- 1College of Clinical Medicine, Jining Medical University, Jining, Shandong, China
- 2Department of Obstetrics, Affiliated Hospital of Jining Medical University, Jining Medical University, Jining, Shandong, China
Fetomaternal hemorrhage (FMH) is a perplexing obstetric condition that predominantly occurs during the third trimester or at the time of delivery. Its insidious and non-specific onset often leads to diagnostic challenges. The underlying pathophysiology of FMH remains incompletely understood, though it is primarily attributed to compromise of the placental barrier. The severity of the condition is intrinsically associated with the volumn of blood loss, the hemorrhage rate, and the presence of alloimmunity. Upon the occurrence of severe FMH, it can rapidly lead to intrauterine distress, fetal anemia, and the possibility of fetal demise, presenting a considerable threat to both maternal and neonatal well-being. In this article, I present a substantial case of FMH and conduct a systematic review of the current scientific literature regarding the etiology, clinical manifestations, diagnostic approaches, treatment highlights, and prognosis of this condition. The objective of this work is to improve clinicians’ comprehension and diagnostic proficiency concerning FMH.
Introduction
Fetomaternal hemorrhage (FMH), first described by Weiner (1) in 1948, refers to a clinical syndrome in which a defined volume of fetal blood enters the maternal circulation via a disrupted placental barrier during pregnancy or childbirth, leading to diverse levels of fetal blood loss or maternal hemolytic reactions. in situations where the maternal and fetal blood types are incompatible, there exists a risk of a hemolytic transfusion reaction occurring between the two circulatory systems (1). Minor bidirectional transfusions may transpire throughout any phase of typical pregnancy (2). Typically, more than 99% of cases of maternal-fetal transfusions involve blood loss of less than 15 milliliters; however, when the blood loss reaches 25–30 ml (which accounts for 20% of the fetal placental blood volume), or in the event of acute hemorrhage, it can lead to severe complications including fetal anemia, edema, and even intrauterine death (3). The incidence of FMH varies according to the volume of bleeding (3). The incidence of FMH in pregnant women is 3 per 1,000 with a blood loss of 30 ml or more, 1 per 1,000 with a blood loss of 80 ml or more, and ranges from 1 per 2,800 to 1 per 3,000 when the blood loss exceeds 150 ml (4–6). Presently, FMH is primarily documented as isolated cases, indicating a deficiency in clinician awareness regarding this medical condition. in situations involving unexplained fetal anemia or intrauterine fetal death, it is prudent to consider the execution of FMH testing to more accurately identify the underlying etiology.
Case data
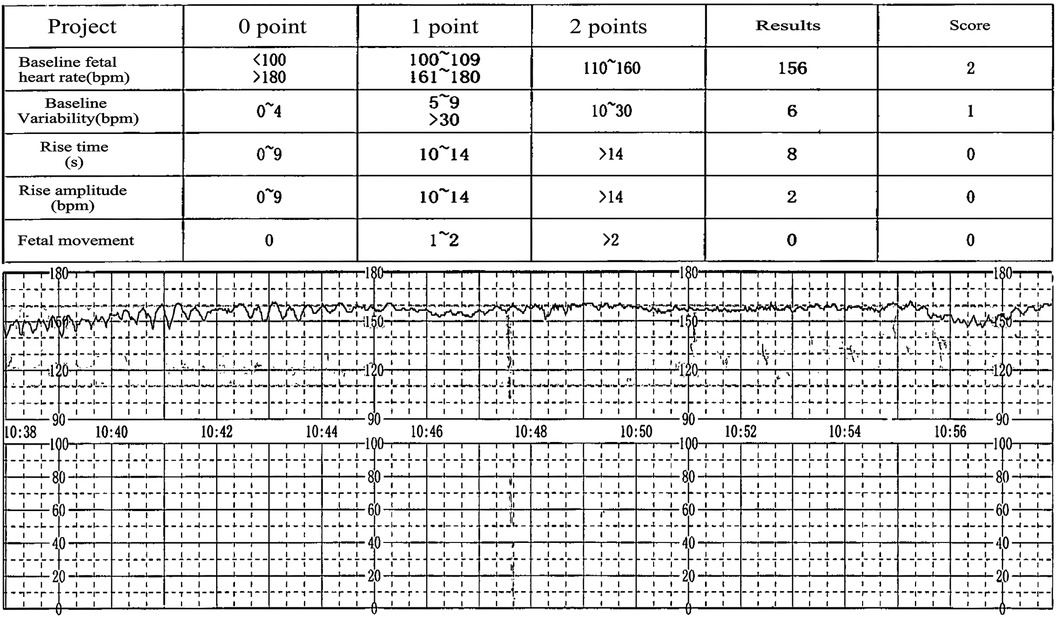
