
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pediatr. , 25 April 2024
Sec. Pediatric Immunology
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1370224
Background: Little is known about the safety of mite extract product Novo-Helisen Depot (NHD) as subcutaneous immunotherapy (SCIT) in the children with mite allergy especially immediate/late local reaction (LRs).
Methods: We conducted a retrospective study analyzing the adverse events of the children undergoing subcutaneous immunotherapy with NHD. Adverse events included local and systemic adverse reactions (SRs) at the very early and late stage. The correlation of the basic characteristics, laboratory analysis results, LRs and SRs were analyzed.
Results: Two hundred and eighty-seven patients received at least 15 months of subcutaneous immunotherapy with NHD were included in the analysis. Skin-prick testing (SPT) results of D. pteronyssinus was associated with an increased risk of immediate LRs in build-up phase (OR = 1.53, 95% CI: 1.02, 2.37) and delayed LRs in maintenance phase (OR = 1.58, 95% CI: 1.05, 2.46), while SPT results of D. farinae was associated with an increased risk of SRs (OR = 3.22, 95% CI: 1.17, 10.00) and severe SRs (OR = 7.68, 95% CI: 1.13, 109.50). Serum IgE level of D. pteronyssinus was associated with an increased risk of SRs (OR = 1.01, 95% CI: 1.00, 1.03). Patients with both asthma and allergic rhinitis was associated with an increased risk of SR, and severe SRs (P < 0.05).
Conclusion: NHD as SCIT is safe. The children with higher SPT level with D. farinae or D. pteronyssinus, higher serum IgE level of D. pteronyssinus, children with both asthma and allergic rhinitis, and the children with treatment interruption had higher risk of adverse events.
Allergic diseases and asthma have been rising for decades (1). The latest national cross-sectional study of China indicated that the prevalence of asthma in is 4.2% in adults and 3.02% in children (2, 3). In China, the prevalence of allergic rhinitis (AR) in children was vary from 9.8% to 22.4% (4). Exposure to inhaled allergens, has an important effect on exacerbations in young children with asthma (5). Mites include Dermatophagoides pteronyssinus (D. pteronyssinus), and Dermatophagoides farinae (D. farinae). Many subsequent studies have confirmed that mites are an essential trigger for many allergic diseases (6, 7). Meanwhile, mite is also the most common indoor allergen and the most common aeroallergen triggers associated with persistent perennial symptoms of patients with AR an asthma in China (4, 8, 9).
AIT has been the only disease-modifying intervention for allergic diseases for over 110 years and has been proven to be a safe treatment for patients (10–14). Conventional AIT includes SCIT, sublingual immunotherapy (SLIT), and oral immunotherapy (OIT). SCIT involves extracts from a single species of allergen (e.g., pollen from grass plants, house dust mites, cat hair epithelial cells, dog hair, Bee Venom, Cockroach Extract, etc.). These allergen extracts are administered in increasing doses, starting at a low dose and steadily increasing the amount during the weekly administration period until a high standard dose after several weeks of treatment and then maintained at a plateau level. This dose is administered monthly for 3–5 years to induce a period of remission of allergic symptoms. The safety of NHD in children and adolescents with allergic rhinitis and asthma has been studied in some researches (15). Although safe, there is still a risk of LRs and SRs and even rare fatal reactions (FRs). This study focused on exploring what factors are associated with the occurrence of adverse reactions so that we can intervene in advance to reduce the occurrence of adverse reaction.
Two hundred and eighty-seven patients received at least 15 months of subcutaneous immunotherapy with NHD were included in the analysis. The mean age of the patients was 7 years-old, with a range of 5–13 years-old. There were 156 (54.36%) patients diagnosed with asthma, 84 (29.27%) patients with rhinitis without asthma, 9 (3.14%) with CVA and 38 (13.24%) with both asthma and rhinitis.
