- 1Department of Public Health, First Capital University of Bangladesh, Chuadanga, Bangladesh
- 2Faculty of Biological Sciences, University of Rajshahi, Rajshahi, Bangladesh
- 3Department of Epidemiology and Preventive Medicine, School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia
- 4Department of Public Health and Sports Science, Faculty of Health and Occupational Studies, University of Gävle, Gävle, Sweden
- 5School of Allied Health, Faculty of Health, Medicine and Social Care, Anglia Ruskin University, Chelmsford, Essex, United Kingdom
- 6School of Science and Technology (SST), Bangladesh Open University (BOU), Dhaka, Bangladesh
- 7Department of Medical Science, School of Health and Welfare, Dalarna University, Falun, Sweden
Background: Fetal macrosomia, marked by excessive birth weight, is a significant public health issue in developing countries, yet it has received less attention compared to low birth weight. This study aims to determine the prevalence of fetal macrosomia in Bangladesh and its associated factors.
Methods: The study utilized data from 4,754 women with complete birth weight information of their children from the Bangladesh Multiple Indicator Cluster Survey (MICS) −2019, defining fetal macrosomia as newborns with a birth weight ≥4,000 g regardless of gestational age. Bivariate logistic regression assessed associations between independent variables and fetal macrosomia, presenting adjusted odds ratios (AOR) and a 95% confidence interval (CI), while controlling for potential confounders such as women's age, wealth index, education, healthcare utilization, comorbidities, newborn sex, and place of residence.
Results: The prevalence of fetal macrosomia was 11.6%. Significant associations with fetal macrosomia included higher maternal age group (30–34 years) (AOR = 1.36, 95% CI = 1.07–1.74), secondary level of mother's education (AOR = 1.95, 95% CI = 1.43–2.66), experienced physical attacks (AOR = 1.41, 95% CI = 1.06–1.88), hypertension during pregnancy (AOR = 1.54, 95% CI = 1.15–2.07), and rural residence (AOR = 1.25, 95% CI = 1.15–1.49). Female infants had 18% lower odds of being macrosomic compared to male infants (AOR = 0.82, 95% CI = 0.72–0.93).
Conclusion: One in ten infants in Bangladesh are born with macrosomia, necessitating a multi-faceted approach involving improving maternal nutrition, promoting healthy lifestyles, enhancing access to quality prenatal care, and addressing socioeconomic, residential, and healthcare system challenges, underlining the importance of further community-based research to expand the study's scope.
Introduction
Abnormal birth weight, encompassing both low birth weight (LBW) and high birth weight (fetal macrosomia), plays a crucial role in predicting children's growth, development, and mortality (1, 2). While most research has focused on LBW, there has been a notable global increase of 15%–25% in fetal macrosomia occurrences in recent decades, observed in both developed (5%–20%) and less developed countries (0.5%–15%) (3, 4). Factors contributing to this increase include sedentary lifestyles, imbalanced nutritional practices during pregnancy, higher rates of maternal obesity, gestational diabetes, and changes in social and demographic patterns (3, 5, 6).
Defining fetal macrosomia, characterized by considerably high birth weight, lacks consensus among researchers and obstetricians, with varying thresholds employed in different studies, such as birth weights exceeding 4,000, 4,200, or 4,500 g (7). Nevertheless, most researchers describe fetal macrosomia as a birth weight of 4,000 g or more, regardless of gestational age (2, 8). Fetal macrosomia significantly impacts maternal and neonatal health, contributing to increased infant and child mortality and morbidity rates (9). During pregnancy, the presence of a macrosomic fetus poses risks for both the newborn and mother, including an elevated likelihood of cesarean section, prolonged labor, postpartum hemorrhage, uterine rupture, puerperal infection, anesthetic complications, and the development of type 2 diabetes after pregnancy (3).
Studies on fetal macrosomia remain limited worldwide. In Uruguay, maternal age and obesity were identified as risk factors, while in China, Ethiopia, and Ghana, various maternal (e.g., age, education, BMI, gestational age, diabetes), child-related (sex), and contextual factors (household wealth index) were associated with fetal macrosomia (9–13). However, in many developing countries like Bangladesh, fetal macrosomia and its risk factors are underreported due to lack of quality data.
In Bangladesh, a few hospital-based studies have reported higher fetal macrosomia rates in mothers with diabetes, gestational diabetes, and pre-pregnancy obesity (14–16). These studies, however, are often small-scale and limited to specific hospital settings and cohorts, potentially failing to provide a comprehensive and representative picture of fetal macrosomia prevalence and its determinants in Bangladesh. Thus, this study aims to investigate the prevalence and determinants of fetal macrosomia in Bangladesh using a nationally representative sample.
Method
Data source: multiple indicator cluster survey
The Multiple Indicator Cluster Survey (MICS) in Bangladesh is a cross-sectional study that provides nationally representative data. The MICS-2019 aimed to gather comprehensive information on 144 key indicators related to maternal reproductive health, child health, development, nutrition, and demographic profiles of respondents. Data collection occurred through face-to-face interviews using a standardized questionnaire between January 19, 2019, and June 1, 2019. The survey covered all eight administrative divisions of Bangladesh, namely the Southern region (Barisal division), Southeastern region (Chittagong), Central region (Dhaka), Western region (Khulna), Upper-central region (Mymensingh), Midwestern region (Rajshahi), Northwestern region (Rangpur), and Eastern region (Sylhet).’
