- 1Department of Neonatology, The Affiliated Hospital of Qingdao University, Qingdao, China
- 2Department of Neonatology, Beijing Jingdu Children’s Hospital, Beijing, China
- 3Qingdao University, Qingdao, China
Objective: This study aimed to investigate the correlation between serum 25-hydroxyvitamin D (25(OH)D) levels and retinopathy of prematurity (ROP) in premature infants one month after birth.
Methods: Preterm infants (gestational age <32 weeks) admitted to the Affiliated Hospital of Qingdao University from 2017 to 2022 were divided into ROP and non-ROP groups based on ROP occurrence any stage. Serum 25(OH)D levels and clinical data were compared between the two groups at 1 month after birth, and the relationship between vitamin D levels and ROP was analyzed.
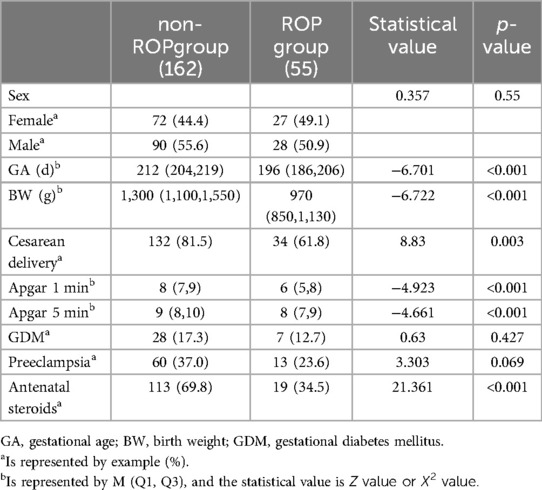
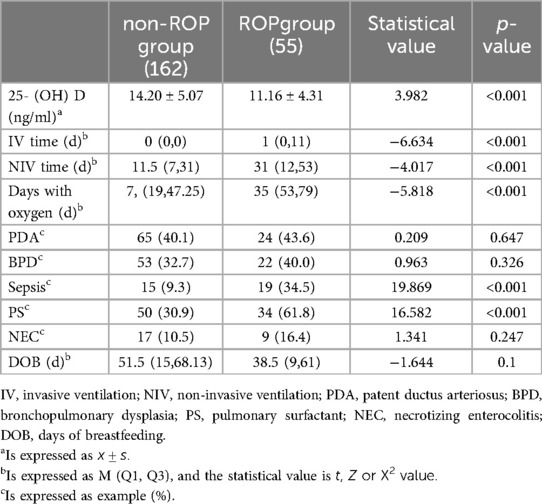
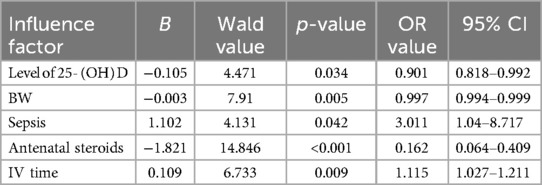
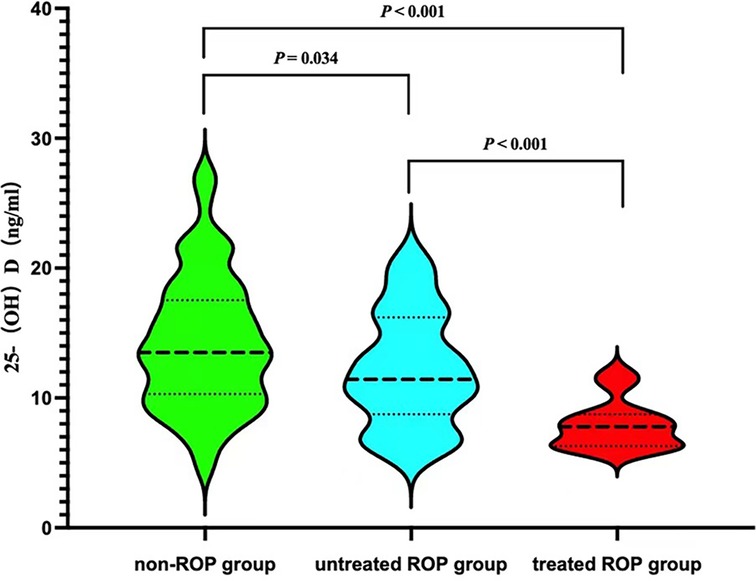
Results: Among the 217 premature infants included, 55 (25.35%) were in the ROP group, and 162 (74.65%) were in the non-ROP group. The ROP group had lower gestational age and birth weight, longer invasive ventilation (IV), non-invasive ventilation (NIV), and oxygen therapy times compared to the non-ROP group. Apgar scores, cesarean delivery, and antenatal steroids ratios were lower in the ROP group, while sepsis and pulmonary surfactant utilization ratios were higher (all p < 0.05). Significant differences in serum 25-(OH)D levels were observed among children in the non-ROP group (14.20 ± 5.07 ng/ml), ROP treated group (7.891 ± 1.878 ng/ml), and untreated group (12.168 ± 4.354 ng/ml) (p < 0.001). Multivariate regression analysis identified antenatal steroids as protective factors and lower birth weight, serum 25-(OH)D levels, long-term invasive mechanical ventilation, and sepsis as independent risk factors for ROP in premature infants.
