- 1Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Obstetric & Gynecologic and Pediatric Diseases and Birth Defects of Ministry of Education, Sichuan University, Chengdu, China
- 3Department of Pediatric Neurology Nursing, West China Second University Hospital, Sichuan University, Chengdu, China
Spinal muscular atrophy (SMA) is an autosomal recessive disease caused by mutations in the survival motor neuron 1 (SMN1) gene on chromosome 5, leading to the degeneration of lower motor neurons. There are few studies on cognitive impairment comorbid with SMA. Here, we report two cases of severe cognitive impairment in Chinese children with SMA type 1, marking the first such reports in this demographic. We propose that severe cognitive dysfunction may be a comorbidity of SMA. Clinicians should consider SMA in patients presenting with severe muscle weakness and atrophy accompanied by cognitive impairments, to avoid misdiagnosis and oversight.
Introduction
Spinal muscular atrophy (SMA) is an autosomal recessive genetic disease affecting motor neurons in the anterior horn caused by survival motor neuron 1 (SMN1) gene (5q11.2) mutation. The main manifestations include progressive muscle weakness and atrophy, primarily in the proximal limbs. Severely affected pediatric patients often succumb to respiratory failure. It has an incidence of approximately 1:10,000 (1, 2). According to the age of onset, motor milestones and the progress of the disease, SMA is divided into 0–4 types. SMA type 0 is defined by prenatal onset and is characterized by limited intrauterine activity. SMA type 1 represents the most serious infant phenotype. Symptoms typically manifest within the first six months after birth, with the maximum motor ability falling short of sitting unassisted. Most children die of respiratory failure within 2 years. SMA type 2 generally presents between 7 and 18 months of age, with patients able to sit independently but unable to stand or walk alone. SMA type 4 starts in adulthood, progresses slowly, and life expectancy is not shortened (3).
Reports often suggest that children with SMA exhibit higher cognitive abilities compared to healthy peers of the same age. Clinicians have noted their keen interest in the environment, sharp mental acuity, and observational skills, despite significant physical limitations (4). The development of cognitive skills may be a creative way to make up for its own limitations (5). For many years, researchers have been focusing on the study of SMA children's motor, breathing, and swallowing system, but cognitive development has not received much attention. SMA (mainly types 1 and 2) is associated with severe weakness, which affects hand coordination and speech acquisition. Limited interaction between speech and sensorimotor skills may lead to cognitive impairments (6, 7).
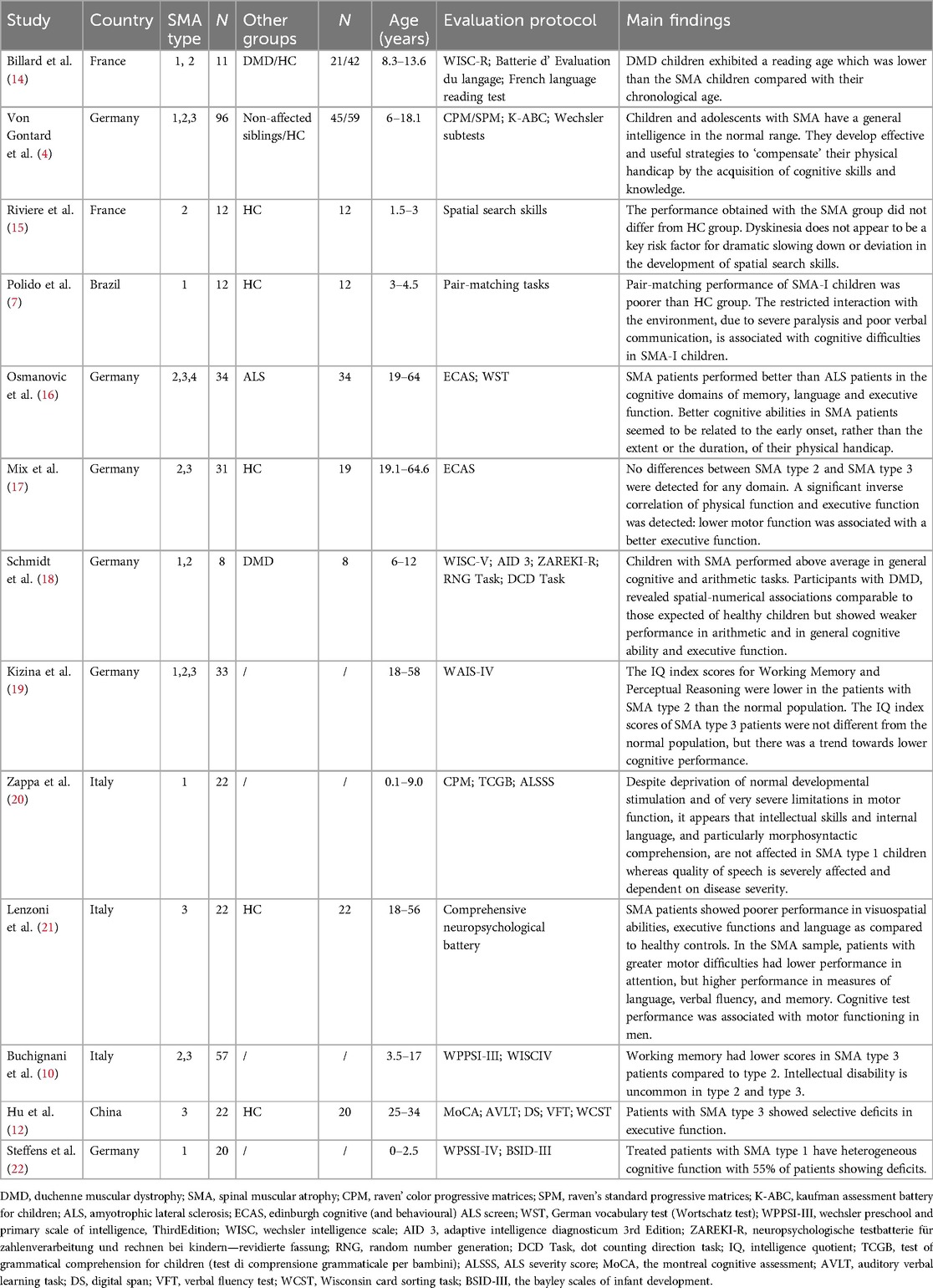
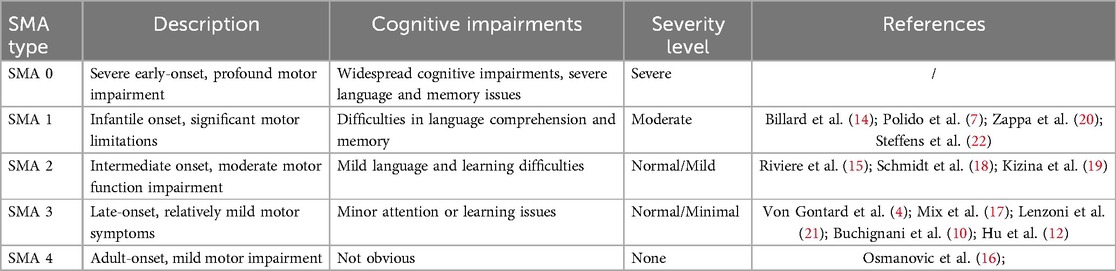
