- 1Department of Neonatology, Guangzhou Key Laboratory of Neonatal Intestinal Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 2Department of Obstetrics and Gynecology, Guangdong Provincial Key Laboratory of Major Obstetric Diseases, The Third Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 3Department of Pediatrics, Peking University Third Hospital, Beijing, China
- 4Department of Neonatology, Sichuan Jinxin Xinan Women & Children’s Hospital, Chengdu, China
- 5Department of Neonatology, The Affiliated Hospital of Guizhou Medical University, Guiyang, Guizhou, China
- 6Department of Neonatology, Xiamen Maternal and Child Health Care Hospital, Xiamen, China
- 7Department of Neonatology, Affiliated Dongguan Shilong People’s Hospital of Southern Medical University, Dongguan, Guangdong, China
- 8Department of Neonatology, First Affiliated Hospital of Shaoyang University, Shaoyang, China
- 9Department of Neonatology, Guangdong Second Provincial General Hospital, Guangzhou, Guangdong, China
- 10Department of Neonatology, Cangzhou People’s Hospital, Cangzhou, Hebei, China
- 11Department of Neonatology, Affiliated Hospital of Yanan University, Yan an, China
- 12Department of Neonatology, Huizhou Central People’s Hospital, Huizhou, China
- 13Department of Neonatology, The Affiliated Yue Bei People’s Hospital of Shantou University Medical College, Shaoguan, China
- 14Department of Neonatology, Xiaogan Hospital Affiliated to Wuhan University of Science and Technology, Xiaogan, China
- 15Department of Neonatology, The Affiliated Hospital of Inner Mongolia Medical University, Hohhot, China
- 16Department of Neonatology, The Second Affiliated Hospital of Shantou University Medical College, Shantou, China
- 17Department of Neonatology, The Third Staff Hospital of Baogang Group Baotou, Baotou, China
- 18Department of Neonatology and Pediatrics, Jinan University First Affiliated Hospital, Guangzhou, China
- 19Department of Neonatology, Lanzhou University Second Hospital, Lanzhou, China
- 20Department of Neonatology, Zhongshan City People’s Hospital, Zhongshan, China
- 21Department of Neonatology, Binhaiwan Central Hospital of Dongguan, Dongguan, China
- 22Department of Neonatology, The First Affiliated Hospital of Shantou University Medical College, Shantou, China
Objective: While prepregnancy overweight or obesity is known to negatively impact maternal health, its effect on twin infants is not well understood. Therefore, we conducted a nationwide, multicenter retrospective study to investigate the association between maternal prepregnancy weight and health outcomes in twins.
Study design: This study collected data from 22 healthcare units across 12 regions in China between January 2018 and December 2020. To control for confounding factors, multiple logistic regression, propensity score matching (PSM), inverse probability of treatment weighting (IPTW), and overlapping weighting models (OW) were applied to explore the effects of prepregnancy BMI on Apgar scores and other outcomes.
Results: After screening, a total of 4,724 women with twin pregnancies and 9,448 newborns were included in the study. Compared to normal prepregnancy weight, prepregnancy overweight/obesity significantly increased the risk of gestational hypertension and gestational diabetes in mothers [adjusted OR (95% CI): 1.85 (1.55–2.21) and 1.49 (1.27–1.74), respectively]. It also increased the incidence of twins with a 1-min Apgar score ≤7, whether they were larger or smaller [1.60 (1.20–2.13) and 1.45 (1.09–1.92), respectively]. Sensitivity analyses using PSM [1.60 (1.20–2.13) and 1.55 (1.07–2.25)], IPTW [1.67 (1.31–2.12) and 1.48 (1.17–1.87)], and OW [1.65 (1.08–2.57) and 1.47 (0.97–2.25)] confirmed the stability of these results. However, it did not affect the likelihood of a 5-min Apgar score ≤7 [adjusted OR (95% CI): 0.82 (0.24–2.17) and 1.40 (0.70–2.73)]. In contrast, prepregnancy underweight was associated with a reduced incidence of twins with a 1-min Apgar score ≤7 [adjusted OR (95% CI): 0.56 (0.32–0.92) and 0.58 (0.34–0.94)], but had no effect on the 5-min Apgar score ≤7 [adjusted OR (95% CI): 0.82 (0.24–2.17) and 0.22 (0.01–1.08)]. Prepregnancy BMI did not significantly affect twin birth weight discordance, NICU admission, preterm birth, or low birth weight.
Conclusion: Maternal overweight/obesity before pregnancy increases the risk of hypertensive disorders and gestational diabetes in twin pregnancies and significantly raises the likelihood of twins having a low 1-min Apgar score. However, no significant impact on 5-min Apgar scores was observed. These findings highlight the importance of managing weight before pregnancy and ensuring readiness for neonatal resuscitation during delivery.
1 Introduction
Maternal overweight and obesity have become pressing global public health concerns, with rates showing a troubling increase in recent years (1). In China, about 17% of adults are classified as obese (2). Maternal overweight and obesity are associated with a greater risk of adverse pregnancy outcomes, such as gestational diabetes (GDM), preeclampsia, preterm birth, and macrosomia (3, 4). These conditions also correlate with elevated risks of neonatal morbidity and mortality (5).
The Apgar score is a widely used assessment tool that evaluates newborns' physical condition immediately after birth based on five key indicators: heart rate, respiratory effort, muscle tone, reflex response, and skin color. A low Apgar score at 1 or 5 min is linked to an increased risk of neonatal asphyxia, long-term developmental disabilities, cerebral palsy, and even neonatal mortality (6, 7). Several studies have investigated the relationship between maternal prepregnancy BMI and Apgar scores in singleton pregnancies (5, 8); however, there is limited evidence regarding twin pregnancies. Twin pregnancies inherently carry a higher risk of adverse outcomes than singleton pregnancies (9). Additionally, research on this subject in China is scarce.
This study aimed to investigate the impact of prepregnancy BMI on outcomes in twin pregnancies, focusing on the 1-min Apgar score. We also examined effects on the 5-min Apgar score, as well as the risks of preterm birth, low birth weight (LBW), NICU admission, and birth weight discordance in twins (BWDT). Our goal is to provide insights that can support improved prepregnancy weight management and early risk assessment during delivery.
2 Materials and methods
2.1 Study population
This study retrospectively analyzed data collected from 22 medical centers across 12 provinces, municipalities, or autonomous regions in China between January 2018 and December 2020. Trained investigators extracted data from electronic medical records for pregnant mothers and their twins. The Third Affiliated Hospital of Guangzhou Medical University served as the coordinating center, overseeing data review, integration, and analysis. Missing prepregnancy BMI data were excluded during data cleaning. To minimize confounding factors, cases with chromosomal abnormalities, twin-to-twin transfusion syndrome, twin anemia-polycythemia sequence, hydrops fetalis, and preconception diabetes were also excluded.
2.2 Outcomes and definitions
The primary outcome were 1-min and 5-min Apgar score ≤7, which serve as key indicators of neonatal asphyxia. Secondary outcomes included NICU admission, BWDT ≥20%, preterm birth, and LBW. Maternal prepregnancy BMI was categorized as underweight (<18.5 kg/m2), normal weight (18.5–23.99 kg/m2), and overweight/obese (24–27.99 kg/m2 or ≥28 kg/m2) based on the Chinese standards (10). Pregnancy weight gain (PWG) was categorized as adequate or inadequate according to the Institute of Medicine 2009 guidelines (11). Women aged 35 years or older were considered to have advanced maternal age. Mode of conception was categorized as natural or assisted, including artificial insemination and in vitro fertilization with embryo transfer. Abnormal thyroid function included both hypothyroidism and hyperthyroidism. Gestational hypertension and diabetes were diagnosed following national guidelines (12, 13), while adverse pregnancy history encompassed stillbirth and miscarriage.
