- Department of Critical Care Medicine, West China Hospital, Sichuan University/West China School of Nursing, Sichuan University, Chengdu, Sichuan, China
Background: Asthma is a common chronic respiratory disease in children. Alongside pharmacological interventions, inspiratory muscle training (IMT) emerges as a complementary therapeutic approach for asthma management. However, the extent of its efficacy in pediatric populations remains uncertain when compared to its benefits in adults. This systematic review aims to evaluate the effectiveness of IMT with threshold loading in children with asthma.
Methods: Randomized controlled trials (RCTs) evaluating the efficacy of inspiratory muscle training in pediatric asthma patients were identified through June 2023 across various literature databases, including PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAL), Web of Science, China Knowledge Resource Integrated Database (CNKI), Wei Pu Database, Wan Fang Database, and Chinese Biomedical Database (CBM). These trials compared inspiratory muscle training against sham inspiratory muscle training and conventional care. Eligible studies were assessed in terms of risk of bias and quality of evidence. Where feasible, data were pooled and subjected to meta-analysis, with results reported as mean differences (MDs) and 95% confidence intervals (CIs).
Results: Six trials involving 333 patients were included in the analysis. IMT demonstrated significant improvements in maximum inspiratory pressure (MIP) (MD 25.36, 95% CI 2.47–48.26, P = 0.03), maximum expiratory pressure (MEP) (MD 14.72, 95% CI 4.21–25.24, P = 0.006), forced vital capacity in percent predicted values [FVC(% pred)] (MD 3.90, 95% CI 1.86–5.93, P = 0.0002), forced expiratory volume in the first second in percent predicted values [FEV1(% pred)] (MD 4.96, 95% CI 2.60–7.32, P < 0.0001), ratio of forced expiratory volume in 1 s to forced vital capacity (FEV1/FVC) (MD 4.94, 95% CI 2.66–7.21, P < 0.0001), and asthma control test (ACT) (MD = 1.86, 95% CI: 0.96–2.75, P < 0.0001).
Conclusions: Findings from randomized controlled trials indicate that inspiratory muscle training enhances respiratory muscle strength and pulmonary function in pediatric asthma patients.
Systematic Review Registration: www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023449918, identifier: CRD42023449918.
Introduction
Asthma poses a serious global health challenge affecting individuals across all age demographics. It is characterized by chronic airway inflammation and variable expiratory airflow limitation, resulting in recurrent wheezing, breathlessness, chest tightness, and coughing, especially during nocturnal and early morning hours (1). The 2019 Global Burden of Disease Collaboration estimated that asthma affected approximately 262 million people worldwide (2), while the 2016 Centers for Disease Control and Prevention reported a pediatric asthma incidence ranging from 9.6%–10.5% (3). Studies indicate that asthma accounts for approximately 14 million missed school days for school-age children annually (4), with healthcare expenditures amounting to billions of dollars (5). Hence, asthma is considered a public health challenge and a major global concern for governments and healthcare professionals.
The key objectives of asthma management, outlined by the Global Initiative for Asthma (GINA) guidelines, include symptom control, prevention of exacerbations, maintenance of near-normal lung function, mitigation of drug-induced side effects, and enabling children to engage in daily activities without hindrance (6). Presently, pharmacologic therapies represent the cornerstone of asthma treatment. However, long-term pharmacological interventions are associated with adverse effects (7); for instance, prolonged corticosteroid usage reduces inspiratory muscle function in patients with asthma (8, 9). Hence, there is a pressing need for safer and more efficacious interventions.
Increased airway resistance and hyperinflation in patients with asthma may lead to respiratory muscle dysfunction (10). Recently, respiratory and physical therapy designed to improve respiratory muscle strength and lung function in children and adults has gradually become a supplementary treatment for patients with asthma (11). Examples of supplementary treatments include physical training (11), breathing exercises (12), and respiratory muscle training (13). Among these, inspiratory muscle training (IMT) stands out as the most available and cost-effectivecost nonpharmacological intervention for supplementary treatment. Previous studies have demonstrated that IMT reduces respiratory muscle weakness and enhances respiratory pressure, exercise capacity, and quality of life (14, 15).
Silva et al. (16), 2013 systematically concluded that IMT significantly increased inspiratory muscle strength in adults with asthma. Lista-Paz et al. (17) reported that IMT can significantly increase maximum inspiratory pressure (MIP) in adults with asthma. While most recent systematic reviews and meta-analyses have predominantly focused on adults with asthma, several RCTs have been conducted on children with asthma (13, 18–22). Therefore, we systematically reviewed the available evidence from RCTs to assess the efficacy of inspiratory muscle training in strengthening the respiratory muscles in children with asthma.
Methods
This systematic review and meta-analysis were conducted in accordance with the “Preferred Reporting Items for Systematic Reviews and Meta-Analyses” (PRISMA) guidelines (23). The protocol for this systematic review was registered in the International Prospective Register of Systematic Reviews (CRD42023449918).