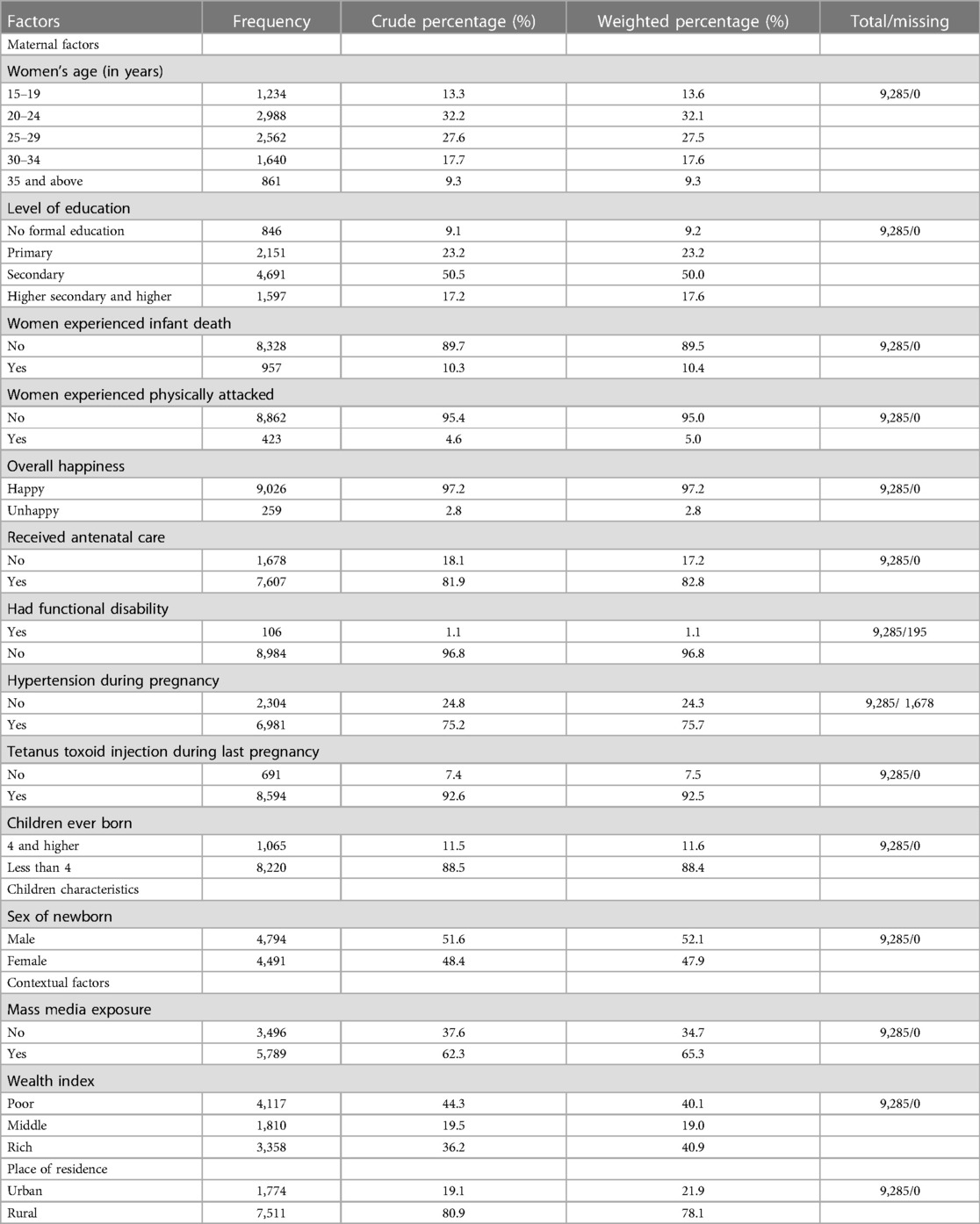
The MICS utilized a two-stage stratified cluster sampling method to select the survey sample. In the first stage, 64,400 households and 3,220 primary sample units (PSUs) were chosen from Enumeration Areas (EAs) using probability proportional to size techniques. These EAs were systematically listed from the Bangladesh Population and Housing Census 2011, which included 65,193 urban areas and 228,340 rural areas. In the second stage, data were collected from 20 households within each PSU using equal probability systematic sampling. This multistage sampling approach, along with sampling weights, aimed to minimize potential sampling bias. Sample weights were calculated at each sampling stage and adjusted for non-response to obtain final standard weights. Additionally, all ever-married women aged 15–49 years with children aged less than 5 years from the selected households were interviewed without replacement to prevent selection bias. From the 64,000 selected households, a total of 64,378 women aged 15–49 were successfully interviewed. Furthermore, 9,285 women who had at least one live birth in the last two years were initially selected to collect birth weight data. Among them, 4,754 women had complete data on their child's birth weight and were retained for the analysis of this study. Further details can be found in the MICS-2019 report (16).
Dependent variable
The dependent variable in this study was baby born with fetal macrosomia. In MICS-2019, live births with a reported birth weight were presented based on either a written record, the mother's report, or a combination of both (16).
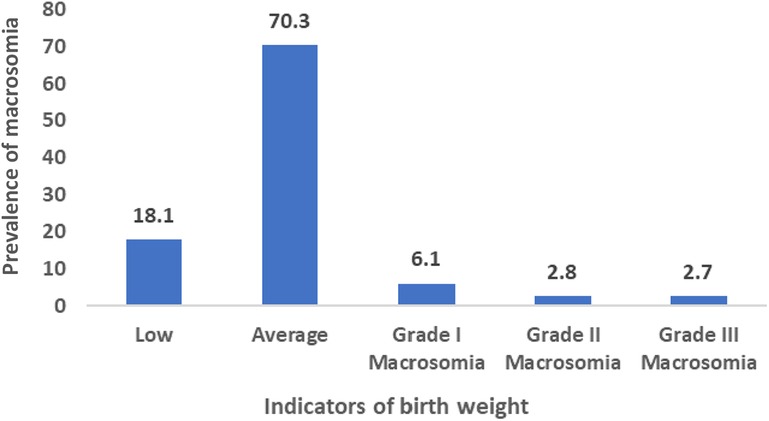
The definition of macrosomia varies among researchers. According to Boulet et al. (2003), birth weights were categorized as low (<2,500 g), average (≥2,500 g and <4,000 g), grade I (4,000–4,499 g) macrosomia, grade II (4,500–4,999 g) macrosomia, and grade III (over 5,000 g) macrosomia (17) Henriksen (2008) suggested that infants born weighing 4,000 g (4 kg) or above, or falling into grades I, II, and III, were considered macrosomic (11). In this study, infant with macrosomia was considered dependent variable and coded 1 for macrosomia, coded 0 for no macrosomia.
