- 1Department of General Surgery, Peking University Third Hospital, Beijing, China
- 2School of Basic Medical Sciences, Health Science Centre, Peking University, Beijing, China
- 3Department of Maternal and Child Health, School of Public Health, Health Science Centre, Peking University, Beijing, China
- 4Department of Ultrasound, Peking University Third Hospital, Beijing, China
- 5National Key Laboratory for Multimedia Information Processing, School of Electronics Engineering and Computer Science, Faculty of Information and Engineering Science, Peking University, Beijing, China
- 6Information Management and Big Data Center, Peking University Third Hospital, Beijing, China
Background: Differentiated thyroid cancer (DTC) has been increasingly common in women of reproductive age. However, the evidence remains mixed regarding the association of DTC with adverse pregnancy outcomes in pregnant women previously diagnosed with DTC.
Methods: We conducted a retrospective cohort study in the Peking University Third Hospital in Beijing, China between January 2012 and December 2022. We included singleton-pregnancy women with a pre-pregnancy DTC managed by surgical treatment (after-surgery DTC) or active surveillance (under-surveillance DTC). To reduce the confounding effects, we adopted a propensity score to match the after-surgery and under-surveillance DTC groups with the non-DTC group, respectively, on age, parity, gravidity, pre-pregnancy weight, height, and Hashimoto's thyroiditis. We used conditional logistics regressions, separately for the after-surgery and under-surveillance DTC groups, to estimate the adjusted associations of DTC with both the composite of adverse pregnancy outcomes and the specific mother-, neonate-, and placenta-related pregnancy outcomes.
Results: After the propensity-score matching, the DTC and non-DTC groups were comparable in the measured confounders. In the after-surgery DTC group (n = 204), the risk of the composite or specific adverse pregnancy outcomes was not significantly different from that of the matched, non-DTC groups (n = 816; P > 0.05), and the results showed no evidence of difference across different maternal thyroid dysfunctions, gestational thyrotropin levels, and other pre-specified subgroup variables. We observed broadly similar results in the under-surveillance DTC group (n = 37), except that the risk of preterm birth, preeclampsia, and delivering the low-birth-weight births was higher than that of the matched, non-DTC group [n = 148; OR (95% CI): 4.79 (1.31, 17.59); 4.00 (1.16, 13.82); 6.67 (1.59, 27.90)].
Conclusions: DTC was not associated with adverse pregnancy outcomes in pregnant women previously treated for DTC. However, more evidence is urgently needed for pregnant women with under-surveillance DTC, which finding will be clinically significant in individualizing prenatal care.
Introduction
Since the implementation of China's two-child policy (1), the proportion of high-risk pregnancies increased from 15.7% in 2008 to 24.7% in 2016 and exceeded 30.0% in 2018 (2). The corresponding increase in adverse pregnancy outcomes has brought new challenges to the reduction of maternal mortality ratio (3–5). Along with this, females have also faced the 2nd most commonly diagnosed cancer related to pregnancy: thyroid cancer. Of all thyroid cancers, differentiated thyroid cancer (DTC) accounts for more than ninety percent. Notably, the incidence of DTC shows an increasing trend among women of reproductive age (6). Recently, a focus on patients with a pre-pregnancy DTC has been strengthened in the Guidelines (2022) for Prevention and Management of Thyroid Diseases During Pregnancy and Perinatal Period in China (7).
Women with DTC are “candidates” for high-risk pregnancies. They would primarily undergo thyroidectomy surgery to manage DTC. They would also receive postoperative thyrotropin (TSH) suppression therapy (the thyroid hormone therapy used to suppress serum TSH levels lower than the normal range) to minimize the risk of DTC recurrence (8). Both surgery and postoperative hormone therapy may result in hypothyroidism or hyperthyroidism (9, 10), and this is more frequently occurring in pregnant women due to the fluctuation of gestational thyroid hormones. As an alternative to immediate surgery, active surveillance is a viable option for appropriately selected patients with low-risk DTC (11). Active surveillance refers to a period of close monitoring of cancer progression without performing immediate surgery. Compared to patients with immediate surgery, patients under active surveillance were shown to be at higher anxiety due to the risk for possible DTC progression (12, 13), predisposing them to adverse pregnancy outcomes.
It is important to clarify whether DTC is associated with the risk of adverse pregnancy outcomes. Notably, multiple risk factors other than DTC have been reported to relate to adverse pregnancy outcomes (14, 15). Theoretically, pregnant women would benefit from all prevention measures for adverse pregnancy outcomes. Practically, it is often not possible to implement such prevention measures at scale due to a lack of resources in real-world settings. To optimize resource allocation for prenatal care, pregnant women are often stratified into high-, moderate-, or low-risk groups and receive targeted interventions corresponding to their risk stratification. Therefore, if DTC is indeed associated with an elevated risk of adverse pregnancy outcomes, DTC should be considered as one risk factor in the management system of risk stratification for pregnant women; if not, clinicians can inform patients that additional prenatal examinations specific to DTC are unnecessary in most cases. This shared decision-making between clinicians and patients is not only helpful in relieving the current strain of medical resources but also in reducing the patients’ psychological burden due to the cancer “label” attached to them.
