
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Pediatr., 10 April 2024
Sec. Pediatric Nephrology
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1401593
This article is part of the Research TopicCystic Kidney Diseases in Children and Adults: From Diagnosis to Etiology and BackView all 8 articles
Editorial on the Research Topic
Cystic kidney diseases in children and adults: from diagnosis to etiology and back
Renal cysts are often regarded as the most common abnormality associated with kidney disease (1, 2). They are encountered in both adults and children, as isolated findings or as part of a more complex clinical condition (3–5). Isolated kidney cysts in adults sometimes require evaluation for kidney cancer or simple cysts may occur as a sign of age-related kidney tissue degeneration in the absence of any underlying specific kidney disease. Recent advances in understanding the underlying mechanisms have led to the concept of renal ciliopathies with more than 100 genes associated with ciliary dysfunction, resulting in conditions such as polycystic kidney disease (PKD), tuberous sclerosis complex (TSC) and nephronophthisis complex (NPHC), which may be associated with various extrarenal phenotypes (Figure 1) (6–8). In addition to progressive CKD, these disorders are characterized by a variety of additional symptoms such as hepatic impairment, vision problems, developmental delays, intellectual disabilities, and skeletal abnormalities, which inconsistently present throughout the course of the disease (4, 5, 7). Furthermore, the significant phenotypic overlap makes it difficult to differentiate specific disorders, often necessitating genetic testing to reach a definite diagnosis (9). Despite a multitude of clinical and translational studies, in the majority of cases it is still challenging or even impossible to predict the individual clinical course, necessitating regular follow-up of the patients and a timely response in terms of treatment, which remains mostly symptomatic (10).
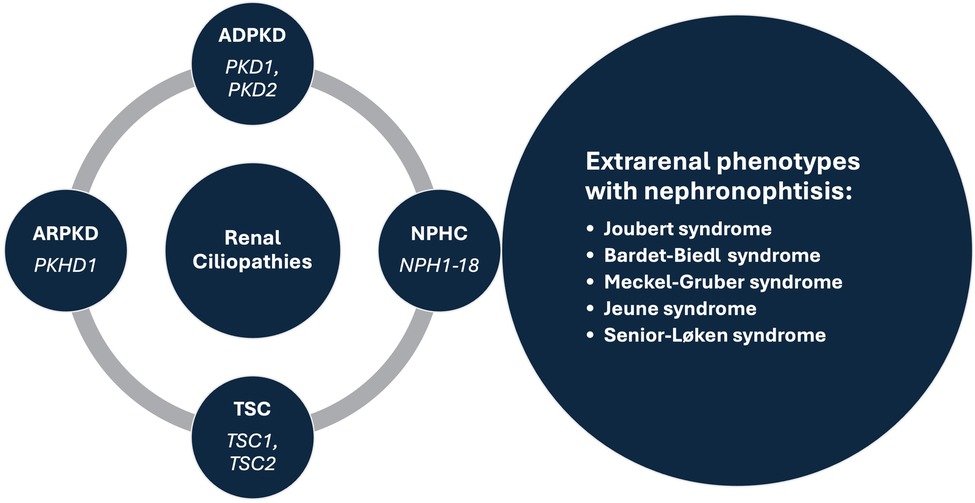
Figure 1. Prominent syndromes and associated genes within the renal ciliopathies concept. ADPKD, autosomal dominant polycystic kidney disease; ARPKD, autosomal recessive polycystic kidney disease; NPHC, nephronophthisis complex; TSC, tuberous sclerosis complex.
The present special issue contains seven noteworthy articles describing engaging cases of children and adults with various disorders having a common denominator in the form of kidney cysts, systematically reviewing the current literature on the clinical characteristics of an HNF1B gene variant and biomarkers of kidney disease progression in autosomal dominant PKD (ADPKD), investigating the outcome of fetal renal cystic disease and exploring the utility of magnetic resonance imaging-based kidney volume assessment for risk stratification in children with ADPKD.
