- 1Mother and Child Medicine Department, “Grigore T. Popa” University of Medicine and Pharmacy, Iasi, Romania
- 2Faculty of Pharmacy, “Grigore T. Popa” University of Medicine and Pharmacy, Iasi, Romania
- 3Faculty of Medical Bioengineering, “Grigore T. Popa” University of Medicine and Pharmacy, Iasi, Romania
Celiac disease, firstly described in children, is a type of T-cell enteropathy that occurs in individuals genetically predisposed to gluten exposure. The estimated global prevalence of celiac disease is continuously increasing. Although, traditionally, celiac disease was diagnosed in children with failure to thrive and digestive issues, it is now recognized that may present with a wide range of symptoms beyond gastrointestinal ones. Celiac disease continues to pose significant challenges due to the continuous advancement of knowledge in understanding its pathophysiology, diagnosing the condition, managing its effects, and exploring potential therapeutic approaches. The prevalence of celiac disease is increased among individuals with chronic kidney disease, also. The most frequent associations are with diabetic nephropathy, IgA nephropathy and urolithiasis. A gut-kidney axis has been recognized to play a significant role in chronic kidney diseases. This literature review aims to review the chronic renal pathology associated with celiac disease, with emphasis on childhood.
1 Introduction
Celiac disease is an autoimmune condition that affects the entire body, causing disruptions in the internal balance and resulting in specific changes in the tissue structure of the small intestine. These changes include significant villi damage with increased crypt formation. Clinically, the disease presents a diverse range of symptoms, spanning from gastrointestinal issues like diarrhea, bloating, weight loss, and abdominal discomfort to non-digestive symptoms such as iron deficiency anemia, delayed puberty, and oral ulcers. These symptoms collectively stem from varying levels of malabsorption (1, 2).
Celiac disease is correlated with a number of autoimmune disorders, including type 1 diabetes and thyroid disease, which are classified as “associated conditions” or conditions that exhibit an elevated incidence but are not directly attributable to gluten consumption (2).
CD is a type of T-cell enteropathy characterized by an abnormal immune response to gluten, a protein found in wheat, barley, and rye. The estimated global prevalence of CD ranges from 0.7%–1.4% (3). CD can affect individuals of all ages and may present with a wide range of symptoms beyond just gastrointestinal problems (4).
CD continues to pose significant challenges due to the continuous advancement of knowledge in understanding its pathophysiology, diagnosing the condition, managing its effects, and exploring potential therapeutic approaches. As awareness of CD has increased, healthcare professionals have become better at identifying atypical and asymptomatic cases. Thus, more adults are now being diagnosed with CD, indicating that the disease's clinical spectrum is broader than previously understood (5).
The identification of tissue transglutaminase as an immunological target has offered a reliable first-line diagnostic test for CD (6). The introduction of screening for CD has enabled the diagnosis of certain cases even in the absence of typical digestive symptoms (7). According to recent studies (8, 9), the occurrence of CD is twice as common in children compared to adults, and 1.5 times higher to women than men. Although small intestinal biopsy remains the “gold standard” in the diagnosis of CD, current serological tests, such as tissue transglutaminase, endomysial gliadin and de-amidated peptide antibodies, have become more important in the diagnosis of the disease (5). The long-term effects of using this new no-biopsy approach on adherence to a gluten-free diet have yet to be evaluated (1, 10).
Currently, the only treatment for CD remains the strict gluten-free diet throughout life, which leads to improved quality of life, relief of symptoms and prevention of reducible complications, such as intestinal lymphoma and intestinal adenocarcinoma (11, 12). CD also presents a variety of extraintestinal manifestations, which affect the skin, liver or joints, nervous system or kidney (13, 14).
The prevalence of CD is increased among individuals with chronic kidney disease (CKD), as evidenced by current studies (15). Also, an increased risk of end-stage renal disease (ESRD) has been associated with CD (16). The most frequent associations are with diabetic nephropathy, IgA nephropathy and reno-urinary lithiasis (17, 18).
A gut-kidney axis has been recognized to play a significant role in chronic kidney diseases. Disruption of the gut barrier can lead to the abnormal entry of bacterial lipo-polysaccharide (LPS) endotoxin into the circulation. This abnormal entry contributes to uremic toxicity and systemic inflammation, which are factors implicated in the development and progression of chronic kidney diseases (19, 10).
This literature review aims to review the chronic renal involvement associated with celiac disease, with emphasis on childhood.
2 Material and methods
The scientific literature was browsed using Scopus, PubMed, and Embase da-ta-bases by searching the following keywords: “celiac disease,” “chronic kidney disease,” “T-cell enteropathy,” and “children,” in various combinations. The search was limited to open-access articles published in English within the last 10 years. Studies that mostly focused on creating a full definition of celiac disease in children were included. These studies were especially interested in the extradigestive symptoms of the disease, with a focus on kidney involvement. Articles that were vague and did not focus on specific aspects of the subject were excluded from the review process, but they were still used for general informational purposes.
