- Department of Pediatric Surgery, Zhujiang Hospital, Southern Medical University, Guangzhou, Guangdong, China
Background: Littoral cell angioma (LCA) is an extremely uncommon benign vascular tumor of the spleen. Cases of LCA in infants are rarely reported, and due to the rarity of the tumor and non-specific symptoms, the diagnosis of LCA is often overlooked in clinical practice.
Case report: We present a 3-year-old girl with pulmonary inflammation who was admitted to the hospital due to the discovery of a space-occupying lesion in the spleen. Pathology after splenectomy confirmed LCA, and there was no recurrence observed at the 5-month follow-up examination.
Conclusion: LCA should be considered when a child shows asymptomatic splenomegaly, with antigen expression indicating dual positivity of endothelial and histiocytic markers. Laparoscopic splenectomy remains the primary method of treating LCA.
1 Introduction
Primary splenic tumors in children are clinically rare, accounting for only 0.03% of all tumors. Splenic tumors can be classified as benign and malignant, with the majority of benign cases including hemangiomas, lymphangiomas, and splenic cysts. In contrast, malignant tumors include lymphoma, leiomyosarcoma, and others. Splenic tumors are usually asymptomatic, with 50% of patients accompanied by splenic enlargement or hypersplenism, leading to anemia or pancytopenia (1). Therefore, its diagnosis is usually incidental.
Littoral cell angioma (LCA) is a rare occurrence in splenic tumors, arising from normal spleen red sinus shore cells and belonging to the reticuloendothelial cell system. Tumor cells exhibit the specific characteristics of double differentiation, including both endothelial and histiocyte antigens (1). Unlike general hemangiomas, LCA has the potential for recurrence and metastasis, necessitating timely surgical intervention. Since LCA tends to strike in middle age, reports of LCA in children are extremely rare. Herein, we present the diagnosis and treatment experience of LCA in a 3-year-old girl, and conduct a literature review of LCA in children, aiming to provide references for the clinical diagnosis and treatment of pediatric surgeons.
2 Case report
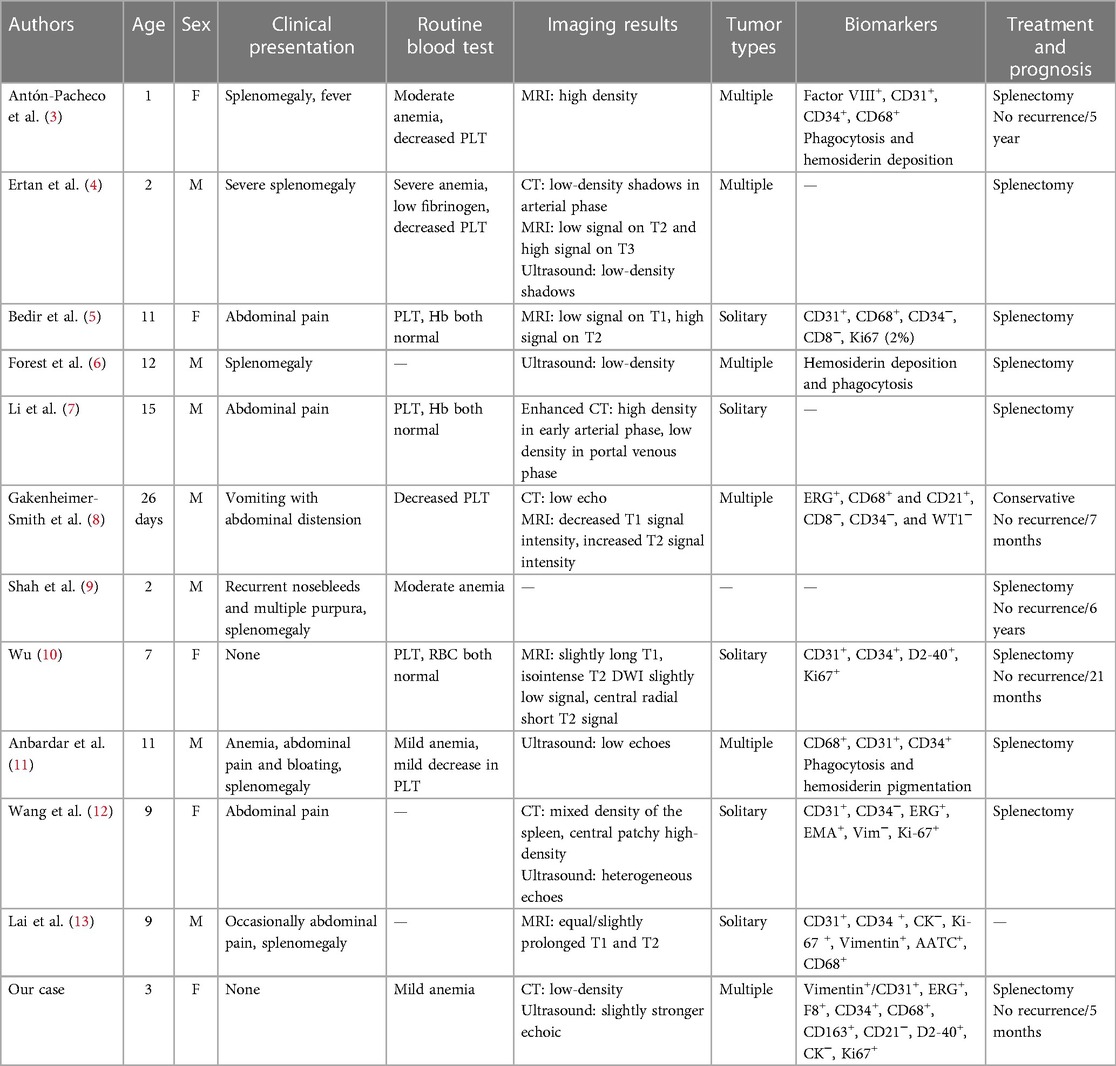
A 3-year-old girl had originally sought treatment at the local hospital 5 months earlier for persistent coughing. A computed tomography (CT) of the abdomen revealed splenomegaly with multiple nodules. Upon physical examination, mild tenderness in the upper left abdomen was noted, along with a palpable spleen enlargement located approximately 5 cm below the costal margin. The spleen exhibited a