Literature search and study inclusion
We systematically searched the following electronic databases for randomized controlled trials indexed through June 2023: PubMed, Embase, Cochrane Central Register of Controlled Trials (CENTRAL), Cumulative Index to Nursing and Allied Health Literature (CINAL), Web of Science, China Knowledge Resource Integrated Database (CNKI), Wan Fang Database, Chinese Biomedical Database (CBM), and Wei Pu Database. The search strings contained the following MeSH and other terms (Supplementary Material S1): (asthma OR asthma* OR bronchial asthma OR wheez*) AND (breathing exercises OR breathing exercise* OR respiratory muscle training OR inspiratory muscle training OR inspiratory muscle train* OR inspiratory muscle strength OR threshold load OR threshold device OR IMT OR RMT). Search strings were adapted to each database as necessary. The reference lists of the relevant articles were manually searched to identify additional publications.
Study selection
Studies were included in our review and meta-analysis if they met the following criteria: (1) they were randomized controlled trials involving children with asthma, regardless of its severity; (2) the intervention group received inspiratory muscle training, while the reference or control group received either sham inspiratory muscle training or usual care, defined as medication, education or traditional physical therapy; (3) data were reported for at least one outcome among maximum inspiratory pressure (MIP), maximal expiratory pressure (MEP), forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), ratio of forced expiratory volume in 1 s to forced vital capacity (FEV1/FVC), peak expiratory flow (PEF), quality of life, safety and asthma symptoms. Studies were excluded if the full text was unavailable. No language restrictions were applied to the eligible studies.
Data extraction
Two authors independently extracted the relevant data, with a third author available to resolve any disagreements. The extracted data included characteristics of the trial, including the first author's name, title, and year of publication; participant demographics, such as age, sex, and sample size; details of inspiratory muscle training in the experimental arm and interventions in the control arm, including method, frequency, duration, and intensity; and outcomes. In cases where data on outcomes were unclear, we contacted corresponding authors to obtain missing information.
Assessment of study quality
The risk of bias (RoB) in the included trials was assessed independently by two investigators using the Cochrane Risk of Bias 2 tool (24). Quality was evaluated across the following domains: randomized sequence generation, allocation concealment, blinding of participants, blinding of therapists, blinding of outcome assessors, incomplete outcome data, bias due to funding, selective reporting of outcomes, and other bias. The risk of bias for each tool item was judged as low, high, or unclear. Visualization of RoB 2 was performed using Robvis software. Any disagreements in quality assessment were resolved through discussion with a third investigator.
Statistical analysis
We used Cochrane Review Manager 5 to combine outcomes when possible. For all continuous outcomes, we extracted the sample size, post-intervention means, and standard deviations (SDs), as well as the sample size and number of events. Mean difference (MD) served as the effect size when studies employed the same tool for outcome assessment. Alternatively, standard mean difference (SMD) was used as the effect size if different tools were employed. All effect sizes were reported with 95% confidence intervals (CI). A significance value of P < 0.05 was considered statistically significant. Heterogeneity within pooled data was assessed using the chi-square test, Cochran's Q test, and inconsistency I2 test. If the heterogeneity was no higher than 50%, meta-analysis was conducted on the pooled data for the entire sample (25); otherwise, sub-group meta-analysis was performed based on age and asthma controls.
Results
Study selection
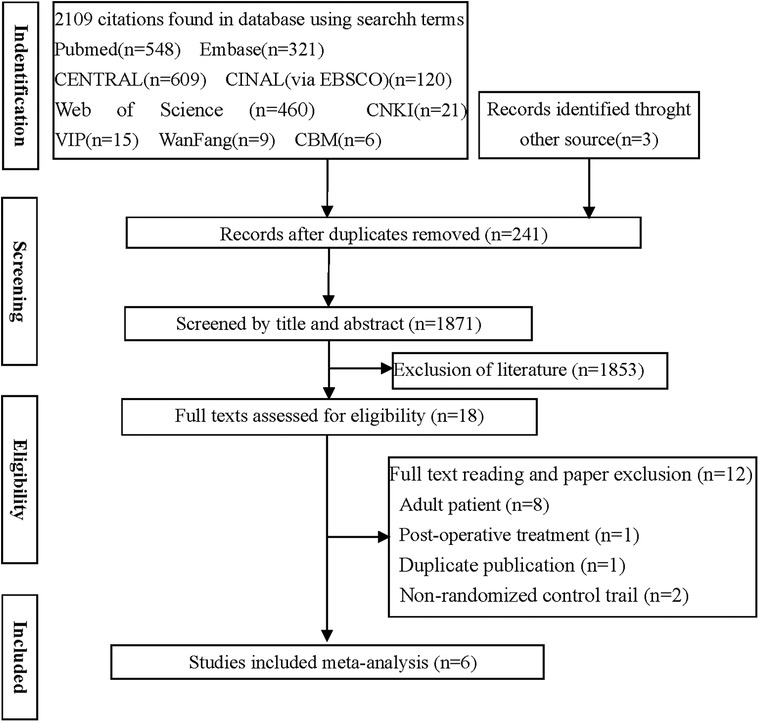
Out of the 2,112 potentially relevant studies, 241 duplicates and 1,853 articles were excluded based on reading of titles and abstracts. The remaining 18 publications were read in full, following which six randomized controlled trials (13, 18–22) were retained for the final analysis (Figure 1, Table 1).