3 Diabetic kidney disease and celiac disease in children
Diabetic kidney disease (DKD) has been observed concurrently with the increased incidence of diabetes mellitus (DM) in children. The kidney disease associated with DM in children and adolescents is represented by persistent albuminuria, arterial hypertension, the progressive decrease in the glomerular filtration rate, with the possibility of evolution towards the final stage (20). The association of type 1 diabetes (T1DM) with CD is known, and its prevalence in children varies from 3%–12% (20). A significant study was developed in Sweden, where patients with T1DM are routinely screened for CD. The risk of kidney disease in patients with type 1 diabetes mellitus and coexisting CD was evaluated in a cohort of 954 patients with both T1DM and CD, comparing the prevalence of CKD with that of patients diagnosed with T1DM alone (21). The authors found similar percentages of patients who developed chronic kidney disease in both groups.
Previous studies discussing the incidence of CKD in patients with T1DM and CD are limited to the adults and report conflicting results. Gopee et al., showed that the gluten-free regimen in children with CD + T1DM has a renoprotective effect, by decreasing albuminuria, compared to those who only have T1DM (22). The same HLA predisposition to CD influences the development and progression of albuminuria (23).
The Genetics of Kidneys in Diabetes (GoKinD) study included 1,879 individuals with long-term evolution of T1DM, half of them with diabetic nephropathy. Involvement of DRB1 is consistent with the known immunological processes in the pathogenesis of diabetic nephropathy, especially in the Late Autoimmune Diabetes of Adults, less in children. There is an inverse and separate relationship between the risk of CD and the age at which diabetes begins, with a greater risk observed in children under 4 years old compared to those over 9 years old. The mechanism of association of these two diseases involves a shared genetic background: HLA genotype DR3-DQ2 and DR4-DQ8 (23, 24).
Another study investigating the risk of ESRD in CD patient showed that the effect of type 1 diabetes on the risk was only marginal (16). The review by Boonpheng et al. (18) demonstrated that CD can be independently related to kidney dysfunction, regardless of the development of type 1 diabetes.
CD is also an independent risk factor for microvascular complications related to diabetes, but also for macrovascular complications (25) especially an increased risk the intima-medial thickness of the carotid arteries (26). The established association between CD and microvascular complications in diabetes is another independent argument in favor of screening for CD in the patient with T1DM (27). However, the mechanism behind how CD increases the risk of microvascular disease in patients with T1DM remains unclear and is likely to involve multiple factors (28). One such factor is the association of unrecognized CD with elevated homocysteine levels, which may be attributed to deficiencies in folic acid and other B vitamins, contributing to endothelial dysfunction (29). A recent study indicated that supplementing vitamin B in individuals with CD was linked to significantly lower homocysteine levels, potentially reducing the risk of vascular disease (30).
4 IgA nephropathy and celiac disease
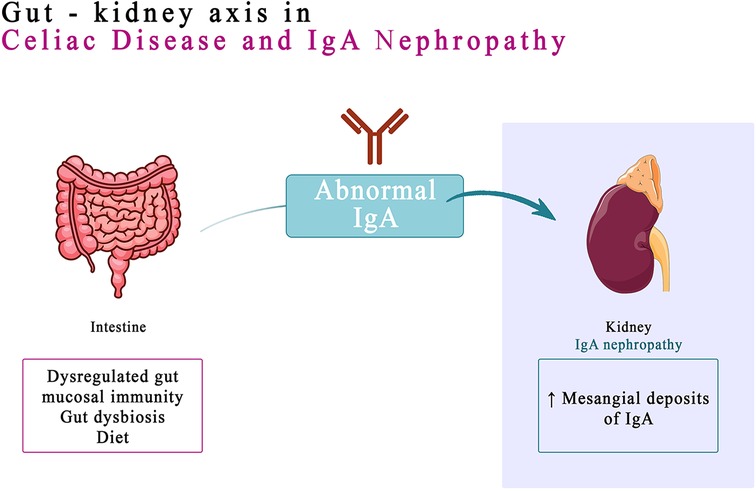
IgA nephropathy (IgAN), identified almost 60 years ago by Berger (31), is one of the most common glomerulonephritis reported worldwide (32). IgA antibodies are produced by B cells in the mucosal tissues, such as the lining of the gut and airways. When these antibodies are activated, they can form immune complexes that can deposit in the kidneys and damage the glomeruli (33). Immunoglobulin A, the hallmark of mesangial deposits of IgAN, is the main immunoglobulin in mucous secretions (34). Recent data suggest that the dysregulated gut mucosal immunity, genetic conditioning, gut dysbiosis, and diet play a combined role in development and progression of IgAN (35). People who have a genetic predisposition to IgA nephropathy in high-risk groups may be able to be prophylactically screened for the CD and closely monitored by immunohistochemical methods or identified by genetic testing. The prognosis for IgA nephropathy varies depending on the severity of the disease. However, with early diagnosis and treatment, most people with IgA nephropathy can live a normal life (36). The link between a dysregulated gut-associated lymphoid tissue (GALT) and IgAN was hypothesized following the observation of a higher association between IgA nephropathy and celiac disease (37). However, in people with IgAN, GALT can become dysregulated, leading to the production of abnormal IgA antibodies. These antibodies can then deposit in the kidneys, causing inflammation and damage. The link between GALT and IgAN has been supported by a number of studies. A genome-wide association study (GWAS) found that most loci associated with the risk of IgAN are also associated with immune-mediated inflammatory bowel diseases, maintenance of the intestinal barrier and regulation of response to gut pathogens. This suggests that exposure to intestinal microbes may play a role in the development of IgAN, particularly in people who are genetically predisposed to the disease (38).