Conclusion: Vitamin D, lower birth weight, long-term invasive mechanical ventilation, and sepsis were associated with incidence of ROP in preterm infants. Vitamin D was associated with the severity of ROP, emphasizing the importance of prudent vitamin D supplementation and regular monitoring of serum 25-(OH)D levels.
1 Introduction
Retinopathy of prematurity (ROP) is characterized by abnormal proliferation of retinal blood vessels in the eyes of premature infants. The incidence and severity of ROP increase with decreasing gestational age and birth weight, posing a significant threat to infant vision. Severe cases may lead to retinal detachment and subsequent blindness (1–4). ROP is linked to elevated levels of vascular endothelial growth factor (VEGF) caused by local hypoxia, promoting aberrant formation of blood vessels in newborns. After birth, premature infants experience a deficiency in the supply of IGF-1 from the placenta and lack autonomous production, thereby impacting the development of retinal blood vessels. Key factors contributing to ROP include premature birth, low birth weight, oxygen therapy, anemia, blood transfusions, sepsis, metabolic acidosis, respiratory distress syndrome (RDS), apnea, and maternal pregnancy complications (5, 6). As our understanding of ultra-premature infants advances, early identification of high-risk factors for ROP becomes crucial for prevention and treatment, ultimately reducing the incidence of blindness and improving the quality of life for premature infants (7).
Vitamin D, a biologically active fat-soluble vitamin synthesized endogenously, not only regulates calcium and phosphorus metabolism but also exhibits antioxidant and anti-inflammatory effects. Additionally, it plays a role in anti-angiogenesis, cell growth, and differentiation. Eye tissues contain vitamin D receptors and 1-hydroxylase, indicating a local regulatory role (8). Given that placental transfer of vitamin D mainly occurs during the third trimester, vitamin D deficiency is a concern, particularly in premature infants with a gestational age <32 weeks (9, 10). However, there is limited research on the association between vitamin D and ROP. Some studies suggested that lower 25(OH)D levels in premature infants with a gestational age <32 weeks after birth might be related to ROP (11–13).
This study aims to collect 25(OH)D levels in premature infants with a gestational age <32 weeks at 1 month after birth and retrospectively analyze clinical data to explore the relationship between serum 25(OH)D levels and ROP. The findings provide valuable insights for the clinical prevention and treatment of ROP in premature infants.
2 Patients and methods
2.1 Preterm infants
A retrospective study was conducted on all preterm infants with a gestational age <32 weeks admitted to the intensive care unit at the Affiliated Hospital of Qingdao University during 2017–2022. All infants in the hospital signed the written consent “clinical samples to carry out scientific research” by parents.
Inclusion criteria: (1) Preterm infants with a gestational age <32 weeks. (2) Premature infants admitted to the hospital within 24 h after birth, with a hospitalization duration exceeding 4 weeks. (3) Infants whose condition improves or is cured upon discharge.
Exclusion criteria: (1) Specific congenital eye diseases, such as retinoblastoma, congenital cataract, glaucoma, etc. (2) Infants with genetic metabolic diseases and congenital developmental malformations. (3) Cases involving death during hospitalization, incomplete clinical data, or family members opting for treatment discontinuation. (4) Individuals with incomplete information.
