
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pediatr. , 10 June 2024
Sec. Pediatric Pulmonology
Volume 12 - 2024 | https://doi.org/10.3389/fped.2024.1358639
This article is part of the Research Topic Reviews in Pediatric Pulmonology View all 5 articles
Background: Acute upper respiratory tract infection (AURI) includes infections caused by a variety of pathogens and is one of the most common diseases in children. Traditional Chinese medicine (TCM) injections are widely used for treating AURI in clinical practice, but their efficacy is unclear because of the lack of clear evidence. In this study, a network meta-analysis (NMA) was used to evaluate the efficacy and safety of TCM injections in the treatment of AURI and to provide a reference for clinical treatment.
Methods: Eight databases were searched, namely, PubMed, Embase, the Cochrane Library, Web of Science, SinoMed, China National Knowledge Infrastructure (CNKI), the Wanfang database, and the Chinese Scientific Journal database (VIP). The search time period was from 1 January 2013 to 1 November 2023. Randomized controlled trials of herbal injections for treating AURI were searched. The Cochrane Risk of Bias 2.0 tool was used to assess the quality of these studies. Review Manager 5.4 and Stata 15.0 were used for the NMA.
Results: A total of 81 papers involving 11,736 patients were included. These involved five different TCM injections, namely, Xiyanping injection (XYPI), Qingkailing injection (QKLI), Reduning injection (RDNI), Yanhuning injection (YHNI), and Tanreqing injection (TRQI). QKLI was most effective in alleviating symptoms of fever and improving overall clinical effectiveness. TRQI was most effective in relieving cough symptoms. YHNI was most effective in alleviating sore throat, runny nose, and nasal congestion. The overall incidence of adverse effects of these herbal injections in the treatment of AURI was lower, and their safety profiles were better.
Conclusions: The herbal injections combined with ribavirin improved clinical outcomes, and were superior to ribavirin injection alone in alleviating clinical symptoms such as fever, cough, sore throat, runny nose, and nasal congestion, and have favorable safety profiles.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023484099, CRD42023484099.
Acute upper respiratory tract infection (AURI) is a general term for acute inflammation of the nasal, pharyngeal, and laryngeal regions that includes a group of diseases, predominantly acute nasopharyngolaryngitis, as well as viral pharyngitis, laryngitis, herpetic pharyngitis, pharyngoconjunctival fever, and tonsillitis (1). It is a common respiratory disease in pediatric outpatient clinics and is mainly characterized by rapid progression and severe fever. Because of children's young age and immaturity, they are susceptible to infections induced by viral and bacterial invasion. If AURI is not controlled in time, it may easily develop into inflammatory lesions in adjacent organs, lymph nodes, etc., and in severe cases inflammation of the lungs may also occur (2, 3). Owing to the unclear symptoms of children's complaints and the rapid progression of the disease, the complication rate is high, and, if not diagnosed early and given symptomatic treatment, delays are easily caused, which adversely affects the physical and mental health of the children (4, 5). The etiology of AURI mostly comprises the invasion of the nasopharynx by viruses such as influenza virus and adenovirus, and the clinical symptoms include headache, fever, sore throat, and runny nose (6). Owing to differences in individual immunity, the disease varies in severity, and, although it is a self-limiting disease with a good prognosis, it may cause complications and further affect health if not treated in time (7). Treatment of this disease in Western medicine is based on antipyretic drugs and antibiotics, and ribavirin is often used in clinical treatment, but its effect is not good.
Traditional Chinese medicine (TCM) is characterized by holistic interventions based on multiple targets and multiple pathways in preventing respiratory diseases in children and improving patients’ prognosis (8). Traditional Chinese medicine injections (TCMIs) are patented traditional Chinese drugs registered by the National Medical Products Administration. Chinese medicine injections are injections prepared under the guidance of Chinese medicinal theory using modern pharmaceutical technology to purify and concentrate the active ingredients in single and compound Chinese medicinal formulations. In recent years, clinical evidence has accumulated for the treatment of AURI with Chinese herbal injections in combination with Western drugs, which can effectively relieve symptoms and improve levels of serum-related factors and blood rheology indices (9–11). However, owing to the large differences in efficacy and the great variety of such drugs, as well as the shortage of studies and evaluations of direct comparisons among TCMIs, there is still a great deal of difficulty in the selection of the optimal treatment regimen in clinical work. Network meta-analysis (NMA) enables quantitative evaluation and ranking of multiple interventions for the same disease on the basis of direct and indirect comparisons (12).
The specific efficacies and therapeutic advantages of TCMIs are unclear, which causes problems in clinical application. This study is the first article to systematically evaluate and compare the clinical efficacies and safety of several commonly used TCMIs in combination with ribavirin. The aim of this study is to provide sufficient evidence-based medical evidence and to inform the use of TCMIs for the treatment of AURI in the clinic.
To make the study more accurate and reproducible, this study refers to the ConPhyMP consensus (13). In addition, we standardized the naming of herbal medicines (14) and validated the names against the Plants of the World Online (http://www.plantsoftheworldonline.org) and the World Flora Online (http://www.worldfloraonline.org/) databases. Summary tables describing the compositions of agents and how they were reported in the original studies were prepared in accordance with the principles described in the four pillars of ethnopharmacology. The composition and standard name of each injection are shown in Table 1.
The NMA was registered with the international prospective register of systematic reviews (PROSPERO) under the registration number CRD42023484099. We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, the associated protocols, and the PRISMA extension statement for network meta-analyses to report the current results (15, 16).
This study searched a total of eight databases, namely, PubMed, Embase, the Cochrane Library, Web of Science, SinoMed, China National Knowledge Infrastructure (CNKI), the Wanfang database, and the Chinese Scientific Journal database (VIP). The main search terms included “Traditional Chinese medicine injections*,” “Respiratory Tract Infections,” “Infection, Respiratory Tract,” “Respiratory Tract Infection,” “Infections, Respiratory,” “Infections, Respiratory Tract,” “Infections, Upper Respiratory,” and “Respiratory Infection, Upper.” References from previous systematic reviews and meta-analyses with similar topics were scanned for supplementation in the preliminary screening stage. References from eligible articles were scanned for supplementation in the full-text screening stage, and unpublished studies were not retrieved. The detailed search strategy is presented in Supplementary Tables S1–S8 (pages 1–S5). The search was limited to the period from 1 January 2013 to 1 November 2023.
Inclusion criteria were devised according to the patient, intervention, comparator, and outcome (PICO) framework: (a) the type of study included comprised randomized controlled trials (RCTs); (b) the type of disease studied was a viral infection of the upper respiratory tract (including viral pharyngitis, laryngitis, herpetic pharyngitis, pharyngoconjunctival fever, and tonsillitis) rather than a bacterial infection (no limitations applied in terms of age, sex, or nationality); (c) in the treatment group, the intervention was TCMIs; and (d) the primary outcome in the study was the total effectiveness rate. The secondary outcomes included the times to resolution of fever, cough, sore throat, runny nose, and nasal congestion.
The following exclusion criteria were used: (a) duplicated articles; (b) incomplete or incorrect data; and (c) nonconforming studies (including reviews, systematic reviews, meta-analyses, animal experiments, conference abstracts, reports, letters, and case reports).
Two researchers (XYG and CXL) from related disciplines independently screened and crosschecked studies for inclusion. In the case of disagreement, a third researcher (QZ or SJYH) adjudicated and provided a solution. Preliminary screening was carried out according to the title and abstract, and the included studies were then selected by reading the full text. Two researchers used uniform criteria for data extraction: the first author, year of publication, duration of AURI, sample size, male-to-female ratio, age, interventions, course of treatment, and outcomes.
The quality of the included studies was assessed by two investigators (XYG and CXL) using the Cochrane Risk of Bias 2.0 tool (17), which includes the randomization process, deviations from the intended interventions, missing outcome data, measurement of outcomes, selection of the reported results, and overall bias. The risk of bias was classified as “low risk,” “high risk,” or “some concerns.” We used the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) method for the entire network to provide a framework for the deterministic rating of each paired comparison, which was classified as high, medium, low, or very low (18, 19).
The NMA was performed using Stata 15.0 for NMA version on the basis of the frequentist framework. Results between pairwise comparisons were reported using the netleague command and were presented in tabular form. For dichotomous variables, the odds ratio (OR) was used as the effect analysis statistic; for continuous variables, the mean difference (MD) was used as the effect analysis statistic. For continuous variables, differences were not statistically significant when the 95% CI included 0. For dichotomous variables, the difference was not statistically significant when the 95% CI included 1.Evidence network diagrams were used to show direct comparisons between different interventions, where the size of each node represented the sample size for the corresponding intervention and the thickness of the line connecting two nodes indicated the number of studies that directly compared the two interventions. The surface area under the cumulative ranking curve (SUCRA) was calculated for each intervention, where SUCRA was expressed as a value in the range of 0%–100% and represented the probability that the treatment was the best choice. A “comparison–correction” funnel plot was drawn to assess the publication bias of the included studies. If a closed loop was formed, an inconsistency test was performed (20).
