
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr. , 18 June 2024
Sec. Pediatric Neurology
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1417873
This article is part of the Research Topic Prenatal diagnosis and follow up of children with CNS abnormalities diagnosed in uterus View all 4 articles
 Iliana Bersani1,†
Iliana Bersani1,† Sara Ronci1,†
Sara Ronci1,† Immacolata Savarese1
Immacolata Savarese1 Fiammetta Piersigilli2*
Fiammetta Piersigilli2* Alessia Micalizzi3
Alessia Micalizzi3 Chiara Maddaloni1
Chiara Maddaloni1 Andrea Dotta1
Andrea Dotta1 Annabella Braguglia1
Annabella Braguglia1 Daniela Longo4
Daniela Longo4 Francesca Campi1
Francesca Campi1
Intracranial hemorrhage may represent a complication of the perinatal period that affects neonatal morbidity and mortality. Very poor data exist about a possible association between mutations of the type IV collagen a1 chain (COL4A1) gene and the development of intracranial hemorrhage, and only sporadic reports focus on intracerebral bleedings already developing in utero or in the neonatal period in infants with such a mutation. This study presents a case series of term neonates affected by intracranial hemorrhage, with no apparent risk factors for the development of this condition, who were carriers of COL4A1 gene variants. This study also provides a review of the most recent scientific literature on this topic, specifically focusing on the available scientific data dealing with the perinatal period.
Intracranial hemorrhage (ICH) develops most often in premature newborns, while its occurrence is relatively uncommon and may represent an incidental finding in term neonates (1, 2). Thanks to increased clinical awareness and improved neuroimaging techniques, however, ICH at term has been diagnosed more often in recent years (1, 3). In some cases, the clinical impact of such a condition may deeply influence the perinatal course and affect neonatal morbidity and mortality (4–7). ICH may develop in utero or after birth. The rates of fetal ICH are 0.46 per 1,000 deliveries and 0.9 per 1,000 pregnancies at referral centers (8). In full-term infants, possible risk factors for the development of perinatal cerebral bleeding include infections, malformations, asphyxia, and thrombophilia (4, 5, 7, 9). Besides these conditions, familial susceptibility with a genetic predisposition to a hereditary form of porencephaly may play a role, but available data are still inconsistent.
Type IV collagen a1 chain (COL4A1) gene mutations are responsible for a hereditary autosomal dominant cerebrovascular disease, characterized by a wide phenotypical spectrum, which may include the development of ICH. To date, only a relatively small number of neonates carrying COL4A1 gene mutations and developing ICHs has been reported (10–15). However, the diagnosis of COL4A1 gene mutations is probably underestimated because of the failure to search for the mutation because of its heterogeneous clinical presentation. When focusing on the perinatal period, the data available are even more infrequent, and the exact clinical features of such disorders are still far from clear.
The aim of this study was to describe the case of two term neonates affected by ICH of apparently unclear origin who showed mutations of the COL4A1 gene, expanding the spectrum of perinatal disease attributable to COL4A1 mutations and adding information in a field where knowledge is still far from clear. This study also provides a review of the currently available scientific literature on this topic, specifically focusing on the perinatal period.
We describe the clinical history of two neonates with apparently unexplainable ICH. A COL4A1 gene mutation was detected in both patients.
Patient 1 (Table 1) was a male neonate born at a gestational age (GA) of 40 weeks by spontaneous delivery, with a birth weight (BW) of 2,900 g. One month before delivery, microcephaly and polihydramnios were detected. Amniocentesis was normal and maternal TORCH assessment was negative for recent infections. At birth, the neonate showed normal cardiorespiratory adaptation (Apgar score at 1 min: 9; 5 min: 10). Cerebral ultrasound (CUS) could not be performed because of the reduced diameter of the front cranial fontanel. Electroencephalography (EEG), performed on day 6 of life, was normal. Magnetic resonance imaging (MRI) of the brain, performed on day 9 of life, showed a volumetric reduction of the brain with a simplification of cortical convolutions, polycystic glio-malacic lesions located in the left parietal and occipital cortical-subcortical region and in the right parietal-mesial region, hemosiderin deposits suggestive of previous hemorrhage in the right posterior paraventricular region, asymmetry of the posterior horns of the lateral ventricles (right > left), and reduced thickness of the intermediate segment and of the splenium of the corpus callosum. Diffusion-weighted imaging (DWI) did not highlight any lesion suggestive of recent ischemic damage (Figure 1). On the day 17 of life, the neonate developed an episode of diffuse muscular hypertonus and gaze deviation. The EEG performed on this occasion showed right focal specific lesions and therapy with barbiturate was therefore begun. The subsequent EEG was normal.
