- 1Department of Pediatrics and Child Health Nursing, School of Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 2Department of Emergency and Critical Care Nursing, School of Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 3Department of Medical Nursing, School of Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 4School of Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 5Department of Clinical Midwifery, School of Midwifery, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
- 6Department of Surgical Nursing, School of Nursing, College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia
Introduction: Neonatal mortality is still a major public health problem in middle- and low-income countries like Ethiopia. Despite strategies and efforts made to reduce neonatal death, the mortality rate declines at a slower pace in the country. Though there are studies conducted on neonatal mortality and its determinants, our searches of the literature have found no study on the extent of mortality of neonates born to mothers of extreme reproductive age in the study area. Therefore, this study aimed to assess the magnitude and factors associated with the mortality of neonates born to mothers of extreme reproductive age in Ethiopia.
Methods: Secondary data analysis was conducted using 2016 Ethiopian Demographic and Health Survey data. The final study contained an overall weighted sample of 2,269 live births. To determine the significant factors in newborn deaths, a multilevel binary logistic regression was fitted. For measuring the clustering impact, the intra-cluster correlation coefficient, median odds ratio, proportional change in variance, and deviation were employed for model comparison. The adjusted odds ratio with a 95% confidence interval was presented in the multivariable multilevel logistic regression analysis to identify statistically significant factors in neonatal mortality. A P-value of less than 0.05 was declared statistically significant.
Results: The neonatal mortality rate of babies born to extreme aged reproductive women in Ethiopia was 34 (95% Cl, 22.2%–42.23%) per 1,000 live birth. Being twin pregnancy (AOR = 10; 95% Cl: 8.61–20.21), being from pastoralist region (AOR = 3.9; 95% Cl: 1.71–8.09), having larger baby size (AOR = 2.93; 95% Cl: 1.4–9.12) increase the odds of neonatal mortality. On the other hand, individual level media exposure (AOR = 0.3; 95% Cl: 0.09–0.91) and community level media exposure (AOR = 0.24; 95% Cl: 0.07–0.83), being term gestation (AOR = 0.14; 95% Cl: 0.01–0.81) decreases the odds of neonatal mortality born to mothers of extreme reproductive age.
Conclusion: Ethiopia had a greater rate of neonatal death among babies born at the extremes of reproductive age than overall reproductive life. Multiple pregnancies, larger baby sizes, emerging regions, term gestation, and media exposure were found to be significant factors associated with the mortality of neonates born to mothers of extreme reproductive age. Therefore, the concerned bodies should give emphasis to mothers giving birth before the age of 20 and above 35, access to media, healthy pregnancy, and special attention to pastoralists to reduce the burden of neonatal mortality.
Introduction
Neonatal mortality is defined as the death of newborn with in the first 28 days of life. Extreme maternal age also refers to maternal age of less than twenty (teenagers) and advanced maternal age (35 and older) (1). Extreme maternal age are associated with a higher risk of neonatal mortality, infant and under five mortality (2–4). The neonatal period is the most vulnerable time for the child's survival (5). The global neonatal mortality decreased by 52% from 36.6 deaths per 1,000 live birth in 1900, to 17.5 in 2019 (2). However, still it is estimated that 6.3 million children death globally occur during the first 28 days of life and almost all (98%) of these occurs in developing countries (6).
For the last few decades, important progress has been made to save the children in the world. Due to this, the rate of neonatal death declined by 54%, from 37 in 1990 to 17 per 1,000 live births in 2020 (7). However, the rate declines at a slower pace in Ethiopia (8, 9), particularly marginally higher in pastoralist/emerging regions (10, 11). The most recent Ethiopian Demographic and Health Survey indicate that the rate of neonatal death decreased from 49 per 1,000 live births to 29 per 1,000 live births. This is less than the rate of decline for infant and under-five mortality, which was 97 to 48 and 166 to 67, respectively (10, 12, 13).
According to different studies conducted, obstetric, socio-demographic, reproductive health, medical, behavioral, and nutritional-related factors are common factors associated with neonatal mortality (14). In addition there exists a relationship between maternal health and neonatal survival. For instance, poor maternal nutritional and health status has been linked to poor neonatal outcome and this is influenced by determinants such as socio-economic, demographic and biologic factors (15).
The Ethiopian government strives to meet Sustainable Development Goal (SDG)-3, which requires all nations to eradicate preventable newborn deaths, with as few as 12 per 1,000 live births being the threshold. Additionally, the nation launched and is currently implementing the Health Sector Transformation Plan, which aims to lower newborn mortality to 10 per 1,000 live births by the program's end (16). Nevertheless, the problem still persists and the declination lag is very slow (17).
Globally, about 25% of women give birth to their first child before turning 20; in developing countries, this percentage is even higher. Early sexual initiation, low self-esteem, and/or isolation from social endeavors can all lead to teenage pregnancy. Unwanted pregnancies were linked to increased rates of school dropout, unemployment, psychological risk, pregnancy termination, and disdain for parental care in this age range. Contrarily, pregnancy in women over 35 is a prominent trend in this era for a number of reasons, such as women's evolving social roles in the workforce and within the home, their desire of financial security, and their accomplishment of a high level of education. Social, economic, and emotional maturity lead to a deeper understanding of the importance of effective pregnancy monitoring. Nevertheless, they became a risk factor for late pregnancy (1, 18). Both young and older maternal ages are associated with poor maternal and newborn outcomes. Thus, the degree and depth of maternal age influence on neonatal health should be understood in order to address the issue and increase neonatal survival.
According to earlier studies, extremes in a mother's age are among the socio-demographic characteristics of mothers that are known to be associated with poor delivery outcomes (19). Extreme maternal age has been linked to poor maternal and perinatal outcomes. As an illustration, being an older mother is linked to negative outcomes for both the mother and the child, including a higher chance of maternal diabetes and hypertension, congenital abnormalities, pregnancy complications, premature birth, and cesarean delivery (20). Conversely, younger women were linked to adverse socioeconomic circumstances, preterm, perinatal mortality, intra-uterine growth restriction (IUGR), low birth weight, and major congenital anomalies (21).