The initial review yielded 2023 relevant papers, and 81 RCTs, which were all two-arm studies, were finally included after the initial screening and rescreening (21–101). These included 11,737 patients: 5,904 in the treatment groups and 5,833 in the control groups. The 81 RCTs included in this study covered a total of six interventions, including five herbal injections, namely, Xiyanping injection (XYPI; 30 RCTs) (21–50); Qingkailing injection (QKLI; 2 RCTs) (51, 52); Reduning injection (RDNI; 22 RCTs) (53–74); Yanhuning injection (YHNI; 22 RCTs) (75–96); and Tanreqing injection (TRQI; 5 RCTs) (97–101). The specific search and screening process is described in Figure 1, and the basic characteristics of the included studies are shown in Table 2.
The quality of the included studies was assessed using the risk assessment tool recommended by the Cochrane Collaboration. Among the 81 included studies, nine studies (55, 58, 63, 65, 67, 80, 85, 90, 100) mentioned the random number table method and were rated as “low risk.” The remaining 72 studies only mentioned randomization and were rated as “some concerns.” All studies were tested according to the established allocation to interventions, and those with no deviations were rated as “low risk.” One study (31) recorded outcome data with omissions and was rated as “high risk,” whereas the others reported complete outcome data with no exclusions or omissions and were rated as “low risk.” All the studies used appropriate measures for the outcome data and had no reporting bias in terms of outcome selection and were rated as “low risk.” The results of the risk of bias assessment of the included studies are shown in Figure 2 and Supplementary Table S10 (pages 11–S74).
Seventy-eight RCTs reported clinical effectiveness and involved five TCMIs and six interventions; 58 RCTs reported time taken to reduce fever and involved five TCMIs and six interventions; 51 RCTs reported time to relief of cough and involved five TCMIs and six interventions; 43 RCTs reported time to relief of sore throat and involved four TCMIs and five interventions; 8 RCTs reported time to relief of runny nose and involved four TCMIs and five interventions; and 21 RCTs reported time to relief of nasal congestion and involved three TCMIs and four interventions. In the evidence networks constructed for the different outcome indicators, the size of a node represents the corresponding study sample size, and the thickness of the line connecting two nodes represents the number of included studies. There was no closed loop between the different interventions, and therefore the consistency model was used for the analysis: see Figure 3.
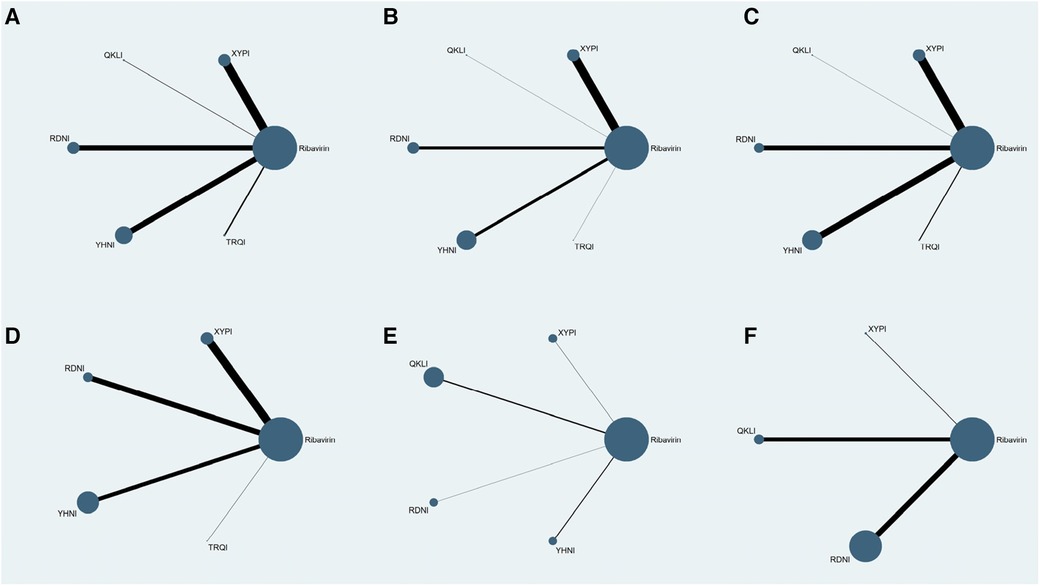
Figure 3. Evidence networks for the outcome indicators. (A) Clinical effectiveness; (B) time to relief of fever; (C) time to relief of cough; (D) time to relief of sore throat; (E) time to relief of runny nose; (F) time to relief of nasal congestion.
A total of 11,396 patients involved in 78 studies (21, 22, 24–47, 49–87, 89–101) were evaluated, and the total effectiveness rate of five TCMIs and six interventions was reported. All five TCMIs were better than ribavirin (P < 0.05), as follows: QKLI [relative risk (RR): 0.21, CI: 0.10–0.33], XYPI (RR: 0.18, CI: 0.15–0.21), RDNI (RR: 0.16, CI: 0.12–0.20), YHNI (RR: 0.16, CI: 0.12–0.20), and TRQI (RR: 0.15, CI: 0.07–0.23), which suggested that they had advantages in alleviating clinical symptoms (Table 3). In addition, none of the differences between groups were statistically significant in pairwise comparisons between the five TCMIs (P > 0.05). According to the results of the SUCRA ranking, QKLI (82.7%) was the best treatment, followed by XYPI (71.8%) and RDNI (51.9%) (Table 4; Figure 4).
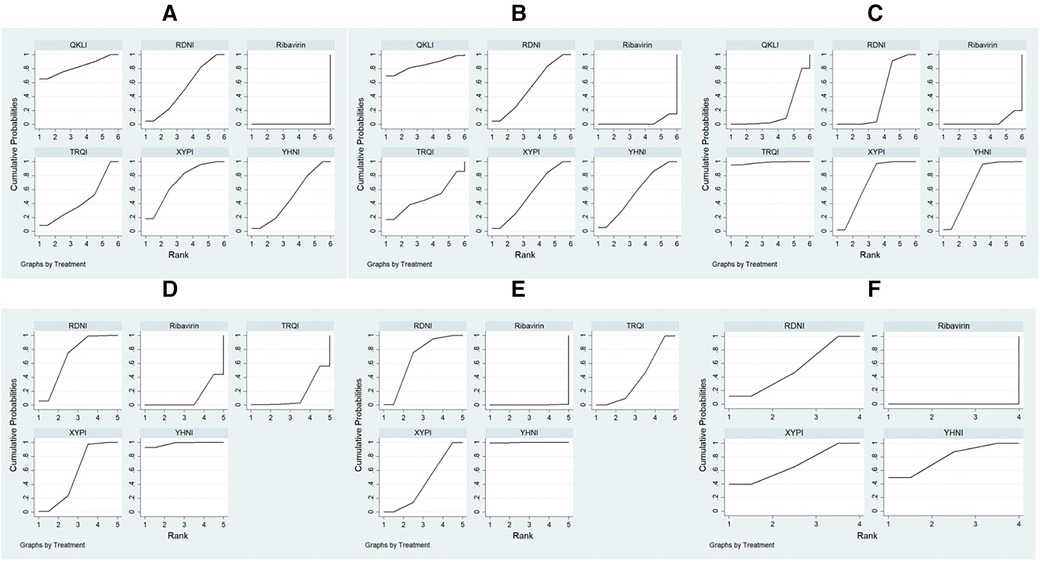
Figure 4. Plots of the surface area under the cumulative ranking curve. (A) Total effectiveness rate; (B) time to relief of fever; (C) time to relief of cough; (D) time to relief of sore throat; (E) time to relief of runny nose; (F) time to relief of nasal congestion.
A total of 9,268 patients involved in 58 studies (21–30, 32, 33, 35, 36, 38–41, 44, 46–48, 50, 52–55, 59–63, 65–71, 73–80, 82, 85–88, 90–96, 98) were evaluated, and the time to relief of fever in the case of five TCMIs and six interventions was reported. Four TCMIs were better than ribavirin (P < 0.05), namely, QKLI [standardized mean difference (SMD): −1.96, CI: −3.51 to −0.21], YHNI (SMD: −1.06, CI: −1.46 to −0.67), XYPI (SMD: −1.04, CI: −1.39 to −0.68), and RDNI (SMD: −1.04, CI: −1.44 to −0.64), in terms of time to relief of fever (Table 3). In addition, none of the differences between groups were statistically significant in pairwise comparisons between the four TCMIs (P > 0.05). According to the results of the SUCRA ranking, QKLI (85.3%) was the best treatment, followed by YHNI (56.2%) and XYPI (53.9%) (Table 4; Figure 4).