Independent variables and operational definitions
This study incorporated variables previously identified as significant in the literatures in associating macrosomia (4, 11, 18, 19). Variables including women's characteristics, such as, women's age (in years) (15–19, 20–24, 25–29, 30–35, 35 and above); level of education (no formal education, primary secondary, higher secondary and higher); women experienced infant death (no, yes), experienced physical attack (yes, no), happiness (happy, unhappy), received antenatal care (no, yes), had functional disability (yes, no), hypertension during pregnancy (no, yes), toxoid injection during last pregnancy (no, yes), and children ever born (4 and higher, less than 4); children characteristics, such as, sex of newborn (male, female); and contextual factors including mass media exposure (no, yes), wealth index (poor, middle, rich), and place of residence (urban, rural) (Supplementary Table 1).
Handling missing data
According to UNICEF and WHO, missing birth weight data can be handled using imputation method (16). Further, the misreporting or heaping cases (e.g., 500 g, 100 g) were replaced by 2,500 g (16) Missing values were imputed using Multiple Imputations by Chained Equations (MICE) (20) MICE has emerged as one of the principal statistical approaches to dealing with missing data, which involves multiple imputations, as opposed to single imputations, in order to account for the statistical uncertainty associated with imputations. The chained equations approach can also handle variables of various types and complexities.
Statistical analysis
Baseline characteristics of the respondents was assessed using descriptive statistics. The Chi-square test was employed to assess the association between various exposure variables and the occurrence of fetal macrosomia. The comparison of prevalence was considered significant based on significant level at p < 0.05 in the Chi-square test. The multivariable logistic regression analysis was carried out to identify the most important variables associated with macrosomia. Variables found significant at the level p < 0.25 in the Chi-square test were entered into the multivariable analysis (21) This choice was made to be more inclusive and capture factors that may have important implications, even if their associations were not considered statistically significant at the traditional significant level (p < 0.05). Odds ratio was assessed to determine the magnitude and direction of the associations, along with their corresponding Confidence Intervals (CIs). Significant value for multivariable logistic regression was set up at p < 0.05. To account for the complex sampling design, including factors such as sampling weight, cluster, and strata, the Stata command “svyset” was employed. This command ensured that the estimates and statistical inferences obtained from the analysis were adjusted to accurately reflect the complex sampling design, enhancing the validity of the findings. Stata version 17 (StataCorp LP, College Station, Texas) was used for entire analyses.
Ethical consideration
The MICS 2019 was administered by the Bangladesh Bureau of Statistics (BBS) and financially supported by the United Nations Children's Fund (UNICEF), enjoyed additional technical backing from the United Nations Population Fund (UNFPA), the Global MICS team, and the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b). The survey protocol adhered to ethical standards, securing verbal consent from all female participants aged 15–49 prior to their involvement.
The present study employed data from the 2019 Bangladesh MICS, publicly accessible via the designated repository at https://mics.unicef.org/surveys. As the dataset is openly available and obtained through a survey with established ethical protocols, no further ethical clearance was deemed necessary for the current research endeavor.
Results
Background characteristics
Around one-third of women (32.2%) were situated within the age bracket of 20–24 years. About 9.2% of mothers who had children had not received any formal education. Hypertension during pregnancy were noted in 75.4% of the women. Of children, slightly over half (51.1%) were male. Approximately 44.1% of the children hailed from a poor socio-economic background, while 78.1% of the total population resided in rural areas. The detailed breakdown is provided in Table 1.
Prevalence of fetal macrosomia
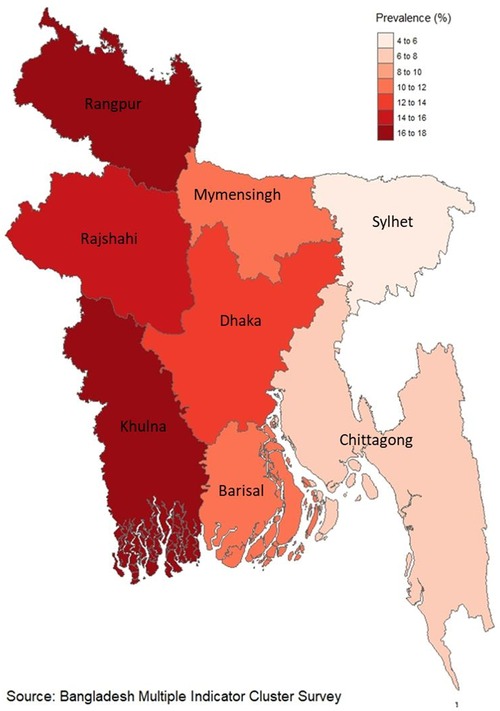
In Bangladesh, the occurrence of fetal macrosomia among newborns stands at 11.6%, as depicted in Figure 1. Moreover, within the Khulna division, which encompasses the western region, a significant prevalence of fetal macrosomia is noted at 16.8%, as illustrated in Figure 2.