Cognitive abilities are frequently impacted in progressive neurodegenerative diseases, yet there is limited knowledge regarding cognitive deficits in SMA patients. Due to the severity of the condition, patients with SMA type 0 may also experience significant impacts on cognitive development. Severe neurological involvement is likely to represent the ultimate manifestation of an extreme phenotype of SMA type 0, resulting from a significant reduction of SMN protein levels in the brain (8). There are very few studies on cognitive impairment in SMA type 0. Although some SMA type 1 patients may not show significant cognitive impairments, early motor function damage can indirectly affect cognitive abilities. SMA type 1 patients often exhibit difficulties in language comprehension and memory (5, 9). Cognitive function in SMA type 2 patients is generally better, but they may still display mild language and learning difficulties (10). Research has found that cognitive impairments in this type are relatively few and less severe. For SMA type 3 patients, cognitive impairments are less common, though some individuals may experience minor attention or learning problems (11, 12). SMA Type 4 usually has minimal or no effect on cognitive function, and there are few studies on cognitive impairment in SMA Type 4 (13).
We aim to describe and discuss the factors and pathogenesis affecting cognitive function in SMA through the study of two cases of 5q- SMA children's cognitive dysfunction. We reviewed the literature to assess the cognitive performance of children with SMA and possible differences in cognitive performance among children with different subtypes of SMA (see Tables 1, 2).
Case presentation
Case 1
The patient was a 6-year-old girl who first presented to our outpatient clinic at 6 months of age, exhibiting an inability to roll over, sit, or stand independently. She was born at full term, and her birth history was unremarkable. Her parents are healthy and unrelated. She was able to raise her head at 3 months, but at 5 months, she could not grasp objects with her hands. After 6 months, her motor function further deteriorated, and she lost the ability to hold her head up, roll over, or sit independently. She also exhibited cognitive dysfunction. By 12 months, she could consciously say “dad” and “mom” and respond to her name. After 18 months, she could only understand simple instructions. She had memory disorders and could not perform counting or simple calculations (see Figure 1A). Neurological examination revealed bilateral flexion of the knees and toes. Neither knee nor Achilles tendon reflexes could be elicited, and there was a decrease in limb muscle tone and strength. At 4.5 years of age, cognitive functioning was assessed using the Wechsler Preschool and Primary Scale of Intelligence, Fourth Edition, Chinese version (WPPSI-IV). The intelligence quotient (IQ) score was ≤40. The Verbal Comprehension Index, Visuospatial Index, Fluid Reasoning Index, Working Memory Index, and Processing Speed Index were all ≤45, indicating low competence in each subscale.
