hosmer–Lemeshow goodness-of-fit statistic, where a non-significant p-value (greater than 0.05) indicates a superior fit. The outcomes of the goodness-of-fit test revealed that the model exhibited commendable calibration for the training dataset (χ2 = 6.2051, df = 8, p = 0.6243) as well as for the validation dataset (χ2 = 11.794, df = 8, p = 0.1606). The graphical representations of the calibration curves for both the training and validation cohorts, derived from the logistic regression analysis, are delineated in Figures 5A,B, respectively.
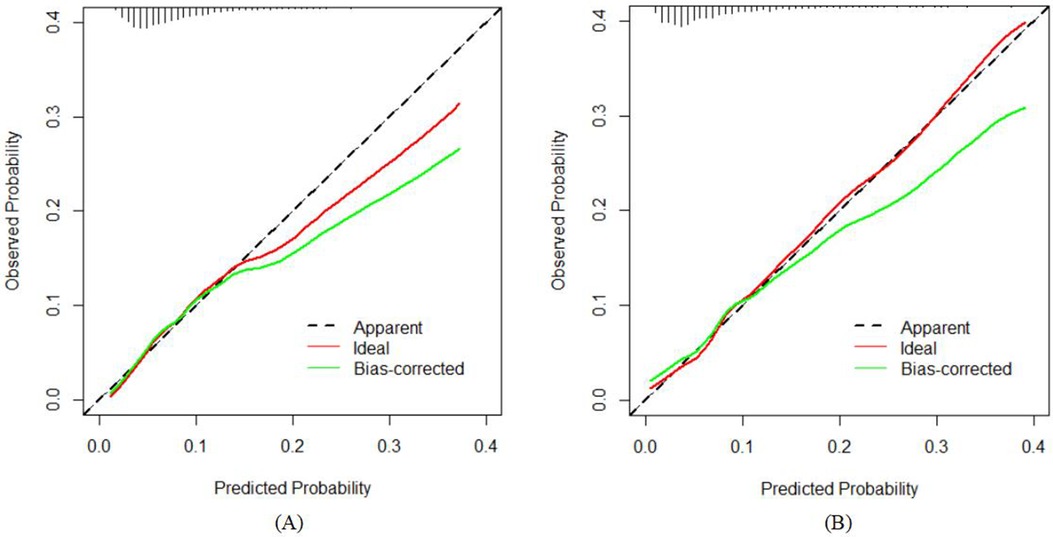
Figure 5. Calibration curves of the nomogram in the study: (A) for the training set; and (B) for the internal validation set.
3.5.3 Clinical validity assessment
The practical applicability of the model was appraised through the Decision Curve Analysis (DCA) framework, with the graphical depictions presented in Figures 6A, B. The DCA demonstrated that the net benefit accruing from the predictive model within the internal validation group markedly surpassed that of the two null hypotheses scenarios. This finding intimates that the nomogram-based model affords a heightened net benefit and precision in predictions, underscoring its clinical significance and practical utility.
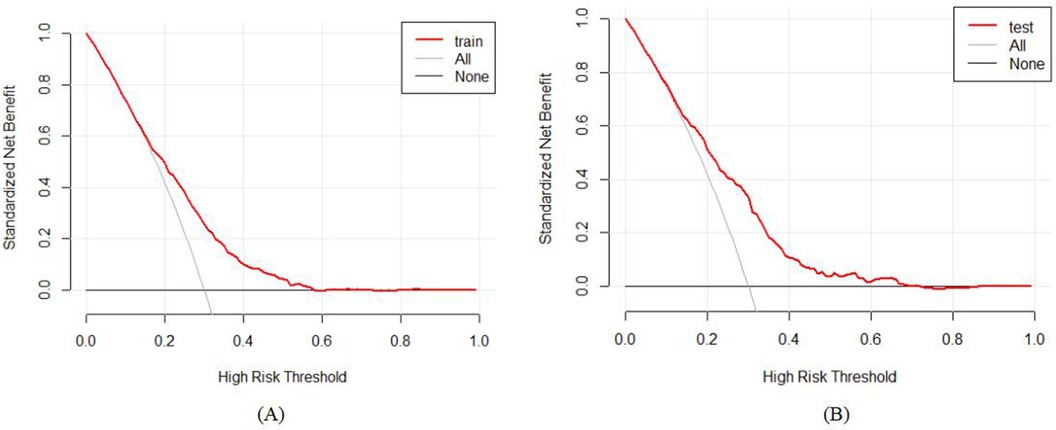
Figure 6. Decision curve analysis (DCA) for the study's nomogram: (A) for the training set; and (B) for the internal validation set.