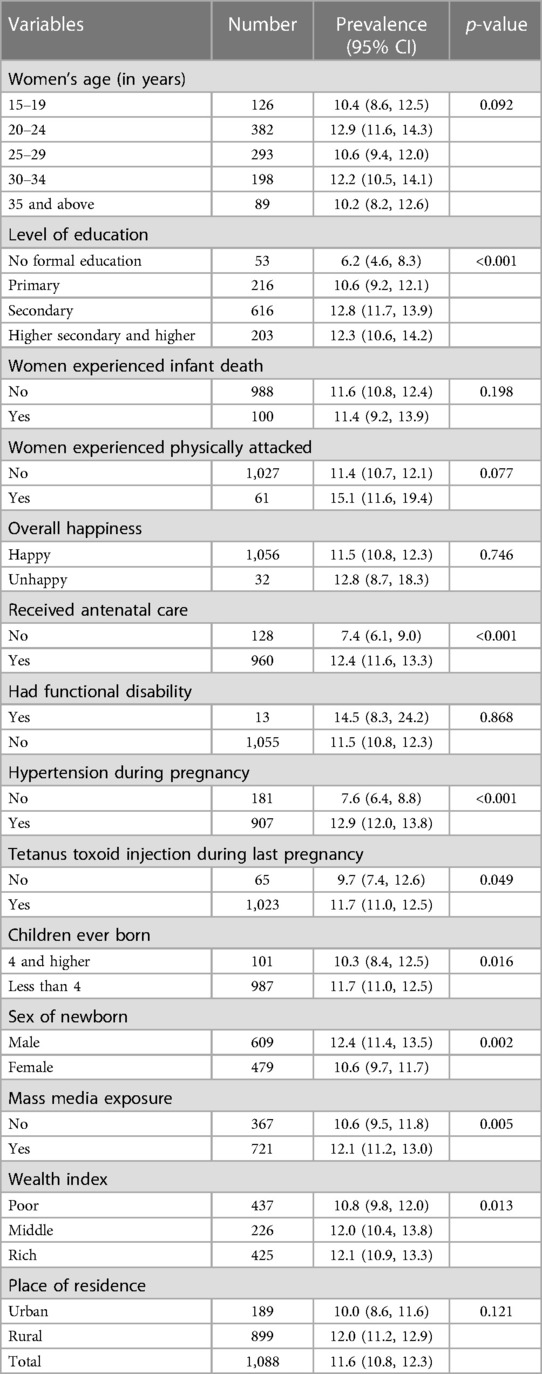
The prevalence of fetal macrosomia is notably higher among women with a history of gestational hypertension, reaching 12.9%. Similarly, those who have received education beyond primary level, specifically secondary education to higher education, demonstrate comparatively higher rates of fetal macrosomia, recorded at 12.8% and 12.3%, respectively. Furthermore, a substantial increase in the prevalence of fetal macrosomia is observed among women who have undergone antenatal care (12.4%), delivered a male infant as their most recent child (12.4%), and belong to a higher socio-economic status (12.1%), as indicated in Table 2.
Determinants
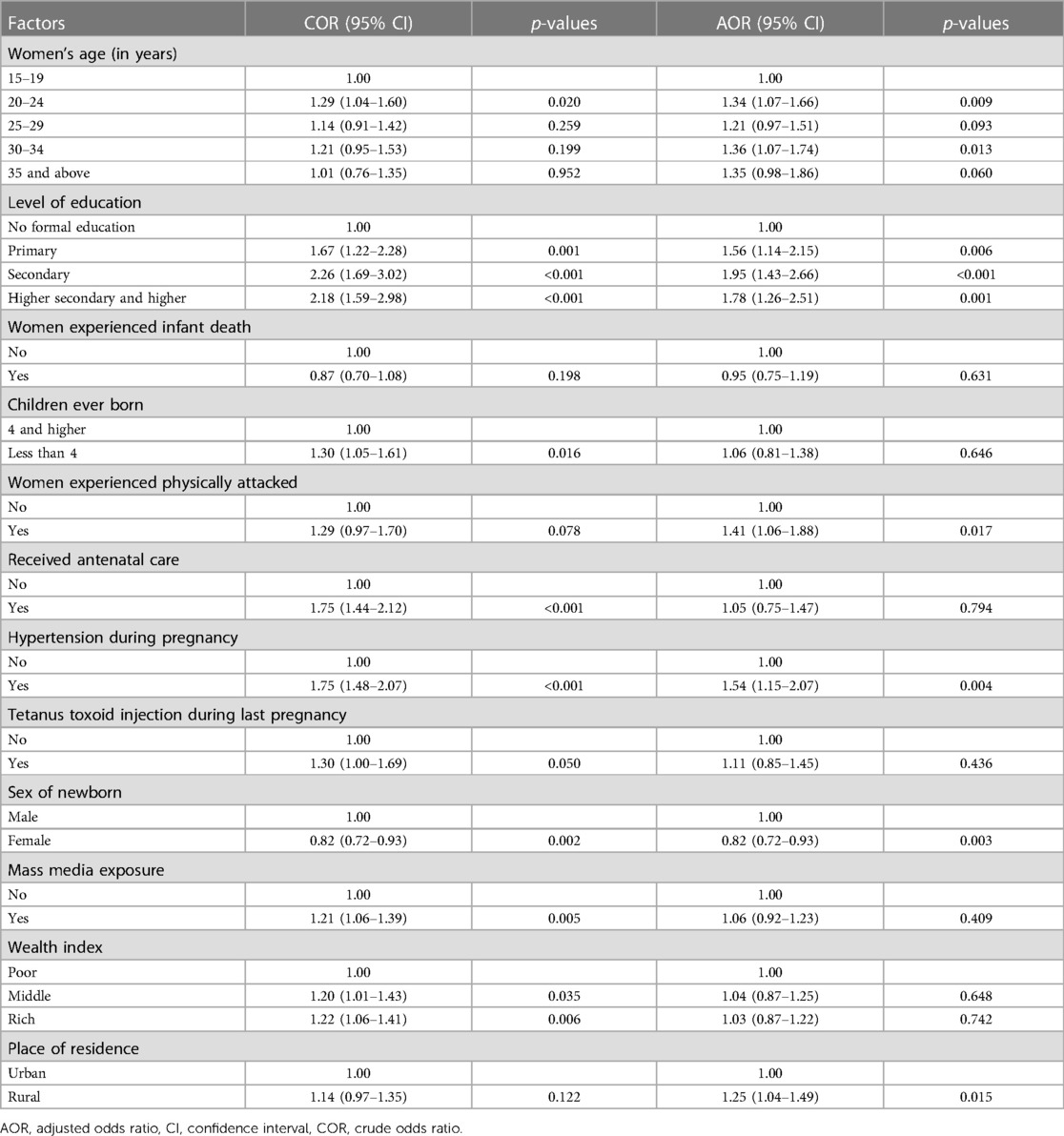
The findings of the study reveal notable associations between various maternal characteristics and the likelihood of fetal macrosomia occurrence. Women aged between 30 and 34 demonstrated a statistically significant 1.36 times higher adjusted odds ratio (AOR) for experiencing fetal macrosomia compared to their counterparts aged 15 to 19 (AOR = 1.36, 95% CI = 1.07–1.74, p = 0.013). Similarly, women with secondary education exhibited a substantially elevated AOR of 1.95 (95% CI = 1.43–2.66, p < 0.001) for fetal macrosomia in contrast to those lacking formal education. Moreover, a history of physical attacks among women was associated with a 1.4 times higher likelihood (AOR = 1.41, 95% CI = 1.06–1.88, p = 0.017) of delivering macrosomic infants. Additionally, mothers with a prior diagnosis of hypertension during pregnancy exhibited a 1.5 times increased probability (AOR = 1.54, 95% CI = 1.15–2.07, p = 0.004) of fetal macrosomia occurrence compared to those without such medical history.
Residential setting also emerged as a significant factor, with women residing in rural areas manifesting a 1.25 times higher probability (AOR = 1.25, 95% CI = 1.15–1.49, p = 0.015) of delivering macrosomic offspring compared to their urban counterparts. Furthermore, a noteworthy gender disparity was observed, wherein female infants exhibited a 12% decreased likelihood (AOR = 0.82, 95% CI = 0.72–0.93, p = 0.003) of being macrosomic in comparison to male infants, as delineated in Table 3.
Discussion
The study aimed to assess fetal macrosomia prevalence in Bangladesh and its associated factors, revealing a prevalence of 11.6%, with higher maternal age group, higher education levels, history of physical attack, hypertension during pregnancy, male gender, and rural residence identified as significant determinants.
Various studies conducted in different regions have reported varying prevalence rates of fetal macrosomia. For instance, in China and Peru, the prevalence was found to be 7.4% and 7.5%, respectively (4, 22). However, there is a scarcity of data regarding nationwide prevalence rates of fetal macrosomia on a global scale. Hospital-based studies have provided insights into regional prevalence rates, with reports indicating rates of 9.4% in India and 7.3% in Pakistan, followed by some African countries where rates were recorded at 6.5% in Ethiopia and 2.3% in Tanzania (2, 7, 23–25). Furthermore, this study reveals that the prevalence of fetal macrosomia was notably higher among women with a history of gestational hypertension. Interestingly, the highest prevalence of fetal macrosomia was observed in the western part (Khulna division) of Bangladesh, potentially attributed to a higher occurrence of abdominal obesity in this population, which could influence post-pregnancy outcomes such as fetal macrosomia (10, 26).