smooth surface and firm texture. Blood routine was as follows: white blood cells 5.38 × 109/L, red blood cells 3.89 × 109/L, hemoglobin 107 g/L, and platelets 179 × 109/L. An ultrasound examination indicated irregular splenic enlargement with uneven internal echoes. Multiple solid slightly hyperechoic masses were diffusely distributed in the spleen. In order to further clarify the mass and vascular distribution, a CT scan was performed, revealing an enlarged spleen with multiple variably sized mass-like and nodular low-density lesions. The large lesion, measuring approximately 44 mm × 23 mm, which was located at the lower pole of the spleen, exhibited uniform density and enhancement on contrast-enhanced scans. Some lesions exhibited obvious enhancement with indistinct edges and were surrounded by splenic vascular branches (Figure 1). Thus, hereditary spherocytosis and portal hypertension (PHT) were excluded as potential causes for splenomegaly. Given the girl's prolonged consumption of raw water, serological testing revealed positive antibodies against Leptospira and Angiostrongylus cantonensis. Additionally, consecutive stool examinations were conducted on three separate occasions, all yielding negative results. To further rule out the association with parasites, the patient underwent magnetic resonance imaging (MRI) of the brain, which revealed there were no space-occupying lesions in the brain. Eventually, the space-occupying lesion in the spleen was considered to be a tumor. A laparoscopic splenectomy was then performed. The pathological examination showed multiple solid nodules of varying sizes evident in the cross-section of the spleen. Broadly speaking, the microscopic picture showed a multifocal tumor. Specifically, the tumor was located within the red pulp and was well demarcated from the surrounding normal tissue without necrosis or hemorrhage. Microscopically, the tumor consisted of vascular lumens of varying sizes, lined with blood vessels and a single layer of proliferating endothelial cells. Papillary-like structures were evident, and the lumens contained decidual cells. The cells were large, with abundant cytoplasm, and iron-containing hemosiderin granules were observed in the cytoplasm. Tumor cells are homogeneous, and nuclear division is rare (Figure 2). Immunohistochemical (IHC) analysis of the tumor cells showed positive staining for vimentin/CD31, ERG, F8, CD34 (partly), with focally positive for CD68, CD163, and D2-40; Ki-67 was approximately 2%. These findings confirmed the diagnosis of LCA. Five months postoperatively, outpatient ultrasonography revealed no tumor residue or recurrence.