A total of 8,382 patients involved in 51 studies (21–25, 27–30, 32, 33, 35, 36, 38–41, 44, 46, 47, 50, 52–55, 58, 60, 62, 65–71, 73, 75, 77, 79, 80, 82, 85, 86, 88, 90, 91, 93–96, 98, 100) were evaluated, and the time to relief of cough in the case of five TCMIs and six interventions was reported. Four TCMIs were better than ribavirin (P < 0.05), namely, XYPI (SMD: −1.46, CI: −1.69 to −1.23), RDNI (SMD: −1.08, CI: −1.35 to −0.82), YHNI (SMD: −1.46, CI: −1.74 to −1.19), and TRQI (SMD: −2.19, CI: −2.93 to −1.46), in terms of the time to relief of cough. Regarding the TCMIs, TRQI was better than RDNI and QKLI (SMD: −1.11, CI: −1.89 to −0.33/SMD: −1.77, CI: −2.97 to −0.58), XYPI was better than RDNI and QKLI (SMD: −0.38, CI: −0.73 to −0.03/SMD: −1.04, CI: −2.01 to −0.88), and YHNI was better than QKLI (SMD: −1.04, CI: −2.03 to −0.06), which suggested that they had advantages in the relief of cough (Table 3). According to the results of the SUCRA ranking, TRQI (98.6%) was the best treatment, followed by XYPI (70.1%) and YHNI (69.8%) (Table 4; Figure 4).
A total of 7,300 patients involved in 43 studies (21, 23–26, 28, 29, 32, 33, 35, 36, 38–40, 44, 46, 47, 50, 53–55, 61, 62, 65–71, 73, 75, 80, 82, 85, 86, 88, 90, 91, 93, 95, 96, 100) were evaluated, and the time to relief of sore throat in the case of four TCMIs and five interventions was reported. Three TCMIs were better than ribavirin (P < 0.05), namely, XYPI (SMD: −1.15, CI: −1.40 to −0.89), RDNI (SMD: −1.29, CI: −1.58 to −1.00), and YHNI (SMD: −1.63, CI: −1.95 to −1.32), in terms of the time to relief of sore throat. Regarding the TCMIs, YHNI was better than XYPI and TRQI (SMD: −0.49, CI: −0.89 to −0.08/SMD: −1.53, CI: −2.65 to −0.41), and RDNI was better than TRQI (SMD: −1.19, CI: −2.30 to −0.08), which suggested that they had advantages in the relief of sore throat (Table 3). According to the results of the SUCRA ranking, YHNI (98.1%) was the best treatment, followed by RDNI (70.1%) and XYPI (55.5%) (Table 4; Figure 4).
A total of 1,036 patients involved in eight studies (22, 38, 61, 66, 67, 80, 98, 100) were evaluated, and the time to relief of runny nose in the case of four TCMIs and five interventions was reported. All four TCMIs were better than ribavirin (P < 0.05), namely, YHNI (SMD: −2.11, CI: −2.82 to −1.04), RDNI (SMD: −1.02, CI: −1.45 to −0.59), XYPI (SMD: −0.69, CI: −1.18 to −0.20), and TRQI (SMD: −0.64, CI: −1.16 to −0.12), in terms of the time to relief of runny nose. Regarding the TCMIs, YHNI was better than RDNI, XYPI, and TRQI (SMD: −1.09, CI: −1.92 to −0.26/SMD: −1.42, CI: −2.28 to −0.56/SMD: −1.47, CI: −2.35 to −0.59), which suggested that it had advantages in the relief of runny nose (Table 3). According to the results of the SUCRA ranking, YHNI (99.8%) was the best treatment, followed by RDNI (67.9%) and XYPI (43.0%) (Table 4; Figure 4).
A total of 4,668 patients involved in 21 studies (22, 29, 53, 54, 58, 65, 68–71, 73, 75, 77, 79, 82, 85, 86, 88, 91, 92, 95) were evaluated, and the time to relief of nasal congestion in the case of four TCMIs and five interventions was reported. Three TCMIs were better than Ribavirin (P < 0.05), namely, YHNI (SMD: −1.22, CI: −1.54 to −0.89), XYPI (SMD: −1.14, CI: −1.82 to −0.45), and RDNI (SMD: −1.02, CI: −1.82 to −0.45), in terms of the time to relief of nasal congestion (Table 3). In addition, none of the differences between groups were statistically significant in pairwise comparisons between the three TCMIs (P > 0.05). According to the results of the SUCRA ranking, YHNI (78.9%) was the best treatment, followed by XYPI (68.3%) and RDNI (52.8%) (Table 4; Figure 4).
The results of evaluation using GRADE-profiler software showed that the levels of evidence for the interventions were low or very low across the studies (Supplementary Table S11: Pages 74–S78). The included studies were only from China, and the risk of bias was increased because most of the studies did not mention blinding and allocation concealment. Moreover, the heterogeneity of the included studies and the differences in sample size caused inconsistency and imprecision, which resulted in serious indirect effects. Therefore, the results reported in this NMA should be viewed with caution.
Twenty-eight RCTs (22–24, 26, 28, 34, 35, 47, 49, 50, 52, 65–69, 71–75, 79, 80, 86, 88, 90, 95, 97, 101) reported the safety of TCMIs and specific adverse reactions, including diarrhea, vomiting, rash at the injection site, loss of appetite, allergies, abdominal pain, laryngitis, and headache. Only a descriptive analysis was performed because the descriptive criteria in the various studies were not uniform. The specific information is given in Table 5.
Comparison–correction funnel plotting for different outcome indicators was performed using Stata 15.0 software. In this study, funnel plots for the total effectiveness rate, time to relief of fever, time to relief of cough, time to relief of sore throat, time to relief of runny nose, and time to relief of nasal congestion were plotted. In combination with the results of Egger's test, the results showed that the distributions of the funnel plots for clinical effectiveness (Figure 5A) and time to relief of fever (Figure 5B) were roughly symmetric, without obvious small-sample effects or publication bias. The symmetries of the studies included with regard to time to relief of cough (Figure 5C), time to relief of sore throat (Figure 5D), time to relief of runny nose (Figure 5E), and time to relief of nasal congestion (Figure 5F) were off-axis, and P < 0.05 was obtained by Egger's test. This suggests that publication bias was present.

Figure 5. Funnel plots for the outcome indicators. OR, odds ratio. (A) Total effectiveness rate; (B) time to relief of fever; (C) time to relief of cough; (D) Time to relief of sore throat; (E) time to relief of runny nose; (F) time to relief of nasal congestion.
The main ingredient of YHNI is dehydrated andrographolide, which is extracted from Andrographis paniculata and can play a very good role in detoxifying and clearing heat. It can therefore be used as a drug for the treatment of children with acute-phase viral upper respiratory tract infections. Modern pharmacological studies have found that YHNI has a strong antiviral effect and is capable of inactivating adenoviruses, influenza viruses, respiratory viruses, etc., and at the same time it can strengthen the body's immune ability to a certain extent (96). The principle behind the antiviral activity of YHNI may be that the monopotassium salt of dehydrated andrographolide succinate occupies the DNA–protein binding site during viral replication and therefore prevents viral replication. As shown in the results of this NMA, intravenous infusion of YHNI for the treatment of children with acute-phase viral upper respiratory tract infections was effective in relieving clinical symptoms such as nasal congestion and runny nose. In addition, some studies have indicated (102) that YHNI can strengthen the immune ability of sick children and avoid the occurrence of febrile convulsions due to hyperthermia, which damage the cerebral nerves of children with acute-phase viral upper respiratory tract infections. Clinically, ribavirin injection is used in combination with YHNI to treat children with acute-phase viral upper respiratory tract infections, which can achieve a better antiviral effect, and the efficacy of YHNI in clearing heat and strengthening the immune system is more suitable for treating sick children.