The production of mucosal IgA is induced both by mechanisms dependent on T lymphocytes and by mechanisms independent of them (39). After priming, B cells migrate to the effector zone of the mucosa-associated lymphoid tissue (MALT), located in the lamina propria of the mucosa, where they release dimeric IgA, composed of two IgA molecules and a connecting chain (35). Secretory IgA has enhanced bacteriostatic effects due to antimicrobial peptides, such as defensins, which are secreted into the intestinal lumen.
In the last decade the role of gut microbiota in patients with IgAN has been intensive investigated (40). Toll-like receptors (TLRs), such as LPS (TLR4) and lipoteichoic acid (TLR2), are significantly expressed on mucosal epithelial cells and recognize the molecular patterns of microbes (41). Gut microbiota signals the gut injury response through TLRs (42). An altered intestinal barrier facilitates increased absorption and circulation of LPS (43). Studies have indicated the presence of increased intestinal permeability in patients with IgAN (35, 40).
The causal association between IgAN and CD was analyzed in prospective study, by screening patients with IgAN for CD and explored the utility of analysis of IgA anti-TG2 antibody deposits. IgA anti tTG2 Ab colocalization study in kidney biopsies of patients with suspected celiac associated IgA nephropathy were found, but a small proportion of patients with IgAN have associated CD (44).
The gluten-free diet has been shown to be effective in treating IgA nephropathy in people with celiac disease. The gluten-free diet helps to reduce the production of IgA antibodies, which can lead to the disappearance of IgA deposits in the glomeruli and improvement of the condition of the glomeruli (45).
Children with IgA Vasculitis or just IgAN have increased expression of TRL4 mRNA in the periphery lymphomononuclear cells, which was correlated with signs of activation of innate immunity and proteinuria (35, 46). These observations lead to the hypothesis that increased intestinal permeability in intestinal microbes it triggers, by activating TLR4, immune system activation detected in IgAN. This mechanism correlates with systemic inflammation and oxidative stress (35).
In celiac disease, increased intestinal permeability is a distinctive feature (47). Many studies suggest the existence of analogies between intestinal barrier disruption in patients with CD and those with IgAN. Gluten aggravates IgAN through the interaction of gliadin with CD89, favoring the formation of the IgA1–sCD89 complex (35, 45). In both IgAN and celiac disease, the transferrin receptor (TFR) is associated with transglutaminase 2 (TG2), both of which are present in enterocytes and mesangial cells.
Tight junctions represent the foremost junctional complex located at the apex of cellular structures, serving to establish a polarized arrangement that segregates the apical and basolateral poles of the cells (48). In celiac disease, increased intestinal permeability results from disruption of intestinal epithelial cell tight junctions (TJ), leading to increased absorption of dietary antigens (48, 49). Gliadin, the lectin component of gluten, is deamidated by TG2 and presented to T cells in the context of HLA-DQ2 or HLA-DQ8 molecules on dendritic cells or macro-phages. Interaction with CD4+ T cells produces a TH1 response, generating proinflammatory cytokines, resulting in mucosal atrophy and a further increase in intestinal permeability. This process also generates a TH2 response, with the production of antibodies by B lymphocytes in the GALT (the lymphoid tissue associated with the intestinal mucosa). Thus, the production of IgA and IgG against gliadin and TG2 is stimulated. Most patients with CD associate the HLA-DQ2/DQ8 haplotype and show anti-gliadin and anti-TG2 antibodies (35) (Figure 1).
In patients with IgAN, it is hypothesized that a similar intestinal permeability mechanism might be active, with the release of anti-gliadin IgA into the circulation to form macromolecular aggregates with IgA1/sCD89, which ultimately deposit in the mesangial tissue (35). The detection of IgA anti-TG2 deposits in the mesangial tissue is a reliable technique for establishing an association with CD in these patients (43). The correlation is also supported by studies that show the remission of IgAN associated with CD in patients subjected to a gluten free diet (GFD) for at least 5 years (49). The link between IgA nephropathy and CD should be kept in mind. CD may be associated with a progressive evolution of CKD towards the end stage (50).
5 Urolithiasis and celiac disease
The first report of the association between CD and urolithiasis in children was made by Ogilvie et al. in the 1970s. The authors found that over half of CD patients had hyperoxaluria (51). Recent studies show increased risks of recurrent lithiasis, especially oxalic, in patients with CD (52, 53).
Urine supersaturation with calcium, phosphates, oxalates or cystine is necessary but not sufficient to explain the formation of calculations. The absorption defect of one or more compounds in the gut, or a defect in the renal handling of these compounds, causes supersaturation, primum movens in initiating the formation of kidney stones. Practically, the imbalance between the factors promoting and inhibiting the formation of stones (malabsorption, increased absorption of oxalates, cystine, calcium, vs. decreased absorption of citrate, magnesium, pyrophosphate at the intestinal level) leads to urinary supersaturation with the possibility of initiating crystallization on a support of cellular detritus. Alternatively, it can be speculated that autoantibodies present in CD may accompany urolithiasis. Relevant for this hypothesis, was a study in which anti-SS-A anti-bodies were detected in patients with urolithiasis, who developed Sjögren's syndrome. In another study, the antinuclear antibodies were positive in patients with hypocitraturia known favoring factor for urolithiasis (53–55).
