- 1Department of Pediatrics, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
- 2Division of Pediatric Stem Cell Transplantation and Immunology, Department of Pediatric Hematology and Oncology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany
Staphylococcus aureus (S. aureus) is a significant human pathogen, in particular in patients with an underlying medical condition. It is equipped with a large variety of virulence factors enabling both colonization and invasive disease. The spectrum of manifestation is broad, ranging from superficial skin infections to life-threatening conditions like pneumonia and sepsis. As a major cause of healthcare-associated infections, there is a great need in understanding staphylococcal immunity and defense mechanisms. Patients with inborn errors of immunity (IEI) frequently present with pathological infection susceptibility, however, not all of them are prone to S. aureus infection. Thus, enhanced frequency or severity of S. aureus infections can serve as a clinical indicator of a specific underlying immunological impairment. In addition, the analysis of immunological functions in patients with susceptibility to S. aureus provides a unique opportunity of understanding the complex interplay between staphylococcal virulence and host immune predisposition. While the importance of quantitatively and qualitatively normal neutrophils is widely known, less awareness exists about the role of specific cytokines such as functional interleukin (IL)-6 signaling. This review categorizes well-known IEI in light of their susceptibility to S. aureus and discusses the relevant associated pathomechanisms. Understanding host-pathogen-interactions in S. aureus infections in susceptible individuals can pave the way for more effective management and preventive treatment options. Moreover, these insights might help to identify patients who should be screened for an underlying IEI. Ultimately, enhanced understanding of pathogenesis and immune responses in S. aureus infections may also be of relevance for the general population.
Introduction
The current list of inborn errors of immunity (IEI) comprises more than 485 monogenetic gene defects (1). Enhanced susceptibility to a specific pathogen such as Staphylococcus aureus (S. aureus) may raise suspicion of a certain type of immunological impairment. Staphylococcus aureus is a great challenge to our health care systems (2). Despite being considered a commensal, with a colonization rate of 20%–30% in the healthy population (3), it can also cause a wide variety of different infections. It is a leading cause of skin and soft tissue infections and abscesses, but may also lead to lung infections, osteomyelitis or endocarditis, in particular in patients with underlying conditions (2). The ability to colonize but also to cause harm to the host, emerges from a complex interaction between the pathogen and its host (4). Staphylococcus aureus is a specialist in adapting to the human host by evading almost every aspect of the immune system (5). In the last decades, changes in strains have led to an increase of S. aureus infections in otherwise healthy individuals (6). Thus, staphylococcal defense in the individual is shaped by both pathogen virulence factors as well as the patient's immune predisposition (4). Recurrent or severe S. aureus infections may both be an indicator of certain IEI and specific IEI can teach us about essential immune functions for staphylococcal defense.
S. aureus immune evasion and host immune response
Staphylococcal infections often arise from asymptomatic colonization and breaches through skin and mucosal barriers (7) (Figure 1). Immune evasion strategies of S. aureus are abundant and tackle particularly innate immunity (8, 9). Examples include inhibition of immune recognition, prevention of complement activation (10), resistance to phagosomal killing (5) and direct killing of immune cells through different leucocidins (7). In addition, presence of peptidoglycan layer, polysaccharide capsule and surface proteins hamper opsonization (7). The most important players in S. aureus defense are phagocytes. In particular neutrophils, along with tissue-resident or monocyte-derived macrophages, are instrumental in identifying, engulfing, and eliminating staphylococci (11). As the first line of innate cellular defense, they also orchestrate subsequent immune responses. The crucial role of neutrophils is clearly evidenced by the enhanced staphylococcal susceptibility of patients with numeric or functional neutrophil defects (12, 13). Staphylococcus aureus has developed numerous mechanisms to reduce neutrophil extravasation, activation, and chemotaxis (9), and may also evade neutrophil extracellular traps using nucleases and proteases (14). Secretion of exopolysaccharides and biofilm formation inhibit phagocytosis (7). When internalized by phagocytes, S. aureus may neutralize reactive oxygen species and employ enzymes for survival (8). Through intracellular survival both in phagocytic and non-phagocytic cells, S. aureus may evade antibiotic killing and facilitate subsequent dissemination (15). Induction of IL-10 by S. aureus may lead to a phenotypic switch in the immune response during persistent staphylococcal infection allowing its persistence as commensal (16). Toxins like Panton–Valentine leucocidin (PVL), which are harbored by some more virulent strains, destroy immune cells and may lead to treatment failure and severe infections even in immunocompetent patients (17, 18). While most virulence factors address innate immunity, S. aureus may also interfere with the adaptive immune response, using proteins like SpA to bind immunoglobulins (19) and superantigens like TSST-1 to induce cytokine release and toxic shock syndrome (20).
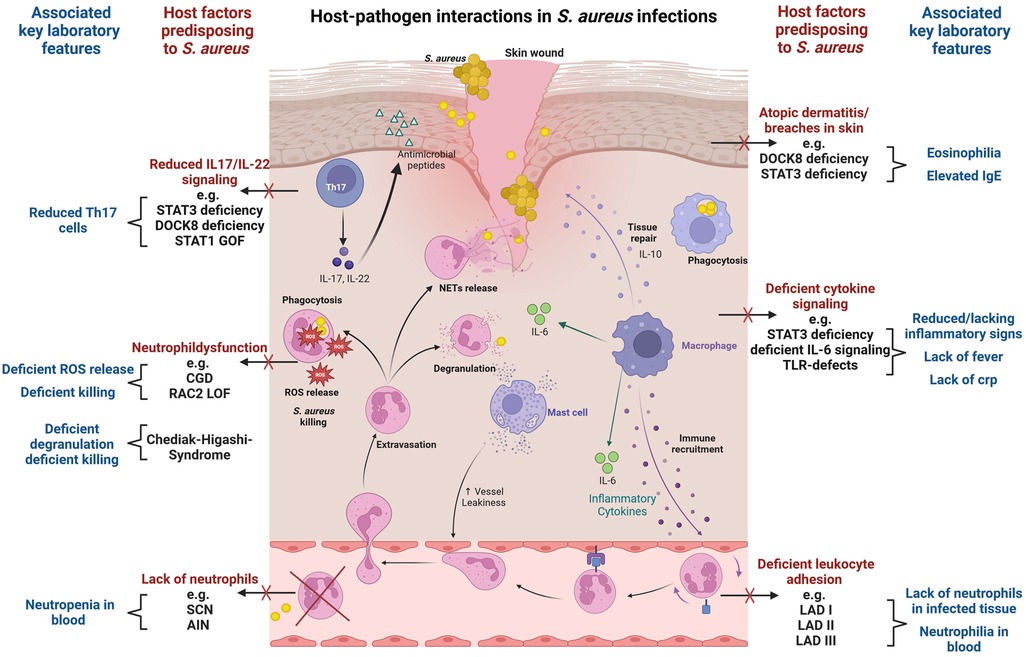
Figure 1. Host-pathogen interactions in S. aureus infections. The figure visualizes key immunological defense mechanisms and highlights host factors predisposing to S. aureus infection in case of deficiency. Commonly associated laboratory findings in the respective setting are also displayed. Selective examples of IEI with susceptibility to S. aureus infection are provided. The figure provides a simplified overview, and displayed host factors and interactions do not claim to be complete. The figure was created with BioRender.com.