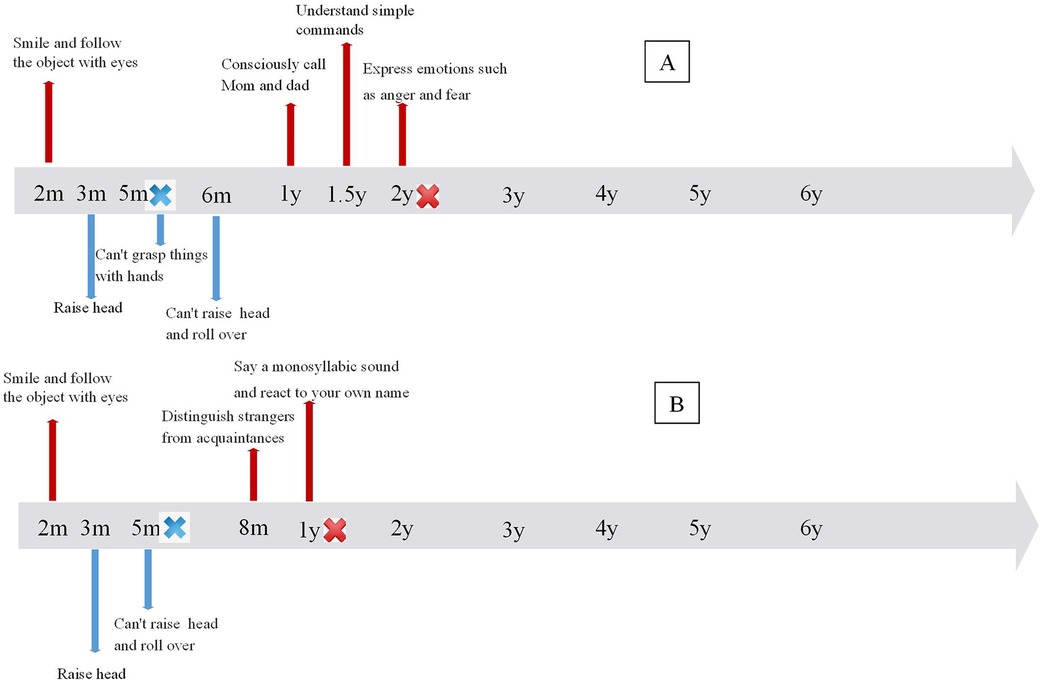
Figure 1. Time line of cognitive and motor development in 2 patients. (A) Timeline of Patient 1, (B) timeline of Patient 2.
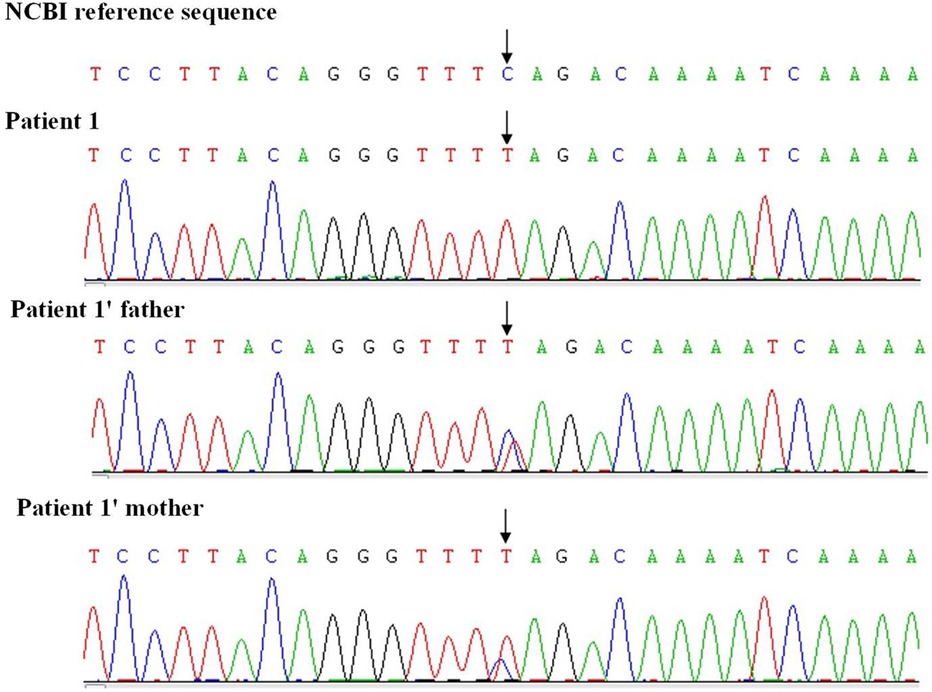
Results of routine blood tests, blood biochemistry, lactic acid, blood ammonia, and muscle enzyme levels were normal. Brain MRI showed slightly enlarged bilateral lateral ventricles. Needle electromyography revealed chronic denervation. MRI of the leg muscles showed atrophy of the bilateral thigh and buttock muscles. Spinal x-ray revealed scoliosis with a Cobb angle of 33 degrees. Whole exome sequencing identified homozygous mutations in exons 7 and 8 (c.840C>T) of the SMN1 gene on chromosome 5q13 (see Figure 2). The MLPA assay revealed a copy number of 0 for exons 7 and 8 of the SMN1 gene and a copy number of 3 for exons 7 and 8 of the SMN2 gene.
At 5 months of age, the patient initially presented with clinical symptoms indicative of a neuromuscular disorder. Whole exome sequencing and MLPA identified an increased copy number of SMN2, leading to a definitive diagnosis of 5q-associated spinal muscular atrophy type 1. She received four loading treatments and three maintenance treatments of nusinersen from 4.8 years of age. Post-treatment WPPSI-IV testing showed an IQ score of ≤40 and scores <45 on all subtests, suggesting no improvement in cognitive function. The Children's Hospital of Philadelphia Infant Test of Neuromuscular Disorders (CHOP-INTEND) showed no significant improvement in motor function.