Study characteristics
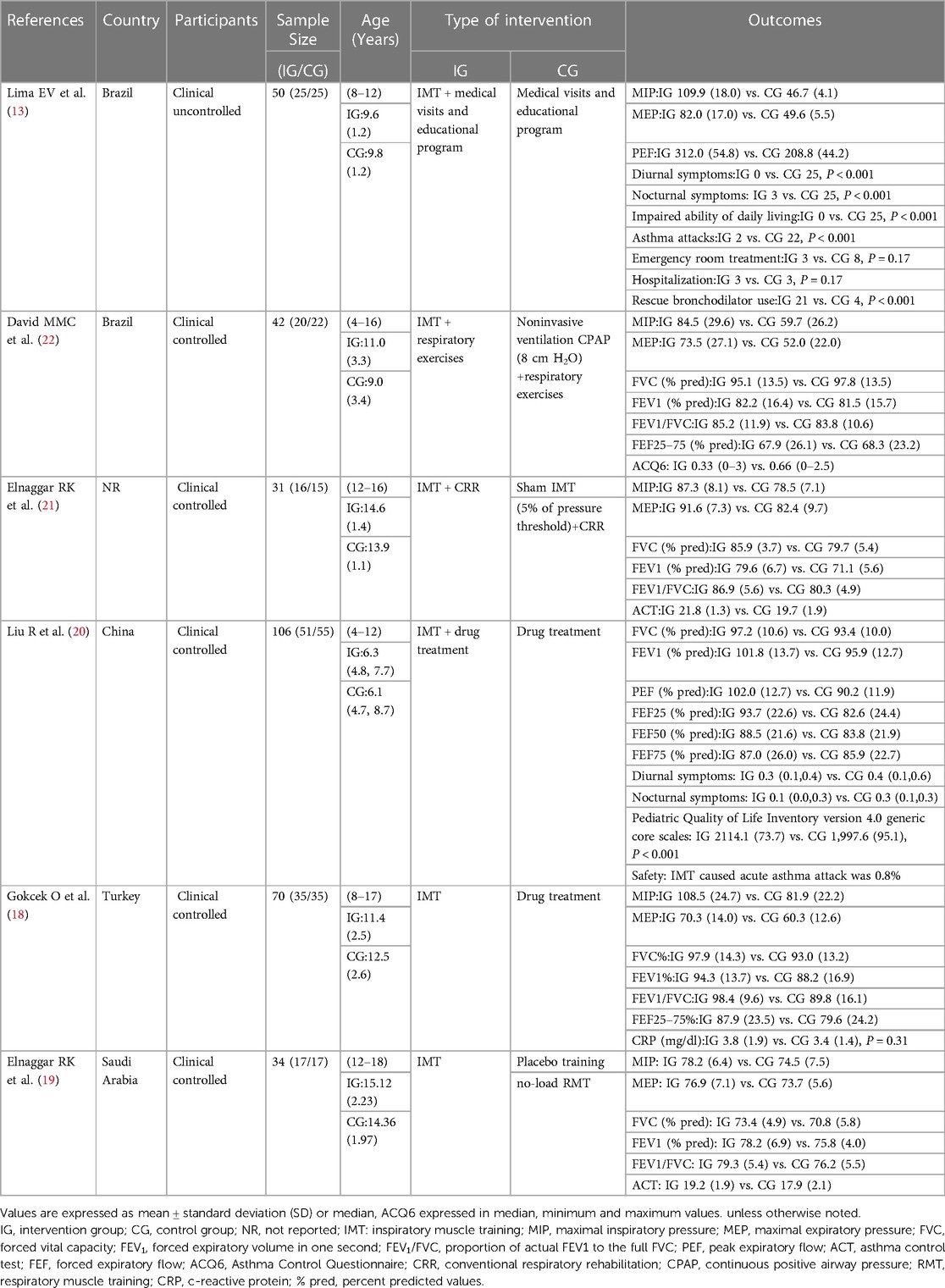
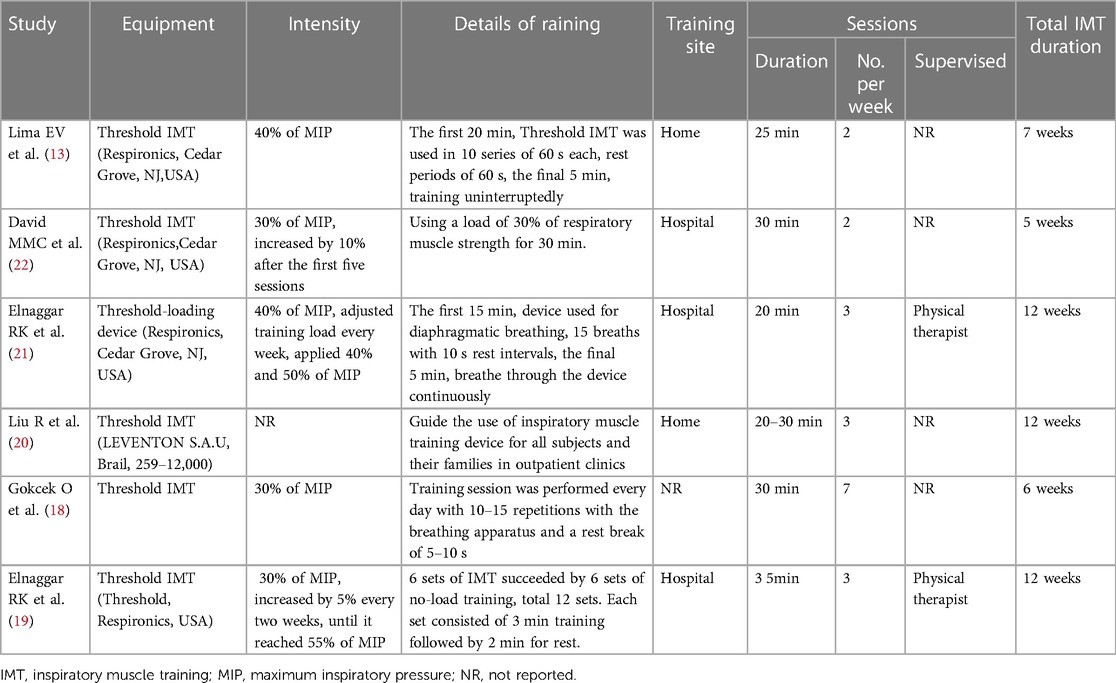
Details of the six included studies are presented in Table 1. One study was published in Chinese (20), while the others were published in English. These six studies included 333 children with asthma, with mean ages ranging from 6 to 15 years. Inspiratory muscle training utilized pressure-threshold loading devices in all studies (Table 2). The training sessions were conducted at 30%–55% of MIP, except in one study (20), which occurred 2–7 times per week, lasting 20–35 min, and spanning 5–12 weeks.