The evasion strategies of S. aureus challenge infection management, prevention and vaccine development (8). We provide an overview of IEI that render individuals susceptible to S. aureus infections (Table 1 and Supplementary Table S1), highlighting key immunological defense mechanism involved in staphylococcal immunity.
IEI with low neutrophil numbers and susceptibility to S. aureus infections
Severe congenital neutropenia (SCN) is usually characterized by severe neutropenia (<500/µl) due to myeloid maturation arrest in the bone marrow. Over 20 different genes have been identified (21). Lack of mature neutrophils leads to a severe infectious phenotype with potentially life-threatening disease in the first months of life. Infections are caused not only by S. aureus but also by gram negative bacteria, and blood stream infections are common. Depending on the underlying gene defect there may be additional somatic features (Supplementary Table S1) (22).
Primary autoimmune neutropenia (AIN) of infancy, which is the most common type of neutropenia in childhood and may also present with nearly absent neutrophils and susceptibility to staphylococcal skin infections (abscesses, furunculosis), needs to be separated from SCN. AIN is typically detected in infancy, frequently as an incidental finding, and shows spontaneous remission in early childhood (23). Neutrophils mature normally in the bone marrow but peripheral numbers may be very low due to the presence of anti-neutrophilic antibodies. Infections are less severe compared to SCN. While the detection of anti-neutrophilic antibodies is suggestive of AIN it does not fully exclude additional SCN. Thus, in cases with severe infections or persistent neutropenia bone marrow evaluation and genetic testing may be indicated. If detected in older children or adults, AIN is more likely to be an immune phenomenon related to another IEI/autoimmune disorders requiring further diagnostic workup (24).
IEI with neutrophil function defects and susceptibility to S. aureus infections
Chronic granulomatous disease (CGD) represents the most common hereditary phagocyte dysfunction with an estimated prevalence of around 1:200,000 (25, 26). CGD leads to deficient reactive oxygen species (ROS) generation due to loss-of-function mutations affecting different aspects of the multicomponent enzyme NADPH oxidase in phagocytes (Nox2) (27). CGD patients experience severe infections accompanied by granuloma and abscess formation. Staphylococcus aureus is the most common pathogen isolated from skin infections/abscesses, liver abscesses and lymphadenitis, but it may also lead to pulmonary infections or sepsis. Patients are also very susceptible to Aspergillus spp. (26). Other characteristic pathogens in CGD include gram negative bacteria (e.g., Salmonella) and catalase positive bacteria (e.g., Burkholderia, Serratia and Nocardia) (12, 28). Additionally, CGD is associated with inflammatory complications like colitis, which might be related to defective T-cell regulation but also hyperactivation of NF-kB and inflammasome pathways (27, 29).
Leukocyte adhesion deficiency (LAD) is characterized by functional defects in neutrophil adhesion, integrin activation or rolling, leading to an inability to migrate effectively to infection sites (30). This results in a striking discrepancy with lack of pus formation at infection sites despite significant leukocytosis with neutrophilia in the blood. LAD patients typically experience recurrent bacterial and fungal infections, delayed wound healing, and other associated features (31). Three different genetic defects affecting neutrophils are known. Associated features are omphalitis and gingivitis (LAD I), developmental impairment and short statue (LAD II), and bleeding tendency (LAD III) (30, 32).
Combined IEI which frequently cause neutropenia or neutrophil dysfunction
Neutropenia has also been described in certain combined immunodeficiencies. Typical examples are CD40Ligand (CD40l) and CD40 deficiency, which are characterized by abnormal serum immunoglobulin levels due to impaired interaction between CD40l on T cells and CD40 on antigen-presenting cells (33, 34). These conditions lead to both impaired cellular and humoral immunity, which results in a broad infection phenotype. Patients frequently present with opportunistic infections (e.g., pneumocystis jirovecii, cryptosporidium, aspergillus spp.) (35). IgM may be elevated concomitantly to low IgA and IgG, which lead to bacterial respiratory and gastrointestinal infections (33). Intermittent or permanent neutropenia might be related to deficient release of growth factors important for granulopoiesis due to impaired CD40-CD40l-interaction (36). Furthermore, functional defects in neutrophils have been described in CD40l deficiency (37).
Mutations in Ras-related C3 botulinum toxin substrate 2 (RAC2) are also typically affecting neutrophil function. RAC2 is an essential regulator of neutrophil chemotaxis and contributes to NADPH oxidase function (38). Autosomal-dominant (AD) RAC2 loss of function (LOF) mutations cause LAD-like disease with neutrophilia and functional neutrophil defects (e.g., deficient chemotaxis and ROS generation) (39). In contrast, AD RAC2 gain of function (GOF) mutations lead to (severe) combined immunodeficiencies with lymphopenia and low immunoglobulins, frequent neutropenia and functional neutrophil abnormalities (38, 40).
Neutropenia has also been reported in some patients with deficiency in phosphoglucomutase 3 (PGM3), a disorder of glycosylation which is currently classified as autosomal-recessive Hyper IgE syndrome (1). PGM3 deficiency presents with eczema, eosinophilia, elevated IgE, but may also display a CID/SCID phenotype, facial dysmorphism and neurocognitive impairment (41).
