
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 25 July 2024
Sec. Neonatology
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1393547
 Ts-Ting Wang1,2,†
Ts-Ting Wang1,2,† Yen-Ju Chen2,†
Yen-Ju Chen2,† Yi-Han Su2
Yi-Han Su2 Yun-Hsiang Yang2
Yun-Hsiang Yang2 Wei-Ying Chu2
Wei-Ying Chu2 Wei-Ting Lin2
Wei-Ting Lin2 Yu-Shan Chang3
Yu-Shan Chang3 Yung-Chieh Lin2
Yung-Chieh Lin2 Chyi-Her Lin4
Chyi-Her Lin4 Yuh-Jyh Lin2*
Yuh-Jyh Lin2* Taiwan Premature Infant Follow-up Network
Taiwan Premature Infant Follow-up Network
Introduction: This study aimed to explore the relationship between the trajectories of body weight (BW) z-scores at birth, discharge, and 6 months corrected age (CA) and neurodevelopmental outcomes at 24 months CA.
Methods: Conducted as a population-based retrospective cohort study across 21 hospitals in Taiwan, we recruited 3,334 very-low-birth-weight (VLBW) infants born between 2012 and 2017 at 23–32 weeks of gestation. Neurodevelopmental outcomes were assessed at 24 months CA. Instances of neurodevelopmental impairment (NDI) were defined by the presence of at least one of the following criteria: cerebral palsy, severe hearing loss, profound vision impairment, or cognitive impairment. Group-based trajectory modeling was employed to identify distinct BW z-score trajectory groups. Multivariable logistic regression was used to assess the associations between these trajectories, postnatal comorbidity, and neurodevelopmental impairments.
Results: The analysis identified three distinct trajectory groups: high-climbing, mid-declining, and low-declining. Significant associations were found between neurodevelopmental impairments and both cystic periventricular leukomalacia (cPVL) [with an adjusted odds ratio (aOR) of 3.59; p < 0.001] and belonging to the low-declining group (aOR: 2.59; p < 0.001).
Discussion: The study demonstrated that a low-declining pattern in body weight trajectory from birth to 6 months CA, along with cPVL, was associated with neurodevelopmental impairments at 24 months CA. These findings highlight the importance of early weight trajectory and specific health conditions in predicting later neurodevelopmental outcomes in VLBW infants.
NDI is a major concern in preterm population (1, 2), ranging from mild to severe, with long-term adverse outcomes. NDI can affect children's cognitive, motor, language, visual, hearing, and behavioural functioning (3); significantly reduce their quality of life; and impair their academic and social functioning. The causes of NDI are complex and multifactorial. Prematurity is one of the most important risk factors. Other known risk factors include male sex (4); lower parental education level; lower birth weight or gestational age (5); central nervous system conditions such as severe intraventricular haemorrhage (IVH) and cPVL; and respiratory distress syndrome (RDS), bronchopulmonary dysplasia (BPD), sepsis, patent ductus arteriosus (PDA), and advanced retinopathy of prematurity (ROP) (1, 3, 6, 7).
Brain development is sensitive to nutrition status, especially in infancy (8). Adequate brain growth and maturation are positively associated with appropriate BW gain (9, 10). Consequently, growth restriction during the neonatal period is associated with elevated risks of adverse neurodevelopmental outcomes in premature infants (2, 4, 11–15). Regarding the associations between BW and neurodevelopmental outcomes, a study found that an BW z-score decrease of more than 1 and 2 standard deviations and a slow weight gain velocity during hospitalisation were associated with NDI incidence at 24 months CA (4). Low BW at a single time point (6, 12, or 24 months CA) may be associated with unfavourable neurodevelopmental outcomes in the VLBW population (12). However, isolated weight assessments, especially for infants below certain weight percentiles, might not offer a comprehensive overview (16). This discrepancy arises because weight measured at specific time points shows weak correlations with longitudinal weight change trends and subsequent neurodevelopmental outcomes (17).
A research gap exists regarding the investigation of the relationship between the longitudinal trajectory assessment of BW increases from birth to the early post discharge period and neurodevelopmental outcomes at 24 months CA. We hypothesised that the patterns of postnatal BW trajectory and neonatal comorbidities was associated with neurodevelopmental outcomes. This study investigated the relationship between the z-score trajectory of BW from birth, discharge, to 6 months CA and neurodevelopment outcomes at 24 months CA.