Controversy remains regarding the association of DTC with adverse pregnancy outcomes (16). This could be interpreted by the study population, controlled confounders, study outcomes, analysis methods, or effect modification. Until now, no studies on the present topic have focused on pregnant women with a pre-pregnancy DTC managed by active surveillance (briefly termed under-surveillance DTC below). To fill the research gap, our study included patients with under-surveillance or after-surgery DTC, adopted propensity-score matching (PSM) to control confounding effects (17) and clarified the association of DTC with the risk of adverse mother-, placental-, or neonatal-related pregnancy outcomes. We also conducted several subgroup analyses to examine the potential factors that modify the exposure-outcome associations. The findings of this study would have important significance for clinical decision-making in individualizing prenatal care.
Methods
Study population
Our multidisciplinary research team included experts in the fields of surgery, pathology, ultrasound, obstetrics, endocrinology, and maternal and child health. We conducted a retrospective cohort study at Peking University Third Hospital in Beijing, China between January 2012 and December 2022.
For the exposed group, we included singleton-pregnancy women with a pre-pregnancy DTC managed by active surveillance (under-surveillance DTC) or surgical treatment (after-surgery DTC). We excluded those with a diagnosis of malignant tumors other than DTC. If women had multiple pregnancies, we only included their first pregnancy. Specifically, women were eligible for the under-surveillance DTC group if they: (1) undergo no less than 2 rounds of examinations of neck ultrasonography during surveillance (before surgical treatment), (2) be under surveillance for at least 6 months, and (3) have no evidence of lymph node metastasis or extrathyroid invasion at baseline examination. Women were eligible for the after-surgery DTC group if they: (1) be treated with total thyroidectomy or lobectomy, and (2) have follow-up (after-surgery) examinations of neck ultrasonography or measurements of serum thyroglobulin (Tg) levels and Tg antibodies.
For the control group, we included singleton-pregnancy women without a pre-conception diagnosis of DTC and further obtained a propensity score (PS) matched population to balance measured confounders between the DTC and non-DTC control groups. We took two steps for the PS matching. First, we used logistic regression to estimate the PS (18), i.e., the probability of an individual included in the exposed group conditional on the observed confounders (X) at baseline: . Based on literature review (16, 19–25) as well as domain knowledge of the present topic, we selected observed confounders including age, parity, gravidity, pre-pregnancy weight, height, and Hashimoto's thyroiditis. Then, we performed the matching at a 1:4 ratio with a caliper of width equal to 0.4 standard deviation of the logit of the PS. This choice of caliper width was made after careful consideration of both the balance of baseline confounders and the sample size, building on our prior work (26, 27). Data were carefully collected from medical records by two researchers with rich experiences in clinical practice (X.L.) and statistical analyses (W.C.X.). This study obtained approval from the Medical Research Ethics Committee of Peking University Third Hospital (No. IRB00006761-M2022721).
Pregnancy outcomes
Taking advantage of well-documented, fine-grained clinical records, we were able to collect a variety of mother-, neonate-, or placenta-related pregnancy outcomes. The primary outcome was the composite of adverse pregnancy outcomes (i.e., the occurrence of any adverse pregnancy outcomes). The secondary outcomes were the specific type of adverse pregnancy outcomes including mother-, neonate-, or placenta-related pregnancy outcomes. Mother-related pregnancy outcomes included assisted reproduction, cesarean delivery, preterm birth, gestational diabetes mellitus, preeclampsia, abortion, premature rupture of membranes, precipitate labor, postpartum anemia, and postpartum hemorrhage. Neonate-related pregnancy outcomes included stillbirth, macrosomia, low birth weight, and small for gestational age. Placenta-related pregnancy outcomes included placental abruption, abnormal aggressive placenta, placenta previa, retained placenta, and battledore placenta.
Statistical analyses
To compare differences between groups, we used the t-test and Kruskal-Wallis rank sum test for normally and abnormally distributed continuous variables, respectively, and the Pearson chi-square test or Fisher exact probability test (when the number of patients ≤ 5) for categorical variables. After the establishment of the PS model, we compared differences in baseline characteristics between the DTC and PS-matched non-DTC groups by using weighted regression based on the matching weight; specifically, we compared means and prevalence of baseline measured characteristics of subjects via the recommended standardized differences for continuous [Formula (1)] and dichotomous [Formula (2)] variables, respectively (28). We used the threshold value of standardized differences higher than 10% to indicate a meaningful imbalance in baseline covariate distribution between the DTC and non-DTC groups. We used conditional logistics regression models, taking into account the matching properties of the data, to assess the associations of DTC with pregnancy outcomes, for the under-surveillance and after-surgery DTC patients, respectively.
( and denote the sample mean of the covariate in exposed and control subjects, respectively; and denote the sample variance of the covariate in exposed and control subjects, respectively; and denote the prevalence of the dichotomous variable in exposed and control subjects, respectively).
We further conducted pre-specified subgroup analyses at two levels. First, in both the DTC and non-DTC groups, we explored whether the exposure-outcome associations differed by maternal advanced age (≥35, <35 years), primipara (yes, no), overweight or obesity (yes, no), assisted reproduction (yes, no), overt hyperthyroidism (yes, no), subclinical hyperthyroidism (yes, no), overt hypothyroidism (yes, no), subclinical hypothyroidism (yes, no), Hashimoto's thyroiditis (yes, no), TSH score (reflecting the average levels of TSH during gestation), and TSH instability (reflecting the degree of fluctuation of TSH during gestation). Second, specifically in the DTC group, we examined whether the risk of adverse pregnancy outcomes differed by tumor type (papillary thyroid carcinoma; follicular thyroid carcinoma), surgery type (total thyroidectomy; lobectomy), response to therapy (excellent; incomplete), TSH suppression therapy (over treatment; mild under treatment; under treatment), the time interval between surgical treatment and conception. Details about the subgroup variables and the cutoffs are shown in Supplementary Table S1. Statistical analyses were performed using R software version 4.3 and Stata software version 16.0. P values less than 0.05 were considered statistically significant.