Disturbances in the gut microbiome and metabolome may thus be determinants of early-onset disease and may explain the association between antibiotics and nephrolithiasis. In addition, decreased butyrate production and decreased oxalate degradation among people with early-onset kidney stones has been proven in studies. Roseburia species, (1% of all bacteria present in the gut microbiome) produce butyrate, a short-chain fatty acid that is a mediator of inflammation, helping to maintain the gut mucosal barrier and regulating the expression of oxalate transporters in the gut (56). Dysbiosis contributes to the loss of this species of beneficial bacteria, leading to the mediation of local inflammation, with increased oxalate absorption, which explains hyperoxalic lithiasis (57).
6 Other nephropathies and celiac disease
A few studies have reported the occurrence of other glomerulopathies (except IgAN) in the context of celiac disease, including membranous nephropathy and membranoproliferative nephropathy (4). Interestingly, in four case reports, membranoproliferative nephropathy showed improvement following a gluten-free diet (18).
The coexistence of CD and nephrotic syndrome is an exceptionally uncommon occurrence (58). In celiac disease, exposure to gliadin triggers the release of zonulin from enterocytes. High serum levels of zonulin activates protease-activated receptor 2 (PAR2) in a paracrine manner, and determine the disruption of the actin cyto-skeleton and cell-cell junctions in the intestinal epithelium (59, 60). PAR2 is also expressed on podocytes. The increased levels of serum zonulin induced by gluten ingestion may result in enhanced ligand binding to the PAR2 receptor on podocytes. Consequently, alterations in the podocyte cytoskeleton can impact cell motility and attachment to the glomerular basement membrane, potentially leading to proteinuria (61). Focal segmental glomerulosclerosis (FSGS) is a cause of nephrotic syndrome in children and adolescents, as well as an important cause of renal failure in adults (62). Isolated cases of membranous nephropathy (MN) associated with CD have been documented. Giménez et al. reported a series of five children with nephrotic syndrome who developed CD. In the renal biopsy performed revealed lesions of the MCD type or mesangial glomerulonephritis with IgM deposits, without IgA deposits, whose coexistence with CD is exceptional (63). The connection between CD and MN is associated with the common autoimmune pathogenesis, proven in studies by the remission of proteinuria simultaneously with the disappearance of IgA anti-tissue transglutaminase antibodies, sometimes without immunosuppression, only with antiproteinuric therapy and a gluten-free regime (64, 65).
Mukta Mantan et al. cited a 2007 study from the Mario Negri Institute for Pharmacological Research in Milan, Italy, which examined the relationship between bread consumption and the risk of kidney cancer. Individuals with the highest bread consumption had a 94% greater likelihood of developing kidney cancer than those with the lowest bread consumption (66).
7 Distal renal tubular acidosis and celiac disease
Renal distal tubular acidosis (RTA) is a significant cause of refractory rickets and stunting in children. In rare cases, distal RTA may be autoimmune in nature, suggested by its association with other autoimmune conditions such as Sjogren's syndrome, systemic lupus erythematosus (SLE), and Hashimoto's thyroiditis (67).
The hereditary form of distal renal tubular acidosis (RTA) is primarily caused by autosomal recessive mutations in genes that encode subunits of the vacuolar H + ATPase. These mutations lead to impaired transporter function in the renal tubules (67, 68). The acquired form of distal RTA is more frequently observed in adults and is often associated with autoimmune conditions, notably Sjögren's syndrome (69, 70).
Although the association of CD with Sjögren's syndrome and distal RTA was previously reported in adults (71), the association of distal tubular acidosis with celiac disease, without other autoimmune determinations, was reported only in a study in children (67).
8 Urinary tract infection and celiac disease
Increased urinary tract infection (UTI) incidence in patients with CD has been attributed to an associated disorder of the urinary system, including tract motility impairment, urinary bladder dysfunction, changes in intestinal bacterial flora that predispose to urinary tract contamination, reduced immune defenses against infections, or altered immunity (72). At the same time, the reverse relationship can also be valid. A simple urinary tract infection can acutely trigger an immune reaction that causes a celiac crisis in a predisposed individual (73). Celiac crisis is the hyperacute manifestation of CD, sometimes the onset of a previously undiagnosed gluten enteropathy.
9 Final considerations
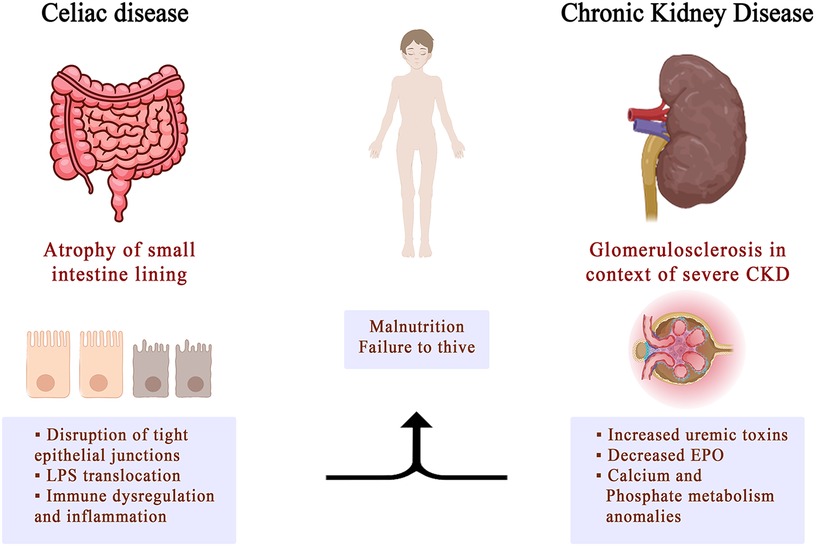
CD is often accompanied by various extraintestinal manifestations, which classifies it as a systemic disease rather than a gastrointestinal condition. Many of the extraintestinal manifestations of CD involve the kidneys. These include conditions like urolithiasis and crystal-induced kidney disease, membranoproliferative glomerulonephritis, IgA nephropathy, or an increased risk for progressing to end-stage renal disease (Figure 2).