Patients with autosomal-recessive deficiency of dedicator of cytokines (DOCK) 8 display severe atopic dermatitis with S. aureus colonization and skin infections (DOCK8 deficiency). Osteomyelitis has also been reported (42). DOCK8 plays a crucial role in lymphocyte proliferation, migration of dendritic cells, and generation of long-term memory in B- and T cells, thus predisposing patients to a mostly severe phenotype regarding viral and mycobacterial infections (43). Dysfunction of regulatory T-cells together with S. aureus exposure have been suggested to drive severe eczema in DOCK8 deficiency (44) and DOCK8-deficient murine neutrophils were prone to undergo S. aureus-induced cell death (45). In addition, reduced signal transducer and activator of transcription 3 (STAT3) signaling and low T helper 17 (Th17) cells have also been reported (46).
IEI with staphylococcal susceptibility associated to defective cytokine signaling
Autosomal-dominant Hyper-IgE syndrome due to dominant-negative mutations in STAT3 (STAT3-HIES) is one of the key IEI associated with a specific susceptibility to S. aureus infections, particularly in the skin and lung (47). Recurrent “cold” abscesses with lacking systemic signs of infections are typical. STAT3 functions as a transcription factor downstream of the tyrosine kinases janus activated kinase (JAK)1, JAK2, and tyrosine kinase 2 (TYK2) and enables signal transduction through various cytokines, such as interleukin-6 (IL-6), IL-10, IL-11, IL-21, and IL-23 (48). STAT3 deficiency results in failure of Th17 cell differentiation (49). Th17 function has been shown to be pivotal in Candida defense (50), explaining the patients' predisposition to mucocutaneous candidiasis. Th17 cells aid epithelial cells to produce neutrophil-recruiting chemokines and antimicrobial factors such as ß-defensins, which may be relevant for staphylococcal defense (51). STAT3-deficient neutrophils display normal functions (52), but are prone to undergo S. aureus-induced cell death (53). Furthermore, STAT3-HIES patients display variable antibody responses and low numbers of memory B cells, which likely contributes to enhanced incidence of respiratory infections with H. influenzae and S. pneumoniae (52). STAT3 is ubiquitously expressed and multisystemic features are present. Thus, deficient epithelial STAT3 signaling may contribute to aberrant staphylococcal control by cytokine dysregulation and aberrant tissue remodeling (54, 55). STAT3 is involved in both pro- and anti-inflammatory signaling which complicates our understanding of single factors for the overall phenotype.
Autosomal-recessive ZNF341 deficiency leads to reduced cytokine signaling via STAT3 and resembles STAT3-HIES by displaying similar multisystemic features (e.g., bone fractures, retention of primary teeth, facial dysmorphism) but also staphylococcal infections (56).
IEI affecting single cytokines may teach us about their individual contribution. Lack of functional IL-6 cytokine family signaling reduces typical local inflammatory reaction, leads to low CRP and reduced systemic symptoms although tissue damage may be considerable. Defective IL-6 signaling either by IL-6 receptor deficiency (57) or by partial IL-6 signal transducer deficiency (IL6ST) (58) also leads to pyogenic infections, cold abscesses and pulmonary S. aureus infections. Additionally, phenocopies of IEI such as autoantibodies against IL-6 show increased susceptibility to S. aureus infection lacking CRP response (59). Staphylococcus aureus infections are also described in ERBIN deficiency which recapitulates some features of STAT3 deficiency (60).
Frequent S. aureus skin infections have also been reported in patients with STAT1GOF who are very susceptible to fungal infections, have low Th17 cells, and display a high rate of autoimmune features (61, 62).
IEI with defects in toll-like receptor (TLR)-signaling and susceptibility to S. aureus
Autosomal-recessive IRAK-4 and MyD88 deficiencies affect TLR and IL-1R induced activation of NF-κB and MAPKs through the classical pathway (63). They disrupt key pathways in the innate immune response and usually present with bacterial pyogenic infections early in life (<2years of age). Most common pathogens are S. pneumoniae, S. aureus and Pseudomonas aeruginosa (64). Lack of TLR-induced signaling affects particularly the production of IL-6 and IL-8, and may lead to severe invasive infections (e.g., meningitis, sepsis, osteomyelitis, arthritis and abscesses), but also localized skin infections, lymphadenitis and ENT infections, usually without marked fever or increase of CRP (64). Still, pus is seen at the site of infection, which underlines that pus formation is not dependent on TLR-related cytokine signaling. As signs of infections may be absent but invasive infection may be rapidly progressing, it is vital to initiate empirical antibiotic treatment as soon as infection is suspected (64).
NEMO deficiency and IκBα GOF, which affect both NF-κB and TRIF-dependent signaling, result in a broad spectrum of immune dysfunctions and present also typically with colitis and ectodermal dysplasia. Apart from pyogenic bacterial infections, patients may also display mycobacterial infections, severe viral infections and opportunistic infections (64). Recently, more rare genetic defects associated to TLR-signaling have been reported, with variable phenotype depending on the protein involved.
Other diseases with susceptibility to S. aureus
Apart from classical IEI, increased susceptibility to S. aureus infections has also been reported in diseases such as cystic fibrosis, HIV infection and/or diabetes mellitus (65–68). In addition to aberrant host immune response, susceptibility to S. aureus may also be enhanced by colonization of multi-resistant strains (MRSA) carrying specific virulence factors.
Discussion: controversies, current knowledge gaps and future perspectives
While the key role of innate immunity for staphylococcal defense is well-established, the contribution of adaptive immunity is less clear.
In regards to B-cell immunity, evidence for a protective role of S. aureus antibodies is scarce. In fact, it has lately been suggested that S. aureus may induce non-protective antibodies, which then interfere with protective immune responses (69) facilitating commensalism and recurrent infections. Furthermore, patients with antibody deficiency do not display a specifically enhanced susceptibility to S. aureus, while they are clearly susceptible to other bacteria with a polysaccharide capsule (e.g., S. pneumoniae, H. influenzae). In contrast to the successful vaccine development for other encapsulated bacteria, there is still no available vaccine against S. aureus, and even adequate antibody induction to relevant S. aureus virulence factors did not lead to protection (70). The ability of anti-TSST-1 antibodies to provide protective immunity against superantigen-driven toxic shock syndrome appears to be an exception to the above, with IVIG being used as potential adjunctive therapy to ameliorate the symptoms (71).