2.3 Covariates
When analysing the association between prepregnancy BMI and neonatal outcomes and complications during pregnancy, potential confounding factors were considered, such as maternal age, hypertensive disorders, mode of conception and delivery, parity, adverse pregnancy history, thyroid function, infections, anaemia, chorionicity, GDM, PWG, and ethnicity. Definitions and categorizations of these covariates are detailed in Supplementary Table S1.
2.4 Ethical approval
This nationwide multicentre study was approved by the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University with the approval number: CERP [2020] No. 097. The study was conducted in accordance with strengthening the reporting of observational studies in epidemiology (STROBE) guidelines.
2.5 Statistical analysis
To compare the effects of different prepregnancy BMI values on outcomes, this study reported baseline demographic characteristics as frequencies (percentages) or mean values with standard deviations. Differences were assessed using Pearson's χ2 test for categorical variables and t-tests for continuous variables. Both univariate and multivariate logistic analyses were employed for primary and secondary outcomes, estimating odds ratios (ORs) with 95% confidence intervals (CIs), with p < 0.05 indicating statistical significance. All analyses were performed using R version 4.2.3. The study utilized five evaluation methods, including unadjusted logistic regression, multivariable logistic regression, propensity score matching (PSM), inverse probability treatment weighting (IPTW), and overlap weighting (OW). The variance inflation factor was used to detect collinearity between variables before subjecting them to multivariate logistic analysis. PSM was applied by calculating the propensity score for each individual based on observed covariates, and then matching treated and control subjects with similar propensity scores to ensure balance in key covariates between the two groups (14). IPTW involved assigning weights to individuals based on their propensity scores, effectively adjusting the sample distribution and reducing sample selection bias (15). OW, which combines the strengths of PSM and IPTW, was used to create a weighted sample that maintains a balanced distribution, thus enhancing the accuracy of causal inference (16). Covariate balance was assessed using standardized mean differences (SMDs), with SMDs <10% indicating good balance. We applied nearest-neighbor matching with a 1:1 ratio and a random-effects model, setting the caliper at a relative coefficient of 0.1 to improve matching precision. R packages such as “MatchIt” and “Tableone” were utilized for conducting analyses and evaluating balance. Loveplot graphs were utilized for visual comparison.
3 Results
3.1 Baseline characteristics of the study population
The dataset initially included 6,720 women with twin pregnancies and clinical data on 14,440 newborns. To reduce confounding factors, cases with chromosomal abnormalities, twin-to-twin transfusion syndrome, twin anemia-polycythemia sequence, hydrops fetalis, or preconception diabetes were excluded. After screening, 4,724 women with twin pregnancies were included in the analysis: 3,053 with normal prepregnancy BMI, 1,123 who were overweight or obese, and 548 who were underweight. The selection process is illustrated in Figure 1.
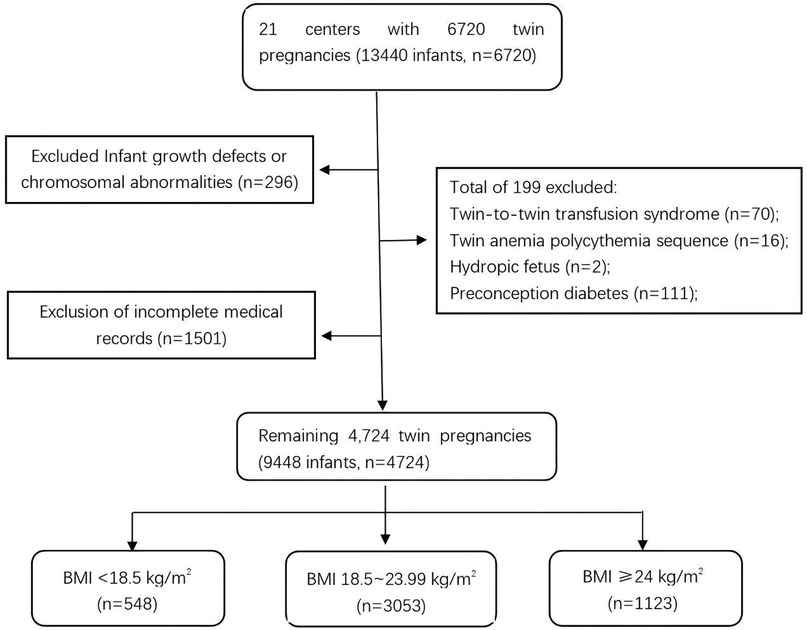
Figure 1. Participant selection process. The flowchart shows the selection of 4,724 twin pregnancies (9,448 infants) from an initial 6,720 pregnancies (13,440 infants). Exclusions included chromosomal abnormalities (n = 296), incomplete records (n = 1,501), and complications (n = 199). Participants were categorized by prepregnancy BMI: < 18.5 kg/m2 (n = 548), 18.5–23.99 kg/m2 (n = 3,053), and ≥24 kg/m2 (n = 1,123).
Table 1 compares the baseline characteristics of women across the BMI categories. Women who were overweight/obese before pregnancy were older, had higher gravidity and parity, and were more likely to conceive through assisted reproduction. This group also had a higher proportion of dichorionic diamniotic twin pregnancies and a higher prevalence of GDM and gestational hypertension. Conversely, they underwent fewer cesarean sections and gained less weight during pregnancy. Similar trends were observed among women with insufficient prepregnancy weight (Supplementary Table S2).
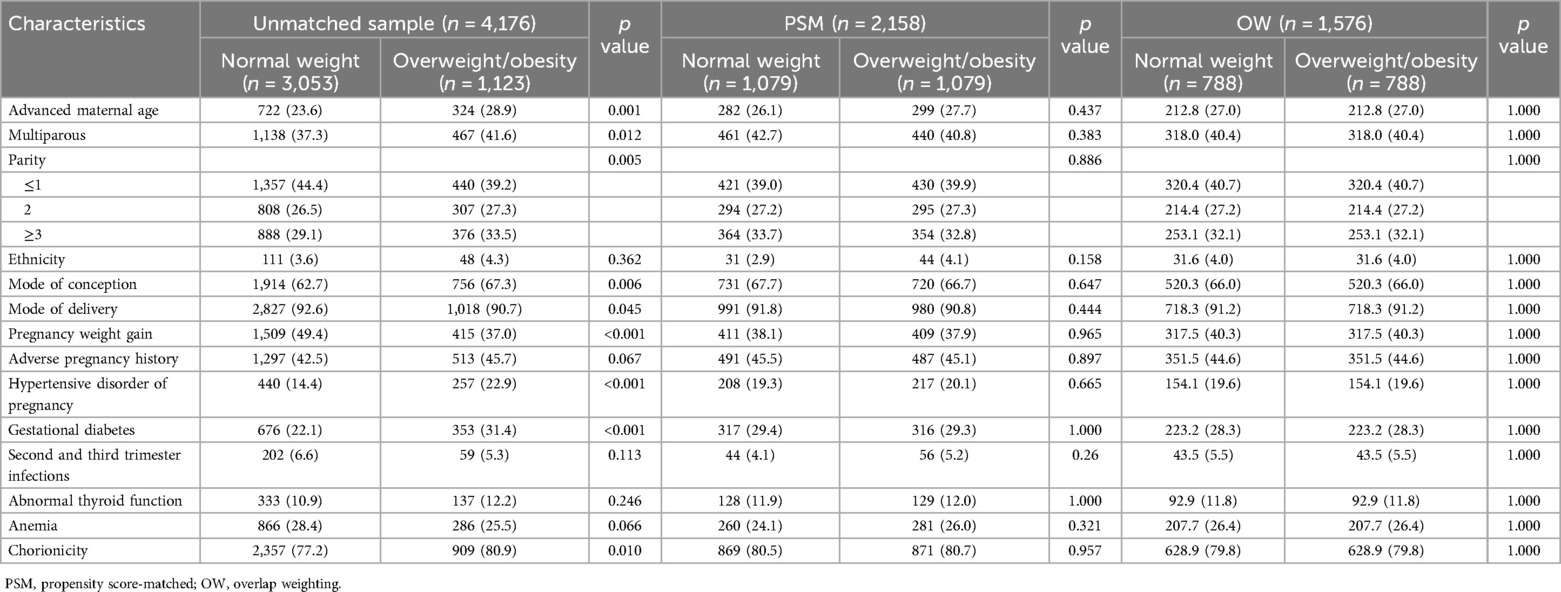
Table 1. Baseline characteristics of the unmatched sample, PSM sample, and OW sample for comparison between prepregnancy normal weight and overweight/obesity.