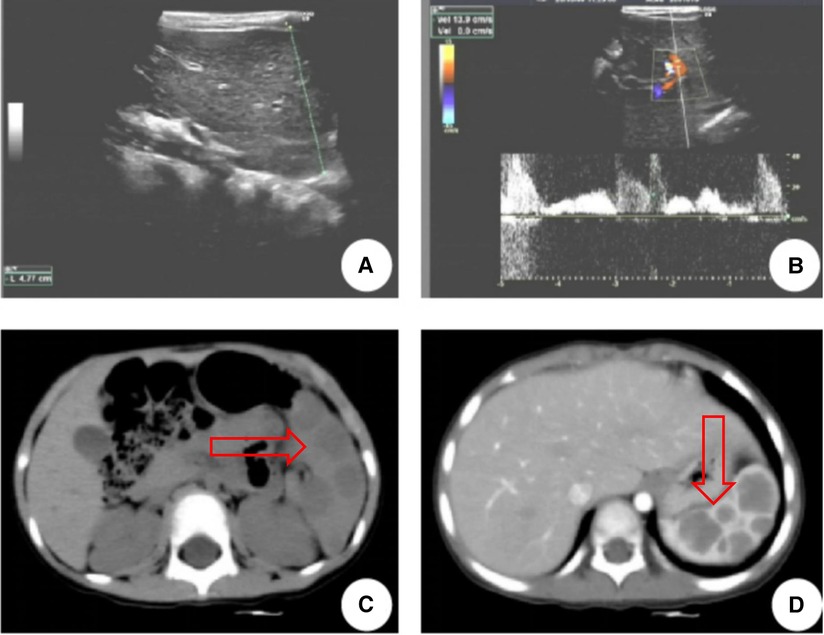
Figure 1. LCA patient's ultrasound and CT images. (A,B) Ultrasound indicated multiple solid slightly hyperechoic masses were diffusely distributed in the spleen, measuring approximately 26 mm × 23 mm, 12 mm × 13 mm, 19 mm × 19 mm, 9 mm × 9 mm, 8 mm × 5 mm, etc. (C) CT revealed multiple nodular lesions in the spleen. (D) Enhancement reveals multiple masses of varying sizes within the spleen, with uneven internal enhancement and weaker than the spleen. The arrows point to the tumor.