Regarding the relevance of T cells, Th17 cells are often suggested to contribute to anti-staphylococcal-response, particularly at mucosa and skin sites (51). In mice, several studies document the importance of functional IL-17 signaling for the protection against mucocutaneous S. aureus infections (72, 73). Patients with IL-17RA deficiency are very prone to mucocutaneous candidiasis but do also display staphylococcal skin infections (74, 75). The initial hypothesis regarding the relevance of Th17 cells to prevent staphylococcal skin infection is closely related to the observed lack of Th17 in STAT3 deficiency (51). While the role of Th17 for candida defense is supported by other IEI with specifically deficient IL-17 signaling such as IL-17 autoantibodies (75), their relevance for S. aureus infections appears less significant. In the context of STAT3-HIES, the abundant changes in different cytokine signaling pathways and the contribution of ubiquitously deficient STAT3 needs to be considered. Of note, deficient IL-6 cytokine signaling is sufficient to predispose to staphylococcal infection even in the setting of normal Th17 cells (58, 76), and mere lack of Th17 cells does not induce susceptibility to S. aureus infection as evidenced in patients with IL12B/IL12RB1 deficiency (77) or CARD9 deficiency (78). Notably, STAT3-deficient patients with somatic mosaicism and normal Th17 compartment may still present with boils and pneumonia (79). Thus, lack of IL-17 signaling alone is likely insufficient in explaining enhanced susceptibility to S. aureus, even though patients may be more prone to folliculitis (74).
IEI with impairments in TLR and NF-κB signaling pathways such as in IRAK-4 or MyD88 deficiency, underline the significance of these pathways in recognizing and responding to S. aureus (80). Patients with STAT3-HIES, ZNF341 deficiency, partial IL6ST deficiency and IL-6 receptor deficiency all share deficient IL-6 signaling and enhanced frequency of “cold” staphylococcal abscesses and lung infections (1). IL-6 is a pleiotropic cytokine that is vital for acute-phase responses, defense against bacterial infections and tissue regeneration (81). The shared phenotype argues for an essential role of IL-6 in staphylococcal defense (82). Still, the precise molecular mechanism behind this particular predisposition and the contribution of other pathways is unknown.
Complement deficiencies might serve as additional risk factors in the context of S. aureus infections due to the crucial role of the complement system in opsonizing pathogens and facilitating their clearance by phagocytes. Susceptibility to S. aureus infections has been described in patients with C2 and C3 deficiencies (83) and complement activation was found to reduce persistent intracellular S. aureus burden in keratinocytes (84). Still, the role of complement in the defense against this pathogen appears less pronounced compared to its critical function in combating other encapsulated bacteria.
More recently, it has been proposed that specific genes may predispose to more severe infections via impairment of selective immune defense mechanism such as the altered response of non-leukocytic cells to staphylococcal alpha-toxin in OTULIN haploinsufficiency (85). With the growing use of NGS our understanding of specific factors in staphylococcal immunity will likely expand further. Still, the rareness of single IEI may hamper reliability of certain genotype-phenotype associations. An example is TYK2 deficiency, where the originally identified patient with susceptibility to S. aureus and hyper-IgE phenotype (86) was later judged to display deficient IL-6 signaling unrelated to TYK2 deficiency (87).
Last, the ability of S. aureus to survive intracellularly, notably within neutrophils, macrophages and as small colony variants in epithelial cells, complicates the immune response and treatment strategies and might facilitate recurrent infections (88). Together with the multiple other evasion strategies this poses significant challenges in vaccine development against S. aureus. In the light of growing rates of MRSA, it therefore remains essential to continue to assess host-pathogen interactions on a functional level and further enhance our understanding about crucial immune defense mechanisms.
Conclusion and diagnostic suggestions
➢ Basic immunological workup in patients with recurrent or severe S. aureus infections should include a differential blood count and IgG, IgA, IgM, IgE
➢ Specific testing for CGD, HIES, complement deficiency, LAD, TLR deficiency, exclusion of secondary immunodeficiencies and assessment for phenocopies of IEI as well as genetic analysis may be warranted
➢ Inconclusive immunological investigation should be complemented by assessment of staphylococcal colonization
Author contributions
HK: Visualization, Writing – original draft, Writing – review & editing. KL: Writing – review & editing, Funding acquisition, Resources. SF: Writing – review & editing, Conceptualization, Supervision, Visualization, Writing – original draft, Methodology.
Funding
The author(s) declare financial support was received for the research and/or publication of this article.
This study was supported by the University Medical Center Hamburg-Eppendorf.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1389650/full#supplementary-material
Abbreviations
AD, autosomal-dominant; AIN, autoimmune neutropenia; AR, autosomal-recessive; C2, C3, complement component 2, complement component 3; CARD9, caspase recruitment domain family member 9; CD40, cluster of differentiation 40; CD40l, cluster of differentiation 40 ligand; CGD, chronic granulomatous disease; CID, combined immunodeficiency; CRP, C-reactive protein; DOCK, dedicator of cytokinesis; G-CSF, granulocyte colony-stimulating factor; GOF, gain of function; HAX1, HCLS1 associated protein X-1; HIES, hyper IgE syndrome; HIV, human immunodeficiency virus; HSCT, hematopoietic stem cell transplantation; IEI, inborn errors of immunity; Ig, immunoglobulin; IL, interleukin; IL-17RA, IL-17 receptor A; IL12B, IL12RB1, IL-12 subunit beta, IL-12 receptor beta 1; IL6ST, IL-6 signal transducer; IRAK-4, IL-1 receptor-associated kinase 4; IVIG, intravenous immunoglobulin; JAK, Janus kinase; LAD, leukocyte adhesion deficiency; LOF, loss of function; MAPK, mitogen-activated protein kinase; MRSA, methicillin-resistant Staphylococcus aureus; MyD88, myeloid differentiation primary response 88; NADPH, nicotinamide adenine dinucleotide phosphate; NEMO, NF-κB essential modulator; NF-kB, nuclear factor kappa B; NGS, next generation sequencing; Nox2, NADPH oxidase 2; OTULIN, OTU deubiquitinase with linear linkage specificity; PGM, phosphoglucomutase; PVL, panton-valentine leucocidin; RAC2, ras-related C3 botulinum toxin substrate 2; ROS, reactive oxygen species; S. aureus, Staphylococcus aureus; SCID, severe combined immunodeficiency; SCN, severe congenital neutropenia; SpA, staphylococcal protein A; spp, species pluralis; STAT3, signal transducer and activator of transcription 3; STAT1, signal transducer and activator of transcription 1; Th17, T helper 17; TLR, toll-like receptor; TMP/SMX, trimethoprim/sulfamethoxazole; TRIF, TIR-domain-containing adapter-inducing interferon-β; TSST-1, toxic shock syndrome toxin-1; TYK2, tyrosine kinase 2; ZNF341, zinc finger protein 341.