A 36-year-old woman, para 1 and gravida 4, with blood type O Rh positive, was referred to our hospital at 36 weeks and 4 days of gestation due to a reported decrease in fetal movements over the past three days. She had no documented history of abdominal trauma during her pregnancy. Prior to this admission, she was diagnosed with gestational diabetes mellitus and undifferentiated connective tissue disease. Prenatal screening tests yielded normal results, and her screening for Group B streptococcus as well as serologies for congenital infections returned negative. Upon admission, her physical examination was largely unremarkable, showing no uterine contractions, stable blood pressure and heart rate, and a long, closed cervix without signs of vaginal bleeding. Ultrasound findings at admission indicated a singleton fetus with growth measurements consistent with 37 weeks gestation, devoid of fetal anomalies or hydramnios, and the placenta exhibited normal morphology and positioning. However, cardiotocography (CTG) revealed significantly reduced baseline variability. In response to fetal intrauterine distress, we executed an urgent cesarean section, resulting in the delivery of a markedly pale female infant. During the operative procedure, the amniotic fluid was found to be contaminated with meconium, while both the placenta and umbilical cord appeared normal. The newborn exhibited pale skin, edema, cyanotic lips, an inability to cry, absence of spontaneous respiration, and a heart rate of 40 beats per minute. Apgar scores recorded at 1, 5, and 10 min postpartum were 1, 5, and 6, respectively. The placental blood gas analysis revealed: pH = 6.88, Pco2 = 108 mmHg, BE = −13.0 mmol/L, and hemoglobin concentration of 32 g/L, indicating decompensated acidosis, which was addressed with alkaline fluid administration. Following resuscitation measures for asphyxia, the neonate was transferred to the NICU for management due to “neonatal asphyxia (severe), neonatal anemia (extremely severe), and neonatal respiratory failure in a preterm infant”. Both the neonatal and maternal blood types were confirmed as RhD type O, and the infant's Coombs test was negative. The complete blood count for the newborn reveals a white blood cell count of 29.76 × 10^9/L, a red blood cell count of 1.09 × 10^12/L, a hemoglobin level of 41 g/L, a hematocrit of 13.5%, a platelet count of 208 × 10^9/L, a neutrophil count of 16.5 × 10^9/L, and reticulocyte count at 556.8 × 10^9/L. The reduction in hemoglobin and elevation in reticulocyte count suggest that the newborn experienced blood loss a few days prior, resulting in severe anemia (7).We hypothesize that the observed blood loss occurred a few days before the cesarean section and may be correlated with a decrease in fetal movements over the past three days. The chest ultrasound of the newborn indicates lung consolidation, neonatal pneumonia, and right pleural effusion, which correlate with the elevated white blood cell and neutrophil counts.The neonate received normal saline for volume expansion and dopamine to enhance circulation. Blood transfusions were administered at 15 ml/kg for three sessions, totaling 120 ml, resulting in significant improvement of severe anemia. (a post-transfusion hemoglobin level of 175 g/L). Subsequent management focused on active infection control, correction of hypoalbuminemia, and maintenance of fluid and electrolyte balance, which progressively stabilized the neonate's condition, leading to discharge after an 11-day hospitalization.