This retrospective cohort study used data from the Taiwan Premature Infant Developmental Collaborative Study Group, which was funded by the Premature Baby Foundation of Taiwan and is collecting follow up data of VLBW infants from 21 hospitals in Taiwan. Infants born between 2012 and 2017 with a birth BW (BBW) <1,500 g, and gestational age (GA) ranged from 23 to 32 weeks were included in this study.
Anthropometric measurements were performed at birth, discharge, and 6 months CA. Neurodevelopmental assessments were performed at 24 months CA. Infants with major anomalies were excluded. To perform group-based trajectory modeling (GBTM), which requires at least three time points of body weight measurements, we excluded patients who died before discharge and those without a BBW record. Patients discharged at a postmenstrual age (PMA) of over 50 weeks (due to the limitations of the Fenton growth chart) were also excluded. During data consolidation, we found that some hospitals had an unusually high proportion of data anomalies for certain years submitted to the Premature Baby Foundation of Taiwan. To enhance the accuracy of our statistics, we removed data from these hospitals (categorized as incomplete data). To improve the precision of follow-up data, we excluded patients who had their follow-ups conducted too early or too late relative to the scheduled age (categorized as follow-up time not within 2 months of the scheduled day). Patients who did not have BSID-III scores at 24 months CA, whether due to death, loss of records from transferring hospitals, or other reasons for loss to follow-up, were also excluded (see online Supplementary Figure S1). This study included 3,334 VLBW newborns. The Institutional Review Board of National Cheng Kung University Hospital approved this study (approval number: ER-109-288).
The collected demographic data included GA, BBW, gender, neonatal morbidities, and post-discharge follow-up information. Anthropometric measurements included BW, body length (BL), and head circumference (HC) at each time point. BW z-scores at birth and discharge were determined by the Fenton growth chart (18), while using the World Health Organization standards for growth measurement at 6 months CA. Risk factors included surfactant-treated RDS, severe IVH, PDA requiring treatment, necrotising enterocolitis (NEC) advanced beyond stage 2, ROP advanced beyond stage 3, BPD, and cPVL. Newborns with respiratory distress syndrome caused by hyaline membrane disease, who require support with a fraction of inspired oxygen above 40%, will be treated with surfactant within 48 h after birth. IVH was graded by the Papile classification, with grades 3 and 4 defined as severe. PDA requiring treatment was defined by hemodynamically significant PDA which was treated by surgical or medical intervention. NEC was defined by modified Bell's staging criteria. The stage of ROP is diagnosed based on the criteria established by the International Committee for the Classification of Retinopathy of Prematurity. BPD was defined according to the 2001 National Institute of Child Health and Human Development criteria (19). cPVL was diagnosed by the neurologist through cranial ultrasound, which brain injury involving periventricular white matter.
A child was considered to have NDI at 24 months CA if at least one of the following criteria was met: cerebral palsy, profound vision impairment, severe hearing loss, or cognitive impairment (cognitive composite score of <85) (20). The BSID-III (21) was used to assess neurodevelopment at 24 months CA, including cognitive composite scores. Any of the following symptoms was regarded as a marker of cerebral palsy: hypotonia, spastic diplegia, spastic tetraparesis, or spastic hemiparesis. Profound vision impairment was defined as amblyopia or blindness in both eyes, and severe hearing loss was defined as hearing loss of less than 60 dB in any ear.
Group-based trajectory modelling (GBTM) identified clusters of BW z-scores at birth, discharge, and 6 months CA. Bayesian information criteria (BIC) determined the optimal cluster count and fittest trajectory shape. Then use average of the posterior probabilities of group membership (APP) to check the modelled trajectories group individuals with similar patterns (22). Parental attributes, neonatal demographics, and postnatal morbidities were compared across three trajectory patterns by using the chi-square or Fisher exact test for categorical variables and the analysis of variance (ANOVA) or Kruskal–Wallis test for continuous variables. Following ANOVA, various trajectory intergroup comparisons were conducted using the Bonferroni post hoc test. The logistic regression model was used to identify the variables associated with a risk of NDI at 24 months CA. after a univariate analysis, covariates were selected on the basis of their clinical relevance and between-variable collinearity. Statistical significance was set at p < 0.05, and all analyses were performed using R-4.0.2, SPSS (Version 29, IBM, Armonk, NY, USA), and SAS (version 9.4, SAS Institute Inc., Cary, NC, USA) software package with accompanying PROC TRAJ application.
