
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pediatr. , 04 July 2024
Sec. Neonatology
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1382133
 Divya Muthiah1
Divya Muthiah1 Ming Chan2
Ming Chan2 Yue Wey Low1
Yue Wey Low1 Sheena Nishanti Ramasamy3
Sheena Nishanti Ramasamy3 Zubair Amin3,4*
Zubair Amin3,4* Pauline Poh Lin Chan-Ng3
Pauline Poh Lin Chan-Ng3 Jeen Liang Low5
Jeen Liang Low5 Jia Ming Low3,4
Jia Ming Low3,4
Introduction: The aim of the study was to summarize and update clinical features and outcomes of multisystem inflammatory syndrome in neonates (MIS-N).
Methods: A systematic literature search was conducted of studies on MIS-N published in PubMed, MEDLINE, EMBASE, CNKI, and WHO COVID-19 databases between 1 December 2019 and 30 June 2023. Reference lists of selected articles, Google Scholar, and pre-print servers were searched for additional studies. The methodological quality of included studies was assessed.
Results: Of 1,572 records screened after the initial search, 35 studies involving a total of 201 neonates with MIS-N were included. One study was retrieved from a pre-print server. For those with available data, 34/47 (78.7%) mothers were infected in the third trimester. Of the 199 mothers (two with twin pregnancies), 183 (92.0%) were from India. The median age of neonates at presentation was 2.0 days (interquartile range 1.0–9.5). Over two-thirds (144/201, 71.6%) presented with respiratory distress, while 112 (55.7%) had cardiac involvement, such as ventricular dysfunctions, involvement of coronary arteries, and atrioventricular blocks. Arrhythmias and thrombosis were reported in 15/201 (7.5%) and 2/201 (3.0%) neonates, respectively. All neonates, except one, required critical care; 64/160 (40.0%) required inotropic support and 105/187 (56.1%) required respiratory support, of whom 59/105 (56.2%) were specified to require intubation. The mortality rate was 5.0% (10/201).
Discussion/Conclusion: MIS-N should be considered in ill neonates presenting with involvement of two or more organ systems, especially among those neonates with cardiorespiratory dysfunctions, in the presence of proven or suspected maternal COVID-19 infection during pregnancy.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021278717, PROSPERO, identifier CRD42021278717.
Transplacental transfer of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) antibodies from mother to fetus has generally been thought to be protective against SARS-CoV-2 infection (1). However, this transfer of antibodies, along with in utero transfer of other inflammatory cytokines (or the response to antibodies mounted by the neonate to SARS-CoV-2 infection) may, in rare cases, trigger a process similar to multisystem inflammatory syndrome in children (MIS-C), with the potential to cause severe immune activation, manifesting as a multisystem inflammatory syndrome in neonates (MIS-N) (2, 3).
In the initial phase of the pandemic, there was no formal or clear definition of MIS-N, in part due to a scarcity of data as well as difficulty in diagnosis due to many overlapping symptoms (2, 4). In the absence of specific laboratory tests for MIS-N, a diagnosis was thus suspected based on clinical signs and symptoms, together with ancillary laboratory findings. Pawar et al. were the first to distinguish MIS-N as a distinct post-infectious immune-mediated syndrome in infants born to mothers with SARS-CoV-2 infection contracted during pregnancy from complications of postnatally acquired primary COVID-19 (5). Although the specific pathogenesis of MIS-N is unknown, two mechanisms have been postulated: (1) transplacental transfer of maternal antibodies; and (2) vertical transmission of maternal infection resulting in endogenous production of antibodies in the fetus (2, 3). These antibodies then initiate a cascade of exaggerated inflammatory responses causing widespread tissue damage in the neonate (3).
This updated systematic review summarizes cases of MIS-N that have been reported in the literature and describes the clinical features of MIS-N.
A systematic review protocol was developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) checklist and registered in the International Prospective Register of Systematic Reviews (PROSPERO; protocol registration number: CRD42021278717) on 14 March 2021.
Articles were retrieved from PubMed/MEDLINE, EMBASE, China National Knowledge Infrastructure (CNKI), and WHO COVID-19 databases. Gray literature, through Google Scholar, pre-print servers (i.e., Research Square, medRxiv), and reference lists of identified articles, was searched for additional studies of interest. Studies published between 1 December 2019 and 30 June 2023 that reported MIS-N secondary to maternal SARS-CoV-2 exposure or infection were included. The search was restricted to articles in the English language.
The search strategy for PubMed/MEDLINE used keywords and MeSH (MEDLINE) terms; this was then adapted accordingly for the other databases. Search terms included neonate, novel coronavirus, COVID-19, 2019-nCoV, SARS-CoV-2, Coronavirus infection, multisystem inflammatory syndrome in children, multisystem inflammatory syndrome in neonates, MIS-C, MIS-N, Kawasaki, Kawasaki-like, hyperinflammation, hyperinflammatory shock, vasculitis, macrophage activation syndrome, hemophagocytic lymphohistiocytosis, pediatric multisystem inflammatory syndrome, PMIS, and toxic shock syndrome. The search was performed in conjunction with a qualified medical librarian experienced in conducting systematic reviews.