4 Discussion
This study revealed that the prevalence of stunting among children under the age of three at 7.14% by employing stratified sampling to select 9,581 study participants. In this retrospective study, we developed and valided a nomogram to predict the stunting among children under the age of three. This model has better discrimination ability, clinical applicability and calibration based on AUC, DCA, Calibration plots and the Hosmer–Lemeshow good-ness-of-fit test. Moreover, the logistic regression analysis demonstrated that age, gender, health status, birth order, feeding patterns, average parent-child reading time per day were the best predictors of stunting.
This research demonstrated a correlation between stunting and gender, highlighting a predisposition towards stunt among the male population, thereby corroborating previous findings reported by Hong Zhou and colleagues (34). The rationale behind the conducted analysis potentially stems from the speculation that stunting is associated with genetic inheritance or mutational alterations within genes. Furthermore, our study revealed no significant association between stunt and factors such as birth weight, maternal gestational diabetes, or gestational hypertension. However, contrasting research has indicated that low birth weight, maternal gestational hypertension, premature rupture of membranes (PROM), and smoking during pregnancy are all contributory factors that elevate the risk of neurodevelopmental impairments (NIs). Conversely, EBF and a higher socioeconomic status appear to be protective factors that mitigate this risk (35). New evidence has emerged, suggesting that antenatal depression, or maternal depression experienced during pregnancy, holds profound implications not only for the mother's own well-being but also for the emotional and behavioral trajectory of the developing child. This finding underscores the intricate link between maternal mental health and the future psychosocial development of offspring (36).
Our research has illuminated the significance of feeding patterns as predictors of stunting, with numerous studies corroborating the unequivocal benefits of EBF on child development. A cluster-randomized controlled trial, conducted in the western Kenyan sub-county of Bondo, revealed noteworthy positive correlations between EBF during the 3- to 6-month age window and various aspects of child development, particularly in the domains of communication, gross motor skills, and problem-solving abilities (37). In a study conducted by Wallenborn JT and his team, it was observed that adhering to the World Health Organization's (WHO) recommendations for EBF remains crucial for promoting healthy physical growth and cognitive development, even in environments where complementary foods are readily accessible (38). A comprehensive cohort analysis conducted in rural South Africa has revealed that EBF is associated with a reduced prevalence of conduct disorders and a modest correlation with enhanced cognitive development among male children (39). A prospective birth cohort study originating from Brazil has unveiled that breastfeeding is positively correlated with superior performance in intelligence assessments administered three decades later, potentially having a significant impact on real-life outcomes, including the augmentation of educational attainment and income during adulthood (40). Furthermore, a Polish Mother and Child Cohort Study failed to identify any significant association between the duration of breastfeeding and child development (41). A study conducted among Chinese toddlers aged 1–3 years revealed a negative correlation between feeding difficulty and their overall health and development (42). For this reason, breastfeeding during infancy is highly emphasized as a pivotal strategy to foster growth and development, even among healthy young children (43).
Concurrently, this study also noted a weak association between the mode of delivery and the incidence of stunting. Prior research has identified certain adverse outcomes associated with cesarean delivery. Specifically, it has been linked to a heightened risk of Attention Deficit Hyperactivity Disorder (ADHD) and Autism Spectrum Disorder (ASD) (44, 45). Despite this, the current research evidence remains inconclusive in determining the precise effect of cesarean section on child health outcomes. For example, certain studies have suggested that cesarean delivery does not markedly increase or decrease the likelihood of suspected developmental delay in children compared to vaginal delivery (46–48). In addition, this study showed that maternal educational level is also associated with stunt. A study conducted in Uganda revealed that children whose mothers possessed secondary education exhibited lower odds of stunting and underweight compared to children whose mothers had no formal education (49). A comparative cross-sectional study has revealed that the prevalence of stunting and wasting is comparatively lower among children of employed mothers than among those of unemployed women (50). In view of this, the reasons for this difference require more intensive research.