From the original pool of 6,535 newborns that fit our study criteria, a series of exclusions narrowed down our final analysis to 3,334 infants (online Supplementary Figure S1). Infants with major anomalies (n = 84), with mortality before discharge (n = 730), no BBW records (n = 1), who were discharged at a PMA of >50 weeks (n = 119), follow up timing beyond 2 months than the regular schedule (n = 749), and who did not have data available on BSID scores at 24 months CA (n = 1,068) were excluded. The reasons for the lack of data on BSID scores at 24 months CA encompassed transfer to another hospital (n = 36) and mortality after discharge but before reaching 24 months CA (n = 26). In addition, one hospital did not provide complete annual data to the foundation because of concerns regarding data integrity and accuracy; therefore, infants from this hospital were excluded from our analysis (n = 450). The average gestational age was 28.45 weeks (28.45 ± 2.26), 50.87% of newborns were male, and 14.97% were small for gestational age. During the neonatal period, 33.62% were diagnosed with RDS and treated with surfactant, 3.57% had severe IVH, 23.94% had PDA and required medication or surgical treatment, 5.01% were diagnosed as having NEC, 24.75% had severe ROP, 37.31% had BPD, and 4.05% had cPVL. At 24 months CA, the mean cognitive, language, and motor composite score were 94.35 (94.35 ± 12.63), 92.29 (92.29 ± 13.79), and 91.74 (91.74 ± 12.41), respectively. NDI was diagnosed in 23.94% of infants (Table 1).
Using GBTM, based on BW z-scores at birth, discharge, and 6 months CA, we found three quadratic shape trajectory group has the highest BIC. Based on the characteristics of the trajectories, the three trajectory groups were named high-climbing, mid-declining, and low-declining groups (Figure 1). These groups comprised 307 (9.2%), 1,737 (52.1%), and 1,290 (38%) of the infants, respectively. On average, the posterior probabilities of group membership were all >0.7 (0.86, 0.84, 0.85, respectively). The odds of correct classification for the three groups were 62.11, 4.85, and 8.86, respectively (online Supplementary Table S1). All trajectories had a z-score nadir upon discharge; thereafter, the z-score improved at 6 months CA. From a trend perspective, the high-climbing group demonstrates that their BW z-score at 6 months CA surpasses their BBW z-score. In contrast, the other two groups do not exhibit this pattern. Notably, the low-declining group shows a BW z-score at 6 months CA that is significantly lower than their BBW z-score. The characteristics and neonatal comorbidities of the three trajectory groups are shown in Table 2. Significant differences were observed in GA, z-scores for anthropometric measurements taken at birth, incidence of small for gestational age (SGA), PDA requiring treatment, NEC, severe ROP, BPD, and cPVL (Table 2).
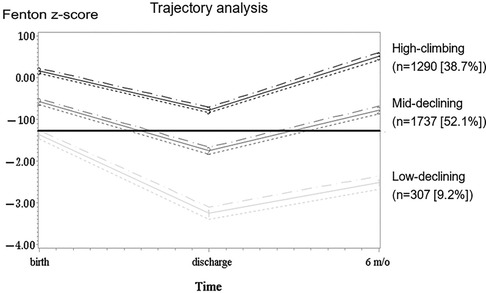
Figure 1 Trajectory patterns of body weight z-scores. The trajectory patterns of body weight z-scores from birth to 6 months corrected age. The body weight z-score trajectory was classified into three trajectory groups: low-declining group [n = 307 (9.2%)], mid-declining group [n = 1,737 (52.1%)], and high-climbing group [n = 1,290 (38.7%)]. The dotted lines represent 95% confidence intervals.