In more detail, Simičić Majce et al. describe a nonconsanguineous family with three members affected by BBS caused by compound heterozygous mutations in the BBS12 gene. Despite identical genotypes, the affected family members demonstrated significant diversity in clinical characteristics (different expressivity) of the BBS phenotype emphasizing the importance of genetic testing for the early diagnosis of this rare ciliopathy. Similarly, Fištrek Prlić et al. present two clinically distinct cases of autosomal dominant tubulointerstitial kidney disease (ADTKD) diagnosed only after genetic testing, along with an extensive review of the literature and a comprehensive overview of the condition. Both patients had uninformative renal ultrasound and urinalysis findings with only elevated serum creatinine levels indicating a kidney disease. An adult patient with a positive family history of CKD had no other symptoms, while an adolescent boy with an unremarkable family history had psychomotor impairment with epilepsy. After the testing they were diagnosed with MUC1-related ADTKD and 17q12 microdeletion syndrome causing the loss of one copy of the transcription factor HNF1B and 14 additional genes, respectively, highlighting the importance of clinical awareness in diagnosing this syndrome. Finally, the third case report by Kasahara et al. advocates an interesting option to treat the chronic pain experienced by more than half of patients with ADPKD. They describe an adolescent girl with persistent pain associated with multiple renal cysts that prevented her from participating in daily activities. After being diagnosed with attention deficit disorder (ADHD) and appropriate treatment for this condition being initiated, she experienced significant pain relief and better control of her hypertension. Therefore, in patients with ADPKD it may be important to recognize concomitant ADHD and consider a trial of ADHD medications when chronic pain associated with ADPKD is present.
In line with the exploration of important associations between cystic kidney disease and other disorders a systematic review by Nittel et al. examined the prevalence of neurodevelopmental disorders (NDD) in patients with 17q12 microdeletions vs. HNF1B point mutations. The results of a diligent literature search revealed that NDDs are frequently observed in HNF1B-associated diseases, especially in the common 17q12 microdeletion, and should hence become a routine part of clinical care for patients with HNF1B-related diseases. On the other hand, a systematic review by Sorić Hosman et al. provided a critical overview of previously examined serum and urine biomarkers with a potential for predicting disease progression or response to therapy in patients with ADPKD. A comprehensive literature review identified several prognostic molecules that are involved in various processes central to the development of the disease, such as tubular injury, inflammation, metabolism, renin-angiotensin, or vasopressin system adjustments. Interestingly, the most accurate predictive models have been achieved when incorporating such serum and urine biomarkers with the Predicting Renal Outcome in Polycystic Kidney Disease (PROPKD) score which combines underlying genetic mutations and clinical risk factors, or with the Mayo Imaging Classification (MIC) which is based on age- and height-adjusted total kidney volume (TKV) measured by magnetic resonance imaging (MRI).
MRI-based kidney volume assessment was further investigated by Yilmaz et al. in a multicenter, cross-sectional, and case-controlled study involving 89 children and adolescents with a genetically confirmed and detailly characterized diagnosis of ADPKD. The study patients were stratified according to the innovative Leuven Imaging Classification (LIC) into different risk categories, with those in the highest risk category having an increased incidence of hypertension and a higher prevalence of PKD1 mutations. Therefore, the study advocates the use of MRI for the measurement of TKV in the pediatric population, in addition to the use of ambulatory blood pressure monitoring to recognize those with hypertension.
Finally, Botero-Calderon et al. presented a retrospective study evaluating clinical and imaging data, genetic testing results and postnatal follow-up outcomes of infants identified in utero with bilateral renal cystic disease at a single referral center over a period of 11 years. Among 17 patients with suspected renal ciliopathy, the most common diagnosis was autosomal recessive PKD (ARPKD, n = 4), followed by Bardet-Biedl syndrome (BBS, n = 3), autosomal dominant polycystic disease (ADPKD, n = 2), HNF1B-related disease (n = 2), and Meckel-Gruber syndrome (MKS, n = 2), while four cases were not genetically resolved. In terms of postnatal management, the study revealed that the vast majority of neonatal survivors with renal ciliopathies are directed to the care of a pediatric nephrologist, while this proportion is much lower in those with genetically unresolved enlarged, echogenic kidneys, stressing the need for structured management programs for prenatally identified kidney disease.