Case 2
The child, an 8-year-old boy, was first admitted to our outpatient clinic at 8 months due to an inability to turn over and sit independently. He was born full term with an unremarkable birth history. His parents are healthy and unrelated. Initially, the patient could hold his head up at 2–3 months, but was unable to hold his head up and roll over after 5 months and unable to crawl or sit down independently after 8 months. He exhibited cognitive dysfunction. After one year, he could only produce monosyllabic sounds and respond to his name. He displayed memory impairment, unable to recognize familiar objects or remember daily routines, and could neither count numbers nor perform simple calculations (see Figure 1B). Neurological examination revealed absent knee and Achilles tendon reflexes, atrophied limb muscles, and reduced muscle tone and strength. Cognitive assessment with the WISC -IV at 6.3 years indicated an IQ score of ≤40, with scores of ≤45 across all subtests, reflecting severe impairment in each domain.
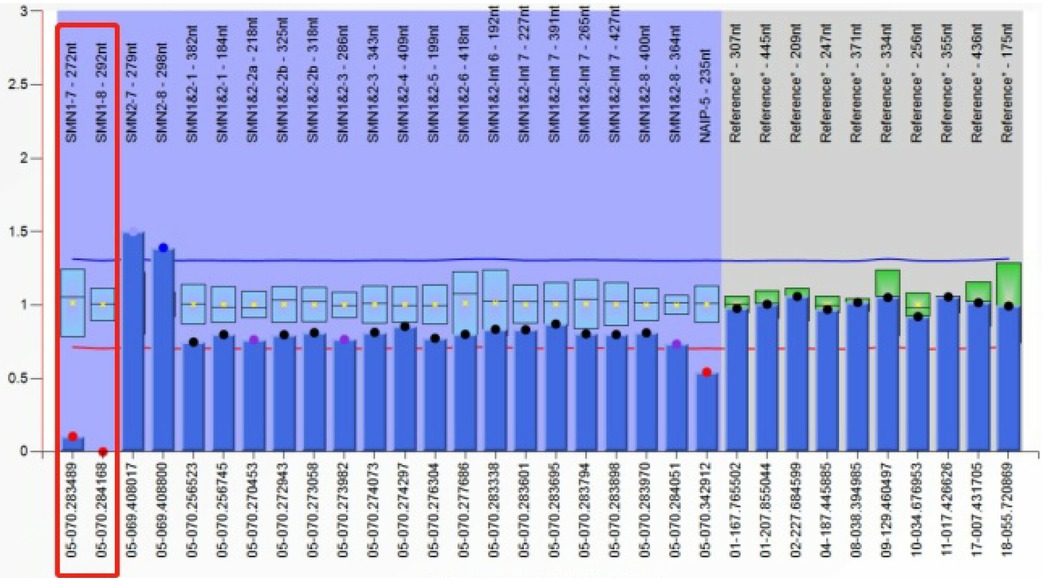
Results from routine blood tests, blood chemistry, lactic acid, ammonia levels, and muscle enzymes were normal. Brain MRI showed scattered abnormal signals in the bilateral subcortical white matter of the frontal, parietal, and occipital lobes. The supratentorial ventricular system was slightly dilated, and the bilateral cerebral sulci were widened. Chronic denervation was noted on needle electromyography. MRI of the leg muscles revealed significant atrophy of the bilateral hip, pelvic girdle, and thigh muscles. Spinal x-ray demonstrated a scoliosis with a Cobb angle of 31 degrees. Whole exome sequencing revealed homozygous mutations in exons 7 and 8 of the SMN1 gene on chromosome 5q13. The MLPA assay indicated a copy number of 0 for exons 7 and 8 of the SMN1 gene and a copy number of 3 for exons 7 and 8 of the SMN2 gene (see Figure 3).
The onset began at 5 months with early motor milestones followed by a progressive decline. Diagnosis of 5q-spinal muscular atrophy type 1 was confirmed through genetic testing and an increased SMN2 copy number. He received four loading treatments and four maintenance treatments of nusinersen from 6.6 years of age. He received four doses of loading therapy and four doses of maintenance therapy with nusinersen. Post-treatment WISC -IV scores showed no improvement in cognitive function, and the CHOP-INTEND score also showed no significant improvement in motor function.