The main components of QKLI are bile acids, mother of pearl powder, porcine deoxycholic acid, Gardenia, buffalo horn powder, Platycodon, baicalin, and honeysuckle. It has antipyretic effects, inhibits bacterial endotoxins and endogenous pyrogens, inhibits inflammatory reactions, improves the circulation in important organs, protects brain tissues, preserves the liver, and lowers levels of enzymes. QKLI has been widely used in febrile illnesses and in the event of dizziness, such as in pediatric febrile convulsions, pneumonia, and upper respiratory tract infections (103, 104). This NMA study showed that QKLI exhibits improved clinical efficacy in alleviating febrile symptoms of AURI in children. Histamine is an autoactive substance that is produced by the enzyme histidine decarboxylase. When the body is stimulated by physical and chemical factors, mast cells degranulate and release histamine (105). Histamine and histamine receptors bind to cause an inflammatory response (106). Relevant experiments confirmed that QKLI could reduce levels of imidazoleacetic acid and thus affect fever caused by inflammation. Elevated levels of imidazoleacetic acid in the urine of rats in a fever group suggested that histamine levels increased in their bodies, and imidazoleacetic acid levels were significantly reduced after the injection of QKLI, which implied that the immunity of the body was improved (107). In conclusion, QKLI was able to significantly affect inflammation-induced fever.
TRQI is a Chinese herbal injection commonly used in China that consists of Scutellaria baicalensis, bear bile powder, goat's horn, honeysuckle, and Forsythia. It has been included in several guidelines, diagnostic and treatment protocols, and expert consensus statements and is recommended for the treatment of a variety of severe types of pneumonia, such as Middle East respiratory syndrome, dengue fever, and human infection with H7N9 avian influenza (108). TRQI can effectively inhibit the growth of Streptococcus pneumoniae and Streptococcus B haemolyticus, markedly reduce hypersensitivity and inflammatory reactions, and indirectly contribute to a decrease in C-reactive protein (CRP) levels in patients (109). Moreover, TRQI resists proinflammatory factors and has antipyretic effects, which improves the antiviral effect, accelerates the excretion of toxins from the body, and reduces the release of procalcitoninogen (PCT) while reducing the stress response of the body. Liu and Qu (110) showed that TRQI could significantly promote the phagocytosis of white blood cells (WBCs) and inhibit the activation of neutrophils in lung tissues. It thus exerts antibacterial and anti-inflammatory effects and improves the respiratory function of patients. This also explains the mechanism by which TRQI, as found in this NMA, can effectively alleviate patients' febrile symptoms and enhance the efficacy of treatment.
Our study showed that XYPI ranked second on the three outcome measures of relieving symptoms of cough and nasal congestion and improving clinical effectiveness. In recent years, Chinese medicinal preparations have achieved remarkable results in the treatment of pediatric diseases. XYPI contains total sulfonated lactones obtained from the Chinese medicinal plant Andrographis paniculata, with a clear composition. Pharmacological studies have shown that it not only has a direct inhibitory effect on a variety of respiratory viruses (111, 112) and bacteria, but also has a significant protective effect on the body. In addition, XYPI can act synergistically with antibiotics and also modulate the inflammatory response (113–116). Clinical studies have shown that the treatment of acute bronchitis with XYPI is superior to conventional or Western drug therapy in terms of overall effectiveness, alleviation of symptoms, and improvements in lung function indices (117–119), and XYPI has a favorable safety profile (120). A recent large-sample RCT study showed that intravenous infusion of XYPI for acute bronchitis significantly reduced median disease duration and median time to relief of cough (117).
The Chinese medicinal preparation RDNI has high application value and is produced from Gardenia, Artemisia, honeysuckle, and other medicinal herbs by the extraction of the effective components of the preparation. RDNI is used to clear heat and disperse wind and has detoxifying effects. It is widely used in the treatment of infectious emergencies, hand, foot, and mouth disease, and influenza, as well as viral infections, in disease rescue, and treatment can be effective in increasing the rate of cure and alleviating the patient's clinical symptoms (121, 122). The results of this study indicated that RDNI can effectively alleviate the symptoms of pediatric AURI such as sore throat and runny nose. Modern pharmacology has found that Artemisia can have anti-inflammatory effects and modulate immunity, Gardenia has significant advantages in antipyretic and anti-inflammatory activity, and Honeysuckle can have antibacterial, anti-inflammatory, and antioxidant effects (123). Relevant online pharmacology and experimental validation has indicated that RDNI can downregulate the expression of inflammatory cells and proinflammatory cytokines such as human interleukin-1β, human interleukin-6, and tumor necrosis factor-α. In addition, the active complex present in honeysuckle (Lonicera) reduces Akt phosphorylation and slows down the onset of inflammation (124).
This study was the first NMA that compared the differences between TCMIs for the treatment of AURI in children. Many previous studies have simply summarized the efficacy and safety of a single TCMI (125–127) for treating AURI or the difference in efficacy between different TCM herbs (128). Those studies could not have stable quality control because of the diversity of ingredients and the variability of doses. The compositions of TCMIs are more stable than those of TCM decoctions, which has quantitative significance. We comprehensively studied the RCTs that used TCMIs in combination with ribavirin in the treatment of AURI and ranked the advantages of the different TCMIs with regard to each outcome index to guide their clinical use.
In this study, we found that QKLI can significantly improve the clinical effectiveness and is effective in alleviating fever symptoms, YHNI is effective in alleviating symptoms such as sore throat, nasal congestion, and runny nose, and TRQI is effective in alleviating cough. These TCMIs can be effective in solving different problems in AURI. No study showed the effects of a combination of multiple TCMIs in the treatment of AURI. This may be related to the complexity of the components, interactions, and other factors, which need to be further investigated in subsequent studies.
By analyzing differences in different indices of children's peripheral blood in a clinical setting, Ding and Qu concluded that the measurement of peripheral WBC counts and the lymphocyte/monocyte ratio is a key test in the diagnosis and treatment of AURI that can guide clinicians to avoid the irrational use of antimicrobial drugs. They also found that a decrease in prenatals is correlated with infection of the body and is a better indicator of the inflammation status of the child's organism than PCT and CRP (129). Therefore, it is essential to investigate the effects of TCMIs on related indices. Hou et al. concluded that RDNI can correct an imbalance in immune responses, improve immunity, reduce inflammatory responses, and thus promote disease regression. This may be related to the strong immunosuppressive and anti-inflammatory effects of Gardenia, honeysuckle, and Artemisia present in this Chinese herbal medicinal preparation (130). Wang et al. concluded that QKLI can effectively reduce WBC counts and levels of CRP, interleukin-18, and other proinflammatory factors, reduce inflammatory responses, and improve the anti-infection effect in children with AURI (131).
Diarrhea and allergic reactions are the most common adverse effects of using TCMIs. Twenty-eight RCTs included in this review described TCMIs as having mild adverse effects (22–24, 26, 28, 34, 35, 47, 49, 50, 52, 65–69, 71–75, 79, 80, 86, 88, 90, 95, 97, 101), which could be mitigated or eliminated by discontinuing the medication, decreasing the dose of the medication, or symptomatic treatment. The safety of TCMIs is greatly improved by standardizing their use in clinical applications (132). Li et al. improved the quality standard for the solubilizer polysorbate 80 in TCMIs to reduce anaphylactic reactions. However, adverse reactions in patients still need to be considered to avoid medical accidents (133).
Despite the widespread and effective use of TCMIs in clinical practice, this study found that the extracted components, complex pharmacological mechanisms, and methodological descriptions of their phytopharmacological properties are still unclear. In the future, more pharmacological and mechanistic studies of TCMIs should be conducted in accordance with the consensus recommendations (134).
The following limitations existed in this study: (a) adverse reactions were poorly reported, and most of the studies did not have a clear safety assessment; (b) most of the studies were rated as “some concerns” in the risk of bias assessment, and the quality of the studies was low; (c) clinical heterogeneity occurred, which was due to differences in the doses of phytomedicine and the course of treatment; (d) all the included studies were from China; (e) some of the outcome indicators were not standardized during this NMA, such as “the unit of measurement of time, including hours and days,” which may have interfered with the final summary of the results.
AURI is treated with inhaled bronchodilators, nebulized adrenaline, systemic steroids, and antibiotics (135). However, because of their side effects, it is particularly important to seek more effective alternative therapies. This study showed that TCMIs provide additional benefits in children. In terms of the different outcome indicators, QKLI was more effective in alleviating fever symptoms, YHNI was more effective in alleviating symptoms of sore throat, runny nose, and nasal congestion, and TRQI was more effective in relieving cough. Despite the low incidence of adverse events, only a few studies have evaluated the safety of TCMIs. Further studies are needed to better understand TCMIs and to guide their clinical application.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
XG: Conceptualization, Data curation, Writing – original draft, Writing – review & editing. CL: Investigation, Methodology, Project administration, Writing – review & editing. QZ: Supervision, Validation, Writing – review & editing. SH: Methodology, Project administration, Resources, Writing – review & editing.