Discussion
The etiology of FMH is complex and associated with numerous obstetric factors, including fetal conditions (such as anomalies, multiple gestations, and intrauterine fetal demise), placental abnormalities (including placenta previa, placental abruption, tumors, and umbilical vein thrombosis), maternal trauma, and obstetric interventions (like amniocentesis) (7). Previous diagnoses of gestational diabetes and undifferentiated connective tissue disease may increase the risk of placental barrier damage; however, clinically, over 80% of cases of FMH with hemorrhage volumes exceeding 30 ml remain idiopathic (8). Massive FMH is a rare occurrence that is primarily documented by case reports. No established risk factors associated with massive FMH have been identified (9). It is often diagnosed in contexts such as severe neonatal anemia, unexplained stillbirth, or non-immune fetal hydrops (10).
The severity of FMH is related to the rate and volume of fetal blood loss (11). Clinical manifestations associated with FMH include neonatal anemia, stillbirth, preterm labor, intrauterine growth restriction, fetal hydrops, reduced fetal movement, and abnormal cardiotocography(CTG) patterns (12). Most patients with FMH initially exhibit reduced fetal movements, accompanied by abnormal CTG patterns, such as bradycardia or tachycardia, diminished variability, and sinusoidal or late deceleration, marking a critical juncture for the identification of FMH (13). A decline or absence of fetal movement, sinusoidal fetal heart rate pattern, and fetal edema constitute the classic triad indicative of massive FMH (14), aligning with the clinical manifestations observed in the patient discussed herein (Figure 1 & Supplementary Figure S1). A decline in fetal movements may serve as an early indicator of FMH, whereas fetal edema indicates a chronic condition stemming from FMH (7).
Based on the Apgar scores and the placental blood gas analysis of the neonate, we determine that the infant is suffering from severe neonatal asphyxia, profound neonatal anemia, and neonatal respiratory failure. It's imperative to recognize that the neonate necessitates not only effective ventilation but also an urgent blood transfusion at this juncture. The identification of meconium-stained amniotic fluid indicates intrauterine hypoxia, which has precipitated acidosis in the neonate. This acidosis stimulates the respiratory center due to increased carbon dioxide levels, potentially leading to significant aspiration of amniotic fluid. Furthermore, substantial blood loss in the neonate poses a risk of hypovolemic shock. These factors are likely critical contributors to the asphyxia observed. According to Clinical Anesthesiology, neonates and preterm infants with a hematocrit lower than 30% are at a higher risk for apnea; thus, it is advised to maintain a hematocrit above 35% for infants younger than three months (15). The infant's hemoglobin level is measured at 41 g/L, with a hematocrit of 13.5%, markedly below the survivable threshold. Therefore, I infer that the neonate has developed a massive FMH, which may elevate the risk of preterm delivery.
The clinical diagnosis of FMH primarily relies on the prompt responses of physicians and auxiliary examinations (6). Common supplementary tests include the Rosettle screen, Kleihauer-Betke test, and flow cytometry, all of which have been approved by the U.S. Food and Drug Administration (16). However, these supplementary tests are not routinely incorporated into standard screenings in many regions, highlighting a significant lack of awareness regarding FMH (6). Furthermore, measurements of fetal hemoglobin and the peak systolic velocity of the middle cerebral artery can provide crucial indications, as these metrics may signal fetal anemia (17, 18). Notably, placentas with FMH often exhibit distinctive characteristics, such as parenchymal pallor, an elevated count of nucleated red blood cells, and the presence of syncytial knots, alongside the expression of vascular endothelial growth factor (VEGF), CD34, and CD31 in capillary endothelial cells (19). Instances of concurrent intraplacental choriocarcinoma and FMH have been documented, prompting placental pathology examinations in FMH cases when feasible to enhance the detection of potential intraplacental choriocarcinoma (20).