A total of 183 (63.76%) patients underwent LRs, 23 patients (8.01%) had SRs, and 7 (2.44%) patients experienced severe SRs after injection. Fifteen patients (65.22%) underwent immediate SRs, 8 (43.48%) patients had late SRs. Sixteen patients (69.56%) had SRs in the build-up phase, while 7 (30.43) in maintenance phase. Eighteen patients developed peripheral rash and edema, and 3 patients developed ocular symptoms. Four patients developed other respiratory symptoms, including nasal and pharyngeal pruritus and cough. Seven (2.44%) patients experienced severe SRs after injection. Six severe SRs occurred in build-up phase, while only one severe systemic adverse reaction occurred in maintenance phase. The symptoms resolved within 1 h after intramuscular injection of epinephrine. There was no fatal reaction.
In patients with immediate LRs in build-up phase, the mean BMI were significantly higher than others (median 16.39 vs. 15.39, P < 0.05). SPT with D. farinae, cat and dog fur and fungal assemblages were associated with immediate LRs in build-up phase (median 3 vs. 3; 0 vs. 0; 0 vs. 0, P < 0.05). In patients with immediate LRs in maintenance phase (18 vs. 8, P < 0.05), the ratio of patient with interruption of the treatment were significantly higher than others (15.38% vs. 4.91%, P < 0.05). SPT with D. farinae, cat and dog fur and fungal assemblages were associated with immediate LRs in maintenance stage (median 3 vs. 3; 0 vs. 0; 0 vs. 0, P < 0.05). In patients with late LRs in build-up phase, the mean age were significantly higher than others (median 8.00 vs. 6.00, P < 0.05). There were statistically significant associations between SPT with D. pteronyssinus, D. farinae, and late LRs in buildup stage (median 3 vs. 3; 3 vs.3, P < 0.05). In patients with late LRs in maintenance phase, the ratio of patient with interruption in the treatment were significantly higher than others (16.53% vs. 3.7% P < 0.05). SPT with D. pteronyssinus was associated with late LRs in maintenance phase (median 3 vs. 3, P < 0.05) (Tables 1, 2).
In patients with SRs, the mean duration of the primary disease were significantly higher than others (median 34.5 vs. 24.0, P < 0.05), while the degree of SPT with D. farinae was also higher (median 3 vs. 3, P < 0.05). In patients with severe SRs, the mean total IgE in serum, sIgE of D. pteronyssinus, SPT with D. pteronyssinus and D. farinae were significantly higher (median 1,190.00 vs. 297.70; 27.69 vs. 11.50; 4 vs. 3; 4 vs.3, P < 0.05). Furthermore, the primary disease was associated with severe SRs (Table 3).
After adjusting for potential confounders, severe SPT with D. pteronyssinus was associated with an increased risk of immediate LRs in buildup stage (OR = 1.53, 95% CI: 1.02–2.37). Application of Omazumab before treatment was associated with a decreased risk of immediate LRs in maintenance phase (OR = 0.23, 95% CI: 0.05, 0.89). On contrary, treatment interruption was associated with an increased risk of immediate LRs in maintenance phase (OR = 8.83, 95% CI: 2.49, 43.36) (Table 4).
SPT with D. pteronyssinus, D. farinae, and cat and dog fur were associated with an increased risk of delayed LRs in build-up phase in model 1 (OR = 1.39, 95% CI: 1.06, 1.86; OR = 1.58, 95% CI: 1.16, 2.18; OR = 1.25, 95% CI: 1.01, 1.55 respectively). After adjusting for potential confounders, there were no significant associations between SPT with D. pteronyssinus, D. farinae, cat and dog fur and delayed LRs in build-up phase in model 3. SPT with D. pteronyssinus and treatment interruption were associated with an increased risk of late LRs in maintenance phase (OR = 1.58, 95% CI: 1.05, 2.46; OR = 6.33, 95% CI: 2.06, 24.03) (Table 5).
Patients with both asthma and allergic rhinitis, sIgE of D. pteronyssinus, SPT with D. farinae were associated with an increased risk of SRs (OR = 8.46, 95% CI: 2.07, 39.09; OR = 1.01, 95% CI: 1.00, 1.03; OR = 3.22, 95% CI: 1.17, 10.00, respectively). Patients with both asthma and allergic rhinitis, SPT with D. farinae were associated with an increased risk of severe SRs (OR = 351.59, 95% CI: 8.30, >999.99; OR = 7.68, 95% CI: 1.13, 109.50, respectively) (Table 6).