However, there is limited knowledge about the risk of kidney disease in children with celiac disease. To date, only a few studies have investigated the risk of renal disease among patients with CD, especially adult patients, and there are no current recommendations for screening for renal involvement in these patients. More studies and pro-longed follow-up are needed to evaluate the connections between celiac chronic kidney diseases and celiac disease, especially in childhood where the experience in limited.
Author contributions
IMS: Conceptualization, Writing – original draft. IM: Supervision, Writing – review & editing. AL: Methodology, Writing – original draft. II: Conceptualization, Writing – original draft. MA: Investigation, Writing – original draft. AA: Investigation, Writing – original draft. IDM: Investigation, Visualization, Writing – review & editing. VM: Visualization, Writing – review & editing. VLL: Project administration, Writing – review & editing. AM: Methodology, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Husby S, Koletzko S, Korponay-Szabó I, Kurppa K, Mearin ML, Ribes-Koninckx C, Shamir R, et al. European society paediatric gastroenterology, hepatology and nutrition guidelines for diagnosing coeliac disease 2020. J Pediatr Gastroenterol Nutr. (2020) 70(1):141–56. doi: 10.1097/MPG.0000000000002497
2. Lupu VV, Jechel E, Mihai CM, Mitrofan EC, Lupu A, Starcea IM, et al. Connection between celiac disease and systemic lupus erythematosus in children—a development model of autoimmune diseases starting from what we inherit to what we eat. Nutrients. (2023) 15(11):2535. doi: 10.3390/nu15112535
3. van Berge-Henegouwen GP, Mulder CJ. Pioneer in the gluten free diet: willem-karel dicke 1905–1962, over 50 years of gluten free diet. Gut. (1993) 34(11):1473–5. doi: 10.1136/gut.34.11.1473
4. Singh P, Arora A, Strand TA, Leffler DA, Catassi C, Green PH, et al. Global prevalence of celiac disease: systematic review and meta-analysis. Clin Gastroenterol Hepatol. (2018) 16(6):823–36. doi: 10.1016/j.cgh.2017.06.037
5. Therrien A, Kelly CP, Silvester JA. Celiac disease: extraintestinal manifestations and associated conditions. J Clin Gastroenterol. (2020) 54(1):8–21. doi: 10.1097/MCG.0000000000001267
6. Caio G, Volta U, Sapone A, Leffler DA, De Giorgio R, Catassi C, Fasano A. Celiac disease: a comprehensive current review. BMC Med. (2019) 17:142. doi: 10.1186/s12916-019-1380-z
7. Jericho H, Sansotta N, Guandalini S. Extraintestinal manifestations of celiac disease: effectiveness of the gluten-free diet. J Pediatr Gastroenterol Nutr. (2017) 65:75–9. doi: 10.1097/MPG.0000000000001420
8. Paez MA, Gramelspacher AM, Sinacore J, Winterfield L, Venu M. Delay in diagnosis of celiac disease in patients without gastrointestinal complaints. Am J Med. (2017) 130:1318–23. doi: 10.1016/j.amjmed.2017.05.027
9. Lupu VV, Trandafir LM, Raileanu AA, Mihai CM, Morariu ID, Starcea IM, et al. Advances in understanding the human gut microbiota and its implication in pediatric celiac disease—a narrative review. Nutrients. (2023) 15:2499. doi: 10.3390/nu15112499
10. Roberts SE, Morrison-Rees S, Thapar N, Benninga MA, Borrelli O, Broekaert I, et al. Systematic review and meta-analysis: the incidence and prevalence of paediatric coeliac disease across Europe. Aliment Pharmacol Ther. (2021) 54:109–28. doi: 10.1111/apt.16337
11. Mocanu A, Bogos RA, Lazaruc TI, Trandafir LM, Lupu VV, Ioniuc I, et al. Exploring a complex interplay: kidney–gut axis in pediatric chronic kidney disease. Nutrients. (2023) 15(16):3609. doi: 10.3390/nu15163609
12. Cheminant M, Bruneau J, Malamut G, Sibon D, Guegan N, van Gils T, et al. NKp46 is a diagnostic biomarker and may be a therapeutic target in gastrointestinal T-cell lymphoproliferative diseases: a CELAC study. Gut. (2018) Nutrients. 2023; 15(16):3609.30448772
13. Caio G, Volta U, Ursini F, Manfredini R, De Giorgio R. Small bowel adenocarcinoma as a complication of celiac disease: clinical and diagnostic features. BMC Gastroenterol. (2019) 19:45. doi: 10.1186/s12876-019-0964-6