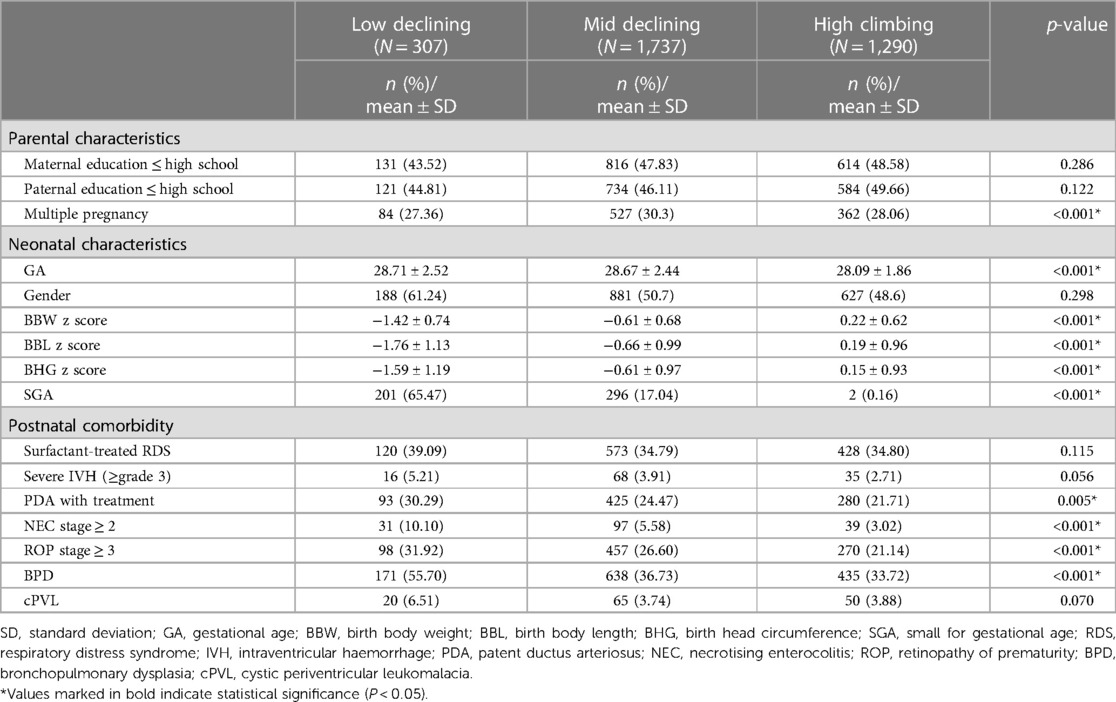
Table 2 Differences in parental and neonatal characteristics and neonatal comorbidities among the 3 body weight growth trajectory groups.
The study used logistic regression to analyse variables, such as BW z-score trajectory, maternal/paternal education level, gender, GA, BBW/BBL/BHC z-score, SGA, surfactant-treated RDS, severe IVH, PDA requiring treatment, NEC advanced beyond stage 2, ROP advanced beyond stage 3, BPD, and cPVL, to determine whether they were significantly associated with NDI at 24 months CA. According to a univariate analysis, there was no significant correlation between the maternal/paternal education level, BBW/BBL/BHCṭ z-score, SGA, and NDI at 24 months CA (p = 0.79, 0.86, 0.56, 0.59, 0.1, 0.28, respectively; online Supplementary Table S2). A multivariate analysis demonstrated that neonatal morbidities, including surfactant-treated RDS, severe IVH, and ROP stage 3, were positively correlated with NDI. However, the cPVL and low-declining groups had the highest aOR associated with NDI at 24 months CA (aOR: 3.59; 95% CI: 2.47–5.24; p < 0.001) and low-declining group (aOR: 2.59; 95% CI: 1.92–3.48; p < 0.001) (Table 3).
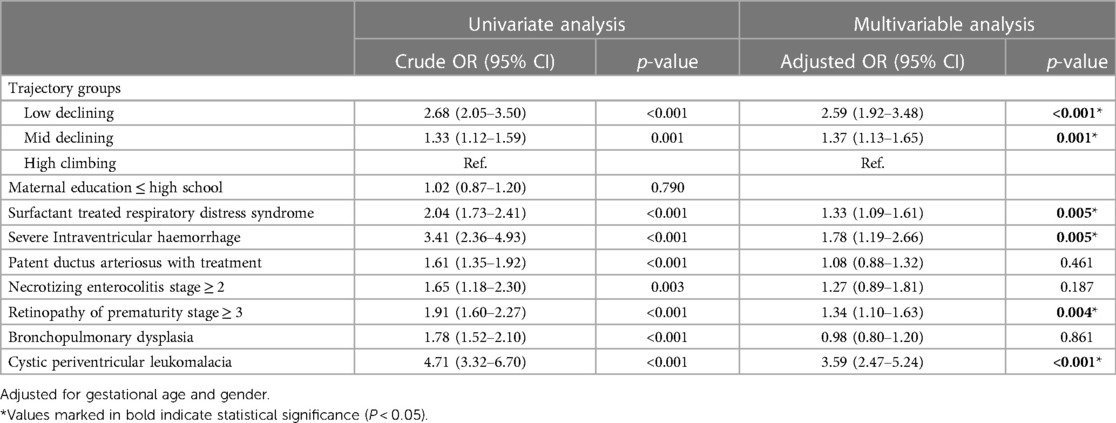
Table 3 Crude and adjusted odds ratios for neurodevelopment impairment at 24 months corrected age stratified by risk factors and z-score body weight trajectory.