Results
Baseline characteristics of the DTC and non-DTC groups
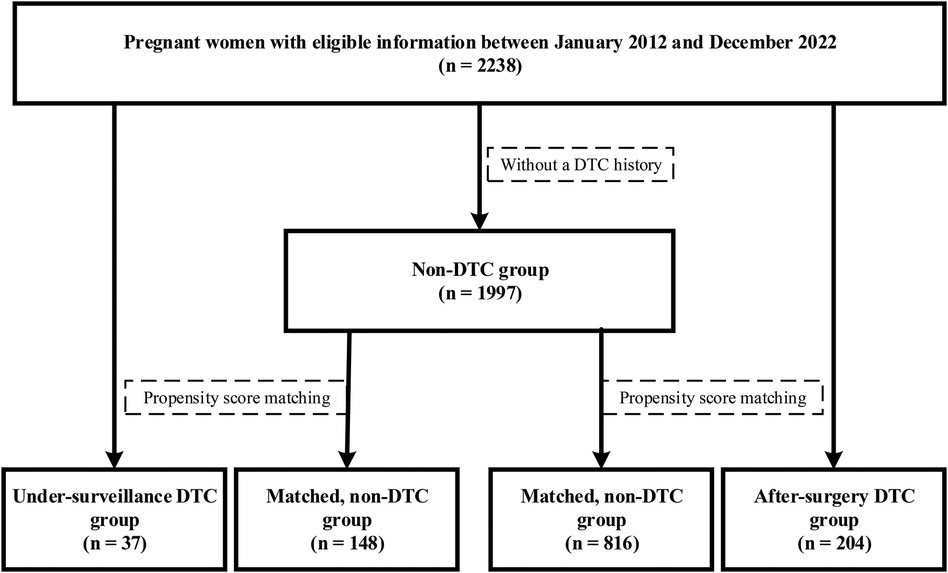
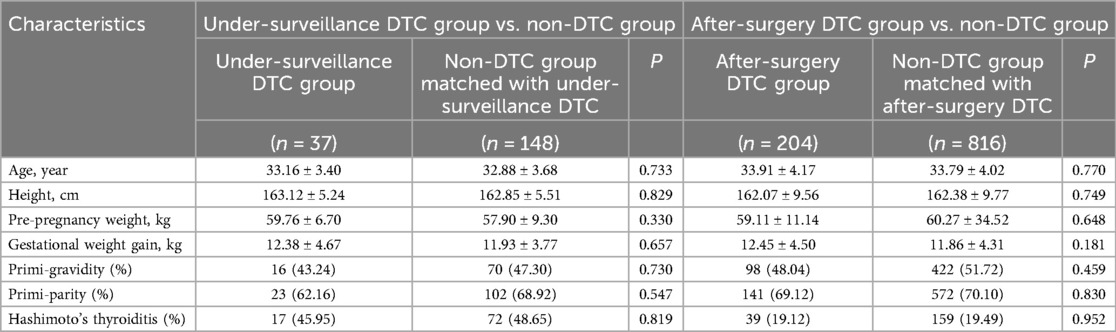
The under-surveillance and after-surgery DTC groups included 37 and 204 pregnant women, respectively, and the correspondingly PS-matched, non-DTC control groups included 148 and 816 pregnant women, respectively (Figure 1). Before PS matching, the DTC and non-DTC groups differed in the distribution of maternal age and occurrence of Hashimoto's thyroiditis (Supplementary Table S2). After PS matching, the DTC group and the matched non-DTC groups were broadly similar in all measured confounders (Table 1; Figure 2).
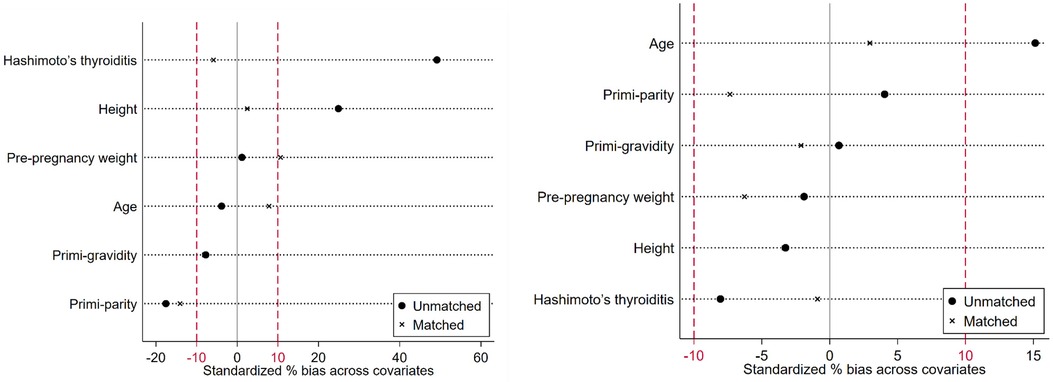
Figure 2. Standardized differences across the baseline measured confounders in the unmatched and matched comparisons (left: comparison between the under-surveillance DTC group and non-DTC group; right: comparison between the after-surgery DTC group and non-DTC group.).