The study revealed that higher maternal age group and higher levels of education among women were significant factors associated with fetal macrosomia (3, 18, 19, 25, 27–29). Women in higher age group is linked to an increased susceptibility to adverse maternal conditions like gestational diabetes mellitus and type 2 diabetes mellitus, both of which are known to be associated with fetal macrosomia (25, 30, 31). Furthermore, older women may be more prone to adopting sedentary lifestyles during pregnancy, which can lead to unfavorable outcomes such as gestational diabetes, excessive weight gain, and ultimately, macrosomia (24). Higher education is often associated with higher socioeconomic status, potentially leading to greater access to resources or increased consumption of energy-rich foods, resulting in higher maternal body mass index (BMI) and excessive weight gain during pregnancy, further increasing the risk of macrosomia (24, 32).
Remarkably, the study also found that women who experienced physical attacks were more likely to deliver macrosomic infants. Physical attacks have not been previously explored as a risk factor for macrosomia in the literature. However, physical or intimate partner violence has been associated with adverse birth or pregnancy outcomes in multiple studies conducted in several developing countries from Asia and Africa, including Ethiopia India and Nepal (33–36). Physical attacks on women can induce both physical and psychological stress, affecting the body's stress response system. This can result in elevated cortisol levels, reduced insulin sensitivity, and increased liver triglycerides, all of which contribute to the risk of gestational and type 2 diabetes mellitus, ultimately leading to fetal macrosomia. The findings suggest that achieving nationwide coverage of reproductive health education, care, and awareness, while incorporating family norms and practices, along with improving the quality and accessibility of antenatal care, could potentially help mitigate adverse pregnancy outcomes such as fetal macrosomia.
The findings indicate that mothers who experienced hypertension during pregnancy had a higher likelihood of delivering macrosomic infants compared to those without hypertension (37, 38). Previous studies in China and Iran have established a link between pregnancy-related hypertension and macrosomia (37, 39). Hypertension during pregnancy may arise from hormonal changes, including estrogen, progesterone, and relaxin, which affect the renin-angiotensin-aldosterone system (RAAS), leading to salt and water retention and increased plasma volume (40). As a result, pregnancy-related hypertension can lead to weight gain and insulin resistance during pregnancy, which in turn contribute to fetal macrosomia (41, 42).