Two reviewers (JML and MC) independently screened titles and abstracts and assessed full-text articles for inclusion. A third reviewer (YWL) resolved any disagreements on study eligibility. Study authors were contacted for clarification if information on eligibility was unavailable or unclear.
We used the following case definition of MIS-N: an inflammatory syndrome affecting neonates (≤28 days of life) with confirmed maternal SARS-CoV-2 exposure or infection during pregnancy in the absence of an alternative diagnosis, whereby there is (1) severe illness requiring hospitalization and (2) two or more organ system involvement or presence of cardiac AV conduction abnormalities/coronary artery dilatation. These had to be accompanied by laboratory evidence of elevated inflammatory markers [C-reactive protein (CRP), procalcitonin, erythrocyte sedimentation rate, lactate dehydrogenase, D-dimer, interleukin-6 (IL-6), ferritin, fibrinogen] with positive SARS-CoV-2 immunoglobulin G (IgG) in the neonate. Maternal SARS-CoV-2 infection or exposure was defined as laboratory-confirmed COVID-19 infection using either quantitative real-time reverse transcription PCR (qRT-PCR) for SARS-CoV-2, immunoassays such as ELISA for SARS-CoV-2 specific IgG/IgM, or clinical history suggestive for SARS-CoV-2 infection. Given the inconsistency of fever in neonates with MIS-N, this was not included as a criterion, unlike in MIS-C. Neonates with postnatal SARS-CoV-2 infection before the development of multisystem inflammation were excluded.
To ensure a comprehensive and up-to-date search on this topic, we determined a priori that the review would include case reports, case series, cohort, case–control, and cross-sectional studies that were published in peer-reviewed journal and pre-print servers. We excluded review articles or articles written based on secondary data.
Two reviewers (DM and JLL) independently extracted individual participant data, such as neonatal and maternal demographics, SARS-CoV-2 test results, clinical manifestations of MIS-N, investigations, findings, treatments, and outcomes.
The quality of included studies was assessed using the framework by Murad et al., which evaluates the methodological quality of studies based on four domains (selection, ascertainment, causality, and reporting) (6). Two reviewers (DM and JLL) completed the quality assessment independently, and a third reviewer (JML) resolved any inconsistencies (Supplementary Material Table S1).
The search yielded 1,572 articles (records identified from databases: 1,559; records identified from citation search: 13). After removing duplicates, 961 articles were screened, with 908 articles excluded after title and abstract screening. Two reports could not be retrieved. Of the remaining 51 articles screened by full text, 35 articles, consisting of 25 case reports (7–30), 9 case series (31–38), and 1 cohort study (39) were selected (Figure 1). One study was identified from a pre-print server (39) and was added to the review in view of the rarity of cases after quality assessment. The total number of MIS-N cases included was 201; 8 additional cases were excluded as they did not meet the study criteria. There were no duplicate cases that appeared in both case reports and case series. Reported denominators in this review represent the cases for which relevant data were available.
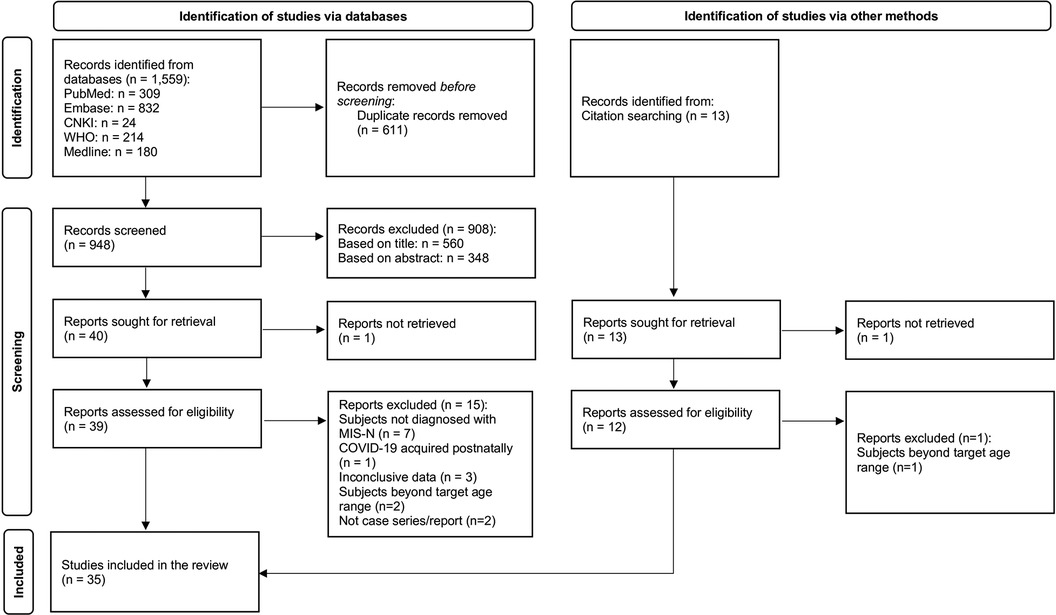
Figure 1 Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram (40).