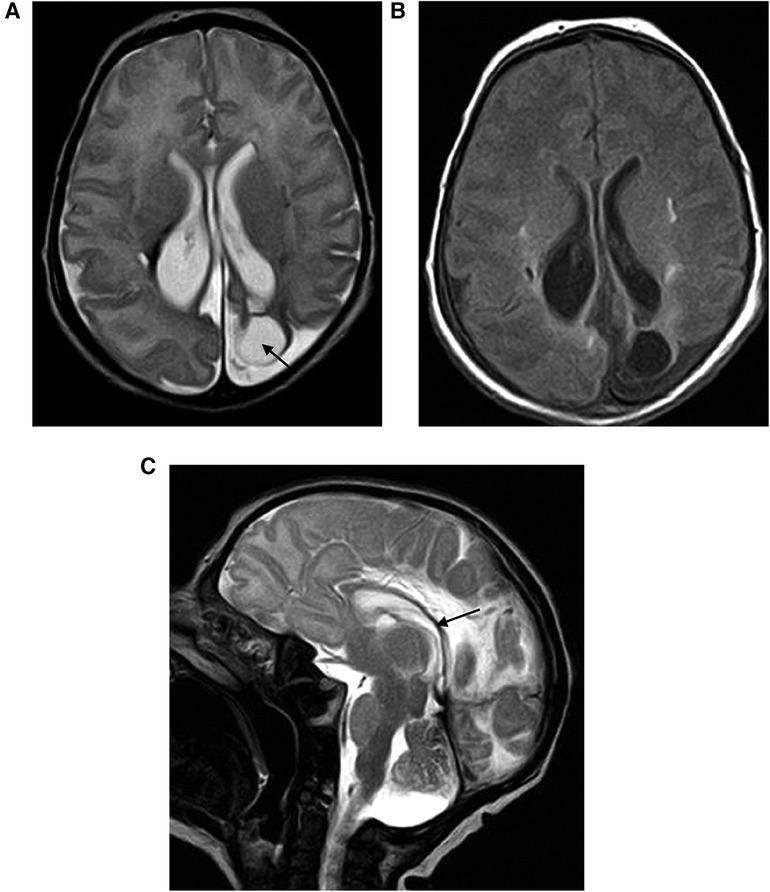
Figure 1 (A,B) Encephalomalacia secondary to parenchymal hemorrhage: axial T2-weighted image shows the limited area of parietal encephalomalacia (black arrow) with ex vacuo enlargement of the lateral ventricle. (C) Corpus callosum thickness reduction (black arrow).
After the first month of life, the infant developed muscular hypertonus of the four limbs, axial hypotonia, reduced reactivity, incomplete Moro reflex, right head deviation, and dysphagia.
The infant’s audiological and ocular assessments were normal. Renal function was normal. Thrombophilia testing was performed; prothrombin time, partial thromboplastin time, bleeding time, fibrinogen, antithrombin III protein C, protein S, activated protein C (APC) resistance, homocysteinemia, and testing for mutations in the prothrombin and factor V were all normal. A heterozygosity of the methyltetrahydrofolate reductase (MTHFR) gene (C677T) was detected. Immunoglobulins for rubeola, herpes viruses 1/2, Toxoplasma gondii, cytomegalovirus, and urinary polymerase chain reaction for cytomegalovirus excluded viral infections.