Methodological quality
A summary and graph of the results of the RoB 2 tool are shown in Figure 2. Five studies featured at least one domain where the risk of bias was judged to be unclear, while three had a high risk of bias in at least one domain. Quality issues were noted in one study regarding subject randomization, another concerning subject allocation, and two in relation to outcome measurements.
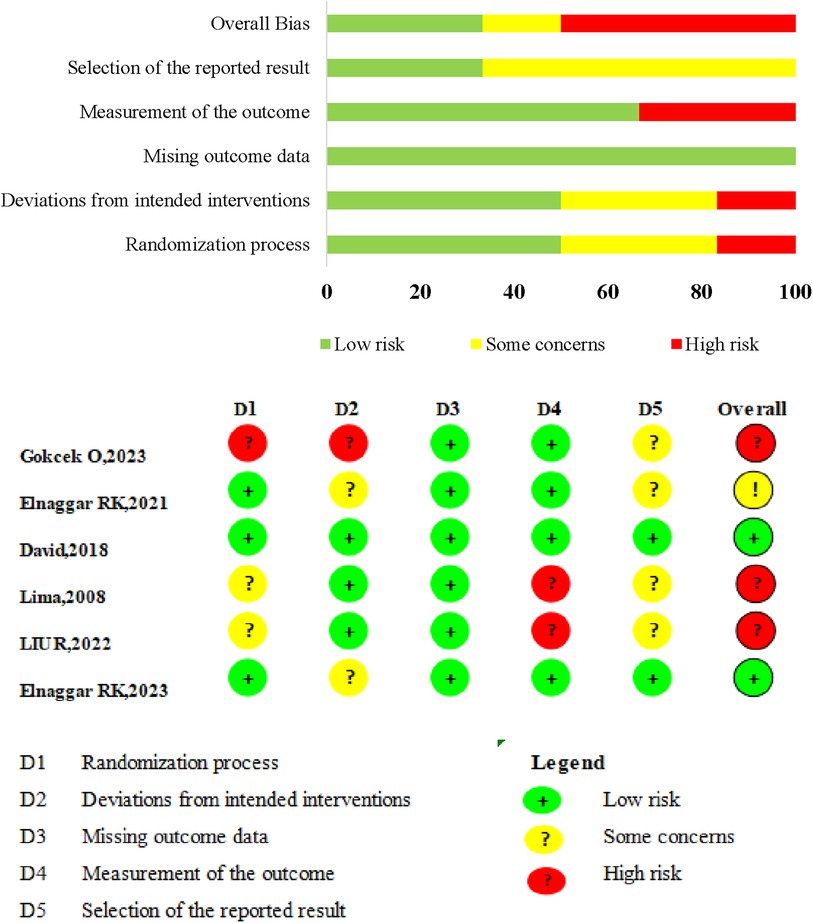
Figure 2. Risk of bias (ROB) summary. ROB 2.0 judgement according to domain and overall risk of bias for each study.