3.2 Primary outcomes
3.2.1 Normal prepregnancy weight vs. overweight/obesity
3.2.1.1 Bivariable analysis
In the unmatched sample of 4,176 twins, those born to mothers who were overweight/obese before pregnancy had a higher incidence of 1-min Apgar scores ≤7 compared to those born to mothers with normal prepregnancy weight (7.7% vs. 5.1% for the larger twin and 7.6% vs. 5.2% for the smaller twin). A similar trend was observed for 5-min Apgar scores ≤7 (1.7% vs. 0.8% for the larger twin and 1.4% vs. 0.8% for the smaller twin) (Model 1).
The crude ORs (95%CIs) from unadjusted logistic regression (Model 1) were 1.57 (1.19–2.06) for the larger twin and 1.49 (1.13–1.95) for the smaller twin in the 1-min Apgar score ≤7 group. After adjusting for confounders using multivariable logistic regression (Model 2), maternal prepregnancy overweight/obesity remained significantly associated with an increased risk of low 1-min Apgar scores, with ORs (95% CIs) of 1.60 (1.20–2.13) for the larger twin and 1.45 (1.09–1.92) for the smaller twin (Table 2).
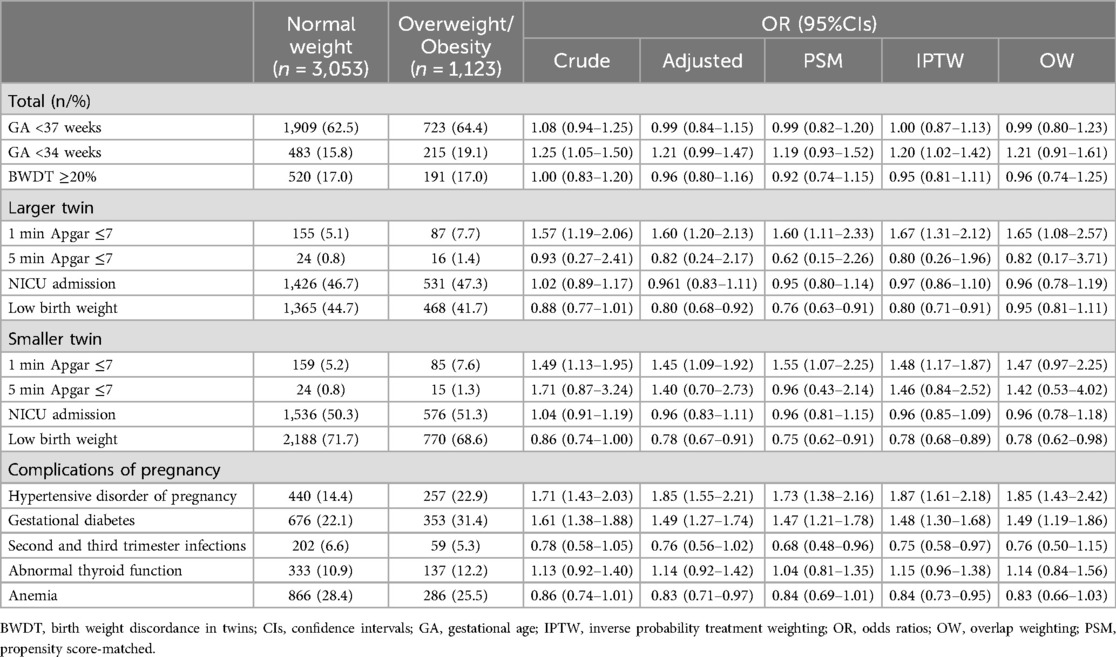
Table 2. The results of OR (95% CIs) for comparisons between prepregnancy normal weight and overweight/obesity in each model.
However, no significant associations were found for 5-min Apgar scores ≤7. The crude ORs (95% CIs) from unadjusted logistic regression (Model 1) were 0.93 (0.27–2.41) for the larger twin and 1.71 (0.87–3.24) for the smaller twin. After adjustment (Model 2), maternal prepregnancy overweight/obesity showed no significant impact on the risk of low 5-min Apgar scores, with ORs (95% CIs) of 0.82 (0.24–2.17) for the larger twin and 1.40 (0.70–2.73) for the smaller twin (Table 2).
3.2.2 Propensity score-matched analysis
To investigate the impact of maternal prepregnancy BMI on the 1-min and 5 min Apgar score of twin infants, it was essential to eliminate confounding factors such as preeclampsia and GDM, which have been indicated to affect Apgar scores (17, 18). A 1:1 PSM analysis (Model 3) was performed. As illustrated in Figure 2, all baseline variables had SMDs of less than 0.1, indicating that the two groups were well balanced after matching.
The results revealed that twin infants born to mothers with prepregnancy overweight/obesity were at a higher risk of having a 1-min Apgar score ≤7 compared to those born to mothers with normal prepregnancy weight, with ORs (95% CIs) of 1.60 (1.11–2.33) for the larger twin and 1.55 (1.07–2.25) for the smaller twin, respectively (Table 2). In contrast, no significant differences were observed in the 5-min Apgar score ≤7 group, with ORs (95% CIs) of 0.62 (0.15–2.26) for the larger twin and 0.96 (0.43–2.14) for the smaller twin.
3.2.3 Inverse probability treatment weighting or overlap weighting analysis
Weighted multivariable logistic regression analyses using IPTW (Model 4) and OW (Model 5) revealed that maternal prepregnancy overweight/obesity increased the risk of a 1-min Apgar score ≤7 in the larger twin, with ORs (95% CI) of 1.67 (1.31–2.12) and 1.65 (1.08–2.57), respectively (Table 2; Supplementary Table S3). Similar results were observed for smaller twin in the analysis with IPTW, while the analysis with OW almost reached significance, with ORs (95% CI, p value) of 1.48 (1.17–1.87, 0.001) and 1.46 (0.97–2.25, 0.07), respectively. In contrast, analyses for 5-min Apgar scores ≤7 confirmed no significant differences using either IPTW or OW (Table 2; Supplementary Table S3). Weighted baseline characteristics were poorly balanced for IPTW but well-balanced for OW (Supplementary Figure S1).