After the proband's parents provided written informed consent, molecular analyses of COL4A1 were performed using a custom panel [NimbleGen SeqCap Exome Enrichment Kit (Roche Sequencing Solution, CA, USA)] according to the manufacturer's protocol and sequenced on the Illumina platform. Annotated data were filtered out to exclude intronic variants, synonymous variants not predicted to affect splice sites, and variants with minor allele frequency (MAF) of ≥0.1% in the Genome Aggregation Database (gnomAD). Trio analysis identified the heterozygous missense variant c.2705C>G (p.Pro902Arg) of the COL4A1 gene (NM_001845) in maternal segregation in our patient.
The COL4A1 gene is an autosomal dominant gene of susceptibility to cerebral hemorrhages. The variant was not known in the scientific literature and was reported in the reference population database (gnomAD AF: 0.0004351; dbSNP: rs146134172; ClinVar ID: 80023). At the comparative genomic hybridization (CGH) array, the infant was also positive for the microduplication 16q24.3 with paternal segregation.
The copy number variants (CNVs) of region 16q24.3 are frequently associated with KBG syndrome. KBG syndrome is often caused by mutations in the AKNRD11 gene, microdeletions of 16q24.3, and, less frequently, by microduplications of this same region (16). Furthermore, the patient did not have phenotypic features peculiar to KBG syndrome, such as triangular face, hypertelorism, brachycephaly, synophyrys, prominent nasal bridge, and elongated philtrum. Furthermore, he had never experienced a single seizure episode that then resolved. Therefore, this diagnosis was excluded. As integration, the analysis of genes associated with microcephaly was performed and was negative.
The patient received rehabilitative physiotherapy during hospitalization and after discharge as well as speech therapy support. Percutaneous endoscopic gastrostomy and jejunostomy were performed because of persistent dysphagia, feeding difficulties, and gastroesophageal reflux. A follow-up at 7 years of age showed that the infant developed severe neurodevelopmental delay, spastic tetraparesis, and relational and language disorders.
Patient 2 (Table 1) was a male neonate born at a GA of 38 weeks by spontaneous delivery, with a BW of 3,870 g and Apgar scores of 9 and 10 at 1 and 5 min, respectively. Pregnancy was uneventful except for placental dysfunction during the first trimester, which was treated with aspirin. The early postnatal period was normal. On day 7 of life, the neonate developed jaundice, multiple vomiting episodes, and irritability. CUS showed asymmetric dilatation of the lateral ventricles with moderate (grade II–III) bilateral intraventricular hemorrhage (IVH) and dilatation of the third ventricle. Brain MRI confirmed these findings and highlighted further hemorrhage in the context of the posterior cranial fossa and small parenchymal hemorrhage in the right cerebellar hemisphere, in the cisterns of the skull base, and in the left hemispheric extra-axial site (Figure 2). EEG showed electric seizures on the left occipital hemisphere, which were treated with barbiturate.
As a result of worsening dilatation of the cerebral ventricles, a ventricular drain connected to a subcutaneous reservoir was inserted 1 week later, which was then replaced with a ventriculo-peritoneal shunt. The patient’s ocular and audiological assessments were normal. Left pyelectasia and megaureter were present and were subsequently treated with endoscopic dilation of the left uretero-vesical joint and ureteral stent placement. Extensive laboratory investigations, including complete blood count, renal function, C-reactive protein, prothrombin time, partial thromboplastin time, protein C, protein S, APC resistance, bleeding time, homocysteinemia, and testing for mutations in the prothrombin and factor V genes were all normal. The heterozygosity of the MTHFR gene (C677T) was highlighted. At the follow-up carried out at 3 years of age, the patient’s neurodevelopment was normal.
After the proband's parents provided written informed consent, a molecular analysis of COL4A1 was performed using the custom panel with the same technique as described in the first case. Trio analysis identified heterozygous missense variant c.413C>G (p.Pro138Arg) of the COL4A1 gene (NM_001845) present in the newborn and in his mother. The variant was neither known in the scientific literature nor reported in the reference population database (gnomAD, HGMD). In silico bioinformatic prediction tools indicated that the variant was pathogenic.