Nevertheless, despite research on neonatal death and its determinants having been conducted in various regions of Ethiopia, the mortality of neonates born to mothers of extreme reproductive age was not well studied. In addition, to the best of the investigator's search of information, the predictors of neonatal mortality born to mothers of extreme reproductive age have not been determined yet. Thus, this study aimed to assess the magnitude and contributing factors of neonatal mortality born to mothers of extreme reproductive age in Ethiopia, which helps responsible bodies make appropriate and timely interventions.
Methods
Study design, period and setting
A secondary analysis of data from the 2016 Ethiopian Demographic and Health Survey was used to conduct a population-based cross-sectional study. The data were collected from nine regional states and two city administrations (Addis Ababa and Dire Dawa). The region was subdivided into 68 zones, 817 districts, and 16,253 Kebele (the lowest administrative units in the country), and the study was conducted from January 18 to June 27, 2016. The 2016 Ethiopian Demographic and Health Survey was a cross-sectional survey that examined population health with a focus on maternal and child health, as well as population health indicators of global interest. The Ministry of Health and Ethiopia's Central Statistical Agency (CSA) worked together to collect the data. A stratified, two-stage cluster sampling technique was applied. In the first stage, 645 enumeration areas (202 urban and 443 rural EAs) were selected, with probability sampling proportional to the size of the EAs. Secondly, fixed numbers of 28 households per cluster were randomly selected. A total of 18,008 households were randomly selected, and 15,683 eligible women were interviewed. Data on 2,269 live births were extracted from Ethiopian Demographic and Health Survey (EDHS) in 2016. The full EDHS 2016 report included information on the sampling process as well as the general data collection (10).
Sample and populations
The source population was all live births among extreme-aged reproductive women (<20 and above 35 years old) within 28 days of life in Ethiopia, whereas all live babies aged 0–28 days in the enumeration areas (EAs) were the study populations. A two-stage stratified cluster sampling technique was employed. Stratification was done by separating each region into urban and rural areas. In stage one, 645 EAs were selected using probability sampling. In the second stage, households were selected systematically in each enumeration area. Then, newborns were selected using the Kids Record (KR) file, and important variables were selected from the data set.
Study variables
The outcome variable of this study was neonatal mortality. Neonatal mortality is defined as the death of a neonate within twenty-eight days of birth, which was dichotomized as yes = 1 for neonates who had died within twenty-eight days of birth, whereas no = 0 for neonates who were alive within one month of life. The neonatal mortality was calculated as the number of infant death that occurs between 0 and 27 days of life divided by the number of life birth, multiplied by 1,000 (22). As reviewed from different literature, extreme maternal age is considered young for mothers less than 20 years old, and older maternal age is considered as maternal age greater than 35 years old (1).
Independent variables
Both individual and community-level factors were reviewed from different literatures, and these include types of pregnancy, maternal age, BMI and educational level, place and mode of delivery, types of gestation, ANC visits, media exposure, and household wealth quintiles. Region, place of residence, community ANC utilization, community women's education, distance to health facilities, community media exposure, and community wealth status were community-level factors aggregated from individual-level factors (23). ANC was classified as optimal if a mother had more than 4 visits during her pregnancy time (24). Maternal education status was categorized as no formal education, primary, secondary and above in our study (25). Region were classified as pastoralist dominant, agrarian dominant and city dwellers (26). Reading newspapers, watching television, and listening to the radio were three ways that media exposure was computed. When there was exposure to any of the three, these variables were combined and classified as yes, meaning that reading newspapers, listening to the radio, or watching television were present. Distance to health facilities was categorized as “yes” for big problems to access and “no” for women to access health facilities without difficulty. Birth size was categorized as small for a weight less than 2,500 g, normal for a weight of 2,500–3,900 g, and large for a weight greater than 4,000 g. Maternal body max index (BMI) was found from DHS recorded data and determined during the survey period as the ratio of weight in kilogram to the square of height in meter (kg/m2) and available in the data as two digit decimal then we categorized the recorded BMI as low for BMI < 18.49 kg/m2, normal for 18.5–24.9 kg/m2, and large for ≥25 kg/m2 (27).
Data collection procedure
The DHS Program granted us permission to collect and use the data from http://www.dhsprogram.com for this study after we asked permission. Before releasing the data collected from the DHS to the public, participant identification was erased, and institutional ethical approval was waived to ensure compliance with the rules governing the protection of human beings.
Data management and model selection
Following extraction from the EDHS portal, data were entered, coded, cleaned, recorded, and analyzed using Stata version 14. In DHS, data variables are nested by clusters, and those within the same cluster show more similarities than those with separate clusters. Consequently, the assumption of independent observation and equal variance across clusters violates the conventional logistic regression model. Hence, there is a need for a more advanced model to account for cluster factors. A model called multi-level multivariable logistic regression was employed to identify factors associated with neonatal mortality. Accordingly, multi-level mixed effect logistic regression uses four models: the null model, model I (individual level factors), model II (community level factors), and model III (which holds both individual and community level factors). The model without an exposure variable (the null model) was used to check the variability of neonatal mortality across the cluster. The associations of individual-level factors with the outcome variable (model II) were examined. In model III, or the final model, the association of both individual and community-level variables was fitted with the outcome variable. Random effects, or measures of variation of the outcome variable, were estimated by the median odds ratio (MOR), intra-class correlation coefficient (ICC), and proportional change in variance (PCV). The intra-class correlation coefficient (ICC) and proportional change in variance (PCV) were computed to measure the variation between clusters. Taking clusters as a random variable, the ICC reveals the variation of early neonatal death between clusters as: ICC = VC/(VC + 3.29) × 100%, where VC is the variance of the cluster. The MOR is the median value of the odds ratio between the area of highest risk and the area of lowest risk for neonatal death when two clusters are randomly selected, using clusters as a random variable; MOR = e95√Vc. Moreover, the PCV demonstrates the variation in the prevalence of neonatal death as explained by factors and computed as PCV = (Vnull−VC)/Vnull × 100%, where Vnull is the variance of the null model and VC is the cluster-level variance. The fixed effects were used to estimate the association between the likelihood of neonatal death and individual and community-level independent variables. It was assessed, and the strength was presented using an adjusted odds ratio (AOR) and 95% confidence intervals with a p-value of 0.05. Because of the nested nature of the model, deviation = −2 (log likelihood ratio) was used to compare models, and the model with the lowest deviance was selected as the best-fit model. The variables used in the models were verified for multi-collinearity by measuring the variance inflation factors (VIF), with the findings falling within acceptable limits of one to ten (28–30).