3.3 Normal prepregnancy weight vs. underweight
In contrast to the increased risk associated with prepregnancy overweight/obesity, maternal prepregnancy underweight was associated with a decreased risk of 1-min Apgar score ≤7 in twins. For the larger twin, the ORs (95% CIs) across the models were 0.56 (0.32–0.92) in Model 1, 0.50 (0.28–0.83) in Model 2, 0.48 (0.24–0.89) in Model 3, 0.49 (0.29–0.78) in Model 4, and 0.50 (0.24–0.98) in Model 5 (Supplementary Table S4). For the smaller twin, the ORs (95% CIs) values in Models 1–5 were 0.58 (0.34–0.94), 0.56 (0.32–0.91), 0.49 (0.25–0.91), 0.55 (0.33–0.86), and 0.55 (0.27–1.07), respectively (Supplementary Table S4). However, no significant differences were observed for 5-min Apgar scores ≤7 across Models 1–5. The baseline characteristics before and after matching or weighting were balanced, as shown in Supplementary Figure S2.
3.4 Other outcomes
Furthermore, the study also examined the impact of different prepregnancy BMI values on various outcomes, including NICU admission, preterm birth (GA <37 weeks), early preterm birth (GA <34 weeks), LBW, and BWDT. Compared to normal prepregnancy BMI, prepregnancy overweight/obesity had a protective effect on smaller twin, reducing the likelihood of LBW. However, maternal normal weight before pregnancy did not have a significant effect on other outcomes, such as NICU admission, preterm birth, early preterm birth, and BWDT, when compared to maternal prepregnancy overweight/obesity or underweight (Table 2; Supplementary Table S4).
3.5 Prepregnancy BMI and pregnancy complications
Table 1 shows the association between different BMI values and maternal pregnancy-related diseases. According to the analysis of Models 1–5, compared with normal prepregnancy weight, maternal overweight/obesity before pregnancy not only increased the risk of developing GDM during pregnancy, with ORs (95% CIs) of 1.61 (1.38–1.88), 1.49 (1.27–1.74), 1.47 (1.21–1.78), 1.48 (1.30–1.68), and 1.49 (1.19–1.86), respectively, but also increased the likelihood of developing gestational hypertension, with ORs (95% CIs) of 1.71 (1.43–2.03), 1.85 (1.55–2.21), 1.73 (1.38–2.16), 1.87 (1.61–2.18), and 1.85 (1.43–2.42) (Table 2; Supplementary Table S5). Conversely, prepregnancy underweight did not present such risks (Supplementary Tables S4–S6). Furthermore, different prepregnancy BMI values did not exhibit significant effects on the occurrence of thyroid function abnormalities, anaemia, and infections during mid-late pregnancy.
3.6 Stratified analysis and sensitivity analysis
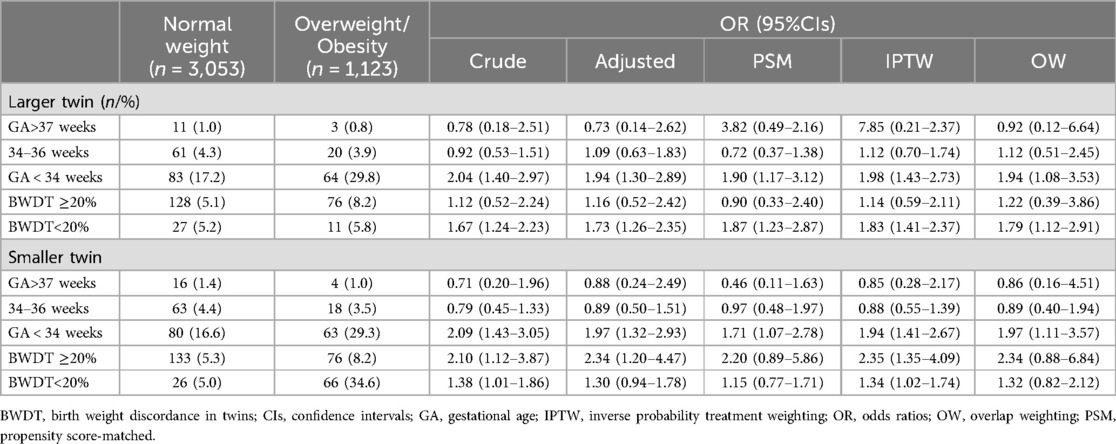
To ensure the reliability of our findings regarding the impact of different prepregnancy BMI categories on 1-min and 5-min Apgar scores, we performed sensitivity analyses by stratifying twins based on GA and BWDT. The results showed that, compared to normal weight, prepregnancy overweight/obesity significantly increased the risk of 1-min Apgar scores ≤7 in twins with a GA less than 34 weeks (Table 3). However, when stratified by BWDT, prepregnancy BMI did not consistently show a significant association with 1-min or 5-min Apgar scores ≤7 (Table 3). Similar results were observed when comparing prepregnancy underweight with normal weight (Supplementary Table S7).
In addition, we conducted subgroup analyses for overweight and obesity. We found that prepregnancy overweight increased the risk of 1-min Apgar scores ≤7 for both larger twins (adjusted OR 1.60, 95% CI: 1.17–2.18) and smaller twins (adjusted OR 1.43, 95% CI: 1.04–1.94). Prepregnancy obesity, however, was associated with an increased risk of 1-min Apgar scores ≤7 only in larger twins (adjusted OR 1.75, 95% CI: 1.03–2.86) but not in smaller twins (adjusted OR 1.58, 95% CI: 0.94–2.54). Additionally, validation methods such as PSM or OW failed to yield consistent results (Supplementary Table S7, S8).
Further sensitivity analyses were performed by adjusting the matching algorithm using various caliper values (0.02, 0.1, 0.2) and matching ratios (1:1, 2:1) or applying a probit model. Regardless of these adjustments, the statistical significance of the results remained consistent, confirming the robustness of our effect estimates (data not shown).
4 Discussion
Our study revealed that maternal prepregnancy overweight/obesity increases the risk of adverse outcomes in twin pregnancies, particularly a higher risk of a 1-min Apgar score ≤7 in both larger and smaller twins, while no significant association was observed with 5-min Apgar scores. Sensitivity analysis further indicated that this effect is primarily evident in twins with a GA of less than 34 weeks. Interestingly, compared to normal prepregnancy weight, maternal underweight was associated with a lower risk of 1-min Apgar scores ≤7, suggesting a progressive increase in risk with rising prepregnancy BMI. Additionally, secondary findings demonstrated that prepregnancy overweight/obesity is associated with a higher risk of gestational hypertension and GDM, emphasizing the importance of maternal weight management before pregnancy.
Our analysis consistently showed that prepregnancy overweight or obesity is associated with adverse outcomes across various models, even after adjusting for multiple prepregnancy covariates. Specifically, our findings align with previous research indicating that, in twin pregnancies, prepregnancy overweight increases the risk of GDM, HDP, and low Apgar scores (8, 19–23). Studies that do not distinguish between singleton and twin pregnancies similarly report that higher prepregnancy BMI is associated with increased risks of GDM, neonatal mortality, preeclampsia, and low Apgar scores (24, 25). Comparable findings have also been observed in singleton pregnancies, where elevated prepregnancy BMI is linked to lower Apgar scores (26). These results suggest that although twin pregnancies are generally at a higher risk for adverse outcomes, prepregnancy overweight or obesity contributes similarly to adverse outcomes in both singleton and twin pregnancies.