To review the literature about COL4A1 and perinatal intracranial hemorrhage in neonates, an extensive literature search in the MEDLINE database (via PubMed) was performed up to the year 2000. The keywords “COL4A1” AND “perinatal intracranial hemorrhage” OR “neonatal intracranial hemorrhage” were also searched as entry terms. All retrieved articles were screened, and then full texts of records deemed eligible for inclusion were assessed. References in the relevant papers were also reviewed and further articles were added if necessary. Papers written in languages other than English were excluded.
In Table 2, we systemically collected and summarized information on patients’ characteristics, age at diagnosis, and genetic diagnosis, and we compared them to our case.
A total of 33 cases were reported in the literature before our case. In 25 of the 33 cases, the onset of ICH was prenatal, and pregnancy was terminated in 8 cases.
COL4A1 and type IV collagen a2 chain (COL4A2) form the (a1)2(a2) IV heterotrimers and represent key components of basement membranes (28–31). COL4A1 is detectable ubiquitously in all tissues, including the vasculature, renal glomeruli, ocular structures, and muscles, and plays a role in the development of basement membrane during embryogenesis, in the cohesiveness of these membranes, and in the maintenance of vascular tone (32–34). The COL4A1 gene is located on the telomeric region of 13q (13q34) and is made up of 52 exons (35). Mutations of the COL4A1 gene may lead to a wide range of abnormalities variably affecting the brain (including the development of ICHs), retinal vasculature, ocular structures, renal glomeruli, and muscles, but no clear genotype–phenotype association has been identified yet, even within the same family (15). COL4A1 mutations are inherited as an autosomal dominant trait and have near 100% penetrance with variable expression; de novo mutations or low-level parental mosaicism have been described (25).
Neonates described in the present case series were both born at term after pregnancies with no history of infections, traumatic injuries, or thrombotic events and had a normal cardiorespiratory adaptation at birth. The heterozygosity of the MTHFR gene was highlighted in both cases, but no other anomalies were detected concerning the coagulative tests, platelet counts, and thrombophilia screening. ICH was diagnosed postnatally in both cases, although the radiologic findings of patient 1 showed the presence of glio-malacic lesions that were presumably of antenatal origin; even if it was not possible to assess the exact onset of the ventricular dilation detected in patient 2, the entity of the lesion was probably consistent with a prenatal origin. In our cases, prenatal MRI was not performed because of the late detection of anomalies during pregnancy in the first case and the low risk of pregnancy in the second case. However, as recently described by George et al. (36), prenatal COL4A1/A2 variations can show a range of ICH affecting the frontal lobes and basal ganglia, such as minor and/or unifocal ICH, as well as multifocal and bilateral lesions. In all cases of prenatal ICH, genetic testing for COL4A1/A2 variations should be taken into consideration because of the wide range of severity observed on fetal brain MRI. However, both patients came to our observation in the postnatal period; therefore, we had no other information regarding the decision-making process that took place during pregnancy.
The neurologic outcome was heterogeneous since it ranged from normal neurodevelopment (patient 2) to severely impaired neurologic development (patient 1).
To date, only a few authors have specifically investigated the role of COL4A1 mutations in the pathophysiologic mechanisms leading to intracerebral bleeding, and most of them focused on pediatric/adult patients rather than on neonates (37–39). Vahedi et al. found that some COL4A1 gene mutations may remain completely asymptomatic for years with no vascular changes on brain MRI, suggesting that the clinical expression may be heterogeneous even within the same family (37). Such findings are in agreement with subsequent data showing that brain MRI is abnormal in the majority of COL4A1-mutated patients (even in asymptomatic ones), but normal brain MRI may also be detectable in some mutation carriers with no brain manifestations (39).
Although there has been increasing interest regarding this condition in recent years, clinical experience about COL4A1-related perinatal ICHs only results from sporadic case reports (Table 1). Gould et al. were the first to demonstrate in both a mice model and in humans that mutations in the COL4A1 gene may compromise the vascular basement membrane and lead to perinatal ICH (10). Since then, other authors reported the association between COL4A1 gene mutation and perinatal ICHs, but no exact genotype–phenotype association has been identified yet.