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2024.1358639/full#supplementary-material
1. Windi R, Efendi F, Qona'ah A, Adnani QES, Ramadhan K, Almutairi WM. Determinants of acute respiratory infection among children under-five years in Indonesia. J Pediatr Nurs. (2021) 60:e54–9. doi: 10.1016/j.pedn.2021.03.010
2. Shapiro DJ, Palmer NP, Bourgeois FT. Factors associated with corticosteroid treatment for pediatric acute respiratory tract infections. J Pediatr Infect Dis Soc. (2021) 10(12):1101–4. doi: 10.1093/jpids/piab082
3. Bhurtel R, Pokhrel RP, Kalakheti B. Acute respiratory infections among under-five children admitted in a tertiary hospital of Nepal: a descriptive cross-sectional study. J Nepal Med Assoc. (2022) 60(245):17–21. doi: 10.31729/jnma.6889
4. Zhu G, Xu D, Zhang Y, Wang T, Zhang L, Gu W, et al. Epidemiological characteristics of four common respiratory viral infections in children. Virol J. (2021) 18(1):10. doi: 10.1186/s12985-020-01475-y
5. González-Ortiz AM, Bernal-Silva S, Comas-García A, Vega-Morúa M, Garrocho-Rangel ME, Noyola DE. Severe respiratory syncytial virus infection in hospitalized children. Arch Med Res. (2019) 50(6):377–83. doi: 10.1016/j.arcmed.2019.10.005
6. Yan YD, Li XQ, Zheng XD, Yang XL, Long T. Application of serum PA, SAA combined with IL-6 detection in diagnosis of acute upper respiratory tract infection in children. J Mol Diagn Ther. (2023) 15(10):1808–11. doi: 10.19930/j.cnki.jmdt.2023.10.023
7. Huo K, Zhao Y, Feng H, Yao M, Sävman K, Wang X, et al. Mortality rates of children aged under five in Henan province, China, 2004–2008. Paediatr Perinat Epidemiol. (2010) 24(4):343–8. doi: 10.1111/j.1365-3016.2010.01126.x
8. Wang SY, Yang X, Wang H, Shao YF. Clinical study on Qingkailing injection combined with cefoxitin in treatment of acute upper respiratory tract infection. Drugs Clin. (2022) 37(12):2786–9. doi: 10.7501/j.issn.1674-5515.2022.12.019
9. Tan NN, Li Y. Meta-analysis of the efficacy of Yanhuning and ribavirin in the treatment of acute upper respiratory tract infection in children. Chin J Drug Eval. (2019) 36(4):316–20.
10. Li TH. Meta-analysis of the treatment of acute upper respiratory tract infections by Reduning and Xiyanping injection. Guide China Med. (2019) 17(16):169–70. doi: 10.15912/j.cnki.gocm.2019.16.129
11. Ren XL, Li AP, Li XH, Wang YP, Wang Q, Zhong ML. Curative effects on Xiyanping injection versus ribavirin for acute upper respiratory infection in children: a meta-analysis. Chin Tradit Pat Med. (2017) 39(3):480–5. doi: 10.3969/j.issn.1001-1528.2017.03.008
12. Song F, Loke YK, Walsh T, Glenny AM, Eastwood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. Br Med J. (2009) 338:b1147. doi: 10.1136/bmj.b1147
13. Heinrich M, Jalil B, Abdel-Tawab M, Echeverria J, Kulić Ž, McGaw LJ, et al. Best practice in the chemical characterisation of extracts used in pharmacological and toxicological research-the ConPhyMP-guidelines. Front Pharmacol. (2022) 13:953205. doi: 10.3389/fphar.2022.953205
14. Rivera D, Allkin R, Obón C, Alcaraz F, Verpoorte R, Heinrich M. What is in a name? The need for accurate scientific nomenclature for plants. J Ethnopharmacol. (2014) 152(3):393–402. doi: 10.1016/j.jep.2013.12.022
15. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. Br Med J. (2015) 350:g7647. doi: 10.1136/bmj.g7647
16. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 Explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. Br Med J. (2021) 372:n160. doi: 10.1136/bmj.n160
17. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
18. Puhan MA, Schünemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. Br Med J. (2014) 349:g5630. doi: 10.1136/bmj.g5630
19. Brignardello-Petersen R, Mustafa RA, Siemieniuk RAC. GRADE Approach to rate the certainty from a network meta-analysis: addressing incoherence. J Clin Epidemiol. (2019) 108:77–85. doi: 10.1016/j.jclinepi.2018.11.025
20. Brignardello-Petersen R, Florez ID, Izcovich A, Santesso N, Hazlewood G, Alhazanni W, et al. GRADE Approach to drawing conclusions from a network meta-analysis using a minimally contextualised framework. Br Med J. (2020) 371:m3900. doi: 10.1136/bmj.m3900
21. Zhao HX. Observation on the efficacy of Xiyanping injection in the treatment of pediatric upper respiratory tract infection. Zhongguancang Med. (2015) 27(10):63–4. doi: 10.3969/j.issn.1672-0369.2015.10.035
22. Li XH. Analysis of the efficacy of Xiyanping injection in the treatment of acute upper respiratory tract infection. Henan Med Res. (2013) 22(5):706–7. doi: 10.3969/j.issn.1004-437X2013.05.028
23. Zheng JX, Hong J. Efficacy and safety evaluation of Xiyanping injection in the treatment of pediatric acute upper respiratory tract infection. Mod J Integr Chin West Med. (2013) 22(5):499–500. 1008-8849(2013)05-0499-02
24. Ma L. Clinical efficacy of Xiyanping injection in the treatment of pediatric acute upper respiratory tract infection. Seek Med Ask Med. (2013) 11(5):31–2. 1672-2523(2013)05-0031-02
25. Zhao RQ, Zhang LH. Comparison of the efficacy of Xiyanping injection and ribavirin in the treatment of pediatric upper respiratory tract infections. Asia-Pac Tradit Med. (2013) 9(11):177–8. 1673-2197(2013)11-0177-01
26. Huang WJ. Clinical application of Xiyanping on pediatric upper respiratory tract infection and nursing experience. Chin Pract Med. (2014) 9(25):204–5. doi: 10.14163/j.cnki.11-5547/r.2014.25.053
27. Zhao YJ, Li NZ, Yang XW. Xiyanping injection combined with ribavirin in the treatment of pediatric acute upper respiratory tract infection with fever in 60 cases. Shaanxi Med J. (2014) 43(10):1405–6. doi: 10.3969/j.issn.1000-7377.2014.10.066
28. Zhang ZH. Observation on the application effect of Xiyanping injection in pediatric upper respiratory tract infection. Tradit Chin Med Herald. (2013) 19(8):104–5. doi: 10.13862/j.cnki.cn43-1446/r.2013.08.026
29. Jiang F. Clinical efficacy analysis of Xiyanping and ribavirin in the treatment of pediatric upper respiratory tract infection. Mod Diagn Treat. (2014) 25(15):3444–5. 1001-8174(2014)15-3444-01
30. Mao LL. Analysis of disease regression of pediatric upper respiratory tract infections treated with Xiyanping. China J Mod Drug Appl. (2021) 15(22):133–5. doi: 10.14164/j.cnki.cn11-5581/r.2021.22.052
31. Ding LH. Clinical efficacy of Xiyanping in the treatment of 50 cases of pediatric upper respiratory tract infection. Everybody Health (Acad Ed). (2014) 8(1):213. 1009-6019(2014)01-0213-01
32. Pan J. Observation on the effect of Xiyanping and ribavirin in the treatment of pediatric upper respiratory tract infection. J Chengde Med Coll. (2017) 34(1):36–7. doi: 10.15921/j.cnki.cyxb.2017.01.017
33. Liu J. Clinical observation on the effect of routine and its combination with Xiyanping in the treatment of pediatric acute upper respiratory tract infection. China Med Guide. (2016) 14(35):255–6. doi: 10.15912/j.cnki.gocm.2016.35.210
34. Liu JR, He Y. Therapeutic efficacy observation of pediatric upper respiratory tract infections treated with Xiyanping injection solution. Everybody Health (Acad Ed). (2016) 10(11):76–7. 1009-6019(2016)17-0076-01
35. Mo GP. Effectiveness of Xiyanping in the treatment of pediatric upper respiratory tract infection with hyperthermia and its effect on symptom disappearance time. Contemp Med. (2016) 22(12):136–7. doi: 10.3969/j.issn.1009-4393.2016.12.090
36. Wu L, Liu JH. Observation on the efficacy of Xiyanping injection in the treatment of pediatric upper respiratory tract infection. Straits Pharm J. (2017) 29(9):187–8. 1006-3765(2017)-09-17949-0187-02
37. Chen HJ. Therapeutic effect of Xiyanping in treating pediatric acute upper respiratory tract infection. Glob Chin Med. (2015) 8(S1):55. 1674-1749(2015)08-0055-01
38. Shen GJ. Analysis of the effect of Xiyanping injection in the treatment of pediatric upper respiratory tract infection. Chin Foreign Med Res. (2017) 15(3):98–100. doi: 10.14033/j.cnki.cfmr.2017.3.054
39. Qin Y. Observations on the efficacy of Xiyanping in the treatment of 42 cases of pediatric upper respiratory tract infection with high fever. Everybody Health (Acad Ed). (2016) 10(5):108–9. 1009-6019(2016)08-0108-02
40. Wang Y. Observation on the efficacy of Xiyanping injection in the treatment of pediatric acute upper respiratory tract infection. J Changzhi Med Coll. (2015) 29(3):203–4. 1006-(2015)03-203-02
41. Zhu LL. Observation on the efficacy of Xiyanping injection in the treatment of pediatric acute upper respiratory tract infection. Chin Pediatr Integr Chin West Med. (2016) 8(5):503–5. doi: 10.3969/j.issn.1674-3865.2016.04.014
42. Liu GF. Application effect of Xiyanping in the treatment of pediatric acute upper respiratory tract infection. China Med Guide. (2015) 13(34):21–2. doi: 10.15912/j.cnki.gocm.2015.34.016
43. Gong TY, Sheng HY. Clinical efficacy observation of Xiyanping injection in the treatment of pediatric upper respiratory tract infection. World Dig Recent Med Inf. (2016) 16(A0):173. doi: 10.3969/j.issn.1671-3141.2016.100.107
44. Gan XH, Chen XL, Huang JB. Observation on the efficacy of Xiyanping in treating pediatric upper respiratory tract infection with high fever. Baiqiu'en Med J. (2014) 12(5):439–40. doi: 10.16485/j.issn.2095-7858.2014.05.053
45. Han CM. Evaluation of clinical effect of Xiyanping injection in the treatment of pediatric acute upper respiratory tract infection. China Prescr Drugs. (2016) 14(9):58–9.