Finally, our study also found that reading time and interacting time with others children was significantly asscociated with the occurrence of stunt, which is consitent with previous research findings. A comprehensive study has revealed a significant positive association between augmented reading time across multiple time intervals and enhanced ASQ-3 scores, encompassing fine motor, gross motor, personal-social, and comprehensive development domains, over a protracted period (26). A prospective longitudinal cohort study in Canada has uncovered that toddlers’ exposure to informal play opportunities, reading picture books, and supervision in childcare centers emerges as protective factors mitigating the risk of delayed speech development (51). The results from the China ECD Program have additionally demonstrated that a deficiency in books and toys (52), coupled with inadequate learning activities (53), substantially elevate the risk of developmental delays in children aged 0–35 months residing in rural areas. Furthermore, a longitudinal birth cohort study has revealed that responsive caregiving and learning opportunities serve as protective factors for young children, mitigating the negative impacts of early adversities on their adolescent human capital development (54).
Constructed through regression analysis, the nomogram integrates diverse predictive indicators to visually represent the inter-variable relationships within the predictive model. The visualization is accomplished via the placement of scaled line segments on a unified plane, adhering strictly to a predetermined ratio. This serves as a predictive instrument that forecasts the likelihood of a specific clinical outcome by aggregating the scores attributed to individual predictors, thereby yielding an overall score. The development of a prediction model for stunting among children under three years of age represents a novel contribution of this study. Notably, the nomogram quantitatively translates hazard ratios into scores, facilitating the straightforward calculation of outcomes. This approach enables an individualized risk assessment for each individual, thereby enhancing both relevance and accuracy.
This study had several limitations. Firstly, the employment of a cross-sectional design in this study limited the capacity to establish definitive causal relationships. Secondly, the nomogram devised in this study is tailored specifically to Chinese data, and its applicability to other regions and countries necessitates further determination through external validation. The data originates from the verbal reports of the infants’ parents; however, the parents may not be fully aware of their child's actual condition, which could result in discrepancies between the data and the actual situation.
This study had some strengths. Firstly, this study has a large and representative sample size. Secondly, we explored the relationship between gestational diseases and developmental delay.
5 Conclusion
Our stunt risk prediction model provides a reliable and accurate tool for children under the age of three in Shenzhen, China. This model serves as a valuable asset to clinical practitioners by furnishing a theoretical foundation and a point of departure for the development of preemptive prevention and intervention strategies.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Shenzhen Health Development and Data Management Center (No.2021006). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
YX: Conceptualization, Formal Analysis, Writing – original draft, Writing – review & editing. XH: Conceptualization, Project administration, Writing – review & editing, Visualization. JC: Methodology, Validation, Writing – review & editing. LS: Data curation, Software, Writing – original draft. BN: Funding acquisition, Resources, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The study is supported by Key Program of National Natural Science Foundation of China (No. 72334004), National Natural Science Foundation of China (No. 71971143), Natural Science Foundation of Guangdong Province (No. 2024A1515011712), Guangdong Provincial Philosophy and Social Sciences Planning Project (No. GD22CGL35), Special Projects in Key Fields of Ordinary Colleges and Universities in Guangdong Province (No. 2022ZDZX2054), University Innovation Team Project of Guangdong Province (No. 2021WCXTD002).