The involvement of the central nervous system in the presence of COL4A1 gene mutations varies widely from case to case in terms of onset timing, injury entity, and location (17–27, 40, 41). Features suggestive of COL4A1 mutations include severe and/or multifocal hemorrhagic or ischemic-hemorrhagic cerebral lesions, especially when such injuries are associated with porencephaly (27).
A deeper understanding of the relationship between COL4A1 mutations and the development of perinatal porencephaly may have important implications for disease prevention and optimization of perinatal care. Some authors suggested a possible interaction between environmental triggers and the genome (10). Although some neonatal cases were diagnosed after birth (as in our cases), the postnatal neuroimaging features were mostly consistent with an antenatal onset of ICH (Table 1).
It is crucial to emphasize the importance of the mother–placental–fetal triad in the developing of illness processes that cause brain abnormalities in fetuses or newborns. Gene–environment interactions affect the brain development of newborns, children, and the mother–placental–fetal triad in both the short and long term. These interactions start at conception (40). During the initial 1,000 days of brain maturation, critical/sensitive periods are more likely to result in lifelong developmental neuroplasticity (40). The hazardous stressor interaction between internal and external sources modifies the neural exposome by means of maladaptive developmental neuroplasticity. The first 1,000 days are when epilepsy and developmental problems mostly manifest (41).
Prenatal environmental factors, such as premature uterine contractions or other traumatic injuries, may activate some triggering mechanisms leading to vascular damage in at-risk patients (17, 42). It was hypothesized that specific preventive strategies, such as cesarean delivery instead of spontaneous delivery in at-risk individuals, might decrease the stress on the abnormal brain vasculature and, therefore, the severity of ICH in high-risk populations (10); however, further studies are needed. Nevertheless, according to the literature, perinatal ICHs occurred in neonates born both by spontaneous and cesarean delivery, and brain injury has sometimes already occurred during pregnancy (Table 2).
Finally, the neonates described in the literature were born either at term or prematurely (Table 2), and it might be put forward that some COL4A1 mutations may carry a higher risk for preterm birth and/or higher susceptibility to environmental factors than others (17).
Regarding the importance of prevention, it is also important to highlight that many children with developmental disabilities live in low-resource countries low or middle income country (LMICs) or high-income desert countries (HICMDs).
In these places, it is even more important to implement strategies aimed at identifying at-risk pregnancies as early as possible to provide families with accurate diagnoses and subsequent effective interventions. To this end, a biosocial model was developed that emphasizes women's reproductive health with trimester-specific maternal and pediatric health interactions (43).
The ability to perform genetic, neurophysiological, and neuroimaging assessments improves clinical decision-making for more effective interventions before pathological expression.
Support for the family, especially in the case of neurological pathologies in which we know how fundamental the first 1,000 days of life are, continues in the postnatal period (40).
Synergy between obstetric and pediatric healthcare providers can reduce neurological morbidities (43).
In conclusion, mutations of the COL4A1 gene as a potential risk factor for perinatal ICH are probably underestimated because of low clinical awareness, poor available data, and wide phenotypical spectrum; COL4A1 mutations should be suspected when otherwise unexplainable ICHs occur prenatally or in the neonatal period; if COL4A1 carriers are identified, familiar genetic counseling is advisable (37, 44); and genetic testing can be performed both prenatally and postnatally (24). There is still a lack of long-term follow-up data about the risk of ICH recurrence as long-term neurologic outcomes are missing. The current gap in knowledge in this field represents a call to action for clinicians/researchers and deserves further investigations.
IB: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. SR: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing. IS: Data curation, Writing – review & editing. FP: Supervision, Validation, Writing – review & editing. AM: Supervision, Validation, Writing – review & editing. CM: Conceptualization, Writing – original draft. AD: Supervision, Validation, Writing – review & editing. AB: Supervision, Validation, Writing – review & editing. DL: Data curation, Methodology, Project administration, Resources, Writing – review & editing. FC: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This work was supported by the Italian Ministry of Health with Current Research funds.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
APC, activated protein C; BW, birth weight; COL4A1, type IV collagen a1 chain; COL4A2, type IV collagen a2 chain; CUS, cerebral ultrasound; EEG, electroencephalography; GA, gestational age; ICH, intracranial hemorrhage; IVH, intraventricular hemorrhage; MTHFR, methyltetrahydrofolate reductase.