In the patients with asthma, SPT with cat and dog fur was associated with a decreased risk of immediate LRs in build-up phase and maintenance phase. The initial treatment month was associated with an increased risk of delayed LRs in build-up phase (OR = 1.02, 95% CI = 1.00, 1.30). sIgE of D. pteronyssinus, SPT with D. farinae were associated with higher risk of severe SRs (P < 0.05). Treatment interruption was associated with a higher risk of immediate and late LRs in maintenance phase and severe SRs (P < 0.05). In the patients without asthma, age was related with a higher risk of late LRs in build-up phase (OR = 1.91, 95% CI = 1.25, 3.24). Treatment interruption was related with a higher risk of delayed LRs in maintenance phase (OR = 9.09, 95% CI = 1.18, 193.28).
The incidence of immediate LRs in build-up phase (50.87%) was significantly higher than that in 0–3rd months (34.29%), 3rd–6th months (32.43%), 6th–12th months (32.24%), 12th–24th month (2.07%) in the maintenance phase (P < 0.01). The incidence of late LRs in build-up phase (49.48%) was significantly higher than that in 0–3rd months (38.21%), 3rd–6th months (25.71%), 6th–12th months (16.36%), 12th–24th month (11.36%) in maintenance phase (P < 0.01) (Figure 1).
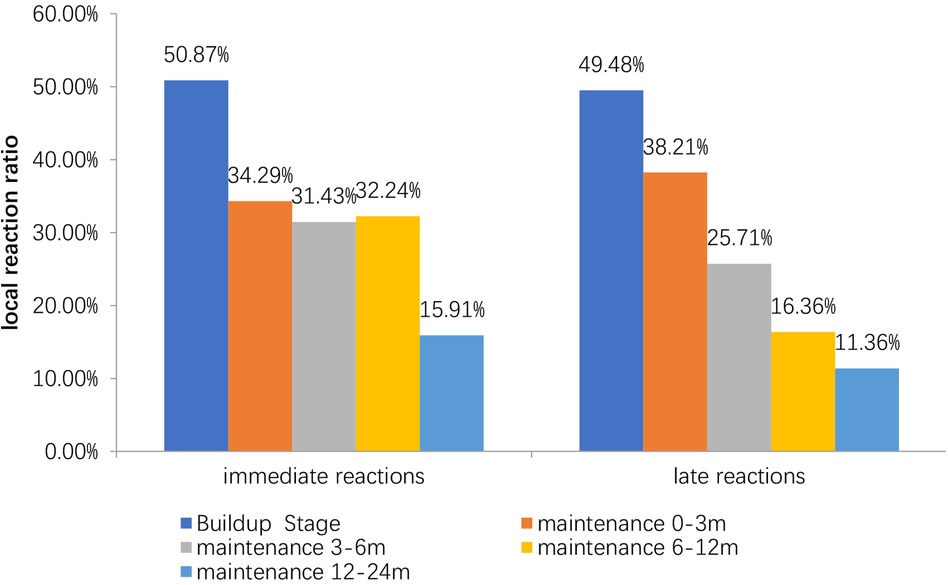
Figure 1. Incidence of local reaction in different stages. The peak incidence of LRss is in the first three months of the treatment. The incidence of delayed LRss decreases gradually with the duration of treatment.
The peak of the adverse events occurred in September–October, while the second peak was March–May. The time of the initial desensitization was associated with both immediate and late adverse reactions during the build-up phase, while the patients who started SCIT in August–November had the highest proportion of adverse reactions during the build-up phase (Figures 2, 3).