Acknowledgments
We thank all the babies and their families for taking part in the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Demilew YM, Alem AT. Food security is not the only solution to prevent under-nutrition among 6–59 months old children in western Amhara Region, Ethiopia. BMC Pediatr. (2019) 19(1):7. doi: 10.1186/s12887-018-1386-2
2. Batiro B, Demissie T, Halala Y, Anjulo AA. Determinants of stunting among children aged 6–59 months at Kindo Didaye woreda, Wolaita Zone, Southern Ethiopia: unmatched case control study. PLoS One. (2017) 12(12):e0189106. doi: 10.1371/journal.pone.0189106
3. Girma A, Woldie H, Mekonnen FA, Gonete KA, Sisay M. Undernutrition and associated factors among urban children aged 24–59 months in northwest Ethiopia: a community based cross sectional study. BMC Pediatr. (2019) 19(1):214. doi: 10.1186/s12887-019-1595-3
4. Black MM. Nurturing care framework and implementation science: promoting nutrition, health and development among infants and toddlers globally. Nestle Nutr Inst Workshop Ser. (2019) 92:53–64. doi: 10.1159/000501660
5. Lu C, Black MM, Richter LM. Risk of poor development in young children in low-income and middle-income countries: an estimation and analysis at the global, regional, and country level. Lancet Glob Health. (2016) 4(12):e916–e22. doi: 10.1016/S2214-109X(16)30266-2
6. Roche ML, Gyorkos TW, Blouin B, Marquis GS, Sarsoza J, Kuhnlein HV. Infant and young child feeding practices and stunting in two highland provinces in Ecuador. Matern Child Nutr. (2017) 13(2):e12324. doi: 10.1111/mcn.12324
7. Otalvaro AMR, Grañana N, Gaeto N, Torres M, Zamblera MN, Vasconez MA, et al. ASQ-3: validation of the ages and stages questionnaire for the detection of neurodevelopmental disorders in Argentine children. Arch Argent Pediatr. (2018) 116(1):7–13. doi: 10.5546/aap.2018.eng.7
8. Gil JD, Ewerling F, Ferreira LZ, Barros AJ. Early childhood suspected developmental delay in 63 low- and middle-income countries: large within- and between-country inequalities documented using national health surveys. J Glob Health. (2020) 10(1):010427. doi: 10.7189/jogh.10.010427
9. Wang K, Qi Y, Wei Q, Shi Y, Zhang Y, Shi H. Responsive caregiving and opportunities for early learning associated with infant development: results from a prospective birth cohort in China. Front Pediatr. (2022) 10:857107. doi: 10.3389/fped.2022.857107
10. McCoy DC, Peet ED, Ezzati M, Danaei G, Black MM, Sudfeld CR, et al. Early childhood developmental status in low- and middle-income countries: national, regional, and global prevalence estimates using predictive modeling. PLoS Med. (2016) 13(6):e1002034. doi: 10.1371/journal.pmed.1002034
11. Zheng S, Fang J, Bai G, He X, Hua M, Zhu B, et al. The association between parental risks and childhood development: findings from a community-based survey in east China. BMC Public Health. (2023) 23(1):878. doi: 10.1186/s12889-023-15702-y
12. Iwayama M, Kira R, Kinukawa N, Sakai Y, Torisu H, Sanefuji M, et al. Parental age and child growth and development: child health check-up data. Pediatr Int. (2011) 53(5):709–14. doi: 10.1111/j.1442-200X.2011.03331.x
13. Gutbrod T, Wolke D, Soehne B, Ohrt B, Riegel K. Effects of gestation and birth weight on the growth and development of very low birthweight small for gestational age infants: a matched group comparison. Arch Dis Child Fetal Neonatal Ed. (2000) 82(3):F208–14. doi: 10.1136/fn.82.3.F208
14. Hanley-Cook G, Argaw A, Dahal P, Chitekwe S, Kolsteren P. Infant and young child feeding practices and child linear growth in Nepal: regression-decomposition analysis of national survey data, 1996–2016. Matern Child Nutr. (2022) 18(Suppl 1):e12911. doi: 10.1111/mcn.12911