The prevalence of obesity is increasing across all age groups and social strata due to environmental and genetic factors (27, 28). While the exact mechanisms by which prepregnancy obesity contributes to low Apgar scores in newborns remain unclear, several factors are generally recognized. Firstly, obesity induces metabolic disorders, such as inflammation and abnormal blood sugar and lipid levels, which may cross the placenta and negatively impact fetal health (29, 30). Additionally, obesity can lead to structural and functional changes in the placenta, potentially restricting fetal nutrition and oxygen supply, which increases the risk of low Apgar scores (3, 31). Moreover, prepregnancy obesity is frequently associated with conditions like hypertension and diabetes, further compounding this risk (18, 32, 33).
Twin pregnancies also introduce additional metabolic challenges, such as vitamin D deficiency and elevated bile acid levels. Vitamin D deficiency, which is more common in twin pregnancies due to the increased nutritional demands, has been associated with impaired fetal development and lower Apgar scores (34, 35). Elevated bile acid levels, often seen in twin pregnancies, can lead to hepatic dysfunction and impaired fetal circulation, further increasing the risk of adverse neonatal outcomes (36, 37). Both of these factors have been linked to lower Apgar scores, which reflect the newborn's immediate post-birth health and response to environmental stress. Finally, the combination of obesity and twin pregnancy substantially increases the complexity of cesarean sections, often resulting in longer surgery and anesthesia times, along with heightened risks of infection and fetal distress—all of which may contribute to lower Apgar scores in newborns (38–40). In summary, prepregnancy obesity and the metabolic challenges associated with twin pregnancies can collectively impact newborn outcomes through multiple pathways.
Our study highlights a significant association between maternal prepregnancy overweight/obesity and low 1-min Apgar scores in twins, particularly those born at gestational ages <34 weeks, while no such association was observed for 5-min Apgar scores. This contrast underscores the immediate impact of maternal obesity-related factors, such as impaired uteroplacental perfusion and fetal hypoxia, which contribute to neonatal distress shortly after birth (41, 42). However, the absence of an association with 5-min Apgar scores suggests that timely and effective perinatal interventions, including resuscitation, oxygen supplementation, and other neonatal care measures, are crucial in mitigating these challenges and facilitating neonatal recovery and stabilization within the first 5 min after birth (43–45).
The differences observed between 1-min and 5-min Apgar scores carry important clinical implications. The association between maternal prepregnancy obesity/overweight and low 1-min Apgar scores highlights the role of obesity-related comorbidities, such as gestational hypertension and diabetes, in impairing uteroplacental perfusion, leading to fetal hypoxia and neonatal distress immediately after birth (5, 46). The lack of association with 5-min Apgar scores underscores the effectiveness of prompt perinatal interventions in alleviating these risks (47). Obesity-related inflammation, oxidative stress, and metabolic disturbances likely exacerbate risks of suboptimal uteroplacental perfusion and fetal oxygenation, increasing the likelihood of low 1-min Apgar scores (48, 49), while rapid stabilization through medical care may explain the absence of a 5-min association (50). Despite the observational nature of this study and potential residual confounding, robust sensitivity analyses strengthen the credibility of these findings. Clinically, these results emphasize the importance of optimizing maternal BMI before pregnancy and implementing targeted interventions for at-risk mothers, particularly those with preterm twins, to mitigate immediate postpartum risks and improve neonatal outcomes.
Interestingly, maternal underweight was associated with a lower risk of low 1-min Apgar scores, suggesting a potential inverse relationship between BMI and neonatal outcomes. This may reflect differences in placental structure or metabolic adaptations across BMI categories, as underweight mothers might experience more efficient placental function, optimizing nutrient and oxygen transfer to the fetus and reducing the risk of immediate neonatal distress (51, 52). Additionally, lower maternal adiposity could minimize inflammatory processes and metabolic disturbances commonly seen in overweight and obese individuals, further contributing to favorable outcomes (53). However, maternal underweight also poses significant risks during pregnancy, including inadequate nutritional reserves, which can lead to fetal growth restriction, low birth weight, and preterm birth, along with potential long-term developmental challenges for the neonate (54, 55). Underweight mothers are also at higher risk of complications such as anemia, reduced immune function, and overall poor maternal health during and after pregnancy (56, 57). These findings emphasize the importance of achieving a balanced and healthy BMI prior to pregnancy to optimize outcomes for both mother and child.
The dangers of low Apgar scores are widely recognized. If left untreated, they can lead to neonatal asphyxia and ischaemic hypoxic encephalopathy, which can adversely affect newborn intellectual development and place significant burdens on families and society. Therefore, it is crucial to identify high-risk factors that may contribute to low Apgar scores in advance. In addition to prepregnancy overweight/obesity, as found in this study, other factors, such as excessive weight gain during pregnancy, infections during pregnancy, and placental abruption, can lead to low Apgar scores (58–60). This article provides evidence of the impact of prepregnancy BMI on the Apgar scores of twins, emphasizing the importance of prepregnancy weight management and preparedness for neonatal resuscitation.
Our study has several strengths (1): To our knowledge, it is one of the few studies in China to investigate the impact of prepregnancy BMI on adverse outcomes such as low Apgar scores in twins; (2) The study included multiple medical units from several provincial administrative regions in China, providing a representative and timely sample; (3) The use of PSM, IPTW, and OW can simulate a randomized controlled trial, thereby reducing the risk of indication bias (61, 62). These techniques are crucial in addressing and reducing the risk of indication bias, which can arise due to non-random assignment of treatment groups in observational studies. By mimicking the conditions of a RCT, we have enhanced the robustness of our findings and provided more reliable estimates of the effect of prepregnancy BMI on twin outcomes. (4) Through strict methods, including five different models and three sensitivity analyses, we obtained consistent results among twin foetuses, and the stability of the results is relatively good.
We acknowledge that our study has several limitations. The inclusion of additional covariates in our analysis led to the exclusion of some data. Nevertheless, the consistency of our results across all models suggests that our findings are robust. Moreover, we combined overweight and obesity into a single group to enhance statistical power. While this approach is supported by the similarity in their mechanisms, it may introduce confounding bias and dilution effects due to differences in the degree of impact associated with these weight categories. Additionally, the relatively small sample size of our study may limit the generalizability of our findings, and future research should aim to incorporate larger-scale datasets to validate these results. Finally, as our study was retrospective in nature, we were unable to control for all potential confounding factors. Despite these limitations, our study provides valuable insights into the impact of prepregnancy BMI on neonatal outcomes in twin pregnancies and underscores the importance of further research in this area.