The definition of extrauterine growth restriction (EUGR) is challenged by the arbitrary cut-off value set at either 36 weeks postmenstrual age or at the time of discharge. This definition may not be useful as weight at a specific time point shows weaker correlations with long-term weight change trends and future neurodevelopment compared to continuous observations (17). Some scholars seem to agree that EUGR associated with poor neurodevelopment outcome. However, there continues to be debate about whether EUGR is predictive of poor neurodevelopment (23, 24). Therefore, different evaluation methods, such as different growth charts for measuring BW (11), BW measurement at a single time point (11, 12, 24), BW z-score differences in each period, or growth velocity (4, 13, 25, 26), were used to analyse the relationship between growth status and neurodevelopment outcomes. In this nationwide retrospective cohort study, we examined the growth trajectory at birth, discharge, and 6 months CA through GBTM. We observed that the weight z-scores of the three patient groups all decreased to their lowest point at discharge after the application of GBTM, followed by an increase at 6 months CA. The low-declining group comprises 65.47% of the SGA population, as indicated by Table 2 and Figure 1. In contrast, the high-climbing group, which began with a z-score of >0, has 0.16% of SGA individuals. The high-climbing group surpasses their birth weight z-score by 6 months CA, whereas the low-declining group remains significantly below their birth weight z-score. Our logistic regression univariate analysis, which combined birth weight z-score and SGA status (Supplementary Table S2), did not reveal a significant association between NDI and either variable (p = 0.562 and p = 0.277, respectively). Our findings indicated that the probability of NDI can be ascertained by analysing long-term weight trends. The NDI at CA 24 months exhibits a substantial correlation with the growth trajectory, particularly the capacity to catch up by CA 6 months, in contrast to the birth weight z-score or SGA status at birth. Poor growth may result from various complications in the early life of preterm infants, ultimately leading to NDI. Therefore, when assessing NDI, various risk factors should be considered (2, 7). Our results indicated that infants with cPVL, severe IVH, RDS requiring surfactant therapy or those with extensive ROP are susceptible to NDI. cPVL and severe IVH are well-documented for their adverse neurological impacts (27, 28), Our findings align with previous research, demonstrating that both cPVL and the low-declining growth trajectory were significant associated with NDI at 24 months CA. Early RDS and advanced ROP are indicators of postnatal growth limitations (29). In addition, research has demonstrated that low parental education, PDA requiring treatment, and NEC are risk factors for poor neurodevelopmental outcomes (1, 6, 30) and may be associated with IVH (11, 31). However, our investigation revealed no correlation between these variables. The reason for the discrepancy between these studies and our findings is unknown, but it may be due to aggressive treatment strategies for PDA in some hospitals, which may prevent patients with hemodynamically significant PDA from experiencing adverse effects on brain development and a weak association between PDA and NDI. Although previous studies have reported an association between NDI and BPD (32–34), we did not find a robust relationship between these conditions. Bauer et al. also found no increase in NDI prevalence among BPD patients (35).
The strength of this study lies in its comprehensive multi-center database. Unlike some studies that excluded (6, 11, 12) conditions like high-grade IVH or cPVL due to their adverse neurodevelopmental impacts (27, 28), we included infants with these two comorbidities given their strong correlation with NDI at 24 months CA. Our study may be among the few to evaluate the association between growth and neurodevelopment outcomes through GBTM.
There are limitations in this cohort study. Sepsis in VLBW neonates often leads to worse neurodevelopmental outcomes (36), achieving full oral nutrition by 40 weeks postmenstrual age is associated with improved neurodevelopmental outcomes (37), administration of antenatal steroid and magnesium sulfate are associated with reduced risk of childhood impairment (38), and delay cord clamping in preterm infants is associated with lower incidence of intraventricular hemorrhage (39). Temperature instability upon NICU admission, including both hyperthermia and hypothermia, can impact adverse neurological development. All of these risk factors are linked to NDI, but we couldn't find that information in our database.
In cases where infants were discharged alive, a loss-to-follow-up rate of 24% was observed at 24 months CA, of which only 2% was due to mortality after discharge. In the future, prospective and longitudinal studies should be conducted on this topic.