15. Parikh P, Semba R, Manary M, Swaminathan S, Udomkesmalee E, Bos R, et al. Animal source foods, rich in essential amino acids, are important for linear growth and development of young children in low- and middle-income countries. Matern Child Nutr. (2022) 18(1):e13264. doi: 10.1111/mcn.13264
16. Bliznashka L, McCoy DC, Siyal S, Sudfeld CR, Fawzi WW, Yousafzai AK. Child diet and mother-child interactions mediate intervention effects on child growth and development. Matern Child Nutr. (2022) 18(2):e13308. doi: 10.1111/mcn.13308
17. Zeng S, Ma L, Mao H, Shi Y, Xu M, Gao Q, et al. Dynamic functional network connectivity in patients with a mismatch between white matter hyperintensity and cognitive function. Front Aging Neurosci. (2024) 16:1418173. doi: 10.3389/fnagi.2024.1418173
18. Del Rosario C, Slevin M, Molloy EJ, Quigley J, Nixon E. How to use the bayley scales of infant and toddler development. Arch Dis Child Educ Pract Ed. (2021) 106(2):108–12. doi: 10.1136/archdischild-2020-319063
19. Flamant C, Branger B, Tich SNTT, de la Rochebrochard E, Savagner C, Berlie I, et al. Parent-completed developmental screening in premature children: a valid tool for follow-up programs. PLoS One. (2011) 6(5):e20004. doi: 10.1371/journal.pone.0020004
20. Kvestad I, Hysing M, Ranjitkar S, Shrestha M, Ulak M, Chandyo RK, et al. The stability of the Bayley scales in early childhood and its relationship with future intellectual abilities in a low to middle income country. Early Hum Dev. (2022) 170:105610. doi: 10.1016/j.earlhumdev.2022.105610
21. Rasheed MA, Kvestad I, Shaheen F, Memon U, Strand TA. The predictive validity of Bayley scales of infant and toddler development-III at 2 years for later general abilities: findings from a rural, disadvantaged cohort in Pakistan. PLoS Glob Public Health. (2023) 3(1):e0001485. doi: 10.1371/journal.pgph.0001485
22. Council on Children With Disabilities; Section on Developmental Behavioral Pediatrics; Bright Futures Steering Committee; Medical Home Initiatives for Children With Special Needs Project Advisory Committee. Identifying infants and young children with developmental disorders in the medical home: an algorithm for developmental surveillance and screening. Pediatrics. (2006) 118(1):405–20. doi: 10.1542/peds.2006-1231
23. Squires J, Bricker D, Potter L. Revision of a parent-completed development screening tool: ages and stages questionnaires. J Pediatr Psychol. (1997) 22(3):313–28. doi: 10.1093/jpepsy/22.3.313
24. Small JW, Hix-Small H, Vargas-Baron E, Marks KP. Comparative use of the ages and stages questionnaires in low- and middle-income countries. Dev Med Child Neurol. (2019) 61(4):431–43. doi: 10.1111/dmcn.13938
25. Alvarez-Nuñez L, González M, Rudnitzky F, Vásquez-Echeverría A. Psychometric properties of the ASQ-3 in a nationally representative sample of Uruguay. Early Hum Dev. (2021) 157:105367. doi: 10.1016/j.earlhumdev.2021.105367
26. Carson V, Zhang Z, Predy M, Pritchard L, Hesketh KD. Longitudinal associations between infant movement behaviours and development. Int J Behav Nutr Phys Act. (2022) 19(1):10. doi: 10.1186/s12966-022-01248-6
27. Peralta CFA, Medrado AP, Botelho RD, da Costa KJR, Imada V, Lamis F. Percutaneous fetal endoscopic third ventriculostomy for severe isolated cerebral ventriculomegaly. Prenat Diagn. (2023) 43(13):1614–21. doi: 10.1002/pd.6465
28. Huang N, Chen W, Jiang H, Yang J, Zhang Y, Shi H, et al. Metabolic dynamics and prediction of sFGR and adverse fetal outcomes: a prospective longitudinal cohort study. BMC Med. (2023) 21(1):455. doi: 10.1186/s12916-023-03134-9
29. Carlos-Oliva D, Vitale MP, Grañana N, Rouvier ME, Zeltman C. Neurodevelopmental development with the use of the ages and stages questionnaire (ASQ-3) in monitoring children’s health. Rev Neurol. (2020) 70(1):12–8. doi: 10.33588/rn.7001.2019169
