- 1Department of Pediatrics, Division of Pediatric Critical Care Medicine, Beatrix Children’s Hospital, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
- 2Department of Neurology, University Medical Center Groningen, University of Groningen, Groningen, Netherlands
Objectives: To describe the use and outcomes of the neurological wake-up test (NWT) in pediatric severe traumatic brain injury (pTBI).
Design: Retrospective single-center observational cohort study.
Setting: Medical-surgical tertiary pediatric intensive care unit (PICU) in a university medical center and Level 1 Trauma Center.
Patients: Children younger than 18 years with severe TBI [i.e., Glasgow Coma Scale (GCS) of ≤8] admitted between January 2010 and December 2020. Subjects with non-traumatic brain injury were excluded.
Measurements and main results: Of 168 TBI patients admitted, 36 (21%) met the inclusion criteria. Median age was 8.5 years [2 months to 16 years], 5 patients were younger than 6 months. Median initial Glasgow Coma Scale (GCS) and Glasgow Motor Scale (GMS) was 6 [3–8] and 3 [1–5]. NWTs were initiated in 14 (39%) patients, with 7 (50%) labelled as successful. Fall from a height was the underlying injury mechanism in those seven. NWT-failure occurred in patients admitted after traffic accidents. Sedation use in both NWT-subgroups (successful vs. failure) was comparable. Cause of NWT-failure was non-arousal (71%) or severe agitation (29%). Subjects with NWT failure subsequently had radiological examination (29%), repeat NWT (43%), continuous interruption of sedation (14%) or intracranial pressure (ICP) monitoring (14%). The primary reason for not doing NWTs was intracranial hypertension in 59%. Compared to the NWT-group, the non-NWT group had a higher PRISM III score (18.9 vs. 10.6), lower GCS/GMS at discharge, more associated trauma, and circulatory support. Nine patients (25%) died during their PICU admission, none of them had an NWT.
Conclusion: We observed limited use of NWTs in pediatric severe TBI. Patients who failed the NWT were indistinguishable from those without NWT. Both groups were more severely affected compared to the NWT successes. Therefore, our results may indicate that only a select group of severe pTBI patients qualify for the NWT.
Introduction
Despite improvements in neurocritical care over the last decades, traumatic brain injury (TBI) remains a leading cause of mortality and significant morbidity. Intensive clinical monitoring, monitoring of intracranial pressure (ICP) and cerebral perfusion pressure (CPP), mechanical ventilation (MV), and continuous sedation are the main objectives of routine management of pediatric patients with severe TBI (1). This approach aims to prevent secondary injury by ensuring adequate brain perfusion and avoiding ischemia. The beneficial effects of sedation include decreasing intracranial pressure and cerebral oxygen consumption, thereby limiting the risk of secondary insults (1–3). However, accurate neurological assessment, which is considered to be the most fundamental clinical monitoring tool in TBI patients, is not possible in sedated patients. Reassessment of the patient's level of consciousness can detect neurological deterioration requiring prompt intervention (4).
Performing a neurological wake-up test (NWT) necessitates sedation interruption. This may be challenging especially in pediatric patients. Since short-acting sedatives such as propofol are contra-indicated in younger children, drugs with a relatively long half-life are used (5). In addition, sedation is also required to facilitate MV and reduce patient stress, which in turn may lead to episodic rises in cerebral blood volume (CBV) and ICP (6–8). This hampers the use of the NWT. Besides these practical considerations, the benefit of doing NWTs in especially severe TBI patients needs to be balanced against the risk of inducing a stress response. It has been suggested that not all patients can safely undergo NWTs due to potential secondary insults and the uncertainty about its clinical relevance in the context of advanced multimodality monitoring (9–14).
To our best of knowledge, there is no data on the usefulness feasibility of the NWT in neuromonitoring in severe pediatric TBI (pTBI). In fact, current international guidelines do not mention using the NWT (1, 15, 16). We therefore sought to characterize the use and outcomes of NWTs in severe pTBI patients admitted to our pediatric critical care unit (PICU).
Patients and methods
Study design and patient selection
We conducted an 11-year retrospective, observational, single-center study at a 20-bed PICU of the University Medical Centre Groningen (the Netherlands). Data from mechanically ventilated, sedated subjects ≤18 years with severe TBI [i.e., Glasgow Coma Scale (GCS) of ≤8] admitted between January 2010 and December 2020 was eligible for analysis. Subjects with non-traumatic brain injury were excluded. The Institutional Review Board approved the study and waived the need for informed consent (IRB UMCG #202200267).
Patient management
Patients with moderate-to-severe TBI, admitted to our PICU are managed according to our protocol based on the most recent international guidelines (1, 17, 18), and with the following objectives: head elevation (30°), PaO2 > 10 kPa, PaCO2 between 4.5 and 6 kPa, targeted temperature <37.5°C, ICP <20 mmHg and CPP target between 40 and 50 mmHg (with infants at the lower end and adolescents at or above the upper end of this range). In the absence of invasive ICP monitoring a mean arterial pressure (MAP) ≥75th percentile per age is being pursued to maintain adequate CPP. If ICP increased, second tier of therapy using practical clinical algorithms included hyperosmolar therapies, mild hyperventilation, late application of hypothermia, barbiturate infusion or decompressive craniectomy surgery. Routine management of sedation includes using a combination of intravenous opioids and benzodiazepines or intravenous opioids and propofol, and neuromuscular blocking agents at the discretion of the bedside team.