In total, 34 studies, including the pre-print (7–22, 24–39), fulfilled all four domains of the quality assessment framework; only one study (23) fulfilled two domains. Inter-rater agreement between the two reviewers was 100%. Quality assessment for the domain of subject selection was high (100%), with a low risk of sampling bias where patients represented the whole experience of the investigator/centers. All studies confirmed maternal SARS-CoV-2 infection or exposure during pregnancy, and accurately ascertained outcome measures while excluding alternative diagnoses. All studies described cases with sufficient details for replication or allowed practitioners to make inferences related to their own practice (high quality) (Supplementary Material Table S1).
Participant data from 201 neonates were extracted and statistically combined for analysis (Supplementary Material Table S2).
Of the 201 neonates, 87 (43.3%) were born at term (≥37 weeks); 37/89 (41.6%) were female. The age of presentation was in the range of 1–28 days of life, with a median time of 2 days [interquartile range (IQR) 1.0–9.5]. For those with available data, 14/74 (18.9%) neonates had low birth weight (i.e., below 2.5 kg). Of 100 neonates, 46 (46%) were delivered by normal vaginal delivery and 54 (54%) by Cesarean section (Supplementary Material Table S2).
Of the 199 mothers (two with twin pregnancies), 183 (92.0%) were from India. For those studies with reported data, over one-third (9/22, 40.9%) had comorbidities: pre-eclampsia (n = 3); diabetes mellitus (n = 4); and diabetes with pregnancy-induced hypertension (n = 2), of which one also had hyperthyroidism (Supplementary Material Table S2).
The clinical manifestations are illustrated in Figure 2. Of the 201 neonates, 144 (71.6%) presented with respiratory distress, of whom 28 (19.4%) had persistent pulmonary hypertension of the newborn. The next most common manifestation was cardiovascular disturbances (112/201, 55.7%), including hypotensive shock. Arrhythmias were present in 15/201 (7.5%) neonates, of whom 10/15 (66.7%) had severe bradycardia with prolonged QTc interval and 2:1 atrioventricular block. Of the 201 infants, 30 (14.9%) had coronary artery involvement: 6 (3.0%) had thrombi (intracardiac (n = 4), aortic (n = 1), and pulmonary trunk (n = 1) (Table 1, Supplementary Material Table S3).
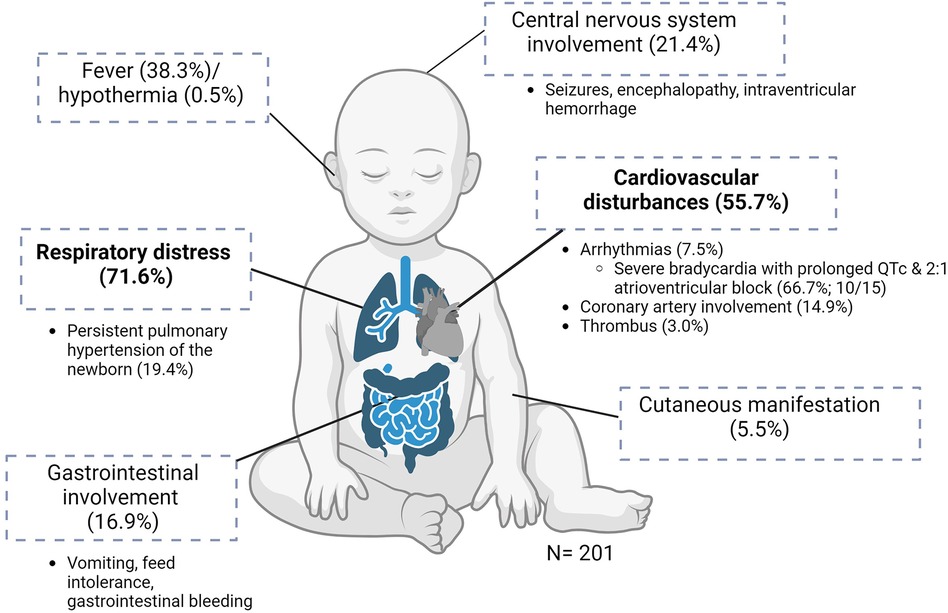
Figure 2 Clinical manifestations of MIS-N (n = 201). Created using BioRender.com.
Of the 201 neonates, 34 (16.9%) had gastrointestinal involvement, mostly in the form of vomiting, feed intolerance, and gastrointestinal tract bleeding. A total of 43/201 (21.4%) infants had neurological involvement in the form of seizures, encephalopathy, or intraventricular hemorrhage. Seven infants (3.5%) had evidence of extravascular fluid leakage, such as pericardial and/or pleural effusions with ascites. A total of 12 (6.0%) infants were reported to have renal impairment. Fever was present in 77/201 (38.3%) neonates and 1 (0.5%) had hypothermia. Of the infants, 11/201 (5.5%) developed cutaneous manifestations during the illness. No musculoskeletal manifestations were reported (Table 1).