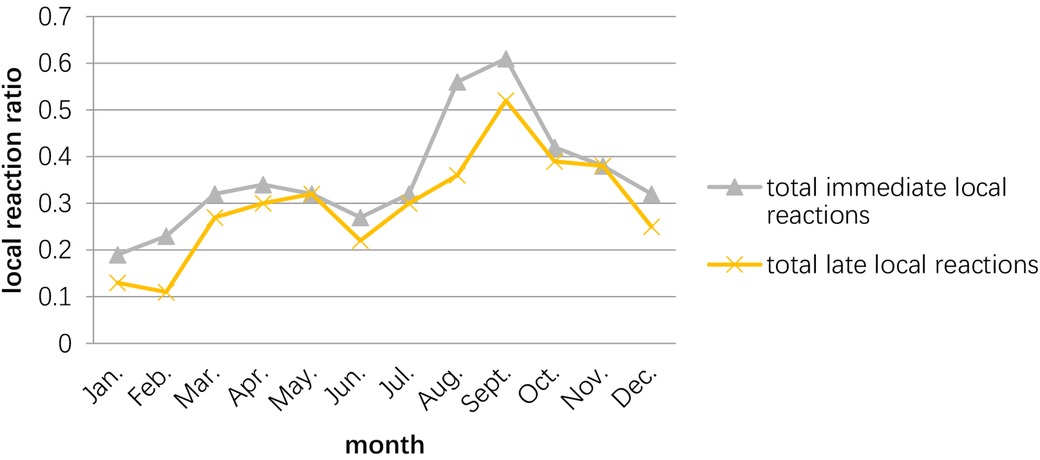
Figure 2. Relationship between the occurrence of adverse reactions and the month in which the child receives desensitization treatment. The peak season for adverse reactions in patients receiving SCIT is September–November, with the 2nd peak occurring in March–May.
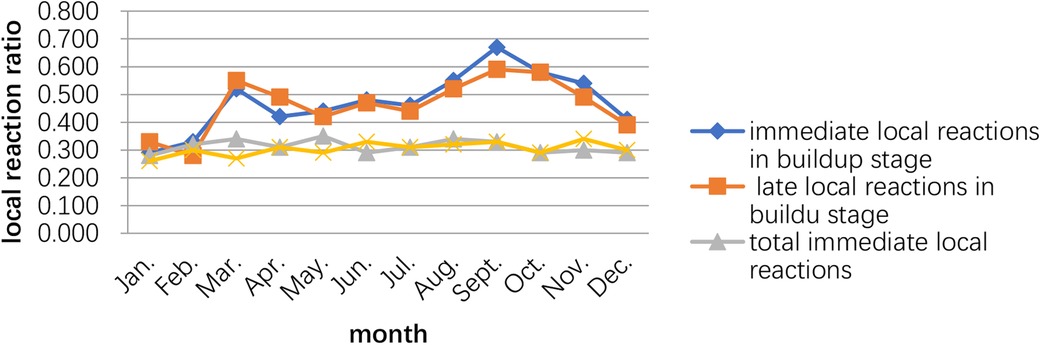
Figure 3. Relationship between the occurrence of adverse reactions and the month the child started subcutaneous immunotherapy.
Children received subcutaneous immunotherapy with purified D. farinae and D. pteronyssinus NHD at the Pediatric Respiratory of Shengjing Hospital of China Medical University from April 2017 to December 2021, were included in this retrospective study.
Inclusion criteria were as follows: (1) age from 5 to 13 years; (2) diagnosis of asthma or cough variant asthma (CAV) according to the Global Initiative for Asthma 2020 (www.ginasthma.org); (3) diagnosis of allergic rhinitis according to Chinese guidelines for diagnosis and treatment of allergic rhinitis (16); (4) allergy to D. pteronyssinus and/or D. farinae identified by SPT or serum specific immunoglobulin E (IgE). The patients with severe immunologic, cardiac, liver, renal, metabolic disease, tumor, chronic infection or allergy to excipients contained in NHD were excluded.