46. Zhang YQ. Observation on the clinical effect of Xiyanping injection in the treatment of acute upper respiratory tract infection in children. North Pharm. (2018) 15(2):151. 672-8351(2018)02-0151-01
47. Yang HY. Observation on the effect of Xiyanping injection in the treatment of pediatric upper respiratory tract infection. Henan Med Res. (2015) 24(2):53–4. doi: 10.3969/j.issn.1004-437X.2015.02.023
48. Zhang J, Wei XL. Observation on the efficacy of Xiyanping in the treatment of pediatric acute upper respiratory tract infection. Clin Med Pract. (2015) 24(1):44–8. doi: 10.16047/j.cnki.cn14-1300/r.2015.01.019
49. Zhu MT. Exploration of the clinical efficacy of Xiyanping injection in the treatment of pediatric upper respiratory tract infection. North Pharm. (2017) 14(6):25. 1672-8351(2017)06-0025-01
50. Yi JT, Liu LQ. Comparison of clinical efficacy of Xiyanping and ribavirin injection in pediatric patients with upper respiratory tract infection. Antiinfect Pharm. (2016) 13(2):432–4. doi: 10.13493/j.issn.1672-7878.2016.02-072
51. Guo YW, Xing JY, Zhang QH. Observation on the efficacy of qingkailing injection in the treatment of acute pediatric upper respiratory tract infection. Zhongguo Nankang Med. (2014) 26(14):94–5. doi: 10.3969/j.issn.1672-0369.2014.14.050
52. Pan H. Qingkailing injection combined with pediatric clearing lung and resolving phlegm granules in the treatment of wind-heat syndrome of pediatric upper respiratory tract infection in 100 cases. Chin Community Physician. (2015) 31(33):78–79+81. doi: 10.3969/j.issn.1007-614x.2015.33.50
53. Liu Q, Zhu B, Wu MH. Comparison of the efficacy of ribavirin and feverfew injection in pediatric patients with acute upper respiratory tract infection. Antiinfect Pharm. (2015) 12(4):532–3. doi: 10.13493/j.issn.1672-7878.2015.04-016
54. Zhang X. Analysis of the efficacy of 196 cases of acute upper respiratory tract infection in children treated with Heat Toxin Ning injection. North Pharm. (2013) 10(8):16–7.
55. Ding P. Treatment of 58 cases of acute viral upper respiratory tract infection in children with heat toxicity. Hunan J Tradit Chin Med. (2013) 29(12):65–6. doi: 10.16808/j.cnki.issn1003-7705.2013.12.033
56. Li H, Liu F, Du LH. Clinical efficacy observation of heat toxin injection in the treatment of pediatric acute upper respiratory tract infection. J Community Med. (2015) 13(18):21–2.
57. Zhou MY, Liu T. Clinical efficacy study of thermotoxin injection in the treatment of acute upper respiratory tract viral infection in children. Drug Eval. (2014) 11(6):35–36+39.
58. Cui BY, Gao KW. Observation on the efficacy of 120 cases of pediatric upper respiratory tract infections treated with Heat Toxin Ning injection. Chin Pediatr Tradit Chin Med West Med. (2013) 5(1):31–2. doi: 10.3969/j.issn.1674-3865.2013.01.010
59. Liu HQ. Analysis of antipyretic efficacy of pyretic injection in the treatment of pediatric acute upper respiratory tract infection. China Med Guide. (2013) 11(27):499–500. doi: 10.15912/j.cnki.gocm.2013.27.398
60. Hu CX, Liang YQ. Observation on the therapeutic effect of Heat Toxin Ning injection in the adjuvant treatment of pediatric acute upper respiratory tract infection. China Disabil Med. (2013) 21(8):291.
61. Liu ZQ, Du QP. Treatment of 208 cases of acute upper respiratory tract infections in children with heat toxin injection. China Med Guide. (2014) 12(13):310–1. doi: 10.15912/j.cnki.gocm.2014.13.024
62. Li MC. Clinical study on the treatment of fever of acute upper respiratory tract infection in pediatric patients with heat toxin. Baiqiu'en Med J. (2014) 12(3):296–7. doi: 10.16485/j.issn.2095-7858.2014.03.007
63. Gu YX, Li P. Clinical efficacy and safety evaluation of thermotoxin in the treatment of pediatric acute upper respiratory tract infection. Chin Pharm. (2013) 16(3):424–5.
64. Miao QJ. Observation on the therapeutic effect of Heat Toxin Ning injection in the treatment of pediatric acute upper respiratory tract infection. Inner Mong J Tradit Chin Med. (2013) 32(32):1–2. doi: 10.16040/j.cnki.cn15-1101.2013.32.195
65. Zhao Q. Clinical evaluation of pyretic injection in the treatment of pediatric viral upper respiratory tract infection. Chin J Mod Drug Appl. (2018) 12(2):73–5. doi: 10.14164/j.cnki.cn11-5581/r.2018.02.043
66. Xu DS JPL, Wang XD. Observation on the therapeutic efficacy of feverfew injection in the treatment of acute upper respiratory tract infection in children. China Prescr Drugs. (2015) 13(6):63–4.
67. Zhuang T. Clinical efficacy observation of thermotoxin injection in the treatment of pediatric viral upper respiratory tract infection. J Pract Heart Cereb Pulm Vasc Dis. (2015) 23(2):110–111+117. doi: 10.3969/j.issn.1008-5971.2015.02.039
68. Fang WF, Liu ZL, Zhao L. Comparison of clinical efficacy of feverfew and ribavirin in the treatment of children with acute upper respiratory tract infection. Chin J Hosp Infect. (2015) 25(15):3572–4. doi: 10.11816/cn.ni.2015-142107
69. Li H, Lu GQ, Yang FL, Chen Q. Observations on the therapeutic effect of thermotoxin injection on pediatric viral upper respiratory tract infection. J Qiqihar Med Coll. (2017) 38(4):446–7.
70. Zhang ZG. Clinical observation on the treatment of pediatric viral upper respiratory tract infections with heat toxin injection. Prim Med Forum. (2019) 23(13):1825–6. doi: 10.19435/j.1672-1721.2019.13.027
71. Zhang J. Analysis of the efficacy of heat toxin injection in the treatment of acute upper respiratory tract infection in children. China Health Stand Admin. (2015) 6(22):152–3. doi: 10.3969/j.issn.1674-9316.2015.22.116
72. Liu Y. Observations on the efficacy and safety of Heat Toxin Ning injection in the treatment of upper respiratory tract infections in children. World Dig Recent Med Inf. (2015) 15(20):65. doi: 10.3969/j.issn.1671-3141.2015.20.051
73. Liu BX. Observations on the efficacy and safety of Heat Toxin Ning injection in the treatment of children’s upper respiratory tract infections. J Qiqihar Med Coll. (2015) 36(16):2416–7.