5 Conclusion
Prepregnancy overweight/obesity is associated with adverse outcomes, including a higher risk of low Apgar scores in twins, as well as HDP and GDM in twin pregnancies. These findings underscore the importance of guiding prepregnancy weight management and advising paediatricians to prepare for the prevention and treatment of neonatal asphyxia caused by low Apgar scores in twins.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Third Affiliated Hospital of Guangzhou Medical University [Clinical Research Review (2020) No. 097]. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants' legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ZF: Conceptualization, Data curation, Investigation, Writing – original draft. XW: Data curation, Software, Validation, Writing – original draft. XT: Data curation, Investigation, Validation, Writing – review & editing. ZH: Data curation, Investigation, Writing – review & editing. CY: Data curation, Investigation, Writing – review & editing. WS: Data curation, Investigation, Writing – review & editing. YD: Data curation, Investigation, Writing – review & editing. JL: Data curation, Investigation, Writing – review & editing. QM: Data curation, Investigation, Writing – review & editing. AZ: Data curation, Investigation, Writing – review & editing. HJ: Data curation, Investigation, Writing – review & editing. WY: Data curation, Investigation, Writing – review & editing. JQ: Data curation, Investigation, Writing – review & editing. XW: Data curation, Investigation, Writing – review & editing. YZ: Data curation, Investigation, Writing – review & editing. XL: Data curation, Investigation, Writing – review & editing. LL: Data curation, Investigation, Writing – review & editing. YJ: Data curation, Investigation, Writing – review & editing. YW: Data curation, Investigation, Writing – review & editing. XY: Data curation, Investigation, Writing – review & editing. YW: Data curation, Investigation, Writing – review & editing. YC: Data curation, Investigation, Writing – review & editing. XL: Project administration, Supervision, Validation, Writing – review & editing. QC: Funding acquisition, Project administration, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the Guangdong Provincial Medical Science and Technology Research Fund (A2022101) and the Guangzhou Municipal Health and Health Technology Project (20221A011089), both of which are public institutions providing research support.
Acknowledgment
We thank American Journal Experts for editing this manuscript. We would like to acknowledge the use of ChatGPT 4.0 (OpenAI) for language translation assistance in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1412975/full#supplementary-material
Supplementary Figure S1 | Love plot of baseline variable balance between normal weight and overweight/obesity groups. This Love plot visualizes the standardized mean difference (SMD) values for baseline variables between normal weight and overweight/obesity groups in unmatched and adjusted models, including propensity score matching (PSM), inverse probability treatment weighting (IPTW), and overlap weighting (OW). Variables assessed include prepregnancy BMI, pregnancy weight gain, hypertensive disorder, gestational diabetes, advanced maternal age, parity, mode of conception, chorionicity, multiparous status, mode of delivery, anemia, adverse pregnancy history, infections in the second and third trimesters, abnormal thyroid function, and ethnicity. An SMD <10% indicates a relatively good balance between groups.
Supplementary Figure S2 | Love plot of baseline variable balance between underweight and normal weight groups. This Love plot depicts the standardized mean difference (SMD) values for baseline variables between underweight and normal weight groups across different models, including the unmatched model and adjusted models: propensity score matching (PSM), inverse probability treatment weighting (IPTW), and overlap weighting (OW). The key variables assessed are prepregnancy BMI, advanced maternal age, mode of conception, gestational diabetes, ethnicity, hypertensive disorders, chorionicity, parity, abnormal thyroid function, multiparity, adverse pregnancy history, pregnancy weight gain, second- and third-trimester infections, mode of delivery, and anemia. An SMD of less than 10% signifies a relatively good balance between the groups. This analysis demonstrates the effectiveness of these models in achieving covariate balance for more reliable comparisons.
Abbreviations
BMI, body mass index; BWDT, birth weight discordance in twins; CIs, confidence intervals; GA, gestational age; IPTW, inverse probability of treatment weighting; ORs, odds ratios; OW, overlapping weighting; PSM, propensity score matching analysis; PWG, pregnancy weight gain; SMDs, standardized mean differences.
References
1. Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. (2015) 33(7):673–89. doi: 10.1007/s40273-014-0243-x
2. Pan XF, Wang L, Pan A. Epidemiology and determinants of obesity in China. Lancet Diabetes Endocrinol. (2021) 9(6):373–92. doi: 10.1016/S2213-8587(21)00045-0
3. Catalano PM, Shankar K. Obesity and pregnancy: mechanisms of short term and long term adverse consequences for mother and child. Br Med J. (2017) 356:j1. doi: 10.1136/bmj.j1
4. Marchi J, Berg M, Dencker A, Olander EK, Begley C. Risks associated with obesity in pregnancy, for the mother and baby: a systematic review of reviews. Obes Rev. (2015) 16(8):621–38. doi: 10.1111/obr.12288
5. Zhu T, Tang J, Zhao F, Qu Y, Mu D. Association between maternal obesity and offspring Apgar score or cord pH: a systematic review and meta-analysis. Sci Rep. (2015) 5:18386. doi: 10.1038/srep18386
6. Cnattingius S, Johansson S, Razaz N. Apgar score and risk of neonatal death among preterm infants. N Engl J Med. (2020) 383(1):49–57. doi: 10.1056/NEJMoa1915075
7. Zhong YJ, Claveau M, Yoon EW, Aziz K, Singhal N, Shah PS, et al. Neonates with a 10-min Apgar score of zero: outcomes by gestational age. Resuscitation. (2019) 143:77–84. doi: 10.1016/j.resuscitation.2019.07.036
8. Schubert J, Timmesfeld N, Noever K, Behnam S, Vinturache A, Arabin B. Impact of maternal body mass index and gestational weight gain on maternal and neonatal outcomes in twin pregnancies. Acta Obstet Gynecol Scand. (2023) 102(2):181–9. doi: 10.1111/aogs.14485
9. B.-O. Committee on Practice and M. Society for Maternal-Fetal. Practice bulletin No. 169: multifetal gestations: twin, triplet, and higher-order multifetal pregnancies. Obstet Gynecol. (2016) 128(4):e131–46. doi: 10.1097/AOG.0000000000001709