Maternal SARS-CoV-2 exposure or infection was diagnosed with qRT-PCR test in 58/199 (29.1%) mothers and by serology in 133/199 (56.8%) mothers; the remainder (28/199, 14.1%) had a diagnosis made based on history of close contact with COVID-19 cases (typically a family member) (Supplementary Material Table S2).
Among those with available data, 25/55 (40.0%) mothers were symptomatic for COVID-19 during pregnancy, with upper respiratory tract symptoms and/or malaise, while 33/55 (60.0%) were asymptomatic. Only one mother had severe illness requiring oxygen supplementation or intensive care. Out of 47 mothers, 37 (78.7%) were infected during their last trimester, 7 (14.9%) in the second trimester, and 3 (6.3%) in the first trimester (Supplementary Material Table S2).
SARS-CoV-2 IgG was detected in 191/201 (95.0%) neonates in the first month of life. Of the 10 that were not detected, 4 had lab-proven maternal history of COVID-19 with placental evidence of infection, 5 had lab-proven maternal history of COVID-19, and 1 was deemed to be positive from suggestive contact tracing; they were culture negative (12/12, 100%) for bacteria and tested negative for other viruses (Supplementary Material Table S2).
We report the following data made available by individual publications, hence the denominators vary. Inflammatory markers were raised in most patients, with high levels of CRP and ferritin reported in 115/185 (62.1%) and 87/161 (54.0%) neonates, respectively. Of the 46/201 (22.8%) neonates who had CRP values available, the median CRP level was 4.25 mg/dl (IQR 1.72–7.6 mg/dl; normal range <1 mg/L). Of the 201 neonates, 50 (24.8%) had ferritin values available: the median ferritin level was 873 ng/ml (IQR 371.2–1,466). Procalcitonin was raised in 19/57 (33.3%) neonates, lactate dehydrogenase in 90/182 (49.5%) neonates, and D-dimer in 123/190 (64.7%). IL-6 was found to be raised in 16/17 (94.1%) neonates who were tested, of whom 13 had the following reported values: median 36.2 (IQR 20.1–69.2; normal range 0–7 pg/ml). Out of 200 neonates, 85 (42.5%) had elevated cardiac enzymes. Hematological abnormalities included thrombocytopenia in 41/70 (58.5%) neonates and thrombocytosis in 19/70 (27.1%) (Supplementary Material Table S3).
ECG findings were reported in 32/105 (30.5%) neonates, of which there were 6 normal sinus rhythms, 12 sinus tachycardia, 2 supraventricular tachycardia, 1 atrial bigeminy, 1 non-specific ST change, and 10 bradycardias with prolonged corrected QT interval, of which 9 had 2:1 AV blocks (Supplementary Material Table S4).
Chest X-ray findings included 10 normal radiographs, 2 with cardiomegaly, 1 with bilateral pulmonary infiltrates, and 1 with diffuse hazy granular opacities. Two patients underwent a CT scan of the thorax, which showed an inflammatory ground-glass pattern in one patient and bilateral reticulonodular opacities in the other (Supplementary Material Table S3).
All neonates, except one, required intensive care. Of 160 neonates, 64 (40%) required inotropic support and 105/187 (56.1%) required respiratory support, of whom 59 (56.2%) were specified to require intubation. Immunomodulatory therapy [intravenous steroids and/or intravenous immunoglobulins (IVIGs)] was used in 125/201 (62.2%) neonates: 104 (51.7%) received IVIG and 107 (53.2%) received steroids. Among the 107 neonates who received steroid therapy, 72 (67.3%) received intravenous methylprednisolone or prednisolone, 22 (20.6%) received dexamethasone, and 4 (3.7%) received hydrocortisone. The type of steroids, however, was not specified for 9 (8.4%) neonates. Anticoagulants were used in 33/201 (16.4%) neonates and aspirin in 30/201 (14.9%). None received biologics or convalescent plasma (Supplementary Material Table S5).
The mean length of stay of the neonates was 21.86 days (range 5–150 days). The mortality rate for MIS-N was 5.0% (10/201). No long-term outcomes were reported for the patients who survived until discharge or follow-up (191/201, 95.0%). The duration of follow-up was up to 3 months (Supplementary Material Table S5).
Our review summarizes the current data available on MIS-N in neonates born to mothers who contracted COVID-19 during pregnancy and distinguishes itself from previous systematic reviews published earlier in the pandemic that summarized both in utero and postpartum transmission of COVID-19 viral-induced, post-infective immune dysregulation in the neonatal population. Our review also serves as an update of the existing literature (2, 4). At the time of writing, another systematic review has been published, which analyzed the clinical features and management strategies of published cases of MIS-N up to 30 September 2022 (41). As there has been an increase in reported cases, we then systematically updated the search and completed a comprehensive review with statistical analyses to provide further insights and understanding of this relatively new condition.