The complications associated with FMH are numerous, including fetal distress, intrauterine growth restriction, postnatal respiratory failure, central nervous system dysfunction, persistent pulmonary hypertension, disseminated intravascular coagulation, pulmonary hemorrhage, and renal failure (8). The prognosis for infants affected by FMH is generally poor, with mortality rates ranging from 8% to 38% (21). Research indicates that the incidence of neurological injuries in FMH is between 4% and 18%, manifesting primarily as intraventricular hemorrhage, cerebral infarction, ventricular enlargement, periventricular white matter softening, cerebral atrophy, and cerebral palsy (22). The hemoglobin levels in the fetus prior to treatment can partially reflect the prognosis of FMH; specifically, lower hemoglobin levels correlate with a worse outcome (23).
Upon receiving a confirmed diagnosis of fetal-maternal hemorrhage (FMH), an early and tailored treatment protocol should be implemented, taking into account gestational age and disease severity.
A. For gestational age ≤ 32 weeks: In cases of mild FMH and slight anemia, observation may continue if the cardiotocography (CTG) and biophysical profile results are within normal limits. For patients at elevated risk for preterm labor, administration of glucocorticoids and magnesium sulfate is advised to enhance fetal pulmonary and neurological development. In cases of severe FMH, intrauterine transfusion (IUT) should be considered while remaining vigilant for the potential recurrence of FMH (24).
B. For gestational age between 32 weeks and 36 weeks: A proficient multidisciplinary team is essential to evaluate the risks associated with IUT and premature delivery (25).
C. For gestational age > 36 weeks: Prompt delivery should be pursued, alongside personalized blood transfusion strategies based on the degree of anemia present in the neonate, with the objective of minimizing maternal and fetal adverse outcomes (26).
Regrettably, we overlooked conducting the FMH testing and placental pathology examination, which necessitates a thorough reflection based on the existing pertinent literature. Although the occurrence of FMH is relatively infrequent, its consequences can be profoundly detrimental for both the mother and the fetus (27). Obstetricians must remain vigilant regarding reduced fetal movements and abnormal cardiotocography (CTG) trace, particularly during the third trimester of pregnancy (28). It is essential to conduct FMH testing promptly when confronted with unexplained anemia and edema.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by The Logic Committee of the Affiliated Hospital of Jining Medical College. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
PLi: Writing – original draft. HS: Writing – review & editing. PLin: Writing – review & editing. JW: Writing – review & editing. DZ: Writing – review & editing. DM: Writing – original draft, Writing – review & editing. FW: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. Funding support from National Natural Science Foundation of China (82201876), Natural Science Foundation of Shandong Province (ZR2021QH114), Natural Science Foundation of Shandong Province (ZR2021LZY001), the Research Fund for Academician Lin He New Medicine, China (JYHL2021MS24), Postdoctoral Program in Affiliated Hospital of Jining Medical University (322155), the Research Fund for Academician Lin He New Medicine, China (JYHL2019FMS14), the Incubation Programme of High-level Scientific Research Projects in Jining Medical University (JYGC2021KJ007), the Key Research and Development Program of Jining Science (2019SMNS015).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1423786/full#supplementary-material
Supplementary Figure S1 | Pre-admission CTG tracing.