Based on the occurrence of retinopathy of prematurity (ROP), participants were divided into the ROP group and non-ROP group. Within the ROP group, two subgroups were identified based on whether ranibizumab or laser treatment was administered: treated ROP group and untreated group. The study received approval from the Affiliated hospital of Qingdao University ethics committee (QYFY WZLL 28884).
2.2 Methods
2.2.1 Vitamin D supplementation method
Enteral feeding should be initiated once the condition of the included children stabilizes. Refer to the “Recommendations for the Prevention and Treatment of Vitamin D Deficiency and Vitamin D Deficiency Rickets” (14) for vitamin D supplementation guidance. For preterm infants with a gestational age <32 weeks, prioritize breast milk when establishing enteral feeding. Breast milk fortifier(Similac Human Milk Fortifier, POWDER) is added when milk intake reaches 80–100 ml/kg. If breast milk is insufficient, formula milk is provided. Daily supplements include 1 Vitamin AD Soft Capsules (DYNE PHARMA, vitamin D 500 IU) and vitamin D3 400 IU per day. Parenteral nutrition for all premature infants follows the 2013 Chinese Clinical Application Guidelines for Neonatal Nutritional Support (15), with the vitamin D content of fat-soluble vitamins in intravenous nutrition at 400 IU/10 ml (neonatal dosage is 1 ml/kg).
2.2.2 ROP screening
Follow the “China Retinopathy of Prematurity Screening Guidelines (2014)” (16). Qualified ophthalmologists use indirect ophthalmoscopy or RetCam fundus cameras to screen infants with a gestational age of 32 weeks or a birth weight of 2,000 g. Premature and low birth weight infants undergo ROP screening, with the first screening performed at 4–6 weeks after birth or 31–32 weeks after menstruation, until corrected the gestational age to 44 weeks, retinal blood vessels should grow until the jagged edge. Indications for early treatment of ROP (17) involve the use of anti-VEGF drug ranibizumab or laser therapy.
2.2.3 Data collection methods: collect data on infants and their mothers through electronic medical records
General information includes sex, gestational age, birth weight, mode of delivery, Apgar scores at 1 and 5 min.
Clinical data encompass oxygen therapy time, invasive ventilation time, non-invasive ventilation time, serum 25-(OH)D level at 1 month after birth, pulmonary surfactant (PS) treatment and breastfeeding time.
Perinatal factors include preeclampsia, gestational diabetes mellitus, and antenatal steroids.
Major complications are defined as follows: Bronchopulmonary dysplasia (BPD), Patent ductus arteriosus (PDA), necrotizing enterocolitis (NEC), and sepsis.
Use of invasive ventilation, non-invasive ventilation, PS, and antenatal steroids aligns with the “European Consensus Guidelines for the Management of Neonatal Respiratory Distress Syndrome (2019 Edition)” (18).
Oxygen therapy time is calculated by summing mechanical ventilation time, hood oxygen inhalation time, and nasal cannula oxygen inhalation time.
3 Statistical methods
SPSS 29.0 statistical software was utilized for comprehensive data analysis, while GraphPad Prism 10 software facilitated graphical representation. For measurement data conforming to a normal distribution, mean ± standard deviation (x ± s) was employed. Group comparisons were conducted using independent samples t-test and single-factor ANOVA. In cases where measurement data deviated from normal distribution, the results are presented as median (interquartile range) M (Q1, Q3), and the Mann–Whitney U rank sum test was applied for between-group comparisons.
Count data was expressed as case (percentage) n (%), and the χ2-test or Fisher's exact probability method was employed for group comparisons. Single-factor analysis helped identify statistically significant influencing factors as independent variables, with ROP occurrence serving as the dependent variable in the logistic regression model for multi-factor analysis. A significance threshold of p < 0.05 was considered indicative of statistically significant differences.
4 Results
4.1 Demographic information
Throughout the study period, 217 premature infants with a gestational age <32 weeks met the inclusion criteria at our hospital. This cohort comprised 162 infants (74.65%) in the non-ROP group and 55 infants (25.35%) in the ROP group. Specifically, among the ROP group, 13 infants underwent treatment with ranibizumab (treated ROP group), constituting 23.6% (13/55), while 42 infants remained untreated, representing 76.4% (42/55).