The BW trajectory pattern before 6 months CA showed a significant association with NDI at 24 months CA. This association suggests that growth reaching normal levels, which means an appropriate growth pattern, should be emphasised in both hospitalisation and early discharge periods before 6 months CA. More RCT are required to determine whether interventions administered to improve BW trajectory in early infancy can improve neurodevelopmental outcomes later in life.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The studies involving humans were approved by The National Cheng Kung University Hospital's Institutional Review Board authorized this study (approval code: ER-109-288. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
T-TW: Data curation, Formal Analysis, Investigation, Visualization, Writing – original draft. Y-JC: Conceptualization, Data curation, Investigation, Methodology, Validation, Writing – original draft. Y-HS: Writing – review & editing. Y-HY: Writing – review & editing. W-YC: Writing – review & editing. W-TL: Supervision, Writing – review & editing. Y-SC: Supervision, Writing – review & editing. Y-CL: Supervision, Writing – review & editing. C-HL: Project administration, Supervision, Writing – review & editing. Y-JL: Conceptualization, Investigation, Supervision, Writing – review & editing.
The author(s) declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by Premature Baby Foundation of Taiwan and supported by the grant of National Cheng Kung University Hospital (NCKUH-T11201003).
The authors thank all parents and infants who participated in this study and all team members in charge of data collection. We are particularly grateful to the Premature Baby Foundation of Taiwan for the support provided to the Taiwan Premature Infant Follow-up Network and for the contribution to the well-being of premature infants in Taiwan. We are also grateful to Dr. Sheng-Hsiang Lin and Ms. Chih-Hui Hsu for providing the statistical consulting services from the Biostatistics Consulting Center, Clinical Medicine Research Center, National Cheng Kung University Hospital. We are also grateful to Ms. Wan-Ting Huang for providing the statistical consulting services from the Clinical Medical Research Center, Ditmanson Medical Foundation Chia-Yi Christian Hospital. Coordinators in the Taiwan Premature Infant Follow-up Network: Jui-Hsing Chang, MD (National coordinator, Mackay Children's Hospital); Kuo-Inn Tsou, MD (Former national coordinator, Cardinal Tien Hospital); Po-Nien Tsao, MD (Regional coordinator, National Taiwan University Hospital); Shu-Chi Mu, MD (Regional coordinator, Shin Kong Wu Ho-Su Memorial Hospital); Chyong-Hsin Hsu, MD (Regional coordinator, Mackay Children's Hospital); Reyin Lien, MD (Regional coordinator, Chang Gung Memorial Hospital); Hung-Chih Lin, MD (Regional coordinator, China Medical University Hospital); Chien-Chou Hsiao, MD (Regional coordinator, Changhua Christian Hospital); Chao-Ching Huang, MD (Regional coordinator, National Cheng Kung University Hospital); and Chih-Cheng Chen, MD (Regional coordinator, Chang Gung Memorial Hospital Kaohsiung Branch).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1393547/full#supplementary-material
1. Lin CY, Hsu CH, Chang JH. Neurodevelopmental outcomes at 2 and 5 years of age in very-low-birth-weight preterm infants born between 2002 and 2009: a prospective cohort study in Taiwan. Pediatr Neonatol. (2020) 61:36–44. doi: 10.1016/j.pedneo.2019.05.006
2. Belfort MB, Ehrenkranz RA. Neurodevelopmental outcomes and nutritional strategies in very low birth weight infants. Semin Fetal Neonatal Med. (2017) 22:42–8. doi: 10.1016/j.siny.2016.09.001
3. Duncan AF, Matthews MA. Neurodevelopmental outcomes in early childhood. Clin Perinatol. (2018) 45:377–92. doi: 10.1016/j.clp.2018.05.001
4. Rafei RE, Jarreau PH, Norman M, Maier RF, Barros H, Van Reempts P, et al. Association between postnatal growth and neurodevelopmental impairment by sex at 2 years of corrected age in a multi-national cohort of very preterm children. Clin Nutr. (2021) 40:4948–55. doi: 10.1016/j.clnu.2021.07.005
5. Lin YC, Lin YJ, Lin CH. Growth and neurodevelopmental outcomes of extremely low birth weight infants: a single center’s experience. Pediatr Neonatol. (2011) 52:342–8. doi: 10.1016/j.pedneo.2011.08.008
6. High Risk Follow-Up Working Group. Neurodevelopmental outcomes of extreme-low-birth-weight infants born between 2001 and 2002. Hong Kong Med J. (2008) 14:21–818239239.