14. Laurikka P, Nurminen S, Kivelä L, Kurppa K. Extraintestinal manifestations of celiac disease: early detection for better long-term outcomes. Nutrients. (2018) 10(8):1015. doi: 10.3390/nu10081015
15. Nurmi R, Pasternack C, Salmi T, Hervonen K, Koskinen I, Järvelin J, et al. The risk of renal comorbidities in celiac disease patients depends on the phenotype of celiac disease. J Intern Med. (2022) 292(5):779–87. doi: 10.1111/joim.13532
16. Sahin I, Eminbeyli L, Andic S, Tuncer I, Koz S. Screening for celiac disease among patients with chronic kidney disease. Ren Fail. (2012) 34:545–9. doi: 10.3109/0886022X.2012.669299
17. Welander A, Prütz KG, Fored M, Ludvigsson JF. Increased risk of end-stage renal disease in individuals with coeliac disease. Gut. (2012) 61:64–8. doi: 10.1136/gutjnl-2011-300134
18. Wijarnpreecha K, Thongprayoon C, Panjawatanan P, Thamcharoen N, Pachariyanon P, Nakkala K, et al. Celiac disease and the risk of kidney diseases: a systematic review and meta-analysis. Dig Liver Dis. (2016) 48:1418–24. doi: 10.1016/j.dld.2016.08.115
19. Boonpheng B, Cheungpasitporn W, Wijarnpreecha K. Renal disease in patients with celiac disease. Minerva Med. (2018) 109:126–40. doi: 10.23736/S0026-4806.17.05403-9
20. Evenepoel P, Poesen R, Meijers B. The gut-kidney axis. Pediatr Nephrol. (2017) 32(11):2005–14. doi: 10.1007/s00467-016-3527-x
21. Muntean C, Starcea IM, Banescu C. Diabetic kidney disease in pediatric patients: a current review. World J Diabetes. (2022) 13(8):587–99. doi: 10.4239/wjd.v13.i8.587
22. Mollazadegan K, Fored M, Lundberg S, Ludvigsson J, Ekbom A, Montgomery SM, et al. Risk of renal disease in patients with both type 1 diabetes and coeliac disease. Diabetologia. (2014) 57(7):1339–45. doi: 10.1007/s00125-014-3223-y
23. Gopee E, van den Oever EL, Cameron F, Thomas MC. Coeliac disease, gluten-free diet and the development and progression of albuminuria in children with type 1 diabetes. Pediatr Diabetes. (2013) 14(6):455–8. doi: 10.1111/pedi.12028
24. Cordovado SK, Zhao Y, Warram JH, Gong H, Anderson KL, Hendrix MM, et al. Nephropathy in type 1 diabetes is diminished in carriers of HLA-DRB1*04: the genetics of kidneys in diabetes (GoKinD) study. Diabetes. (2008) 57(2):518–22. doi: 10.2337/db07-0826
25. Mueller PW, Rogus JJ, Cleary PA, Zhao Y, Smiles AM, Steffes MW, et al. Genetics of kidneys in diabetes (GoKinD) study: a genetics collection available for identifying genetic susceptibility factors for diabetic nephropathy in type 1 diabetes. J Am Soc Nephrol. (2006) 17:1782–90. doi: 10.1681/ASN.2005080822
26. Ludvigsson JF. Mortality and malignancy in celiac disease. Gastrointest Endosc Clin N Am. (2012) 22:705–22. doi: 10.1016/j.giec.2012.07.005
27. Pitocco D, Giubilato S, Martini F, Zaccardi F, Pazzano V, Manto A, et al. Combined atherogenic effects of celiac disease and type 1 diabetes mellitus. Atherosclerosis. (2011) 217:531–5. doi: 10.1016/j.atherosclerosis.2011.04.042
28. Rohrer TR, Wolf J, Liptay S, Zimmer KP, Fröhlich-Reiterer E, Scheuing N, et al. Microvascular complications in childhood-onset type 1 diabetes and celiac disease: a multicenter longitudinal analysis of 56,514 patients from the German-Austrian DPV database. Diabetes Care. (2015) 38(5):801–7. doi: 10.2337/dc14-0683
29. Leeds JS, Hopper AD, Hadjivassiliou M, Tesfaye S, Sanders DS. High prevalence of microvascular complications in adults with type 1 diabetes and newly diagnosed celiac disease. Diabetes Care. (2011) 34(10):2158–63. doi: 10.2337/dc11-0149
30. Saibeni S, Lecchi A, Meucci G, Cattaneo M, Tagliabue L, Rondonotti E, et al. Prevalence of hyperhomocysteinemia in adult gluten-sensitive enteropathy at diagnosis: role of B12, folate, and genetics. Clin Gastroenterol Hepatol. (2005) 3:574–80. doi: 10.1016/S1542-3565(05)00022-4
31. Hadithi M, Mulder CJ, Stam F, Azizi J, Crusius JB, Peña AS, et al. Effect of B vitamin supplementation on plasma homocysteine levels in celiac disease. World J Gastroenterol. (2009) 15:955–60. doi: 10.3748/wjg.15.955