4.2 Single factor analysis of ROP
4.2.1 Analysis of perinatal factors
In the ROP group, the gestational age, birth weight, Apgar score, rate of cesarean section, and antenatal steroid usage were all notably lower than those observed in the non-ROP group. These differences reached statistical significance (p < 0.05). In contrast, when comparing sex, gestational diabetes mellitus, and preeclampsia between the two groups, no statistically significant differences were identified (p > 0.05) (refer to Table 1).
4.2.2 Clinical data analysis
The serum 25-(OH)D level in the ROP group was observed to be lower than that in the non-ROP group. Additionally, the ROP group exhibited significantly higher values in terms of invasive ventilation time, non-invasive ventilation time, oxygen therapy time, sepsis incidence, and pulmonary surfactant (PS) treatment compared to the non-ROP group, with all differences proving statistically significant (p < 0.05). In contrast, there were no statistically significant differences noted in the occurrences of Patent Ductus Arteriosus (PDA), Bronchopulmonary Dysplasia (BPD), and Necrotizing Enterocolitis (NEC) between the two groups (p > 0.05) (refer to Table 2).
4.2.3 Multifactor analysis of ROP risk factors
Multivariate logistic regression analysis was employed on variables that exhibited statistical significance in the univariate analysis. The outcomes revealed that antenatal steroids serve as a protective factor for ROP in premature infants. Conversely, lower vitamin D, lower birth weight, prolonged invasive mechanical ventilation time, and sepsis were identified as independent risk factors for the development of retinopathy of prematurity (ROP) in preterm infants (refer to Table 3).
4.2.4 Comparison of serum 25-(Oh)D levels among non-ROP, treated ROP, and untreated ROP groups and correlation analysis with ROP severity
Significant differences in serum 25-(OH)D levels were observed among the three groups of children (p < 0.001). Specifically, the treated ROP group exhibited the lowest serum 25-(OH)D levels (see Figure 1).
5 Discussion
ROP is one of the leading causes of blindness in preterm infants. The development of fetal retinal blood vessels initiates from the optic disc at approximately 16 weeks of gestation and progresses towards the periphery. By about 32 weeks of gestation, these vessels reach the lateral peripheral part. Notably, the temporal retinal blood vessels do not achieve full maturity until the fetus reaches full term. The immaturity of retinal blood vessels is more pronounced in infants with younger gestational ages, correlating with an increased likelihood of ROP development. In this study, the incidence of ROP in premature infants with a gestational age <32 weeks was found to be 25.35%, a figure that aligns closely with the results reported in both domestic and foreign studies (16, 19–21).
The etiology and pathogenesis of Retinopathy of Prematurity (ROP) involve the intricate interplay of multiple factors. Notably, preterm birth and low body weight stand out as foundational contributors to the development of ROP (19, 21). Cytokines that play a crucial role in promoting retinal development are relatively scarce in early pregnancy and do not witness a significant increase until the later stages of pregnancy. Among these cytokines, insulin-like growth factor-1 (IGF-1) emerges as a key regulator of the vascular endothelial system, playing a pivotal role in the occurrence and progression of ROP (22, 23). After birth, premature infants experience a deficiency in the supply of IGF-1 from the placenta and lack autonomous production, thereby impacting the development of retinal blood vessels. The findings of this study corroborate these insights, revealing that children in the ROP group had lower gestational ages and birth weights compared to those in the non-ROP group. It is evident that low birth weight serves as a high-risk factor for the onset of ROP.
Due to the underdeveloped respiratory system and nervous system of premature infants, they often experience frequent apnea or neonatal respiratory distress syndrome, necessitating high-concentration oxygen therapy for survival. The immature retinal blood vessels in these infants are highly sensitive to oxygen, and exposure to high concentrations can lead to vasoconstriction and, in severe cases, occlusion, resulting in relative hypoxia in the retina. The intricate interplay of various active factors under these conditions promotes the generation of numerous new blood vessels, culminating in the development of Retinopathy of Prematurity (ROP) (24, 25). The findings of this study align with these mechanisms, indicating that children with ROP had higher durations of invasive mechanical ventilation, non-invasive mechanical ventilation, and oxygen therapy compared to those without ROP. Notably, invasive ventilation time emerged as an independent risk factor for the occurrence of ROP. de las Rivas Ramírez et al. retrospectively collected ROP data from preterm infants treated in Regional University Hospital of Málaga, in the multivariate analysis, weight and mechanical ventilation duration, and late-onset sepsis were independently associated with the development of ROP (26).