Result
Socio-demographic characteristics
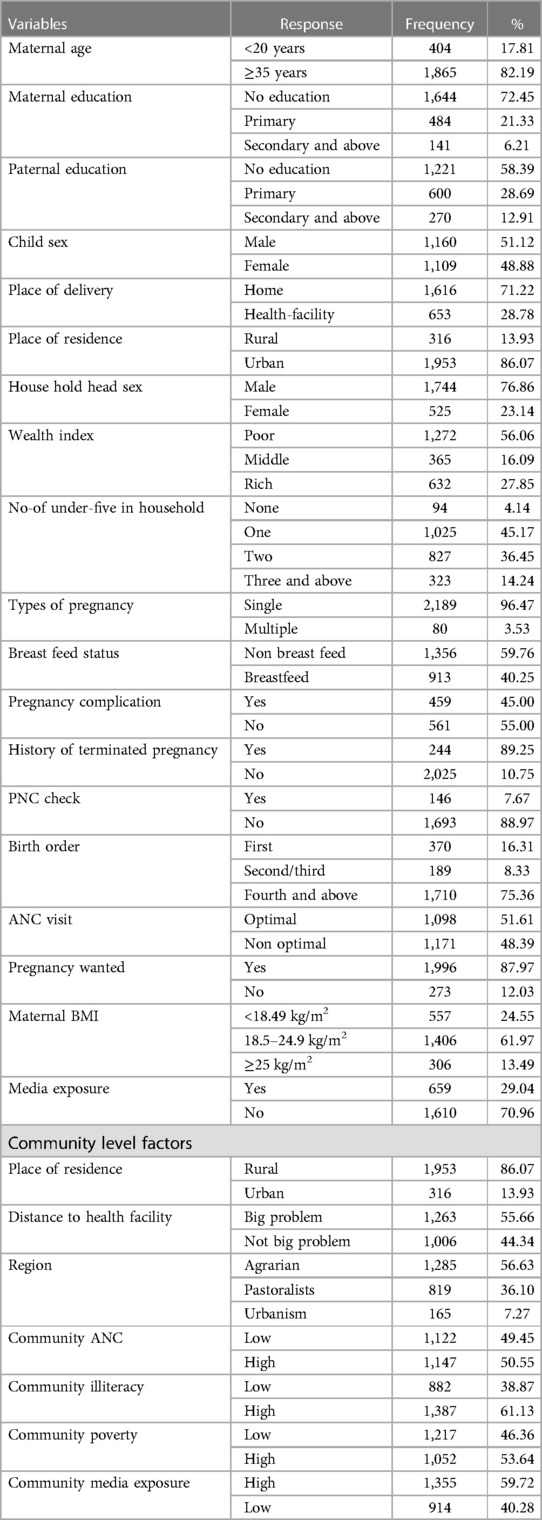
A total of 2,269 mothers with extreme maternal age (less than twenty and above thirty-five) were included in the study. The majority (82.19%) of mothers were aged above 35 years (older mothers), and nearly one fifth (17.81%) of mothers were aged below 20 years old (teenagers). Nearly two-thirds (72.45%) of mothers hadn't received formal education. More than half of the participants’ partners or husbands (58.39%) lacked formal education. The majority of participants (86%) reside in rural areas. Majorities (97%) of children's were delivered by normal vaginal delivery and were born at home (71.22%). The majority of newborns (98.5%) were born at nine months (term) Table 1.
Prevalence of neonatal mortality among extreme reproductive aged women’s in Ethiopia
According to this study, neonatal mortality among extreme-aged mothers in Ethiopia was 34 (95% Cl, 27.2–42.2) per 1,000 live births. This newborn mortality rate is higher than Ethiopia's general reproductive age women's neonatal death rate, which is 29 per 1,000 live births and 30 per 1,000 live births, according to the 2016 and 2019 EDHS reports, respectively (31).
Random effect and model comparison
Random effect or community variation was examined by ICC, MOR, and PCV. The ICC value in model I was 0.8, indicating that 80% of neonatal mortality among the extremes of reproductive age in Ethiopia was attributable to the differences at the cluster level. The median odds ratio in the null model was 2.33, which indicated that neonatal mortality was different between clusters. The PCV in the final model indicates that 56.25% of neonatal mortality was attributable to both individual and community-level factors. Finally, the four models were compared to select the best fit model, and the final model with the lowest deviance was considered the best fit model, which is model IV (149.52) (Table 2).
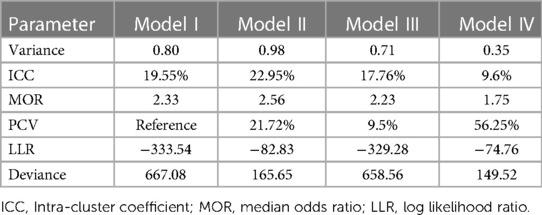
Table 2 Random effect and model fit statistics of neonatal mortality among extreme reproductive age in Ethiopia.