Outcome measures
Respiratory muscle strength (MIP and MEP)
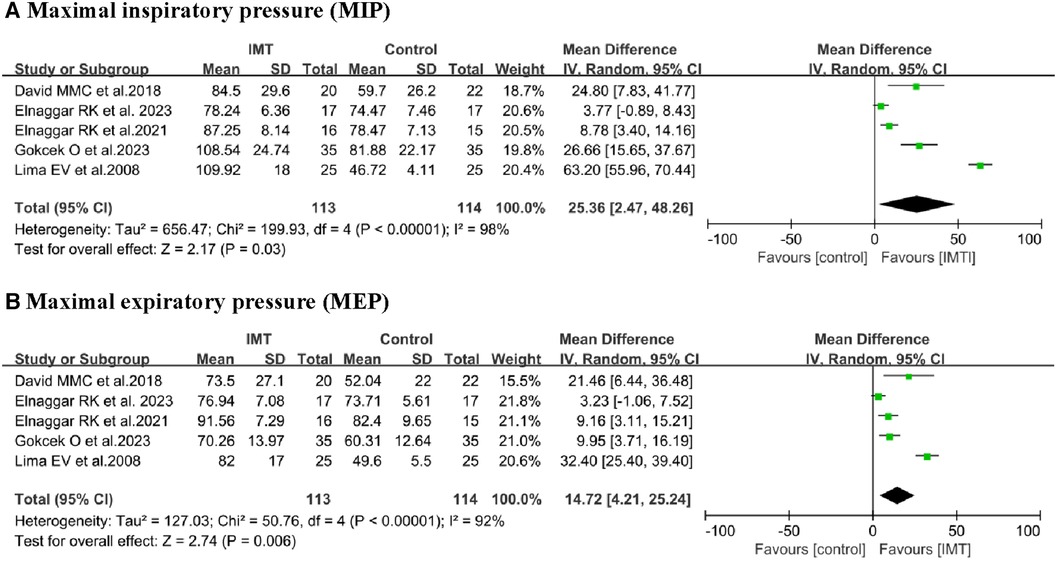
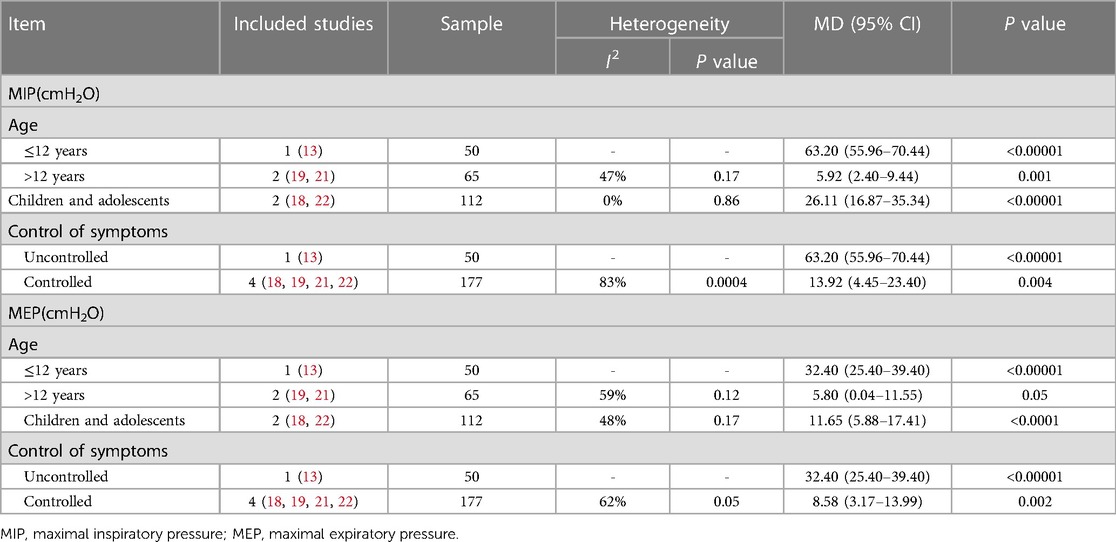
MIP: Data from five studies (13, 18, 19, 21, 22) involving 227 children were extracted for meta-analysis to assess the effects of IMT on MIP. The training led to significant increase in MIP compared to control interventions (MD 25.36 cmH2O, 95% CI 2.47–48.26, P = 0.03; Figure 3). However, this outcome was associated with high heterogeneity (I2 = 98%). Subgroup analysis confirmed a significant difference when participants were stratified by age or asthma control (Table 3).
MEP: Data from five studies (13, 18, 19, 21, 22) involving 227 children were extracted for meta-analysis to assess the effects of IMT on MEP. Due to the high level of homogeneity (I2 = 92%), a random-effects model was employed. The training led to significant improvement in MEP (MD 14.72 cmH2O, 95% CI: 4.21–25.24, P = 0.006; Figure 3). Subgroup analyses based on age and asthma control confirmed a significant difference (Table 3).
Pulmonary function
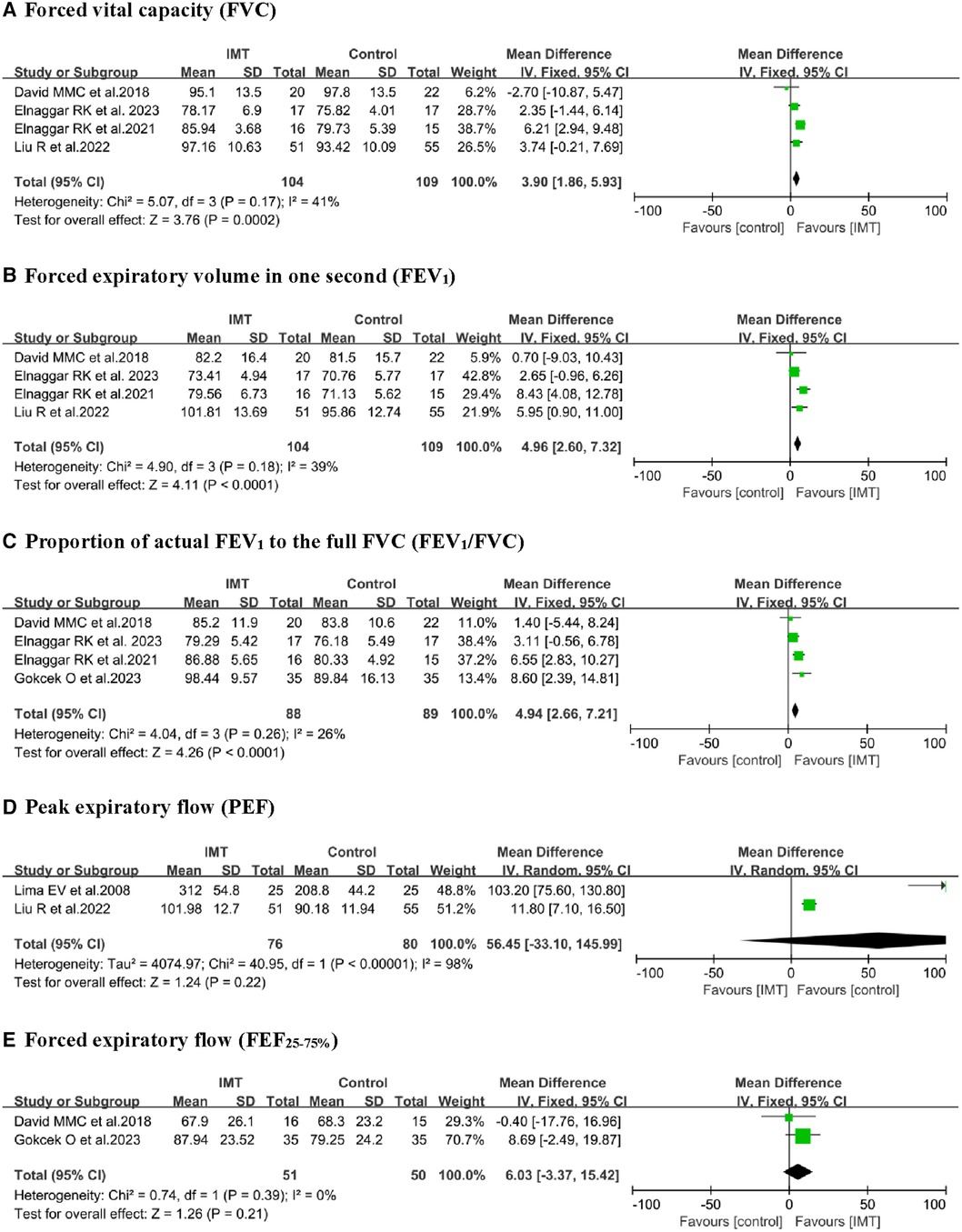
Five studies (18–22) reported the effect of IMT on FVC and FEV1, with four of them undergoing meta-analysis (19–22). A fixed-effect model was used to analyze and compare the IMT groups to control groups, where IMT was significantly associated with increased FVC (% predicted, MD 3.90, 95% CI: 1.86–5.93, P = 0.0002) and FEV1 (% predicted, MD 4.96, 95% CI: 2.60–7.32, P < 0.0001; Figure 4). Pooled analysis of FEV1/FVC revealed significant differences (MD 4.94, 95% CI: 2.66–7.21, P < 0.0001) between the IMT and control group. FVC, FEV1, and FEV1/FVC pooled estimates showed with low heterogeneity. Additionally, IMT did not significantly differ from control interventions in terms of peak expiratory flow in percent predicted values [PEF (% predicted)] or forced expiratory flow from 25% to 75% of vital capacity [FEF25–75 (% predicted)].