Initial reviews of MIS-N reported a mortality rate in the range of 8.2%–11% (42); however, our current review with almost double the number of cases reported a lower mortality rate of 5% (10/201). This difference could be related to probable reporting bias for critical neonates with MIS-N in the literature in earlier part of epidemic.
We defined MIS-N as a distinct entity from neonates who develop MIS-C after COVID-19 infections contracted after birth (2, 4). This distinction from MIS-C was needed given the differences in clinical presentations between MIS-C and MIS-N. It is critical to recognize MIS-N, as all neonates afflicted with MIS-N had a more severe clinical course with almost all requiring intensive care.
We observed certain features unique to MIS-N. Unlike postnatally acquired SARS-CoV-2 infection or MIS-C, less than half of the affected neonates with MIS-N had fever (43, 44). More than two-thirds of neonates with MIS-N typically presented with cardiorespiratory dysfunction within 2 days of birth. MIS-N–affected neonates had high levels of inflammatory cytokines, such as IL-6, and other inflammatory markers, such as CRP and ferritin. However, there are no specific biomarkers for MIS-N (3). Nonetheless, these findings should be interpreted with caution as there was no control group of infants born to women without COVID-19 during pregnancy and hence cannot be concluded to be causal. Understandably, this area of work would be challenging to study given the uptake in COVID-19 vaccination status among pregnant women and the added complexity of interpreting SARs-CoV-2 antibody levels in neonates whose mothers have been vaccinated. While we continue to evaluate whether these findings are attributable to SARS-CoV2 in pregnancy, having a low threshold for ECG and ECHO in ill neonates with perplexing diagnoses seems reasonable.
We also attempted to evaluate the vaccination status of mothers of neonates with MIS-N; however, the numbers were too small to be conclusive. While the risk of MIS-N in neonates born to vaccinated mothers has not been extensively reviewed (44), emerging evidence supports the protective effect of maternal mRNA COVID-19 vaccinations during pregnancy against SARS-CoV-2 infection and its complications, including MIS-N among infants in the first 6 months of life (45). The role of transplacental transfer of antibodies in protecting infants is also seen with other recommended antenatal vaccine-preventable diseases, such as influenza (46). Future work involving neonates afflicted with MIS-N should investigate maternal vaccination status as a potential protective mechanism against MIS-N.
From a clinical perspective, the diagnosis in newborns is based on clinical suspicion, suggestive history from the mother, and suggestive lab findings. Given the rarity of this condition, a high index of suspicion is needed. If the diagnosis is suspected, we propose that a transthoracic echocardiogram, a non-invasive and relatively easily test, should be performed and infants should be monitored closely for multisystem involvement. It is imperative that concurrent diagnoses, such as neonatal sepsis and other viral infections (e.g., enterovirus infection), should be entertained and additional specific therapy, such as empirical antibiotics, should be added.
In this review, we observed that the principles of treating MIS-C were used in the treatment of MIS-N. At present, there is insufficient evidence to conclude that immunomodulatory agents, such as early initiation of steroids and IVIG, play a beneficial role in the treatment of MIS-N. More work is required to determine the efficacy of immunomodulatory treatment. Therefore, the mainstay of therapy is supportive. It is essential that adequate cardiorespiratory support, fluid and nutrition, and thermoregulation are provided. In the absence of a proper clinical trial, it is imprudent to suggest routine use of systemic steroids or other immune modulatory therapy for all cases of MIS-N, especially because of the risk of adverse side effects from systemic steroids in neonates including possible long-term neurodevelopmental impairment.
The strength of this review is the inclusion of a larger number of cases with a detailed statistically combined data analysis. This review has some limitations. First, data on some variables of interest, such as maternal COVID-19 vaccination status, were not available for all included cases. Second, the lack of universal definition could lead to a misdiagnosis or underreporting of MIS-N cases. Third, there could be reporting bias as most cases were case reports and case series from India, which form the current predominant available literature for MIS-N. We have included one study, a prospective cohort, from pre-print servers (47); which contributed to 100 cases (out of 201). Finally, the long-term outcomes of MIS-N are unknown. Further studies with larger cohorts over an extended period of time are necessary to investigate the full spectrum of clinical features and short- and long-term outcomes.
In conclusion, healthcare professionals looking after neonates born to mothers with SARS-CoV-2 infection or significant exposure should remain cognizant of MIS-N as a possible differential diagnosis in seriously unwell babies as MIS-N appears to have distinct clinical presentation, laboratory profiles, and outcomes.
DM: Conceptualization, Data curation, Formal Analysis, Writing – original draft, Writing – review & editing. MC: Writing – original draft. YL: Writing – original draft, Data curation, Methodology. SR: Writing – review & editing. ZA: Writing – original draft, Writing – review & editing. PC-N: Writing – review & editing. JLL: Data curation, Formal Analysis, Methodology, Writing – original draft, Writing – review & editing. JML: Conceptualization, Data curation, Methodology, Writing – original draft, Writing – review & editing.
The authors declare financial support was received for the research, authorship, and/or publication of this article.