74. Liu ZY, Zeng R, Huang Z. Observations on the efficacy of fever-toxin injection in the treatment of pediatric upper respiratory tract infection with fever. Hubei J Tradit Chin Med. (2015) 37(6):39–40.
75. Wei YR. Comparison of the effects of influnin and ribavirin in the treatment of pediatric acute upper respiratory tract infection. Chin Contemp Med. (2014) 21(26):96–8.
76. Wang GY. Observation on the efficacy of Yansuning injection in the treatment of pediatric upper respiratory tract infection. Chin J Clin Ration Use Drugs. (2014) 7(3):63–4. doi: 10.15887/j.cnki.13-1389/r.2014.03.091
77. Wu GB, Ma YR, Guan XT. Analysis of the effect of Yansuning injection in the adjuvant treatment of pediatric upper respiratory tract infection in 50 cases. China Pharm Sci. (2013) 3(18):95–7.
78. Sai Q, Yin YY. Clinical observation on the treatment of acute upper respiratory tract infection in pediatric patients with Yansuning and tanshinone. Clin Res Tradit Chin Med. (2015) 7(24):78–9. doi: 10.3969/j.issn.1674-7860-2015.34.037
79. Mu SY. Analysis of the effect of Yansuning injection in the adjuvant treatment of pediatric upper respiratory tract infection in 100 cases. J Clin Exp Med. (2013) 12(4):309–11.
80. Wang XL. Clinical observation on the adjuvant treatment of pediatric acute viral upper respiratory tract infection with Yansuning injection. China Naturopathy. (2019) 27(21):52–3. doi: 10.19621/j.cnki.11-3555/r.2019.2127
81. Miao CJ. Observation on the efficacy of Yansuning injection in the treatment of pediatric acute upper respiratory tract infection. Guangming Tradit Chin Med. (2013) 28(12):2563–4. doi: 10.3969/J.issn.1003-914.2013.12.050
82. Tang LQ. Comparison of the efficacy of influnin and ribavirin in the treatment of pediatric acute upper respiratory tract infection. Chin Foreign Med Treat. (2013) 32(13):115–6. doi: 10.16662/j.cnki.1674-0742.2013.13.099
83. Kuang ZB. Analysis of the effect of injectable influnin in the treatment of pediatric upper respiratory tract infection. Jilin Med J. (2014) 35(30):6693–4.
84. Tang HJ. Treatment of 82 cases of pediatric upper respiratory tract infections with fever by Yansuning injection. Health All (Acad Ed.). (2015) 9(6):160–1.
85. Ji FY. Observation on the efficacy of Yansuning injection in the treatment of acute upper respiratory tract infection in children. Hebei Med. (2014) 20(1):141–3. doi: 10.3969/j.issn.1006-6233.2014.01.053
86. Liu AP. Analysis of the efficacy and safety of Yansuning injection in the treatment of pediatric upper respiratory tract infection. Contemp Med. (2014) 20(19):134–5. doi: 10.3969/j.issn.1009-4393.2014.19.096
87. Zhang JJ. Comparison of the efficacy of Yansuning and ribavirin in the treatment of pediatric acute upper respiratory tract infection. China Rural Health. (2017) (14):87.
88. Bao L, Wu HN, Yuan HZ, Tang FL. Comparative observation on the effect of Yansuning and ribavirin in the treatment of pediatric severe upper respiratory tract infection. People’s Mil Med. (2016) 59(6):587–8.
89. Zhao N. Efficacy observation and nursing care of Yansuning injection in pediatric upper respiratory tract infection. Chin Med Clin Res. (2015) 7(24):80–1. doi: 10.3969/j.issn.1674-7850.2015.24.038
90. Lu HM, Zhao LQ, Cai HX, Liang JX. Clinical efficacy of Yansuning injection combined with ribavirin in the treatment of pediatric acute viral upper respiratory tract infection. Chin J Clin Ration Use Drugs. (2019) 12(33):81–2. doi: 10.15887/j.cnki.13-1389/r.2019.33.040
91. Zhu YM. Observation on the efficacy of Yansuning injection combined with ribavirin in the treatment of pediatric acute viral upper respiratory tract infection. Hebei J Tradit Chin Med. (2017) 39(9):1332–4. doi: 10.3969/j.issn.1002-2619.2017.09.013
92. Li XL. Analysis of the effect of influnin in the treatment of pediatric acute upper respiratory tract infection. J Clin Med Lit. (2018) 5(55):80–2. doi: 10.16281/j.cnki.jocml.2018.55.069
93. Yang HG. Clinical efficacy observation of 86 cases of acute upper respiratory tract infection in pediatric patients treated with Yanhuning. China Pract Med. (2015) 10(11):145–6. doi: 10.14163/j.cnki.11-5547/r.2015.11.099
94. Li JH. Clinical analysis of yansuning injection combined with ribavirin in the treatment of acute upper respiratory tract infection in children. Mod Diagn Treat. (2015) 26(1):124–5.
95. Hao BF, Li PF. Observations on the efficacy of injectable Yanhuning in the treatment of pediatric acute upper respiratory tract infection. Clin Med Res Pract. (2017) 2(24):116–7. doi: 10.19347/j.cnki.2096-1413.201724057
96. Yang HZ. Application of ribavirin combined with Yanhuning injection in the treatment of children with acute-phase viral upper respiratory tract infection. Pract Chin West Med Comb Clin. (2023) 23(17):110–2. doi: 10.13638/j.issn.1671-4040.2023.17.031
97. Cui BZ, Chen XT, Duan MT. Therapeutic effect of phlegm-heat-clearing injection in the treatment of upper respiratory tract infection in children. China Pract Med. (2013) 8(16):70–1. doi: 10.14163/j.cnki.11-5547/r.2013.16.010
98. Wang CY. Analysis of clinical effects of phlegm-heat clearing in the treatment of acute upper respiratory tract infection in children. Chin J Mod Drug Appl. (2014) 8(10):125–6. doi: 10.14164/j.cnki.cn11-5581/r.2014.10.024
99. Wan M. Observations on the treatment of pediatric upper respiratory tract infection with phlegm-heat clearing combined with ribavirin. Jiangxi Med. (2016) 51(10):1093–5. doi: 10.3969/j.issn.1006-2238.2016.10.040
100. Kong SS. Clinical observation on acute upper respiratory tract infection in pediatric patients with phlegm-heat-clearing injection. Shenzhen J Integr Chin West Med. (2018) 28(1):34–5. doi: 10.16458/j.cnki.1007-0893.2018.01.017
101. Guo H, Song YS. Clinical efficacy of phlegm-heat clear injection in the treatment of pediatric upper respiratory tract infection with fever. Chin J Mod Drug Appl. (2017) 11(11):130–1. doi: 10.14164/j.cnki.cn11-5581/r.2017.11.065
102. Hu Y, Hu Y, Sun XY. Progress of pharmacological effects and application of Yansunin. Specialty Res. (2023) 45(3):150–6. doi: 10.16720/j.cnki.tcyj.2023.092
103. Zhang ZZ, Liang Y, Cui Y, Cao JL. Progress of clinical application and safety of Qingkailing injection. Jilin Chinese Edicine. (2017) 37(03):320–4. doi: 10.13463/j.cnki.jlzyy.2017.03.030
104. Ma WN, Meng CJ. Mechanism of action and progress of clinical application of Qingkailing. Med Rev. (2016) 22(23):4664–7. doi: 10.3969/j.issn.1006-2084.2016.23.023
105. Feng SQ, Wu X, Tan YH. Research progress of histamine and histamine receptors. Chin J Lung Dis. (2015) 8(2):234–7. 3877/cma.j.issn.1674-6902.2015.02.023105
106. Kinbara M, Bando K, Shiraishi D, Kuroishi T, Nagai Y, Ohtsu H, et al. Mast cell histamine-mediated transient inflammation following exposure to nickel promotes nickel allergy in mice. Exp Dermatol. (2016) 25(6):466–71. doi: 10.1111/exd.12985
107. Li H, Zhao LF, Hao YQ, Yin L, Zhao YC, Han DW. Effects of antihistamine treatment on mast cell infiltration and expression of c-kit and SCF in rats with experimental hepatitis. Chin J Pathophysiol. (2013) 29(9):1609–14. doi: 10.3969/j.issn.1000-4718.2013.09.012