Collection of data variables
We collected baseline patient characteristics, information on trauma mechanism, presence of associated lesions, radiological examination within the first 24 h, information concerning neuromonitoring, neurosurgery, clinical and physiological data from the first 7 days of PICU admission. Radiological examinations were assessed by the attending radiologist. Ventilation time ≤24 h is described in actual hours, being easily deduced from the patients' records. When ventilation time exceeded 24 h, hours were simplified to whole day hours. The Pediatric RISk of Mortality [PRISMII]—24 h score and the Pediatric Index of Mortality [PIM] were calculated to assess patient status (19, 20). The GCS at onset was used to assess TBI and trauma severity (21).
NWT
The NWT was defined as a sedation interruption allowing for a clinical examination by the attending pediatric neurologist within the first 24 h of PICU admission. The NWT was labelled as successful if the neurological evaluation could be completed without signs of increasing ICP allowing arousal or even detubation. Failure criteria for the NWT were defined as hemodynamic instability, respiratory distress, increasing ICP or other neurological distress. In addition, the NWT was also labelled as failed in the absence of any arousal [i.e., present or absent neurological deterioration (e.g., decreasing level of consciousness defined by a decrease in the GCS-M score of ≥2 points (16))].
Outcomes of interest
The primary outcome of our study was the number of subjects in whom the NWT was performed, and to identify reasons why the NWT was not performed. Secondary outcomes included identifying which (type of) physician initiated the NWT, and the outcome of patients who underwent the NWT.
Statistical methods
For analytical purposes, we stratified patients by NWT-outcome [i.e., NWT- success, NWT failure and non-NWT (i.e., NWT not performed)]. Categorical variables were described as absolute number and percentage (%) of total, and continuous variables as median and interquartile range [IQR]. Continuous variables were analyzed with the Mann-Whitney U test and Kruskal–Wallis test, categorical data was analyzed using the χ2 test (Fisher exact test if the value of any cell was <5). All analyses were performed with SPSS v23.0 [IBM Statistical Package for the Social Sciences (SPSS) for Windows, Armonk, NY: IBM Corp]. P values < 0.05 were accepted as statistically significant.
Results
Study population
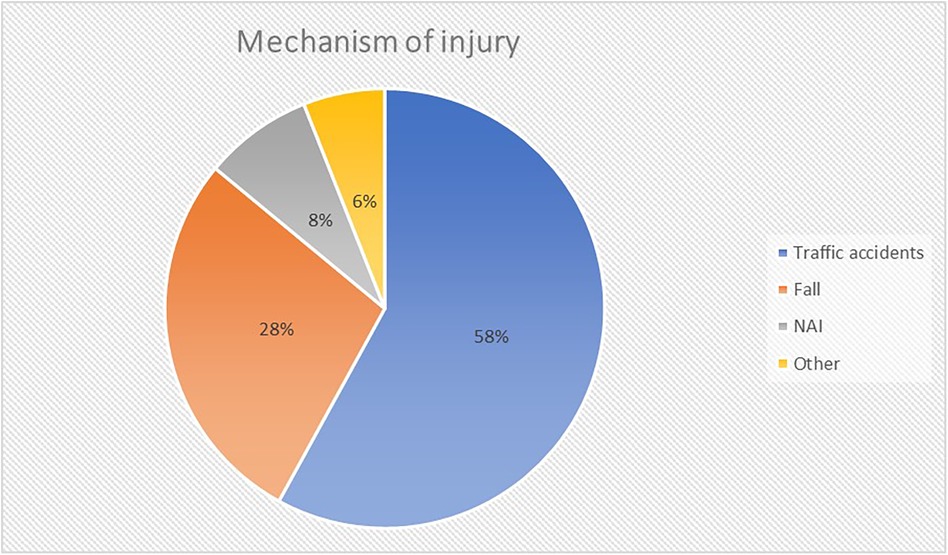
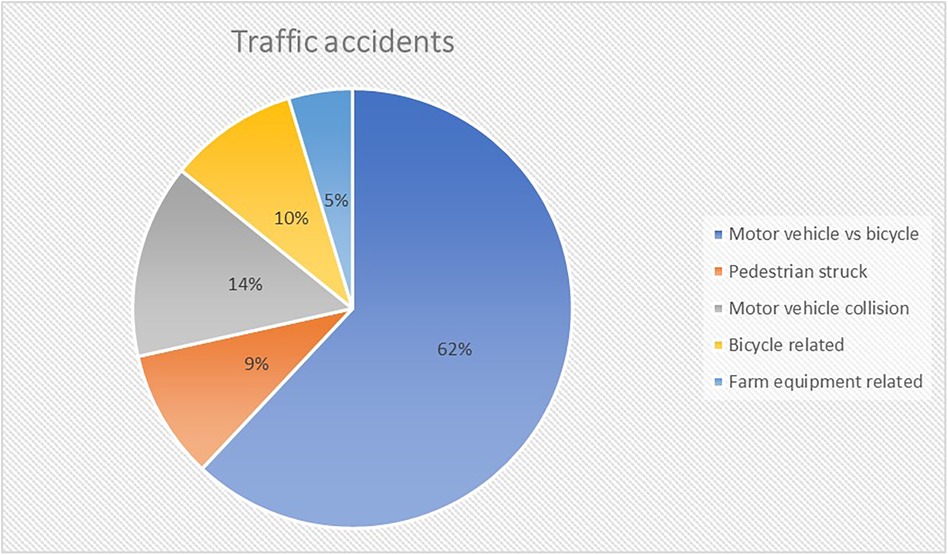
Out of 168 TBI patients admitted, 39 (23%) had a GCS ≤8. Subsequently, a further three patients were excluded: one who with anoxia following cardiac arrest and two patients who were not mechanically ventilated. Thus, data from 36 patients (21 male gender; 58%) with a median age of 8.5 years [2 months to 16 years] was available for analysis (Supplementary Table A). Median PRISM and PIM score was 14 [2–48] and −2.798 [−3.468 to 2.671]. The most common mechanism of injury were traffic accidents (TA; 58%) (Figures 1, 2). Median initial GCS and GMS was 6 [3–8] and 3 [1–5].