1. Luciano R, Bersani I, Mancini G, Vento G, Mercuri E. Cranial ultrasound evaluation in term neonates. Early Hum Dev. (2020) 143:104983. doi: 10.1016/j.earlhumdev.2020.104983
2. Sirgiovanni I, Avignone S, Groppo M, Bassi L, Passera S, Schiavolin P, et al. Intracranial haemorrhage: an incidental finding at magnetic resonance imaging in a cohort of late preterm and term infants. Pediatr Radiol. (2014) 44:289–96. doi: 10.1007/s00247-013-2826-7
3. Brouwer AJ, Groenendaal F, Koopman C, Nievelstein RJ, Han SK, de Vries LS. Intracranial hemorrhage in full-term newborns: a hospital-based cohort study. Neuroradiology. (2010) 52:567–76. doi: 10.1007/s00234-010-0698-1
4. Shah NA, Wusthoff CJ. Intracranial hemorrhage in the neonate. Neonatal Netw. (2016) 35:67–71. doi: 10.1891/0730-0832.35.2.67
5. Novak CM, Ozen M, Burd I. Perinatal brain injury: mechanisms, prevention, and outcomes. Clin Perinatol. (2018) 45:357–75. doi: 10.1016/j.clp.2018.01.015
6. Gupta SN, Kechli AM, Kanamalla US. Intracranial hemorrhage in term newborns: management and outcomes. Pediatr Neurol. (2009) 40:1–12. doi: 10.1016/j.pediatrneurol.2008.09.019
7. Hong HS, Lee JY. Intracranial hemorrhage in term neonates. Childs Nerv Syst. (2018) 34:1135–43. doi: 10.1007/s00381-018-3788-8
8. Elchalal U, Yagel S, Gomori JM, Porat S, Beni-Adani L, Yanai N, et al. Fetal intracranial hemorrhage (fetal stroke): does grade matter? Ultrasound Obstet Gynecol. (2005) 26:233–43. doi: 10.1002/uog.1969
9. Perlman JM. Brain injury in the term infant. Semin Perinatol. (2004) 28:415–24. doi: 10.1053/j.semperi.2004.10.003
10. Gould DB, Phalan FC, Breedveld GJ, van Mil SE, Smith RS, Schimenti JC, et al. Mutations in COL4A1 cause perinatal cerebral hemorrhage and porencephaly. Science. (2005) 308:1167–71. doi: 10.1126/science.1109418
11. Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, et al. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. (2006) 354:1489–96. doi: 10.1056/NEJMoa053727
12. Yurchenco PD, Amenta PS, Patton BL. Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol. (2004) 22:521–38. doi: 10.1016/j.matbio.2003.10.006
13. Timpl R. Structure and biological activity of basement membrane proteins. Eur J Biochem. (1989) 180:487–502. doi: 10.1111/j.1432-1033.1989.tb14673.x
14. Kefalides NA. Isolation of a collagen from basement membranes containing three identical α-chains. Biochem Biophys Res Commun. (1971) 45:226–34. doi: 10.1016/0006-291X(71)90073-8
15. Vahedi K, Alamowitch S. Clinical spectrum of type IV collagen (COL4A1) mutations: a novel genetic multisystem disease. Curr Opin Neurol. (2011) 24:63–8. doi: 10.1097/WCO.0b013e32834232c6
16. Sirmaci A, Spiliopoulos M, Brancati F, Powell E, Duman D, Abrams A, et al. Mutations in ANKRD11 cause KBG syndrome, characterized by intellectual disability, skeletal malformations, and macrodontia. Am J Hum Genet. (2011) 89(2):289–94. doi: 10.1016/j.ajhg.2011.06.007
17. De Vries LS, Koopman C, Groenendaal F, Van Schooneveld M, Verheijen FW, Verbeek E, et al. COL4A1 mutation in two preterm siblings with antenatal onset of parenchymal hemorrhage. Ann Neurol. (2009) 65:12–8. doi: 10.1002/ana.21525