The likelihood of fetal macrosomia, a condition characterized by excessive birth weight, was found to be higher among male infants. This observation aligns with previous research conducted in various regions. Studies from Asian countries, including Malaysia (18) and China (19), as well as those from the Middle East, such as Turkey (30), and African nations like Ethiopia (3) and Cameroon (43), have consistently reported a higher incidence of fetal macrosomia among male babies. One contributing factor to this phenomenon is the inherent biological differences between male and female infants. Genetically, male fetuses tend to exhibit slightly larger body weight, length, and head circumference compared to their female counterparts at the same gestational age (31, 44, 45). These differences in size can predispose male fetuses to an increased risk of macrosomia. Additionally, maternal behavior during pregnancy may play a role in the development of fetal macrosomia, particularly in pregnancies carrying male fetuses. Expectant mothers, upon learning they are carrying male babies, may inadvertently engage in excessive food consumption in an attempt to ensure a healthy outcome for their infants (39). This behavior can lead to disproportionate maternal weight gain and subsequently elevate the likelihood of fetal macrosomia (28, 45).
Furthermore, women residing in rural areas had a higher possibility of delivering macrosomic children compared to their counterparts. Women residing in rural areas had less access to proper dietary planning and prenatal care, resulting in abnormal weight gain during pregnancy (46). Moreover, rural women were more vulnerable to intimate partner violence, increasing the possibility of fetal macrosomia (34–36, 47). To reduce the occurrence of macrosomia, it is crucial to implement early screening, careful monitoring, and appropriate management strategies addressing the reproductive health of women. In addition, developing a local surveillance system collaborating with public and private healthcare providers might be effective for the management of adverse reproductive complications, like gestational hypertension. Promoting nutritional education and healthy lifestyle practices among women residing in rural areas might help to shrink the disparities in access to appropriate care and resources.
The study exhibits several notable strengths, notably its utilization of a nationally representative large sample size and the application of appropriate methodological frameworks. However, the investigation is not devoid of limitations. Principally, the present dataset may not faithfully encapsulate the entirety of the population of children under the age of five, owing to its reliance on a restricted dataset comprising children aged 2–3 years. Furthermore, the cross-sectional design of the study precludes the establishment of causal relationships between macrosomia and associated exposures. Moreover, the retrospective and self-reported nature of data collection introduces the possibility of underreporting, as well as information and recall biases. Several important mother's characteristics associated with macrosomia, such as gestational week or gestational age were not adjusted in the model due to their unavailability. Lastly, but not exhaustively, the study's scope is confined in terms of its generalizability to low- and middle-income countries.
Conclusions
The prevalence of macrosomia among infants in Bangladesh stands at a noteworthy one in ten. This phenomenon has been linked to several significant determinants, including higher maternal age group, characterized by a higher level of education, a history of physical assault, hypertension during pregnancy, the birth of a male child, and residing in rural areas. Mitigating the escalating incidence of macrosomia demands a multifaceted strategy, encompassing enhancements in maternal nutrition, advocacy for healthy lifestyles, augmentation of access to quality prenatal care services, and the remediation of underlying socioeconomic, residential, and healthcare system hurdles. Moreover, the expansion of the study's purview necessitates further community-based investigations to glean comprehensive insights into this issue.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by The United Nations Population Fund (UNFPA), the Global MICS team, and the International Centre for Diarrheal Disease Research, Bangladesh (icddr,b). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
ZI: Conceptualization, Methodology, Writing – original draft, Data curation, Formal analysis, Investigation, Project administration, Resources, Software, Validation, Visualization. MC: Conceptualization, Writing – original draft, Data curation, Formal analysis, Investigation, Project administration, Resources, Software, Validation, Visualization. BB: Writing – review & editing. MR: Writing – review & editing. RK: Writing – review & editing. MH: Data curation, Formal analysis, Methodology, Software, Validation, Writing – review & editing. MK: Conceptualization, Data curation, Methodology, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
The authors express their gratitude to UNICEF for providing permission to utilize the data for this study, which was obtained from the UNICEF MICS Archive.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1405442/full#supplementary-material
References