The indications and contraindications for SCIT followed the recommendations of the European Academy of Allergy and Clinical Immunology (EAACI) guidelines (17), and children received subcutaneous immunotherapy with NHD, with the vaccine administered by a specialist nurse. The build-up phase of SCIT has been carried out according to the routine schedule provided by the manufacturer. All injectable penicillin-bottles are 4.5 ml and the SCIT kit for each patient consists of 3 penicillin-bottles (50 TU/ml = green, 500 TU/ml = yellow, 5,000 TU/ml = red). The build-up phase of SCIT with bivalve allergens lasted for approximately three months, with weekly injections starting with 0.1 ml of the 50 TU/ml green penicillin bottle and gradually increasing until the 5000 TU/ml red penicillin bottle reaches 1.0 ml for the maintenance phase. The patient entered the maintenance phase for approximately 33 months (36 months). The maintenance phase consisted of a 5000 TU/ml red penicillin bottle at 0.5–1.0 ml, with the next injection one week apart for the last 0.5 or 0.75 ml and four weeks apart for the last 1.0 ml.
The SCIT program was conducted under controlled conditions in a clinical setting where the physician assessed the patient and provided safety measures.
Before each infusion, the nurse asked and recorded whether the patient had symptoms of infection in the last two weeks. Patients with a diagnosis of asthma had their PEF recorded, and the injection was withheld if the patient had an infected fever, wheezing episode, or FEV1 <80% within two weeks. After each injection, patients were observed by the clinic nurse and doctor for at least 30 min. NHD concentration and dose, topical and SR, and treatment of such reactions were recorded in the patient record sheet. The amount is reduced in the case of SR or beyond the interval between two series of injections. In the event of SRs, resuscitation treatment is administered by a specialist physician.
Adverse events (AE) were divided into LRs and SRs. LRs referred to skin symptoms of acupuncture site. SRs included skin symptoms (generalized pruritus, urticaria, flushing, and angioedema), rhinoconjunctivitis, asthma, cardiovascular symptoms, and nonspecific systemic symptoms (headache, cough, vomiting, chest tightness, chest discomfort, etc.) (12). Serious systematic adverse reactions referred to that two or more systems were involved and norepinephrine treatment was required. By the time of the onset of AEs were divided into immediate adverse reactions (occurring within 30 min) and late adverse reactions (first episode >30 min after injection). The patients were monitored during injections following EAACI guidelines (12).
Criteria of allergen skin test:
Negative: wheal diameter <1/3 wheal diameter of the positive control [histamine];
1+: 2/3> wheal diameter ≥1/3 wheal diameter of the positive control;
2+: wheal diameter ≥2/3 wheal diameter of the positive control;
3+: equal to the diameter of the positive control;
4+: wheal diameter > the diameter of the positive control.
The characteristics including gender, age, date of the treatment initiation, season, disease (rhinitis or asthma), phase of treatment (build-up or maintenance), and whether the patient discontinued treatment before reaching the maintenance dose were collected. The laboratory results including total serum IgE levels, specific IgE levels, blood eosinophil levels, SPT levels of different allergens, treatment interruption for at least 3 months were collected.
The normality of all continuous variables was evaluated through the Shapiro-Wilk test. The student's t-tests and the chi-square tests were used for comparison of continuous variables and categorical variables respectively. Results of continuous variables were expressed as means ± standard deviation (SD) and categorical variables were expressed as count with percentage. An unconditional multivariable logistic regression model was used to calculate odds ratios (ORs) and the corresponding 95% confidence intervals (CIs). Model 1 was the crude model. Model 2 was adjusted for age (years), gender, BMI (kg/m2), primary disease (asthma, allergic rhinitis, CVA or asthma with allergic rhinitis), application of Omazumab before the treatment (yes or no), initial month of the treatment (January–December), treatment interruption (yes or no). Model 3 was further adjusted for specific IgE (sIgE) of D. pteronyssinus (IU/ml), SPT with D. pteronyssinus, D. farinae, cat and dog fur, fungal assemblages, grass and tree pollen (1–4 grades). In addition, subgroup analysis stratified by diagnosis with or without asthma were performed. All analyses were performed with SAS version 9.4 (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at p < 0.05 and was based on a two-sided test.
SCIT is considered safe when the patient selection is appropriate, the clinic facilities are suitable, the injections are administered by trained staff, and the emergency treatment is provided (18).