32. Berger J, Hinglais N. Les depots intercapillaires d’IgAIgG. J Urol Nephrol. (1968) 74:694–5. 4180586.
33. Gutiérrez E, Carvaca-Fontán F, Luzardo L, Morales E, Alonso M, Praga M. A personalized update on IgA nephropathy: a new vision and new future challenges. Nephron. (2020) 144:555–71. doi: 10.1159/000509997
34. Coppo R, Amore A, Hogg R, Emancipator S. Idiopathic nephropathy with IgA deposits. Ped Nephrol. (2000) 15:139–50. doi: 10.1007/s004670000309
35. Wyatt RJ, Julian BA. Iga nephropathy. N Engl J Med. (2013) 368:2402–14. doi: 10.1056/NEJMra1206793
36. Coppo R. The intestine–renal connection in IgA nephropathy. Nephrol Dial Transplant. (2015) 30:360–6. doi: 10.1093/ndt/gfu343
37. Stanley JC, Deng H. Progress in pathogenesis of immunoglobin A nephropathy. Cureus. (2020) 12(6):e8789. doi: 10.7759/cureus.8789
38. Coppo R. The gut-renal connection in IgA nephropathy. Semin Nephrol. (2018) 38(5):504–12. doi: 10.1016/j.semnephrol.2018.05.020
39. Kiryluk K, Li Y, Scolari F, Sanna-Cherchi S, Choi M, Verbitsky M, et al. Discovery of new risk loci for IgA nephropathy implicates genes involved in immunity against intestinal pathogens. Nat Genet. (2014) 46:1187–96. doi: 10.1038/ng.3118
40. Kiryluk K, Novak J. The genetics and immunobiology of IgA nephropathy. J Clin Invest. (2014) 124:2325–32. doi: 10.1172/JCI74475
41. De Angelis M, Montemurno E, Piccolo M, Vannini L, Lauriero G, Maranzano V, et al. Microbiota and metabolome associated with immunoglobulin A nephropathy (IgAN). PLoS One. (2014) 9:e99006. doi: 10.1371/journal.pone.0099006
42. Yiu JH, Dorweiler B, Woo CW. Interaction between gut microbiota and toll-like receptor: from immunity to metabolism. J.Mol.Med. (2017) 95(1):13–20. doi: 10.1007/s00109-016-1474-4
43. Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. (2005) 122:107–18. doi: 10.1016/j.cell.2005.05.007
44. Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. (2013) 83:1010–9. doi: 10.1038/ki.2012.440
45. Dutta R, Rawat R, Das P, Singh G, Kumari A, Ahmad M, et al. Identification of celiac disease associated IgA nephropathy by IgA anti-tissue transglutaminase2 antibody deposits in archived formalin-fixed tissues. Saudi J Gastroenterol. (2023) 29(1):59–65. doi: 10.4103/sjg.sjg_326_22
46. Dutta R, Rawat R, Das P, Singh G, Kumari A, Ahmad M, et al. Gluten exacerbates IgA nephropathy in humanized mice through gliadin-CD89 interaction. Kidney Int. (2015) 88:276–85. doi: 10.1038/ki.2015.94
47. Donadio ME, Loiacono E, Peruzzi L, Amore A, Camilla R, Chiale F, et al. Toll-like receptors, immunoproteasome and regulatory T cells in children with Henoch–Schönlein purpura and primary IgA nephropathy. Pediatr Nephrol. (2014) 29:1545–51. doi: 10.1007/s00467-014-2807-6
48. Valitutti F, Fasano A. Breaking down barriers: how understanding celiac disease pathogenesis informed the development of novel treatments. Dig Dis Sci. (2019) 64(7):1748–58. doi: 10.1007/s10620-019-05646-y
49. Koning F. Pathophysiology of celiac disease. J Pediatr Gastroenterol Nutr. (2014) 59(Suppl 1):S1–4. doi: 10.1097/01.mpg.0000450391.46027.48
50. Slavin SF. IgA nephropathy as the initial presentation of celiac disease in an adolescent. Pediatrics. (2021) 148:e2021051332. doi: 10.1542/peds.2021-051332
51. Nurmi R, Metso M, Pörsti I, Niemelä O, Huhtala H, Mustonen J, et al. Celiac disease or positive tissue transglutaminase antibodies in patients undergoing renal biopsies. Dig Liver Dis. (2018) 50(1):27–31. doi: 10.1016/j.dld.2017.09.131
52. Ogilvie D, McCollum JP, Packer S, Manning J, Oyesiku J, Muller DP, et al. Urinary outputs of oxalate, calcium and magnesium in children with intestinal disorders. Potential cause of renal calculi. Arch Dis Child. (1976) 51:790–5. doi: 10.1136/adc.51.10.790
53. Menekşe E, Selimoşlu MA, Temel I, Tabel Y, Gürgöze M. Celiac disease in children with urolithiasis. Turk J Pediatr. (2012) 54(4):382–6.
54. Ludvigsson JF, Zingone F, Fored M, Ciacci C, Cirillo M. Moderately increased risk of urinary stone disease in patients with biopsy-verified coeliac disease. Aliment Pharmacol Ther. (2012) 35:477–84. doi: 10.1111/j.1365-2036.2011.04968.x
55. Eriksson P, Denneberg T, Eneström S, Johansson B, Lindström F, Skogh T. Urolithiasis and distal renal tubular acidosis preceding primary Sjögren’s syndrome: a retrospective study 5–53 years after the presentation of urolithiasis. J Intern Med. (1996) 239:483–8. doi: 10.1046/j.1365-2796.1996.487829000.x
56. Eriksson P, Denneberg T, Lundström I, Skogh T, Tiselius HG. Autoantibodies and primary Sjögren’s syndrome in a hypocitraturic stone population. Scand J Urol Nephrol. (1997) 31:73–80. doi: 10.3109/00365599709070306
57. Denburg MR, Koepsell K, Lee JJ, Gerber J, Bittinger K, Tasian GE. Perturbations of the gut microbiome and metabolome in children with calcium oxalate kidney stone disease. J Am Soc Nephrol. (2020) 31(6):1358–69. doi: 10.1681/ASN.2019101131
58. Landry GM, Hirata T, Anderson JB, Cabrero P, Gallo CJ, Dow JA, et al. Sulfate and thiosulfate inhibit oxalate transport via a dPrestin (Slc26a6)-dependent mechanism in an insect model of calcium oxalate nephrolithiasis. Am J Physiol Renal Physiol. (2016) 310:F152–9. doi: 10.1152/ajprenal.00406.2015
59. Ghavidel A, Ghavidel A. Minimal change disease in association with celiac disease; a case report and review of the literature. J Renal Endocr. (2020) 6(1):e10.