References
1. Tangye SG, Al-Herz W, Bousfiha A, Cunningham-Rundles C, Franco JL, Holland SM, et al. Human inborn errors of immunity: 2022 update on the classification from the international union of immunological societies expert committee. J Clin Immunol. (2022) 42(7):1473–507. doi: 10.1007/s10875-022-01289-3
2. Tong SY, Davis JS, Eichenberger E, Holland TL, Fowler VG Jr. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. (2015) 28(3):603–61. doi: 10.1128/CMR.00134-14
3. Wertheim HF, Melles DC, Vos MC, van Leeuwen W, van Belkum A, Verbrugh HA, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. (2005) 5(12):751–62. doi: 10.1016/S1473-3099(05)70295-4
4. Kwiecinski JM, Horswill AR. Staphylococcus aureus bloodstream infections: pathogenesis and regulatory mechanisms. Curr Opin Microbiol. (2020) 53:51–60. doi: 10.1016/j.mib.2020.02.005
5. Thammavongsa V, Kim HK, Missiakas D, Schneewind O. Staphylococcal manipulation of host immune responses. Nat Rev Microbiol. (2015) 13(9):529–43. doi: 10.1038/nrmicro3521
6. David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. (2010) 23(3):616–87. doi: 10.1128/CMR.00081-09
7. Cheung GYC, Bae JS, Otto M. Pathogenicity and virulence of Staphylococcus aureus. Virulence. (2021) 12(1):547–69. doi: 10.1080/21505594.2021.1878688
8. Wójcik-Bojek U, Różalska B, Sadowska B. Staphylococcus aureus-a known opponent against host defense mechanisms and vaccine development-do we still have a chance to win? Int J Mol Sci. (2022) 23(2). doi: 10.3390/ijms23020948
9. de Jong NWM, van Kessel KPM, van Strijp JAG. Immune evasion by Staphylococcus aureus. Microbiol Spectr. (2019) 7(2).30927347
10. Loh JM, Aghababa H, Proft T. Eluding the immune system’s frontline defense: secreted complement evasion factors of pathogenic gram-positive cocci. Microbiol Res. (2023) 277:127512. doi: 10.1016/j.micres.2023.127512
11. Miller LS, Cho JS. Immunity against Staphylococcus aureus cutaneous infections. Nat Rev Immunol. (2011) 11(8):505–18. doi: 10.1038/nri3010
12. Marciano BE, Spalding C, Fitzgerald A, Mann D, Brown T, Osgood S, et al. Common severe infections in chronic granulomatous disease. Clin Infect Dis. (2014) 60(8):1176–83. doi: 10.1093/cid/ciu1154
13. van den Berg JM, Kuijpers TW. Educational paper: defects in number and function of neutrophilic granulocytes causing primary immunodeficiency. Eur J Pediatr. (2011) 170(11):1369–76. doi: 10.1007/s00431-011-1584-5
14. Berends ET, Horswill AR, Haste NM, Monestier M, Nizet V, von Köckritz-Blickwede M. Nuclease expression by Staphylococcus aureus facilitates escape from neutrophil extracellular traps. J Innate Immun. (2010) 2(6):576–86. doi: 10.1159/000319909
15. Löffler B, Tuchscherr L, Niemann S, Peters G. Staphylococcus aureus persistence in non-professional phagocytes. Int J Med Microbiol. (2014) 304(2):170–6. doi: 10.1016/j.ijmm.2013.11.011
16. Li Z, Peres AG, Damian AC, Madrenas J. Immunomodulation and disease tolerance to Staphylococcus aureus. Pathogens. (2015) 4(4):793–815. doi: 10.3390/pathogens4040793
17. Missiakas D, Winstel V. Selective host cell death by Staphylococcus aureus: a strategy for bacterial persistence. Front Immunol. (2020) 11:621733. doi: 10.3389/fimmu.2020.621733
18. Rao Q, Shang W, Hu X, Rao X. Staphylococcus aureus ST121: a globally disseminated hypervirulent clone. J Med Microbiol. (2015) 64(12):1462–73. doi: 10.1099/jmm.0.000185
19. Kobayashi SD, DeLeo FR. Staphylococcus aureus protein A promotes immune suppression. mBio. (2013) 4(5). doi: 10.1128/mbio.00764-13
20. Miethke T, Duschek K, Wahl C, Heeg K, Wagner H. Pathogenesis of the toxic shock syndrome: t cell mediated lethal shock caused by the superantigen TSST-1. Eur J Immunol. (1993) 23(7):1494–500. doi: 10.1002/eji.1830230715
21. Skokowa J, Dale DC, Touw IP, Zeidler C, Welte K. Severe congenital neutropenias. Nat Rev Dis Primers. (2017) 3:17032. doi: 10.1038/nrdp.2017.32