In this study, pre-treatment mite-specific IgE and skin-spotting mite allergy levels were associated with late LRs during the build-up phase. The highest value of other allergen positivity was associated with tachyphylaxis in children with concomitant skin prick allergens other than mite allergy. It delayed local adverse reactions during the build-up phase. However, as the duration of treatment increased, the effect of this pre-treatment highly sensation on the children gradually decreased and did not affect the overall rate of adverse reactions during the 21-month maintenance period.
In this study, serum IgE level of D. pteronyssinus was associated with an increased risk of SRs and higher risk of severe SRs. SPT with D. pteronyssinus was associated with an increased risk of immediate LRs in build-up phase and late LRs in maintenance phase. It seemed that the level of SPT with D. farinae was associated with a decreased risk of immediate LRs in build-up phase. However, in this study the level of SPT with D. farinae was associated with an increased risk of SRs and severe SRs.
Although house dust mite allergy is a perennial allergen, it can be characterized by typical seasonal changes. Within a given geographical region, dust mites vary in their seasonal distribution. A previous study in the Beijing of northern China have shown that the number of indoor mite species peaks in the spring and autumn, with a peak season in October (19). Patients who started SCIT in February–March and August–October had the highest rate of adverse reactions during the next dose escalation period of approximately three months. Still, the overall rate of adverse reactions over two years was not related to the timing of desensitization. When desensitization was initiated was not relevant. Clinicians can choose whether they need to start mite desensitization before the mite seasons. The peak incidence of both rapid and late reactions are in the first 3 months of the SCTI, after which the incidence of allergic reactions decreases as the treatment duration increases and the child becomes tolerant to the mites.
Asthma biologics, especially Omalizumab, had been reported that it could improve outcomes in severe, controlled asthmatic patients with SCIT (20). In our study the application of Omazumab before treatment was associated with a decreased risk of immediate LRs in maintenance phase. But it was not associated with SRs and severe SRs.
In current study, affected by COVID-19 epidemic, some patients discontinued SCIT for at least 3 months. Most of the interruption occurred in maintenance phase, coursing a restarting with 0.1 ml of the 5,000 TU/ml red penicillin bottle and gradually increasing until reaching 1.0 ml. This interruption was associated with an increased risk of immediate and late LRs in maintenance phase. Therefore, treatment interruption should be paid attention to avoid the possible increased LRs risks.
SRs' risk factors were identified as symptomatic asthma, high sensitivity, cumulative injection, dosing error, the use of β-blockers, injection from a new vial, and injection during worsening symptoms (21, 22). Patients with asthma are at the highest risk of severe reactions as these patients had previous systemic reactions (23). In this study, all the severe systemic adverse reaction occurred in children with asthma. Moreover, patients with both asthma and allergic rhinitis, sIgE of D. pteronyssinus, SPT with D. farinae were associated with an increased risk of SRs, while patients with both asthma and allergic rhinitis, SPT with D. farinae, were associated with an increased risk of severe SRs.
The development of epinephrine autoinjector prescriptions for children may be a good option. All seven cases of systemic serious adverse reactions in the study occurred in autumn (September–November), which is similar with previous studies showing that pollen allergic patients (especially highly sensitized individuals) are more likely to experience SRs, even fatal SRs, after injections during the peak pollen season (24, 25). The occurrence of SRs should be more vigilant during the mite epidemic season.
The hospital where the study was conducted is a national regional medical center. Most of the children receiving SCIT had severe diseases, and some of the children with moderate to severe asthma or even refractory asthma, thus the incidence of SRs is higher than that reported in previous studies.