30. Ahmed M, Zepre K, Lentero K, Gebremariam T, Jemal Z, Wondimu A, et al. The relationship between maternal employment and stunting among 6–59 months old children in Gurage Zone Southern Nation Nationality People’s Region, Ethiopia: a comparative cross-sectional study. Front Nutr. (2022) 9:964124. doi: 10.3389/fnut.2022.964124
31. Li W, Wang J, Liu W, Xu C, Li W, Zhang K, et al. Machine learning applications for the prediction of bone cement leakage in percutaneous vertebroplasty. Front Public Health. (2021) 9:812023. doi: 10.3389/fpubh.2021.812023
32. Adanikin A, Lawlor DA, Pell JP, Nelson SM, Smith GCS, Iliodromiti S. Association of birthweight centiles and early childhood development of singleton infants born from 37 weeks of gestation in Scotland: a population-based cohort study. PLoS Med. (2022) 19(10):e1004108. doi: 10.1371/journal.pmed.1004108
33. Schonhaut L, Armijo I, Schönstedt M, Alvarez J, Cordero M. Validity of the ages and stages questionnaires in term and preterm infants. Pediatrics. (2013) 131(5):e1468–74. doi: 10.1542/peds.2012-3313
34. Zhou H, Ding Y, Yang Y, Zou S, Qu X, Wang A, et al. Effects on developmental outcomes after cesarean birth versus vaginal birth in Chinese children aged 1–59 months: a cross-sectional community-based survey. PeerJ. (2019) 7:e7902. doi: 10.7717/peerj.7902
35. Lee S, Han Y, Lim MK, Lee HJ. Impact of moderate-to-late preterm birth on neurodevelopmental outcomes in young children: results from retrospective longitudinal follow-up with nationally representative data. PLoS One. (2023) 18(11):e0294435. doi: 10.1371/journal.pone.0294435
36. Milgrom J, Hirshler Y, Holt C, Skouteris H, Galbally M, East C, et al. Early intervention to prevent adverse child emotional and behavioural development following maternal depression in pregnancy: study protocol for a randomised controlled trial. BMC Psychol. (2023) 11(1):222. doi: 10.1186/s40359-023-01244-w
37. Onyango S, Kimani-Murage E, Kitsao-Wekulo P, Langat NK, Okelo K, Obong'o C, et al. Associations between exclusive breastfeeding duration and children’s developmental outcomes: evidence from Siaya county, Kenya. PLoS One. (2022) 17(3):e0265366. doi: 10.1371/journal.pone.0265366
38. Wallenborn JT, Levine GA, dos Santos AC, Grisi S, Brentani A, Fink G. Breastfeeding, physical growth, and cognitive development. Pediatrics. (2021) 147(5):e2020008029. doi: 10.1542/peds.2020-008029
39. Rochat TJ, Houle B, Stein A, Coovadia H, Coutsoudis A, Desmond C, et al. Exclusive breastfeeding and cognition, executive function, and behavioural disorders in primary school-aged children in rural South Africa: a cohort analysis. PLoS Med. (2016) 13(6):e1002044. doi: 10.1371/journal.pmed.1002044
40. Victora CG, Horta BL, de Mola CL, Quevedo L, Pinheiro RT, Gigante DP, et al. Association between breastfeeding and intelligence, educational attainment, and income at 30 years of age: a prospective birth cohort study from Brazil. Lancet Glob Health. (2015) 3(4):e199–205. doi: 10.1016/S2214-109X(15)70002-1
41. Stelmach I, Kwarta P, Jerzyńska J, Stelmach W, Krakowiak J, Karbownik M, et al. Duration of breastfeeding and psychomotor development in 1-year-old children - Polish mother and child cohort study. Int J Occup Med Environ Health. (2019) 32(2):175–84. doi: 10.13075/ijomeh.1896.01328
42. Ren Z, Lan H, Szeto IM, Yang C, Zhang J, Li P, et al. Feeding difficulty among Chinese toddlers aged 1–3 years and its association with health and development. Front Pediatr. (2021) 9:758176. doi: 10.3389/fped.2021.758176