In the under-surveillance DTC group, 91.7% and 8.3% of them were diagnosed with papillary thyroid cancer and follicular thyroid cancer, respectively; for the clinical TNM stage at the diagnosis, all were N0, and 73.0%, 21.6%, and 5.4% of them were T1a, T1b, and T2, respectively. In this study setting, no levothyroxine medication was administered to under-surveillance individuals to maintain low TSH levels. This was following the current Guidelines for the diagnosis and management of thyroid nodules and differentiated thyroid cancer in China (29).
In the after-surgery DTC group, 96.3% and 3.7% of them were diagnosed with papillary thyroid cancer and follicular thyroid cancer, respectively; for the pathological TNM stage, 58.1%, 30.2%, 3.5%, 3.5%, and 4.7% of them were T1a, T1b, T2, T3a, and T3b, respectively, and 79.8%, 14.6%, and 5.6% of them were N0, N1a, and N1b, respectively; for those with complete treatment information at follow-up (n = 70), 12.9% of them received the radioactive iodine (RAI) treatment.
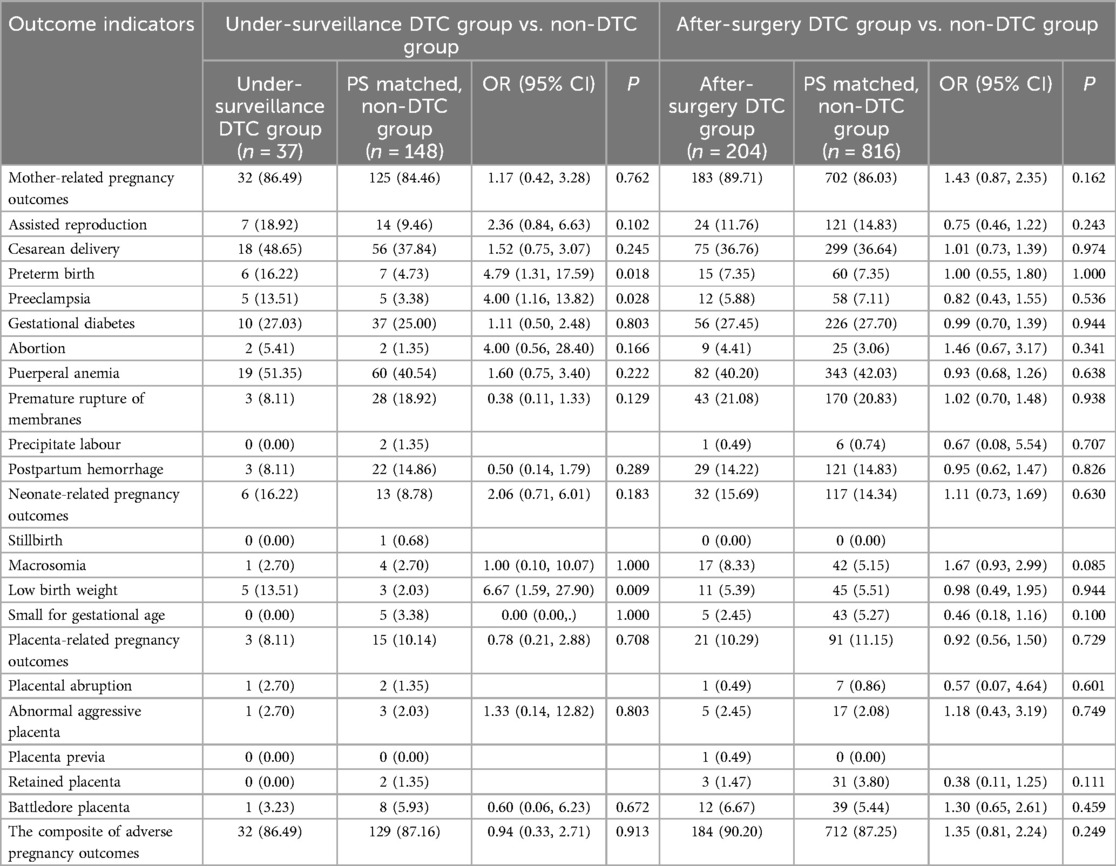
Pregnancy outcomes in the under-surveillance DTC group and PS-matched, non-DTC group
We compared the risk of adverse pregnancy outcomes between the under-surveillance DTC (n = 37) and PS-matched non-DTC (n = 148) groups in Table 2. There was no evidence of difference between the two groups in the composite of adverse pregnancy outcomes [OR (95% CI): 0.94 (0.33, 2.71); P = 0.913], mother- [OR (95% CI): 1.17 (0.42, 3.28); P = 0.762], neonate- [OR (95% CI): 2.06 (0.71, 6.01); P = 0.183], or placenta-related [OR (95% CI): 0.78 (0.21, 2,88); P = 0.708] adverse pregnancy outcomes. However, the under-surveillance DTC was associated with a higher risk of preterm birth [OR (95% CI): 4.79 (1.31, 17.59); P = 0.018], preeclampsia [OR (95% CI): 4.00 (1.16, 13.82); P = 0.028] and having a low-birth-weight baby [OR (95% CI): 6.67 (1.59, 27.90); P = 0.009], compared with the matched, non-DTC group.