Discussion
SMN2 gene is one of the important modification genes that affect the progression of SMA disease. Most SMA type 1 patients have 2 copies of SMN2, type 2 patients have 3 copies of SMN2, and type 3 patients have 3–4 copies of SMN2 (23). The number of SMN2 gene copies is negatively correlated with the severity of SMA. However, it does not fully correspond to the clinical phenotype because not all SMN2 copies are functionally identical. Intragenic SMN2 mutations, partial SMN2 deletions or duplications, and different degrees of SMN2 promoter methylation may further modify the functionality of the SMN2 gene (24, 25). Some studies that conducted sequencing analysis of the SMN2 gene found that c.859G>C mutations are associated with milder cases. Because the SMN2 genes that contain this rare variant would produce a higher number of full-length transcripts and thus of functional protein. However, the SMN2 c.859C>G variant is present in a few patients with chronic SMA but not in type 1 (26). The two patients in this study had 3 SMN2 copies, but still showed severe clinical symptoms and cognitive impairment, which may be related to these factors. Recent studies have revealed the existence of new modifier genes associated with SMA. Neuronal Apoptosis Inhibitory Protein gene (NAIP) and Small EDRK-rich factor 1A (SERF1A) are located in the 5q13.2 region. SERF1A gene can regulate the aggregation of SMN proteins. The function of NAIP gene is related to negative regulatory factors of motor neuron apoptosis. About half of patients with severe SMA lack the NAIP and SERF1A genes (27). According to previous studies, the NAIP gene is deleted in more than 50% of patients with type 1, but the frequency of this gene deletion is much lower in patients with type 2 and 3 (28). Medrano et al. (29) found in their study of SMA phenotypes in children that nearly 73% of children with type 1 lacked NAIP gene and 35% of children with type 1 lacked the SERF1A gene.
Both patients we reported had copy number of 3 copies of the SMN2 gene but were clinically typed as type 1. Detection of the patients’ SMN1 and SMN2 genes by MLPA did not reveal other SMA modifier genes. SMA patients are less likely to have genetic alterations that lead to phenotypic inconsistencies. However, further genetic testing is required to check for alterations such as mutations, partial deletions, or duplications within the SMN2 gene in these two patients.
MRI studies have revealed changes in brain white matter and brain volume in patients with SMA, which may be associated with cognitive impairment. Some studies have identified diffuse abnormal signals in the white matter of SMA patients, consistent with the MRI findings in patient 2 of this study (30). Additionally, other research has indicated that SMA patients may experience a reduction in brain volume, potentially reflecting abnormalities in neurodevelopment or neurodegenerative changes (31). These imaging findings support the notion that SMA is not only a motor system disorder but also involves alterations in cognitive function and brain structure. Therefore, a more detailed assessment and monitoring of cognitive function in SMA patients are necessary. This approach can enhance our understanding of the comprehensive impact of the disease and help optimize treatment strategies.
Although the clinical phenotype and natural history of SMA type 1 are well known in terms of motor, breathing, and swallowing functions, cognitive development in children and adolescents with this chronic disease has not received much attention. Communication has important effects on neurodevelopment, especially socialization, learning and education. Respiratory muscles and medulla oblongata muscles are the engines of speech function, and the respiratory muscles of SMA type 1 patients are seriously affected (32). Speech development is often absent or very limited in SMA type 1 patients, which significantly limits social interaction in children with SMA type 1. There are few studies on cognitive dysfunction in 5q-SMA patients. Existing studies evaluating cognitive function in SMA patients often do not specifically report or analyze those with cognitive impairment. The severely impaired motor and speech abilities in SMA children hinder accurate assessment of cognitive function, which may lead to an underestimation of their cognitive abilities (33). This is the first report of cognitive impairment in 5q-SMA patients in which two SMA type 1 patients developed cognitive impairment at an early stage.
The loss of motor ability may lead to a selective development of learning skills and cognitive abilities in SMA patients, potentially making them appear more intellectually capable compared to healthy individuals. However, SMA patients (mainly types 1 and 2) experience severe weakness that affects hand coordination and speech acquisition. The limited interaction between sensory motor skills and speech development can contribute to cognitive impairment (6). Two patients with SMA had significant cognitive impairment in our study, with severe motor limitations and lack of speech at an early stage. Children with SMA type 1 have difficulty communicating due to their inability to speak and poor motor control. Severe motor paralysis in SMA type 1 may be related to cognitive delays (11, 34). The cognitive abilities of SMA patients are related to motor dysfunction, with those experiencing greater motor difficulties showing poorer performance in attention (21). Studies on cognitive function and disease severity in SMA have found that, in patients with higher disease severity, there is lower attention and working memory ability, but better performance in verbal and verbal fluency tests (13). Motor dysfunction in SMA patients may not have a clear correlation with visual-spatial cognitive ability. A study on the cognitive and visual-spatial abilities of children with SMA type 2 found that these children did not have difficulty with complex spatial relations, and motor disorders were not a key risk factor for the significant slowdown in the development of spatial search skills (15).
The cognitive function in SMA patients may be related to the age of onset. The influence of cognitive factors may not be related to the early disease itself, the degree and duration of physical disability, but to the onset of movement disorders in early life. Children with early-onset SMA can compensate for their physical disabilities through cognitive development, which may result in higher scores in various cognitive abilities (16). As SMA patients reach adolescence, they may “compensate” for their physical deficits by acquiring cognitive skills and knowledge, with their environment facilitating higher levels of intelligence (4). The two patients described in this study were both SMA type 2 and exhibited cognitive impairment in infancy. This may be attributed to early motor disorders leading to reduced social communication and a lack of formal education, resulting in noticeable cognitive dysfunction. Family background, social factors, and access to appropriate education are likely to play a significant role in improving cognitive function in SMA patients. Without strong support and encouragement in these areas, SMA children may fail to develop compensatory mechanisms, leading to cognitive impairment (16).