43. Lorthe E, Torchin H, Delorme P, Ancel PY, Marchand-Martin L, Foix-L'Hélias L, et al. Preterm premature rupture of membranes at 22–25 weeks’ gestation: perinatal and 2-year outcomes within a national population-based study (EPIPAGE-2). Am J Obstet Gynecol. (2018) 219(3):298.e1–e14. doi: 10.1016/j.ajog.2018.05.029
44. Talge NM, Allswede DM, Holzman C. Gestational age at term, delivery circumstance, and their association with childhood attention deficit hyperactivity disorder symptoms. Paediatr Perinat Epidemiol. (2016) 30(2):171–80. doi: 10.1111/ppe.12274
45. Curran EA, Dalman C, Kearney PM, Kenny LC, Cryan JF, Dinan TG, et al. Association between obstetric mode of delivery and autism Spectrum disorder: a population-based sibling design study. JAMA Psychiatry. (2015) 72(9):935–42. doi: 10.1001/jamapsychiatry.2015.0846
46. Li HT, Luo S, Trasande L, Hellerstein S, Kang C, Li JX, et al. Geographic variations and temporal trends in cesarean delivery rates in China, 2008–2014. JAMA. (2017) 317(1):69–76. doi: 10.1001/jama.2016.18663
47. Finder M, Boylan GB, Twomey D, Ahearne C, Murray DM, Hallberg B. Two-year neurodevelopmental outcomes after mild hypoxic ischemic encephalopathy in the era of therapeutic hypothermia. JAMA Pediatr. (2020) 174(1):48–55. doi: 10.1001/jamapediatrics.2019.4011
48. Dekeunink GM, Goossens SM, Matthijs V, Senden RH, Beckers CM, Roumen FJ. Neurodevelopmental outcome of twins at two years of age according to the planned mode of delivery. J Matern Fetal Neonatal Med. (2016) 29(2):303–8. doi: 10.3109/14767058.2014.999232
49. Nankinga O, Kwagala B, Walakira EJ. Maternal employment and child nutritional status in Uganda. PLoS One. (2019) 14(12):e0226720. doi: 10.1371/journal.pone.0226720
50. Zewdu D, Halala Handiso Y. Under-nutrition of 2–5 years old children and associated factor among employed and unemployed women: comparative cross-sectional study. Cogent Food Agric. (2020) 6(1):1801215. doi: 10.1080/23311932.2020.1801215
51. Collisson BA, Graham SA, Preston JL, Rose MS, McDonald S, Tough S. Risk and protective factors for late talking: an epidemiologic investigation. J Pediatr. (2016) 172:168–74.e1. doi: 10.1016/j.jpeds.2016.02.020
52. Wu TC, Shi HF, Du YF, Zhang JX, Zhao CX, Huang XN, et al. Effect of books and toys on early childhood development in poor rural areas of China. Zhonghua er ke za zhi. (2019) 57(3):187–93. doi: 10.3760/cma.j.issn.0578-1310.2019.03.006
53. Wei QW, Zhang JX, Scherpbier RW, Zhao CX, Luo SS, Wang XL, et al. High prevalence of developmental delay among children under three years of age in poverty-stricken areas of China. Public Health. (2015) 129(12):1610–7. doi: 10.1016/j.puhe.2015.07.036
54. Trude ACB, Richter LM, Behrman JR, Stein AD, Menezes AMB, Black MM. Effects of responsive caregiving and learning opportunities during pre-school ages on the association of early adversities and adolescent human capital: an analysis of birth cohorts in two middle-income countries. Lancet Child Adolesc Health. (2021) 5(1):37–46. doi: 10.1016/S2352-4642(20)30309-6
Keywords: stunting, ages and stages questionnaires, clinical prediction models, nomogram, LASSO
Citation: Xiong Y, Hu X, Cao J, Shang L and Niu B (2024) A predictive model for stunting among children under the age of three. Front. Pediatr. 12:1441714. doi: 10.3389/fped.2024.1441714
Received: 31 May 2024; Accepted: 16 August 2024;
Published: 3 September 2024.
Edited by:
Wencai Liu, Shanghai Jiao Tong University, ChinaReviewed by:
Dehua Hu, Central South University, ChinaXiaolu Fang, Hubei University of Medicine, China
Copyright: © 2024 Xiong, Hu, Cao, Shang and Niu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ben Niu, ZHJuaXViZW5AZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
 Yuxiang Xiong
Yuxiang Xiong Xuhuai Hu2,†
Xuhuai Hu2,† Ben Niu
Ben Niu