Factors associated with neonatal mortality among extreme age of reproductive age
Socio-demographic, obstetric, and child-related factors were analyzed in the bivariate multiple logistic regression model. The variables associated with neonatal mortality among women of extreme reproductive age at a p-value of 0.2 were further analyzed in multi-variable multilevel mixed effect models (model III and model IV). In the final model (model IV), both individual and community-level factors were fitted to control confounders and identify statistically significant factors of neonatal mortality among women of extreme reproductive age.
Accordingly, the final model revealed that mothers having term pregnancy had 86% (AOR: 0.14, 95% CI, 0.01–0.81) lower odds of neonatal mortality than mothers having non-term pregnancy. Similarly, women's having media exposure was 70% (AOR: 0.3, 95% CI: 0.09–0.91) less likely to have neonatal mortality than women's not exposed to media. On the other hand, children with a large birth size had almost three times (AOR: 2.93, 95% CI, 1.4–9.12) higher odds of neonatal mortality than normal birth-sized babies’. Mothers having multiple gestations had a tenfold (AOR: 10.5% CI, 8.61–20.21) more likely neonatal death than singleton gestation. Among community-level factors, Ethiopian pastoralist regions were almost fourfold (AOR: 3.94, 95% CI: 1.71–8.09) more likely to have neonatal mortality than mothers who reside in the city. On the other hand, communities’ having media exposure had 76% (AOR: 0.24 95% CI, 0.07–0.83) less likely neonatal morality than communities with no media exposure (Table 3).
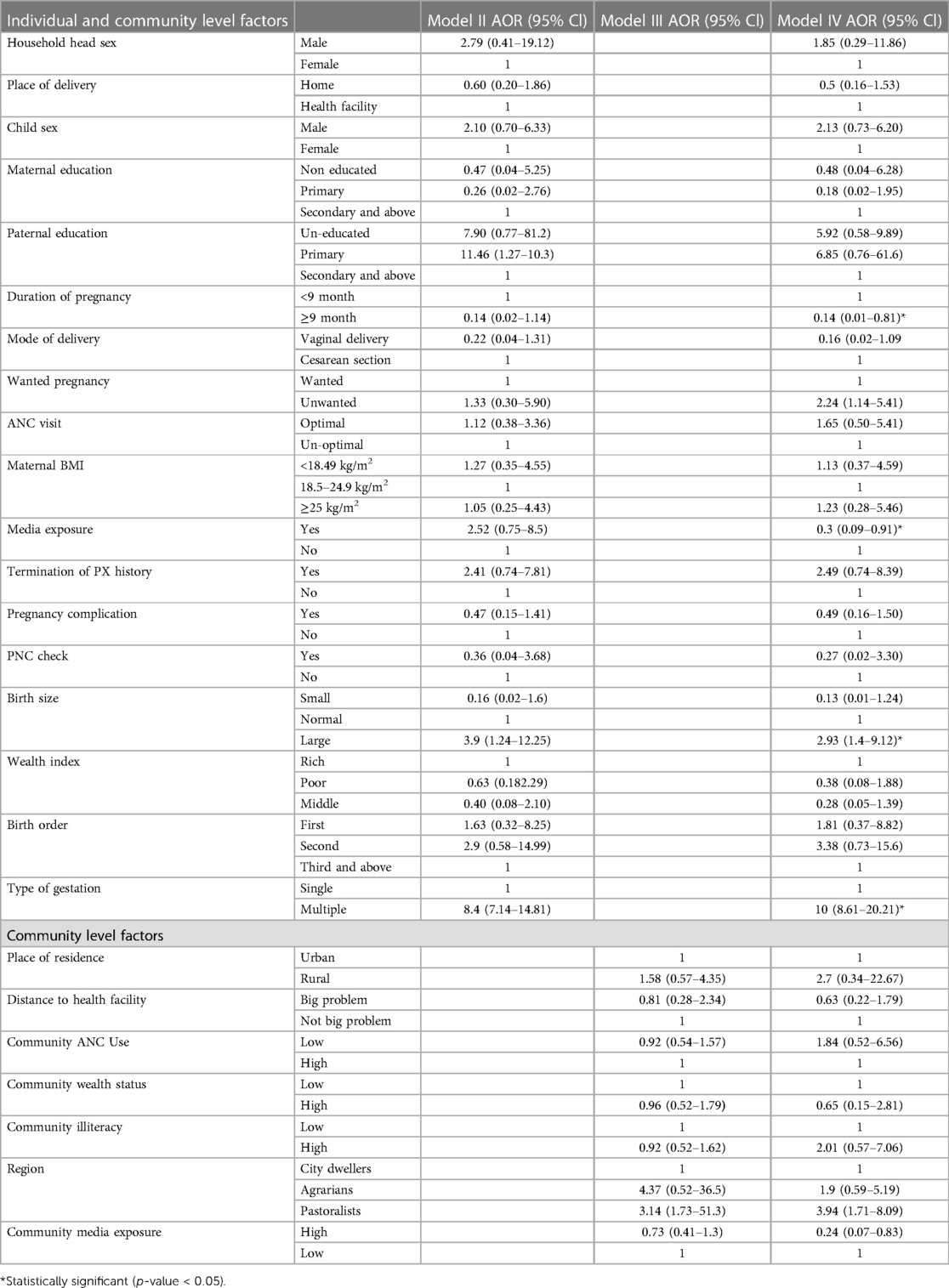
Table 3 Multivariable multi-level logistic regression analysis of individual and community level factors associated with neonatal mortality among extreme reproductive age women in Ethiopia, 2016.