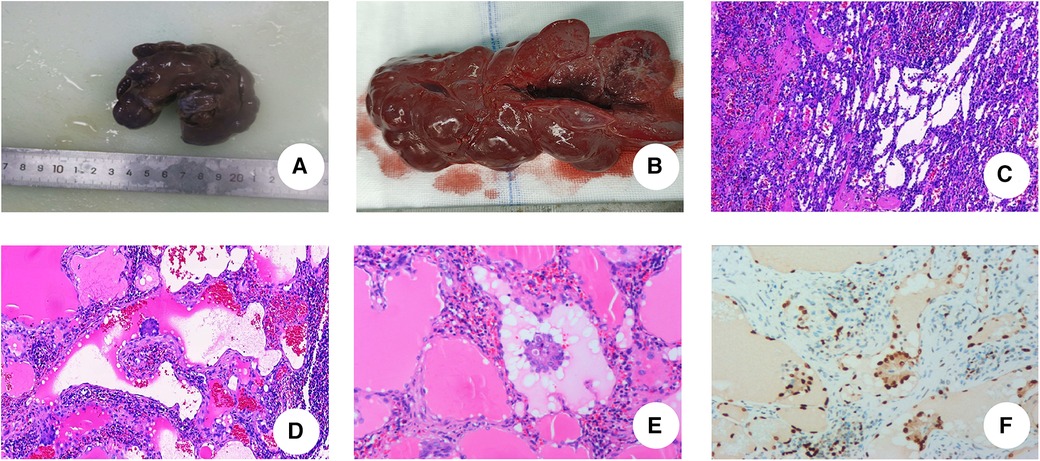
Figure 2. Postoperative pathological examinations. (A) The spleen significantly enlarged, with measured 10.5 cm × 5 cm × 3 cm. (B) The spleen is grayish-red, with nodular cut surfaces, solid, and slightly firm in consistency. (C) Hematoxylin and eosin staining of LCA. The tumor consists of an interlacing vascular network similar to splenic sinus, with papillary projections and cystic cavities (IHC × 5). (D) Papillary structures are seen in the lumen of the blood vessels (IHC × 10). (E) Nuclei with no obvious heterogeneity. (IHC × 20). (F) ERG staining: ERG(+) (IHC × 20).
3 Discussion
LCA is a relatively uncommon benign tumor of the spleen, primarily affecting individuals aged 40–60 years (2). It is notably scarce in the pediatric population, with the existing literature predominantly consisting of case reports. Since its initial identification by Falk et al. (1) in 1991, 11 cases have been reported in children younger than 16 years old (Table 1). The cohort comprised seven boys and five girls (age range 26 days–15 years) who were incidentally diagnosed due to splenomegaly and hyperfunction of the spleen.
LCA was categorized as a type of spleen-specific vascular interstitial tumor (14). The exact pathogenetic mechanisms responsible for LCA are unknown but dysregulation of the immune system is likely. Li et al. (15) revealed that one-third of their cases were accompanied by visceral malignancies, whereas another third of their patients suffered from immune system diseases or immune dysfunctions. Due to the characteristics of the pediatric group, there were fewer tumors among the accompanying diseases in the pediatric patients with LCA. Occasionally, LCA occurs in children due to immune dysfunction caused by congenital diseases of the immune system or immunosuppressive treatment (16). In addition, as with other diseases, there appears to be a correlation between LCA and the inflammatory factor storm (17). Given occasional individual reports of siblings in a family having both LCA and littoral cell angiosarcoma (LCAS) (18), this disease may have genetic susceptibility or familial clustering. Notably, a pediatric patient with LCA also had Gaucher's disease, an autosomal recessive genetic disorder, which may also support the possibility of the genetic connection in pediatric cases of LCA. Interestingly, not all researchers agree that LCA is composed of tumor cells. After reviewing 17 cases of LCA pathology, Bi et al. (19) proposed that proliferating endothelial cells are a benign granulomatous response triggered by circulating tumor cell-immune cell complexes during the blood-filtering process in the splenic sinuses.
Imaging examinations hold certain diagnostic significance for this condition, although they lack specificity. CT scans typically reveal low-density splenic masses, marked by delayed enhancement. These characteristics were observed in 10 children with LCA. MRI can show a distinctive speckled sign, the extent of which depends on the amount of hemosiderin in the tumor's endothelial cells. Due to the paramagnetic effect of iron, the increased deposition of hemosiderin within cells can reduce the signal on T2-weighted imaging (T2WI). This characteristic aids in differentiating LCA from other vascular tumors and serves as a specific manifestation of LCA (20). In pediatric cases, MRI was performed in six cases, with five cases showing high signal intensity in the T2 phase of the lesion, displaying uneven internal signals mixed with speckled low signals, known as the speckled sign.