NWT
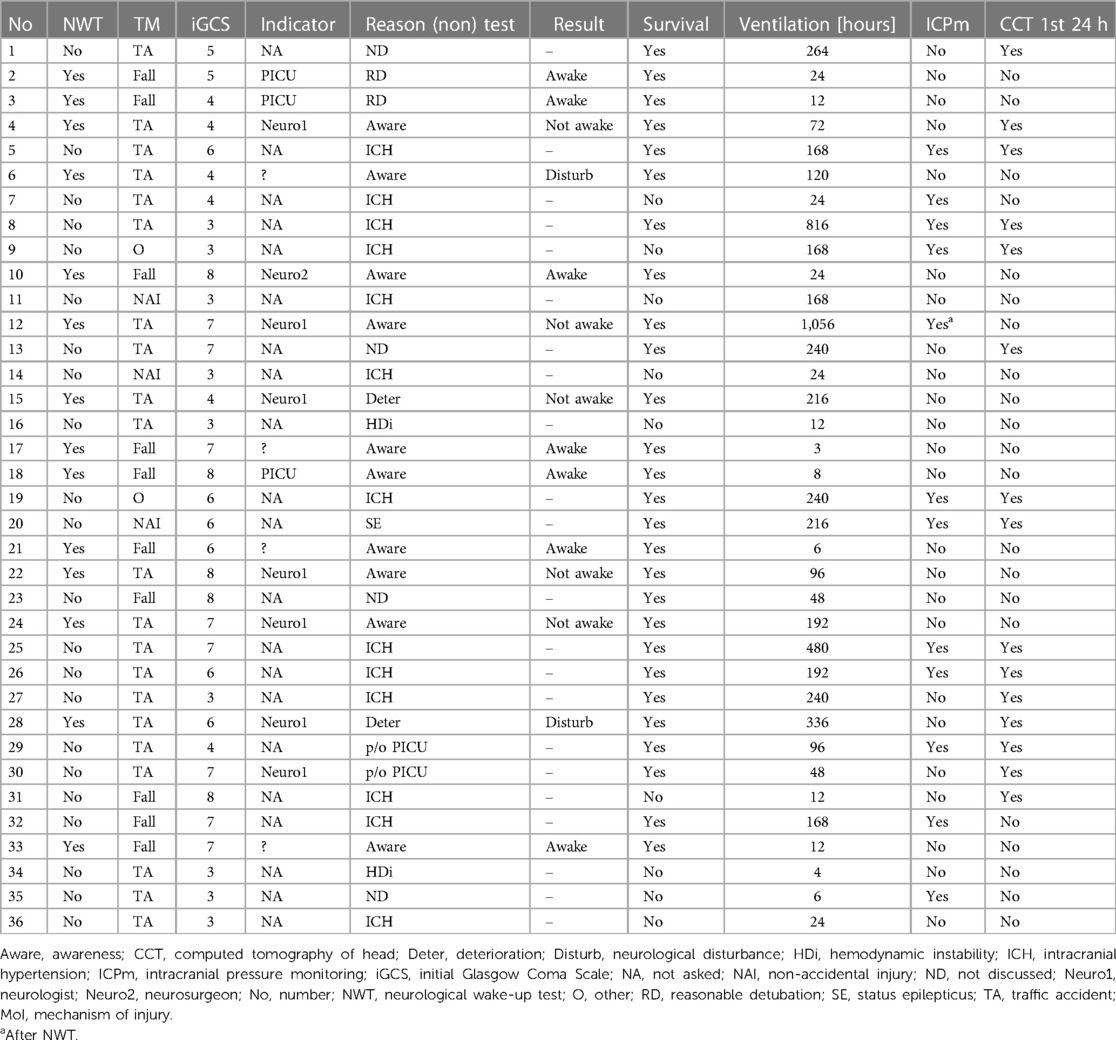
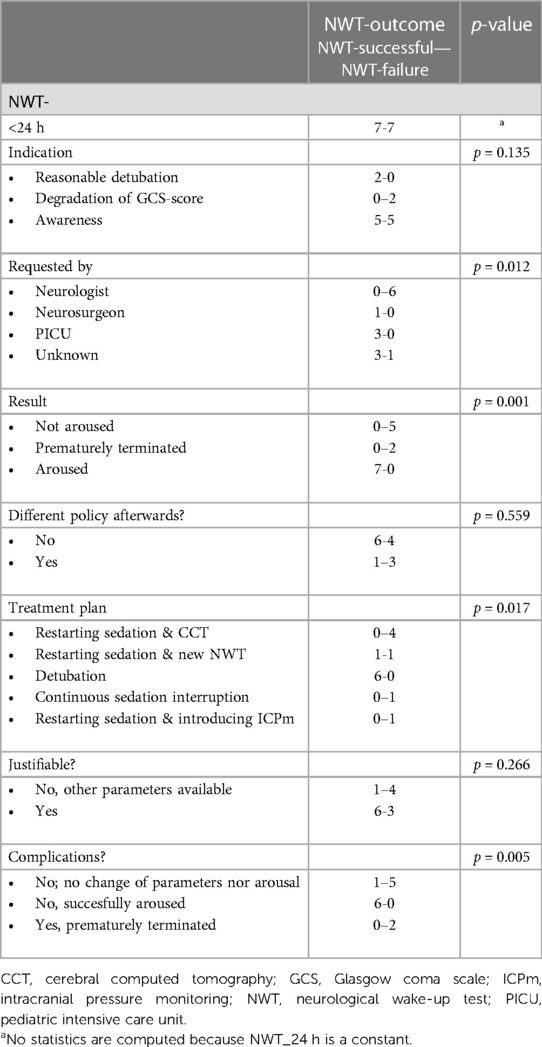
NWTs were performed in 14 patients (39%), with seven (50%) labelled as successful (Table 1). NWTs were requested by either the pediatric intensivist, neurologist or neurosurgeon. Assessing awareness (57%) or neurological deterioration (43%) were mentioned as NWT-indications. The distribution of NWTs during the study period was equal (Supplementary Table B). No intracranial pressure monitoring was present at the time of the first NWT in any of the 14 patients.
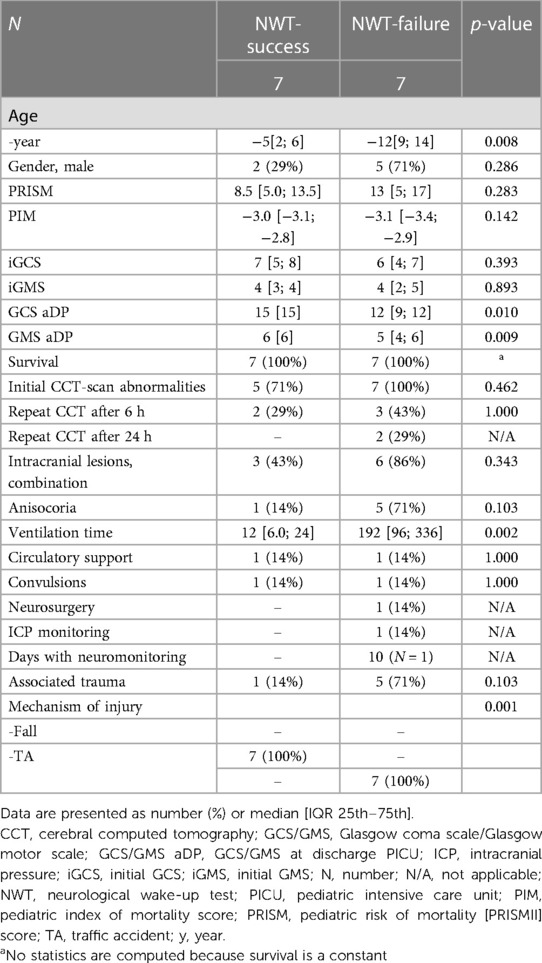
Patients in whom the NWT was successful were significantly younger (5 [2–6] vs.12 [9–14] years (p = 0.008) and all had a fall from height as injury mechanism compared with all patients having a TA in the failure group, (p = 0.001) (Table 2). Overall GCS and GMS scores at onset were similar, but patients in whom the NWT was stopped had significant lower GCS/GMS scores at discharge PICU (12/5 vs.15/6, p = 0.010/p = 0.009). Patient characteristics were similar for associated trauma, initial cerebral computed tomography (CCT)-scan abnormalities, convulsions, circulatory support or survival. Patients in whom the NWT was successful were extubated shortly hereafter. They had a significantly shorter MV duration (12 [6–24] vs. 192 h [96–336] (p = 0.002) compared to the failed NWTs.