Discussion
This study was done on newborns in Ethiopia whose moms were at the upper end of the reproductive age range; less than twenty and older than thirty-five. Thus, the study found that the neonatal mortality rates among extremes of reproductive age in Ethiopia were 34 (95% Cl, 27.2–42.2) per 1,000 live birth. This newborn mortality rate is higher than Ethiopia's general reproductive age women's neonatal mortality, which is 29 per 1,000 live births and 30 per 1,000 live births, according to the 2016 and 2019 EDHS reports, respectively (31). This is due to the fact that young mothers are more biologically vulnerable to unfavorable birth outcomes, such as physical immaturity causing pregnancy complications, and competition between the mother and the fetus for nutrients can exacerbate the effects of chronic malnutrition, leading to poorer outcomes for the health of the infant and child (32–35). On the other hand, older mothers are at a higher risk of fetal death at term from intra-partum asphyxia (36), more likely to experience unfavorable labor and delivery outcomes, obstetric difficulties, and preexisting medical conditions (37), including preterm delivery, perinatal mortality, low birth weight and macro-somia (38, 39). Therefore, older women and adolescent mothers deserve special consideration.
This study also found factors significantly associated with the mortality of neonates born to mothers of extreme age. Accordingly, media exposure, multiple gestations, larger birth size, duration of pregnancy, community media exposure, and region were significantly associated with neonatal death among the extremes of maternal and reproductive life.
The odds of neonatal death were higher among multiple births compared with singleton births. This is supported by study done in Korea (40). One possible explanation is that preterm births and low birth weight are commonly associated with twin pregnancies, which is the primary cause of neonatal mortality. Additionally, twin pregnancies expose the fetus to infection and twin-twin transfusion syndrome, which reduces infant survival and eventually causes death (35, 41, 42). It is consistent with findings in Ghana (43). This could be because preterm delivery and fetal growth restriction are associated with a higher likelihood of twin births, which in turn raises the risk of hypothermia, sepsis, and hypoglycemia, all of which may increase the risk of neonatal mortality (44). Thus, clinicians shall give special attention to multiple births through early detection of plurality by ultrasonography, give special care during delivery, and provide counseling and support to mothers on handling twin pregnancies during ANC and PNC visits.
Compared to full-term deliveries, non-term pregnancies experienced greater increases in neonatal, postnatal, and infant death rates (45, 46). An investigation conducted in New York supports the notion that preterm neonates were significantly more likely than full-term infants to experience hypoglycemia, respiratory distress, transient tachypnea, NICU need, respiratory intubation, intravenous fluid requirements, mechanical ventilation, and cesarean delivery, all of which could lead to serious complications or even death (47, 48). Thus, government and policymakers should pay attention to preterm births. Intensive care unit should be available for all preterm births.
According to this study, media exposure was significantly associated with neonatal mortality. Babies from parents with high media exposure at the individual and at the community level decreased the odds of neonatal death compared to newborns with low media exposure. This is in line with the study done in Bangladesh (49). One plausible explanation could be that moms and the general community, who were exposed to the media, had greater knowledge about the use of ANC, institutional delivery, and pediatric illnesses (50). Thus, the government and other relevant authorities should promote media exposure and increasing accesses to media.
Baby size at birth was also significantly associated with neonatal mortality. This is supported by study done in London (51) by which pregnancy with macrosomia increases the risk of maternal and neonatal complications, such as the necessity for an emergency cesarean section, obstetric brachial plexus injury, and birth fractures that compromise the newborn's quality of life (51). Furthermore, larger newborns are linked to other maternal co-morbidities including gestational diabetes, which puts the mother's and the child's lives in danger (52). Therefore, special consideration should be given to macrocosmic babies.
Regions in Ethiopia also significantly associated with neonatal mortality. Ethiopian regions can be classified as Agrarian, Pastoralist, and city administration.This is in line with the pervious study in Ethiopia (53). A plausible rationale could be because earlier research conducted in Ethiopia was conducted in pastoralist areas, where the availability of mother and child health services is relatively low and the population is economically disadvantaged compared to other regions (24). Moreover, these regions exhibit poorer vaccination rates, limited healthcare accessibility, lower urbanization, and higher incidence of infectious diseases such as acute respiratory infections and malaria in comparison to other regions of the country (54, 55). Hence, priority shall be given to pastoralists in increasing access to information regarding maternal and newborn care and in increasing access to basic health services to minimize the burden of neonatal mortality in these emerging regions of Ethiopia.
This study found that the newborn mortality rate is higher in extreme-aged reproductive women as compared to general reproductive-age women's neonatal deaths. The study also found that for babies born at extreme maternal age, factors at the individual and community levels were associated with neonatal mortality. Thus, responsible bodies shall give special attention to extremely aged reproductive women in Ethiopia and its predictors.
Conclusion
The study found that for babies born at extreme maternal age, factors at the individual and community levels were associated with neonatal mortality. The main factors that determined neonatal death in our study were multiple pregnancies, larger babies, pastoral region, term gestation, and media exposure. Policy makers, researchers, health planners, and implementers must therefore place attention on promoting healthy pregnancy, promoting media access, and paying special attention to mothers in low and high quartiles of age.
Strength and limitation
This study's strength was the use of multi-level modeling to make meaningful inferences and findings while accounting for the clustering effect in EDHS. The study has the potential to assist programmers and policymakers in developing effective national interventions because it is based on data from a countrywide survey. Due to the DHS nature of the data, variables like infectious illness, congenital abnormalities, and respiratory state after deliveries, which are thought to be the most common causes of neonatal mortality, were left out of the analysis. Furthermore, recall bias may exist in this study due to the cross-sectional nature of DHS and its reliance on respondents’ self-reports. Due to our small sample size of teenage mothers and our relatively larger sample of older mothers, which makes our sample inconsistent, we couldn't perform comparisons independently among these age groups; thus, a future comparative study on neonatal mortality between extreme maternal age and normal maternal age or between early (teenager maternal age) and older (advanced maternal age) shall be done.