Currently, there are no standard diagnostic guidelines for LCA, as it lacks specific clinical symptoms and imaging features. The definitive diagnosis primarily relies on pathological examination. LCA usually presents multiple lesions in the spleen, whereas in children, it exhibits varied of types. Our review revealed an equal occurrence of single and multiple lesions, comprising five cases of multiple nodular lesions, five cases of solitary nodular lesions, and one case with an unspecified type of lesion. Even though the main biological behavior of LCA is benign, caution must be taken due to its close relationship with malignant masses. Malignant transformation occurs when the spleen weighs more than 1,500 g or its diameter exceeds 20 cm (21). Compared to normal sinus endothelial cells, a littoral cell is characterized by dual antigen expression, with both endothelial and histiocytic markers being positive, occasionally showing positivity for transferrin receptors, distinguishing it from hemangiomas. IHC shows a positive staining for endothelial markers [such as Factor VIII (FVIII), CD31, von Willebrand Factor (vWF), CD34] and histiocytic markers (CD 68) (1). Although patients with LCA generally do not express CD34, recent studies have suggested the possibility of LCA malignancy in cases where CD34 is positive (22). In this cohort of children, CD68 was positive in five cases. The patient in our hospital showed dual differentiation in immunohistochemical staining results, with Ki-67 positivity (approximately 2%), and pathology revealed phagocytosis and hemosiderin pigment deposition, consistent with previous reports. The primary components of the differential diagnosis include lymphomas, tumors of vascular origin, and LCAS, which appear to be easier to distinguish than the former two because of the markedly heterogeneous nature of its cells, active nuclear division, and frequent necrosis. In fact, LCA uniquely exhibits as double antibody-positive to distinguish it from the vast majority of diseases, which is the basis of the pathological diagnosis for LCA.
Surgeons often grapple with fundamental questions related to the management of LCA. When a splenic mass is discovered, it is crucial to accurately determine the degree of benignity or malignancy of the tumor. If there is a potential for malignancy within a benign mass, a rational treatment plan should be selected. In the pediatric population in particular, the spleen functions as an immune organ. Explaining the need for splenectomy to guardians and taking measures to prevent and manage post-splenectomy infections (OPS) play essential roles. Surgeons should determine the most beneficial treatment for their patients through a careful evaluation process. Here, some evidence suggests that conservative treatment has no potentially malignant effects on patients (8). While Li et al. suggested close monitoring (12), others advocated radical splenectomy instead, considering that LCA poses potential risks of malignancy, recurrence, and metastasis (23). Susan et al. suggested that progressive splenic enlargement can also lead to a decrease in blood cells and even rupture, causing catastrophic hemorrhage. Eventually, after discussions with our medical team, splenectomy was cautiously recommended, considering that malignant transformation can be easily overlooked. Methods of splenectomy include laparoscopic splenectomy and splenectomy. Nowadays, total splenectomy under laparoscopy is generally carried out due to its advantages of being safe, effective, and minimally invasive (24, 25). In fact, the final choice for total or partial splenectomy depends on the patient's age and the size of the splenic tumor; partial splenectomy can be performed when there is a small, limited lesion within the spleen (16). Surgery is not the only treatment for the disease, and medication such as etoposide, paclitaxel, and vincristine may sometimes play a role (26). Although chemotherapy showed a significant anti-tumor effect, it is still not the main treatment for LCA. In all cases of children with LCA, splenectomy was performed in most cases, and only one case received conservative treatment. No recurrence was observed in all cases during follow-up.
Following pediatric splenectomy guidelines (27), the patient received a prompt vaccination with a triple vaccine upon discharge. In addition, a long-term anticoagulation therapy was initiated consisting of warfarin and regular injections of long-acting penicillin. Notably, no recurrence was observed during the 5-month follow-up period.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Zhujiang Hospital of Southern Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
YM: Formal Analysis, Visualization, Writing – original draft. LY: Supervision, Writing – review & editing. JW: Writing – review & editing, Conceptualization. QC: Data curation, Writing – original draft. MZ: Writing – original draft, Resources. XZ: Writing – original draft, Methodology. RT: Writing – original draft, Investigation. DA: Writing – original draft, Data curation. KW: Project administration, Supervision, Writing – review & editing.