60. Guandalini S, Assiri A. Celiac disease. A review. JAMA Pediatr. (2014) 168:272–8. doi: 10.1001/jamapediatrics.2013.3858
61. Trachtman H, Gipson DS, Lemley KV, Troost JP, Faul C, Morrison DJ, et al. Plasma zonulin levels in childhood nephrotic syndrome. Front Pediatr. (2019) 7:197. doi: 10.3389/fped.2019.00197
62. Schell C, Huber TB. The evolving complexity of the podocyte cytoskeleton. J Am Soc Nephrol. (2017) 28:3166–74. doi: 10.1681/ASN.2017020143
63. Harbia H, Abdelsalamb MS, Abdulshakoura A, Alfurayhc O. Celiac disease and focal segmental glomerulosclerosis: is it a fortuity finding? World J Nephrol Urol. (2015) 4(4):264–6. doi: 10.14740/wjnu242w
64. Gimenez Llort A, Vila Cots J, Camacho Diaz JA, Vila Santandreu A, Concheiro Guisan A, Garcia Garcia L. Nephrotic syndrome associated with celiac disease. A report of five cases. Nephron. (2002) 92:950. doi: 10.1159/000065576
65. Soro S, Sánchez de la Nieta MD, Rivera F. Coeliac disease and membranous nephropathy. Nefrología (English Edition). (2009) 29(5):479–81. doi: 10.3265/Nefrologia.2009.29.5.5324.en.full
66. Pestana N, Vida C, Vieira P, Durães J, Silva G. Celiac disease as a rare cause of membranous nephropathy: a case report. Cureus. (2021) 13(2):e13312.33738158
67. Ji S. The Dark Side of Wheat. England: National Library of Medicine Available online at: https://detoxnutrition.com/wp-content/uploads/SayerJi-DarkSideWheat.pdf (Accessed December 15, 2023).
68. Satapathy AK, Mittal S, Jain V. Distal renal tubular acidosis associated with celiac disease and thyroiditis. Indian Pediatr. (2016) 53:1013–4. doi: 10.1007/s13312-016-0978-x
69. Karet FE. Inherited distal renal tubular acidosis. J Am Soc Nephrol. (2002) 13:2178–84. doi: 10.1097/01.ASN.0000023433.08833.88
70. Takemoto F, Hoshino J, Sawa N, Tamura Y, Tagami T, Yokota M, et al. Autoantibodies against carbonic anhydrase II are increased in renal tubular acidosis associated with Sjogren syndrome. Am J Med. (2005) 118:181–4. doi: 10.1016/j.amjmed.2004.07.049
71. Fracchia M, Galatola G, Corradi F, Dall'Omo AM, Rovera L, Pera A, et al. Coeliac disease associated with Sjögren’s syndrome, renal tubular acidosis, primary biliary cirrhosis and autoimmune hyperthyroidism. Dig Liver Dis. (2004) 36:489–91. doi: 10.1016/j.dld.2003.10.022
72. Kalyoncu D, Urgancı N. Urinary tract infections in pediatric patients with celiac disease. Eurasian Journal of Medical Investigation. (2018) 2(4):224–6. doi: 10.14744/ejmi.2018.97268
Keywords: celiac disease, chronic kidney disease, children, IgA nephropathy, diabetic nephropathy
Citation: Starcea IM, Miron I, Lupu A, Ioniuc I, Alecsa M, Azoicai A, Morariu ID, Munteanu V, Lupu VV and Mocanu A (2024) Unraveling chronic kidney disease in children: a surprising manifestation of celiac disease. Front. Pediatr. 12:1384591. doi: 10.3389/fped.2024.1384591
Received: 9 February 2024; Accepted: 5 April 2024;
Published: 24 April 2024.
Edited by:
Francesco Valitutti, University of Perugia, ItalyReviewed by:
Angela Pepe, Ospedale San Carlo, Italy© 2024 Starcea, Miron, Lupu, Ioniuc, Alecsa, Azoicai, Morariu, Munteanu, Lupu and Mocanu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ancuta Lupu YW5jYV9pZ25AeWFob28uY29t Ileana Ioniuc aWxlYW5haW9uaXVjQHlhaG9vLmNvbQ==
†These authors have contributed equally to this work
 Iuliana Magdalena Starcea
Iuliana Magdalena Starcea Ingrith Miron1,†
Ingrith Miron1,† Ancuta Lupu
Ancuta Lupu Ileana Ioniuc
Ileana Ioniuc Mirabela Alecsa
Mirabela Alecsa Vasile Valeriu Lupu
Vasile Valeriu Lupu Adriana Mocanu
Adriana Mocanu