Reasons for cessation of the NWT were non-arousal (71%) or severe agitation (29%). No clinical signs of increasing ICP during the NWT were observed. All but one of the NWTs in this subgroup were requested by the attending neurologist (Table 3). Subjects who failed the NWT subsequently had radiological examination (57%), request for a new NWT (14%), continuous interruption of sedation (14%) or intracranial pressure monitoring (14%). Although a significant difference (p = 0.036) in sedation use between both NWT-subgroups (successful vs. failure) was shown, both subgroups were considered comparable (Supplementary Table C-III) due to equal use of propofol with or without a combination of intravenous opioids and benzodiazepines, albeit in different combinations.
No significant complications as a result of the NWTs were observed due to cessation of the test when failure criteria were met.
Non-NWT
Descriptive characteristics of the non-NWTs (n = 22) are summarized in Table 4. Patients with NWTs had less severe head injury marked by higher GCS/GMS scores upon admission (6–7 vs. 4–5; p = 0.045/4 vs. 2; p = 0.019) as well at discharge (13–14 vs. 9; p = 0.014/6 vs. 5; 0.070) and significant lower median PRISM (11 vs. 15.5; p = 0.014) and PIM scores (−3.1 vs. −2.5; p = 0.008). Patients without NWT more often required circulatory support (82% vs. 14%, p < 0.001), ICP-monitoring (45% vs. 7%, p = 0.025) or neurosurgical interventions (68% vs. 7%, p < 0.001) and they had higher mortality (59% vs. 100%, p = 0.006). Intracranial pressure monitoring was used in 11 (50%) of patients without NWT. All ICP-monitoring devices were placed within the first 24 h.