Funding
The authors declare financial support was received for the research, authorship, and/or publication of this article.
This study was supported by Guangdong Basic and Applied Research Foundation (2019A1515011086).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Falk S, Stutte HJ, Frizzera G. Littoral cell angioma. A novel splenic vascular lesion demonstrating histiocytic differentiation. Am J Surg Pathol. (1991) 15:1023–33. doi: 10.1097/00000478-199111000-00001
2. Weijie W, Guangzhao Q, Xiangtian Z, Yanping Z, Rongtao Z, Ruopeng L. Clinical landscape of littoral cell angioma in the spleen based on a comprehensive analysis. Front Oncol. (2022) 12:790332. doi: 10.3389/fonc.2022.790332
3. Antón-Pacheco J, Ayuso RM, Cano I, Martinez MA, Cuadros J, Berchi FJ. Splenic littoral cell angioma in an infant. J Pediatr Surg. (2000) 35(3):508–9. doi: 10.1016/S0022-3468(00)90225-2
4. Ertan G, Tekes A, Mitchell S, Keefer J, Huisman TA. Pediatric littoral cell angioma of the spleen: multimodality imaging including diffusion-weighted imaging. Pediatr Radiol. (2009) 39(10):1105–9. doi: 10.1007/s00247-009-1339-x
5. Bedir R, Sehitoğlu I, Calapoğlu AS, Yurdakul C. A rare case of splenic littoral cell angioma in a child. J Lab Physicians. (2014) 6(2):117–20. doi: 10.4103/0974-2727.141511
6. Forest F, Duband S, Clemenson A, Peoc'h M. Traumatic subcapsular splenic hematoma revealing littoral cell angioma and Gaucher’s disease. Ann Hematol. (2010) 89(10):1061–2. doi: 10.1007/s00277-010-0909-1
7. Li Y, Wang X, Cai Y, Peng B. Laparoscopic central splenectomy for littoral cell angioma. J Gastrointest Surg. (2021) 25(2):576–7. doi: 10.1007/s11605-020-04829-7
8. Gakenheimer-Smith L, Mohlman J, VandenHeuvel K, Jackson WD, Thomsen W, Stevenson A, et al. A novel presentation of littoral cell angioma and lymphatic malformations in a neonate. Pediatrics. (2018) 141(Suppl 5):S520–5. doi: 10.1542/peds.2017-2782
9. Shah A, Jasani M, Shah A. Littoral cell angioma: a rare cause of pediatric thrombocytopenia. J Indian Assoc Pediatr Surg. (2018) 23(3):156–7. doi: 10.4103/jiaps.JIAPS_214_17
10. Wu R. Littoral Cell Angioma of the Spleen: Report of 10 Cases and Literature Review. Jinan: Shandong University (2020).
11. Anbardar MH, Perikala VK, Hamid RF. Littoral cell angioma of the spleen: cytological findings and review of the literature. J Cytol. (2017) 34(2):121–4. doi: 10.4103/JOC.JOC_118_15
12. Wang C, Tian Y, Cai W, Li N. Littoral cell angioma of spleen complicated with hemorrhage—2019 film reading window (9). Anhui Yixue. (2019) 40(9):1076–7. doi: 10.3969/j.issn.1000-0399.2019.09.036
13. Lai B, Zhong Q, Peng J. Case 149 answer of diagnose please: rare single littoral cell angioma of spleen in children. Radiol Pract. (2021) 36(3):419–20. doi: 10.13609/j.cnki.1000-0313.2021.03.026
14. Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms. Leukemia. (2022) 36:1720–48. doi: 10.1038/s41375-022-01620-2
15. Li J, Wang Q. Advances in diagnosis and treatment of littoral cell angioma in spleen. Medical Recapitulate. (2011) 17:3429–31.