18. Bilguvar K, DiLuna ML, Bizzarro MJ, Bayri Y, Schneider KC, Lifton RP, et al. COL4A1 mutation in preterm intraventricular hemorrhage. J Pediatr. (2009) 155:743–5. doi: 10.1016/j.jpeds.2009.04.014
19. Vermeulen RJ, Peeters-Scholte C, Van Vugt JJ, Barkhof F, Rizzu P, van der Schoor SR, et al. Fetal origin of brain damage in 2 infants with a COL4A1 mutation: fetal and neonatal MRI. Neuropediatrics. (2011) 42:1–3. doi: 10.1055/s-0031-1275343
20. Meuwissen ME, de Vries LS, Verbeek HA, Lequin MH, Govaert PP, Schot R, et al. Sporadic COL4A1 mutations with extensive prenatal porencephaly resembling hydranencephaly. Neurology. (2011) 76:844–6. doi: 10.1212/WNL.0b013e31820e7751
21. Lichtenbelt KD, Pistorius LR, De Tollenaer SM, Mancini GM, De Vries LS. Prenatal genetic confirmation of a COL4A1 mutation presenting with sonographic fetal intracranial hemorrhage. Ultrasound Obstet Gynecol. (2012) 39:726–7. doi: 10.1002/uog.11070
22. Takenouchi T, Ohyagi M, Torii C, Kosaki R, Takahashi T, Kosaki K. Porencephaly in a fetus and HANAC in her father: variable expression of COL4A1 mutation. Am J Med Genet A. (2015) 167A:156–8. doi: 10.1002/ajmg.a.36823
23. Colin E, Sentilhes L, Sarfati A, Mine M, Guichet A, Ploton C, et al. Fetal intracerebral hemorrhage and cataract: think COL4A1. J Perinatol. (2014) 34:75–7. doi: 10.1038/jp.2013.135
24. Meuwissen ME, Halley DJ, Smit LS, Lequin MH, Cobben JM, de Coo R, et al. The expanding phenotype of COL4A1 and COL4A2 mutations: clinical data on 13 newly identified families and a review of the literature. Genet Med. (2015) 17:843–53. doi: 10.1038/gim.2014.210
25. Durrani-Kolarik S, Manickam K, Chen B. COL4A1 mutation in a neonate with intrauterine stroke and anterior segment dysgenesis. Pediatr Neurol. (2017) 66:100–3. doi: 10.1016/j.pediatrneurol.2016.04.010
26. Grego L, Pignatto S, Rassu N, Passone E, Cogo P, Lanzetta P. Optic nerve hypoplasia, corpus callosum agenesis, cataract, and lissencephaly in a neonate with a novel COL4A1 mutation. Case Rep Ophthalmol. (2019) 10:424–30. doi: 10.1159/000505017
27. Maurice P, Guilbaud L, Garel J, Mine M, Dugas A, Friszer S, et al. Prevalence of COL4A1 and COL4A2 mutations in severe fetal multifocal hemorrhagic and/or ischemic cerebral lesions. Ultrasound Obstet Gynecol. (2021) 57:783–9. doi: 10.1002/uog.22106
28. Mayne R. Collagenous proteins of blood vessels. Arteriosclerosis. (1986) 6:585–93. doi: 10.1161/01.ATV.6.6.585
29. Urabe N, Naito I, Saito K, Yonezawa T, Sado Y, Yoshioka H, et al. Basement membrane type IV collagen molecules in the choroid plexus, pia mater and capillaries in the mouse brain. Arch Histol Cytol. (2002) 65:133–43. doi: 10.1679/aohc.65.133
30. Van Agtmael T, Bruckner-Tuderman L. Basement membranes and human disease. Cell Tissue Res. (2010) 339:167–88. doi: 10.1007/s00441-009-0866-y
31. Shekhonin BV, Domogatsky SP, Muzykantov VR, Idelson GL, Rukosuev VS. Distribution of type I, III, IV and V collagen in normal and atherosclerotic human arterial wall: immunomorphological characteristics. Coll Relat Res. (1985) 5:355–68. doi: 10.1016/S0174-173X(85)80024-8
32. Van Agtmael T, Bailey MA, Schlotzer-Schrehardt U, Craigie E, Jackson IJ, Brownstein DG, et al. COL4A1 mutation in mice causes defects in vascular function and low blood pressure associated with reduced red blood cell volume. Hum Mol Genet. (2010) 19:1119–28. doi: 10.1093/hmg/ddp584