References
1. Piva I, Iannone P, Morano D, Greco P. Untimely diagnosis of fetomaternal hemorrhage: what went wrong? J Perinat Med. (2018) 47(1):61–7. doi: 10.1515/jpm-2017-034430052523
2. Bianchi DW, Romero R. Biological implications of bi-directional fetomaternal cell traffic: a summary of a national institute of child health and human development-sponsored conference. J Matern Fetal Neonatal Med. (2003) 14(2):123–9. doi: 10.1080/jmf.14.2.123.12914629094
3. Stefanovic V. Fetomaternal hemorrhage complicated pregnancy: risks, identification, and management. Curr Opin Obstet Gynecol. (2016) 28(2):86–94. doi: 10.1097/GCO.000000000000024826866844
4. Schmit M, Duminil L, Loron G, Bednarek N, Graesslin O, Raimond E. Massive feto-maternal hemorrhage: two cases. J Gynecol Obstet Hum Reprod. (2019) 48(7):533–5. doi: 10.1016/j.jogoh.2019.03.01230898631
5. Sueters M, Arabin B, Oepkes D. Doppler Sonography for predicting fetal anemia caused by massive fetomaternal hemorrhage. Ultrasound Obstet Gynecol. (2003) 22(2):186–9. doi: 10.1002/uog.19012905516
6. Stroustrup A, Plafkin C, Savitz DA. Impact of physician awareness on diagnosis of fetomaternal hemorrhage. Neonatology. (2014) 105(4):250–5. doi: 10.1159/00035779724526231
7. Wylie BJ, D'Alton ME. Fetomaternal hemorrhage. Obstet Gynecol. (2010) 115(5):1039–51. doi: 10.1097/AOG.0b013e3181da792920410781
8. Giacoia G P. Severe fetomaternal hemorrhage: a review. Obstet Gynecol Surv. (1997) 52(6):372–80. doi: 10.1097/00006254-199706000-000229178311
9. Akorsu EE, Acquaye JK, Benneh AA, Oppong SA, Olayemi E. Fetomaternal hemorrhage among pregnant women in Accra, Ghana. Int J Gynaecol Obstet. (2019) 146(3):333–8. doi: 10.1002/ijgo.1289031206635
10. Tao E, Ye D, Long G, Hu Y, Fu Q, Yuan T, et al. Severe neonatal anemia affected by massive fetomaternal hemorrhage: a single-center retrospective observational study. J Matern Fetal Neonatal Med. (2022) 35(20):3972–8. doi: 10.1080/14767058.2020.184531333183095
11. Markham LA, Charsha DS, Perelmuter B. Case report of massive fetomaternal hemorrhage and a guideline for acute neonatal management. Adv Neonatal Care. (2006) 6(4):197–205. 206-207. doi: 10.1016/j.adnc.2006.04.00716890132
12. Weinhold V, Rauber S, Hollatz-Galuschki E, Seybold M, Kainer F, Kouskouti C. Massive fetomaternal hemorrhage: a case report. Z Geburtshilfe Neonatol. (2024) 228(4):382–5. doi: 10.1055/a-2238-321738286411
13. Ishihara H, Takahashi H, Takeuchi Y, Kigawa J, Sawazumi K, Ito T, et al. Massive fetomaternal hemorrhage: case report. Asia Oceania J Obstet Gynaecol. (1990) 16(3):225–8. doi: 10.1111/j.1447-0756.1990.tb00230.x1708232
14. Stroustrup A, Plafkin C, Tran T-A, Savitz DA. Demographic and behavioral predictors of severe fetomaternal hemorrhage: a case-control study. Neonatology. (2016) 109(4):248–54. doi: 10.1159/00044208226859152
15. Strauss RG. Anaemia of prematurity: pathophysiology and treatment. Blood Rev. (2010) 24(6):221–5. doi: 10.1016/j.blre.2010.08.00120817366
16. Dziegiel MH, Nielsen LK, Berkowicz A. Detecting fetomaternal hemorrhage by flow cytometry. Curr Opin Hematol. (2006) 13(6):490–5. doi: 10.1097/01.moh.0000245687.09215.c417053464
17. Bellussi F, Perolo A, Ghi T, Youssef A, Pilu G, Simonazzi G. Diagnosis of severe fetomaternal hemorrhage with fetal cerebral Doppler: case series and systematic review. Fetal Diagn Ther. (2017) 41(1):1–7. doi: 10.1159/00044610927174184