108. Jiang YH, Ni SH, Zhang JJ, Huang J, Liu HH, Xu L, et al. Meta-analysis and trial sequential analysis of phlegm-heat clear injection for the treatment of severe pneumonia in the elderly. Chin J Tradit Chin Med. (2023) 21:1–14. doi: 10.19540/j.cnki.cjcmm.20231025.501
109. Hu C, Li J, Tan Y, Liu Y, Bai C, Gao J, et al. Tanreqing injection attenuates macrophage activation and the inflammatory response via the lncRNA-SNHG1/HMGB1 axis in lipopolysaccharide-induced acute lung injury. Front Immunol. (2022) 13:820718. doi: 10.3389/fimmu.2022.820718
110. Liu H, Qu NN. Clinical efficacy of phlegm-heat-clearing injection combined with Western medicine in the treatment of elderly patients with multi-drug-resistant bacterial pneumonia. Chin J Exp Tradit Med Formulae. (2021) 27(24):125–30. doi: 10.13422/j.cnki.syfjx.20212495
111. Che S, Zhou N, Liu Y, Xie J, Liu E. Andrographolide exerts anti-respiratory syncytial virus activity by up-regulating heme oxygenase-1 independent of interferon responses in human airway epithelial cells. Mol Biol Rep. (2023) 50(5):4261–72. doi: 10.1007/s11033-023-08346-z
112. Wang SY, Cheng P, Xie N. Pharmacodynamic study on in vitro antiinfluenza virus resistance of Andrographis paniculata lactone sulfonate. Zhongnan Pharm. (2013) 11(5):331–4. doi: 10.7539/j.issn.1672-2981.2013.05.003
113. Ping J, Wang SY, Xie N. In vitro anti-adenoviral pharmacodynamics of Andrographis paniculata lactone sulfonate. Chin J Exp Formulas. (2012) 18(21):175–9. doi: 10.13422/j.cnki.syfjx.2012.21.070
114. Zhang LL, Bao M, Yang WF. Synergistic inhibition of methicillin-resistant Staphylococcus aureus by andrographolide and cefoxitin. World Sci Technol Mod Tradit Chin Med. (2020) 22(7):2556–62. doi: 10.11842/wst.20190430002
115. Zhang L, Wen B, Bao M. Andrographolide Sulfonate Is a Promising Treatment to Combat Methicillin-resistant Staphylococcus aureus and Its Biofilms. Front Pharmacol. (2021) 12:720685. Published 2021 Sep 16. doi: 10.3389/fphar.2021.720685
116. Gu X, Gao R, Li Y, Liu J, Wu Y, Xu H. Combination effect of azithromycin with TCM preparation Xiyanping injection against Klebsiella pneumoniae infection in rats. Phytomedicine. (2022) 104:154332. doi: 10.1016/j.phymed.2022.154332
117. Ding Y, Liu WY, Liu JF. A multicenter, double-blind, randomized controlled trial on the efficacy and safety of Xiyanping injection in the treatment of acute bronchitis. China J Basic Chin Med. (2023) 29(3):432–7. doi: 10.19945/j.cnki.issn.1006-3250.2023.03.032
118. Sun MH, Lv J, Zhang YL, Wang ZF, Yang J, Xie YM. Systematic evaluation and meta-analysis of the efficacy and safety of Xiyanping injection in the treatment of pediatric acute bronchitis. Chin J Tradit Chin Med. (2019) 44(20):4387–96. doi: 10.19540/j.cnki.cjcmm.20190730.501
119. Tan XY, Mou BH, Ma LF. Systematic evaluation of clinical efficacy and safety of Xiyanping and Yansuning in the treatment of pediatric acute bronchitis. J Southwest Med Univ. (2021) 44(1):1–7. doi: 10.3969/j.issn.2096-3351.2021.01.001
120. Deng JX, Wang ZF, Xie YM. Post-marketing safety re-evaluation of Xiyanping injection. J Adverse Drug React. (2018) 20(1):15–22.
121. Liu L, Men P, Zou BZ, Cao MM, Zhao RS, Yang YH. Evidence-based evaluation of feverfew injection for the treatment of respiratory system infections. Eval Anal Hosp Medicat China. (2021) 21(2):185–188+194. doi: 10.14009/j.issn.1672-2124.2021.02.015
122. Zhang YF, Lin C, Zhang HJ. Efficacy and effect on IFN-γ, TNF-ɑ, and IL-6 of Mycoplasma pneumonia in children adjuvanted with thermotoxin. J Binzhou Med Coll. (2021) 44(1):53–6. doi: 10.19739/j.cnki.issn1001-9510.2021.01.011
123. Jia-Xing W, Chao-Yi L, Wei-Ya C, Yi-Jun C, Chun-Yu L, Fei-Fei Y. The pulmonary biopharmaceutics and anti-inflammatory effects after intratracheal and intravenous administration of Re-Du-Ning njection. Biomed Pharmacother. (2023) 160:114335. doi: 10.1016/j.biopha.2023.114335
124. Yang C, Song C, Wang Y, Zhou W, Zheng W, Zhou H, et al. Re-Du-Ning injection ameliorates radiationinduced pneumonitis and fibrosis by inhibiting AIM2 inflammasome and epithelialmesenchymal transition. Phytomedicine. (2022) 102:154184. doi: 10.1016/j.phymed.2022.154184
125. David S, Cunningham R. Echinacea for the prevention and treatment of upper respiratory tract infections: a systematic review and meta-analysis. Complementary Ther Med. (2019) 44:18–26. doi: 10.1016/j.ctim.2019.03.011
126. Tan NN, Li Y. Meta-analysis of the efficacy of influnin and ribavirin in the treatment of pediatric acute upper respiratory tract infection. Drug Eval China. (2019) 36(4):316–20.
127. Lin MJ, Wu JR, Zhang XM, Liu S, Zhang B. A study on the clinical evaluation of Qingkailing injection for the treatment of acute upper respiratory tract infection based on meta-analysis. Chin J Pharmacoepidemiol. (2016) 25(12):763–72. doi: 10.19960/j.cnki.issn1005-0698.2016.12.006
128. Li H, Ren LT, Li JY, Guo YY, Wei ZY, Chen H, et al. Network meta-analysis of four commonly used proprietary Chinese medicines for the adjunctive treatment of pediatric acute upper respiratory tract infections. Eval Anal Hosp Medicat China. (2022) 22(1):77–82. doi: 10.14009/j.issn.1672-2124.2022.01.016
129. Ding Y, Qu CH. Characterization and significance of various inflammatory indexes in peripheral blood in children with acute upper respiratory tract infection of unknown pathogen. Lab Med Clin. (2023) 20(6):823–7. doi: 10.3969/j.issn.1672-9455.2023.06.026
130. Hou J, Wang SP, Zhu BH, Li B. Clinical efficacy of feverfew injection combined with oseltamivir phosphate granules in pediatric patients with acute viral upper respiratory tract infection. Propr Chin Med. (2023) 45(10):3261–5. doi: 10.3969/j.issn.1001-1528.2023.10.017
131. Wang SY, Yang X, Wang H, Shao YF. Clinical study of Qingkailing injection combined with cefoxitin in the treatment of acute upper respiratory tract infection. Mod Drugs Clin. (2022) 37(12):2786–9. doi: 10.7501/j.issn.1674-5515.2022.12.019
132. Li W. Application actuality of polysorbate 80 (twain 80) in traditional Chinese medicine injections. Chin J Ethnomedicine Ethnopharmacy. (2018) 27(23):69–72.
133. Li H, Ma S, Wang L. Current situation and thinking on the safety of traditional Chinese medicine injection and its asepsis guarantee system. Chin Tradit Pat Med. (2022) 44(9):2939–43.
134. Heinrich M, Appendino G, Efferth T, Fürst R, Izzo AA, Kayser O, et al. Best practice in research-overcoming common challenges in phytopharmacological research. J Ethnopharmacol. (2020) 246:112230. doi: 10.1016/j.jep.2019.112230
Keywords: acute upper respiratory tract infection in children, traditional Chinese medicine, randomized controlled trial, network meta-analysis, injection
Citation: Guo X, Liu C, Zhao Q and Huang S (2024) Efficacy of five different traditional Chinese medicine injections in acute upper respiratory tract infection in children: a network meta-analysis and systematic review. Front. Pediatr. 12:1358639. doi: 10.3389/fped.2024.1358639
Received: 20 December 2023; Accepted: 10 May 2024;
Published: 10 June 2024.
Edited by:
Francesca Santamaria, University of Naples Federico II, Italy© 2024 Guo, Liu, Zhao and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiong Zhao, emhhb3Fpb25nbEAxMjYuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.