22. Han X, Lu S, Gu C, Bian Z, Xie X, Qiao X. Clinical features, epidemiology, and treatment of Shwachman-Diamond syndrome: a systematic review. BMC Pediatr. (2023) 23(1):503. doi: 10.1186/s12887-023-04324-3
23. Bux J, Behrens G, Jaeger G, Welte K. Diagnosis and clinical course of autoimmune neutropenia in infancy: analysis of 240 cases. Blood. (1998) 91(1):181–6. doi: 10.1182/blood.V91.1.181
24. Farruggia P, Dufour C. Diagnosis and management of primary autoimmune neutropenia in children: insights for clinicians. Ther Adv Hematol. (2015) 6(1):15–24. doi: 10.1177/2040620714556642
25. Buvelot H, Posfay-Barbe KM, Linder P, Schrenzel J, Krause KH. Staphylococcus aureus, phagocyte NADPH oxidase and chronic granulomatous disease. FEMS Microbiol Rev. (2017) 41(2):139–57.27965320
26. van den Berg JM, van Koppen E, Ahlin A, Belohradsky BH, Bernatowska E, Corbeel L, et al. Chronic granulomatous disease: the European experience. PLoS One. (2009) 4(4):e5234. doi: 10.1371/journal.pone.0005234
27. Roos D. Chronic granulomatous disease. Br Med Bull. (2016) 118(1):50–63. doi: 10.1093/bmb/ldw009
28. Slack MA, Thomsen IP. Prevention of infectious complications in patients with chronic granulomatous disease. J Pediatric Infect Dis Soc. (2018) 7(suppl_1):S25–30. doi: 10.1093/jpids/piy016
29. Meissner F, Seger RA, Moshous D, Fischer A, Reichenbach J, Zychlinsky A. Inflammasome activation in NADPH oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood. (2010) 116(9):1570–3. doi: 10.1182/blood-2010-01-264218
30. Hanna S, Etzioni A. Leukocyte adhesion deficiencies. Ann N Y Acad Sci. (2012) 1250:50–5. doi: 10.1111/j.1749-6632.2011.06389.x
31. Roos D, van Leeuwen K, Madkaikar M, Kambli PM, Gupta M, Mathews V, et al. Hematologically important mutations: leukocyte adhesion deficiency (second update). Blood Cells Mol Dis. (2023) 99:102726. doi: 10.1016/j.bcmd.2023.102726
32. Gazit Y, Mory A, Etzioni A, Frydman M, Scheuerman O, Gershoni-Baruch R, et al. Leukocyte adhesion deficiency type II: long-term follow-up and review of the literature. J Clin Immunol. (2010) 30(2):308–13. doi: 10.1007/s10875-009-9354-0
33. Banday AZ, Nisar R, Patra PK, Kaur A, Sadanand R, Chaudhry C, et al. Clinical and immunological features, genetic variants, and outcomes of patients with CD40 deficiency. J Clin Immunol. (2023) 44(1):17. doi: 10.1007/s10875-023-01633-1
34. Ferrari S, Giliani S, Insalaco A, Al-Ghonaium A, Soresina AR, Loubser M, et al. Mutations of CD40 gene cause an autosomal recessive form of immunodeficiency with hyper IgM. Proc Natl Acad Sci U S A. (2001) 98(22):12614–9. doi: 10.1073/pnas.221456898
35. Ferrua F, Galimberti S, Courteille V, Slatter MA, Booth C, Moshous D, et al. Hematopoietic stem cell transplantation for CD40 ligand deficiency: results from an EBMT/ESID-IEWP-SCETIDE-PIDTC study. J Allergy Clin Immunol. (2019) 143(6):2238–53. doi: 10.1016/j.jaci.2018.12.1010
36. Mavroudi I, Papadaki HA. The role of CD40/CD40 ligand interactions in bone marrow granulopoiesis. ScientificWorldJournal. (2011) 11:2011–9. doi: 10.1100/2011/671453
37. Cabral-Marques O, França TT, Al-Sbiei A, Schimke LF, Khan TA, Feriotti C, et al. CD40 ligand deficiency causes functional defects of peripheral neutrophils that are improved by exogenous IFN-γ. J Allergy Clin Immunol. (2018) 142(5):1571–88.9. doi: 10.1016/j.jaci.2018.02.026
38. Hsu AP, Donkó A, Arrington ME, Swamydas M, Fink D, Das A, et al. Dominant activating RAC2 mutation with lymphopenia, immunodeficiency, and cytoskeletal defects. Blood. (2019) 133(18):1977–88. doi: 10.1182/blood-2018-11-886028
39. Ambruso DR, Knall C, Abell AN, Panepinto J, Kurkchubasche A, Thurman G, et al. Human neutrophil immunodeficiency syndrome is associated with an inhibitory Rac2 mutation. Proc Natl Acad Sci U S A. (2000) 97(9):4654–9. doi: 10.1073/pnas.080074897
40. Donkó A, Sharapova SO, Kabat J, Ganesan S, Hauck F, Marois L, et al. Clinical and functional spectrum of RAC2-related immunodeficiency. Blood. (2024).