Asthma control test (ACT)
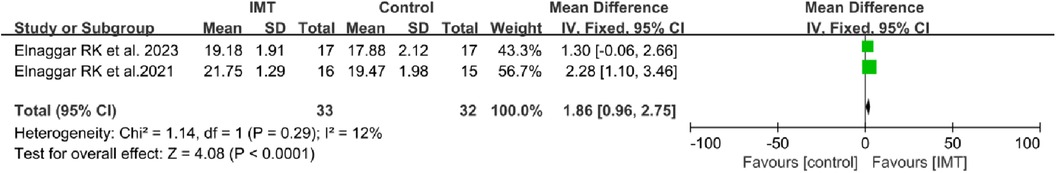
Two of the included studies assessed the impact of IMT on the ACT (19, 21). The pooled analysis revealed a statistically difference in ACT scores between the IMT group and the control group (MD 1.86, 95% CI: 0.96–2.75, P < 0.0001; Figure 5).
Qualitative analysis of other outcomes
Quality of life (QoL): Liu (20). observed a significant increase in pediatric QoL inventory scores associated with IMT.
Severity of asthma: Lima et al. (13) linked asthma severity with significant improvements in diurnal and nocturnal asthma symptoms, daily living abilities, asthma attacks, and rescue use of bronchodilators.
Safety: Liu (20). reported that the incidence of acute asthma attacks due to IMT was 0.8%.
Inflammatory markers: Gokcek et al. (18) detected no significant differences in the levels of the inflammatory marker, C-reactive protein, between children who underwent inspiratory muscle training and those who received control interventions.
Discussion
Our review and meta-analysis of six randomized controlled trials suggest that IMT can significantly improve MIP and MEP, as well as FVC, FEV1, and FEV1/FVC of pulmonary function in children with asthma. We also observed significant differences in Asthma Control Test (ACT) between the IMT and control groups. In other words, available evidence supports the use of respiratory training in children with asthma. Additionally, we did not find extensive evidence either supporting or refuting the safety of IMT or its efficacy in improving quality of life or mitigating asthma severity. More evidence is needed to clarify the effect of IMT on quality of life, severity of asthma, and safety.