Pregnancy outcomes in the after-surgery DTC group and PS-matched, non-DTC group
We compared the risk of adverse pregnancy outcomes between the after-surgery DTC (n = 204) and PS-matched non-DTC (n = 816) groups in Table 2. There was no evidence of difference between the two groups in the composite of any adverse pregnancy outcomes (OR (95% CI): 1.35 (0.81, 2.24); P = 0.249), mother- (OR (95% CI): 1.43 (0.87, 2.35); P = 0.162), neonate- (OR (95% CI): 1.11 (0.73, 1.69); P = 0.630), or placenta-related OR (95% CI): 0.92 (0.56, 1.50); P = 0.729) adverse pregnancy outcomes.
Subgroup analyses
The associations of after-surgery DTC with adverse pregnancy outcomes showed no differences across all prespecified subgroups (P for interaction > 0.05), except that the exposure-outcome association was stronger (P for interaction < 0.05) among those with maternal advanced age (≥35 years) than those with younger age (Figure 3).
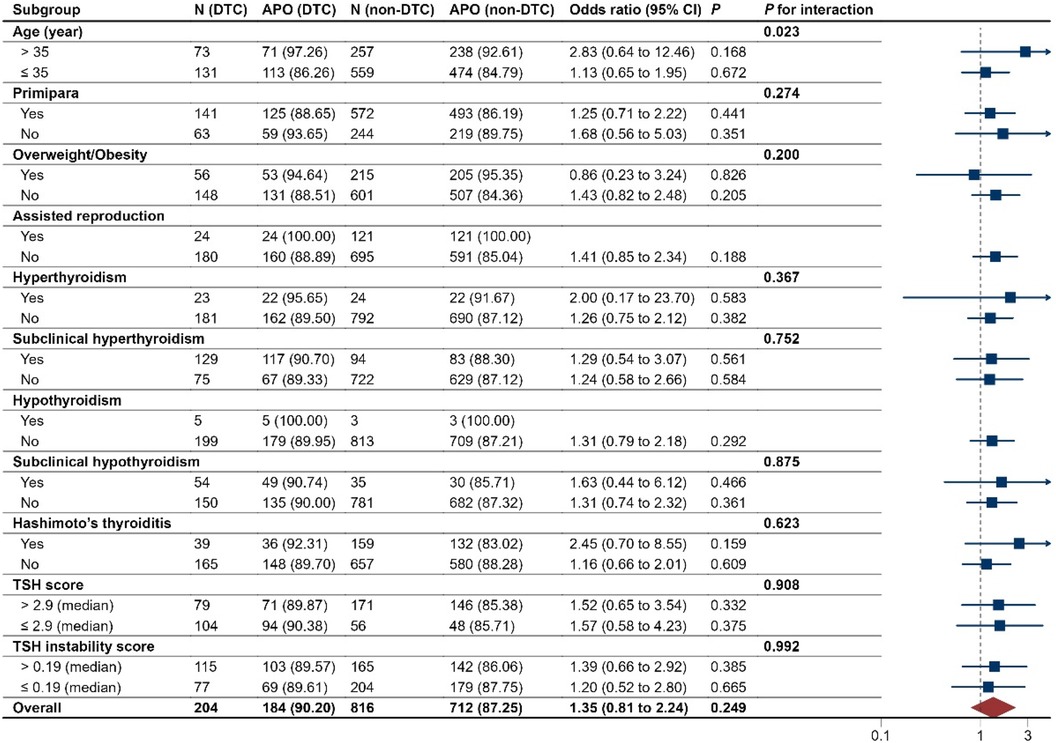
Figure 3. Subgroup analyses of pregnancy outcomes in the after-surgery DTC group and PS matched, non-DTC group. DTC, differentiated thyroid cancer; APO, adverse pregnancy outcomes. The median values of TSH in the after-surgery DTC group and PS matched, non-DTC group were 1.33 and 1.50 uIU/ml, respectively. The median values of FT4 in the two groups were 1.29 and 1.23 ng/dl, respectively.
Among pregnant women with after-surgery DTC, the risk of adverse pregnancy outcomes was shown no differences across tumor type, surgery type, response to therapy, TSH suppression therapy, and the time interval between surgical treatment and conception (all P > 0.05; Supplementary Figure S1).
Discussion
This study was the first to examine the associations of DTC with adverse pregnancy outcomes, with a focus on both the under-surveillance and after-surgery patients. Taking advantage of the method of PS matching, we found that the DTC was not associated with the risk of adverse pregnancy outcomes in most cases, except for a higher risk of preterm birth, preeclampsia, and having a low-birth-weight baby in the under-surveillance DTC group.
The observed higher risk of some adverse pregnancy outcomes in the under-surveillance DTC group compared with that in the non-DTC group might be interpreted by patients’ poor psychological status during the surveillance period. For example, a prospective cohort study in China showed that patients with highly suspicious DTC had overall elevated anxiety and worse emotional function (13). Additionally, other studies indicated that higher levels of anxiety during pregnancy were associated with an increased risk of preterm birth (30), preeclampsia (31), and having a low-birth-weight baby (32). Thus, anxiety during pregnancy might bridge the association of under-surveillance DTC with some adverse pregnancy outcomes. Nevertheless, we should interpret this finding cautiously due to the modest sample size of patients with under-surveillance DTC in this study.