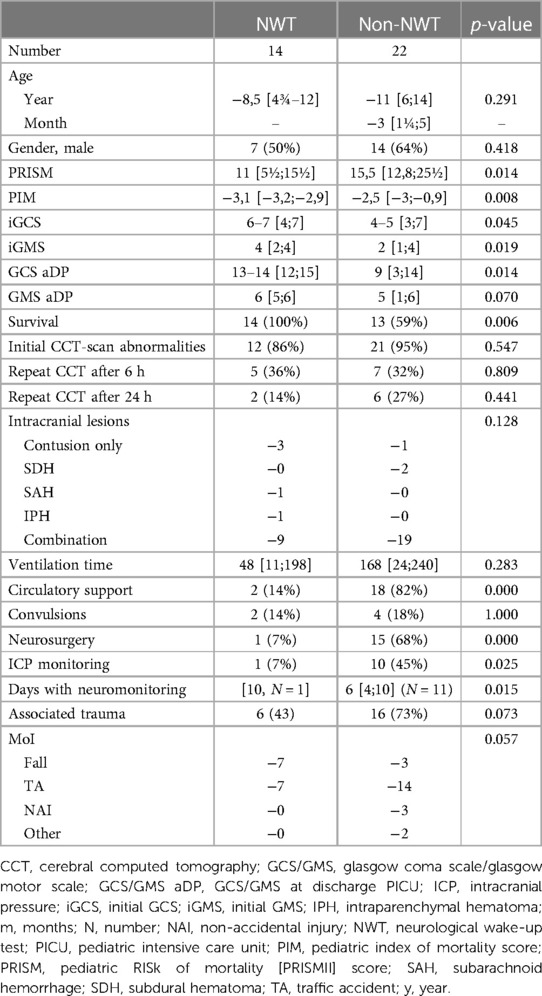
Table 4. NWT-outcome of NWT-group vs non-NWT group. Data are presented as number (%) or median (IQR 25th–75th).
Patients without NWTs had a significantly higher PIM score (−2.5 vs. −3.1, p = 0.017), and more often required circulatory support (82% vs. 14%, p = 0.003) and neurosurgical intervention (68% vs. 14%, p = 0.026) compared to the patients in whom the NWT was defined as failed (Table 5). GCS/GMS scores upon admission and discharge and initial CCT-scan abnormalities between non-NWTs and patients in whom the NWT failed were comparable, as were age, ventilation time, associated trauma and underlying mechanism of injury. The successful NWTs were less similar to the non-NWTs (Supplementary Table D): patients without NWT had significantly longer ventilation time (168 vs.12 h, p = 0.004), more circulatory support (82% vs. 14%, p = 0.003), intracranial lesions (82% vs. 43%, p = 0.045), neurosurgery (68% vs. 0%, p = 0.002), associated trauma (73% vs. 14%, p = 0.011) and underlying mechanism of injury (several mechanisms vs. 100% fall, p = 0.001) (Supplementary Table E).
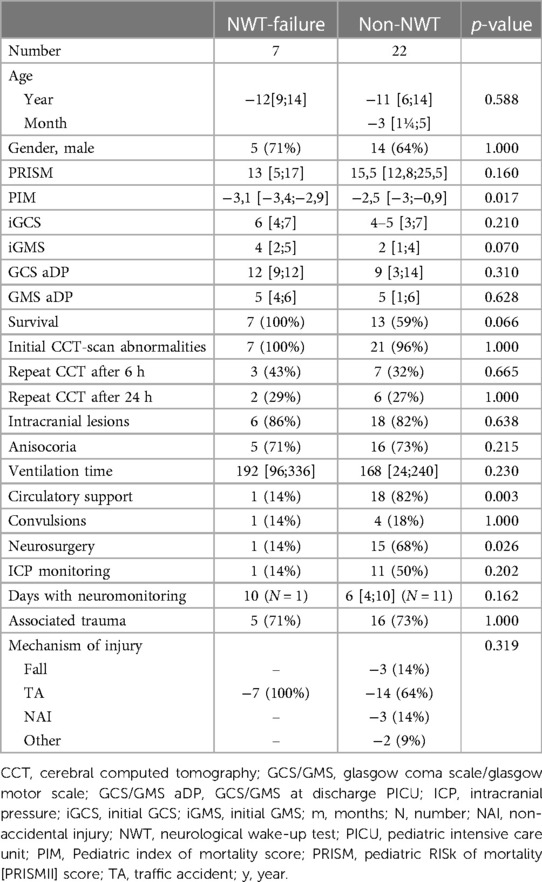
Table 5. NWT-outcome of NWT-failure group vs non-NWT group. Data are presented as number (%) or median (IQR 25th–75th).
In two patients the NWT was not performed, despite being requested. Reasons for not doing NWTs were severe trauma requiring neurosurgical interventions and refractory intracranial hypertension (ICH). No NWT was requested in the remaining 20 patients, mainly because of ICH (62%).