Asthma treatment comprises both pharmacological and non-pharmacological interventions. While medication therapy has long been used for asthma control, non-pharmacological approaches, such as educational programs (26), self-management (27), breathing exercises (12), and physical training (28), have been highlighted as adjuvant therapies for children undergoing pharmacological asthma treatment and are widely used worldwide. IMT is a therapeutic modality aimed at strengthening respiratory muscles and has some applications in children with asthma (13, 18–22).
To our knowledge, this is the first meta-analysis evaluating the efficacy of IMT in children with asthma. Our findings align with the results of IMT in asthmatic patients reported by Lista-Paz et al., 2023 (17), which included 11 studies, 10 with adults and only one study with children (13). These findings demonstrated a significant increase in MIP after IMT in adults with asthma, thereby reinforcing the relevance of the dose-response principle of training. Castilho et al. (12), published in 2020, investigated the effects of physical therapy on lung function in children with asthma across 18 studies; only two of these studies included IMT and conducted a qualitative analysis.
Training the respiratory muscles, particularly the inspiratory muscles, is recommended as part of the pulmonary rehabilitation program used in adults with asthma and chronic obstructive pulmonary disease (COPD) (16, 17, 29, 30). Research evidence indicates that IMT enhances inspiratory muscle strength and endurance, functional exercise capacity, and quality of life while decreasing dyspnea in COPD patients (29, 30). However, our meta-analysis was unable to determine the optimal duration of intervention or training intensity. A pressure threshold device was used for a session duration of 20–35 min. Regarding IMT intensity, 30%–55% of MIP was employed, and children reported that training with 40% of MIP (moderate intensity) was perceived more comfortable (21). Notably, only two studies reported training under the supervision of a physical therapist (19, 21). Additionally, no studies have reported adherence to IMT programs.
Limitations
The findings of our review should be interpreted with caution, given several limitations of the included studies, primarily heterogeneity in participant age (4–18 years), variability in asthma control status, interventions utilized in the control arm, and inconsistencies in IMT intervention intensity. We found that IMT programs utilized external loads ranging from 30% to 55% of MIP. We attempted to address heterogeneity through subgroup analysis, which resulted in insufficient studies in some subgroups. The meta-analyses included no more than six trials, which precluded the assessment of publication bias using funnel plots. Due to the limited number of studies and small sample sizes, we recommend that future studies develop standardized protocols for IMT. Large randomized, placebo-controlled, double-blind trials would be a significant step forward in elucidating the effectiveness and safety of IMT in children and adolescents with asthma.
Conclusion
Evidence from randomized controlled trials suggests that inspiratory muscle training can potentially strengthen respiratory muscles and improve pulmonary function in children with asthma. The efficacy and safety of such training should be explored in larger multicenter trials. Future research should explore whether inspiratory muscle training improves inspiratory muscle endurance, quality of life, asthma control, symptoms, severity, and safety.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YX: Writing – original draft, Software. TL: Writing – review & editing. XC: Writing – review & editing. HZ: Writing – review & editing. LZ: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1367710/full#supplementary-material
References
1. van Beveren GJ, Said H, van Houten MA, Bogaert D. The respiratory microbiome in childhood asthma. J Allergy Clin Immunol. (2023) 152:1352–67. doi: 10.1016/j.jaci.2023.10.001
2. The global asthma report 2022. Int J Tuberc Lung Dis. (2022) 26:1–104. doi: 10.5588/ijtld.22.1010
3. Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital signs: asthma in children—united States, 2001–2016. MMWR Morb Mortal Wkly Rep. (2018) 67:149–55. doi: 10.15585/mmwr.mm6705e1
4. Centers for Disease Control and Prevention (CDC). Vital signs: asthma prevalence, disease characteristics, and self-management education: United States, 2001–2009. MMWR Morb Mortal Wkly Rep. (2011) 60:547–52.21544044
5. Gaillard EA, Kuehni CE, Turner S, Goutaki M, Holden KA, de Jong C, et al. European respiratory society clinical practice guidelines for the diagnosis of asthma in children aged 5–16 years. Eur Respir J. (2021) 58:1–22. doi: 10.1183/13993003.04173-2020
6. Levy ML, Bacharier LB, Bateman E, Boulet LP, Brightling C, Buhl R, et al. Key recommendations for primary care from the 2022 global initiative for asthma (gina) update. NPJ Prim Care Respir Med. (2023) 33:7. doi: 10.1038/s41533-023-00330-1
7. Reddy AP, Gupta MR. Management of asthma: the current US and European guidelines. Adv Exp Med Biol. (2014) 795:81–103. doi: 10.1007/978-1-4614-8603-9_6
8. Perez T, Becquart LA, Stach B, Wallaert B, Tonnel AB. Inspiratory muscle strength and endurance in steroid-dependent asthma. Am J Respir Crit Care Med. (1996) 153:610–5. doi: 10.1164/ajrccm.153.2.8564106