We did not observe evidence of differences in the risk of adverse pregnancy outcomes between the after-surgery DTC and non-DTC groups. This is potentially interpreted by China's highly effective and efficient management of high-risk pregnancies during prenatal care (33). Prenatal care provided for pregnant women is often more intensive in the study setting as a modernized and comprehensive upper first-class hospital in Beijing, China. However, we should note that patients with DTC and maternal advanced age were more susceptible to adverse pregnancy outcomes compared to those with younger age, as shown in our subgroup analysis results.
Our study contributes to the research field from at least two aspects. First, previous studies have been only focused on patients receiving surgical treatment for DTC but little attention has been paid to those receiving active surveillance, for the present topic (16). It should be noted that the underlying mechanisms for the association of DTC with pregnancy outcomes probably differ between individuals with surgical treatment and those with active surveillance. The former was more likely to experience thyroid dysfunction due to thyroidectomy or the postoperative TSH suppression treatment, while the latter was more likely to experience psychological burdens such as anxiety due to the risk of disease progression. We have preliminarily filled this research gap by conducting analyses separately for the two groups of individuals, and we indeed observed distinct findings between them.
Second, the associations of DTC with adverse pregnancy outcomes have been reported inconsistently across the previous studies (16, 19–25). One potential interpretation is that some factors might modify the exposure-outcome association. Taking advantage of our data with detailed clinical, surgical, pathological, and endocrinological records, we have conducted multiple subgroup analyses across patient-, disease-, treatment-, hormone-, and other variables based on the literature review, previous research work (26, 27), and domain knowledge of our multidisciplinary research team.
Our study had several limitations. The type of DTC included in this study was mainly papillary thyroid carcinoma and thus the finding may not be directly translated to other types of DTC. We are conducting a prospective study on other types of DTC including follicular, medullary, and poorly differentiated thyroid carcinoma and the findings will be reported in the future. Additionally, the study was based on a single institution in China, limiting the generalizability to other populations. Also, some results in this study might not have enough statistical power due to the moderate sample size and relatively low incidence of some adverse pregnancy outcomes.
Our study also had advantages. First, our study was based on well-documented, fine-grained clinical records, and before data analyses, all of the diagnosis and treatment information was carefully checked by the senior clinicians. This strict quality control for study data ensured the accuracy and reliability of our study findings. Second, we have used the PS matching method to obtain a matched comparison group and reduce the confounding bias in this observational cohort study, making our findings less likely to be biased (17). Third, we have conducted several subgroup analyses, findings of which could not only elucidate the robustness and consistency across subgroups but also clarify the potentially important modification effects such as the maternal advanced age.
Our study had important implications for future research. First, active surveillance has become gradually adopted worldwide to manage low-risk DTC in properly selected patients, in addition to immediate surgery (34–36). Our study is among the first attempts to explore the association of under-surveillance DTC with adverse pregnancy outcomes. We called for future studies to prospectively observe pregnant women with under-surveillance DTC to assess their risk of adverse pregnancy outcomes and validate the findings from our study. Further, it is important to carefully assess whether the DTC influences long-term outcomes for mothers and their offspring. Moreover, as the PS matching could not control the unmeasured confounders (17), it is important to collect baseline lifestyle factors in the exposed and controlled group before we can make a firm conclusion for the present topic.
Our study also had important significance for clinical practice. Our results suggested that women of reproductive age who have a pregnancy plan might, in most cases, not need to be very anxious about the elevated risk of adverse pregnancy outcomes. However, more intensive prenatal care might be considered to provide for patients with DTC who were receiving active surveillance (13) and those with maternal advanced age.
Conclusion
The risk of adverse pregnancy outcomes in women previously treated for DTC did not differ from that in the well-matched, non-DTC women. However, our findings highlight the need to closely monitor the risk of adverse pregnancy outcomes among DTC patients under active surveillance in future studies.
Data availability statement
The raw data supporting the conclusions of this article are available upon request from the corresponding author.
Ethics statement
The studies involving humans were approved by the Medical Research Ethics Committee of Peking University Third Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study was based on the analysis of historical hospital data, and informed consent of the subjects could not be obtained.