This study identifies sepsis as a significant risk factor for Retinopathy of Prematurity (ROP). Sepsis poses an increased risk for ROP, with inflammatory factors playing a pivotal role in this association (27, 28). During infections, premature infants release substantial amounts of cytokines, including IL-6 and TNF-α. These cytokines can inflict damage on endothelial cells or disrupt vascular function, subsequently stimulating retinal vascular endothelial cells to produce vascular endothelial growth factor (VEGF), thereby contributing to the onset of ROP (29–31). Infections in premature infants may also induce hemodynamic changes, influencing retinal blood perfusion and resulting in heightened retinal ischemia, ultimately leading to the development of ROP.
Antenatal steroids emerge as protective factors against Retinopathy of Prematurity (ROP), effectively reducing the risk of its occurrence (32, 33). Notably, this study revealed a notably low rate of antenatal steroid administration in the ROP group, standing at only 34.5%. The “European Guidelines for the Management of Neonatal Respiratory Distress Syndrome” advocate for the administration of antenatal steroids to all pregnant women with a gestational age of less than 34 weeks and those at risk for preterm birth. The administration of glucocorticoids aids in promoting fetal lung maturation, decreasing the postpartum oxygen requirement, and consequently mitigating the risk of ROP. Additionally, antenatal steroids exhibit inhibitory effects on the production of oxidative stress and inflammatory factors, indirectly contributing to the prevention of ROP.
Vitamin D undergoes hydroxylation in the human liver to form 25-(OH)D. This metabolite, characterized by a stable structure and a long half-life, serves as the optimal indicator for assessing the nutritional status of vitamin D (34). Studies by Alsalem et al. (8) have identified the presence of vitamin D receptors and hydroxylase in ocular cells, including corneal epithelial and retinal cells. These cells have the capacity to convert vitamin D from an inactive form to its active form. Given that intrauterine placental transport of vitamin D predominantly occurs in the third trimester, vitamin D deficiency is prevalent among premature infants. Despite routine vitamin D supplementation, our study reveals an overall deficiency in 25-(OH)D levels among premature infants in our hospital. The serum 25-(OH)D concentration in the ROP group at one month is significantly lower than that in the non-ROP group, exhibiting a consistent pattern, and that had significant difference between treated group and untreated group. A study conducted in Iran (35) found an association between low vitamin D levels in premature infants on the first day after birth and ROP. The severity of ROP increased with greater vitamin D deficiency. Another study from India (36) demonstrated persistent vitamin D deficiency in premature infants with ROP at 4 weeks after birth compared to those without ROP. The current understanding of the pathogenesis of vitamin D in retinopathy encompasses several aspects: Antioxidant effect: Vitamin D's antioxidant properties mitigate oxidative stress damage to the retina and optic nerve by preserving mitochondrial function and neutralizing free radicals (37). Anti-inflammatory and immunomodulatory effects: Studies have demonstrated that vitamin D can modulate T cell subset proportions in retinopathy, inhibit inflammatory response signaling pathways, and decrease inflammatory cell infiltration, thereby reducing damage to the retina and optic nerve (38, 39). Anti-angiogenic effect: Deregulation of vascular endothelial growth factor (VEGF) levels leads to (ROP). VIT-D binding domains are present in the promoter regions of VEGF, thus, VIT-D supplementation might aid in restoring VEGF levels, especially in pathologic conditions secondary to low VEGF levels (40). Jamali et al. (41) have demonstrated that vitamin D's inhibitory effect on retinal neovascularization is dependent on the expression of vitamin D receptors. Experimental studies in a mouse model of oxygen-induced ischemic retinopathy revealed that mice treated with calcitriol exhibited a significant decrease in VEGF expression, retinal neovascularization, and blood-retinal barrier permeability (42, 43). Investigation of serum vitamin D levels is recommended, Early correction of vitamin D deficiency may lead to reduction of RoP in premature infants (35).
6 Conclusion
In conclusion, this study highlights that Vitamin D, lower birth weight, long-term invasive mechanical ventilation, and sepsis were associated with incidence of ROP in preterm infants.