Some studies suggest that physical activity in healthy teenagers positively impacts cognition. However, physical disability in early life might also positively influence cognitive function in other ways (35, 36). The severe physical disability in childhood and adolescence experienced by SMA patients often leads them to focus more on education. As a result, their cognitive function, which relies heavily on knowledge and education, may improve compensatorily. Severe dyskinesia may lead to educational disadvantages, but early educational support appears to stimulate compensatory development (16).
The two patients in our study are SMA type 1, with early onset and noticeable cognitive impairment in infancy. They did not receive formal education after birth. The gradual lack of stimulation and limited social experiences in older children with SMA type 1 may contribute to a gap between cognitive ability and language comprehension. A study on task performance in SMA type 1 patients found that these patients had poorer task completion. Although children with SMA type 1 may attend school regularly and receive formal education, they might still experience difficulties in remembering, processing, or expressing cognitive information. Additionally, children with SMA type 1 may start school later and experience more comorbidities and absences, which can affect their learning and contribute to cognitive impairments (7). SMA primarily affects motor function and usually has minimal impact on cognitive function. Few studies have shown that SMA can impact cognitive function, though it typically does not result in severe cognitive impairment. Cognitive dysfunction may be a co-morbidity in these two SMA patients, and the underlying causes of their severe cognitive impairment require further investigation.
There is little evidence in studies about cognitive performance in children with SMA, but children with SMA type 1 are more likely to be affected. Even children who are cognitively capable at birth may experience cognitive delays due to a lack of cognitive stimulation (7). Current research suggests that cognitive outcomes may be related to the copy number of the SMN2 gene. The deficiency of SMN protein not only impacts spinal motor neurons but may also affect other cellular components of the central nervous system. Severe reductions in SMN protein levels may lead to progressive brain dysfunction and degeneration. Some cases of SMA without SMN2 (SMA type 0) exhibit atrophy of the white matter and hippocampus, with high signals observed in the thalamus and basal ganglia on magnetic resonance imaging (8). Wishart et al. (37) demonstrated that alterations in brain development processes were associated with low SMN protein levels in severe SMA mouse models. Studies on brain structure changes in adults with SMA and healthy controls found cerebellar atrophy and increased gray matter density in the motor cortex. Cortical hypertrophy in motor areas has been interpreted as a form of cortical reorganization following lower motor neuron degeneration (38). It remains unclear whether SMN2 deficiency in the central nervous system is the primary cause of reduced intelligence quotient (IQ), or if muscle weakness limits patients’ ability to explore their surroundings, thereby hindering IQ development (19). The abnormal cranial MRI findings in patient 2 suggest that atypical brain development in SMA patients may be linked to cognitive dysfunction.
Conclusion
Cognitive impairment may occur in SMA patients, but relevant studies are limited. Cognitive impairment in SMA patients might be associated with motor impairment, age of onset, and education, rather than the underlying gene mutation. The pathogenesis may involve brain developmental disorders and SMN protein deficiency. SMA patients typically exhibit mild cognitive changes, but this study reports two SMA type 1 patients with severe cognitive dysfunction, which may be a co-morbidity of SMA. Clinicians should be cautious not to underdiagnose SMA or misdiagnose it as another condition, especially in patients with muscle weakness and atrophy. Early cognitive intervention is recommended to prevent progressive cognitive impairment in SMA patients. The relationship between cognitive dysfunction and SMA is under-researched, and there is no evidence linking cognitive characteristics with nusinersen treatment. Further research and exploration are needed in these areas in the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by West China Second University Hospital, Sichuan University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
Author contributions