Author contributions
XL: Investigation, Methodology, Writing – original draft. FM: Investigation, Validation, Writing – original draft. W-CX: Data curation, Formal Analysis, Investigation, Writing – review & editing. FZ: Conceptualization, Formal Analysis, Investigation, Writing – review & editing. SZ: Investigation, Methodology, Writing – review & editing. PF: Investigation, Methodology, Writing – original draft. JC: Investigation, Writing – original draft. RS: Investigation, Writing – original draft. B-KS: Data curation, Writing – review & editing. S-BS: Investigation, Writing – review & editing. CY: Project administration, Writing – review & editing. ZL: Formal Analysis, Investigation, Methodology, Project administration, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1377061/full#supplementary-material
References
1. China TSCotPsRo. Decision on Implementing Universal Two-Child Policy Reform and Perfecting Family Planning Service Management (2015). https://www.gov.cn/gongbao/content/2016/content_5033853.htm
2. Liu J, Song L, Qiu J, Jing WZ, Wang L, Dai Y, et al. Reducing maternal mortality in China in the era of the two-child policy. BMJ Glob Health. (2020) 5(2):e002157. doi: 10.1136/bmjgh-2019-002157
3. Frick AP. Advanced maternal age and adverse pregnancy outcomes. Best Pract Res Cl Ob. (2021) 70:92–100. doi: 10.1016/j.bpobgyn.2020.07.005
4. Li H, Nawsherwan , Fan C, Yin S, Haq IU, Mubarik S, et al. Changes in adverse pregnancy outcomes in women with advanced maternal age (ama) after the enactment of China’s universal two-child policy. Sci Rep. (2022) 12(1):5048. doi: 10.1038/s41598-022-08396-6
5. Tian ML, Ma GJ, Du LY, Jin Y, Zhang C, Xiao YG, et al. The effect of 2016 Chinese second-child policy and different maternal age on pregnancy outcomes in Hebei province, China. BMC Pregnancy Childbirth. (2023) 23(1):267. doi: 10.1186/s12884-023-05552-2
6. Douglas EH, Rhoads A, Thomas A, Aloi J, Suhl J, Lycan T Jr, et al. Incidence and survival in reproductive-aged women with differentiated thyroid cancer: United States seer 18 2000–2016. Thyroid. (2020) 30(12):1781–91. doi: 10.1089/thy.2020.0152
7. Writing Committee for Guidelines for Prevention and Management of Thyroid Diseases During Pregnancy and Perinatal Period CSoE, Chinese Medical Association, Women’s Health Care Branch of Chinese Preventive Medicine Association. Guidelines for prevention and management of thyroid diseases during pregnancy and perinatal period. Chin J Endocrinol Metab. (2022) 38(7):539–51. doi: 10.19757/j.cnki.issn1674-7763.2022.04.001
8. McGriff NJ, Csako G, Gourgiotis L, Guthrie LC, Pucino F, Sarlis NJ. Effects of thyroid hormone suppression therapy on aciverse clinical outcomes in thyroia cancer. Ann Med. (2002) 34(7-8):554–64. doi: 10.1080/078538902321117760
9. Verloop H, Louwerens M, Schoones JW, Kievit J, Smit JW, Dekkers OM. Risk of hypothyroidism following hemithyroidectomy: systematic review and meta-analysis of prognostic studies. J Clin Endocrinol Metab. (2012) 97(7):2243–55. doi: 10.1210/jc.2012-1063
10. Lemieux P, Yamamoto JM, Nerenberg KA, Metcalfe A, Chin A, Khurana R, et al. Thyroid laboratory testing and management in women on thyroid replacement before pregnancy and associated pregnancy outcomes. Thyroid. (2021) 31(5):841–9. doi: 10.1089/thy.2020.0609
11. Chou RG, Dana T, Haymart M, Leung AM, Tufano RP, Sosa JA, et al. Active surveillance versus thyroid surgery for differentiated thyroid cancer: a systematic review. Thyroid. (2022) 32(4):351–67. doi: 10.1089/thy.2021.0539
12. Yoshida Y, Horiuchi K, Okamoto T. Patients’ view on the management of papillary thyroid microcarcinoma: active surveillance or surgery. Thyroid. (2020) 30(5):681–7. doi: 10.1089/thy.2019.0420
13. Liu C, Zhao H, Xia Y, Cao Y, Zhang L, Zhao Y, et al. Active surveillance of highly suspicious thyroid nodules cohort in China shows a worse psychological Status in younger patients. Front Oncol. (2022) 12:981495. doi: 10.3389/fonc.2022.981495
14. Gurung S, Tong HH, Bryce E, Katz J, Lee AC, Black RE, et al. A systematic review on estimating population attributable fraction for risk factors for small-for-gestational-age births in 81 low- and middle-income countries. J Glob Health. (2022) 12:04024. doi: 10.7189/jogh.12.04024
15. Yang L, Feng LF, Huang LJ, Li XJ, Qiu WW, Yang KH, et al. Maternal factors for intrauterine growth retardation: systematic review and meta-analysis of observational studies. Reprod Sci. (2023) 30(6):1737–45. doi: 10.1007/s43032-021-00756-3
16. Moon S, Yi KH, Park YJ. Risk of adverse pregnancy outcomes in young women with thyroid cancer: a systematic review and meta-analysis. Cancers (Basel). (2022) 14(10):2382. doi: 10.3390/cancers14102382
17. Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardio-Thorac. (2018) 53(6):1112–7. doi: 10.1093/ejcts/ezy167
18. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. (1983) 70(1):41–55. doi: 10.1093/biomet/70.1.41