10. Wang Y, Mi J, Shan XY, Wang QJ, Ge KY. Is China facing an obesity epidemic and the consequences? The trends in obesity and chronic disease in China. Int J Obes (Lond). (2007) 31(1):177–88. doi: 10.1038/sj.ijo.0803354
11. Fox NS, Rebarber A, Roman AS, Klauser CK, Peress D, Saltzman DH. Weight gain in twin pregnancies and adverse outcomes: examining the 2009 institute of medicine guidelines. Obstet Gynecol. (2010) 116(1):100–6. doi: 10.1097/AOG.0b013e3181e24afc
12. C. S. o. O. Obstetrics Subgroup, C. M. A. Gynecology, C. S. o. P. M. C. M. A, Group of Pregnancy with Diabetes Mellitus, O. Obstetrics Subgroup Chinese Society of, A. Gynecology Chinese Medical and A. Group of Pregnancy with Diabetes Mellitus Chinese Society of Perinatal Medicine Chinese Medical. Diagnosis and therapy guideline of pregnancy with diabetes mellitus. Zhonghua Fu Chan Ke Za Zhi. (2014) 49(8):561–9.25354853
13. C. S. o. O. Hypertensive Disorders in Pregnancy Subgroup, C. M. A. Gynecology, O. Hypertensive Disorders in Pregnancy Subgroup Chinese Society of and A. Gynecology Chinese Medical. Diagnosis and treatment guideline of hypertensive disorders in pregnancy (2015). Zhonghua Fu Chan Ke Za Zhi. (2015) 50(10):721–8.26675569
14. Su Z, Lin L, Fan X, Jia C, Shi B, Huang X, et al. Increased risk for respiratory complications in male extremely preterm infants: a propensity score matching study. Front Endocrinol (Lausanne). (2022) 13:823707. doi: 10.3389/fendo.2022.823707
15. Kuss O, Blettner M, Börgermann J. Propensity score: an alternative method of analyzing treatment effects. Dtsch Arztebl Int. (2016) 113(35–36):597–603. doi: 10.3238/arztebl.2016.0597
16. Cheng C, Li F, Thomas LE, Li FF. Addressing extreme propensity scores in estimating counterfactual survival functions via the overlap weights. Am J Epidemiol. (2022) 191(6):1140–51. doi: 10.1093/aje/kwac043
17. Ajibo BD, Wolka E, Aseffa A, Nugusu MA, Adem AO, Mamo M, et al. Determinants of low fifth minute Apgar score among newborns delivered by cesarean section at Wolaita Sodo university comprehensive specialized hospital, southern Ethiopia: an unmatched case control study. BMC Pregnancy Childbirth. (2022) 22(1):665. doi: 10.1186/s12884-022-04999-z
18. Fuka F, Osuagwu UL, Agho K, Gyaneshwar R, Naidu S, Fong J, et al. Factors associated with macrosomia, hypoglycaemia and low Apgar score among Fijian women with gestational diabetes mellitus. BMC Pregnancy Childbirth. (2020) 20(1):133. doi: 10.1186/s12884-020-2821-6
19. Kivela J, Sormunen-Harju H, Girchenko PV, Huvinen E, Stach-Lempinen B, Kajantie E, et al. Longitudinal metabolic profiling of maternal obesity, gestational diabetes, and hypertensive pregnancy disorders. J Clin Endocrinol Metab. (2021) 106(11):e4372–88. doi: 10.1210/clinem/dgab475
20. Raatikainen K, Heiskanen N, Heinonen S. Transition from overweight to obesity worsens pregnancy outcome in a BMI-dependent manner. Obesity (Silver Spring). (2006) 14(1):165–71. doi: 10.1038/oby.2006.20
21. Sweeting A, Wong J, Murphy HR, Ross PG. A clinical update on gestational diabetes mellitus. Endocr Rev. (2022) 43(5):763–93. doi: 10.1210/endrev/bnac003
22. Liu X, Wang H, Yang L, Zhao M, Magnussen CG, Xi B. Associations between gestational weight gain and adverse birth outcomes: a population-based retrospective cohort study of 9 million mother-infant pairs. Front Nutr. (2022) 9:811217. doi: 10.3389/fnut.2022.811217
23. Mitha A, Chen R, Johansson S, Razaz N, Cnattingius S. Maternal body mass index in early pregnancy and severe asphyxia-related complications in preterm infants. Int J Epidemiol. (2020) 49(5):1647–60. doi: 10.1093/ije/dyaa088
24. Shirvanifar M, Ahlqvist VH, Lundberg M, Kosidou K, Herraiz-Adillo Á, Berglind D, et al. Adverse pregnancy outcomes attributable to overweight and obesity across maternal birth regions: a Swedish population-based cohort study. Lancet Public Health. (2024) 9(10):e776–86. doi: 10.1016/S2468-2667(24)00188-9
25. Mackeen AD, Boyd VE, Schuster M, Young AJ, Gray C, Angras K. The impact of prepregnancy body mass index on pregnancy and neonatal outcomes. J Osteopath Med. (2024) 124(10):447–53. doi: 10.1515/jom-2024-0025
26. Steffen HA, Swartz SR, Kenne KA, Wendt LH, Jackson JB, Rysavy MB. Increased maternal BMI at time of delivery associated with poor maternal and neonatal outcomes. Am J Perinatol. (2024) 41(14):1908–17. doi: 10.1055/a-2274-0463
27. Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the global burden of disease study 2013. Lancet. (2014) 384(9945):766–81. doi: 10.1016/S0140-6736(14)60460-8
28. Wang Y, Zhao L, Gao L, Pan A, Xue H. Health policy and public health implications of obesity in China. Lancet Diabetes Endocrinol. (2021) 9(7):446–61. doi: 10.1016/S2213-8587(21)00118-2
29. Huda SS, Brodie LE, Sattar N. Obesity in pregnancy: prevalence and metabolic consequences. Semin Fetal Neonatal Med. (2010) 15(2):70–6. doi: 10.1016/j.siny.2009.09.006
30. Sureshchandra S, Marshall NE, Messaoudi I. Impact of pregravid obesity on maternal and fetal immunity: fertile grounds for reprogramming. J Leukoc Biol. (2019) 106(5):1035–50. doi: 10.1002/JLB.3RI0619-181R
31. Higgins L, Mills TA, Greenwood SL, Cowley EJ, Sibley CP, Jones LR. Maternal obesity and its effect on placental cell turnover. J Matern Fetal Neonatal Med. (2013) 26(8):783–8. doi: 10.3109/14767058.2012.760539
32. Lowe WL Jr, Scholtens DM, Lowe LP, Kuang A, Nodzenski M, Talbot O, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood adiposity. JAMA. (2018) 320(10):1005–16 doi: 10.1001/jama.2018.11628
33. Thrift AP, Callaway LK. The effect of obesity on pregnancy outcomes among Australian indigenous and non-indigenous women. Med J Aust. (2014) 201(10):592–5. doi: 10.5694/mja13.11170
34. Amjadi N, Pooransari P, Mirzamoradi M, Gargari SS, Naeiji Z, Rahmati N, et al. Association of maternal serum vitamin D level with fetal pulmonary artery Doppler indices and neonatal respiratory distress syndrome. J Clin Ultrasound. (2024) 52(7):913–22. doi: 10.1002/jcu.23734
35. Li X, Yu J, Wen L, Li Q, Yan J, Tian J, et al. Vitamin D status in women with dichorionic twin pregnancies and their neonates: a pilot study in China. BMC Pregnancy Childbirth. (2021) 21(1):279. doi: 10.1186/s12884-021-03707-7
36. Feng F, Li J, Liao J, Qin S, Liu Y, Che X, et al. Associations of clinical subtypes and bile acid levels of intrahepatic cholestasis of pregnancy with pregnancy outcomes. Sci Rep. (2024) 14(1):12185. doi: 10.1038/s41598-024-63183-9
37. Huang L, Li X, Liu T, Wei L, Fan C, Tang D, et al. Effect of intrahepatic cholestasis of pregnancy on infantile food allergy: a retrospective longitudinal study cohort in southwest China. Eur J Obstet Gynecol Reprod Biol. (2022) 272:110–5. doi: 10.1016/j.ejogrb.2022.03.026