HY: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Resources, Visualization, Writing – original draft, Writing – review & editing. JY: Investigation, Resources, Writing – original draft, Writing – review & editing. YX: Data curation, Writing – review & editing. LL: Data curation, Writing – review & editing. QC: Validation, Writing – review & editing. RL: Conceptualization, Resources, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors thank the patients’ parents for providing permission to use the information of their children.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Darras BT. Spinal muscular atrophies. Pediatr Clin North Am. (2015) 62(3):743–66. doi: 10.1016/j.pcl.2015.03.010
2. Shababi M, Lorson CL, Rudnik-Schoneborn SS. Spinal muscular atrophy: a motor neuron disorder or a multi-organ disease? J Anat. (2014) 224(1):15–28. doi: 10.1111/joa.12083
3. Kolb SJ, Kissel JT. Spinal muscular atrophy. Neurol Clin. (2015) 33(4):831–46. doi: 10.1016/j.ncl.2015.07.004
4. von Gontard A, Zerres K, Backes M, Laufersweiler-Plass C, Wendland C, Melchers P, et al. Intelligence and cognitive function in children and adolescents with spinal muscular atrophy. Neuromuscul Disord. (2002) 12(2):130–6. doi: 10.1016/s0960-8966(01)00274-7
5. Polido GJ, de Miranda MMV, Carvas N, Mendonça RH, Caromano FA, Reed UC, et al. Cognitive performance of children with spinal muscular atrophy: a systematic review. Dement Neuropsychol. (2019) 13(4):436–43. doi: 10.1590/1980-57642018dn13-040011
6. Sieratzki JS, Woll B. Cognitive function in children with spinal muscular atrophy. Neuromuscul Disord. (2002) 12(7–8):693–4. doi: 10.1590/1980-57642018dn13-040011
7. Polido GJ, Barbosa AF, Morimoto CH, Caromano FA, Favero FM, Zanoteli E, et al. Matching pairs difficulty in children with spinal muscular atrophy type I. Neuromuscul Disord. (2017) 27(5):419–27. doi: 10.1590/1980-57642018dn13-040011
8. Mendonça RH, Rocha AJ, Lozano-Arango A, Diaz AB, Castiglioni C, Silva AMS, et al. Severe brain involvement in 5q spinal muscular atrophy type 0. Ann Neurol. (2019) 86(3):458–62. doi: 10.1002/ana.25549
9. Giannotta G, Ruggiero M, De Rinaldis M, Trabacca A. Exploring variability in cognitive functioning in patients with spinal muscular atrophy: a scoping review. Neurol Sci. (2024) 45(8):3699–710. doi: 10.1007/s10072-024-07503-x
10. Buchignani B, Cicala G, Moriconi F, Ricci M, Capasso A, Coratti G, et al. Profile of cognitive abilities in spinal muscular atrophy type II and III: what is the role of motor impairment? Neuromuscul Disord. (2023) 33(10):711–7. doi: 10.1016/j.nmd.2023.08.005
11. Qian Y, McGraw S, Henne J, Jarecki J, Hobby K, Yeh WS. Understanding the experiences and needs of individuals with spinal muscular atrophy and their parents: a qualitative study. BMC Neurol. (2015) 15:217. doi: 10.1186/s12883-015-0473-3
12. Hu Y, Wei L, Li A, Liu T, Jiang Y, Xie C, et al. Cognitive impairment in Chinese adult patients with type III spinal muscular atrophy without disease-modifying treatment. Front Neurol. (2023) 14:1226043. doi: 10.3389/fneur.2023.1226043
13. Rochmah M A, Shima A, Harahap NIF, Niba ETE, Morisada N, Yanagisawa S, et al. Gender effects on the clinical phenotype in Japanese patients with spinal muscular atrophy. Kobe J Med Sci. (2017) 63(2):E41–4.29434173
14. Billard C, Gillet P, Barthez M, Hommet C, Bertrand P. Reading Ability and processing in duchenne muscular dystrophy and spinal muscular atrophy. Dev Med Child Neurol. (1998) 40(1):12–20. doi: 10.1111/j.1469-8749.1998.tb15351.x
15. Riviere J, Lecuyer R. Spatial cognition in young children with spinal muscular atrophy. Dev Neuropsychol. (2002) 21(3):273–83. doi: 10.1207/S15326942DN2103_4
16. Osmanovic A, Wieselmann G, Mix L, Siegler HA, Kumpe M, Ranxha G, et al. Cognitive performance of patients with adult 5q-spinal muscular atrophy and with amyotrophic lateral sclerosis. Brain Sci. (2020) 11(1):8. doi: 10.3390/brainsci11010008
17. Mix L, Schreiber-Katz O, Wurster CD, Uzelac Z, Platen S, Gipperich C, et al. Executive function is inversely correlated with physical function: the cognitive profile of adult spinal muscular atrophy (SMA). Orphanet J Rare Dis. (2021) 16(1):10. doi: 10.1186/s13023-020-01661-9
18. Schmidt H, Felisatti A, von Aster M, Wilbert J, von Moers A, Fischer MH. Neuromuscular diseases affect number representation and processing: an exploratory study. Front Psychol. (2021) 12:697881. doi: 10.3389/fpsyg.2021.697881
19. Kizina K, Akkaya Y, Jokisch D, Stolte B, Totzeck A, Munoz-Rosales J, et al. Cognitive impairment in adult patients with 5q-associated spinal muscular atrophy. Brain Sci. (2021) 11(9):1184. doi: 10.3390/brainsci11091184
20. Zappa G, LoMauro A, Baranello G, Cavallo E, Corti P, Mastella C, et al. Intellectual abilities, language comprehension, speech, and motor function in children with spinal muscular atrophy type 1. J Neurodev Disord. (2021) 13(1):9. doi: 10.1186/s11689-021-09355-4
21. Lenzoni S, Semenza C, Calligaro D, Turcano P, Caumo L, Pegoraro E, et al. Cognitive profiles and clinical factors in type III spinal muscular atrophy: a preliminary study. Neuromuscul Disord. (2022) 32(8):672–7. doi: 10.1016/j.nmd.2022.05.005