33. Tarasov KV, Sanna S, Scuteri A, Strait JB, Orru M, Parsa A, et al. COL4A1 is associated with arterial stiffness by genome-wide association scan. Circ Cardiovasc Genet. (2009) 2:151–8. doi: 10.1161/CIRCGENETICS.108.823245
34. Poschl E, Schlotzer-Schrehardt U, Brachvogel B, Saito K, Ninomiya Y, Mayer U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development. (2004) 131:1619–28. doi: 10.1242/dev.01037
35. Emanuel BS, Sellinger BT, Gudas LJ, Myers JC. Localization of the human procollagen alpha 1(IV) gene to chromosome 13q34 by in situ hybridization. Am J Hum Genet. (1986) 38:38–44.3753820
36. George E, Vassar R, Mogga A, Li Y, Norton ME, Gano D, et al. Spectrum of fetal intraparenchymal hemorrhage in COL4A1/A2-related disorders. Pediatr Neurol. (2023) 147:63–7. doi: 10.1016/j.pediatrneurol.2023.07.008
37. Vahedi K, Boukobza M, Massin P, Gould DB, Tournier-Lasserve E, Bousser MG. Clinical and brain MRI follow-up study of a family with COL4A1 mutation. Neurology. (2007) 69:1564–8. doi: 10.1212/01.wnl.0000295994.46586.e7
38. Decio A, Tonduti D, Pichiecchio A, Vetro A, Ciccone R, Limongelli I, et al. A novel mutation in COL4A1 gene: a possible cause of early postnatal cerebrovascular events. Am J Med Genet A. (2015) 167A:810–5. doi: 10.1002/ajmg.a.36907
39. Alamowitch S, Plaisier E, Favrole P, Prost C, Chen Z, Van Agtmael T, et al. Cerebrovascular disease related to COL4A1 mutations in HANAC syndrome. Neurology. (2009) 73:1873–82. doi: 10.1212/WNL.0b013e3181c3fd12
40. Scher MS. “The first thousand days” define a fetal/neonatal neurology program. Front Pediatr. (2021) 9:683138. doi: 10.3389/fped.2021.683138
41. Scher MS. Interdisciplinary fetal-neonatal neurology training applies neural exposome perspectives to neurology principles and practice. Front Neurol. (2024) 14:1321674. doi: 10.3389/fneur.2023.1321674
42. Mine M, Tournier-Lasserve E. Intracerebral hemorrhage and COL4A1 mutations, from preterm infants to adult patients. Ann Neurol. (2009) 65:1–2. doi: 10.1002/ana.21607
43. Scher MS. A bio-social model during the first 1000 days optimizes healthcare for children with developmental disabilities. Biomedicines. (2022) 10(12):3290. doi: 10.3390/biomedicines10123290
Keywords: collagen type 4, COL4A1 gene, neonate, intracerebral hemorrhage, porencephaly
Citation: Bersani I, Ronci S, Savarese I, Piersigilli F, Micalizzi A, Maddaloni C, Dotta A, Braguglia A, Longo D and Campi F (2024) COL4A1 gene mutations and perinatal intracranial hemorrhage in neonates: case reports and literature review. Front. Pediatr. 12:1417873. doi: 10.3389/fped.2024.1417873
Received: 15 April 2024; Accepted: 27 May 2024;
Published: 18 June 2024.
Edited by:
Barbara Scelsa, Buzzi Children’s Hospital, ItalyReviewed by:
Mark Steven Scher, Case Western Reserve University, United States© 2024 Bersani, Ronci, Savarese, Piersigilli, Micalizzi, Maddaloni, Dotta, Braguglia, Longo and Campi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fiammetta Piersigilli, ZmlhbW1ldHRhLnBpZXJzaWdpbGxpQHNhaW50bHVjLnVjbG91dmFpbi5iZQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.