Standardizing the administration of antenatal steroids, providing judicious vitamin D supplementation, and closely monitoring serum 25-(OH)D levels can be instrumental in mitigating the incidence and severity of ROP in this vulnerable population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Medical Ethics Committee of the Affiliated Hospital of Qingdao University. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from primarily isolated as part of your previous study for which ethical approval was obtained. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
XY: Conceptualization, Writing – original draft. SX: Formal Analysis, Methodology, Writing – review & editing. XZ: Data curation, Investigation, Writing – review & editing. LL: Investigation, Writing – review & editing. HX: Data curation, Writing – review & editing. LM: Investigation, Writing – review & editing. MS: Funding acquisition, Writing – review & editing. PY: Writing – review & editing. XL: Supervision, Visualization, Writing – review & editing. HJ: Supervision, Visualization, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by a grant from the Youth Project of Natural Science Foundation of Shandong Province [grant number ZR2020QH054].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sabri K, Ells AL, Lee EY, Dutta S, Vinekar A. Retinopathy of prematurity: a global perspective and recent developments. Pediatrics. (2022) 150(3):e2021053924. doi: 10.1542/peds.2021-053924
2. Xun X, Hospital SG, Jiao S. Expert consensus on the classification and treatment of retinopathy of prematurity in China (2023 edition). Chinese J Ocul Fundus Dis. (2023) 39:720–7.
3. Jianhong L. Expert consensus on the treatment of retinopathy of prematurity. Chinese J Ocul Fundus Dis. (2022) 38:10–3.
4. Trzcionkowska K, Termote JU, Böhringer S, van Sorge AJ, Schalij-Delfos N. Nationwide inventory on retinopathy of prematurity screening in The Netherlands. Br J Ophthalmol. (2023) 107:712–6. doi: 10.1136/bjophthalmol-2021-319929
5. Dammann O, Hartnett ME, Stahl A. Retinopathy of prematurity. Dev Med Child Neurol. (2023) 65:625–31. doi: 10.1111/dmcn.15468
7. Gilbert C, Foster A. Blindness in children : control priorities and research opportunities. Br J Ophthalmol. (2001) 85:1025–7. doi: 10.1136/bjo.85.9.1025
8. Alsalem JA, Patel D, Susarla R, Coca-Prados M, Bland R, Walker EA, et al. Characterization of vitamin D production by human ocular barrier cells. Invest Ophthalmol Vis Sci. (2014) 55:2140–7. doi: 10.1167/iovs.13-13019
9. Kanike N, Hospattankar KG, Sharma A, Worley S, Groh-Wargo S. Prevalence of vitamin D deficiency in a large newborn cohort from Northern United States and effect of intrauterine drug exposure. Nutrients. (2020) 12:1–9. doi: 10.3390/nu12072085
10. Yang C, Jing W, Ge S, Sun W. Vitamin D status and vitamin D deficiency risk factors among pregnancy of Shanghai in China. BMC Pregnancy Childbirth. (2021) 21:431. doi: 10.1186/s12884-021-03889-0
11. Kabataş EU, Dinlen NF, Zenciroğlu A, Dilli D, Beken S, Okumuş N. Relationship between serum 25-hydroxy vitamin D levels and retinopathy of prematurity. Scott Med J. (2017) 62:129–35. doi: 10.1177/0036933017701867
12. Gverović Antunica A, Znaor L, Ivanković M, Puzović V, Marković I, Kaštelan S. Vitamin D and diabetic retinopathy. Int J Mol Sci. (2023) 24(15):12014. doi: 10.3390/ijms241512014
13. Chan H-N, Zhang X-J, Ling X-T, Bui CH-T, Wang Y-M, Ip P, et al. Vitamin D and ocular diseases: a systematic review. Int J Mol Sci. (2022) 23:1–58. doi: 10.3390/ijms23084226
14. National Scientific Research Group on Prevention and Treatment of Rickets CNPC of CEA. Recommendations for the prevention and treatment of vitamin D deficiency and vitamin D deficient rickets. Chin J Child Heal Care. (2015) 23:781–2.
15. Cai W, Tang Q, Wang Y, Feng Y, Wu J, Qian L, et al. Clinical application guidelines for neonatal nutritional support in China. J Clin Pediatr. (2013) 31:1177–82.