1. Gage TB, Fang F, O’Neill E, DiRienzo G. Maternal education, birth weight, and infant mortality. Demography. (2013) 50(2):615–35. doi: 10.1007/s13524-012-0148-2
2. Jeyaseelan L, Yadav B, Silambarasan V, Vijayaselvi R, Jose R. Large for gestational age births among south Indian women: temporal trend and risk factors from 1996 to 2010. J Obstet Gynecol India. (2016) 66(1):42–50. doi: 10.1007/s13224-015-0765-y
3. Woltamo DD, Meskele M, Workie SB, Badacho AS. Determinants of fetal macrosomia among live births in southern Ethiopia: a matched case–control study. BMC Pregnancy Childbirth. (2022) 22(1):1–10. doi: 10.1186/s12884-022-04734-8
4. Shen L, Wang J, Duan Y, Yang Z. Prevalence of low birth weight and macrosomia estimates based on heaping adjustment method in China. Sci Rep. (2021) 11(1):15016. doi: 10.1038/s41598-021-94375-2
5. Wang YW, Chen Y, Zhang YJ. Risk factors combine in a complex manner in assessment for macrosomia. BMC Public Health. (2023) 23(1):1–11. doi: 10.1186/s12889-022-14670-z
6. Fazzi C, Saunders DH, Linton K, Norman JE, Reynolds RM. Sedentary behaviours during pregnancy: a systematic review. Int J Behav Nutr Phys Act. (2017) 14(1):1–13. doi: 10.1186/s12966-017-0485-z
7. Adugna DG, Enyew EF, Jemberie MT. Prevalence and associated factors of macrosomia among newborns delivered in university of gondar comprehensive specialized hospital, Gondar, Ethiopia: an institution-based cross-sectional study. Pediatr Heal Med Ther. (2020) 11:495–503. doi: 10.2147/PHMT.S289218
8. Dube Jara TW. Factors associated with macrosomia among neonates delivered at Debre Markos referral hospital, northwest Ethiopia, 2014: a case control study. J Diabetes Metab. (2014) 5(12). doi: 10.4172/2155-6156.1000468
9. Mengesha HG, Wuneh AD, Weldearegawi B, Selvakumar DL. Low birth weight and macrosomia in Tigray, Northern Ethiopia: who are the mothers at risk? BMC Pediatr. (2017) 17(1):1–9. doi: 10.1186/s12887-017-0901-1
10. Pereda J, Bove I, Pineyro MM. Excessive maternal weight and diabetes are risk factors for macrosomia: a cross-sectional study of 42,663 pregnancies in Uruguay. Front Endocrinol (Lausanne). (2020) 11:588443. doi: 10.3389/fendo.2020.588443
11. Abubakari A, Kynast-Wolf G, Jahn A. Prevalence of abnormal birth weight and related factors in northern region, Ghana. BMC Pregnancy Childbirth. (2015) 15(1):1–8. doi: 10.1186/s12884-015-0790-y
12. Lei F, Zhang L, Shen Y, Zhao Y, Kang Y, Qu P, et al. Association between parity and macrosomia in Shaanxi Province of Northwest China. Ital J Pediatr. (2020) 46(1):1–7. doi: 10.1186/s13052-019-0764-1
13. Rao J, Fan D, Wu S, Lin D, Zhang H, Ye S, et al. Trend and risk factors of low birth weight and macrosomia in South China, 2005–2017: a retrospective observational study. Sci Rep. (2018) 8(1):3393. doi: 10.1038/s41598-018-21771-6
14. Yesmin K, Begum F, Islam MR. Nutritional status in a patient with gestational diabetes mellitus and pregnancy outcome. J Bangladesh Coll Physicians Surg. (2023) 41(1):63–74. doi: 10.3329/jbcps.v41i1.63261
15. Islam MN, Tazmin T, Siddika M, Bhuiyan MKJ. Morbidities and mortalities among infant of diabetic mother in a newly established scabu of a tertiary care hospital, Bangladesh. J Nepal Paediatr Soc. (2015) 35(3):253–6. doi: 10.3126/jnps.v35i3.14004
17. Boulet SL, Alexander GR, Salihu HM, Pass MA. Macrosomic births in the United States: determinants, outcomes, and proposed grades of risk. Am J Obstet Gynecol. (2003) 188(5):1372–8. doi: 10.1067/mob.2003.302
18. Yadav H, Lee N. Factors influencing macrosomia in pregnant women in a tertiary care hospital in Malaysia. J Obstet Gynaecol Res. (2014) 40(2):439–44. doi: 10.1111/jog.12209
19. Li G, Kong L, Li Z, Zhang L, Fan L, Zou L, et al. Prevalence of macrosomia and its risk factors in China: a multicentre survey based on birth data involving 101,723 singleton term infants. Paediatr Perinat Epidemiol. (2014) 28(4):345–50. doi: 10.1111/ppe.12133
20. Azur MJ, Stuart EA, Frangakis C, Leaf PJ. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res. (2011) 20(1):40. doi: 10.1002/mpr.329
21. Bursac Z, Gauss CH, Williams DK, Hosmer DW. Purposeful selection of variables in logistic regression. Source Code Biol Med. (2008) 3(1):1–8. doi: 10.1186/1751-0473-3-17
22. Canchucaja AR, Cardenas LAC. Maternal factors associated with fetal macrosomia according to the national survey of demographics and family health 2020. Rev la Fac Med Humana. (2022) 22(3):6. doi: 10.25176/RFMH.v22i3.4795
23. Shafqat T, Zeb L, Yasmin S. Fetal macrosomia among non-diabetic women: our experience in a developing country. Cureus. (2022) 14(7):e26763. doi: 10.7759/cureus.26763
24. Bedu-Addo K, Ephraim RKD, Tanoe-Blay C, Ahenkorah-Fondjo L, Osei-Darkwah K, Ephraim M, et al. Prevalence and associated factors of fetal macrosomia in a rural community in Ghana. Cogent Med. (2020) 7(1):1746602. doi: 10.1080/2331205X.2020.1746602
25. Said AS, Manji KP. Risk factors and outcomes of fetal macrosomia in a tertiary centre in Tanzania: a case-control study. BMC Pregnancy Childbirth. (2016) 16(1):1–8. doi: 10.1186/s12884-015-0735-5
26. Ali N, Mohanto NC, Nurunnabi SM, Haque T, Islam F. Prevalence and risk factors of general and abdominal obesity and hypertension in rural and urban residents in Bangladesh: a cross-sectional study. BMC Public Health. (2022) 22(1):1707. doi: 10.1186/s12889-022-14087-8