JML was supported by the National University Health System (NUHS), Singapore, Clinician Scientist Program 2.0 (grant reference no.: NCSP2.0/2023/PVO/LJM) and the National Medical Research Council (NMRC) research training fellowship (MOH-RTF23jan-0004/MOH-001381-00). The funders had no role in study design, data collection, analysis, interpretation and decision to publish. The other authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1382133/full#supplementary-material.
1. Otero S, Miller ES, Sunderraj A, Shanes ED, Sakowicz A, Goldstein JA, et al. Maternal antibody response and transplacental transfer following severe acute respiratory syndrome coronavirus 2 infection or vaccination in pregnancy. Clin Infect Dis. (2023) 76(2):220–8. doi: 10.1093/cid/ciac793
2. Shaiba LA, More K, Hadid A, Rana A, Munira AM, Mahdi A, et al. Multisystemic inflammatory syndrome in neonates: a systematic review. Neonatology. (2022) 119(4):405–17. doi: 10.1159/000524202
3. Gee S, Chandiramani M, Seow J, Pollock E, Modestini C, Das A, et al. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat Immunol. (2021) 22(12):1490–502. doi: 10.1038/s41590-021-01049-2
4. De Rose DU, Pugnaloni F, Calì M, Ronci S, Caoci S, Maddaloni C, et al. Multisystem inflammatory syndrome in neonates born to mothers with SARS-CoV-2 infection (MIS-N) and in neonates and infants younger than 6 months with acquired COVID-19 (MIS-C): a systematic review. Viruses. (2022) 14(4):750. doi: 10.3390/v14040750
5. Pawar R, Gavade V, Patil N, Mali V, Girwalkar A, Tarkasband V, et al. Neonatal multisystem inflammatory syndrome (MIS-N) associated with prenatal maternal SARS-CoV-2: a case series. Children (Basel). (2021) 8(7):572. doi: 10.3390/children8070572
6. Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. (2018) 23(2):60–3. doi: 10.1136/bmjebm-2017-110853
7. Divekar AA, Patamasucon P, Benjamin JS. Presumptive neonatal multisystem inflammatory syndrome in children associated with coronavirus disease 2019. Am J Perinatol. (2021) 38(6):632–6. doi: 10.1055/s-0041-1726318
8. Lima ARO, Cardoso CC, Bentim PRB, et al. Maternal SARS-CoV-2 infection associated to systemic inflammatory response and pericardial effusion in the newborn: a case report. J Pediatric Infect Dis Soc. (2021) 10(4):536–9. doi: 10.1093/jpids/piaa133
9. Kappanayil M, Balan S, Alawani S, Mohanty S, Leeladharan SP, Gangadharan S, et al. Multisystem inflammatory syndrome in a neonate, temporally associated with prenatal exposure to SARS-CoV-2: a case report. Lancet Child Adolesc Health. (2021) 5(4):304–8. doi: 10.1016/S2352-4642(21)00055-9
10. McCarty KL, Tucker M, Lee G, Pandey V. Fetal inflammatory response syndrome associated with maternal SARS-CoV-2 infection. Pediatrics. (2021) 147(4):e2020010132. doi: 10.1542/peds.2020-010132
11. Schoenmakers S, Snijder P, Verdijk RM, Kuiken T, Kamphuis SSM, Koopman LP, et al. SARS-CoV-2 placental infection and inflammation leading to fetal distress and neonatal multi-organ failure in an asymptomatic woman. J Pediatric Infect Dis Soc. (2020) 10(5):556–61. doi: 10.1093/jpids/piaa153
12. Borkotoky RK, Banerjee Barua P, Paul SP, Heaton PA. COVID-19-related potential multisystem inflammatory syndrome in childhood in a neonate presenting as persistent pulmonary hypertension of the newborn. Pediatr Infect Dis J. (2021) 40(4):e162–4. doi: 10.1097/INF.0000000000003054
13. Shaiba LA, Hadid A, Altirkawi KA, Bakheet HM, Alherz AM, Hussain SA, et al. Case report: neonatal multi-system inflammatory syndrome associated with SARS-CoV-2 exposure in two cases from Saudi Arabia. Front Pediatr. (2021) 9:652857. doi: 10.3389/fped.2021.652857
14. Amonkar PS, Gavhane JB, Kharche SN, Kadam SS, Bhusare DB. Aortic thrombosis in a neonate with COVID-19-related fetal inflammatory response syndrome requiring amputation of the leg: a case report. Paediatr Int Child Health. (2021) 41(3):211–6. doi: 10.1080/20469047.2021.1968596