7. Rogers EE, Hintz SR. Early neurodevelopmental outcomes of extremely preterm infants. Semin Perinatol. (2016) 40:497–509. doi: 10.1053/j.semperi.2016.09.002
8. Skinner AM, Narchi H. Preterm nutrition and neurodevelopmental outcomes. World J Methodol. (2021) 11:278–93. doi: 10.5662/wjm.v11.i6.278
9. Coviello C, Keunen K, Kersbergen KJ, Groenendaal F, Leemans A, Peels B, et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr Res. (2018) 83:102–10. doi: 10.1038/pr.2017.227
10. Schneider J, Fischer Fumeaux CJ, Duerden EG, Guo T, Foong J, Graz MB, et al. Nutrient intake in the first two weeks of life and brain growth in preterm neonates. Pediatrics. (2018) 141:e20172169. doi: 10.1542/peds.2017-2169
11. Chien HC, Chen CH, Wang TM, Hsu YC, Lin MC. Neurodevelopmental outcomes of infants with very low birth weights are associated with the severity of their extra-uterine growth retardation. Pediatr Neonatol. (2018) 59:168–75. doi: 10.1016/j.pedneo.2017.08.003
12. Hsu CT, Chen CH, Lin MC, Wang TM, Hsu YC. Post-discharge body weight and neurodevelopmental outcomes among very low birth weight infants in Taiwan: a nationwide cohort study. PLoS One. (2018) 13:e0192574. doi: 10.1371/journal.pone.0192574
13. Maruyama H, Yonemoto N, Kono Y, Kusuda S, Fujimura M. Neonatal research network of Japan. Weight growth velocity and neurodevelopmental outcomes in extremely low birth weight infants. PLoS One. (2015) 10:e0139014. doi: 10.1371/journal.pone.0139014
14. Chan SH, Johnson MJ, Leaf AA, Vollmer B. Nutrition and neurodevelopmental outcomes in preterm infants: a systematic review. Acta Paediatr. (2016) 105:587–99. doi: 10.1111/apa.13344
15. Levitsky DA, Strupp BJ. Malnutrition and the brain: changing concepts, changing concerns. J Nutr. (1995) 125:2212S–20. doi: 10.1093/jn/125.suppl_8.2212S
16. Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr. (2012) 161:222–8.e3. doi: 10.1016/j.jpeds.2012.01.057
17. Fenton TR, Cormack B, Goldberg D, Nasser R, Alshaikh B, Eliasziw M, et al. “Extrauterine growth restriction” and “postnatal growth failure” are misnomers for preterm infants. J Perinatol. (2020) 40:704–14. doi: 10.1038/s41372-020-0658-5
18. Fenton TR, Kim JH. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. (2013) 13:59. doi: 10.1186/1471-2431-13-59
19. Jobe AH, Bancalari E. Bronchopulmonary dysplasia. Am J Respir Crit Care Med. (2001) 163:1723–9. doi: 10.1164/ajrccm.163.7.2011060
20. Younge N, Goldstein RF, Bann CM, Hintz SR, Patel RM, Smith PB, et al. Survival and neurodevelopmental outcomes among periviable infants. N Engl J Med. (2017) 376:617–28. doi: 10.1056/NEJMoa1605566
21. Albers CA, Grieve AJ, Bayley N. Bayley scales of infant and Toddler Development, 3rd edn. San Antonio, TX: Harcourt Assessment (2006). p. 180–190. doi: 10.1037/t14978-000
22. Baumgartner SE, Leydesdorff L. Group-based trajectory modeling (GBTM) of citations in scholarly literature: dynamic qualities of “transient” and “sticky knowledge claims”. J Assoc Inf Sci Technol. (2014) 65(4):797–811. doi: 10.1002/asi.23009
23. Zozaya C, Díaz C, Saenz de Pipaón M. How should we define postnatal growth restriction in preterm infants? Neonatology. (2018) 114:177–80. doi: 10.1159/000489388
24. Rahman A, Kase JS, Murray YL, Parvez B. Neurodevelopmental outcome of extremely low birth weight infants fed an exclusive human milk diet is not affected by growth velocity. Breastfeed Med. (2020) 15:362–9. doi: 10.1089/bfm.2019.0214
25. Belfort MB, Rifas-Shiman SL, Sullivan T, Collins CT, McPhee AJ, Ryan P, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. (2011) 128:e899–906. doi: 10.1542/peds.2011-0282
26. Frondas-Chauty A, Simon L, Branger B, Gascoin G, Flamant C, Ancel PY, et al. Early growth and neurodevelopmental outcome in very preterm infants: impact of gender. Arch Dis Child Fetal Neonatal Ed. (2014) 99:F366–72. doi: 10.1136/archdischild-2013-305464