41. Fallahi M, Jamee M, Enayat J, Abdollahimajd F, Mesdaghi M, Khoddami M, et al. Novel PGM3 mutation in two siblings with combined immunodeficiency and childhood bullous pemphigoid: a case report and review of the literature. Allergy Asthma Clin Immunol. (2022) 18(1):111. doi: 10.1186/s13223-022-00749-0
42. Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. (2009) 361(21):2046–55. doi: 10.1056/NEJMoa0905506
43. Engelhardt KR, McGhee S, Winkler S, Sassi A, Woellner C, Lopez-Herrera G, et al. Large deletions and point mutations involving the dedicator of cytokinesis 8 (DOCK8) in the autosomal-recessive form of hyper-IgE syndrome. J Allergy Clin Immunol. (2009) 124(6):1289–302.4. doi: 10.1016/j.jaci.2009.10.038
44. Wilkie H, Das M, Pelovitz T, Bainter W, Woods B, Alasharee M, et al. Regulatory T-cell dysfunction and cutaneous exposure to Staphylococcus aureus underlie eczema in DOCK8 deficiency. J Allergy Clin Immunol. (2024).38185418
45. Wilkie H, Timilshina M, Rahmayanti S, Das M, Pelovitz T, Geha RS. DOCK8 is essential for neutrophil mediated clearance of cutaneous S. aureus infection. Clin Immunol. (2023) 254:109681. doi: 10.1016/j.clim.2023.109681
46. Keles S, Charbonnier LM, Kabaleeswaran V, Reisli I, Genel F, Gulez N, et al. Dedicator of cytokinesis 8 regulates signal transducer and activator of transcription 3 activation and promotes T(H)17 cell differentiation. J Allergy Clin Immunol. (2016) 138(5):1384–94.2. doi: 10.1016/j.jaci.2016.04.023
47. Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. (2007) 357(16):1608–19. doi: 10.1056/NEJMoa073687
48. Farmand S, Sundin M. Hyper-IgE syndromes: recent advances in pathogenesis, diagnostics and clinical care. Curr Opin Hematol. (2015) 22(1):12–22. doi: 10.1097/MOH.0000000000000104
49. Milner JD, Brenchley JM, Laurence A, Freeman AF, Hill BJ, Elias KM, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. (2008) 452(7188):773–6. doi: 10.1038/nature06764
50. Puel A, Cypowyj S, Maródi L, Abel L, Picard C, Casanova JL. Inborn errors of human IL-17 immunity underlie chronic mucocutaneous candidiasis. Curr Opin Allergy Clin Immunol. (2012) 12(6):616–22. doi: 10.1097/ACI.0b013e328358cc0b
51. Minegishi Y, Saito M, Nagasawa M, Takada H, Hara T, Tsuchiya S, et al. Molecular explanation for the contradiction between systemic Th17 defect and localized bacterial infection in hyper-IgE syndrome. J Exp Med. (2009) 206(6):1291–301. doi: 10.1084/jem.20082767
52. Chandesris MO, Melki I, Natividad A, Puel A, Fieschi C, Yun L, et al. Autosomal dominant STAT3 deficiency and hyper-IgE syndrome: molecular, cellular, and clinical features from a French national survey. Medicine (Baltimore). (2012) 91(4):e1–19. doi: 10.1097/MD.0b013e31825f95b9
53. Farmand S, Kremer B, Häffner M, Pütsep K, Bergman P, Sundin M, et al. Eosinophilia and reduced STAT3 signaling affect neutrophil cell death in autosomal-dominant hyper-IgE syndrome. Eur J Immunol. (2018) 48(12):1975–88. doi: 10.1002/eji.201847650
54. Zhang Y, Lin T, Leung HM, Zhang C, Wilson-Mifsud B, Feldman MB, et al. STAT3 mutation-associated airway epithelial defects in job syndrome. J Allergy Clin Immunol. (2023) 152(2):538–50. doi: 10.1016/j.jaci.2022.12.821
55. Myles IA, Anderson ED, Earland NJ, Zarember KA, Sastalla I, Williams KW, et al. TNF overproduction impairs epithelial staphylococcal response in hyper IgE syndrome. J Clin Invest. (2018) 128(8):3595–604. doi: 10.1172/JCI121486
56. Béziat V, Li J, Lin JX, Ma CS, Li P, Bousfiha A, et al. A recessive form of hyper-IgE syndrome by disruption of ZNF341-dependent STAT3 transcription and activity. Sci Immunol. (2018) 3(24). doi: 10.1126/sciimmunol.aat4956
57. Spencer S, Köstel Bal S, Egner W, Lango Allen H, Raza SI, Ma CA, et al. Loss of the interleukin-6 receptor causes immunodeficiency, atopy, and abnormal inflammatory responses. J Exp Med. (2019) 216(9):1986–98. doi: 10.1084/jem.20190344
58. Béziat V, Tavernier SJ, Chen YH, Ma CS, Materna M, Laurence A, et al. Dominant-negative mutations in human IL6ST underlie hyper-IgE syndrome. J Exp Med. (2020) 217(6). doi: 10.1084/jem.20191804
59. Puel A, Picard C, Lorrot M, Pons C, Chrabieh M, Lorenzo L, et al. Recurrent staphylococcal cellulitis and subcutaneous abscesses in a child with autoantibodies against IL-6. J Immunol. (2008) 180(1):647–54. doi: 10.4049/jimmunol.180.1.647
60. Lyons JJ, Liu Y, Ma CA, Yu X, O’Connell MP, Lawrence MG, et al. ERBIN deficiency links STAT3 and TGF-β pathway defects with atopy in humans. J Exp Med. (2017) 214(3):669–80. doi: 10.1084/jem.20161435
61. Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. (2016) 127(25):3154–64. doi: 10.1182/blood-2015-11-679902
62. Liu L, Okada S, Kong XF, Kreins AY, Cypowyj S, Abhyankar A, et al. Gain-of-function human STAT1 mutations impair IL-17 immunity and underlie chronic mucocutaneous candidiasis. J Exp Med. (2011) 208(8):1635–48. doi: 10.1084/jem.20110958
63. Picard C, von Bernuth H, Ghandil P, Chrabieh M, Levy O, Arkwright PD, et al. Clinical features and outcome of patients with IRAK-4 and MyD88 deficiency. Medicine (Baltimore). (2010) 89(6):403–25. doi: 10.1097/MD.0b013e3181fd8ec3
64. Picard C, Casanova JL, Puel A. Infectious diseases in patients with IRAK-4, MyD88, NEMO, or IκBα deficiency. Clin Microbiol Rev. (2011) 24(3):490–7. doi: 10.1128/CMR.00001-11
65. Blanchard AC, Waters VJ. Microbiology of cystic fibrosis airway disease. Semin Respir Crit Care Med. (2019) 40(6):727–36. doi: 10.1055/s-0039-1698464
66. Dde C F, Silva GR, Cavalcante FS, Carmo FL, Fernandes LA, Moreira S, et al. Methicillin-resistant Staphylococcus aureus in HIV patients: risk factors associated with colonization and/or infection and methods for characterization of isolates—a systematic review. Clinics (Sao Paulo). (2014) 69(11):770–6. doi: 10.6061/clinics/2014(11)11