In conclusion, our research topic provides a contemporary overview of current practices, unmet clinical needs and research gaps regarding the broad spectrum of renal ciliopathies that may be useful to a wide range of physicians and researchers dealing with these complex disorders.
LL: Writing – review & editing, Writing – original draft, Visualization, Conceptualization. IV: Writing – review & editing, Conceptualization. MF: Writing – review & editing, Conceptualization. BB: Writing – review & editing, Conceptualization.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Kwatra S, Krishnappa V, Mhanna C, Murray T, Novak R, Sethi SK, et al. Cystic diseases of childhood: a review. Urology. (2017) 110:184–91. doi: 10.1016/j.urology.2017.07.040
2. De Groof J, Dachy A, Breysem L, Mekahli D. Cystic kidney diseases in children. Archives de Pédiatrie. (2023) 30(4):240–6. doi: 10.1016/j.arcped.2023.02.005
3. Mensel B, Kühn JP, Kracht F, Völzke H, Lieb W, Dabers T, et al. Prevalence of renal cysts and association with risk factors in a general population: an MRI-based study. Abdominal Radiology. (2018) 43(11):3068–74. doi: 10.1007/s00261-018-1565-5
4. McConnachie DJ, Stow JL, Mallett AJ. Ciliopathies and the kidney: a review. Am J Kidney Dis. (2021) 77(3):410–9. doi: 10.1053/j.ajkd.2020.08.012
5. Gambella A, Kalantari S, Cadamuro M, Quaglia M, Delvecchio M, Fabris L, et al. The landscape of HNF1B deficiency: a syndrome not yet fully explored. Cells. (2023) 12(2):307. doi: 10.3390/cells12020307
6. Modarage K, Malik SA, Goggolidou P. Molecular diagnostics of ciliopathies and insights into novel developments in diagnosing rare diseases. Br J Biomed Sci. (2022) 79. doi: 10.3389/bjbs.2021.10221
7. Devlin LA, Sayer JA. Renal ciliopathies. Curr Opin Genet Dev. (2019) 56:49–60. doi: 10.1016/j.gde.2019.07.005
8. Kurschat CE, Müller RU, Franke M, Maintz D, Schermer B, Benzing T. An approach to cystic kidney diseases: the clinician’s view. Nat Rev Nephrol. (2014) 10(12):687–99. doi: 10.1038/nrneph.2014.173
9. Lam BL, Leroy BP, Black G, Ong T, Yoon D, Trzupek K. Genetic testing and diagnosis of inherited retinal diseases. Orphanet J Rare Dis. (2021) 16(1):514. doi: 10.1186/s13023-021-02145-0
Keywords: cystic kidney disease, autosomal dominant polycystic kidney disease (ADPKD), autosomal recessive polycystic kidney disease (ARPKD), nephronophtisis complex (NPHC), Bardet Biedl syndrome (BBS)
Citation: Lamot L, Vuković Brinar I, Fištrek Prlić M and Beck B (2024) Editorial: Cystic kidney diseases in children and adults: from diagnosis to etiology and back. Front. Pediatr. 12:1401593. doi: 10.3389/fped.2024.1401593
Received: 15 March 2024; Accepted: 29 March 2024;
Published: 10 April 2024.
Edited and Reviewed by: Michael L. Moritz, University of Pittsburgh, United States
© 2024 Lamot, Vuković Brinar, Fištrek Prlić and Beck. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lovro Lamot bG92cm8ubGFtb3RAa2JjLXphZ3JlYi5ocg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.