In four patients (19%) we could not retrieve the rationale for not performing the NWT in the first 24 h. Patients without NWTs received significant more long half-life sedatives and more often a combination of several sedatives when compared to the patients with NWTs (p = 0.003, Supplementary Table C-II).
Outcome
Nine patients (25%) died during their PICU admission, none of them had an NWT. Three of them were younger than 6 months. Fourteen patients were ventilated <24 h, with seven of them having a complete NWT. The other seven patients died. Five of them had TA, 2 non-accidental injury (NAI), 1 other and 1 fall from height.
Discussion
To our best knowledge, this is the first study reporting the feasibility of the neurological wake-up test (NWT) in critically ill children with severe TBI. In the described cohort, NWTs were infrequently performed (mainly in those with less severe manifestations of the pTBI) and were successful in about half of these patients.
NWTs are considered gold standard in the evaluation of patients with severe TBI, despite the fact that NWTs are being absent in international (pediatric) guidelines. However, we found that NWTs were performed in less than half of our cohort. Strikingly, no NWTs were performed in children <6 months of age. In retrospect, no clear explanation could be identified. One of the possible reasons for a low use of NWTs might be the confusion about terminology. It has been proposed that the response to sedation interruption should be addressed as “arousal” and not to “awakening” (22). As such, the term “wake-up test” is confusing and this could result in different predetermined goals. As Table 1 shows, there was a difference in predetermined goals in our NWT-group. When a reasonable detubation was considered, the NWT was mostly suggested by the pediatric intensivist. In the other cases, “awareness” and “deterioration” were the indications by the neurologist. This emphasizes the importance of speaking the same language in a multidisciplinary team. Another explanation could be that performing the NWT requires an immediate disruption in administration of sedato-analgesic drugs. This may be a barrier particularly in the PICU because in general there is little use of sedato-analgesic drugs with short half-lives and a low potential of inducing tolerance and withdrawal symptoms, which will hamper the NWT (23). Short-acting sedatives such as propofol are contra-indicated in younger children, resulting in the use of drugs with a relatively long half-life (8).
Interestingly, while all patients with NWT-success had an initial GCS ≤8, they were extubated very rapidly after completing the NWT. No doubt, these patients benefited from the NWT, but it might also be surmised that the initial GCS assessment was incorrect. It is known that about 20% of TBI patients are misclassified as severe (4, 24). We found no differences in use of sedatives in the NWT-group, thereby not explaining the non-arousal in the failure-group. The frequent use of propofol in both NWT-groups suggests that these patients were deemed to have less severe brain injury based on the higher initial GCS/GMS scores upon PICU admission and lower PRISM II score.
Importantly, there was no standardized protocol on when or how to use the NWT. Therefore, the actual performance of the NWT was dependent on the discretion of the attending physician. No time-interval of sedation interruption to clinical evaluation was defined and this could not be distracted from patient records. This may have resulted in premature ending of the NWT, classifying it as a failure. Hence, our study suffers from confounding by indication. It may be surmised that NWTs were not performed in patients with more severe brain injury. We observed no differences in clinical characteristics between patients without NWT and those in whom the NWT failed except for higher PRISM/PIM scores, more additional trauma and circulatory support and more invasive monitoring. All non-survivors were non-NWTs. Therefore, we cannot rule out a clinical preference for not doing NWTs in those with the most severe brain injury because of the supposed risks such as increases in ICP. While awaiting arousal a variable response can occur due to ongoing pharmacological effects and alterations in behavior resulting from brain damage (22). The NWT procedure induces a biochemical response and short duration of increase in ICP and CPP (11–13). Agitation is a common finding in TBI patients and is associated with poor functional outcome depending on severity and duration of the agitation (25). Pulmonary edema has also been described as a consequence of prolonged agitation (26). Recurrent agitation as a result of frequent NWT-trials therefore should be prevented.
Further studies are indicated to identify patients in whom the NWT is indicated. We observed some interesting differences between the NWT-successes vs. failures which might guide future decision-making. We found differences in age and mechanism of injury. Furthermore, NWT-failures had a significant lower GCS/GMS score at discharge. All 7 NWT-failures had high impact TAs (mean velocity 75 km/h) caused by pedestrian (n = 1) or (non-helmeted) cyclist (n = 6) vs. car collision and all of them showed ongoing reduced levels of consciousness (GCS <8) and were diagnosed as having diffuse axonal injury (DAI). It may therefore be postulated that vulnerable road users (VRU's) (pedestrians, cyclists e.g.,) with a known high impact TA and consequently risk of DAI in the absence of focal deficits should not be selected for the NWT (27, 28). Patients in this group do not benefit from the NWT within the first 24 h and clinical evaluations that could cause secondary insults should be postponed. In this setting, the clinician should rely on neuroimaging and multimodality monitoring (18, 29).
Our study had several additional limitations. First, our study was designed as a single center retrospective study, limiting generalizability of our findings. Second, we have studied a heterogeneous population with a small total number of included patients. Third, there was no standardized protocol for performing the NWT. Fourth, limited data concerning the involvement of CCT interpretation in performing NWTs could be retrieved from our data (30). Therefore, we do not know to what extent the decision to perform NWTs or not was influenced by this interpretation or by the clinical presentation at onset. Such information should be incorporated into future studies. Nonetheless, our data can be interpreted as a small but hopefully important contribution in gaining knowledge regarding NWTs in critically ill children with severe TBI.