18. Gielezynska A, Stachurska A, Fabijanska-Mitek J, Debska M, Muzyka K, Kraszewska E. Quantitative fetomaternal hemorrhage assessment with the use of five laboratory tests. Int J Lab Hematol. (2016) 38(4):419–25. doi: 10.1111/ijlh.1251827320948
19. Zheng Y, Li D, Li X, Zheng A, Wang F. Spontaneous massive fetomaternal hemorrhage: two case reports and a literature review of placental pathology. BMC Pregnancy Childbirth. (2023) 23(1):530. doi: 10.1186/s12884-023-05826-937480031
20. She Q, Cheng Z, El-Chaar D, Luo F, Guo X, Wen SW. Intraplacental choriocarcinoma coexisting with fetomaternal hemorrhage: case report, chemotherapy management, and literature review. Medicine (Baltimore). (2018) 97(14):e9977. doi: 10.1097/MD.000000000000997729620671
21. Ladhani S, Fox G. Massive foetomaternal haemorrhage associated with a favourable outcome after two years. Acta Paediatr. (2002) 91(8):983–4. doi: 10.1080/08035250276014876612222728
22. De Luca D, Pietrini D, Piastra M, Tiberi E, Romiti A, Bernardini T, et al. Successful resuscitation of unexpected neonatal hemorrhagic shock due to massive feto-maternal hemorrhage. Paediatr Anaesth. (2008) 18(10):1004–6. doi: 10.1111/j.1460-9592.2008.02581.x18811852
23. Rubod C, Deruelle P, Le Goueff F, Tunez V, Fournier M, Subtil D. Long-term prognosis for infants after massive fetomaternal hemorrhage. Obstet Gynecol. (2007) 110(2 Pt 1):256–60. doi: 10.1097/01.AOG.0000271212.66040.7017666598
24. Troìa L, Al-Kouatly HB, McCurdy R, Konchak PS, Weiner S, Berghella V. The recurrence risk of fetomaternal hemorrhage. Fetal Diagn Ther. (2019) 45(1):1–12. doi: 10.1159/000491788
25. Williams J E, Pugh Y. The late preterm: a population at risk. Crit Care Nurs Clin North Am. (2018) 30(4):431–43. doi: 10.1016/j.cnc.2018.07.00130447804
26. Lubusky M, Simetka O, Studnickova M, Prochazka M, Ordeltova M, Vomackova K. Fetomaternal hemorrhage in normal vaginal delivery and in delivery by cesarean section. Transfusion. (2012) 52(9):1977–82. doi: 10.1111/j.1537-2995.2011.03536.x22313121
27. Meleti D, De Oliveira LG, Araujo JE, Caetano ACR, Boute T, Nardozza LMM, et al. Evaluation of passage of fetal erythrocytes into maternal circulation after invasive obstetric procedures. J Obstet Gynaecol Res. (2013) 39(9):1374–82. doi: 10.1111/jog.1207323822541
Keywords: fetomaternal hemorrhage, severe neonatal anemia, pregnancy, obstetrics, case report
Citation: Li P, Shu H, Lin P, Wang J, Zhang D, Man D and Wang F (2024) Case Report: Fetomaternal hemorrhage and its association with pronounced neonatal anemia. Front. Pediatr. 12:1423786. doi: 10.3389/fped.2024.1423786
Received: 24 June 2024; Accepted: 10 September 2024;
Published: 8 October 2024.
Edited by:
Margherita Neri, University of Ferrara, ItalyReviewed by:
Gabriela Corina Zaharie, University of Medicine and Pharmacy Iuliu Hatieganu, RomaniaSachin Gajanan Damke, Dr Rajendra Gode Medical College, India
Copyright: © 2024 Li, Shu, Lin, Wang, Zhang, Man and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongmei Man, bWFuZG9uZ21laUAxNjMuY29t; Fengge Wang, d2FuZ2ZnMTZAbWFpbHMuamx1LmVkdS5jbg==
 Peng Li
Peng Li Hua Shu1,2
Hua Shu1,2 Peng Lin
Peng Lin Fengge Wang
Fengge Wang