27. Njom Nlend AE, Gwodog J, Sandie AB. Fetal macrosomia and associated factors to perinatal adverse outcomes, in Yaounde, Cameroon: a case control study. Preprints. (2022):2022080261. doi: 10.20944/preprints202208.0261.v1
28. Tela FG, Bezabih AM, Adhanu AK, Tekola KB. Fetal macrosomia and its associated factors among singleton live-births in private clinics in Mekelle city, Tigray, Ethiopia. BMC Pregnancy Childbirth. (2019) 19(1):1–6. doi: 10.1186/s12884-018-2145-y
29. Luo L, Zeng H, Zeng M, Liu X, Xu X, Wang L, et al. The second pregnancy has no effect in the incidence of macrosomia: a cross-sectional survey in two western Chinese regions. J Heal Popul Nutr. (2021) 40(1):19. doi: 10.1186/s41043-021-00244-z
30. Usta A, Usta CS, Yildiz A, Ozcaglayan R, Dalkiran ES, Savkli A, et al. Frequency of fetal macrosomia and the associated risk factors in pregnancies without gestational diabetes mellitus. Pan Afr Med J. (2017) 26:62. doi: 10.11604/pamj.2017.26.62.11440
31. Wong PY, To WW. Risk factors and pregnancy outcomes of macrosomia: a retrospective cohort study. Obstet Gynaecol Soc. (2018) 18(1):18–23. doi: 10.12809/hkjgom.18.1.238
32. Rao J, Fan D, Wu S, Lin D, Zhang H, Ye S, et al. Trend and risk factors of low birth weight and macrosomia in South China, 2005–2017: a retrospective observational study. Sci Rep. (2018) 8(1):1–8. doi: 10.1038/s41598-018-21771-6
33. Berhanie E, Gebregziabher D, Berihu H, Gerezgiher A, Kidane G. Intimate partner violence during pregnancy and adverse birth outcomes: a case-control study. Reprod Health. (2019) 16(1):1–9. doi: 10.1186/s12978-019-0670-4
34. Krishnamoorthy Y, Ganesh K. Prevalence and determinants of physical violence and its impact on birth outcomes during pregnancy in India: evidence from a nationally representative survey. J Interpers Violence. (2022) 37(5-6):2615–32. doi: 10.1177/0886260520938509
35. Laelago T, Belachew T, Tamrat M. Effect of intimate partner violence on birth outcomes. Afr Health Sci. (2017) 17(3):681–9. doi: 10.4314/ahs.v17i3.10
36. Pun KD, Rishal P, Darj E, Infanti JJ, Shrestha S, Lukasse M, et al. Domestic violence and perinatal outcomes - a prospective cohort study from Nepal. BMC Public Health. (2019) 19(1):671. doi: 10.1186/s12889-019-6967-y
37. Zhang S, Wang L, Leng J, Liu H, Li W, Zhang T, et al. Hypertensive disorders of pregnancy in women with gestational diabetes Mellitus on overweight Status of their children. J Hum Hypertens. (2017) 31(11):731. doi: 10.1038/jhh.2017.17
38. Xiong X, Demianczuk NN, Buekens P, Saunders LD. Association of preeclampsia with high birth weight for gestational age. Am J Obstet Gynecol. (2000) 183(1):148–55. doi: 10.1016/S0002-9378(00)24793-5
39. Mohammadbeigi A, Farhadifar F, Soufi Zadeh N, Mohammadsalehi N, Rezaiee M, Aghaei M. Fetal macrosomia: risk factors, maternal, and perinatal outcome. Ann Med Health Sci Res. (2013) 3(4):546–50. doi: 10.4103/2141-9248.122098
40. Braunthal S, Brateanu A. Hypertension in pregnancy: pathophysiology and treatment. SAGE Open Med. (2019) 7:2050312119843700. doi: 10.1177/2050312119843700
41. Kalupahana NS, Moustaid-Moussa N. The renin-angiotensin system: a link between obesity, inflammation and insulin resistance. Obes Rev. (2012) 13(2):136–49. doi: 10.1111/j.1467-789X.2011.00942.x
42. Tiruneh T, Shiferaw E, Enawgaw B. Prevalence and associated factors of anemia among full-term newborn babies at University of Gondar comprehensive specialized hospital, Northwest Ethiopia: a cross-sectional study. Ital J Pediatr. (2020) 46(1):495–503. doi: 10.1186/s13052-019-0764-1
43. Nkwabong E, Nzalli Tangho GR. Risk factors for macrosomia. J Obstet Gynaecol India. (2015) 65(4):226–9. doi: 10.1007/s13224-014-0586-4
44. Al-Qashar F, Al-Ghamdi M, Agab W, Al-Sayed M, Jabari M, Al-Shehri H, et al. Prevalence and outcomes of macrosomic infants born to non-diabetic mothers: a ten years’ experience at tertiary care center. J Am Sci. (2016) 12(12):89. doi: 10.7537/marsjas121216.12
45. Lampl M, Gotsch F, Kusanovic JP, Gomez R, Nien JK, Frongillo EA, et al. Sex differences in fetal growth responses to maternal height and weight. Am J Hum Biol. (2010) 22(4):431. doi: 10.1002/ajhb.21014
46. Gallagher A, Liu J, Probst JC, Martin AB, Hall JW. Maternal obesity and gestational weight gain in rural versus urban dwelling women in South Carolina. J Rural Heal. (2013) 29(1):1–11. doi: 10.1111/j.1748-0361.2012.00421.x
Keywords: infant, birth weight, macrosomia, determinants, Bangladesh
Citation: Islam MZ, Chowdhury MRK, Billah B, Rashid M, Kabir R, Hasan M and Kader M (2024) Prevalence and determinants of fetal macrosomia in Bangladesh. Front. Pediatr. 12:1405442. doi: 10.3389/fped.2024.1405442
Received: 22 March 2024; Accepted: 11 July 2024;
Published: 2 August 2024.
Edited by:
Carmen Rubio, Manuel Velasco Suárez National Institute of Neurology and Neurosurgery, MexicoReviewed by:
Bryanne Colvin, Washington University in St. Louis, United StatesYong Guo, Guangdong Women and Children Hospital, China
Antonella Poloniato, San Raffaele Hospital (IRCCS), Italy
© 2024 Islam, Chowdhury, Billah, Rashid, Kabir, Hasan and Kader. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manzur Kader, bWFrZEBkdS5zZQ==
†These authors have contributed equally to this work
‡ORCID:
Mamunur Rashid
orcid.org/0000-0001-7558-4168
 Md. Zahidul Islam
Md. Zahidul Islam Mohammad Rocky Khan Chowdhury
Mohammad Rocky Khan Chowdhury Baki Billah
Baki Billah Mamunur Rashid
Mamunur Rashid Russell Kabir
Russell Kabir Mehedi Hasan6
Mehedi Hasan6 Manzur Kader
Manzur Kader