Conclusion
We observed a low use of NWTs in children with severe TBI. Patients in whom the NWT was successful had relatively low-impact injury and where extubated very rapidly after the NWT. Those in whom the NWT-failed showed similar characteristics to those in whom an NWT was not performed. As many factors may play a role in the success and yield of an NWT, the decision to perform this test should be based on multidisciplinary evaluation. The timing of the NWT within the first 24 h of admission seems appropriate, as it may expose those patients initially misclassified as severe. Although no complications were observed, no conclusions can be made about the safety of the NWT based on our results.
Further research is much needed to truly establish the feasibility, safety of and indications for the NWT in severe pTBI. A future prospective study should not only include factors such as age, GCS-score at onset or mechanism of injury, but also include CCT-interpretations (Rotterdam and Marshall score) to determine in which subgroups of patients an NWT is of value.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by University Medical Center Groningen (IRB UMCG #202200267). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
HM: Writing – original draft, Writing – review & editing. JH: Writing – review & editing. MK: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1367337/full#supplementary-material
References
1. Kochanek PM, Tasker RC, Carney N, Totten MA, Adelson PD, Selden NR, et al. Guidelines for the management of pediatric severe traumatic brain injury, third edition: update of the brain trauma foundation guidelines. Pediatr Crit Care Med. (2019) 20(Suppl 1):S1–82. doi: 10.1097/PCC.0000000000001735
2. Shein SL, Ferguson NM, Kochanek PM, Bayir H, Clark RSB, Fink EL, et al. Effectiveness of pharmacological therapies for intracranial hypertension in children with severe traumatic brain injury–results from an automated data collection system time-synched to drug administration. Pediatr Crit Care Med. (2016) 17:236–45. doi: 10.1097/PCC.0000000000000610
3. Rhoney DH, Parker D. Use of sedative and analgesic agents in neurotrauma patients: effects on cerebral physiology. Neurol Res. (2001) 23:237–59. doi: 10.1179/016164101101198398
4. Esnault P, Montcriol A, D’Aranda E, Bordes J, Goutorbe P, Boret H, et al. Early neurological wake-up test in intubated brain-injured patients: a long-term, single-centre experience. Aust Crit Care. (2017) 30:273–8. doi: 10.1016/j.aucc.2016.10.002
5. Felmet K, Nguyen T, Clark RS, Orr D, Carcillo J. The FDA warning against prolonged sedation with propofol in children remains warranted. Pediatrics. (2003) 112:1002–3. doi: 10.1542/peds.112.4.1002
6. Minardi C, Sahillioğlu E, Astuto M, Colombo M, Ingelmo PM. Sedation and analgesia in pediatric intensive care. Curr Drug Targets. (2012) 13:936–43. doi: 10.2174/138945012800675740
7. Kerr ME, Weber BB, Sereika SM, Darby J, Marion DW, Orndoff PA, et al. Effect of endotracheal suctioning on cerebral oxygenation in traumatic brain-injured patients. Crit Care Med. (1999) 27:2776–81. doi: 10.1097/00003246-199912000-00028
8. Tume LN, Baines PB, Lisboa PJ. The effect of nursing interventions on the intracranial pressure in paediatric traumatic brain injury. Nurs Crit Care. (2011) 16:77–84. doi: 10.1111/j.1478-5153.2010.00412.x
9. Helbok R, Kurtz P, Schmidt MJ, Stuart MR, Fernandez L, Connolly SE, et al. Effects of the neurological wake-up test on clinical examination, intracranial pressure, brain metabolism and brain tissue oxygenation in severely brain-injured patients. Crit Care. (2012) 16:R226. doi: 10.1186/cc11880
10. Marklund N. The neurological wake-up test-a role in neurocritical care monitoring of traumatic brain injury patients? Front Neurol. (2017) 8:540. doi: 10.3389/fneur.2017.00540
11. Skoglund K, Enblad P, Hillered L, Marklund N. The neurological wake-up test increases stress hormone levels in patients with severe traumatic brain injury. Crit Care Med. (2012) 40:216–22. doi: 10.1097/CCM.0b013e31822d7dbd
12. Skoglund K, Hillered L, Purins K, Tsitsopoulos PP, Flygt J, Engquist H. The neurological wake-up test does not alter cerebral energy metabolism and oxygenation in patients with severe traumatic brain injury. Neurocrit Care. (2014) 20:413–26. doi: 10.1007/s12028-013-9876-4
13. Skoglund K, Enblad P, Marklund N. Effects of the neurological wake-up test on intracranial pressure and cerebral perfusion pressure in brain-injured patients. Neurocrit Care. (2009) 11:135–42. doi: 10.1007/s12028-009-9255-3
14. Prisco L, Citerio G. To wake-up, or not to wake-up: that is the hamletic neurocritical care question!. Crit Care. (2012) 16:190. doi: 10.1186/cc11891
15. Carney N, Totten AM, O’Reilly C, Ullman JS, Hawryluk GWJ, Bell MJ, et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery. (2017) 80:6–15. doi: 10.1227/NEU.0000000000001432