Data availability statement
The datasets presented in this study can be found in online repositories. The data can be found here: https://www.dhsprogram.com/data/available-datasets.cfm.
Ethics statement
The studies involving humans were approved by this study is a secondary analysis of the DHS data, so it does not require ethical approval. For conducting our study, we registered and requested the datasets from DHS, which were publically available, and received approval to access and download the data files. According to the DHS report, all participant data were anonymized during the collection of the survey data. More details regarding DHS data and ethical standards are available online at http://www.dhsprogram.com. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin.
Author contributions
BT: Writing – original draft, Validation, Conceptualization. MT: Writing – review & editing, Validation. BW: Writing – review & editing, Formal Analysis. AZ: Writing – original draft, Supervision, Formal Analysis. AG: Writing – review & editing, Investigation. TA: Writing – review & editing, Formal Analysis. MW: Writing – review & editing, Visualization. AK: Writing – review & editing, Validation, Formal Analysis. MA: Writing – original draft, Investigation. EM: Writing – review & editing, Formal Analysis, Data curation. TT: Writing – review & editing, Validation.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ANC, antenatal care; EA, enumeration area; EDHS, Ethiopian demographic health survey; ICC, inter cluster correlation; NMR, neonatal Mortality; LR, logistic regression; MOR, median odds ratio; PCH, Primary Health Care; PCV, proportional change in variance.
References
1. Ribeiro FD, Ferrari RAP, Sant'Anna FL, Dalmas JC, Girotto E. Extremes of maternal age and child mortality: analysis between 2000 and 2009. Rev Paul Pediatr. (2014) 32:381–8. doi: 10.1016/j.rpped.2014.05.002
2. Nove A, Matthews Z, Neal S, Camacho AV. Maternal mortality in adolescents compared with women of other ages: evidence from 144 countries. Lancet Glob Health. (2014) 2(3):e155–e64. doi: 10.1016/S2214-109X(13)70179-7
3. Noori N, Proctor JL, Efevbera Y, Oron AP. The effect of adolescent pregnancy on child mortality in 46 low-and middle-income countries. BMJ Global Health. (2022) 7(5):e007681. doi: 10.1136/bmjgh-2021-007681
4. Laopaiboon M, Lumbiganon P, Intarut N, Mori R, Ganchimeg T, Vogel J, et al. Advanced maternal age and pregnancy outcomes: a multicountry assessment. BJOG. (2014) 121:49–56. doi: 10.1111/1471-0528.12659
5. UNICEF. United nations children’s fund. The State of the World’s Children. New York: UNICEF–NY (2006). p. 10017.
6. Ezbakhe F, Pérez-Foguet A. Child mortality levels and trends. Demogr Res. (2020) 43:1263–96. doi: 10.4054/DemRes.2020.43.43
7. Sharrow D, Hug L, You D, Alkema L, Black R, Cousens S, et al. Global, regional, and national trends in under-5 mortality between 1990 and 2019 with scenario-based projections until 2030: a systematic analysis by the UN inter-agency group for child mortality estimation. Lancet Glob Health. (2022) 10(2):e195–206. doi: 10.1016/S2214-109X(21)00515-5
8. Mekonnen Y, Tensou B, Telake DS, Degefie T, Bekele A. Neonatal mortality in Ethiopia: trends and determinants. BMC public Health. (2013) 13(1):1–14. doi: 10.1186/1471-2458-13-483
9. Wang H, Liddell CA, Coates MM, Mooney MD, Levitz CE, Schumacher AE, et al. Global, regional, and national levels of neonatal, infant, and under-5 mortality during 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet. (2014) 384(9947):957–79. doi: 10.1016/S0140-6736(14)60497-9
10. Csa I. Central Statistical Agency (CSA) [Ethiopia] and ICF. Ethiopia Demographic and Health Survey, Addis Ababa, Ethiopia and Calverton, Maryland, USA (2016):1.
11. Negera A, Abelti G. An Analysis of the Trends, Differentials and Key Proximate Determinants of Infant and Under-five Mortality in Ethiopia: Further Analysis of the 2000, 2005, and 2011 Demographic and Health Surveys (2013).
12. Demographic N. Health Survey 2011. Central Statistical Agency Addis Ababa Ethiopia ICF International Calverton, Maryland, USA (2012) 2016(1).
13. Arimond M, Ruel MT. Progress in Developing an Infant and a Child Feeding index: An Example Using the Ethiopia Demographic and Health Survey 2000. Washington, DC: Research in Agriculture & Applied Economics (2002).
14. Lawn JE, Cousens S, Zupan J. 4 Million neonatal deaths: when? where? why? Lancet. (2005) 365(9462):891–900. doi: 10.1016/S0140-6736(05)71048-5
15. Imbo AE, Mbuthia EK, Ngotho DN. Determinants of neonatal mortality in Kenya: evidence from the Kenya demographic and health survey 2014. Int J MCH AIDS. (2021) 10(2):287. doi: 10.21106/ijma.508
16. UNICEF.org. Progress on Children’s Well-Being: Centring Child Rights in the 2030 Agenda. New York: UNICEF.org (2023). Available online at: https://data.unicef.org (Accessed November 11, 2023).