67. Crum-Cianflone NF, Burgi AA, Hale BR. Increasing rates of community-acquired methicillin-resistant Staphylococcus aureus infections among HIV-infected persons. Int J STD AIDS. (2007) 18(8):521–6. doi: 10.1258/095646207781439702
68. Shahrokh S, Tabatabaee A, Yazdi M, Siavash M. Proportion of toxin and non-toxin virulence factors of Staphylococcus aureus isolates from diabetic foot infection: a systematic review and meta-analysis. BMC Microbiol. (2024) 24(1):1. doi: 10.1186/s12866-023-03142-y
69. Tsai CM, Caldera JR, Hajam IA, Chiang AWT, Tsai CH, Li H, et al. Non-protective immune imprint underlies failure of Staphylococcus aureus IsdB vaccine. Cell Host Microbe. (2022) 30(8):1163–72.6. doi: 10.1016/j.chom.2022.06.006
70. Hassanzadeh H, Baber J, Begier E, Noriega DC, Konishi H, Yato Y, et al. Efficacy of a 4-antigen Staphylococcus aureus vaccine in spinal surgery: the STaphylococcus aureus suRgical inpatient vaccine efficacy (STRIVE) randomized clinical trial. Clin Infect Dis. (2023) 77(2):312–20. doi: 10.1093/cid/ciad218
71. Yanagisawa C, Hanaki H, Natae T, Sunakawa K. Neutralization of staphylococcal exotoxins in vitro by human-origin intravenous immunoglobulin. J Infect Chemother. (2007) 13(6):368–72. doi: 10.1007/s10156-007-0551-6
72. Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, et al. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. (2009) 30(1):108–19. doi: 10.1016/j.immuni.2008.11.009
73. Cho JS, Pietras EM, Garcia NC, Ramos RI, Farzam DM, Monroe HR, et al. IL-17 is essential for host defense against cutaneous Staphylococcus aureus infection in mice. J Clin Invest. (2010) 120(5):1762–73. doi: 10.1172/JCI40891
74. Lévy R, Okada S, Béziat V, Moriya K, Liu C, Chai LY, et al. Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency. Proc Natl Acad Sci U S A. (2016) 113(51):E8277–85. doi: 10.1073/pnas.1618300114
75. Puel A, Cypowyj S, Bustamante J, Wright JF, Liu L, Lim HK, et al. Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science. (2011) 332(6025):65–8. doi: 10.1126/science.1200439
76. Schwerd T, Twigg SRF, Aschenbrenner D, Manrique S, Miller KA, Taylor IB, et al. A biallelic mutation in IL6ST encoding the GP130 co-receptor causes immunodeficiency and craniosynostosis. J Exp Med. (2017) 214(9):2547–62. doi: 10.1084/jem.20161810
77. de Beaucoudrey L, Puel A, Oe F-S, Al C, Ghandil P, Chrabieh M, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17–producing T cells. J Exp Med. (2008) 205(7):1543–50. doi: 10.1084/jem.20080321
78. Corvilain E, Casanova JL, Puel A. Inherited CARD9 deficiency: invasive disease caused by ascomycete fungi in previously healthy children and adults. J Clin Immunol. (2018) 38(6):656–93. doi: 10.1007/s10875-018-0539-2
79. Hsu AP, Sowerwine KJ, Lawrence MG, Davis J, Henderson CJ, Zarember KA, et al. Intermediate phenotypes in patients with autosomal dominant hyper-IgE syndrome caused by somatic mosaicism. J Allergy Clin Immunol. (2013) 131(6):1586–93. doi: 10.1016/j.jaci.2013.02.038
80. Bucciol G, Moens L, Bosch B, Bossuyt X, Casanova JL, Puel A, et al. Lessons learned from the study of human inborn errors of innate immunity. J Allergy Clin Immunol. (2019) 143(2):507–27. doi: 10.1016/j.jaci.2018.07.013
81. Rose-John S, Winthrop K, Calabrese L. The role of IL-6 in host defence against infections: immunobiology and clinical implications. Nat Rev Rheumatol. (2017) 13(7):399–409. doi: 10.1038/nrrheum.2017.83
82. Puel A, Casanova JL. The nature of human IL-6. J Exp Med. (2019) 216(9):1969–71. doi: 10.1084/jem.20191002
83. Ram S, Lewis LA, Rice PA. Infections of people with complement deficiencies and patients who have undergone splenectomy. Clin Microbiol Rev. (2010) 23(4):740–80. doi: 10.1128/CMR.00048-09
84. Abu-Humaidan AH, Elven M, Sonesson A, Garred P, Sorensen OE. Persistent intracellular Staphylococcus aureus in keratinocytes lead to activation of the complement system with subsequent reduction in the intracellular bacterial load. Front Immunol. (2018) 9:396. doi: 10.3389/fimmu.2018.00396
85. Spaan AN, Neehus AL, Laplantine E, Staels F, Ogishi M, Seeleuthner Y, et al. Human OTULIN haploinsufficiency impairs cell-intrinsic immunity to staphylococcal α-toxin. Science. (2022) 376(6599):eabm6380. doi: 10.1126/science.abm6380
86. Minegishi Y, Saito M, Morio T, Watanabe K, Agematsu K, Tsuchiya S, et al. Human tyrosine kinase 2 deficiency reveals its requisite roles in multiple cytokine signals involved in innate and acquired immunity. Immunity. (2006) 25(5):745–55. doi: 10.1016/j.immuni.2006.09.009
87. Kreins AY, Ciancanelli MJ, Okada S, Kong XF, Ramírez-Alejo N, Kilic SS, et al. Human TYK2 deficiency: mycobacterial and viral infections without hyper-IgE syndrome. J Exp Med. (2015) 212(10):1641–62. doi: 10.1084/jem.20140280
Keywords: S. aureus, inborn errors of immunity (IEI), immunodeficiency, STAT3 deficiency, neutrophil dysfunction, chronic granulomatous disease (CGD), neutropenia, IL-6 deficiency
Citation: Kurz H, Lehmberg K and Farmand S (2024) Inborn errors of immunity with susceptibility to S. aureus infections. Front. Pediatr. 12:1389650. doi: 10.3389/fped.2024.1389650
Received: 21 February 2024; Accepted: 25 March 2024;
Published: 24 April 2024.
Edited by:
Sara Sebnem Kilic, Bursa Uludağ University, TürkiyeReviewed by:
Lina Maria Castano-Jaramillo, Fundación Hospital Pediátrico la Misericordia, Colombia© 2024 Kurz, Lehmberg and Farmand. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Susan Farmand cy5mYXJtYW5kQHVrZS5kZQ==
 Hannah Kurz1
Hannah Kurz1 Susan Farmand
Susan Farmand