15. Diwakar K, Gupta BK, Uddin MS, Sharma A, Jhajra S. Multisystem inflammatory syndrome with persistent neutropenia in neonate exposed to SARS-CoV-2 virus: a case report and review of literature. J Neonatal Perinatal Med. (2022) 15(2):373–7. doi: 10.3233/NPM-210839
16. Costa S, Delogu AB, Bottoni A, Purcaro V, D’Andrea V, Paladini A, et al. COVID-19-associated multisystem inflammatory syndrome in a neonate with atypical coronary artery involvement. Am J Perinatol. (2022) 29(14):1514–8. doi: 10.1055/a-1733-4163
17. Amulya GI, Kaur A, Sharma M. Multisystem inflammatory syndrome in a neonate with prenatal exposure to COVID-19. Indian J Pediatr. (2022) 89(3):314. doi: 10.1007/s12098-021-03948-6
18. Agrawal G, Wazir S, Arora A, Sethi SK. Multisystem inflammatory syndrome in a neonate masquerading as surgical abdomen. BMJ Case Rep. (2021) 14(10):e246579. doi: 10.1136/bcr-2021-246579
19. Bakhle A, Sreekumar K, Baracho B, Sardessai S, Silveira MP. Cavitary lung lesions in a neonate: potential manifestation of COVID-19 related multisystem inflammatory syndrome. Pediatr Pulmonol. (2022) 57(1):311–4. doi: 10.1002/ppul.25732
20. Nitya US, Devassy BM, Joseph S, Anila AP, George R, Sreenivasan VK. Intrauterine onset of multisystem inflammatory syndrome in a neonate temporally associated with maternal COVID-19. Indian Journal of Rheumatology. (2022) 17(3):289–93. doi: 10.4103/injr.injr_4_22
21. Sojisirikul N, Lapphra K, Ngerncham S, Charuvanij S, Durongpisitkul K, Curlin ME, et al. Neonatal multisystem inflammatory syndrome (MIS-N): the first case report in Thailand. COVID. (2022) 2(9):1265–9. doi: 10.3390/covid2090093
22. Voddapelli SK, Murki S, Rao VP. Neonatal multisystem inflammatory syndrome (MIS-N) presenting as necrotizing enterocolitis and cardiac dysfunction. Indian Pediatr. (2022) 59(6):502–3. doi: 10.1007/s13312-022-2544-z
23. Gupta P, Arun S, Tamatam PR, Dhulipudi B, Vardhelli V, Deshabhotla S, et al. Neonatal multisystem inflammatory syndrome (MIS-N) associated with maternal SARS-CoV-2 exposure. Indian J Pediatr. (2022) 89:827–8. doi: 10.1007/s12098-022-04167-3
24. Malek A, Khadga M, Zahid MN, Mojib S, Debnath R, Khan S, et al. Multisystem inflammatory syndrome of a neonate from a COVID-19-infected mother: a case report. Cureus. (2022) 14(3):e23046. doi: 10.7759/cureus.23046
25. Shinde MD, Khamkar AM, Pote PD, Suryawanshi PB. Fetal inflammatory response syndrome associated with SARS-CoV-2 exposure in utero. Pediatric Oncall J. (2022) 19(4). doi: 10.7199/ped.oncall.2022.45
26. Aguilar-Caballero D, Capcha JMC, Caballero V, Young KC, Duara S, Borchetta M, et al. Case report: fatal lung hyperinflammation in a preterm newborn with SARS-CoV-2 infection. Front Pediatr. (2023) 11:1144230. doi: 10.3389/fped.2023.1144230
27. Arun S, Cherian TG, Philip C. Multisystem inflammatory syndrome in a neonate with severe hemophilia-a diagnostic challenge in COVID times: a case report. BMC Pediatr. (2022) 22(1):1–4. doi: 10.1186/s12887-022-03463-3
28. Ragireddy A, Das RK, Mallick B, Nanda D. An unusual course of pneumonia in a term neonate with suspected multisystem inflammatory syndrome secondary to severe acute respiratory syndrome coronavirus 2 infection. J Clin Neonatol. (2023) 12:34–7. doi: 10.4103/jcn.jcn_96_22
29. Rackauskaite S, Lipnevicius A, Sendzikaitė S. Paediatric inflammatory multisystem syndrome in a neonate with CHD: case description and current issues in children with CHD. Cardiol Young. (2023) 33(2):321–2. doi: 10.1017/S1047951122001718
30. Abdulaziz-Opiela G, Sobieraj A, Sibrecht G, Bajdor J, Mrozinski B, Kozlowska Z, et al. Prenatal and neonatal pulmonary thrombosis as a potential complication of SARS-CoV-2 infection in late pregnancy. Int J Mol Sci. (2023) 24(8):7629. doi: 10.3390/ijms24087629
31. Shanker V, Chaudhary M, Shanker P. Antenatal SARS-COV-2 exposure leading to multisystem inflammatory syndrome (MIS-N) presenting with neonatal encephalopathy. J Clin Pediatr Neonatol. (2021) 1(3):41–4.