19. Cohen N, Levy A, Wiznitzer A, Sheiner E. Perinatal outcomes in post-thyroidectomy pregnancies. Gynecol Endocrinol. (2011) 27(5):314–8. doi: 10.3109/09513590.2010.491166
20. Hartnett KP, Ward KC, Kramer MR, Lash TL, Mertens AC, Spencer JB, et al. The risk of preterm birth and growth restriction in pregnancy after cancer. Int J Cancer. (2017) 141(11):2187–96. doi: 10.1002/ijc.30914
21. Beksaç K, Aktoz F, Örgül G, Çelik HT, Özgü-Erdinç AS, Beksaç MS. Pregnancy in papillary thyroid cancer survivors. J Turk Ger Gynecol Assoc. (2018) 19(2):94–7. doi: 10.4274/jtgga.2017.0057
22. Cao Q, Zhu H, Zhang J, Li Y, Huang W. Pregnancy outcomes in thyroid cancer survivors: a propensity score-matched cohort study. Front Endocrinol (Lausanne). (2022) 13:816132. eCollection 2022. doi: 10.3389/fendo.2022.816132
23. Blackburn BE, Ganz PA, Rowe K, Snyder J, Wan Y, Deshmukh V, et al. Reproductive and gynecological complication risks among thyroid cancer survivors. J Cancer Surviv. (2018) 12(5):702–11. doi: 10.1007/s11764-018-0707-7
24. Cho GJ, Kim SY, Lee HC, Lee KM, Han SW, Oh MJ, et al. Risk of adverse obstetric outcomes and the abnormal growth of offspring in women with a history of thyroid cancer. Thyroid. (2019) 29(6):879–85. doi: 10.1089/thy.2018.0283
25. Spiegel E, Spence AR, Czuzoj-Shulman N, Abenhaim HA. Pregnancy outcomes after thyroid cancer. J Perinat Med. (2019) 47(7):710–6. doi: 10.1515/jpm-2019-0039
26. Li X, Xiao WC, Mei F, Shan R, Song SB, Sun BK, et al. The association of pregnancy with disease progression in patients previously treated for differentiated thyroid cancer: a propensity score-matched retrospective cohort study. J Womens Health (Larchmt). (2023) 32(11):1174–81. doi: 10.1089/jwh.2023.0172
27. Xiao WC, Li X, Shan R, Mei F, Song SB, Chen J, et al. Pregnancy and progression of differentiated thyroid cancer: a propensity score-matched retrospective cohort study. J Clin Endocr Metab. (2023) 109:837–43. doi: 10.1210/clinem/dgad557
28. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. (2009) 28(25):3083–107. doi: 10.1002/sim.3697
29. Chinese Society of Endocrinology, Thyroid and Metabolism Surgery Group of the Chinese Society of Surgery, China Anti-Cancer Association, Chinese Association of Head and Neck Oncology, Chinese Society of Nuclear Medicine, China Anti-Cancer Association, et al. Guidelines for the diagnosis and management of thyroid nodules and differentiated thyroid cancer (second edition). Chin J Endocrinol Metab. (2023) 39(03):181–226. doi: 10.3760/cma.j.cn311282-20221023-00589
30. Tomfohr-Madsen L, Cameron EE, Dunkel Schetter C, Campbell T, O'Beirne M, Letourneau N, et al. Pregnancy anxiety and preterm birth: the moderating role of sleep. Health Psychol. (2019) 38(11):1025–35. doi: 10.1037/hea0000792
31. Kurki T, Hiilesmaa V, Raitasalo R, Mattila H, Ylikorkala O. Depression and anxiety in early pregnancy and risk for preeclampsia. Obstet Gynecol. (2000) 95(4):487–90. doi: 10.1016/s0029-7844(99)00602-x
32. Bandyopadhyay A, Jones H, Parker M, Marchant E, Evans J, Todd C, et al. Weighting of risk factors for low birth weight: a linked routine data cohort study in Wales, UK. BMJ Open. (2023) 13(2):e063836. doi: 10.1136/bmjopen-2022-063836
33. Obstetrics Subgroup, Chinese Society of Obstetrics and Gynecology, Chinese Medical Association. Guideline of preconception and prenatal care (2018). Zhonghua Fu Chan Ke Za Zhi. (2018) 53(1):7–13. doi: 10.3760/cma.j.issn.0529-567X.2018.01.003
34. Leboulleux S, Tuttle RM, Pacini F, Schlumberger M. Papillary thyroid microcarcinoma: time to shift from surgery to active surveillance? Lancet Diabetes Endo. (2016) 4(11):933–42. doi: 10.1016/S2213-8587(16)30180-2
35. Liu W, Cao W, Dong Z, Cheng R. Can active surveillance management be developed for patients with low-risk papillary thyroid microcarcinoma? A preliminary investigation in a Chinese population. Endocr Pract. (2022) 28(4):391–7. doi: 10.1016/j.eprac.2022.01.013
Keywords: differentiated thyroid cancer, pregnancy outcomes, propensity score matching, cohort, real-world data
Citation: Li X, Mei F, Xiao W-C, Zhang F, Zhang S, Fu P, Chen J, Shan R, Sun B-K, Song S-B, Yuan C and Liu Z (2024) Differentiated thyroid cancer and adverse pregnancy outcomes: a propensity score-matched retrospective cohort study. Front. Pediatr. 12:1377061. doi: 10.3389/fped.2024.1377061
Received: 6 February 2024; Accepted: 30 August 2024;
Published: 12 September 2024.
Edited by:
Nan Zhao, Peking Union Medical College Hospital, ChinaReviewed by:
Zheng Ding, Shanghai Jiao Tong University, ChinaMirjana D. Stojkovic, University of Belgrade, Serbia
Copyright: © 2024 Li, Mei, Xiao, Zhang, Zhang, Fu, Chen, Shan, Sun, Song, Yuan and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Liu, bGl1emhlbmdAYmptdS5lZHUuY24=
†These authors have contributed equally to this work
‡ORCID:
Zheng Liu
orcid.org/0000-0002-0405-2348
 Xin Li1,†
Xin Li1,† Fang Mei
Fang Mei Fan Zhang
Fan Zhang Chunhui Yuan
Chunhui Yuan Zheng Liu
Zheng Liu