9. Akkoca O, Mungan D, Karabiyikoglu G, Misirligil Z. Inhaled and systemic corticosteroid therapies: do they contribute to inspiratory muscle weakness in asthma? Respiration. (1999) 66:332–7. doi: 10.1159/000029403
10. Stell IM, Polkey MI, Rees PJ, Green M, Moxham J. Inspiratory muscle strength in acute asthma. Chest. (2001) 120:757–64. doi: 10.1378/chest.120.3.757
11. Zhang W, Wang Q, Liu L, Yang W, Liu H. Effects of physical therapy on lung function in children with asthma: a systematic review and meta-analysis. Pediatr Res. (2021) 89:1343–51. doi: 10.1038/s41390-020-0874-x
12. Castilho T, Itaborahy BDH, Hoepers A, Brito JND, Almeida ACDS, Schivinski CIS. Effects of inspiratory muscle training and breathing exercises in children with asthma: a systematic review. J Hum Growth Dev. (2020) 30:291–300. doi: 10.7322/JHGD.V30.10381
13. Lima EV, Lima WL, Nobre A, Dos SA, Brito LM, Costa MR. Inspiratory muscle training and respiratory exercises in children with asthma. J Bras Pneumol. (2008) 34:552–8. doi: 10.1590/s1806-37132008000800003
14. Sogard AS, Mickleborough TD. The therapeutic role of inspiratory muscle training in the management of asthma: a narrative review. Am J Physiol Regul Integr Comp Physiol. (2023) 325:645–63. doi: 10.1152/ajpregu.00325.2022
15. Shei RJ, Paris HL, Sogard AS, Mickleborough TD. Time to move beyond a “one-size fits all” approach to inspiratory muscle training. Front Physiol. (2021) 12:766346. doi: 10.3389/fphys.2021.766346
16. Silva IS, Fregonezi GA, Dias FA, Ribeiro CT, Guerra RO, Ferreira GM. Inspiratory muscle training for asthma. Cochrane Database Syst Rev. (2013) 2013:CD3792. doi: 10.1002/14651858.CD003792
17. Lista-Paz A, Bouza CL, Jácome C, Fregonezi G, Labata-Lezaun N, Llurda-Almuzara L, et al. Effect of respiratory muscle training in asthma: a systematic review and meta-analysis. Ann Phys Rehabil Med. (2023) 66:101691. doi: 10.1016/j.rehab.2022.101691
18. Gokcek O, Yurdalan U, Tugay BU, El C, Dogan S. Evaluation of the possible effect of inspiratory muscle training on inflammation markers and oxidative stress in childhood asthma. Eur J Pediatr. (2023) 182:3713–22. doi: 10.1007/s00431-023-05047-4
19. Elnaggar RK, Osailan AM, Elbanna MF. The rationale of applying inspiratory/expiratory muscle training within the same respiratory cycle in children with bronchial asthma: a placebo-controlled randomized clinical investigation. J Asthma. (2023) 60:900–11. doi: 10.1080/02770903.2022.2103708
20. Liu R. Prospective cohort study of inspiratory muscle training in children with bronchial asthma. Chong Qing Medical University. (2022):1–31. doi: 10.27674/d.cnki.gcyku.2022.000872
21. Elnaggar RK. A randomized placebo-controlled study investigating the efficacy of inspiratory muscle training in the treatment of children with bronchial asthma. J Asthma. (2021) 58:1661–9. doi: 10.1080/02770903.2020.1821058
22. Cabral DMM, Dantas GELD, Carvalho MM, Dirceu C. Noninvasive ventilation and respiratory physical therapy reduce exercise-induced bronchospasm and pulmonary inflammation in children with asthma: randomized clinical trial. Ther Adv Respir Dis. (2018) 12:327051276. doi: 10.1177/1753466618777723
23. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The prisma 2020 statement: an updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89. doi: 10.1186/s13643-021-01626-4
24. Sterne J, Savovic J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
25. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
26. Mccallum GB, Morris PS, Brown N, Chang AB. Culture-specific programs for children and adults from minority groups who have asthma. Cochrane Database Syst Rev. (2017) 8:CD6580. doi: 10.1002/14651858.CD006580.pub5
27. Harris K, Kneale D, Lasserson TJ, Mcdonald VM, Grigg J, Thomas J. School-based self-management interventions for asthma in children and adolescents: a mixed methods systematic review. Cochrane Database Syst Rev. (2019) 1:1–332. doi: 10.1002/14651858.CD011651.pub2
28. Mcloughlin RF, Clark VL, Urroz PD, Gibson PG, Mcdonald VM. Increasing physical activity in severe asthma: a systematic review and meta-analysis. Eur Respir J. (2022) 60:1–15. doi: 10.1183/13993003.00546-2022
29. Gosselink R, De Vos J, van den Heuvel SP, Segers J, Decramer M, Kwakkel G. Impact of inspiratory muscle training in patients with copd: what is the evidence? Eur Respir J. (2011) 37:416–25. doi: 10.1183/09031936.00031810
Keywords: inspiratory muscle training, children, asthma, maximum inspiratory pressure, systematic review
Citation: Xiang Y, Luo T, Chen X, Zhang H and Zeng L (2024) Effect of inspiratory muscle training in children with asthma: a systematic review and meta-analysis of randomized controlled trials. Front. Pediatr. 12:1367710. doi: 10.3389/fped.2024.1367710
Received: 9 January 2024; Accepted: 4 March 2024;
Published: 18 March 2024.
Edited by:
Ren-Jay Shei, Coherus BioSciences, United StatesReviewed by:
John Lowman, University of Alabama at Birmingham, United StatesAbigail S. Sogard, Indiana University, United States
Noppawan Charususin, Thammasat University, Thailand
© 2024 Xiang, Luo, Chen, Zhang and Zeng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ling Zeng emVuZ2xpbmc1MTBAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yuping Xiang
Yuping Xiang Tianhui Luo†
Tianhui Luo†