In conclusion, the safety profile of NHD in Chinese children with mite allergy is favorable. Understanding the risk factors and temporal patterns that influence adverse reactions to SCIT is needed to reduce further the occurrence of adverse reactions and discomfort in children with SCIT. The children with higher SPT level with D. farinae or D. pteronyssinus, higher serum IgE level of D. pteronyssinus, children with both asthma and allergic rhinitis, and the children with treatment interruption had higher risk of adverse events.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
QZ: Conceptualization, Investigation, Methodology, Writing – original draft. SL: Conceptualization, Data curation, Formal Analysis, Writing – original draft. BD: Data curation, Investigation, Writing – original draft. LC: Conceptualization, Data curation, Formal Analysis, Writing – original draft. LH: Data curation, Methodology, Writing – original draft. QZ: Formal Analysis, Visualization, Writing – original draft. WS: Formal Analysis, Visualization, Writing – original draft. LS: Conceptualization, Investigation, Methodology, Supervision, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors are grateful who have participated in this research work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1370224/full#supplementary-material
1. Sinyor B, Concepcion Perez L. Pathophysiology of Asthma. Treasure Island (FL): StatPearls (2022).
2. Huang K, Yang T, Xu J, Yang L, Zhao J, Zhang X, et al. Prevalence, risk factors, and management of asthma in China: a national cross-sectional study. Lancet. (2019) 394(10196):407–18. doi: 10.1016/S0140-6736(19)31147-X
3. National Cooperative Group on Childhood Asthma; Institute of Environmental Health and Related Product Safety, Chinese Center for Disease Control and Prevention. Third nationwide survey of childhood asthma in urban areas of China. Zhonghua Er Ke Za Zhi. (2013) 51(10):729–35.24406223
4. Zhang Y, Zhang L. Increasing prevalence of allergic rhinitis in China. Allergy Asthma Immunol Res. (2019) 11(2):156–69. doi: 10.4168/aair.2019.11.2.156
5. Dick S, Doust E, Cowie H, Ayres JG, Turner S. Associations between environmental exposures and asthma control and exacerbations in young children: a systematic review. BMJ Open. (2014) 4(2):e003827. doi: 10.1136/bmjopen-2013-003827
6. Wilson JM, Platts-Mills TAE. Home environmental interventions for house dust mite. J Allergy Clin Immunol Pract. (2018) 6(1):1–7. doi: 10.1016/j.jaip.2017.10.003
8. Feng M, Sun W, Cheng X. Seasonal dynamics and distribution of house dust mites in China. Biosci Trends. (2009) 3(6):210–5.20103849
9. Guan K, Liu B, Wang M, Li Z, Chang C, Cui L, et al. Principles of allergen immunotherapy and its clinical application in China: contrasts and comparisons with the USA. Clin Rev Allergy Immunol. (2019) 57(1):128–43. doi: 10.1007/s12016-019-08751-y
10. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, et al. Allergic rhinitis and its impact on asthma (ARIA) 2008 update [in collaboration with the world health organization, GA(2)LEN and AllerGen]. Allergy. (2008) 63(Suppl 86):8–160. doi: 10.1111/j.1398-9995.2007.01620.x
11. Curin M, Khaitov M, Karaulov A, Namazova-Baranova L, Campana R, Garib V, et al. Next-generation of allergen-specific immunotherapies: molecular approaches. Curr Allergy Asthma Rep. (2018) 18(7):39. doi: 10.1007/s11882-018-0790-x
12. Dhami S, Kakourou A, Asamoah F, Agache I, Lau S, Jutel M, et al. Allergen immunotherapy for allergic asthma: a systematic review and meta-analysis. Allergy. (2017) 72(12):1825–48. doi: 10.1111/all.13208
13. Agache I, Lau S, Akdis CA, Smolinska S, Bonini M, Cavkaytar O, et al. EAACI guidelines on allergen immunotherapy: house dust mite-driven allergic asthma. Allergy. (2019) 74(5):855–73. doi: 10.1111/all.13749