27. Vohr BR. Neurodevelopmental outcomes of premature infants with intraventricular hemorrhage across a lifespan. Semin Perinatol. (2022) 46:151594. doi: 10.1016/j.semperi.2022.151594
28. Mukerji A, Shah V, Shah PS. Periventricular/intraventricular hemorrhage and neurodevelopmental outcomes: a meta-analysis. Pediatrics. (2015) 136:1132–43. doi: 10.1542/peds.2015-0944
29. Griffin IJ, Tancredi DJ, Bertino E, Lee HC, Profit J. Postnatal growth failure in very low birthweight infants born between 2005 and 2012. Arch Dis Child Fetal Neonatal Ed. (2016) 101:F50–5. doi: 10.1136/archdischild-2014-308095
30. Choi IR, Lee JH, Park MS, Kim JY, Park KH, Kim GH, et al. Early neurodevelopment in very low birth weight infants with mild intraventricular hemorrhage or those without intraventricular hemorrhage. Korean J Pediatr. (2012) 55:414–9. doi: 10.3345/kjp.2012.55.11.414
31. Madan JC, Kendrick D, Hagadorn JI, Frantz ID III, National Institute of Child Health and Human Development Neonatal Research Network. Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome. Pediatrics. (2009) 123:674–81. doi: 10.1542/peds.2007-2781
32. Trittmann JK, Nelin LD, Klebanoff MA. Bronchopulmonary dysplasia and neurodevelopmental outcome in extremely preterm neonates. Eur J Pediatr. (2013) 172:1173–80. doi: 10.1007/s00431-013-2016-5
33. Malavolti AM, Bassler D, Arlettaz-Mieth R, Faldella G, Latal B, Natalucci G. Bronchopulmonary dysplasia-impact of severity and timing of diagnosis on neurodevelopment of preterm infants: a retrospective cohort study. BMJ Paediatr Open. (2018) 2:e000165. doi: 10.1136/bmjpo-2017-000165
34. Cheong JLY, Doyle LW. An update on pulmonary and neurodevelopmental outcomes of bronchopulmonary dysplasia. Semin Perinatol. (2018) 42:478–84. doi: 10.1053/j.semperi.2018.09.013
35. Bauer SE, Schneider L, Lynch SK, Malleske DT, Shepherd EG, Nelin LD. Factors associated with neurodevelopmental impairment in bronchopulmonary dysplasia. J Pediatr. (2020) 218:22–7.e2. doi: 10.1016/j.jpeds.2019.11.016
36. Shim SY, Cho SJ, Park EA. Neurodevelopmental outcomes at 18–24 months of corrected age in very low birth weight infants with late-onset sepsis. J Korean Med Sci. (2021) 36:e205. doi: 10.3346/jkms.2021.36.e205
37. Fenton TR, Dai S, Lalari V, Alshaikh B. Neonatal and preterm infant growth assessment. Clin Perinatol. (2022) 49:295–311. doi: 10.1016/j.clp.2022.02.001
38. Mactier H, Bates SE, Johnston T, Lee-Davey C, Marlow N, Mulley K, et al. Perinatal management of extreme preterm birth before 27 weeks of gestation: a framework for practice. Arch Dis Child Fetal Neonatal Ed. (2020) 105(3):232–9. doi: 10.1136/archdischild-2019-318402
Keywords: neurodevelopment outcome, very-low-birth-weight preterm infants, growth trajectory, extrauterine growth retardation, neurodevelopmental impairment, group-based trajectory modelling
Citation: Wang T-T, Chen Y-J, Su Y-H, Yang Y-H, Chu W-Y, Lin W-T, Chang Y-S, Lin Y-C, Lin C-H, Lin Y-J and Taiwan Premature Infant Follow-up Network (2024) Associations between body weight trajectories and neurodevelopment outcomes at 24 months corrected age in very-low-birth-weight preterm infants: a group-based trajectory modelling study. Front. Pediatr. 12: 1393547. doi: 10.3389/fped.2024.1393547
Received: 29 February 2024; Accepted: 15 July 2024;
Published: 25 July 2024.
Edited by:
Mohamed E. Abdel-Latif, The Canberra Hospital, AustraliaReviewed by:
Balaji Govindaswami, Frontiers Media SA, Switzerland© 2024 Wang, Chen, Su, Yang, Chu, Lin, Chang, Lin, Lin, Lin and Taiwan Premature Infant Follow-up Network. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yuh-Jyh Lin, cGVkMUBtYWlsLm5ja3UuZWR1LnR3
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.