16. Fundus Disease Group of the Ophthalmology Branch of the Chinese Medical Association. Chinese Guidelines for screening retinopathy of prematurity (2014). Chin J Ophthalmol. (2014) 50:933–5.
17. Early Treatment for Retinopathy of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. (2003) 121:1684–94. doi: 10.1001/archopht.121.12.1684
18. Sweet DG, Carnielli V, Greisen G, Hallman M, Ozek E, Plavka R, et al. European consensus guidelines on the management of respiratory distress syndrome—2016 update. Neonatology. (2017) 111:107–25. doi: 10.1159/000448985
19. Edy Siswanto J, Sauer PJJ. Retinopathy of prematurity in Indonesia: incidence and risk factors. J Neonatal Perinatal Med. (2017) 10:85–90. doi: 10.3233/NPM-915142
20. Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. (2013) 74(Suppl 1 (Suppl 1)):35–49. doi: 10.1038/pr.2013.205
21. Siswanto JE, Bos AF, Dijk PH, Rohsiswatmo R, Irawan G, Sulistijono E, et al. Multicentre survey of retinopathy of prematurity in Indonesia. BMJ Paediatr Open. (2021) 5:e000761. doi: 10.1136/bmjpo-2020-000761
22. Yenice O, Cerman E, Ashour A, Firat R, Haklar G, Sirikci O, et al. Serum erythropoietin, insulin-like growth factor 1, and vascular endothelial growth factor in etiopathogenesis of retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina. (2013) 44:549–54. doi: 10.3928/23258160-20131105-05
23. Yu C, Yi J, Yin X, Deng Y, Liao Y, Li X. Correlation of interactions between NOS3 polymorphisms and oxygen therapy with retinopathy of prematurity susceptibility. Int J Clin Exp Pathol. (2015) 8:15250–4. http://www.ncbi.nlm.nih.gov/pubmed/2682387526823875
24. Chen J, Stahl A, Hellstrom A, Smith LE. Current update on retinopathy of prematurity: screening and treatment. Curr Opin Pediatr. (2011) 23:173–8. doi: 10.1097/MOP.0b013e3283423f35
25. Enomoto H, Miki A, Matsumiya W, Honda S. Evaluation of oxygen supplementation status as a risk factor associated with the development of severe retinopathy of prematurity. Ophthalmologica. (2015) 234:135–8. doi: 10.1159/000433565
26. de Las Rivas Ramírez N, Luque Aranda G, Rius Díaz F, Pérez Frías FJ, Sánchez Tamayo T. Risk factors associated with retinopathy of prematurity development and progression. Sci Rep. (2022) 12(1):21977. doi: 10.1038/s41598-022-26229-4
27. Silveira RC, Filho JBF, Procianoy RS. Assessment of the contribution of cytokine plasma levels to detect retinopathy of prematurity in very low birth weight infants. Invest Opthalmol Vis Sci. (2011) 52:1297. doi: 10.1167/iovs.10-6279
28. Yu H, Yuan L, Zou Y, Peng L, Wang Y, Li T, et al. Serum concentrations of cytokines in infants with retinopathy of prematurity. APMIS. (2014) 122:818–23. doi: 10.1111/apm.12223
29. Zhou Y, Jiang Y, Bai Y, Wen J, Chen L. Vascular endothelial growth factor plasma levels before and after treatment of retinopathy of prematurity with ranibizumab. Graefes Arch Clin Exp Ophthalmol. (2016) 254:31–6. doi: 10.1007/s00417-015-2996-0
30. Hong HK, Lee HJ, Ko JH, Park JH, Park JY, Choi CW, et al. Neonatal systemic inflammation in rats alters retinal vessel development and simulates pathologic features of retinopathy of prematurity. J Neuroinflammation. (2014) 11:87. doi: 10.1186/1742-2094-11-87
31. Ma X, Bi H, Qu Y, Xie X, Li J. The contrasting effect of estrogen on mRNA expression of VEGF in bovine retinal vascular endothelial cells under different oxygen conditions. Graefes Arch Clin Exp Ophthalmol. (2011) 249:871–7. doi: 10.1007/s00417-010-1594-4