16. Swadron SP, LeRoux P, Smith WS, Weingart SD. Emergency neurological life support: traumatic brain injury. Neurocrit Care. (2012) 17:S112–21. doi: 10.1007/s12028-012-9760-7
17. Kneyber MCJ, de Luca D, Calderini E, Jarreau PH, Javouhey E, Lopez-Herce J, et al. Recommendations for mechanical ventilation of critically ill children from the paediatric mechanical ventilation consensus conference (PEMVECC). Intensive Care Med. (2017) 43:1764–80. doi: 10.1007/s00134-017-4920-z
18. Kochanek PM, Tasker RC, Bell MJ, Adelson PD, Carney N, Vaillant MS, et al. Management of pediatric severe traumatic brain injury: 2019 consensus and guidelines-based algorithm for first and second tier therapies. Pediatr Crit Care Med. (2019) 20:269–79. doi: 10.1097/PCC.0000000000001737
19. Pollack MM, Ruttimann UE, Getson PR. Pediatric risk of mortality (PRISM) score. Crit Care Med. (1988) 16:1110–6. doi: 10.1097/00003246-198811000-00006
20. Straney L, Clements A, Parslow RC, Pearson G, Shann F, Alexander J, et al. Paediatric index of mortality 3: an updated model for predicting mortality in pediatric intensive care. Pediatr Crit Care Med. (2013) 14:673–81. doi: 10.1097/PCC.0b013e31829760cf
21. Teasdale G, Maas A, Lecky F, Manley G, Stocchetti N, Murray G, et al. The Glasgow coma scale at 40 years: standing the test of time. Lancet Neurol. (2014) 13:844–54. doi: 10.1016/S1474-4422(14)70120-6
22. Stover JF. Arousal from sedation in wake-up tests requires careful risk stratification. Crit Care Med. (2012) 40:338–40. doi: 10.1097/CCM.0b013e31823291bf
23. Franck LS, Naughton I, Winter I. Opioid and benzodiazepine withdrawal symptoms in paediatric intensive care patients. Intensive Crit Care Nurs. (2004) 20:344–51. doi: 10.1016/j.iccn.2004.07.008
24. Stocchetti N, Pagan F, Calappi F, Canavesi K, Beretta L, Citerio G, et al. Inaccurate early assessment of neurological severity in head injury. J Neurotrauma. (2004) 21:1131–40. doi: 10.1089/neu.2004.21.1131
25. Singh R, Venkateshwara G, Nair KPS, Khan M, Saad R. Agitation after traumatic brain injury and predictors of outcome. Brain Inj. (2014) 28:336–40. doi: 10.3109/02699052.2013.873142
26. Reznik ME, Mahta A, Schmidt JM, Frey HP, Park S, Roh DJ, et al. Duration of agitation, fluctuations of consciousness, and associations with outcome in patients with subarachnoid hemorrhage. Neurocrit Care. (2018) 29:33–9. doi: 10.1007/s12028-017-0491-7
27. Baker CE, Martin P, Wilson MH, Ghajari M, Sharp DJ. The relationship between road traffic collision dynamics and traumatic brain injury pathology. Brain Commun. (2022) 4:fcac033. doi: 10.1093/braincomms/fcac033
28. Javouhey E, Guérin AC, Chiron M. Incidence and risk factors of severe traumatic brain injury resulting from road accidents: a population-based study. Accid Aal Prev. (2006) 38:225–33. doi: 10.1016/j.aap.2005.08.001
29. Figaji AA, Fieggen AG, Argent AC, Leroux PD, Peter JC. Does adherence to treatment targets I children with severe traumatic brain injury avoid brain hypoxia? A brain tissue oxygenation study. Neurosurgery. (2008) 63:83–91. doi: 10.1227/01.NEU.0000313113.43447.0C
Keywords: traumatic brain injury, pediatric, neurocritical care, wake-up test, NWT
Citation: Mulder HD, Helfferich J and Kneyber MCJ (2024) The neurological wake-up test in severe pediatric traumatic brain injury: a long term, single-center experience. Front. Pediatr. 12:1367337. doi: 10.3389/fped.2024.1367337
Received: 8 January 2024; Accepted: 12 February 2024;
Published: 23 February 2024.
Edited by:
Christian Dohna-Schwake, Essen University Hospital, GermanyReviewed by:
Markus Lehner, Lucerne Children’s Hospital, SwitzerlandFelix Neunhoeffer, University Children’s Hospital Tübingen, Germany
© 2024 Mulder, Helfferich and Kneyber. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hilde D. Mulder aC5kLm11bGRlckB1bWNnLm5s
Abbreviations CBV, cerebral blood volume; CCT scan, cerebral computed tomography; CPP, cerebral perfusion pressure; DAI, diffuse axonal injury; GCS, Glasgow coma scale; GMS, Glasgow motor scale; ICH, intracranial hypertension; ICP, intracranial pressure; IQR, inter quartile range; MAP, mean arterial pressure; MV, mechanical ventilation; MMM, multi modality monitoring; NAI, non-accidental injury; NWT, neurological wake-up test; PaO2, partial pressure of oxygen; PaCO2, partial pressure of carbon dioxide; PbtiO2, brain tissue oxygenation; PICU, pediatric critical care unit; PIM, pediatric index of mortality; PRISM III, pediatric risk of mortality score; pTBI, pediatric traumatic brain injury; SPSS, statistical package for the social sciences; TA, traffic accident; TBI, traumatic brain injury; VRU's, vulnerable road users.
 Hilde D. Mulder
Hilde D. Mulder Jelte Helfferich
Jelte Helfferich Martin C. J. Kneyber
Martin C. J. Kneyber