17. Haileamlak A. Is neonatal mortality rate in Ethiopia going from bad to worse? Ethiop J Health Sci. (2022) 32(3):472. doi: 10.4314/ejhs.v32i3.1
18. de Oliveira Caminha N, Freitas LV, Herculano MMS, de Castro Damasceno AK. Pregnancy in adolescence: from planning to the desire to become pregnant-descriptive study. Online Braz J Nurs. (2010) 9(1):1–24. doi: 10.5935/1676-4285.20102872
19. Houweling TA, van Klaveren D, Das S, Azad K, Tripathy P, Manandhar D, et al. A prediction model for neonatal mortality in low-and middle-income countries: an analysis of data from population surveillance sites in India, Nepal and Bangladesh. Int J Epidemiol. (2019) 48(1):186–98. doi: 10.1093/ije/dyy194
20. Leader J, Bajwa A, Lanes A, Hua X, White RR, Rybak N, et al. The effect of very advanced maternal age on maternal and neonatal outcomes: a systematic review. J Obstet Gynaecol Can. (2018) 40(9):1208–18. doi: 10.1016/j.jogc.2017.10.027
21. Kang G, Lim JY, Kale AS. Adverse effects of young maternal age on neonatal outcomes. Singapore Med J. (2015) 56(3):157. doi: 10.11622/smedj.2014194
22. Bitew FH. Neonatal Mortality Rate (0 to 27 days) Per 1000 Live Births) (SDG 3.2.2). (2023). Available online at: https://www.who.int/data/gho/indicator-metadata-registry/imr-details/67 (Accessed February 2023).
23. Bitew FH. Spatio-Temporal Inequalities and Predictive Models for Determinants of Undernutrition among Women and Children in Ethiopia. Hoboken: The University of Texas at San Antonio (2020).
24. Belay AT, Fenta SM, Birhan Biresaw H, Abebaw Moyehodie Y, Melkam Yelam M, Mekie M. The magnitude of optimal antenatal care utilization and its associated factors among pregnant women in South Gondar zone, Northwest Ethiopia: a cross-sectional study. Int J Reprod Med. (2022) 2022:1–6. doi: 10.1155/2022/1415247
25. Berhe T, Gebreyesus H, Desta H. Determinants of preterm birth among mothers delivered in central zone hospitals, Tigray, Northern Ethiopia. BMC Res Notes. (2019) 12(1):1–6. doi: 10.1186/s13104-018-4038-6
26. Tassew AA, Tekle DY, Belachew AB, Adhena BM. Factors affecting feeding 6–23 months age children according to minimum acceptable diet in Ethiopia: a multilevel analysis of the Ethiopian demographic health survey. PLoS One. (2019) 14(2):e0203098. doi: 10.1371/journal.pone.0203098
27. Neupane S KCP, Doku DT. Overweight and obesity among women: analysis of demographic and health survey data from 32 Sub-Saharan African countries. BMC public Health. (2015) 16:1–9. doi: 10.1186/s12889-015-2639-8
28. Lehtonen L, Gimeno A, Parra-Llorca A, Vento M. Early neonatal death: a challenge worldwide. Semin Fetal Neonatal Med. (2017) 15:1–12. doi: 10.1016/j.siny.2017.02.006
29. Akinyemi JO, Bamgboye EA, Ayeni O. Trends in neonatal mortality in Nigeria and effects of bio-demographic and maternal characteristics. BMC Pediatr. (2015) 15(1):1–12. doi: 10.1186/s12887-015-0349-0
30. Reyes J, Ramírez RP, Ramos LL, Ruiz LG, Vázquez EB, Patino VR. Neonatal mortality and associated factors in newborn infants admitted to a neonatal care unit. Arch Argent Pediatr. (2018) 116(1):42–8. Available online at: https://ephi.gov.et/wp-content/uploads/2021/05/Final-Mini-DHS-report-FR36329333811
31. Ephi I. Ethiopia Mini Demographic and Health Survey 2019: Key Indicators. Rockville, Maryland, USA: EPHI and ICF (2019).
32. Sharma V, Katz J, Mullany LC, Khatry SK, LeClerq SC, Shrestha SR, et al. Young maternal age and the risk of neonatal mortality in rural Nepal. Arch Pediatr Adolesc Med. (2008) 162(9):828–35. doi: 10.1001/archpedi.162.9.828
33. Kamal SM. What is the association between maternal age and neonatal mortality? An analysis of the 2007 Bangladesh demographic and health survey. Asia Pac J Public Health. (2015) 27(2):NP1106–NP17. doi: 10.1177/1010539511428949
34. Conde-Agudelo A, Belizán JM, Lammers C. Maternal-perinatal morbidity and mortality associated with adolescent pregnancy in Latin America: cross-sectional study. Am J Obstet Gynecol. (2005) 192(2):342–9. doi: 10.1016/j.ajog.2004.10.593
35. Ghosh R, Bharati P. Determinants of infant and child mortality in Periurban areas of Kolkata city, India. Asia Pac J Public Health. (2010) 22(1):63–75. doi: 10.1177/1010539509350758
36. Smith G, Pasupathy D, Wood AM, Pell JP, Fleming M. Advanced maternal age and the risk of perinatal death due to intrapartum anoxia at term. J Epidemiol Community Health. (2011) 65(3):241–5. doi: 10.1136/jech.2009.097170
37. Ludford I, Scheil W, Tucker G, Grivell R. Pregnancy outcomes for nulliparous women of advanced maternal age in South Australia, 1998–2008. Aust N Z J Obstet Gynaecol. (2012) 52(3):235–41. doi: 10.1111/j.1479-828X.2012.01442.x
38. Kozuki N, Lee AC, Silveira MF, Sania A, Vogel JP, Adair L, et al. The associations of parity and maternal age with small-for-gestational-age, preterm, and neonatal and infant mortality: a meta-analysis. BMC public Health. (2013) 13:1–10. doi: 10.1186/1471-2458-13-1