16. Mac New HG, Fowler CL. Partial splenectomy for littoral cell angioma. J Pediatr Surg. (2008) 43(12):2288–90. doi: 10.1016/j.jpedsurg.2008.07.031
17. Cao Z, Wei J, Cen H, Yuan X, Zhou G, Zhao J, et al. 13 cases of littoral cell angioma in spleens. Beijing Da Xue Xue Bao Yi Xue Ban. (2017) 49:495–500. doi: 10.3969/j.issn.1671-167X.2017.03.020
18. Michael K, Margit B, Thomas R, Martina R, Martin H, Peter W, et al. Littoral cell angioma and angiosarcoma of the spleen: report of two cases in siblings and review of the literature. J Gastrointest Surg. (2012) 16(4):863–7. doi: 10.1007/s11605-011-1773-6
19. Bi C, Jiang L, Li Z, Liu W. Littoral cell angioma of spleen: a clinicopathologic study of 17 cases. Zhonghua Bing Li Xue Za Zhi. (2007) 4(36):239–43.
20. Ding Y, Zeng M, Sheng R, Chen C. Littoral cell angioma of the spleen: correlation between magnetic resonance imaging and pathology features. J Radiol Pract. (2012) 27(07):761–4. doi: 10.13609/j.cnki.1000-0313.2012.07.013
21. Sarandria JJ, Escano M, Kamangar F, Farooqui S, Montgomery E, Cunningham SC. Massive splenomegaly correlates with malignancy: 180 cases of splenic littoral cell tumors in the world literature. Minerva Chir. (2014) 69(4):229–37.24987971
22. Li Q, Wang L, Zhou X, Wang D, Li X. Spontaneous rupture of splenic littoral cell angioma: a case report. J Binzhou Med Coll. (2015) 5:395–7.
23. Susan F, George WC, Daniel AA. Metastasizing splenic littoral cell hemangioendothelioma. Am J Surg Pathol. (2006) 30(8):1036–40. doi: 10.1097/00000478-200608000-00016
24. Yan Z, Wu X, Zhan H, Zhang G, Hu S. Laparoscopic splenectomy for littoral cell angioma of the spleen: a report of 3 cases and review of the literature. J Laparoscopic Surg. (2017) 22(8):588–91. doi: 10.13499/j.cnki.fqjwkzz.2017.08.588
25. Shao F, Yu Q, Xiang L, Hua Y, Chen W, Jun Y, et al. Laparoscopic versus open splenectomy in children: a systematic review and meta-analysis. Pediatr Surg Int. (2016) 32(3):253–9. doi: 10.1007/s00383-015-3845-2
26. Kotoe T, Goro D, Nobuhiro T, Tomoyasu Y, Kenta N, Kenji T, et al. Successful chemotherapeutic treatment for metastatic littoral cell angioma: a case report. Medicine (Baltimore). (2018) 97(15):e0378. doi: 10.1097/MD.0000000000010378
Keywords: littoral cell angioma, splenic tumor, children, treatment, diagnosis
Citation: Mou Y, Yang L, Wang J, Chen Q, Zhang M, Zhang X, Tan R, Adam Mahamat D and Wu K (2024) Case report and literature review: Asymptomatic littoral cell angioma in a 3-year-old girl. Front. Pediatr. 12:1383015. doi: 10.3389/fped.2024.1383015
Received: 6 February 2024; Accepted: 27 March 2024;
Published: 18 April 2024.
Edited by:
Luca Giacomelli, Polistudium srl, ItalyReviewed by:
Ismail Saygın, Karadeniz Technical University, TürkiyeAhu Demiröz, Istanbul University-Cerrahpasa, Türkiye
© 2024 Mou, Yang, Wang, Chen, Zhang, Zhang, Tan, Adam Mahamat and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kai Wu d3VrYWlAc211LmVkdS5jbg==
 Yanling Mou
Yanling Mou Kai Wu
Kai Wu