32. More K, Aiyer S, Goti A, Parikh M, Sheikh S, Patel G, et al. Multisystem inflammatory syndrome in neonates (MIS-N) associated with SARS-CoV2 infection: a case series. Eur J Pediatr. (2022) 181(5):1883–98. doi: 10.1007/s00431-022-04377-z
33. Tambekar HJ, Ashtekar SD, Mirgunde SP, Khot S, Mane S. Neonatal multisystem inflammatory syndrome associated with prenatal maternal SARS-CoV-2 exposure: a case series. Inter J Contemp Pediatr. (2022) 9(4):381. doi: 10.18203/2349-3291.ijcp20220766
34. Saeedi M, Mirnia K, Sangsari R, Jannatmakan Z, Ziaee V. Neonatal multisystem inflammatory syndrome associated with COVID-19 exposure in two cases from Iran. J Compr Ped. (2023) 14(2):e134897. doi: 10.5812/compreped-134897
35. Balleda L, Pasupula S, Kolla S, Thimmapuram CR. Clinical profile, laboratory parameters, management and outcomes of newborns with multisystem inflammatory syndrome (MIS-N) due to transplacental transfer of SARS-CoV 2 antibodies: a study from a tertiary care institute. J Clin Neonatol. (2022) 11(2):65. doi: 10.4103/jcn.jcn_1_22
36. Chaudhuri M, Tomar M, Gaonkar S, Rastogi A, Shenoi A. Pilot study analyzing combination of point-of-care echocardiography and clinical correlation in unveiling cryptic multi-inflammatory syndrome in neonates during coronavirus disease 2019 pandemic. J Indian Acad Echocardio Cardiovasc Imag. (2022) 6(2):89–99. doi: 10.4103/jiae.jiae_64_21
37. Hashiq N, Nigade R, Amith K, Kurane AB. Multisystem inflammatory syndrome in a neonate, temporally associated with prenatal exposure to SARS-COV-2: case series. I J Paediatr Geriatrics. (2021) 4(1):148–50. doi: 10.33545/26643685.2021.v4.i1c.146
38. Gamez-Gonzalez LB, Escrcega-Jurez AS, Aguilar-Soto DE, Colmenero Rascon M, Garcia Espinosa AC, Yamazaki-Nakashimada MA. Multisystem inflammatory syndrome in neonates associated with SARS-CoV-2 infection, a different entity? J Neonatal Perinat Med. (2022) 16:169–77. doi: 10.3233/NPM-220990
39. Charki S, Patil V, Lavanya P, Nandakishore K. Clinical spectrum and outcome of neonatal multi-system inflammatory syndrome (MIS-N)—A prospective cohort study. Authorea. (2022). doi: 10.22541/au.166979176.66104074/v1
40. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
41. Lakshminrusimha S, More K, Shah PS, Wynn JL, Sánchez PJ. Multisystem inflammatory syndrome in neonates (MIS-N) associated with perinatal SARS CoV-2 infection: does it exist? Semin Fetal Neonatal Med. (2023) 28(2):101433. doi: 10.1016/j.siny.2023.101433
42. Centers for Disease Control and Prevention. Multisystem Inflammatory Syndrome in Children (MIS-C) Associated with Coronavirus Disease 2019 (COVID-19) (2020). Available online at: https://emergency.cdc.gov/han/2020/han00432.asp (accessed March 15, 2023).
43. Trevisanuto D, Cavallin F, Cavicchiolo ME, Borellini M, Calgaro S, Baraldi E. Coronavirus infection in neonates: a systematic review. Arch Dis Child Fetal Neonatal Ed. (2021) 106(3):330–5. doi: 10.1136/archdischild-2020-319837
44. De Rose DU, Salvatori G, Dotta A, Auriti C. SARS-CoV-2 vaccines during pregnancy and breastfeeding: a systematic review of maternal and neonatal outcomes. Viruses. (2022) 14(3):539. doi: 10.3390/v14030539
45. Mangat C, Yarrarapu SNS, Singh G, Bansal P. Maternal COVID-19 vaccine may reduce the risk of MIS-C in infants: a narrative review. Vaccines (Basel). (2022) 10(9):1454. doi: 10.3390/vaccines10091454
46. Marchant A, Sadarangani M, Garand M, Dauby N, Verhasselt V, Pereira L, et al. Maternal immunisation: collaborating with mother nature. Lancet Infect Dis. (2017) 17(7):e197–208. doi: 10.1016/S1473-3099(17)30229-3
Keywords: multisystem inflammatory syndrome, COVID-19, neonates, pregnancy, cardiorespiratory dysfunction
Citation: Muthiah D, Chan M, Low YW, Ramasamy SN, Amin Z, Chan-Ng PPL, Low JL and Low JM (2024) Multisystem inflammatory syndrome in neonates (MIS-N): an updated systematic review. Front. Pediatr. 12:1382133. doi: 10.3389/fped.2024.1382133
Received: 5 February 2024; Accepted: 11 June 2024;
Published: 4 July 2024.
Edited by:
Muhammad Waseem, Lincoln Medical Center, United StatesReviewed by:
Domenico Umberto De Rose, Bambino Gesù Children’s Hospital (IRCCS), Italy© 2024 Muthiah, Chan, Low, Ramasamy, Amin, Chan-Ng, Low and Low. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zubair Amin, cGFlemFAbnVzLmVkdS5zZw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.