39. Cleary-Goldman J, Malone FD, Vidaver J, Ball RH, Nyberg DA, Comstock CH, et al. Impact of maternal age on obstetric outcome. Obstet Gynecol. (2005) 105(5 Part 1):983–90. doi: 10.1097/01.AOG.0000158118.75532.51
40. Ko HS, Wie JH, Choi SK, Park IY, Park Y-G, Shin JC. Multiple birth rates of Korea and fetal/neonatal/infant mortality in multiple gestation. PLoS One. (2018) 13(8):e0202318. doi: 10.1371/journal.pone.0202318
41. Hehir MP, Mctiernan A, Martin A, Carroll S, Gleeson R, Malone FD. Improved perinatal mortality in twins—changing practice and technologies. Am J Perinatol. (2016) 2(01):084–9. doi: 10.1055/s-0035-1559807
42. Townsend R, Khalil A. Fetal growth restriction in twins. Best Pract Res Clin Obstet Gynaecol. (2018) 49:79–88. doi: 10.1016/j.bpobgyn.2018.02.004
43. Kayode GA, Ansah E, Agyepong IA, Amoakoh-Coleman M, Grobbee DE, Klipstein-Grobusch K. Individual and community determinants of neonatal mortality in Ghana: a multilevel analysis. BMC Pregnancy Childbirth. (2014) 14:1–12. doi: 10.1186/1471-2393-14-165
44. Marchant T, Willey B, Katz J, Clarke S, Kariuki S, Kuile F, et al. Neonatal mortality risk associated with preterm birth in East Africa, adjusted by weight for gestational age: individual participant level meta-analysis. PLoS Med. (2012) 9(8):e1001292. doi: 10.1371/journal.pmed.1001292
45. Reddy UM, Bettegowda VR, Dias T, Yamada-Kushnir T, Ko C-W, Willinger M. Term pregnancy: a period of heterogeneous risk for infant mortality. Obstet Gynecol. (2011) 117(6):1279. doi: 10.1097/AOG.0b013e3182179e28
46. Reddy U, Ko C-W, Willinger M. “Early” term births (37–38 weeks) are associated with increased mortality. Am J Obstet Gynecol. (2006) 195(6):S202. doi: 10.1016/j.ajog.2006.10.725
47. Sengupta S, Carrion V, Shelton J, Wynn RJ, Ryan RM, Singhal K, et al. Adverse neonatal outcomes associated with early-term birth. JAMA Pediatr. (2013) 167(11):1053–9. doi: 10.1001/jamapediatrics.2013.2581
48. McIntire DD, Leveno KJ. Neonatal mortality and morbidity rates in late preterm births compared with births at term. Obstet Gynecol. (2008) 111(1):35–41. doi: 10.1097/01.AOG.0000297311.33046.73
49. Nilima S, Sultana R, Ireen S. Neonatal, infant and under-five mortality: an application of cox proportional hazard model to BDHS data. J Asiat Soc Bangladesh Sci. (2018) 44(1):7–14. doi: 10.3329/jasbs.v44i1.46541
50. Agha S, Carton TW. Determinants of institutional delivery in rural Jhang, Pakistan. Int J Equity Health. (2011) 10(1):1–12. doi: 10.1186/1475-9276-10-1
51. Beta J, Khan N, Khalil A, Fiolna M, Ramadan G, Akolekar R. Maternal and neonatal complications of fetal macrosomia: systematic review and meta-analysis. Ultrasound Obstet Gynecol. (2019) 54(3):308–18. doi: 10.1002/uog.20279
52. Dahiru T. Determinants of early neonatal mortality in Nigeria: results from 2013 Nigeria DHS. J Pediatr Neonatal Care. (2015) 2(5):00089. doi: 10.15406/jpnc.2015.02.00089
53. Tesema GA, Worku MG. Individual-and community-level determinants of neonatal mortality in the emerging regions of Ethiopia: a multilevel mixed-effect analysis. BMC Pregnancy Childbirth. (2021) 21:1–11. doi: 10.1186/s12884-020-03485-8
54. Admassie A, Abebaw D. Rural poverty and marginalization in Ethiopia: a review of development interventions. In: von Braun J, Gatzweiler F, editors. Marginality. Dordrecht: Springer (2014). p. 269–300. doi: 10.1007/978-94-007-7061-4_17
Keywords: neonatal mortality, extreme reproductive age, multilevel mixed effect analysis, demographic and health survey, Ethiopia
Citation: Tekeba B, Techane MA, Workneh BS, Zegeye AF, Gonete AT, Alemu TG, Wassie M, Kassie AT, Ali MS, Mekonen EG and Tamir TT (2024) Mortality of neonates born to mothers of extreme reproductive age in Ethiopia; multilevel mixed effect analysis of Ethiopian demographic and health survey data of 2016. Front. Pediatr. 12:1390952. doi: 10.3389/fped.2024.1390952
Received: 24 February 2024; Accepted: 12 June 2024;
Published: 28 June 2024.
Edited by:
Zhangbin Yu, The Second Clinical Medical College of Jinan University, First Affiliated Hospital of Southern University of Science and Technology, ChinaReviewed by:
Hugo Martinez-Rojano, Escuela Superior de Medicina (IPN), MexicoAristide Romaric Bado, Research Institute for Health Sciences (IRSS), Burkina Faso
© 2024 Tekeba, Techane, Workneh, Zegeye, Gonete, Alemu, Wassie, Kassie, Ali, Mekonen and Tamir. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Berhan Tekeba, berishboss7@gmail.com
 Berhan Tekeba
Berhan Tekeba Masresha Asmare Techane
Masresha Asmare Techane Belayneh Shetie Workneh
Belayneh Shetie Workneh Alebachew Ferede Zegeye
Alebachew Ferede Zegeye Almaz Tefera Gonete
Almaz Tefera Gonete Tewodros Getaneh Alemu
Tewodros Getaneh Alemu Mulugeta Wassie
Mulugeta Wassie Alemneh Tadesse Kassie
Alemneh Tadesse Kassie Mohammed Seid Ali
Mohammed Seid Ali Enyew Getaneh Mekonen
Enyew Getaneh Mekonen Tadesse Tarik Tamir
Tadesse Tarik Tamir