14. Pfaar O, Agache I, de Blay F, Bonini S, Chaker AM, Durham SR, et al. Perspectives in allergen immunotherapy: 2019 and beyond. Allergy. (2019) 74(Suppl 108):3–25. doi: 10.1111/all.14077
15. Xiang L, Liu F, Zhi L, Jiang W, Liu C, Xie H, et al. Safety of semi-depot house dust mite allergen extract in children and adolescents with allergic rhinitis and asthma. Immunotherapy. (2021) 13(3):227–39. doi: 10.2217/imt-2020-0232
16. Subspecialty Group of Rhinology, Editorial Board of Chinese Journal of Otorhinolaryngology Head and Neck Surgery; Subspecialty Group of Rhinology, Society of Otorhinolaryngology Head and Neck Surgery, Chinese Medical Association. Chinese Guidelines for diagnosis and treatment of allergic rhinitis. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. (2016) 51(1):6–24. doi: 10.3760/cma.j.issn.1673-0860.2016.01.004
17. World Health Organisation/International Union of Immunological Societies Working Group. Position paper: immunotherapy. Allergy. (1993) 14(Supp):9–35. doi: 10.1111/j.1398-9995.1993.tb04754.x
18. Scadding GK, Kariyawasam HH, Scadding G, Mirakian R, Buckley RJ, Dixon T, et al. BSACI guideline for the diagnosis and management of allergic and non-allergic rhinitis (revised edition 2017; first edition 2007). Clin Exp Allergy. (2017) 47(7):856–89. doi: 10.1111/cea.12953
19. Sun JL, Shen L, Chen J, Yu JM, Yin J. Species diversity of house dust mites in Beijing, China. J Med Entomol. (2013) 50(1):31–6. doi: 10.1603/ME12036
20. Epstein TEG, Calabria CW. Is immunotherapy safe for treatment of severe asthma. Curr Opin Allergy Clin Immunol. (2022) 22(6):396–401. doi: 10.1097/ACI.0000000000000853
21. Epstein TG, Liss GM, Berendts KM, Bernstein DI. AAAAI/ACAAI subcutaneous immunotherapy surveillance study (2013–2017): fatalities, infections, delayed reactions, and use of epinephrine autoinjectors. J Allergy Clin Immunol Pract. (2019) 7(6):1996–2003.e1. doi: 10.1016/j.jaip.2019.01.058
22. Epstein TG, Murphy-Berendts K, Liss GM, Bernstein DI. Risk factors for fatal and nonfatal reactions to immunotherapy (2008–2018): postinjection monitoring and severe asthma. Ann Allergy Asthma Immunol. (2021) 127(1):64–9.1. doi: 10.1016/j.anai.2021.03.011
23. Bernstein DI, Epstein TG. Safety of subcutaneous allergen immunotherapy. Allergy Asthma Proc. (2022) 43(4):267–71. doi: 10.2500/aap.2022.43.220035
24. Epstein TG, Liss GM, Murphy-Berendts K, Bernstein DI. AAAAI/ACAAI surveillance study of subcutaneous immunotherapy, years 2008–2012: an update on fatal and nonfatal systemic allergic reactions. J Allergy Clin Immunol Pract. (2014) 2(2):161–7. doi: 10.1016/j.jaip.2014.01.004
25. Albuhairi S, Sare T, Lakin P, El Khoury K, Crestani E, Schneider LC, et al. Systemic reactions in pediatric patients receiving standardized allergen subcutaneous immunotherapy with and without seasonal dose adjustment. J Allergy Clin Immunol Pract. (2018) 6(5):1711–6.4. doi: 10.1016/j.jaip.2017.11.040
Keywords: subcutaneous immunotherapy, D. farinae, D. pteronyssinus, children, local reactions, systematic adverse reactions
Citation: Zhou Q, Liu S, Dai B, Chen L, Han L, Zhang Q, Shen W and Shan L (2024) Safety of subcutaneous immunotherapy with Novo-Helisen-Depot in the children: a retrospective analysis from a single center in Northern China. Front. Pediatr. 12:1370224. doi: 10.3389/fped.2024.1370224
Received: 14 January 2024; Accepted: 19 March 2024;
Published: 25 April 2024.
Edited by:
Luis Ignacio Gonzalez-Granado, University Hospital October 12, SpainReviewed by:
Wayne Robert Thomas, University of Western Australia, Australia© 2024 Zhou, Liu, Dai, Chen, Han, Zhang, Shen and Shan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lishen Shan c2hhbmxzQHNqLWhvc3BpdGFsLm9yZw==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.