38. Oltean I, Tran J, Mavedatnia D, Lawrence S, Tristani L, Moretti F, et al. Acute chorioamnionitis in pregnancies complicated by placental abruption and short-term neonatal outcomes: a retrospective cohort study. Placenta. (2022) 130:67–9. doi: 10.1016/j.placenta.2022.11.005
39. Gao Y, Song Y, Miao J, Lei X, Liu H, Gan L, et al. Correlation between anesthetic concentration and low Apgar scores in neonates born via cesarean sections under general anesthesia. BMC Pediatr. (2024) 24(1):571. doi: 10.1186/s12887-024-05041-1
40. Wang L, Liu C, Wang X, Zhu S, Zhang L, Wang B, et al. The impact of general anesthesia on the outcomes of preterm infants with gestational age less than 32 weeks delivered via cesarean section. Front Pharmacol. (2024) 15:1360691. doi: 10.3389/fphar.2024.1360691
41. Zong X, Wang H, Yang L, Guo Y, Zhao M, Magnussen CG, et al. Maternal pre-pregnancy body mass index categories and infant birth outcomes: a population-based study of 9 million mother-infant pairs. Front Nutr. (2022) 9:789833. doi: 10.3389/fnut.2022.789833
42. Minsart A-F, Buekens P, De Spiegelaere M, Englert Y. Neonatal outcomes in obese mothers: a population-based analysis. BMC Pregnancy Childbirth. (2013) 13(1):36. doi: 10.1186/1471-2393-13-36
43. Ringer SA, Aziz K. Neonatal stabilization and postresuscitation care. Clin Perinatol. (2012) 39(4):901–18. doi: 10.1016/j.clp.2012.09.007
44. Ladelund AK, Bruun FJ, Slavensky JA, Ladelund S, Kesmodel US. Association of Apgar score at 5 min with academic performance and intelligence in youth: a cohort study. Acta Obstet Gynecol Scand. (2022) 101(3):303–12. doi: 10.1111/aogs.14320
45. Selvaratnam RJ, Wallace EM, Davis PG, Rolnik DL, Fahey M, Davey MA. The 5-minute apgar score and childhood school outcomes. Acta Paediatr. (2022) 111(10):1878–84. doi: 10.1111/apa.16443
46. Yeagle KP, O’Brien JM, Curtin WM, Ural HS. Are gestational and type II diabetes mellitus associated with the Apgar scores of full-term neonates? Int J Womens Health. (2018) 10:603–7. doi: 10.2147/IJWH.S170090
47. Nguyen TC, Madappa R, Siefkes HM, Lim MJ, Siddegowda KM, Lakshminrusimha S. Oxygen saturation targets in neonatal care: a narrative review. Early Hum Dev. (2024) 199:106134. doi: 10.1016/j.earlhumdev.2024.106134
48. Marseglia L, Manti S, D’Angelo G, Nicotera A, Parisi E, Di Rosa G, et al. Oxidative stress in obesity: a critical component in human diseases. Int J Mol Sci. (2015) 16(1):378–400 doi: 10.3390/ijms16010378
49. Desoye G, Carter MA. Fetoplacental oxygen homeostasis in pregnancies with maternal diabetes mellitus and obesity. Nat Rev Endocrinol. (2022) 18(10):593–607. doi: 10.1038/s41574-022-00717-z
50. Huang S, Yitayew M, Rozycki JH. The contribution of low Apgar scores in identifying neonates with short-term morbidities in a large single center cohort. J Perinatol. (2024) 44(6):865–72. doi: 10.1038/s41372-024-01944-0
51. Scott H, Grynspan D, Anderson LN, Connor LK. Maternal underweight and obesity are associated with placental pathologies in human pregnancy. Reprod Sci. (2022) 29(12):3425–48. doi: 10.1007/s43032-022-00983-2
52. Zhou S, Yang Y, Zhang X, Mu X, Quan Q, Zhong Q. Perinatal outcomes of twin pregnancies with preterm premature rupture of the membranes at 24–34 weeks’ gestation. Sci Rep. (2021) 11(1):23419. doi: 10.1038/s41598-021-02884-x
53. Heslehurst N, Ngongalah L, Bigirumurame T, Nguyen G, Odeniyi A, Flynn A, et al. Association between maternal adiposity measures and adverse maternal outcomes of pregnancy: systematic review and meta-analysis. Obes Rev. (2022) 23(7):e13449. doi: 10.1111/obr.13449
54. Han Z, Mulla S, Beyene J, Liao G, Mcdonald DS. Maternal underweight and the risk of preterm birth and low birth weight: a systematic review and meta-analyses. Int J Epidemiol. (2011) 40(1):65–101. doi: 10.1093/ije/dyq195
55. Liu L, Ma Y, Wang N, Lin W, Liu Y, Wen D. Maternal body mass index and risk of neonatal adverse outcomes in China: a systematic review and meta-analysis. BMC Pregnancy Childbirth. (2019) 19(1):105. doi: 10.1186/s12884-019-2249-z
56. Tang J, Zhu X, Chen Y, Huang D, Tiemeier H, Chen R, et al. Association of maternal pre-pregnancy low or increased body mass index with adverse pregnancy outcomes. Sci Rep. (2021) 11(1):3831. doi: 10.1038/s41598-021-82064-z
57. Muglia LJ, Benhalima K, Tong S, Ozanne S. Maternal factors during pregnancy influencing maternal, fetal, and childhood outcomes. BMC Med. (2022) 20(1):418. doi: 10.1186/s12916-022-02632-6
58. Ferrari N, Mallmann P, Brockmeier K, Struder HK, Graf C. Secular trends in pregnancy weight gain in German women and their influences on foetal outcome: a hospital-based study. BMC Pregnancy Childbirth. (2014) 14:228. doi: 10.1186/1471-2393-14-228
59. Kojima T, Takami M, Shindo R, Saigusa Y, Miyagi E, Aoki S. Perinatal outcomes of recurrent placental abruption. J Matern Fetal Neonatal Med. (2021) 34(13):2192–6. doi: 10.1080/14767058.2019.1660766
60. Schneeberger C, Geerlings SE, Middleton P, Crowther CA. Interventions for preventing recurrent urinary tract infection during pregnancy. Cochrane Database Syst Rev. (2015) 2015(7):CD009279. doi: 10.1002/14651858.CD009279.pub3
61. D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. (1998) 17(19):2265–81. doi: 10.1002/(sici)1097-0258(19981015)17:19%3C2265::aid-sim918%3E3.0.co;2-b
Keywords: pregnancy, twins, Apgar score, overweight, neonatology
Citation: Feng Z, Wu X, Tong X, He Z, Yang C, Shen W, Ding Y, Liu J, Meng Q, Zhang A, Jiang H, Yan W, Qiu J, Wei X, Zhang Y, Lin X, Liu L, Jin Y, Wei Y, Yang X, Wang Y, Cai Y, Lin X and Cui Q (2025) Maternal prepregnancy overweight/obesity increase the risk of low Apgar scores in twins: a population-based cohort study in China. Front. Pediatr. 12:1412975. doi: 10.3389/fped.2024.1412975
Received: 6 April 2024; Accepted: 18 December 2024;
Published: 17 January 2025.
Edited by:
Guanghui Li, Beijing Maternal and Child Health Care Hospital, ChinaReviewed by:
Li Cai, Sun Yat-sen University, ChinaXin Li, Chengdu Women and Children's Central Hospital, China
Oluwasegun Akinyemi, Howard University, United States
Copyright: © 2025 Feng, Wu, Tong, He, Yang, Shen, Ding, Liu, Meng, Zhang, Jiang, Yan, Qiu, Wei, Zhang, Lin, Liu, Jin, Wei, Yang, Wang, Cai, Lin and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinzhu Lin, eGluemh1ZmpAMTYzLmNvbQ==; Qiliang Cui, Z3ljdWlxaWxpYW5nQDEyNi5jb20=
†These authors have contributed equally to this work
 Zhoushan Feng
Zhoushan Feng Xiaohong Wu1,†
Xiaohong Wu1,† Xiaomei Tong
Xiaomei Tong Chunxia Yang
Chunxia Yang Wei Shen
Wei Shen Yayu Zhang
Yayu Zhang Xiufang Yang
Xiufang Yang Qiliang Cui
Qiliang Cui