22. Steffens P, Weiss D, Perez A, Appel M, Weber P, Weiss C, et al. Cognitive function in SMA patients with 2 or 3 SMN2 copies treated with SMN-modifying or gene addition therapy during the first year of life. Eur J Paediatr Neurol. (2024) 51:17–23. doi: 10.1016/j.ejpn.2024.05.002
23. Calucho M, Bernal S, Alías L, March F, Venceslá A, Rodríguez-Álvarez FJ, et al. Correlation between SMA type and SMN2 copy number revisited: an analysis of 625 unrelated spanish patients and a compilation of 2834 reported cases. Neuromuscul Disord. (2018) 28(3):208–15. doi: 10.1016/j.nmd.2018.01.003
24. Feldkötter M, Schwarzer V, Wirth R, Wienker TF, Wirth B. Quantitative analyses of SMN1 and SMN2 based on real-time lightCycler PCR: fast and highly reliable carrier testing and prediction of severity of spinal muscular atrophy. Am J Hum Genet. (2002) 70(2):358–68. doi: 10.1086/338627
25. Hauke J, Riessland M, Lunke S, Eyüpoglu IY, Blümcke I, El-Osta A, et al. Survival motor neuron gene 2 silencing by DNA methylation correlates with spinal muscular atrophy disease severity and can be bypassed by histone deacetylase inhibition. Hum Mol Genet. (2009) 18(2):304–17. doi: 10.1093/hmg/ddn357
26. Bernal S, Alías L, Barceló MJ, Also-Rallo E, Martínez-Hernández R, Gámez J, et al. The c.859G > C variant in the SMN2 gene is associated with types II and III SMA and originates from a common ancestor. J Med Genet. (2010) 47(9):640–2. doi: 10.1136/jmg.2010.079004
27. Brkušanin M, Kosać A, Jovanović V, Pešović J, Brajušković G, Dimitrijević N, et al. Joint effect of the SMN2 and SERF1A genes on childhood-onset types of spinal muscular atrophy in serbian patients. J Hum Genet. (2015) 60(11):723–8. doi: 10.1038/jhg.2015.104
28. Amara A, Adala L, Ben Charfeddine I, Mamaï O, Mili A, Lazreg TB, et al. Correlation of SMN2, NAIP, p44, H4F5 and occludin genes copy number with spinal muscular atrophy phenotype in Tunisian patients. Eur J Paediatr Neurol. (2012) 16(2):167–74. doi: 10.1016/j.ejpn.2011.07.007
29. Medrano S, Monges S, Gravina LP, Alías L, Mozzoni J, Aráoz HV, et al. Genotype-phenotype correlation of SMN locus genes in spinal muscular atrophy children from Argentina. Eur J Paediatr Neurol. (2016) 20(6):910–7. doi: 10.1016/j.ejpn.2016.07.017
30. Scoto M, Rossor AM, Harms MB, Cirak S, Calissano M, Robb S, et al. Novel mutations expand the clinical spectrum of DYNC1H1-associated spinal muscular atrophy. Neurology. (2015) 84(7):668–79. doi: 10.1212/WNL.0000000000001269
31. Rubboli G, Veggiotti P, Pini A, Berardinelli A, Cantalupo G, Bertini E, et al. Spinal muscular atrophy associated with progressive myoclonic epilepsy: a rare condition caused by mutations in ASAH1. Epilepsia. (2015) 56(5):692–8. doi: 10.1016/j.ajhg.2012.05.001
32. LoMauro A, Aliverti A, Mastella C, Arnoldi MT, Banfi P, Baranello G. Spontaneous breathing pattern as respiratory functional outcome in children with spinal muscular atrophy (SMA). PLoS One. (2016) 11(11):e0165818. doi: 10.1371/journal.pone.0165818
33. Geytenbeek J, Harlaar L, Stam M, Ket H, Becher JG, Oostrom K, et al. Utility of language comprehension tests for unintelligible or non-speaking children with cerebral palsy: a systematic review. Dev Med Child Neurol. (2010) 52(12):1098. doi: 10.1111/j.1469-8749.2010.03833.x
34. Kubota M, Sakakihara Y, Uchiyama Y, Nara A, Nagata T, Nitta H, et al. New ocular movement detector system as a communication tool in ventilator-assisted werdnig-hoffmann disease. Dev Med Child Neurol. (2000) 42(1):61–4. doi: 10.1017/s0012162200000116
35. Esteban-Cornejo I, Tejero-Gonzalez CM, Sallis JF, Veiga OL. Physical activity and cognition in adolescents: a systematic review. J Sci Med Sport. (2015) 18(5):534–9. doi: 10.1016/j.jsams.2014.07.007
36. Erickson KI, Hillman C, Stillman CM, Ballard RM, Bloodgood B, Conroy DE, et al. Physical activity, cognition, and brain outcomes: a review of the 2018 physical activity guidelines. Med Sci Sports Exerc. (2019) 51(6):1242–51. doi: 10.1249/MSS.0000000000001936
37. Wishart TM, Huang JP, Murray LM, Lamont DJ, Mutsaers CA, Ross J, et al. SMN Deficiency disrupts brain development in a mouse model of severe spinal muscular atrophy. Hum Mol Genet. (2010) 19(21):4216–28. doi: 10.1093/hmg/ddq340
Keywords: spinal muscular atrophy type 1, motor neuron disease, cognitive impairment, children, case report
Citation: Yang H, Yang J, Xue Y, Liao L, Cai Q and Luo R (2024) Cognitive impairment in children with 5q-associated spinal muscular atrophy type 1: two case reports and the review of the literature. Front. Pediatr. 12:1407341. doi: 10.3389/fped.2024.1407341
Received: 26 March 2024; Accepted: 16 September 2024;
Published: 27 September 2024.
Edited by:
Andrea Domenico Praticò, University of Catania, ItalyReviewed by:
Lorenzo Pavone, University of Catania, ItalyMatthew S. Alexander, University of Alabama at Birmingham, United States
Copyright: © 2024 Yang, Yang, Xue, Liao, Cai and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rong Luo, bHJzY3VAc2N1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Hua Yang
Hua Yang Jie Yang
Jie Yang Yawen Xue3
Yawen Xue3 Rong Luo
Rong Luo