32. Yim C-L, Tam M, Chan H-L, Tang S-M, Au SCL, Yip WWK, et al. Association of antenatal steroid and risk of retinopathy of prematurity: a systematic review and meta-analysis. Br J Ophthalmol. (2018) 102:1336–41. doi: 10.1136/bjophthalmol-2017-311576
33. Zeng Y, Ge G, Lei C, Zhang M. Beyond fetal immunity: a systematic review and meta-analysis of the association between antenatal corticosteroids and retinopathy of prematurity. Front Pharmacol. (2022) 13:759742. doi: 10.3389/fphar.2022.759742
34. Sung C-C, Liao M-T, Lu K-C, Wu C-C. Role of vitamin D in insulin resistance. J Biomed Biotechnol. (2012) 2012:634195. doi: 10.1155/2012/634195
35. Boskabadi H, Abrishami M, Shoeibi N, Sanei Z, Moradi A, Zakerihamidi M. Comparison of vitamin D levels in premature infants with and without retinopathy of prematurity. Arch Iran Med. (2022) 25:209–13. doi: 10.34172/aim.2022.36
36. Deb D, Annamalai R, Muthayya M. Correlation of vitamin D levels with low gestational age and low birth weight in babies developing retinopathy of prematurity. Indian J Public Health. (2022) 66:531–2. doi: 10.4103/ijph.ijph_1044_22
37. Skowron K, Pawlicka I, Gil K. The role of vitamin D in the pathogenesis of ocular diseases. Folia Med Cracov. (2018) 58:103–18. doi: 10.24425/fmc.2018.124662
38. Fogagnolo P, De Cilla’ S, Alkabes M, Sabella P, Rossetti L. A review of topical and systemic vitamin supplementation in ocular surface diseases. Nutrients. (2021) 13:1–12. doi: 10.3390/nu13061998
39. Gorimanipalli B, Shetty R, Sethu S, Khamar P. Vitamin D and the eye: current evidence and practice guidelines. Indian J Ophthalmol. (2023) 71:1127–34. doi: 10.4103/IJO.IJO_3174_22
40. Murugeswari P, Vinekar A, Grace Prakalapakorn S, Anandula VR, Subramani M, Vaidya TA, et al. Correlation between tear levels of vascular endothelial growth factor and vitamin D at retinopathy of prematurity stages in preterm infants. Sci Rep. (2023) 13(1):16175. doi: 10.1038/s41598-023-43338-w
41. Jamali N, Wang S, Darjatmoko SR, Sorenson CM, Sheibani N. Vitamin D receptor expression is essential during retinal vascular development and attenuation of neovascularization by 1, 25(OH)2D3. PLoS One. (2017) 12:e0190131. doi: 10.1371/journal.pone.0190131
42. Albert DM, Scheef EA, Wang S, Mehraein F, Darjatmoko SR, Sorenson CM, et al. Calcitriol is a potent inhibitor of retinal neovascularization. Invest Ophthalmol Vis Sci. (2007) 48:2327–34. doi: 10.1167/iovs.06-1210
Keywords: retinopathy of prematurity, serum 25-hydroxyvitamin D, lower birth weight infants, preterm infant, sepsis
Citation: Yin X, Xu S, Zhang X, Li L, Xi H, Ma L, Sun M, Yang P, Li X and Jiang H (2024) The association between serum 25-hydroxyvitamin D levels and retinopathy of prematurity in preterm infants. Front. Pediatr. 12:1404196. doi: 10.3389/fped.2024.1404196
Received: 20 March 2024; Accepted: 22 July 2024;
Published: 2 August 2024.
Edited by:
Minesh Khashu, University Hospitals Dorset NHS Foundation Trust, United KingdomReviewed by:
Lingkong Zeng, Huazhong University of Science and Technology, ChinaKarel Allegaert, KU Leuven, Belgium
© 2024 Yin, Xu, Zhang, Li, Xi, Ma, Sun, Yang, Li and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xianghong Li, bGl4aWFuZ2hvbmcwMzI5QDEyNi5jb20=; Hong Jiang, amlhbmdob25nQHFkdS5lZHUuY24=
†These authors contributed equally to this work and share first authorship
 Xiangyun Yin
Xiangyun Yin Shimin Xu2,†
Shimin Xu2,† Xianghong Li
Xianghong Li Hong Jiang
Hong Jiang